1. Introduction
Non-toxic plant extracts with high dying power and ample colour are excellent for use in colouring food, products, cosmetics, pharmaceuticals and textiles. One of these plants, annatto (
Bixa orellana) which originated from Central America and now widely grown throughout the tropical regions of the world, including West Africa, has its seeds as the raw material for pigment extraction [
15]. The chemical constituents of the pigment include bixin which represents more than 80% of the total fat-soluble carotenoids, norbixin and norbixinate, whose levels are variable according to seeds maturation [
16]. Bixin is responsible for imparting reddishness and norbixin yellow.
The main product from
B. orellana is an organic dye present in the seed coat called "annatto," lipid-soluble and widely used in the food industry for its red to orange-yellow colours (cheese, butter, oils, margarine, ice-cream, pastries) [
12]. Next to caramel, it is the world's second most important food colourant. Besides providing an attractive colour to meat and other dishes, it also imparts a subtle and distinctive flavour. In the cosmetic industry, it finds use in hair, nail and soap products, and also in the many of the household products – floor wax, shoe polish, russet leather, wood stains [
14].
The dye obtained from the pulp of the
B. orellana seed is used all over the world as a red-orange dye for colouring rice, cheeses, soft drinks, oil, butter, and soup [
16].
Bixin was isolated for the first time from the seeds of
Bixa orellana in 1875 and in 1961 its complete chemical structure and stereochemistry were determined by
1H and
13C-NMR [
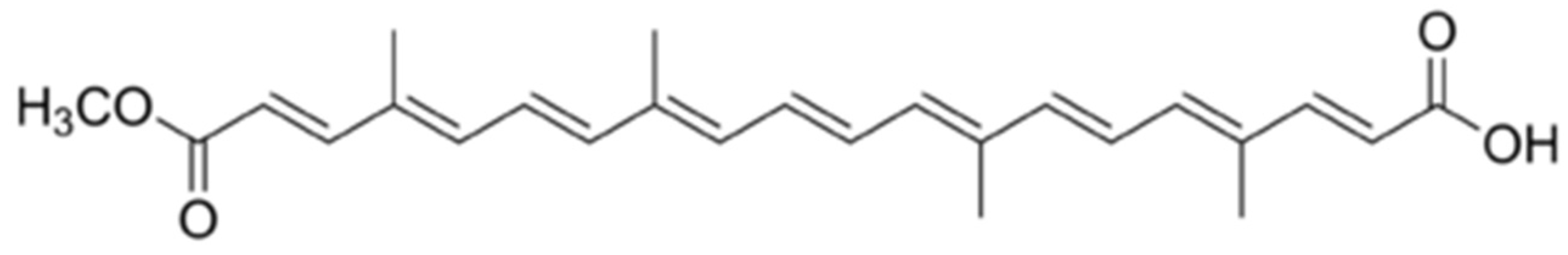
6]. Bixin as shown in
Figure 2.1 belongs to the small class of natural apocarotenoids, whose formation occurs by the oxidative degradation of C40 carotenoids [
6].
Bixin (2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-methoxy-4,8,13,17-tetramethyl-20-oxoicosa 2,4,6,8,10,12,14,16,18-nonaenoic acid) is a polyunsaturated, norcarotenoid, red dye from the main fruit of annatto, its seeds reduced to powder are widely used to colour food and sunscreen [
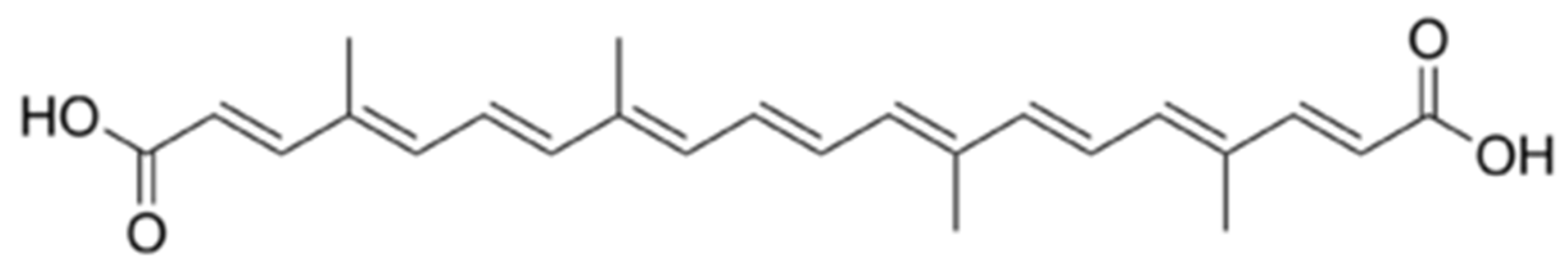
3]. The annatto seeds contain about 5% pigment, which consist of 70-80% of bixin. The bixin is soluble in fats but insoluble in water. When exposed to alkalis, the methyl ester is hydrolyzed and produces the norbixin dicarboxylic acid, a water soluble derivative. Norbixin (
Figure 2.2) is a demethylated derivative of bixin and although it is a naturally occurring compound, it is almost always referred to as a saponification product of bixin. [
4]. This is the form used for commercial purposes. It is a chemically unstable compound when isolated and is converted via It is a chemically unstable compound when isolated and is isomerization, in bixin trans-(β-bixin), the cis-trans isomer of bixin. Carotenoids bixin and norbixin have two stereo configurations, i.e., cis and trans. In extracts, under normal conditions, the cis-bixin or cis-norbixin are more unstable. The cis-bixin or cis-norbixin solution under heating are partially converted into the trans configuration, which is more stable and known as isobixin and isonorbixin [
3].
Currently, more than two dozen substances have been isolated from the seeds of Bixa orellana. Besides bixin and norbixin, other compounds such as isobixin, beta-carotene, cryptoxanthin, lutein, zeaxanthin, orellin, bixein, bixol, crocetin, ishwarane, ellagic acid, salicylic acid, threonine, tomentosic acid, tryptophan, and phenylalanine have been found in the seeds of annatto [
6]. In addition, the following compounds, in their respective concentrations, are found in these seeds: 40 to 45% cellulose, 3.5 to 5.5% sugars, 0.3 to 0.9% essential oils, 3% fixed oils, 1.0 to 4.5% pigments, and 13 to 16 % proteins and alpha and beta-carotene, as well as tannins and saponins [
10].
2. Materials and Methods
Bixa orellana seed and pod were harvested at Fiwasaye Girls’ Grammar School, Akure. Identification was done by the Biology Department of The Federal University of Technology, Akure. The samples were washed with distilled water to remove dusts and air dried for 4 days. The dried samples were blended and kept for analysis.
Approximately 200 g of the seed sample was extracted for dye by Soxhlet extraction method. Approximately 1000 ml of commercial ethanol degree at 96% ethanol was used for the extraction. Temperature of the Soxhlet Apparatus was maintained between 50
0C and 60
0C [
9]. Several cycles of solvent were run so as to extract all the dye present in the seed. The extract obtained was distilled of ethanol and concentrated.
Approximately 50 ml of n-hexane was added to the concentrate with rigorous stirring for 30 minutes and left to settle for 4 hours to remove fat after which filtration was carried out. The dye crystals were obtained as the residue. Test sample was prepared for UV visible spectroscopy which was done at the Central Laboratory of The Federal University of Technology, Akure.
Recrystallization of the crystal was done using chloroform and ethanol. The dye crystals was dissolved in chloroform in a 250 ml beaker at room temperature and filtered when warmed. The filtrate was left to cool and the dye which crystallised out was obtained by filtration as the residue.
Ethanol was used to recrystallize some of the dye by dissolving in ethanol in a 250 ml beaker and heating on water bath at 700C for 20 minutes. Filtration was carried out using Whatmann filter paper while hot. The filtrate was allowed to cool and the dye crystallised out. Filtration was also carried out to obtain the dye as residue. Test sample of the recrystallized dye was analysed for UV Spectroscopy at the Central Laboratory of The Federal University of Technology, Akure while the FTIR was done at Ladoke Akintola Akintola University of Technology, Ogbomoso.
The percentage yield of the dye was calculated at the end of the recrystallization using the equation below:
To about 2g of the dye extract was added 20 ml of 0.1M NaOH and the mixture was heated on water bath to hydrolyse the colouring matter and cooled. The aqueous solution was filtered and acidified with concentrated hydrochloric acid, a few drops slowly from sides to precipitate norbixin. The precipitate is filtered, washed, dried, and milled to give a granular powder [
13]. The norbixin was prepared for characterisation by UV Spectroscopy and FTIR. The UV Spectroscopy was done at the Central Laboratory of The Federal University of Technology, Akure while the FTIR was carried out at Ladoke Akintola University of Technology, Ogbomoso.
A thin layer chromatography plate was activated with very thick film to 500 μm with silica gel and kept in hot air oven for one hour at 1100 C. A 10% solution of the powder dye extract was dissolved in 95% ethanol and applied to the plate and allowed it to dry. The chromatography was developed using a solvent comprising a mixture of benzene, ethyl acetate methanol in the ratio 3:4:2 by volume until solvent front has ascended about 10 cm.
Solubility test and colour change characteristics was carried out on the dye with water, acetone, acetic acid, hexane, carbonated soft drink, thinner, palm wine, ethanol, sodium hydroxide and hydrochloric acid. About 0.1 g of dye was used with 10 ml each of test reagents at room temperature.
3. Results and Discussion
The Bixa seed was well grown and readily available at the chosen site. This shows that
Bixa orellana is a plant that thrives well in tropical region having originated from South and Central America and widely grown in Africa over the years [
6].
Bixa orellana dye obtained as a solid is red powder with a melting point of 190
0C and rarely soluble in water as shown in
Table 3.1. The poor solubility in water is due to the small proportion of norbixin, the water soluble constituent in comparison with bixin which is fat soluble. The available structures for the two compounds revealed that norbixin has two hydroxyl end-groups while bixin has just one of its hydroxyls methylated. A percentage yield of 4.3% was obtained by the Soxhlet extraction used.
Table 3.
1. Physical Properties of Extracted Bixa orellana Dye.
Table 3.
1. Physical Properties of Extracted Bixa orellana Dye.
| Dye |
Bixa orellana |
| Appearance |
Red powder |
| Solubility in Water |
Slightly |
| Melting Point |
1900C |
| Yield |
4.3% |
As given in
Table 3.2 below, the dye was soluble in only ethanol, thinner, acid and alkaline solutions. The solubility of the dye in the mentioned solvents can be accounted for by the polarisation, though weak of the bixin and norbixin molecules. The carboxylic ends of the two compounds ionize partially to form polar ends which associate with polar compounds. The hydroxyl in the carboxylic acid is responsible for its solubility in ethanol. Its solubility in ethanol explains why ethanol is a suitable solvent for its extraction and a medium for dissolution its solubility in alkaline and acidic medium suggests that the dye is pH sensitive.
It can therefore be used as an acid-base indicator. Annatto extract, even as a food dye is insoluble in carbonated drink and palm wine. This leads to a conclusion that the dye may not be used directly on food wherein it is insoluble, but may be dissolved first in a food grade soluble medium before addition. The solubility in paint thinner also suggests that paint thinner can be used to thin or clean up the dye when applied to hard surfaces such as wood. Paint thinner is a non-polar organic solvent hence able to dissolve the non-polar Bixa orellana dye.
Table 3.
2: Solubility and Colour Change Characteristics of Extracted Bixa orellana Dye.
Table 3.
2: Solubility and Colour Change Characteristics of Extracted Bixa orellana Dye.
| S/N |
Reagent/Solvent |
Solubility/Colour |
| 1 |
Water |
Slightly soluble |
| 2 |
Ethanol |
Light yellow |
| 3 |
n-hexane |
Insoluble |
| 4 |
Palm wine |
Insoluble |
| 5 |
Thinner |
Soluble |
| 6 |
Carbonated drink |
Insoluble |
| 7 |
Aq. Sodium hydroxide |
Light yellow |
| 8 |
Hydrochloric acid |
Greenish yellow |
| 9 |
Acetic acid |
Intense yellow |
As shown in
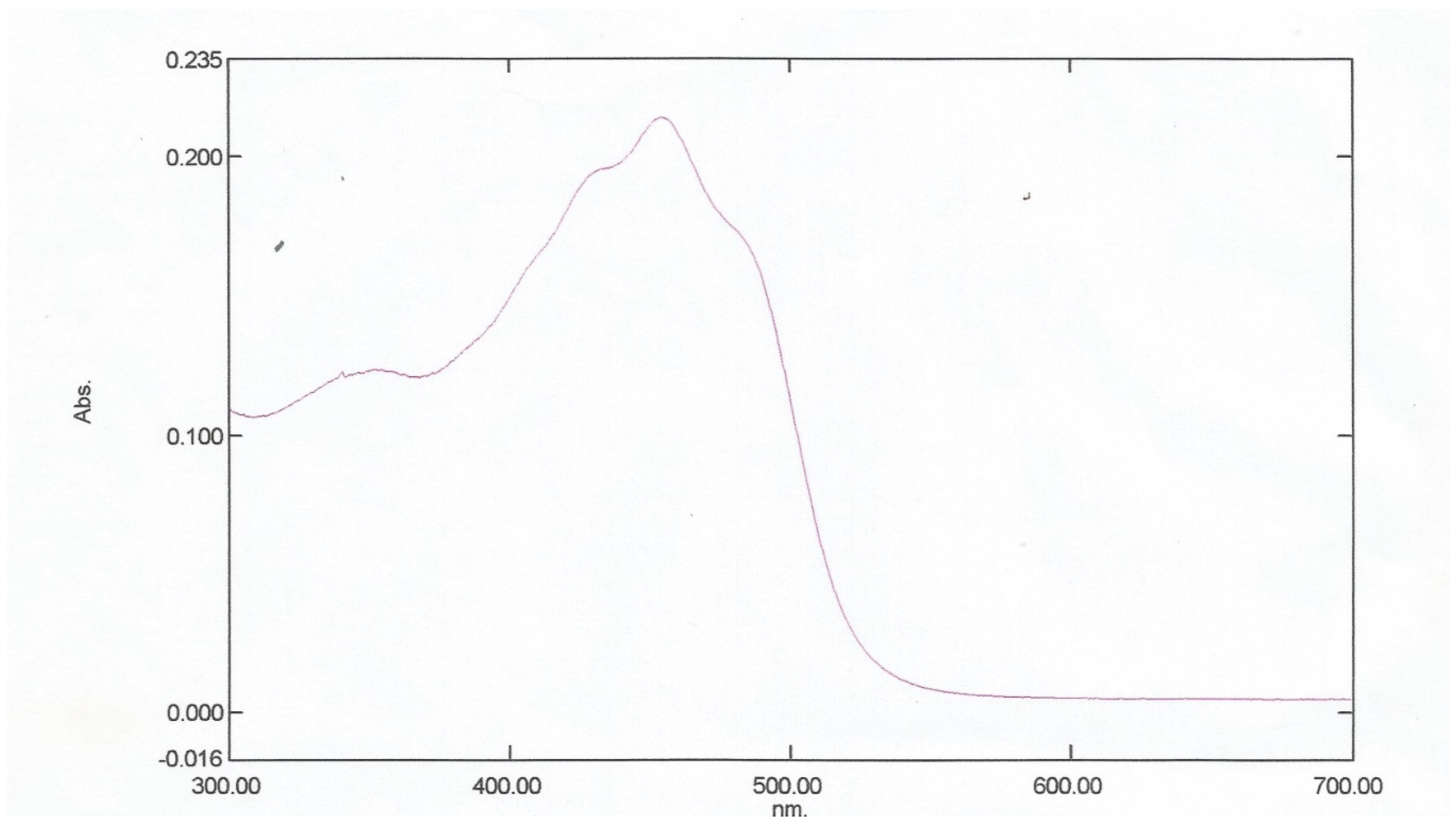
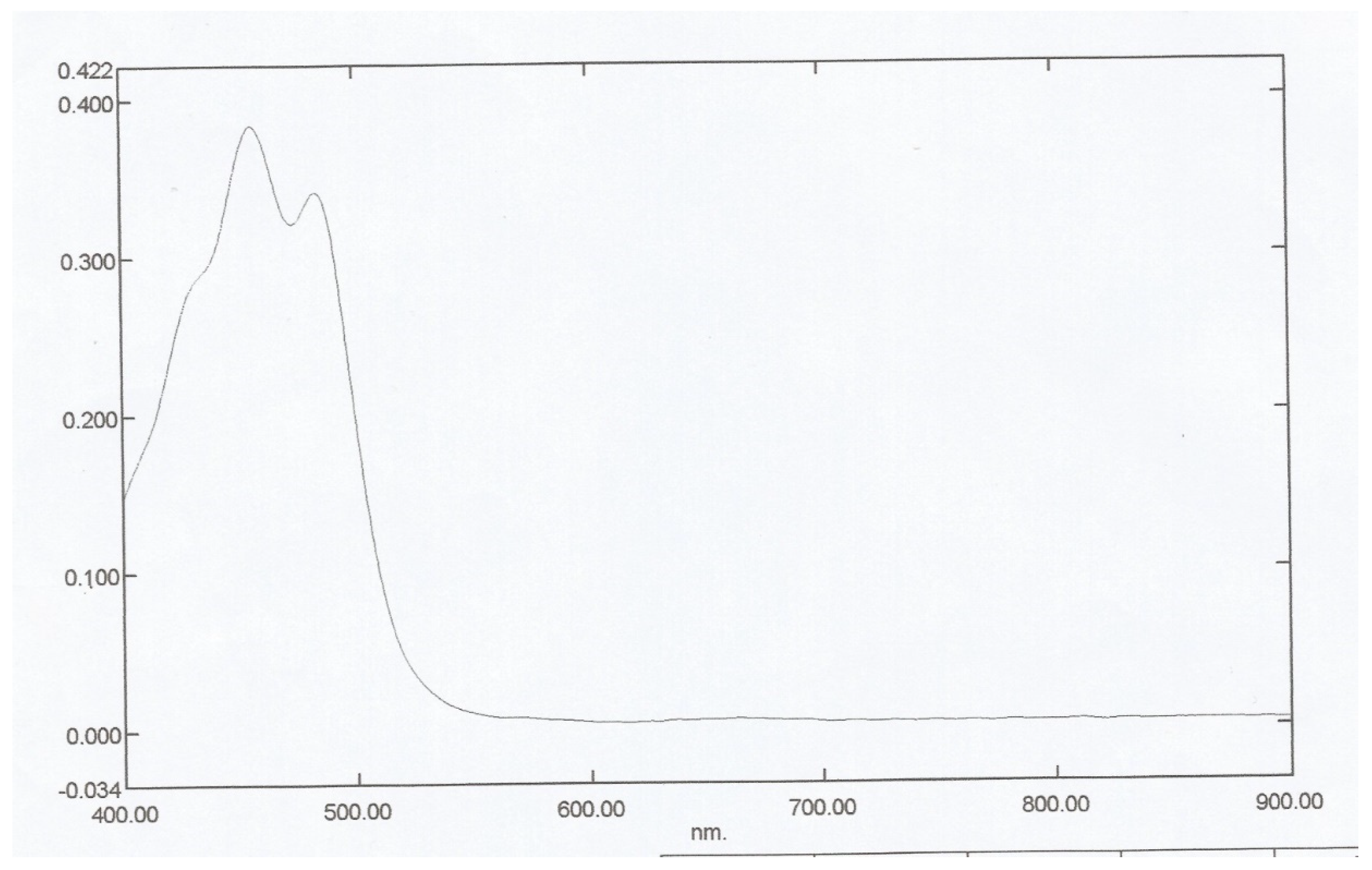
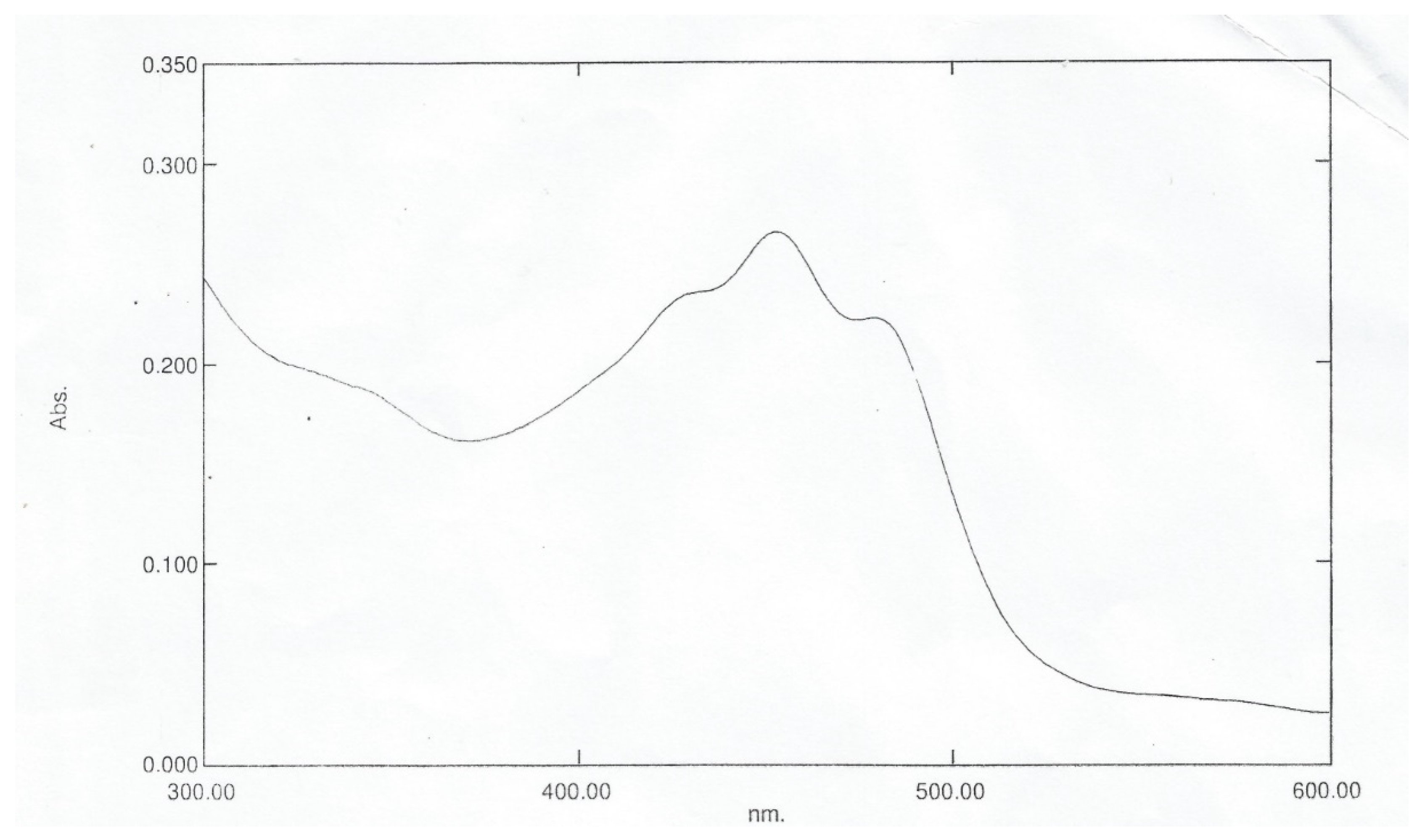
Figure 3.1, maximum absorption of the Bixa orellana dye extracted occurs at wavelength 454nm. Adetuyi [
1] recorded a similar maximum absorption wavelength of 454nm. In
Figure 3.2, the ethanol recrystallized dye had maximum absorptions at 457nm and 484nm in agreement with previous work done in literatures [1-2] [
7]. The chloroform recrystallized has maximum absorptions at 471nm and 503nm as seen in
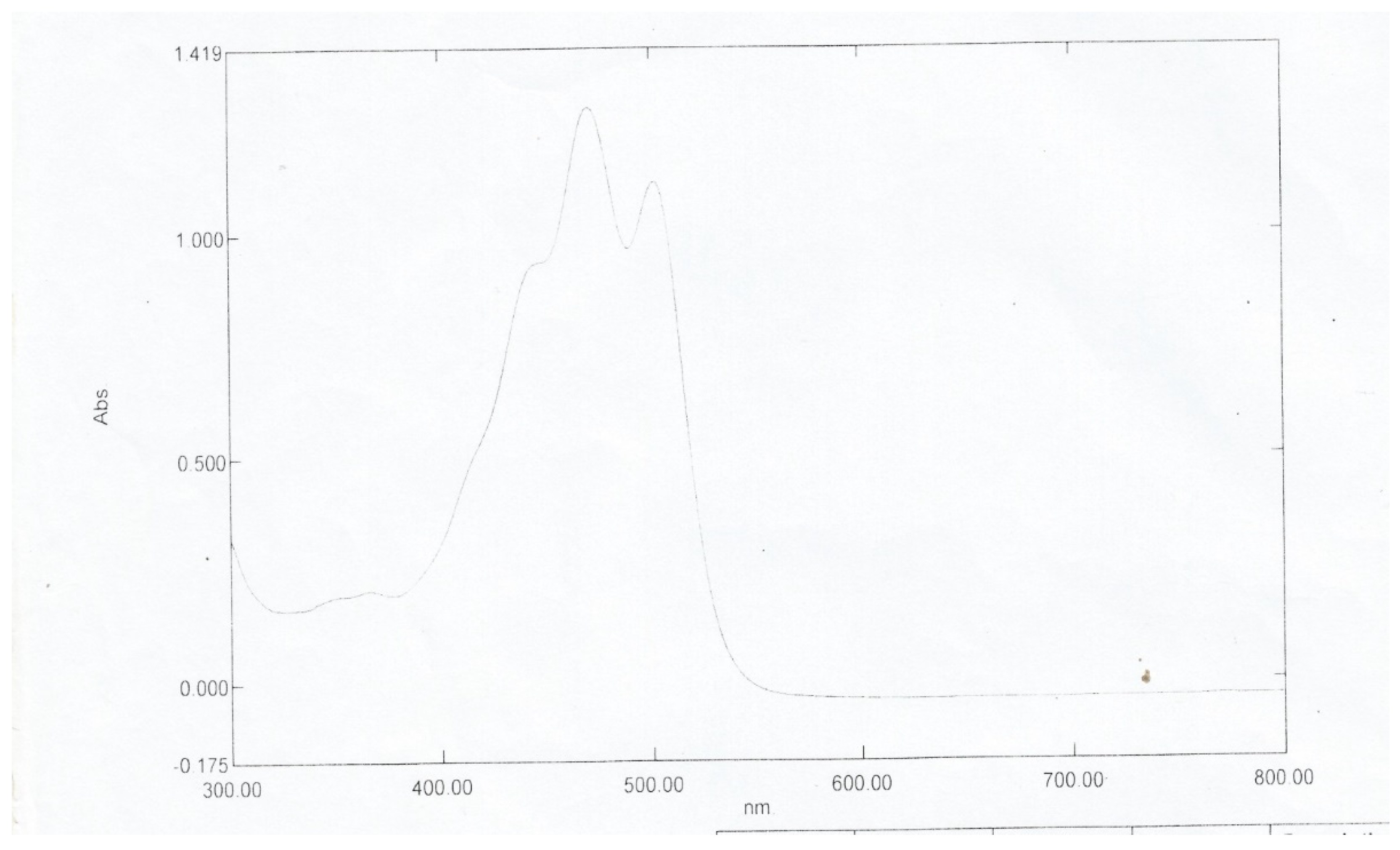
Figure 3.3. The close maximum wavelengths of 484nm and 471nm with ethanol and chloroform recrystallized respectively revealed that either solvent can be used to purify the dye sample. Maximum absorption of the Norbixin compound occurs at 453nm as given by
Figure 3.4 [
11]. This value, close to the 457nm of the pure dye, shows that Norbixin is a significant constituent of the colour offered by
Bixa orellana dye.
Figure 3.
1: UV Spectrum of Extracted Bixa orellana Dye.
Figure 3.
1: UV Spectrum of Extracted Bixa orellana Dye.
Figure 3.
2: UV Spectrum of Ethanol Recrystallised Bixa orellana Dye.
Figure 3.
2: UV Spectrum of Ethanol Recrystallised Bixa orellana Dye.
Figure 3.
3: UV spectrum of Chloroform Recrystallised Bixa orellana Dye.
Figure 3.
3: UV spectrum of Chloroform Recrystallised Bixa orellana Dye.
Figure 3.
4: UV Spectrum for Norbixin.
Figure 3.
4: UV Spectrum for Norbixin.
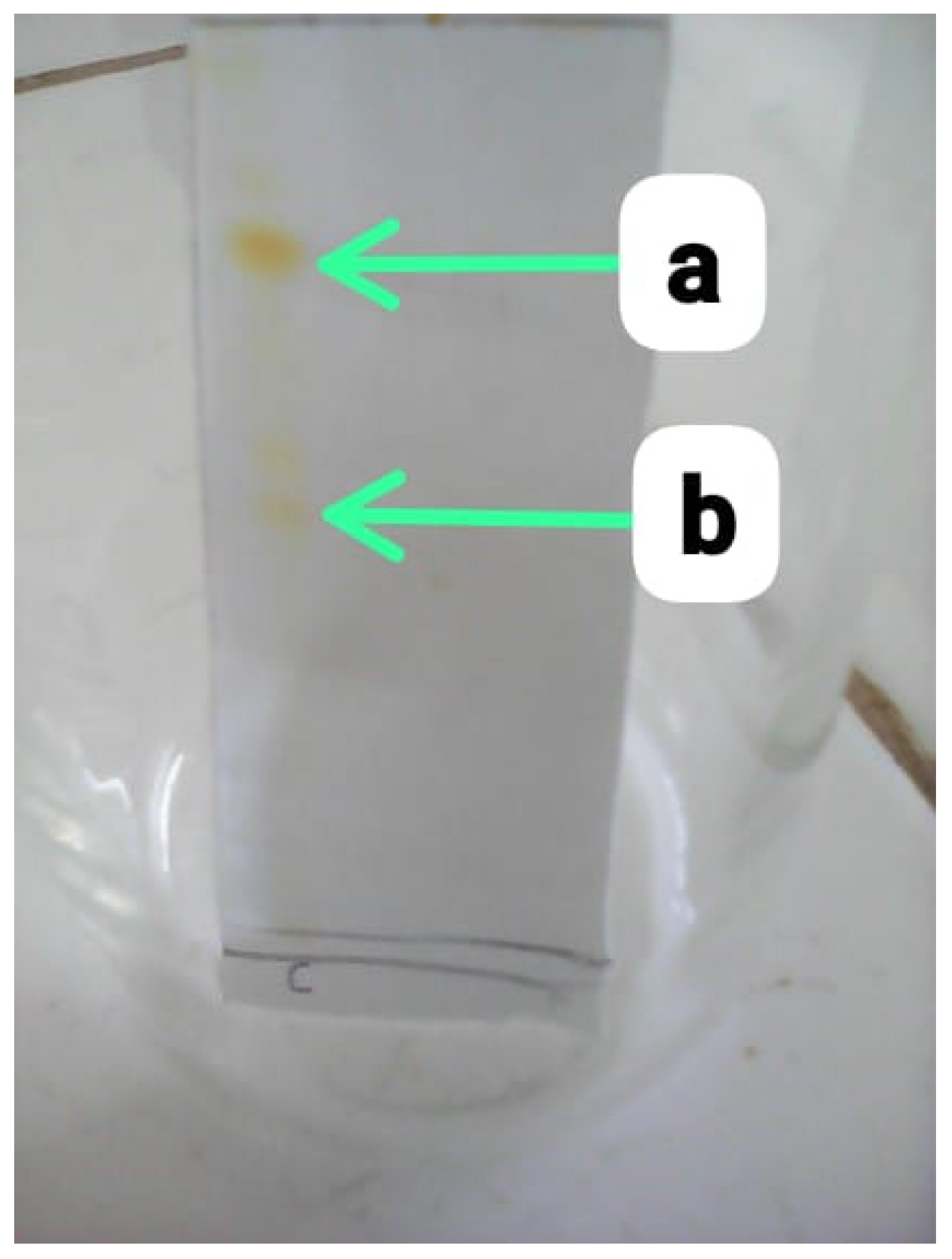
Two distinct yellow spots were seen on the plate (
Figure 3.5). The two spots correspond to the bixin and norbixin which are the pigment constituents of
Bixa orellana. The different elution of the constituents of on the plate is as a result of the variations in their polarities. The R
f values of about 0.52 and 0.46 respectively corresponding to bixin and norbixin respectively [
5,
8] was calculated as shown in
Table 3.3. The different R
f values further proves the existence of Bixin and Norbixin as the major pigment constituents of
Bixa orellana. The benzene-ethyl acetate-methanol solvents developed for the chromatography is able to elute the bixin higher than the norbixin because the bixin is less polar than the norbixin.
Figure 3.
5: TLC for Extracted Bixa orellana Dye. (a): Bixin spot (b): Norbixin
Figure 3.
5: TLC for Extracted Bixa orellana Dye. (a): Bixin spot (b): Norbixin
Table 3.
3: Rf values for Bixin and Norbixin.
Table 3.
3: Rf values for Bixin and Norbixin.
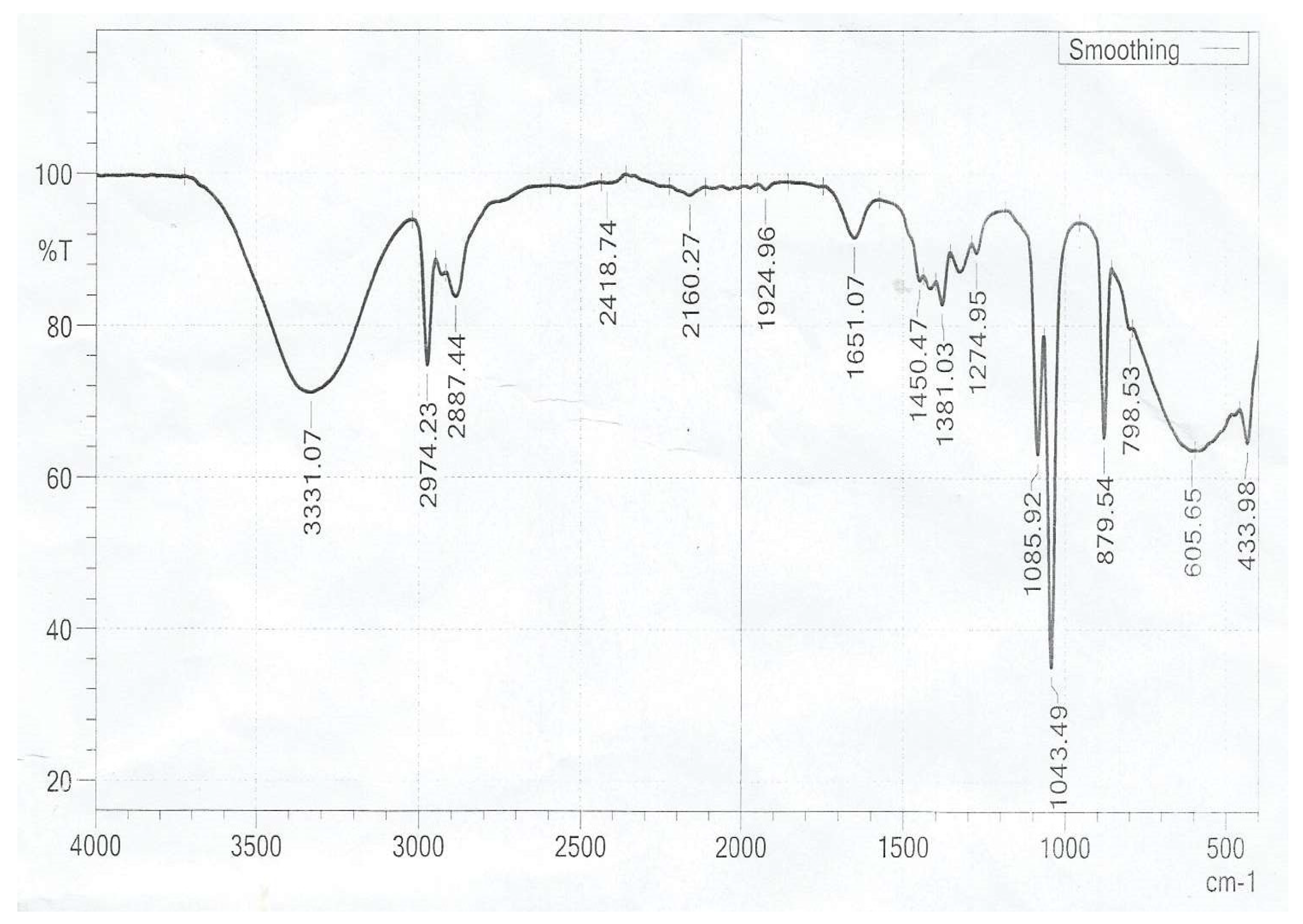
The FTIR spectrum of annatto extract (Fig. 3.5) shows the following bands: at 3331 cm–1 the -O-H stretching vibration is observed, at 2 974 cm–1 and 2 887 cm–1 the H-C-H bending vibration, at 1651 cm–1 the carboxylic C=O group, at 1 450 cm-1 the alkene C=C streching, at 1 381cm–1 the C–H bending of the methyl groups, at 1 274 cm–1 the C=O streching, at 1085 cm–1 and 1043cm–1 symmetric and asymmetric vibrations of the C–O–C ester group, 879 cm–1 the methylene rocking vibration of trans-carotenoid, at 846 cm–1 the coupling of the -C-O stretching vibrations and at 798 cm–1 the methylene rocking vibration of cis-carotenoid.
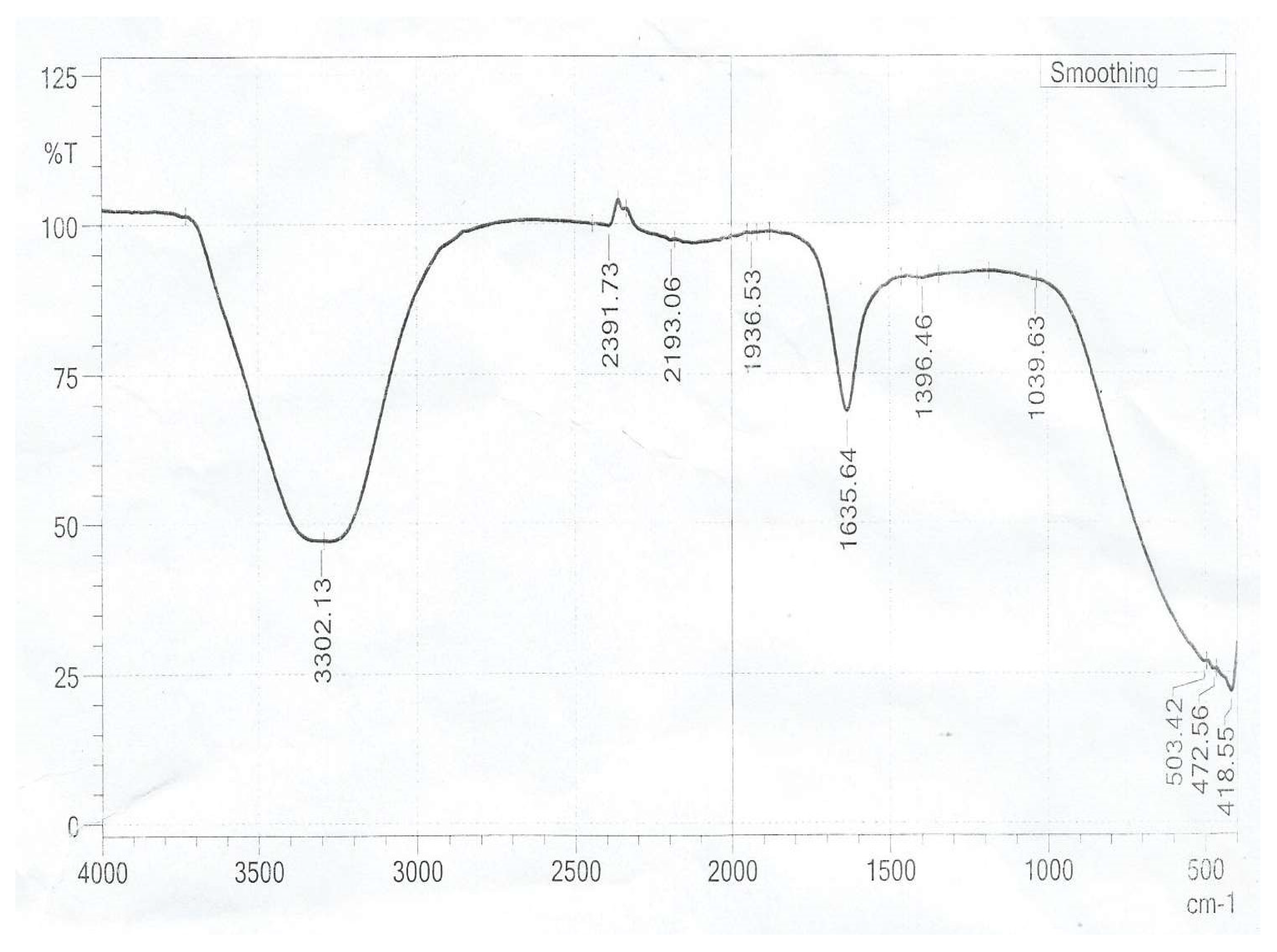
The FTIR spectrum of norbixin (
Figure 3.6) shows the following bands: at 3 302 cm
–1 the -O-H stretching vibration is observed, at 2 391 cm
–1 and 2 193 cm
–1 the H-C-H bending vibration, at 1 635 cm
–1 the carboxylic C=O group, at 1 396 cm–1 the alkene C=C streching, at 1 396cm
–1 the C–H bending of the methyl groups, at 1 396 cm
–1 the C=O stretching.
The functional groups mentioned in both the dye sample and norbixin component except for the –CO- stretching and methylene vibration which were absent in the norbixin. The absence of these functional groups in the norbixin is as a result of the conversion of the carboxylic acid in the bixin of the dye to norbixin by saponification with caustic alkaline. This further helps in elucidating the structure available for the bixin and norbixin components of Bixa orellana dye.
Figure 3.
5: FTIR Spectra for Bixa orellana Extract.
Figure 3.
5: FTIR Spectra for Bixa orellana Extract.
Figure 3.
6: FTIR Spectra for Norbixin.
Figure 3.
6: FTIR Spectra for Norbixin.
4. Conclusion
Bixa orellana dye is a vegetable dye with fascinating physico-chemical properties, making it suitable for use as food dye on wide range of substrates. The pigment contains essentially the fat soluble bixin and water soluble norbixin. Alkali hydrolysis of the bixin yields norbixin. Appreciable yield is obtained with the ethanol used for the extraction, showing that ethanol is an excellent solvent for the extraction of Bixa orellana from its seed. In addition, annatto dye may be used with suitable solvent for use as acid-base indicator as a result of its varying colour with pH. The sensitivity of the dye to different pH media makes it stimuli sensitive and a potential chemical sensor.
And in the world of nanoscience, bixin and norbixin potentials as nanocomposites open a wide variety of studies and applications.
Acknowledgements
Authors acknowledged the Department of Chemistry, School of Science as well as School of Postgraduate Studies, Federal University of Technology, Akure, Nigeria for the success of this work.
References
- Adetuyi, A. O. (2004). Extraction, Spectroscopic Studies and Uses of Some Natural Dyes- (Ph.D.).
- Adetuyi, A. O., Lajide, L., and Popoola A. V. (2005). Spectroscopic and Dyeing Characteristics of the Yellow Dye from Morinda lucida (Brimstone Tree). Pakistan Journal of Scientific and Industrial Research, Vol. 48, No. 6.
- Aline I.A., Afonso M.R. and Paulo S. (2013). Carotenoids: Food Sources. Production and Health Benefits.
- Aluísio, M.D. (2022). Combined Study of Docking and Molecular Dynamics Against DNV-3 SN1 Protein By Bixinoids. [CrossRef]
- Amusa, S.A. (2014). In vitro Antioxidant Activity of Bixa orellana (Annatto) Seed Extract. [CrossRef]
- Daniela de Araújo Vilar, L. (2014). Traditional Uses, Chemical Constituents, and Biological Activities of Bixa orellana L.: A Review. [CrossRef]
- Dolores J.Y., María T. D., Ivan L. V., Antonio F. B., Sofia V. and Laura F. (2008). Characterization of Colouring Compounds In Annatto (Bixa Orellana L.) Used In Historic Textiles By Means Of UV-Vis Spectrophotometry and FT-IR Spectroscopy.
- FAO-JECFA (2006). Monographs 3. Compendium of food additive specifications. Sixty-seventh meeting. FAO JECFA Monograph: Rome.
- Handayani R. and Setyawati R. (2019). Evaluation of Types of Solvent on the Extraction of Bixa Orellana and Application of Extract on a Chicken Sauage Product as A Natural Colour and Antioxidant Sources. [CrossRef]
- Hirwani R.R. (2019). Pharmacological and Cosmeceutical Applications of Bixa Orellana L: A review of the scientific and patient Literature. [CrossRef]
- Marta G.D., Adão L.G, Enieluce S. B., Paulo R. N. (2017). The Annatto Carotenoids and the Norbixin Absorption Coefficient Carotenoides.
- Satyanarayana Akula, Prabhakara Rao Pamidighantam and Rao D.G. (2003). Chemistry, Processing and Toxicology of Annatto (Bixa Orellana L.) Journal of Food Science and Technology- Mysore- 40(2):131-141.
- Silva G.F., Felix M. C., Gamarra, A. L., Oliveira and Cabral F.A., (2008). Extraction of Bixin From Annatto Seeds Using Supercritical Carbon Dioxide. [CrossRef]
- Sindhu P., Seema S., Sivakami D. and Hirwani R.R (2018). Pharmacological and Cosmeceutical Applications of Bixa Orellana: A Review of the Scientific and Patent Literature. [CrossRef]
- Tamil S.A., Dinesh M.G., Satyan R.S., Chandrasekaran B., and Rose C. (2011). Leaf and Seed Extracts of Bixa Orellana L. Exert Anti-Microbial Activity Against Bacterial Pathogens.
- Valerio M.A. (2015). Annatto Seed Residue (Bixa orellana L.): Nutritional Quality. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).