Submitted:
19 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
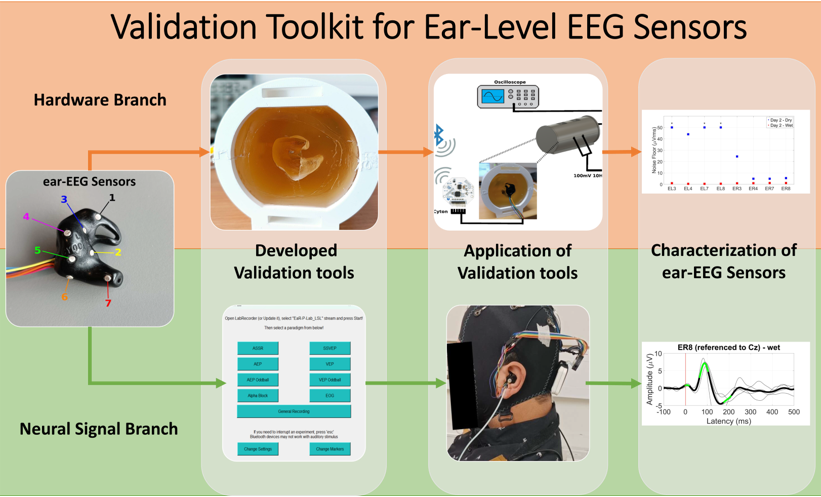
- A software framework ("EaR-P Lab") that allows the user to readily make a validation test battery for the characterization of ear-EEG devices at the neural signal acquisition level.
- The design and prototyping of an ear-EEG suitable physical phantom for systematic characterization of in-ear sensors, allowing controlled comparison of fit form factors for ear-EEG acquisition.
2. Materials and Methods: Ear-EEG Toolkit Design and Validation
2.1. EaR-P Lab - Design and Validation
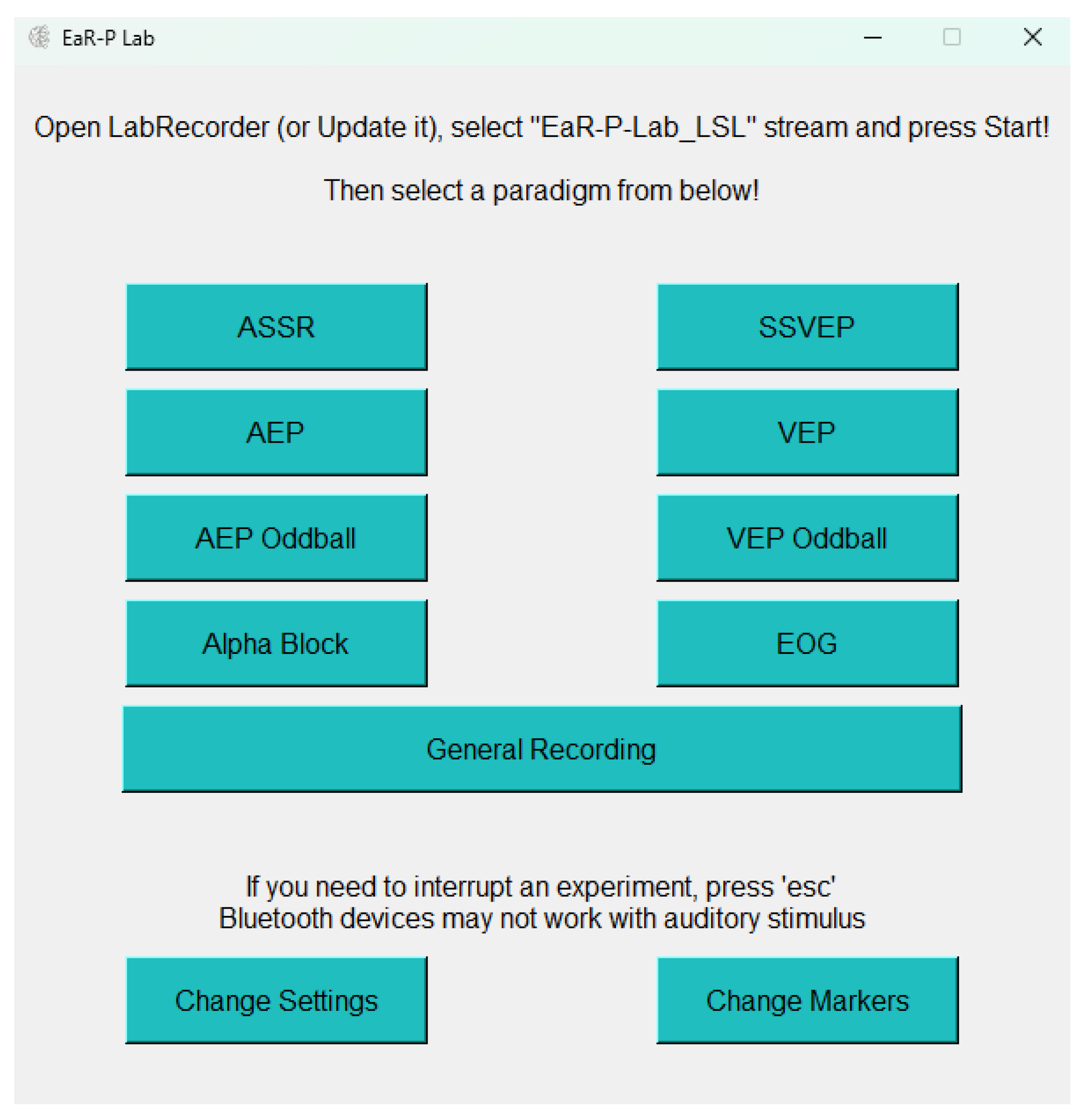
2.1.1. GUI - Main Menu
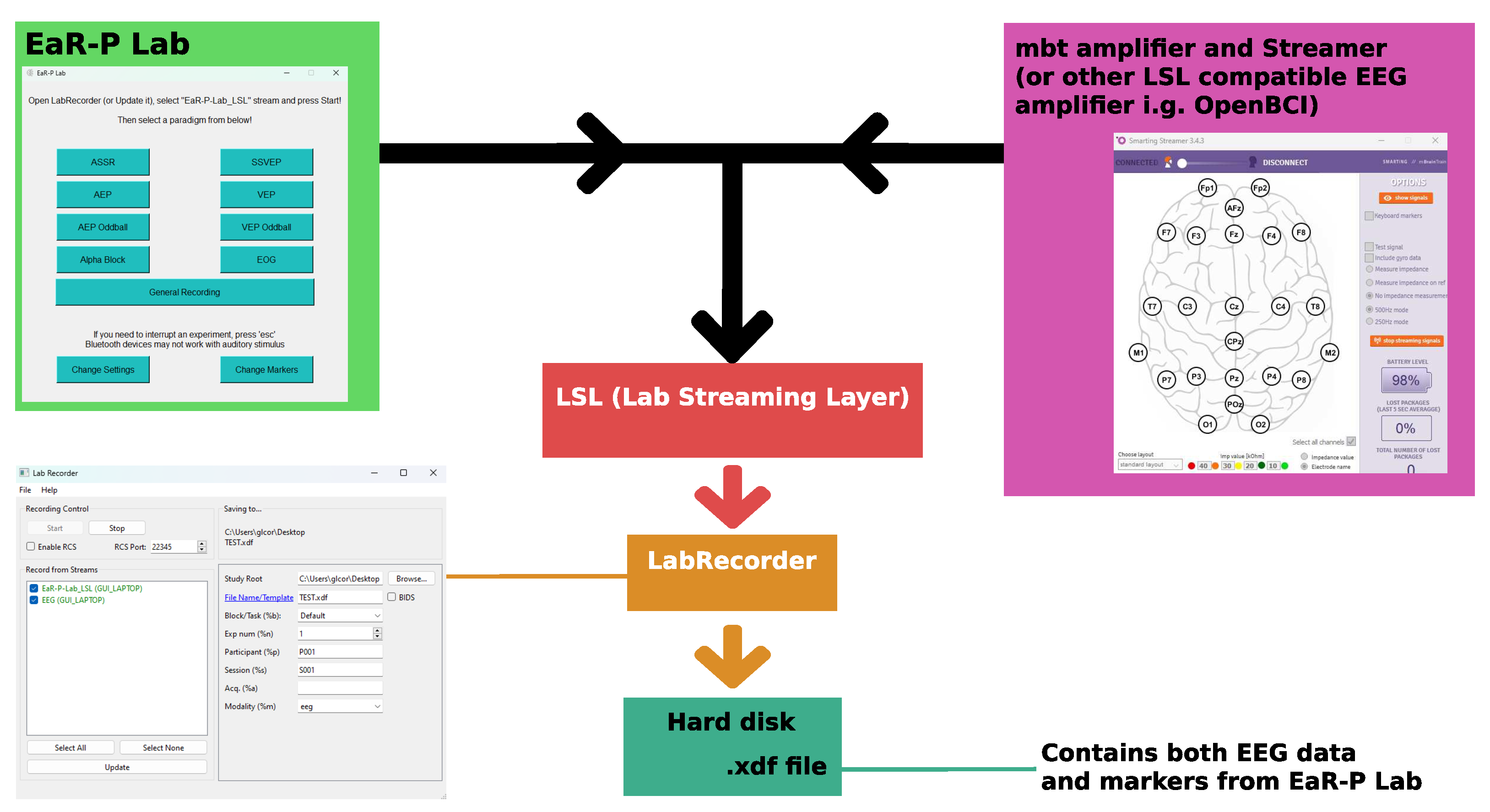
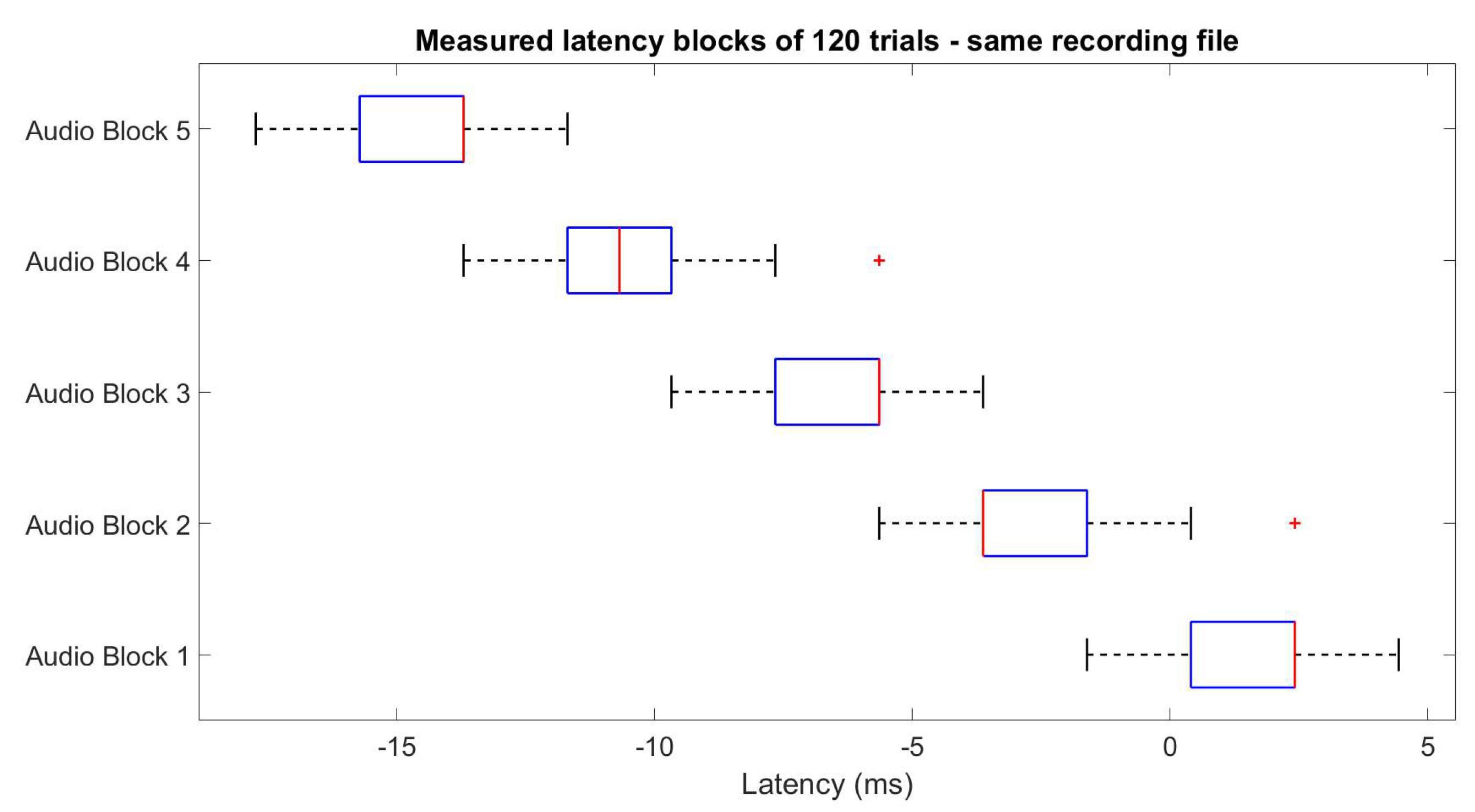
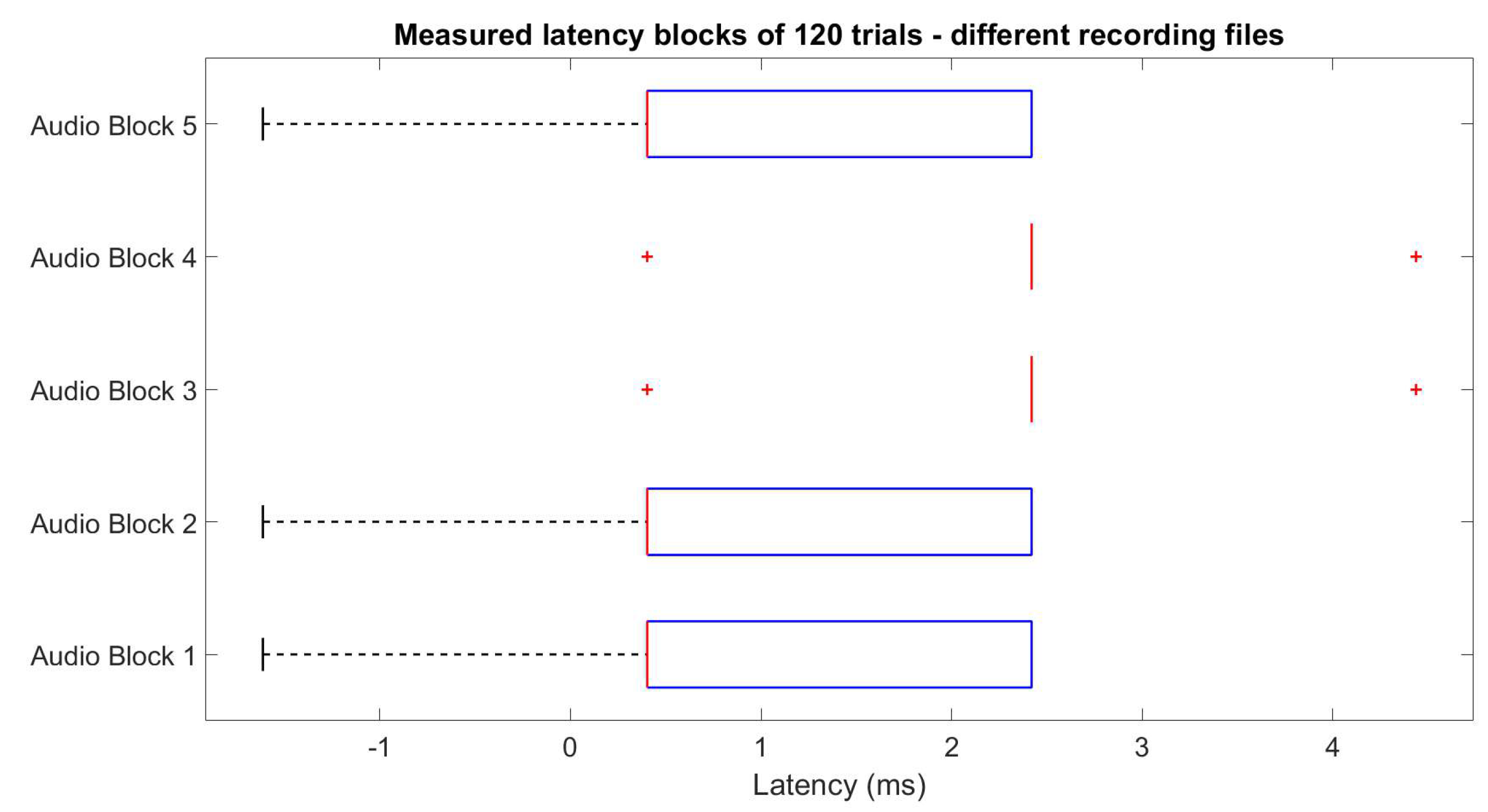
2.1.2. Stimuli and Trigger Latency
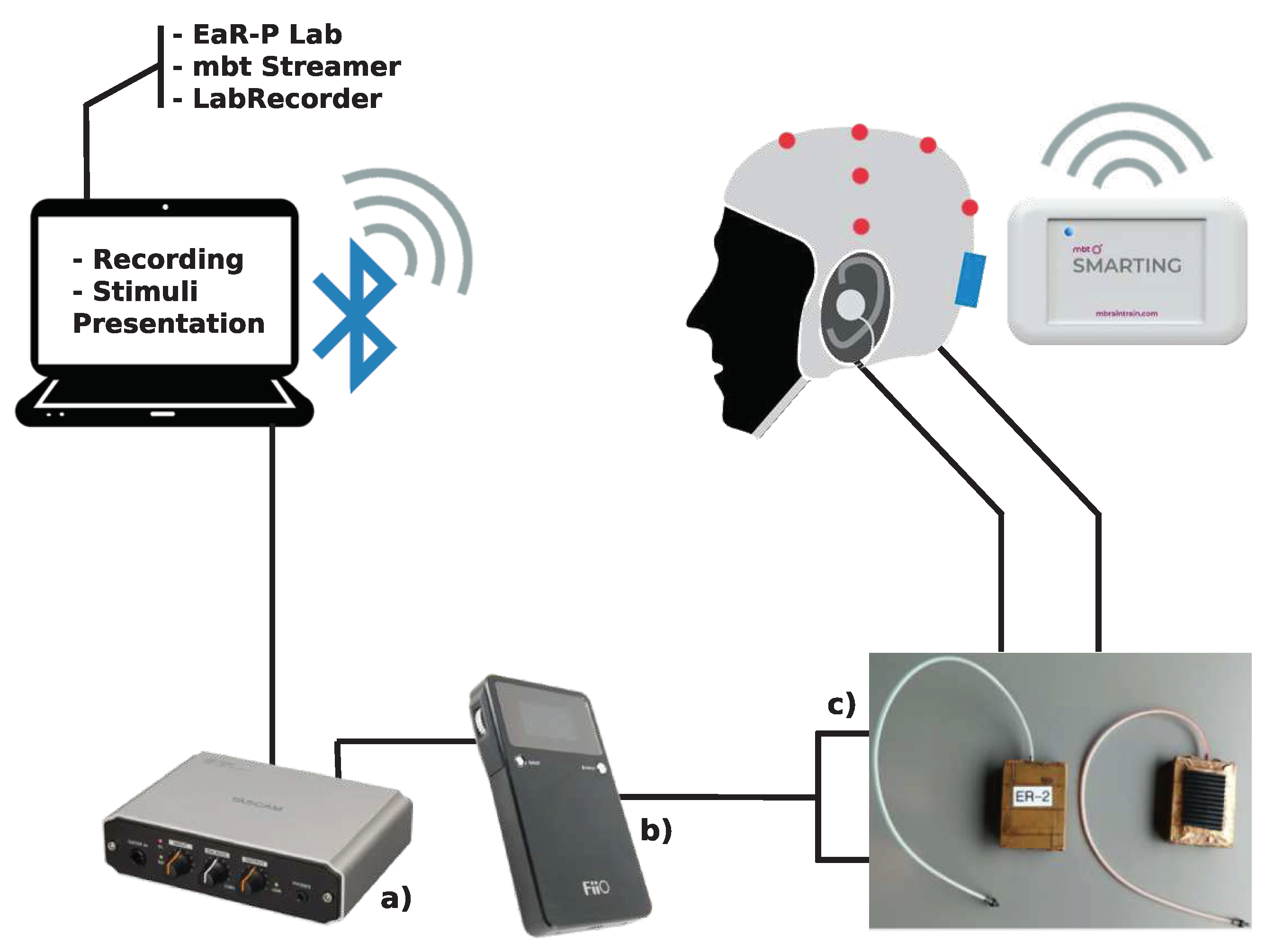
2.1.3. Acquisition Setup and Test Battery
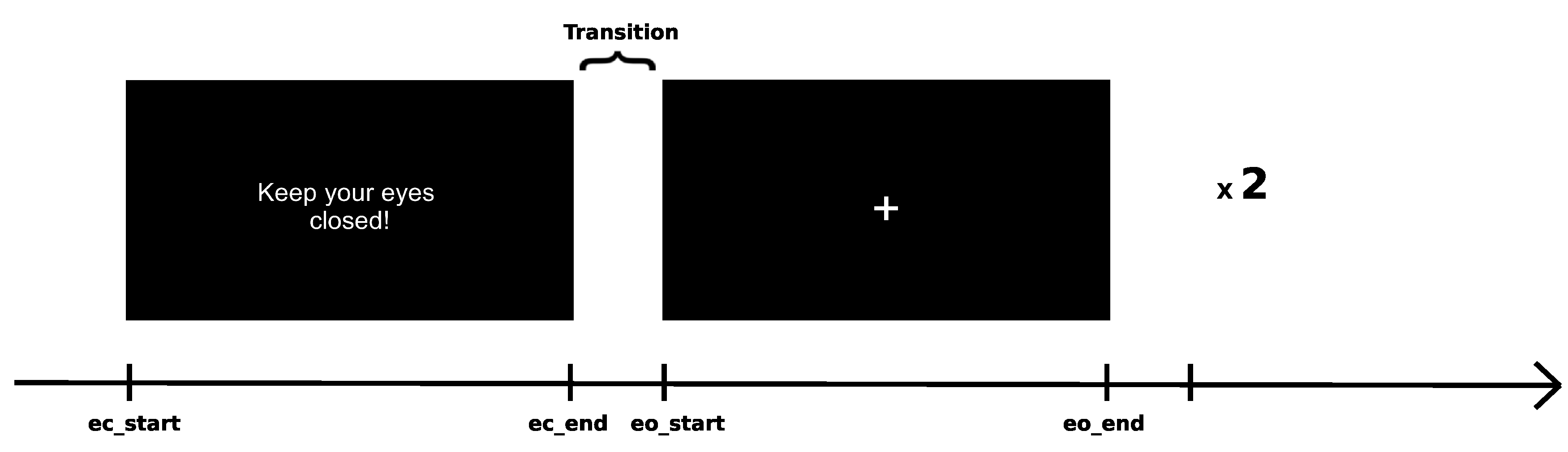
- General Recording - 4 minutes of resting state pre-testing
- Alpha Block - 1 minute per section, 4 minutes total
- ASSR - 4 minutes of continuous 1 kHz carrier 40 Hz modulated sound wave
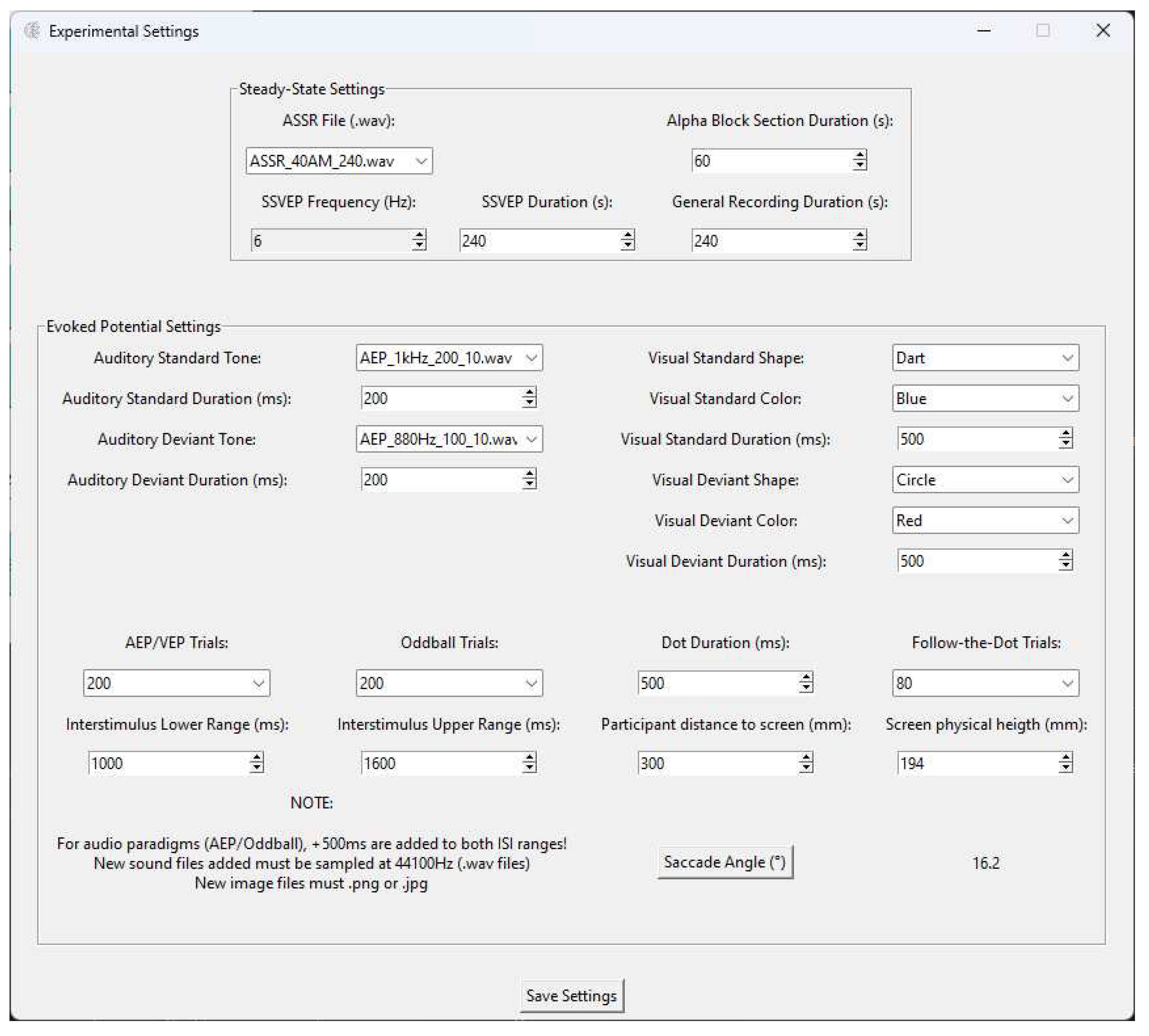
- SSVEP - 4 minutes of continuous 10 Hz visual stimulation, subject at a distance of 60 cm from the middle of the screen, measured from the point in between the eyes, during this paradigm, the room’s light was turned off, to maximize the flickering
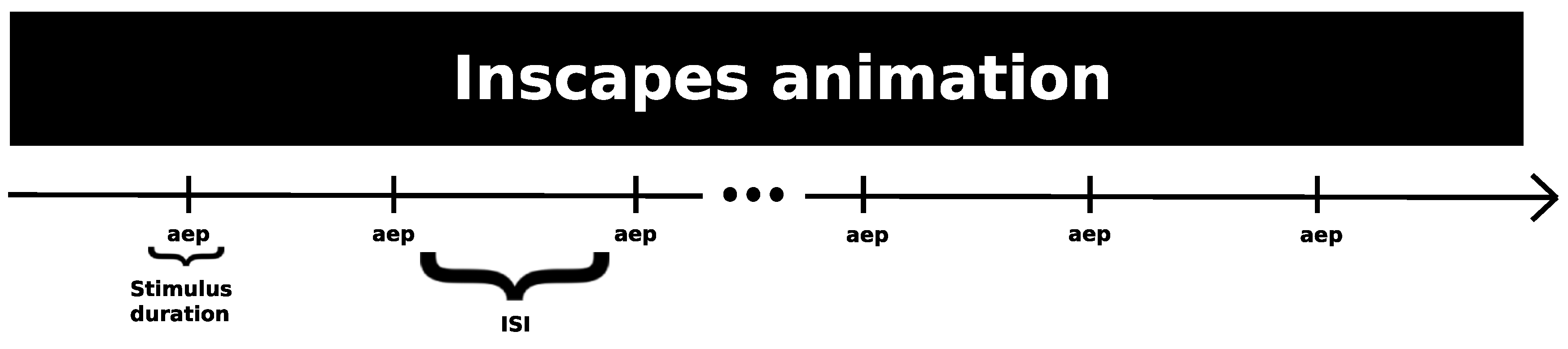
- AEP - 200 trials of discreet 1 kHz sound stimulus, with 200 ms of duration and a 10 ms ramp up/fall off, Interstimulus Interval (ISI) between 1200 ms and 1800 ms, for a total duration of around 7 to 8 minutes
- VEP - 200 trials of onset-offset pattern reversal with the dartboard target being shown for 500 ms of duration and a 500 ms ISI, taking about 5 minutes to complete
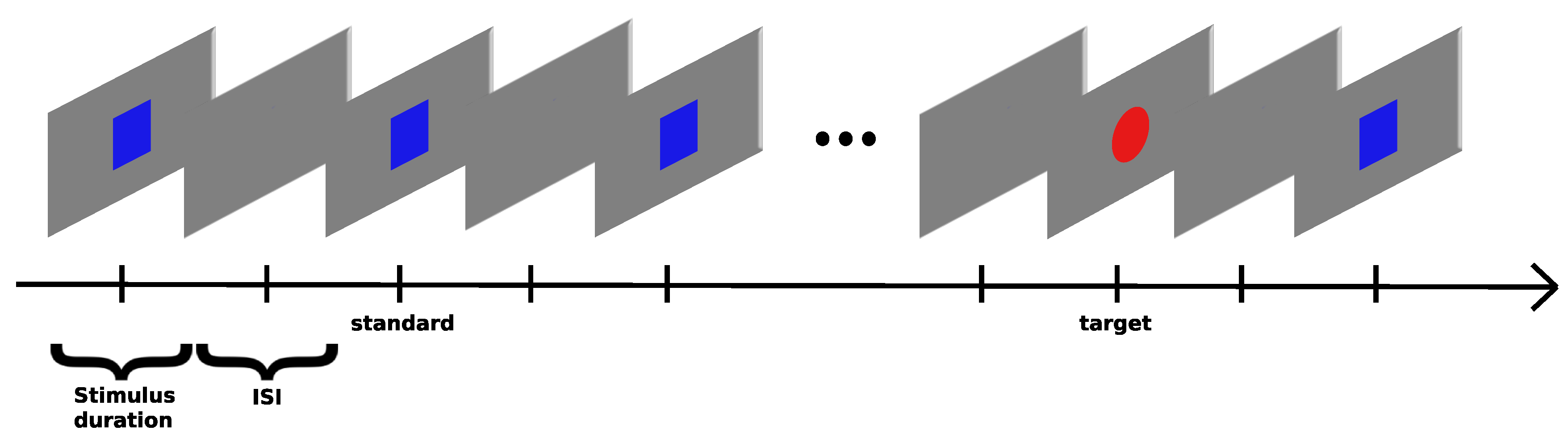
- AEP Oddball - Standard: 440 Hz sound wave | Target: 880 Hz sound wave - a total of 200 oddball/target events were presented to the subject, with every sound having a duration of 100 ms with a 10ms ramp up/fall off and ISI between 1200 ms and 1800 ms, for a total length of around 15 minutes
- VEP Oddball - Standard: Blue Square | Target: Red Circle - a total of 200 oddball/target events were presented to the subject, stimuli on screen for 500 ms, ISI between 600 ms and 700 ms, subjects were told beforehand to press "SPACE" once the target stimuli appear, for a total length of around 18 minutes
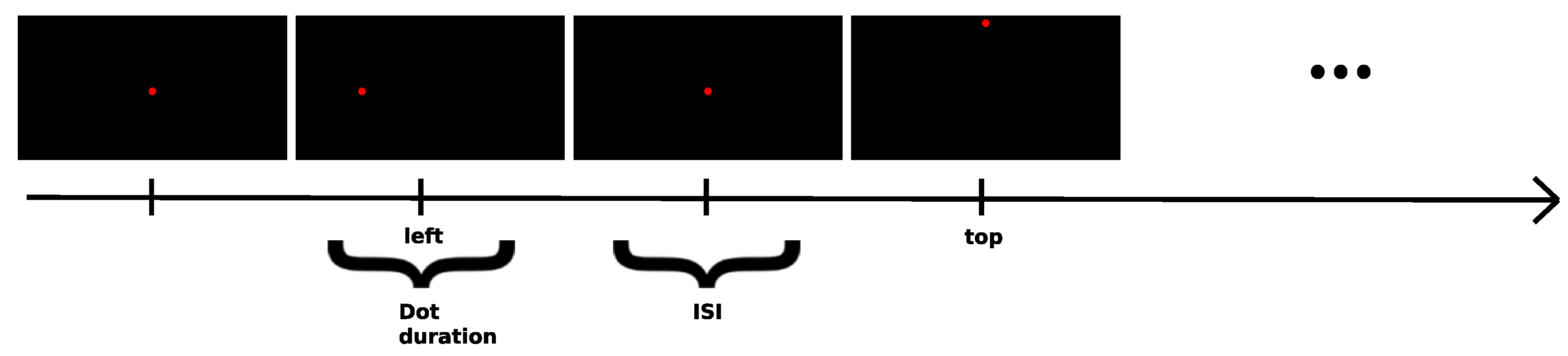
- EOG - 80 trials per saccade, dot moved for 500 ms, ISI between 1000 ms and 1600 ms, subject at a distance of 30 cm from the middle of the screen, measured from the point in between the eyes, which translates to 16.2° angle for each saccade, for the follow-the-dot paradigm, the subject’s head was stabilized using an adjustable chin rest and the monitor was leveled and centered with the subject’s eyes by being placed on a box. This section had a total duration of about 10 minutes
2.1.4. Processing and Statistical Analysis
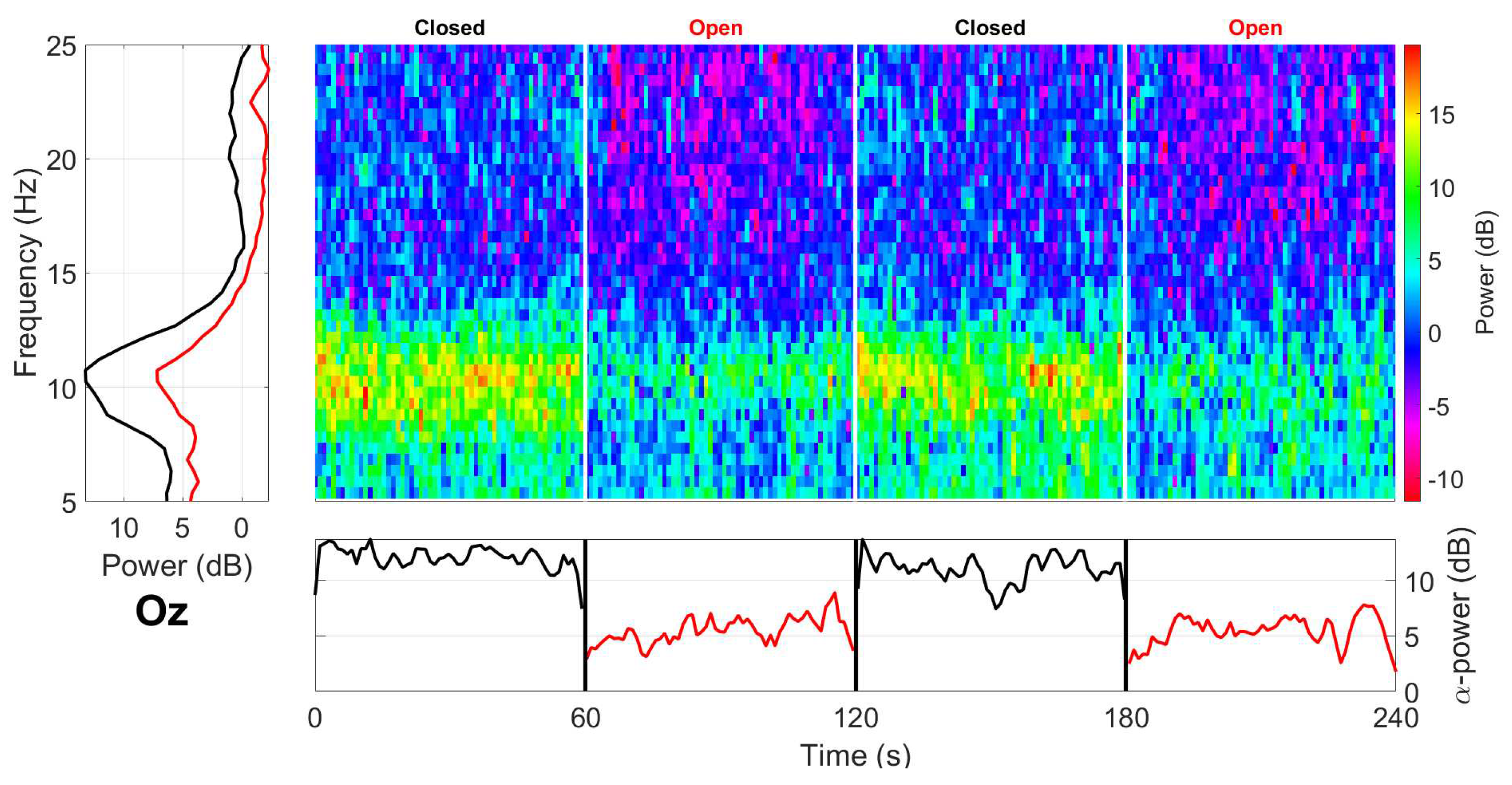
2.1.5. Alpha Block
2.1.6. ASSR
2.1.7. SSVEP
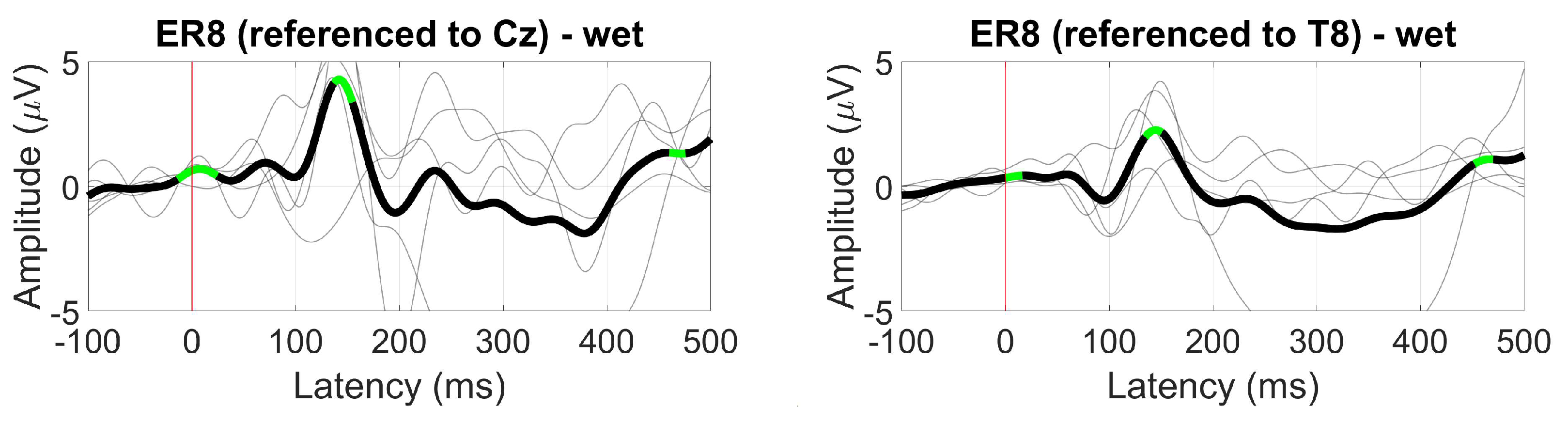
2.1.8. AEP (N100)
2.1.9. VEP
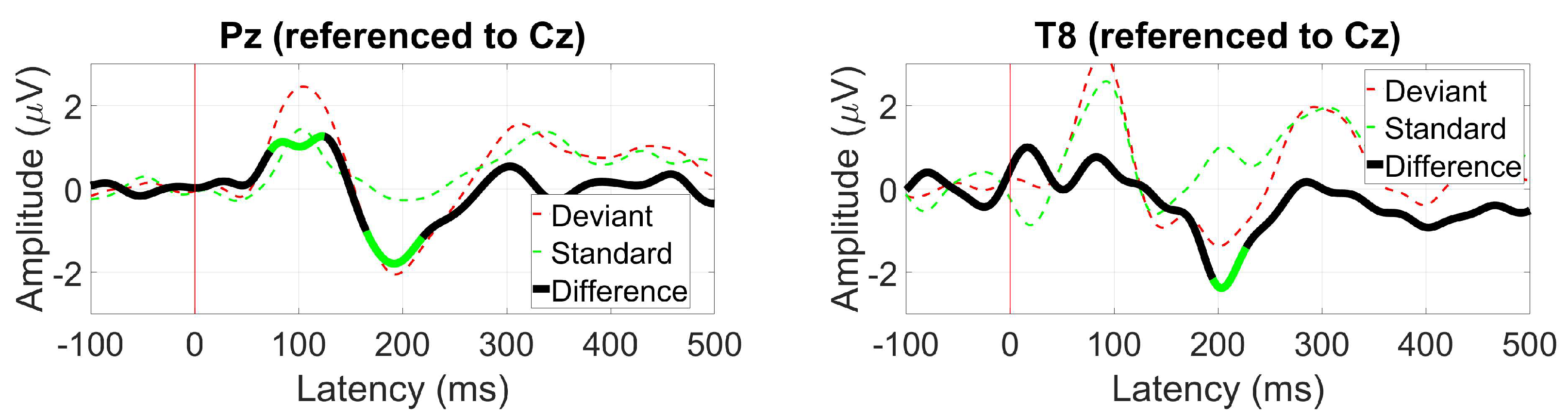
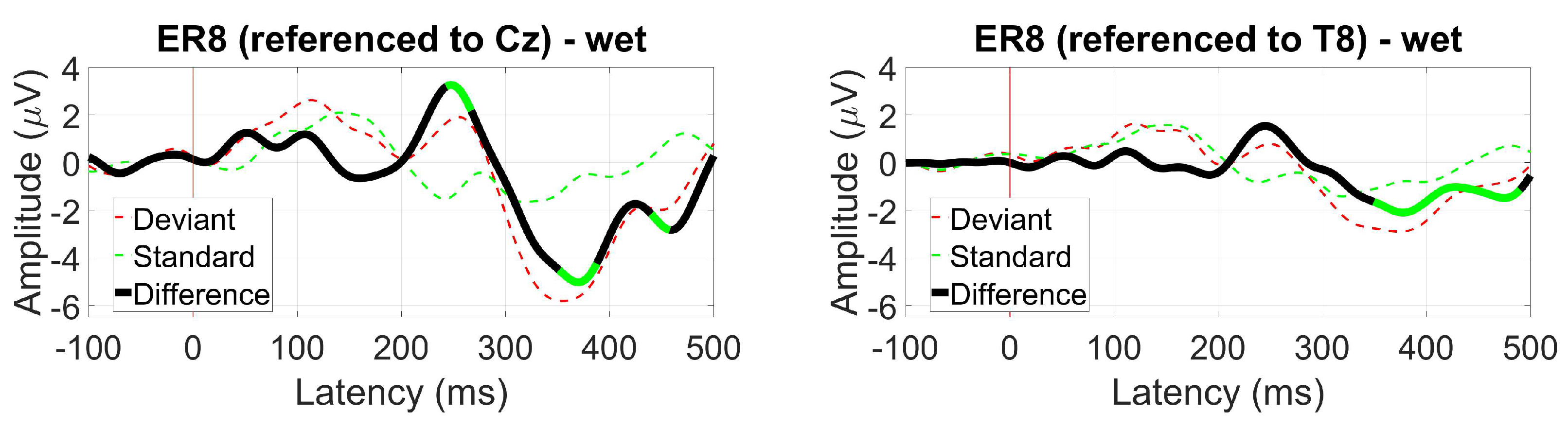
2.1.10. AEP Oddball (MMN)
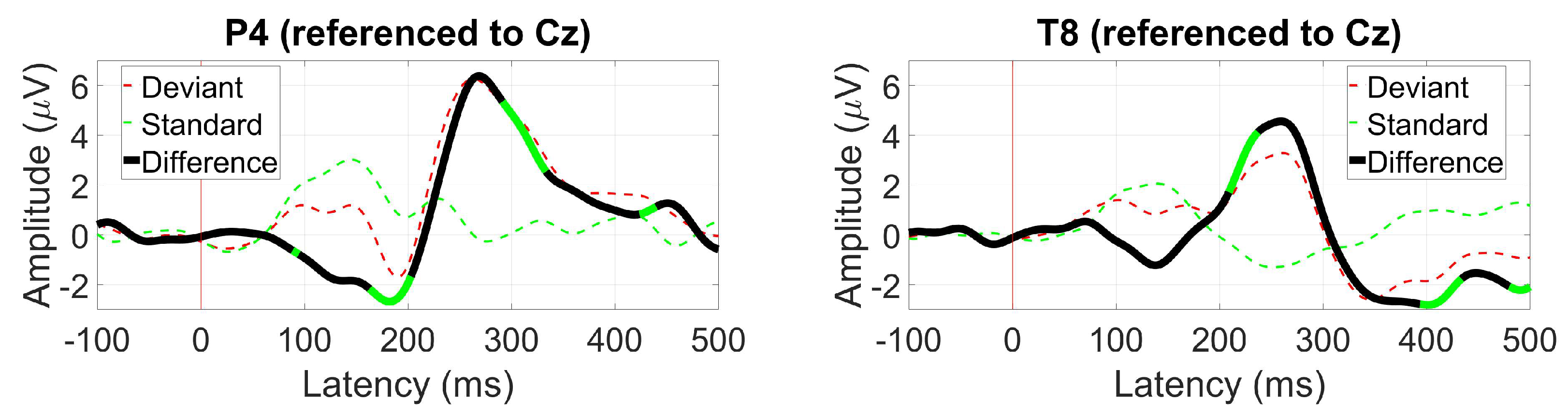
2.1.11. VEP Oddball (P300)
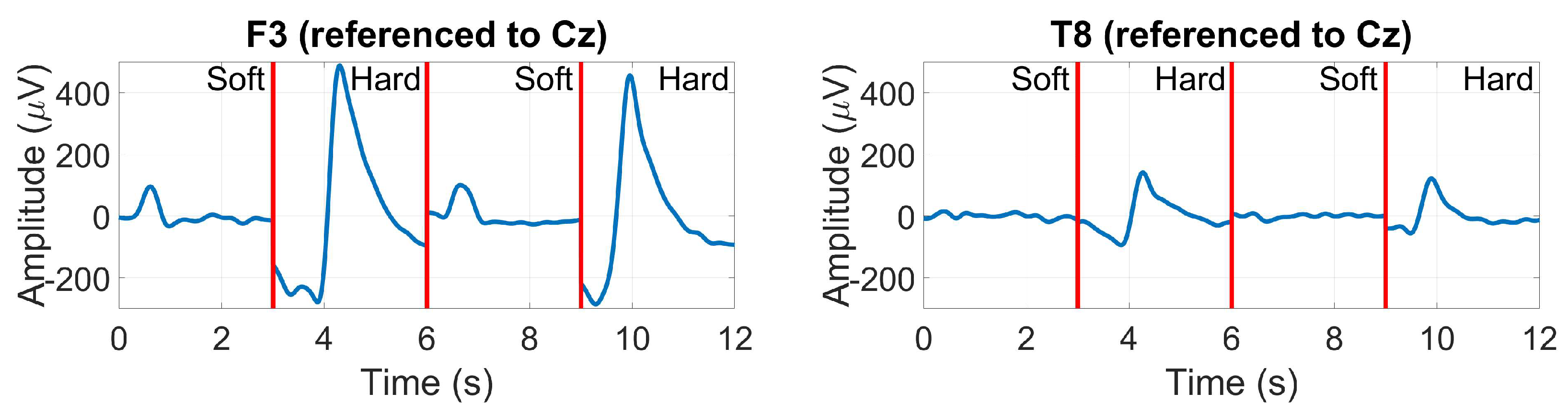
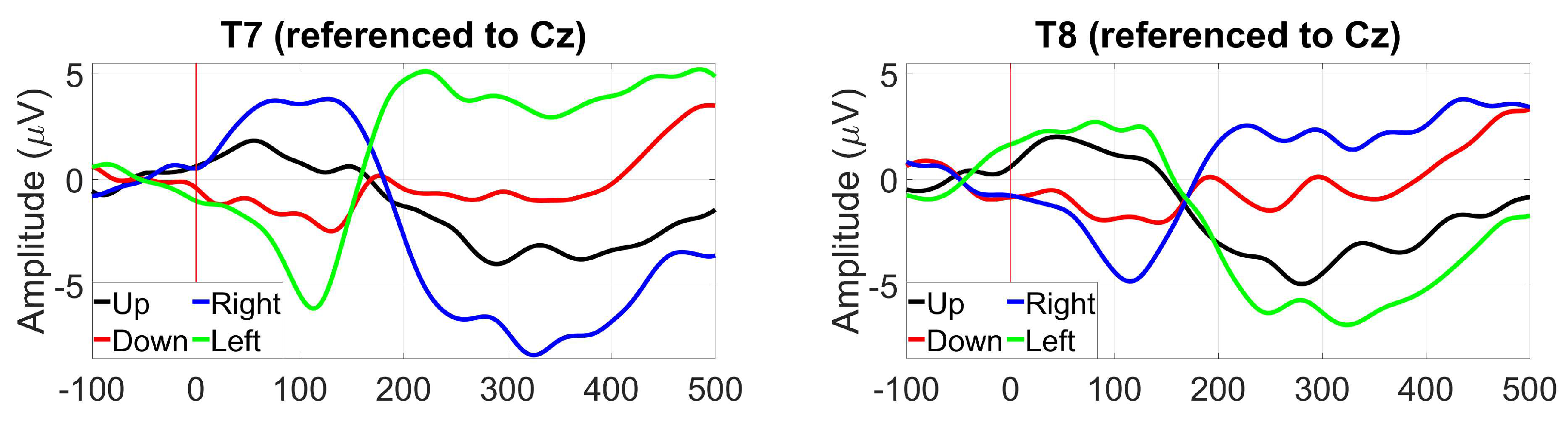
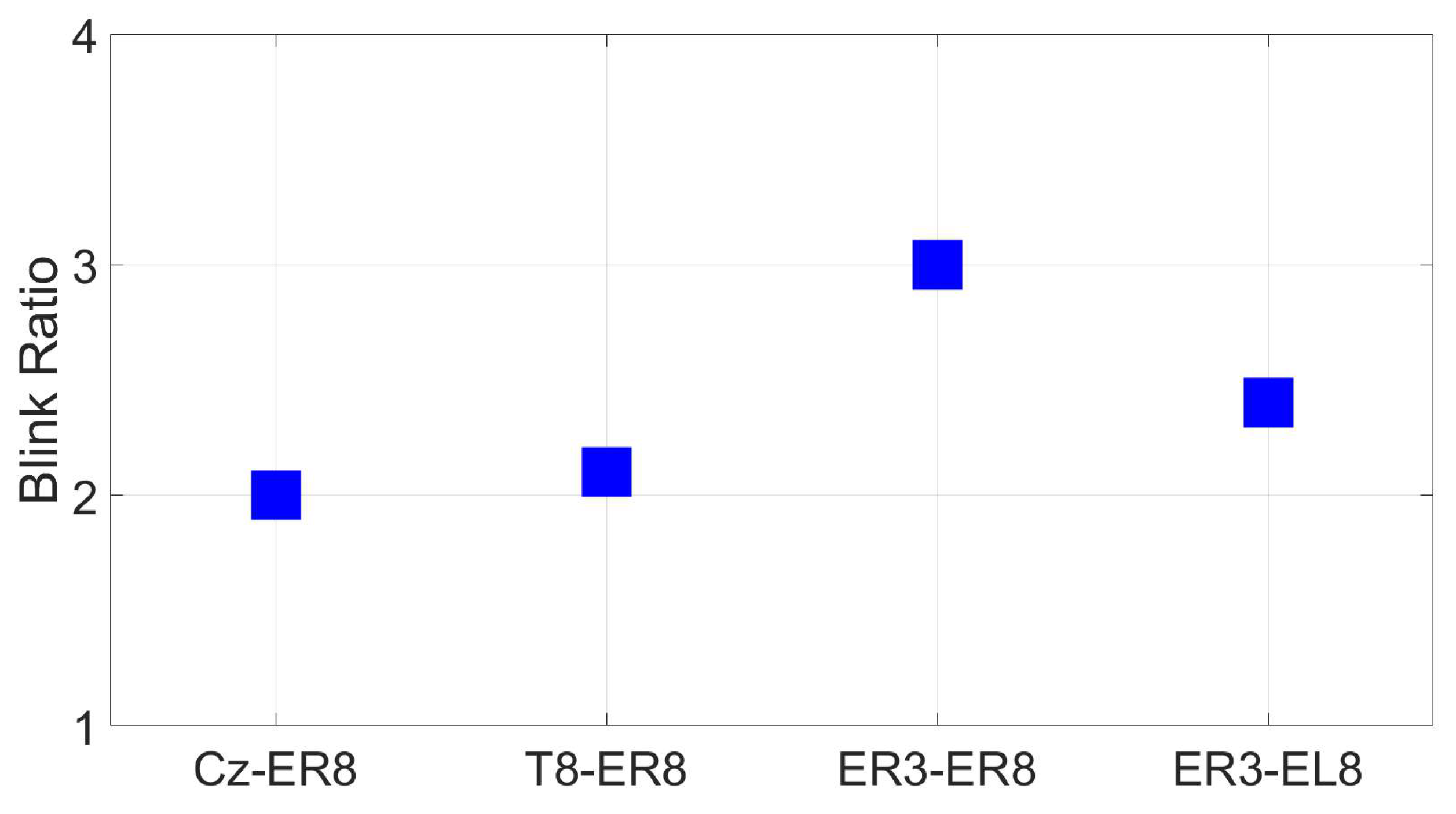
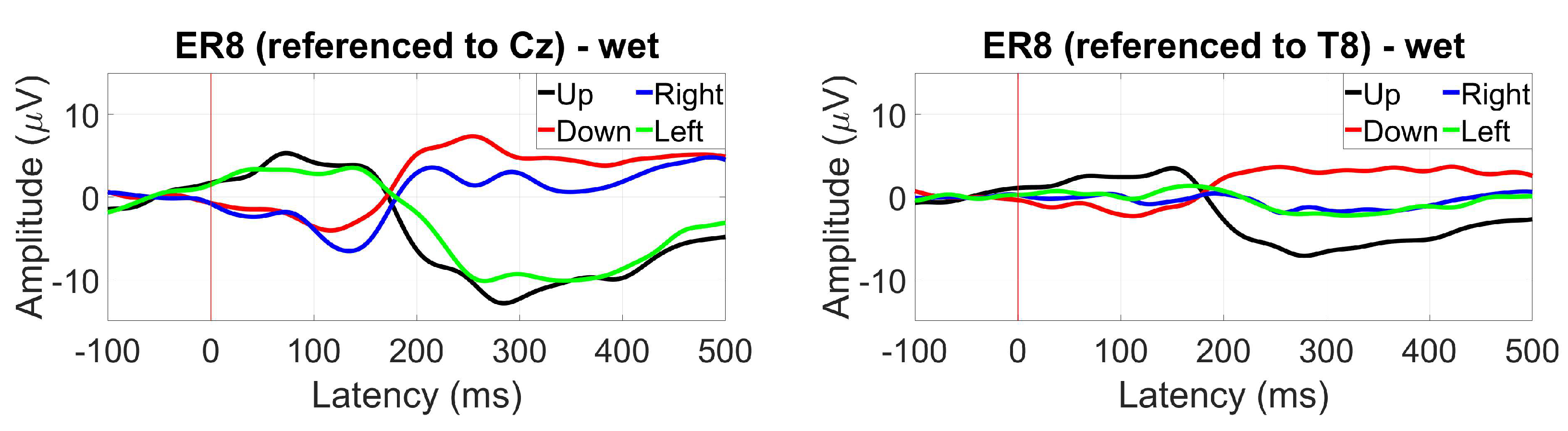
2.1.12. EOG (Blinks and saccades)
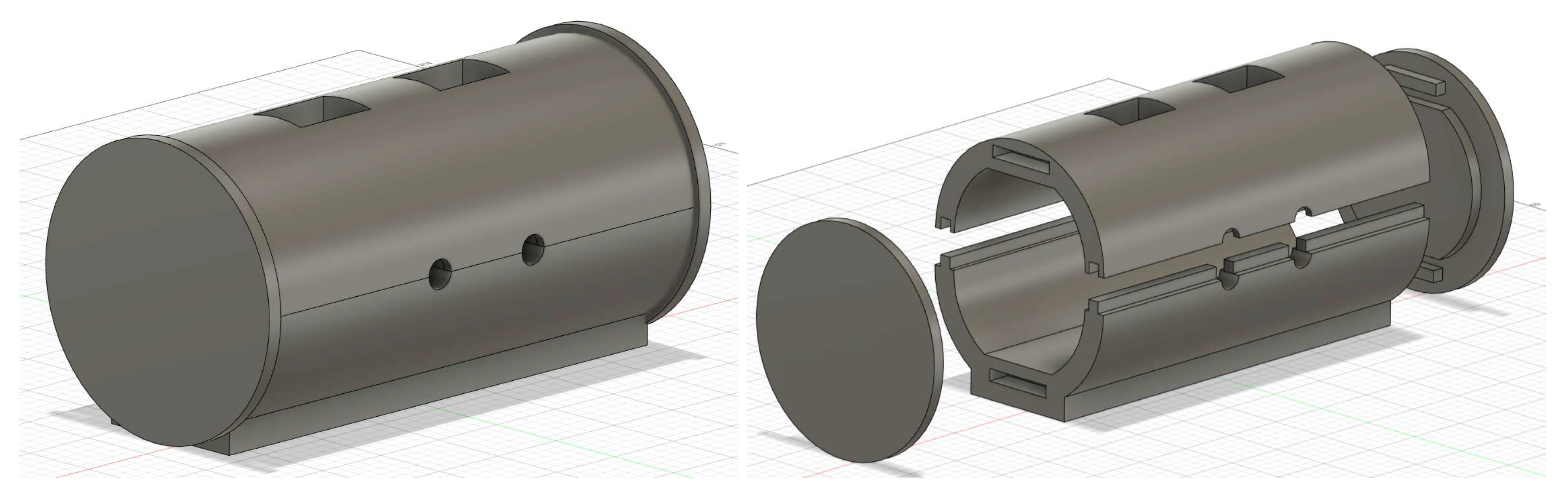
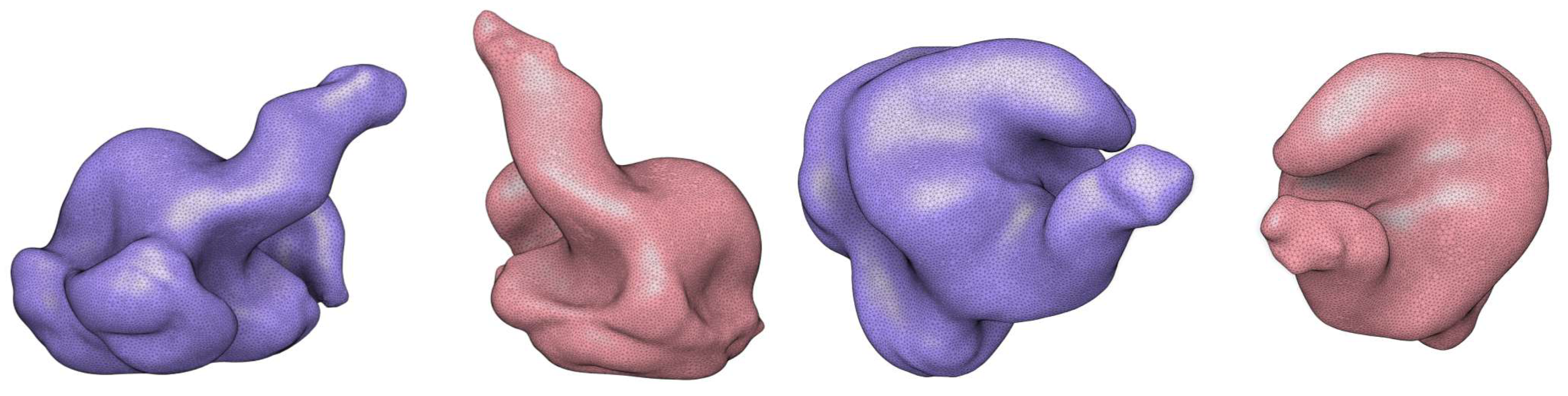
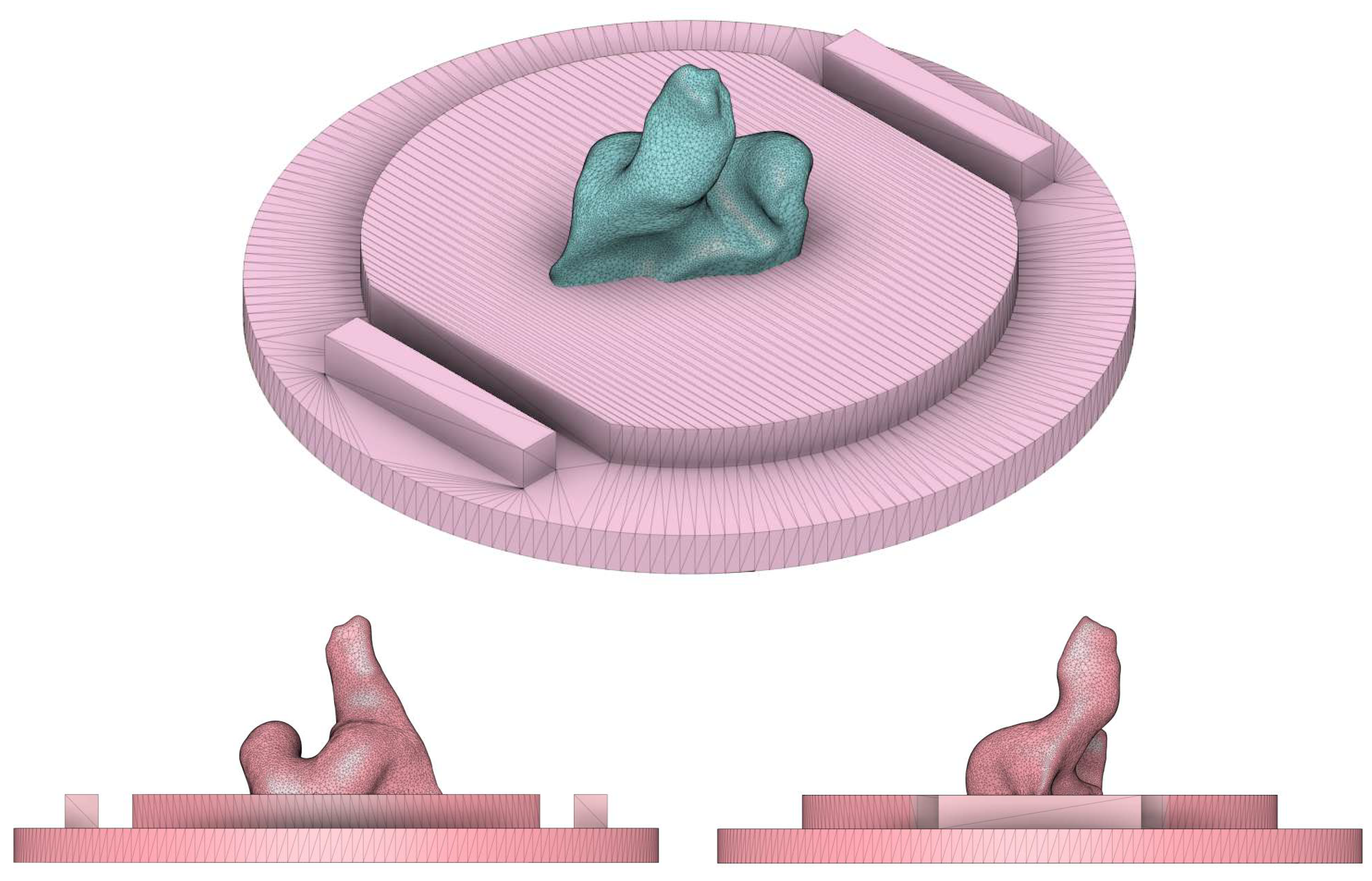
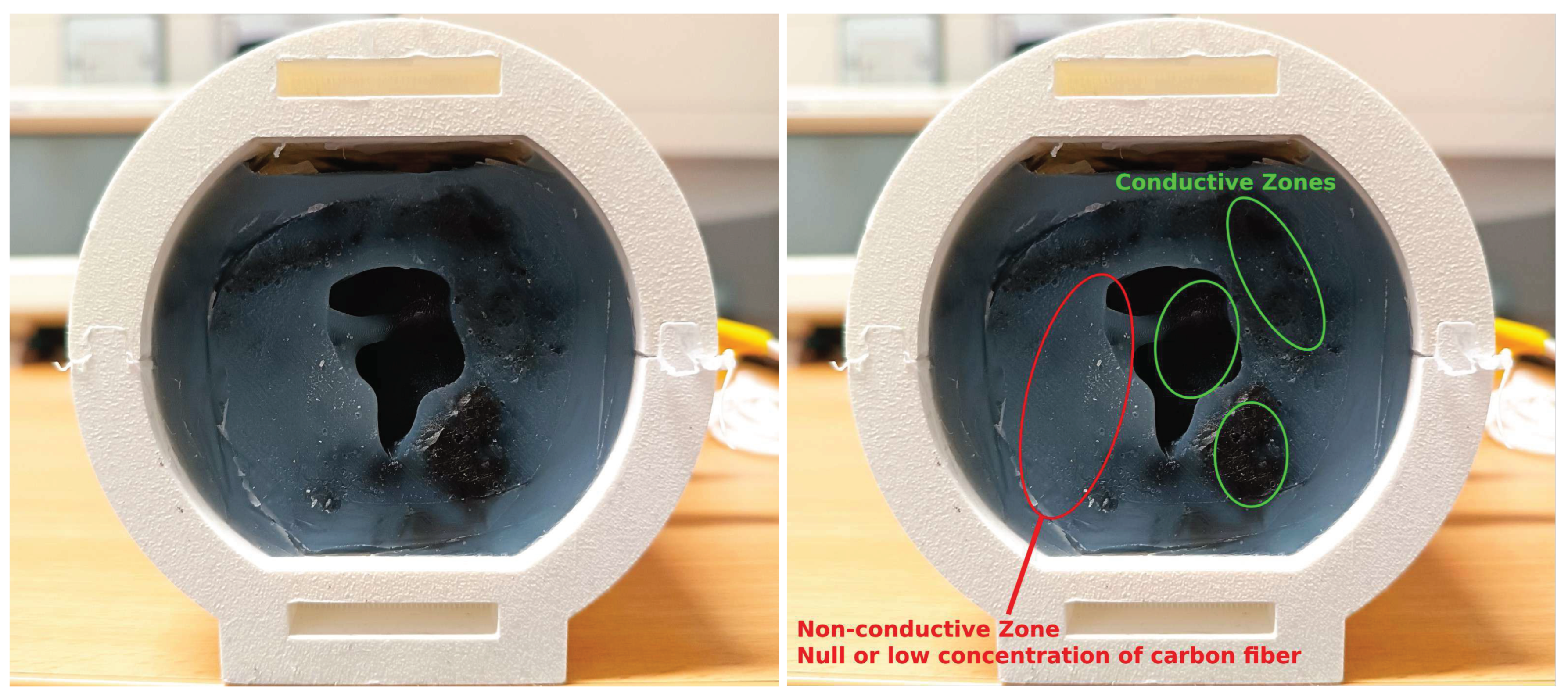
2.2. Ear-EEG Phantom - Design and Validation
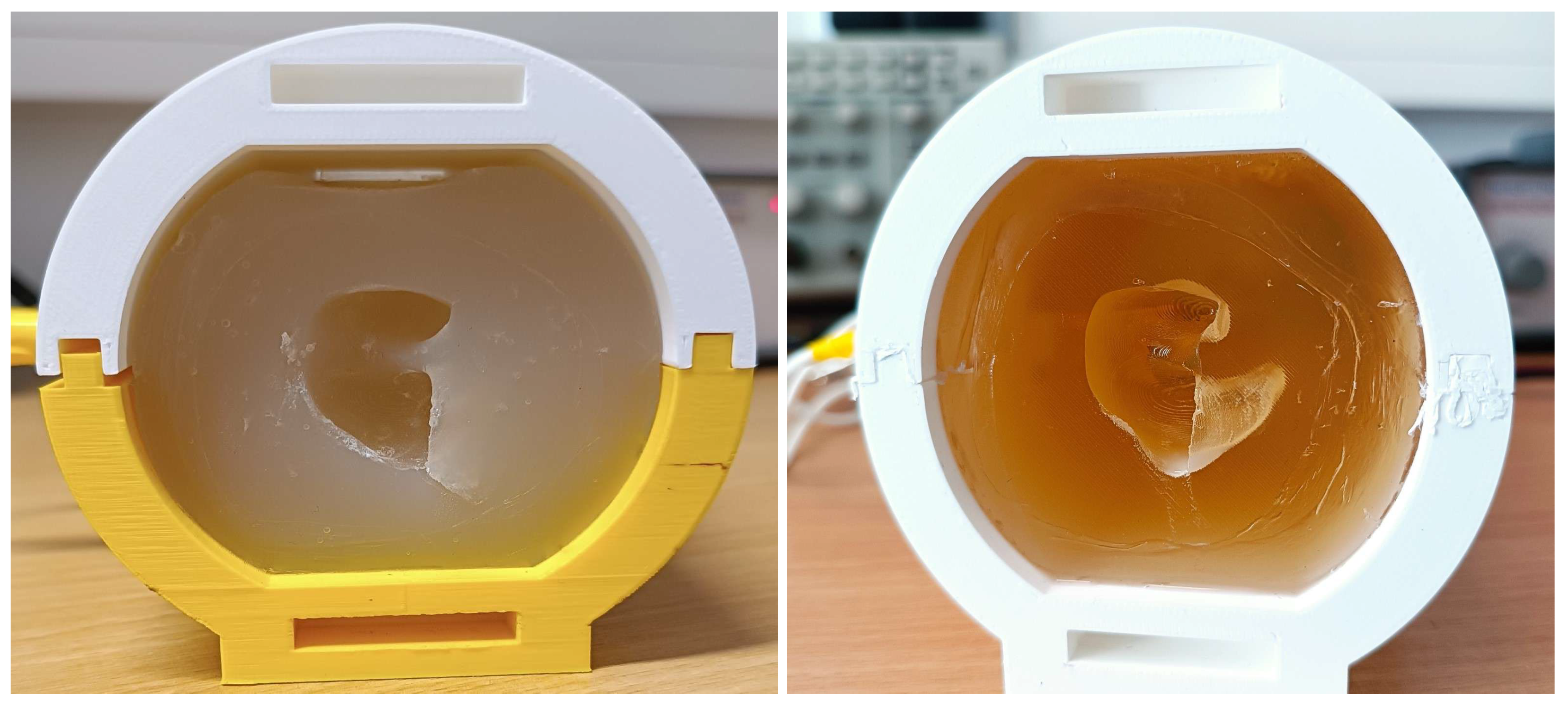
2.2.1. Phantom assembly and bulk materials
Agar and BG
- Boil 700 ml of regular tap water (or deionized water)
- Add 30 g of agar slowly while stirring the mixture
- Add 4 g of table salt while stirring until no granules are present - keep mixing while letting it cool down at room temperature for 10 minutes
- Pour the mix into the assembled phantom through the top vents until the liquid reaches half the vent’s height, let it sit in a refrigerator until it fully solidifies (minimum 2 hours, preferably overnight), which can be checked through the top
Silicone doped with carbon fiber
- Chopped carbon fibers, 3 mm in length, 30€ for 500 g, on Amazon
- Two-part A/B system platinum curable silicone, mixing ratio of 1:1, 23€ for around 630 ml, on Amazon (two were acquired for the phantom)
- Measure 8 g of carbon fibers into a disposable cup (use a mask/gloves when handling carbon fibers)
- Wet the carbon fibers with a small amount of rubbing alcohol, spread them around, and let almost entirely evaporate (to release strands of hair that surround the carbon fibers)
- Add the carbon fibers to 350 ml of part A silicone and mix thoroughly (an electric mixer with a wider spatula attachment was used) until the mix presents a grey/blueish tint
- Add 350 ml of part B silicone and keep mixing for up to 25 minutes to the same tint
- Pour into the phantom, equally through each vent, and let cure for 6 hours
2.2.2. Ear-EEG phantom testing protocol and setup
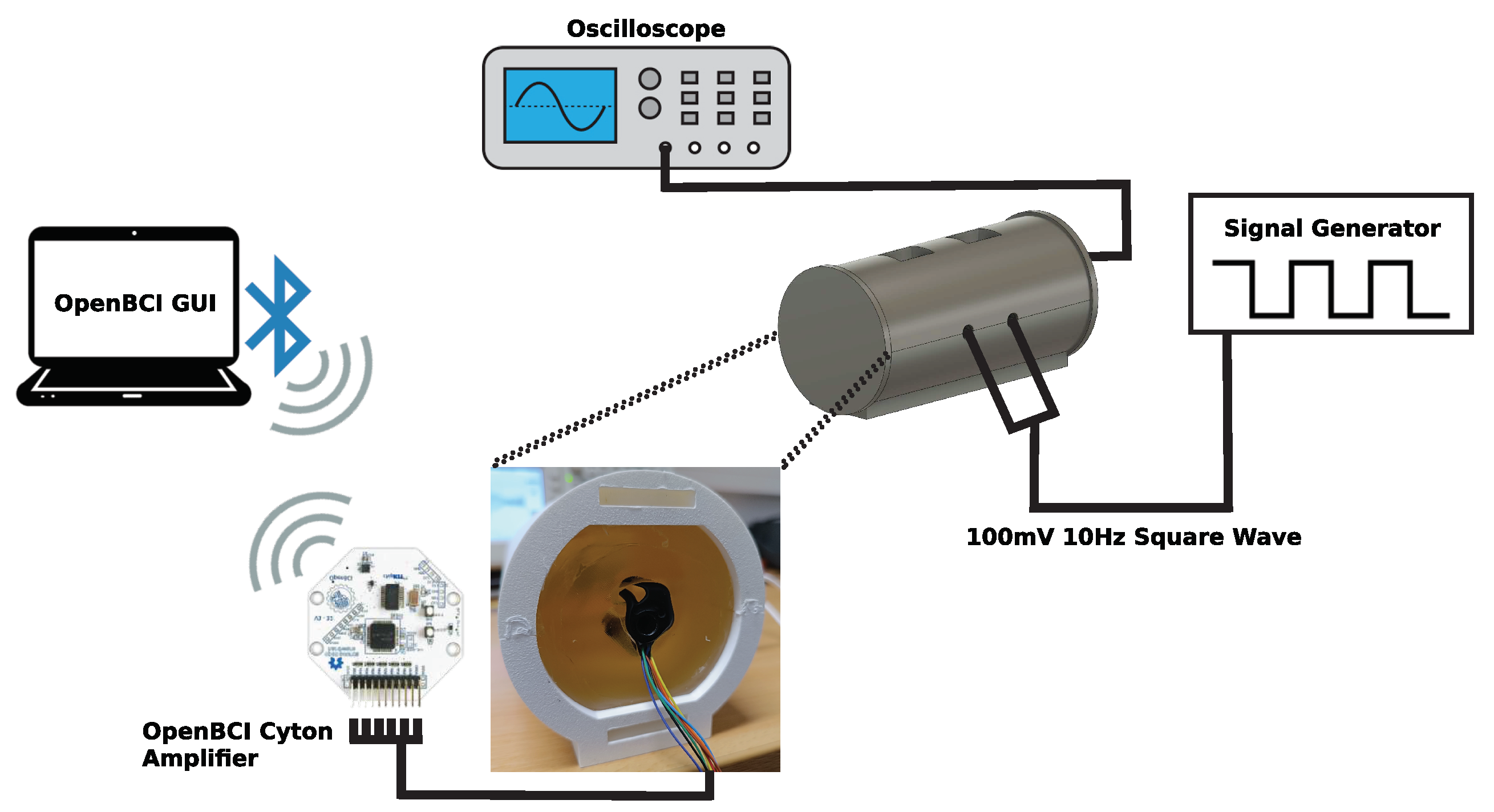
2.2.3. Phantom integrity and durability
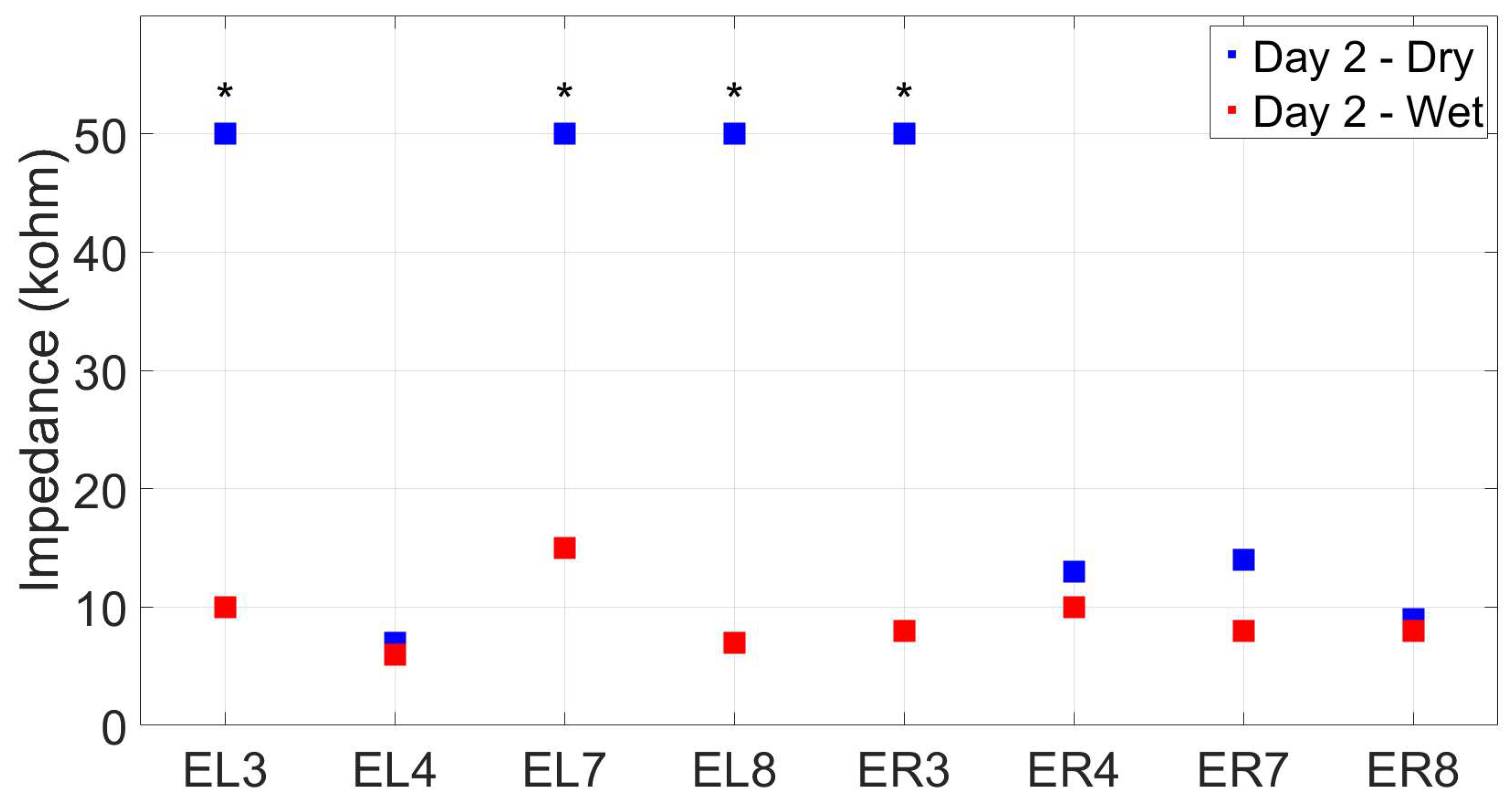
2.2.4. Electrode impedance
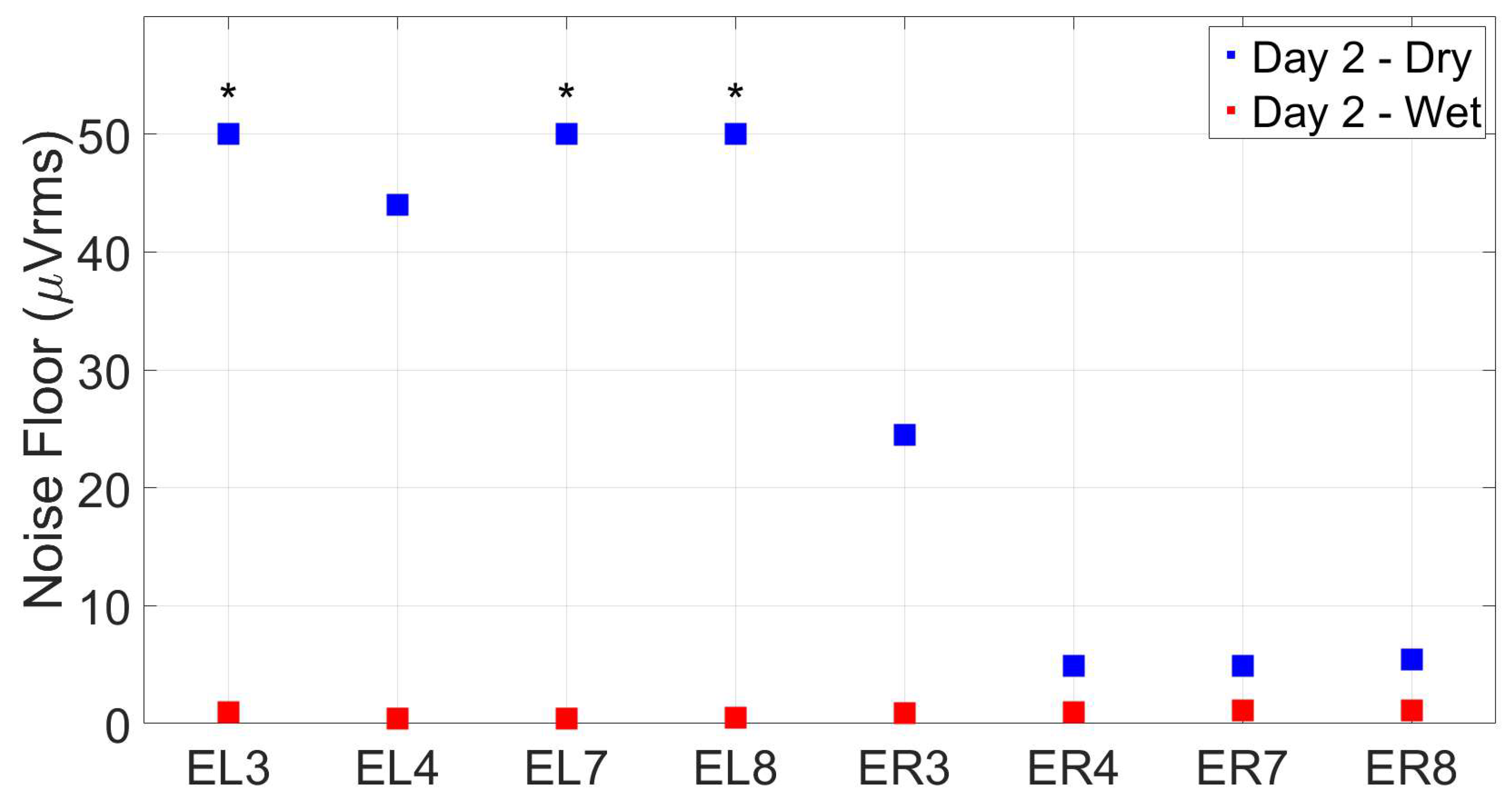
2.2.5. Noise floor measurements
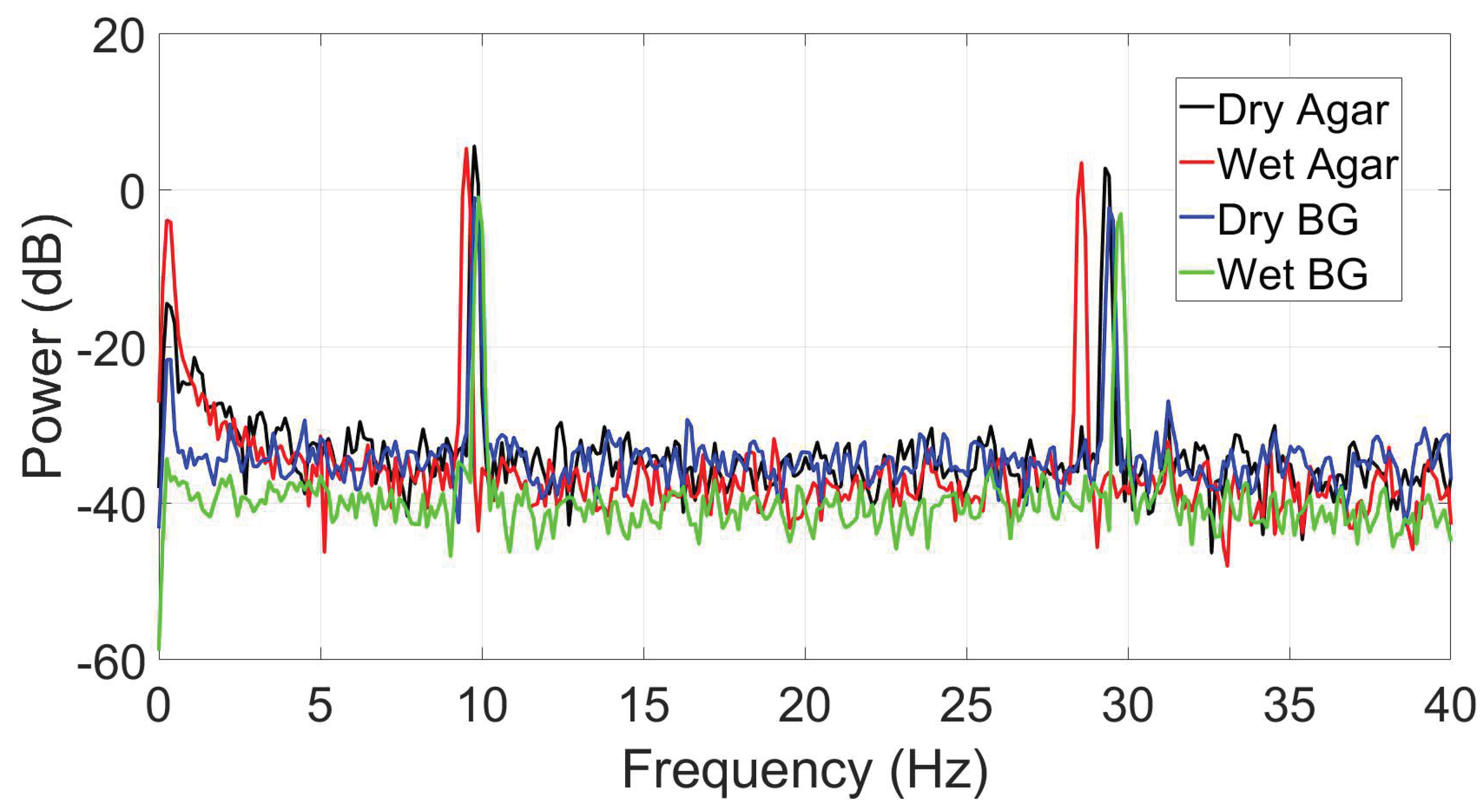
2.2.6. Alpha wave simulation
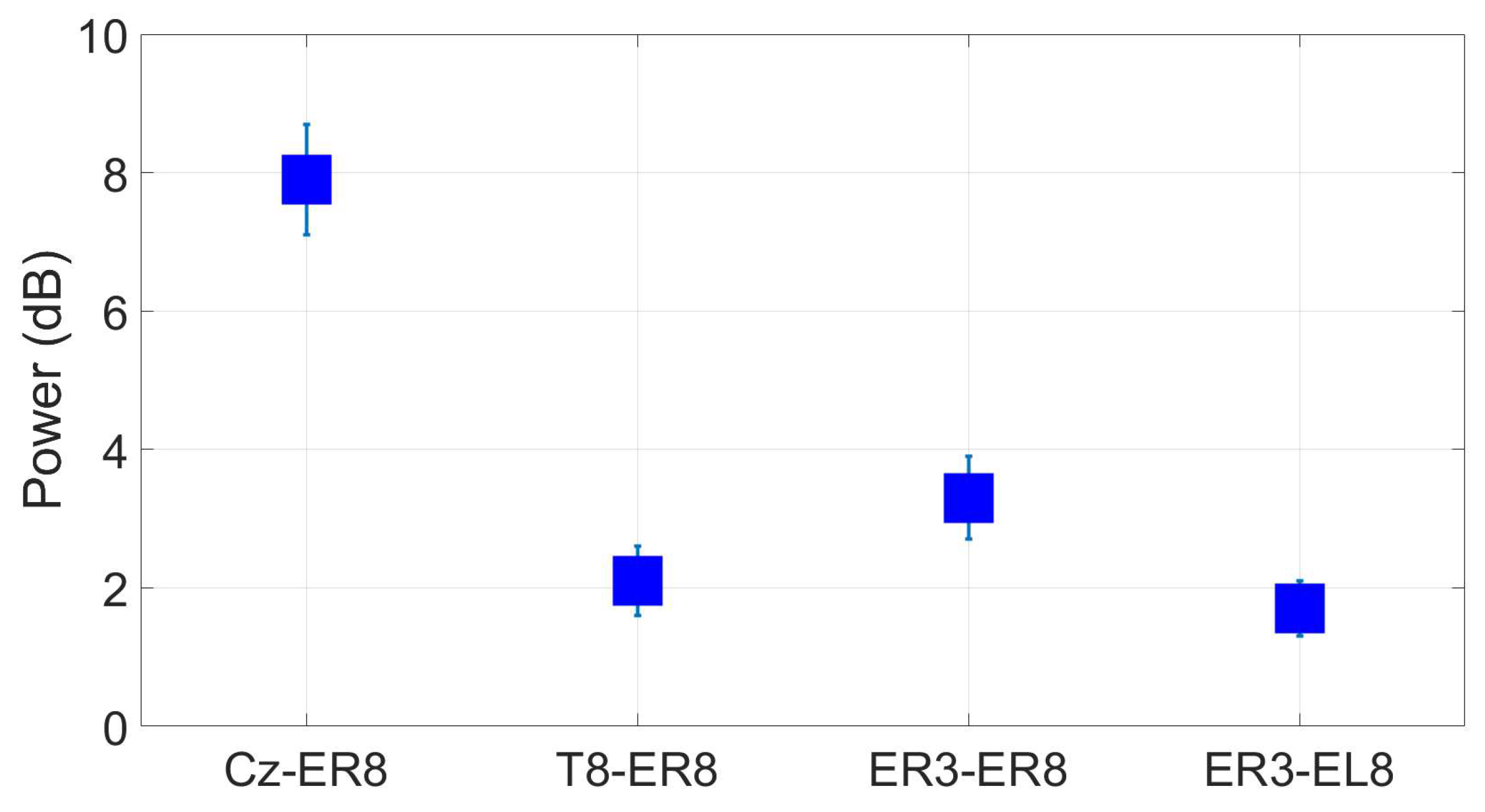
3. Results: Toolkit Use Case: Validation of an ear-EEG sensor
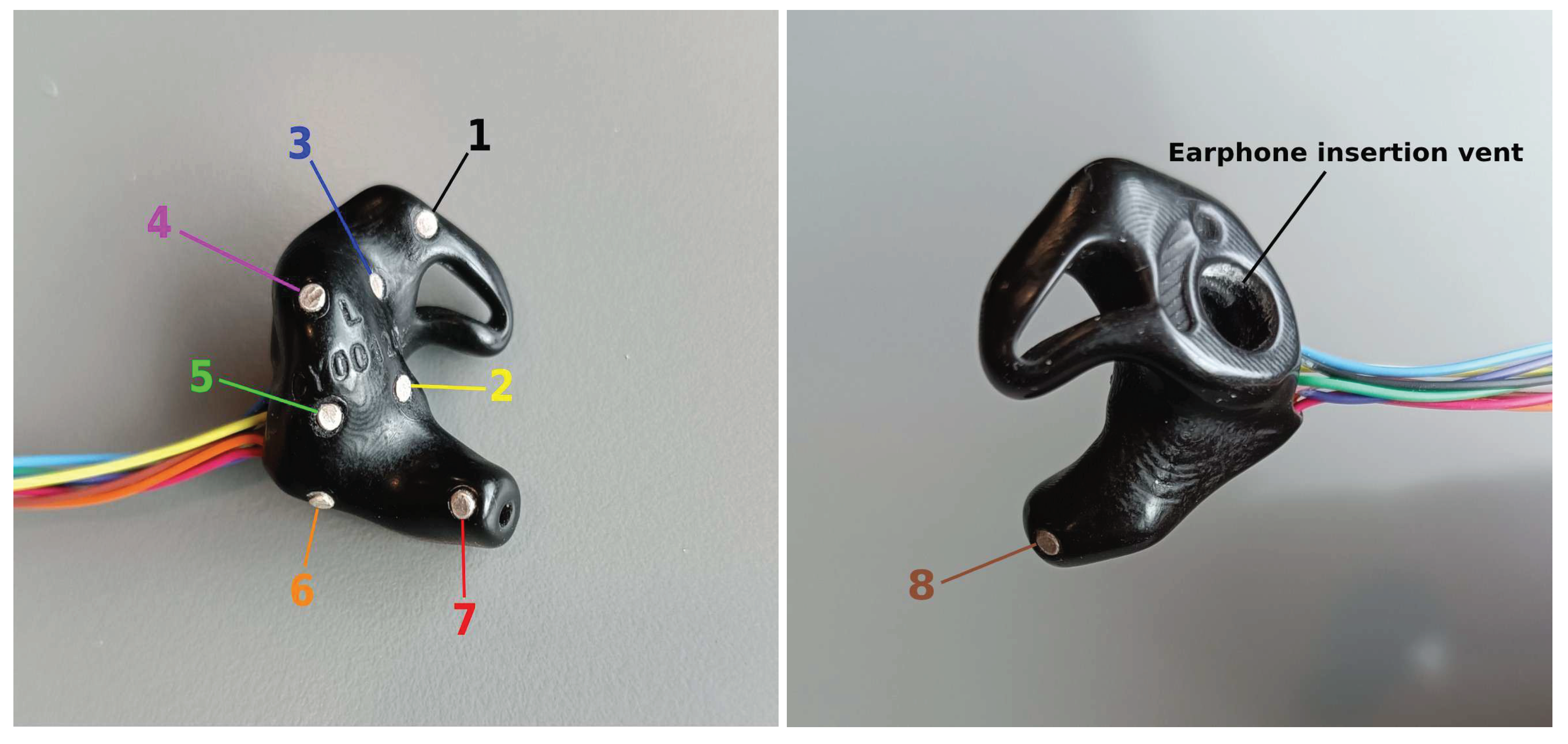
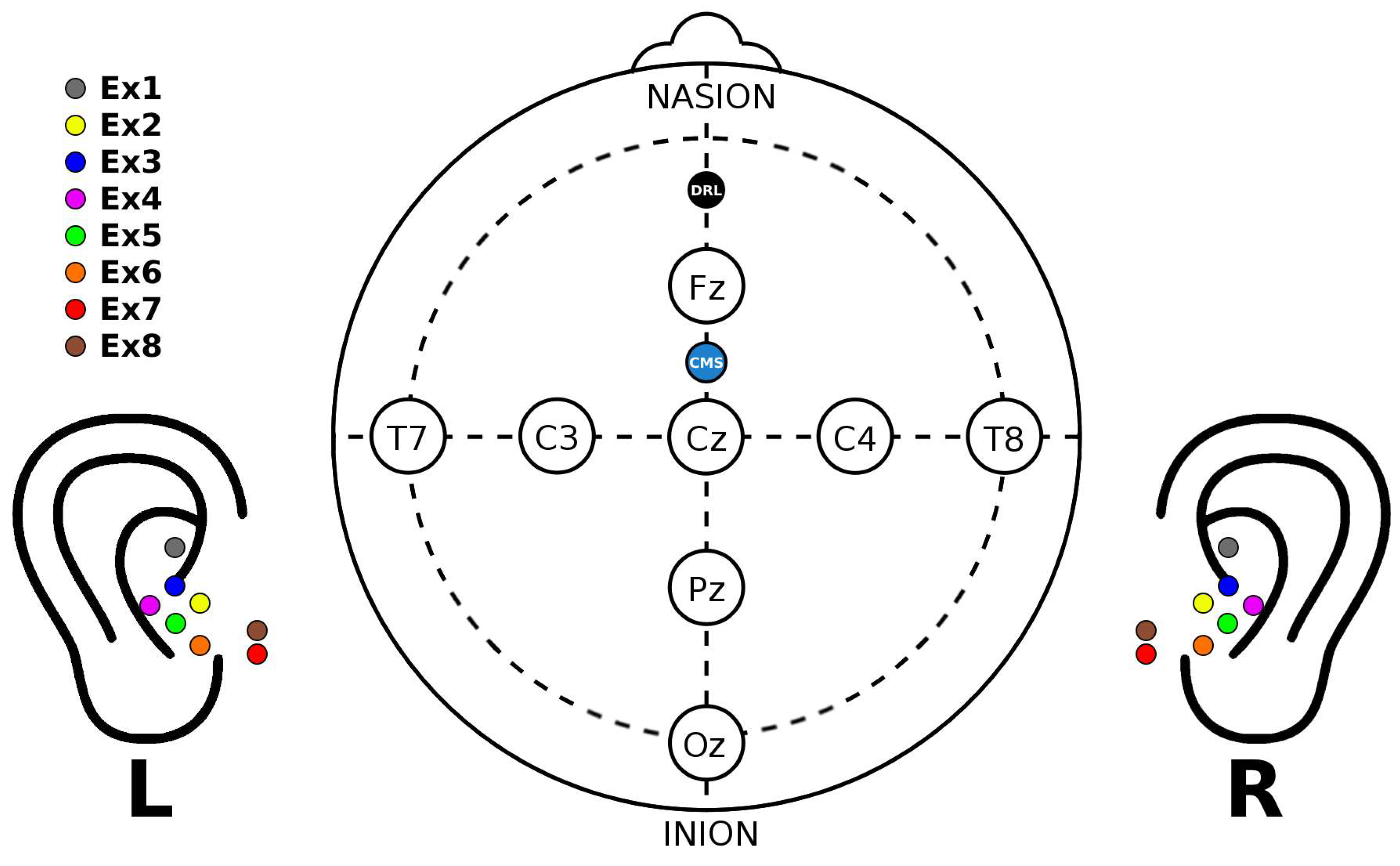
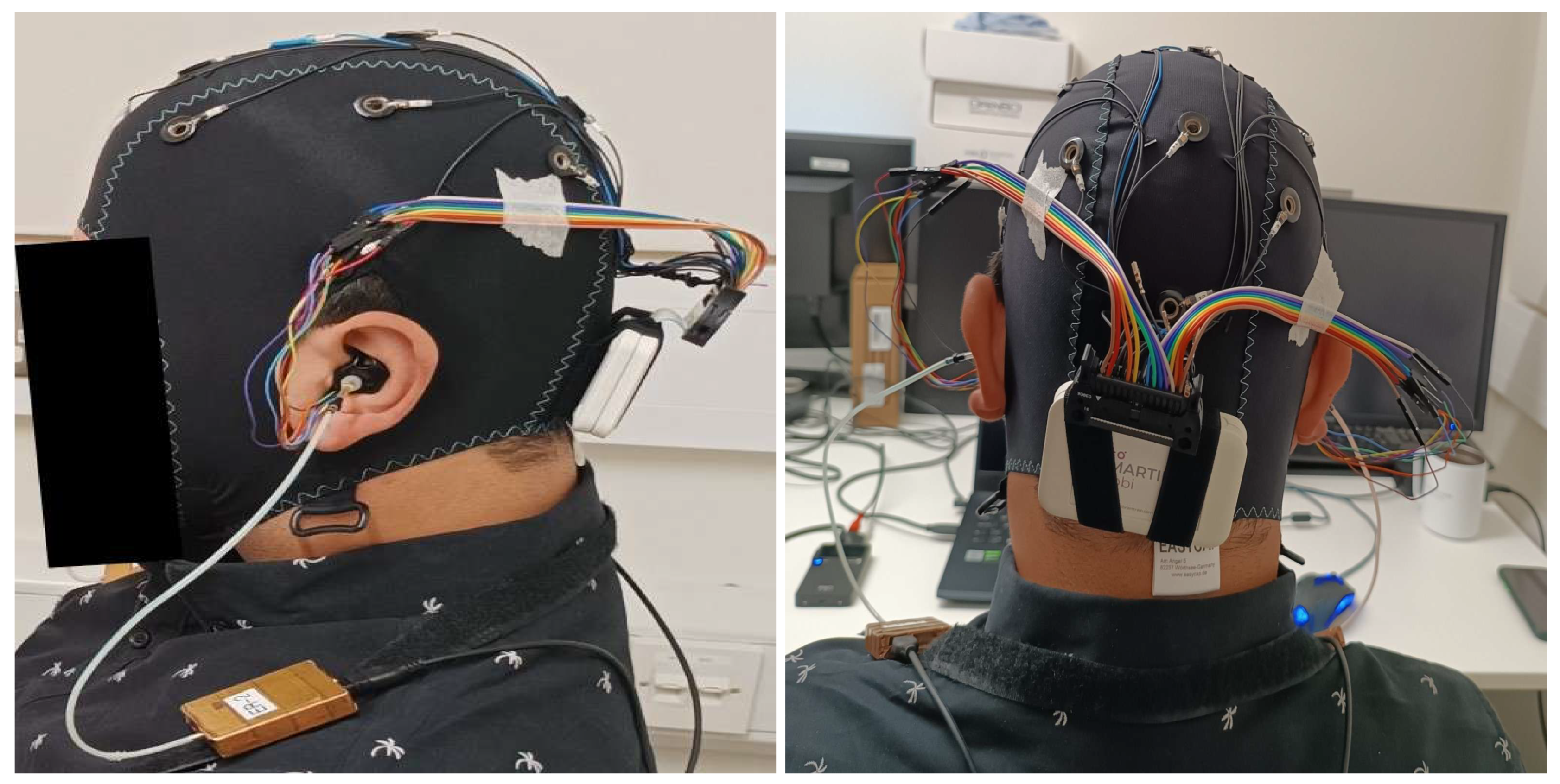
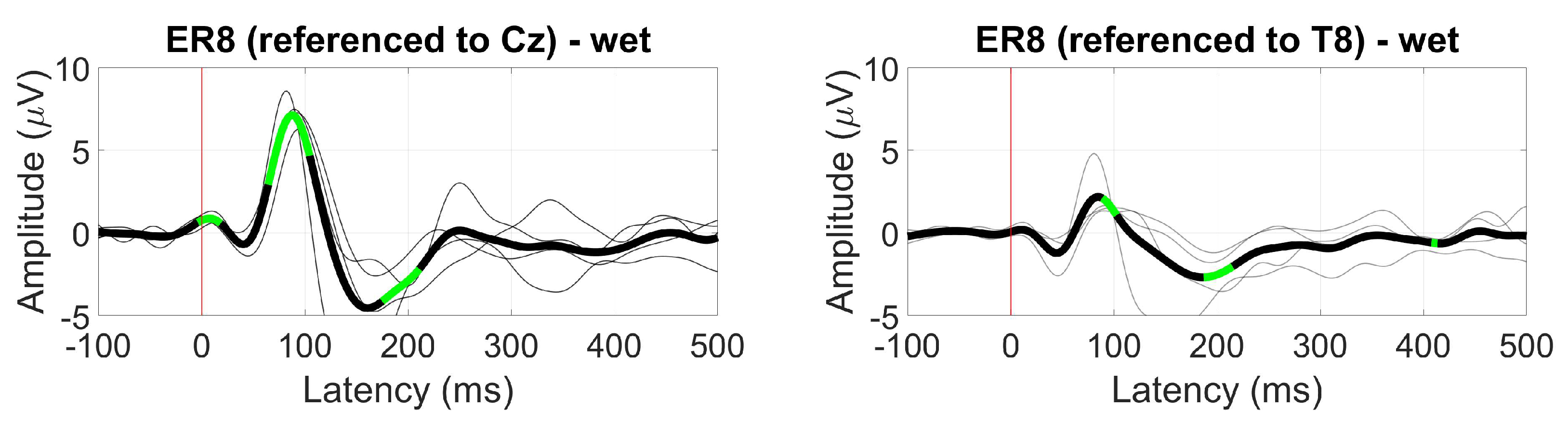
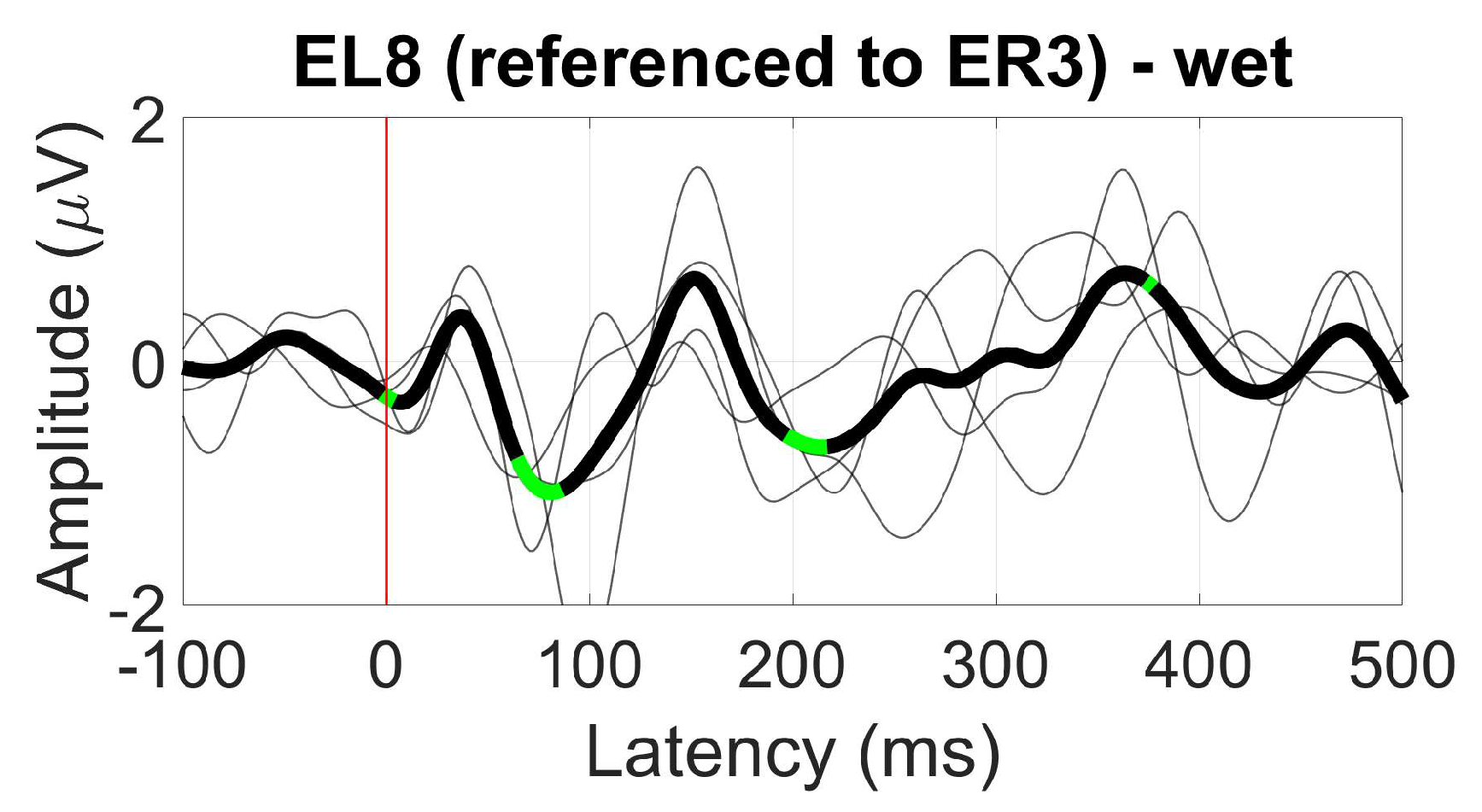
3.1. Tested ear-EEG devices and setup
- Cz, as a standard scalp reference
- T8, as a scalp but closer to the ear reference
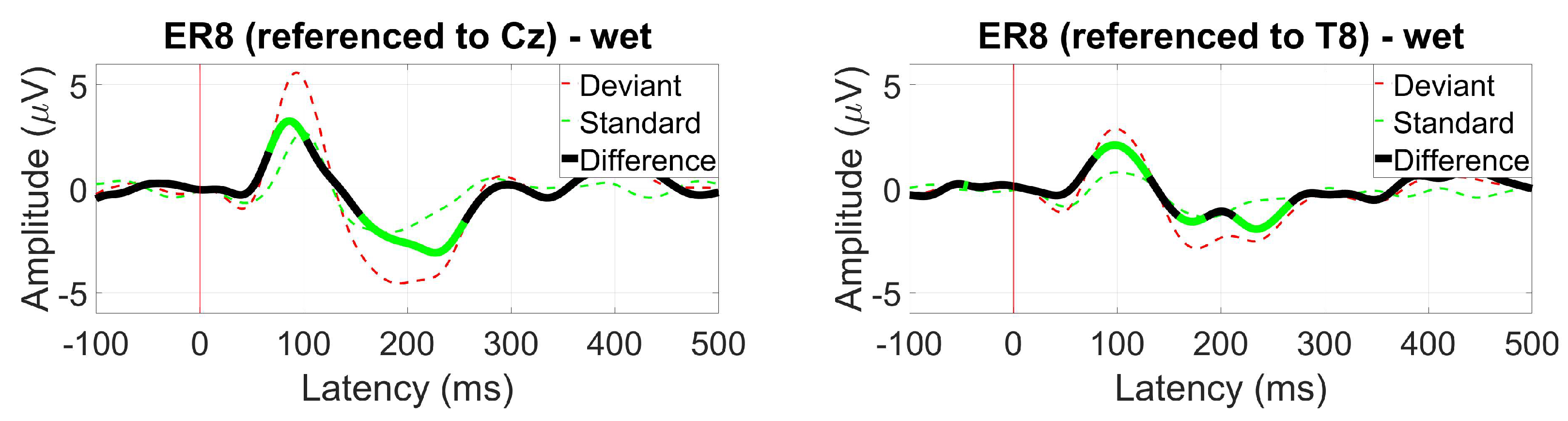
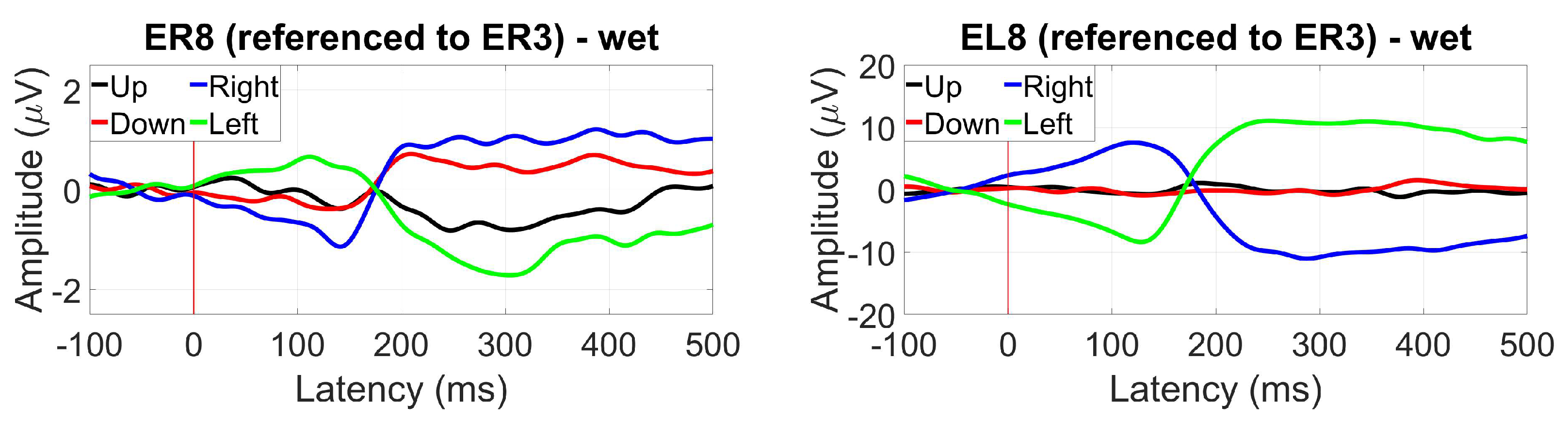
- ER3, as a within-ear (assessing ER8, for example) and between-ears reference (assessing EL8, for instance)
3.2. EaR-P Lab Applied to Ear-EEG Study
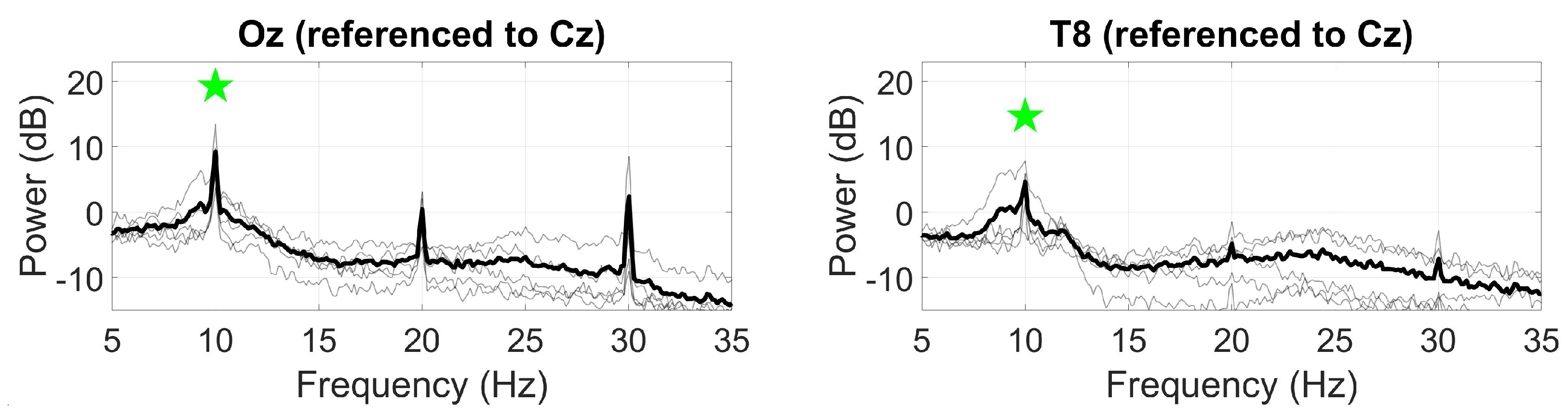
3.2.1. Alpha block
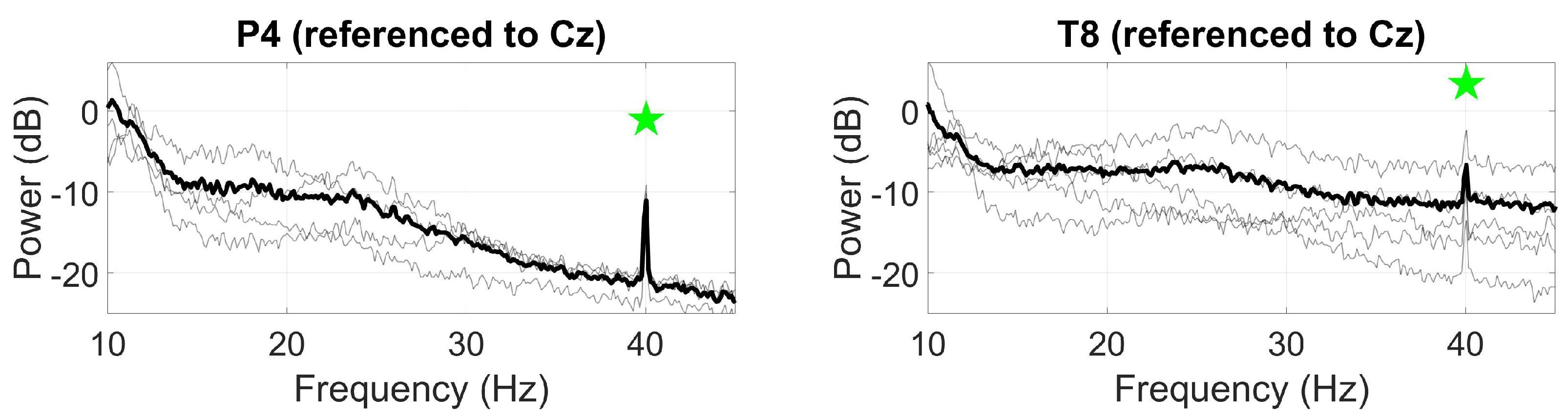
3.2.2. ASSR
3.2.3. SSVEP
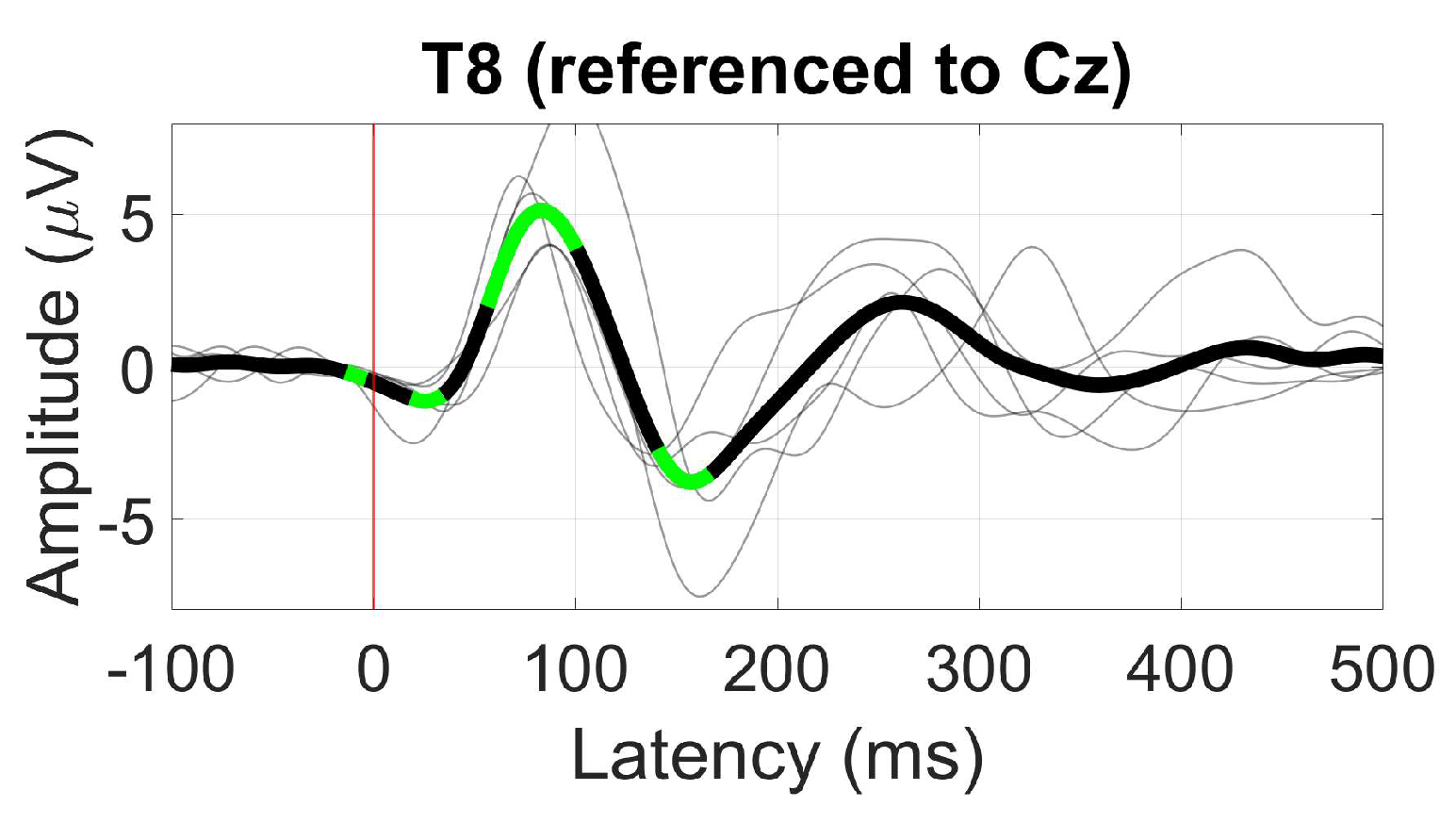
3.2.4. AEP (N100)
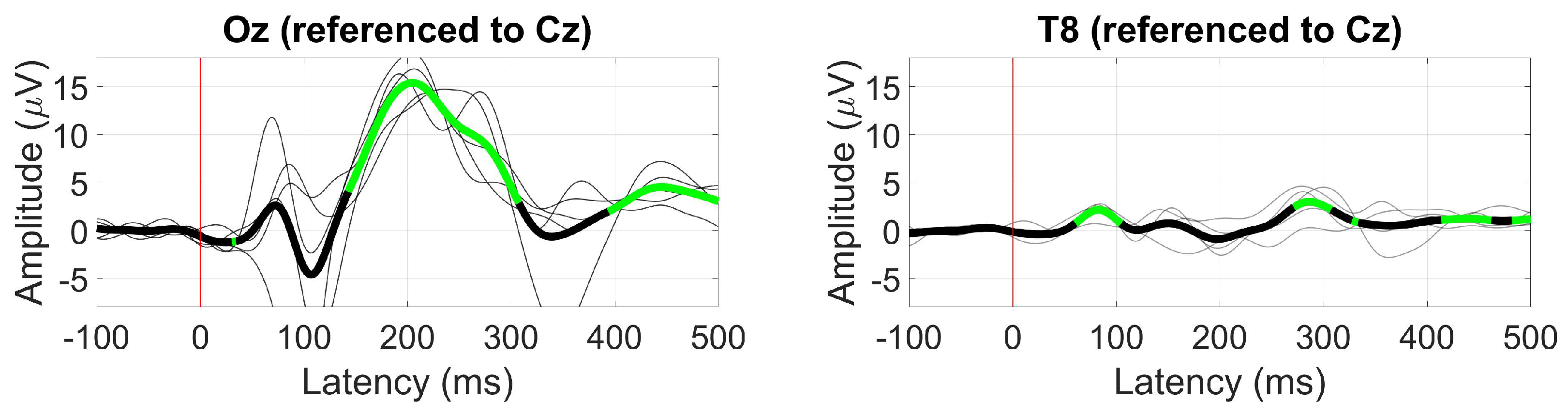
3.2.5. VEP
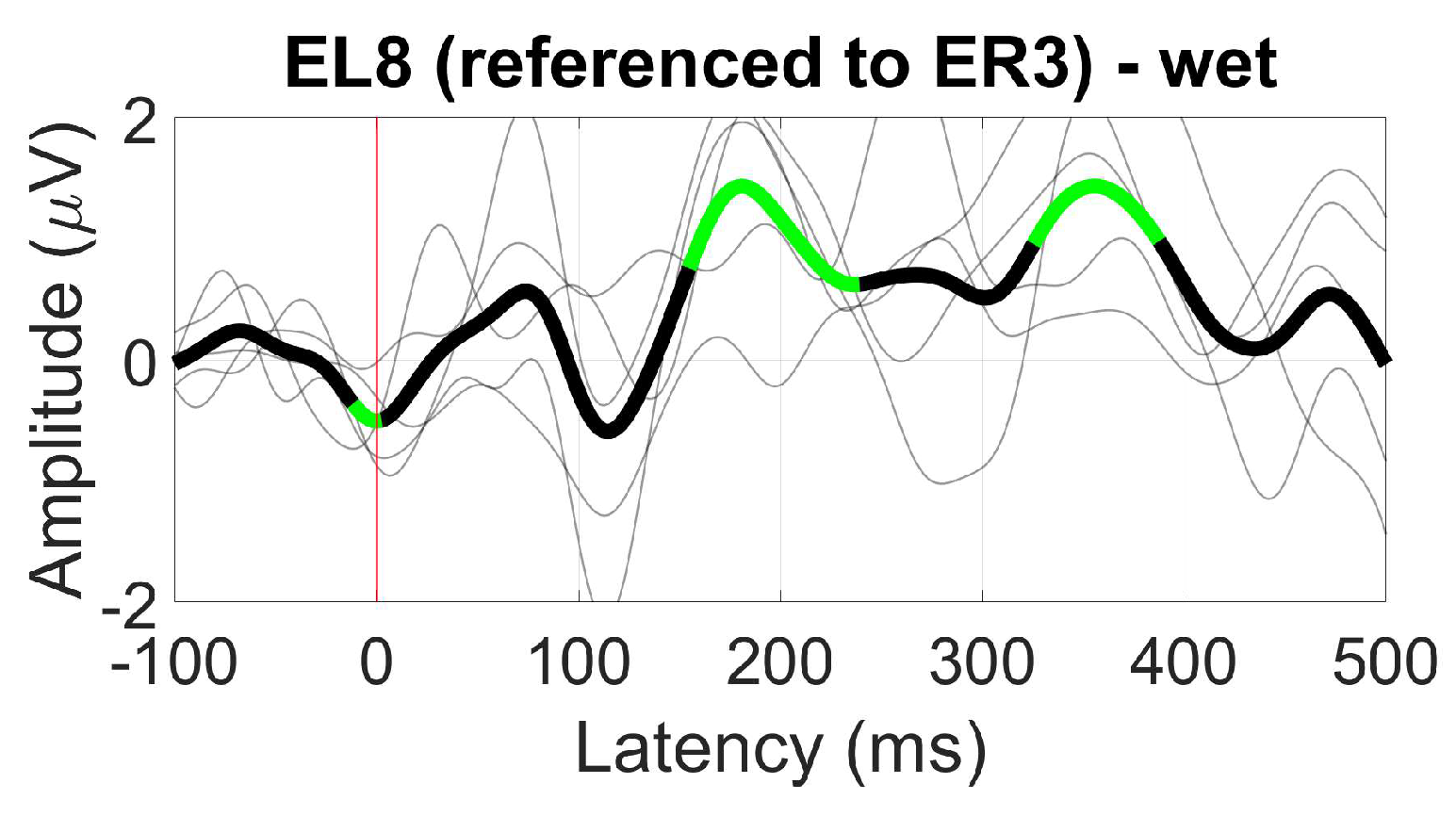
3.2.6. AEP Oddball (MMN)
3.2.7. VEP Oddball (P300)
3.2.8. EOG (Blinks and saccades)
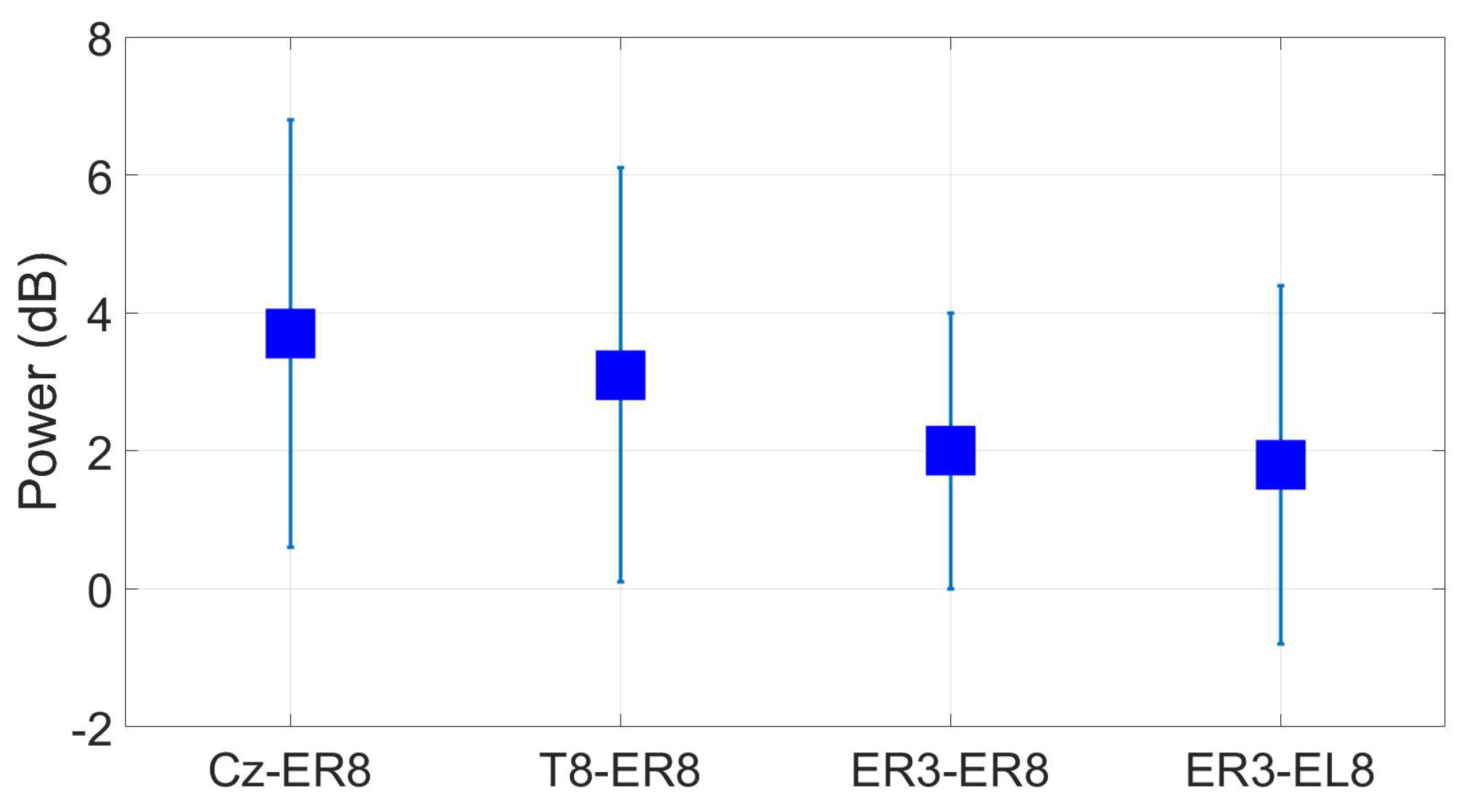
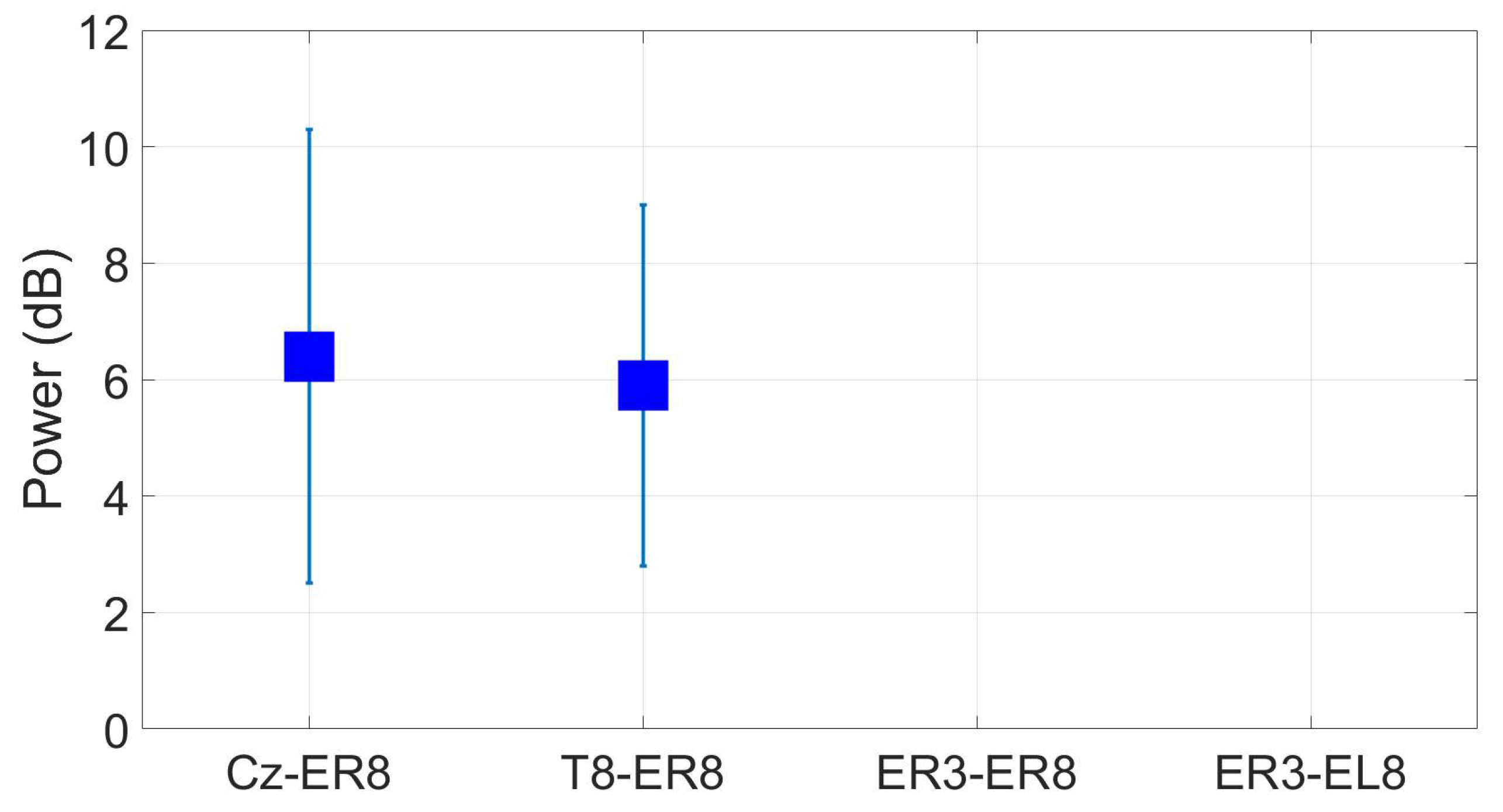
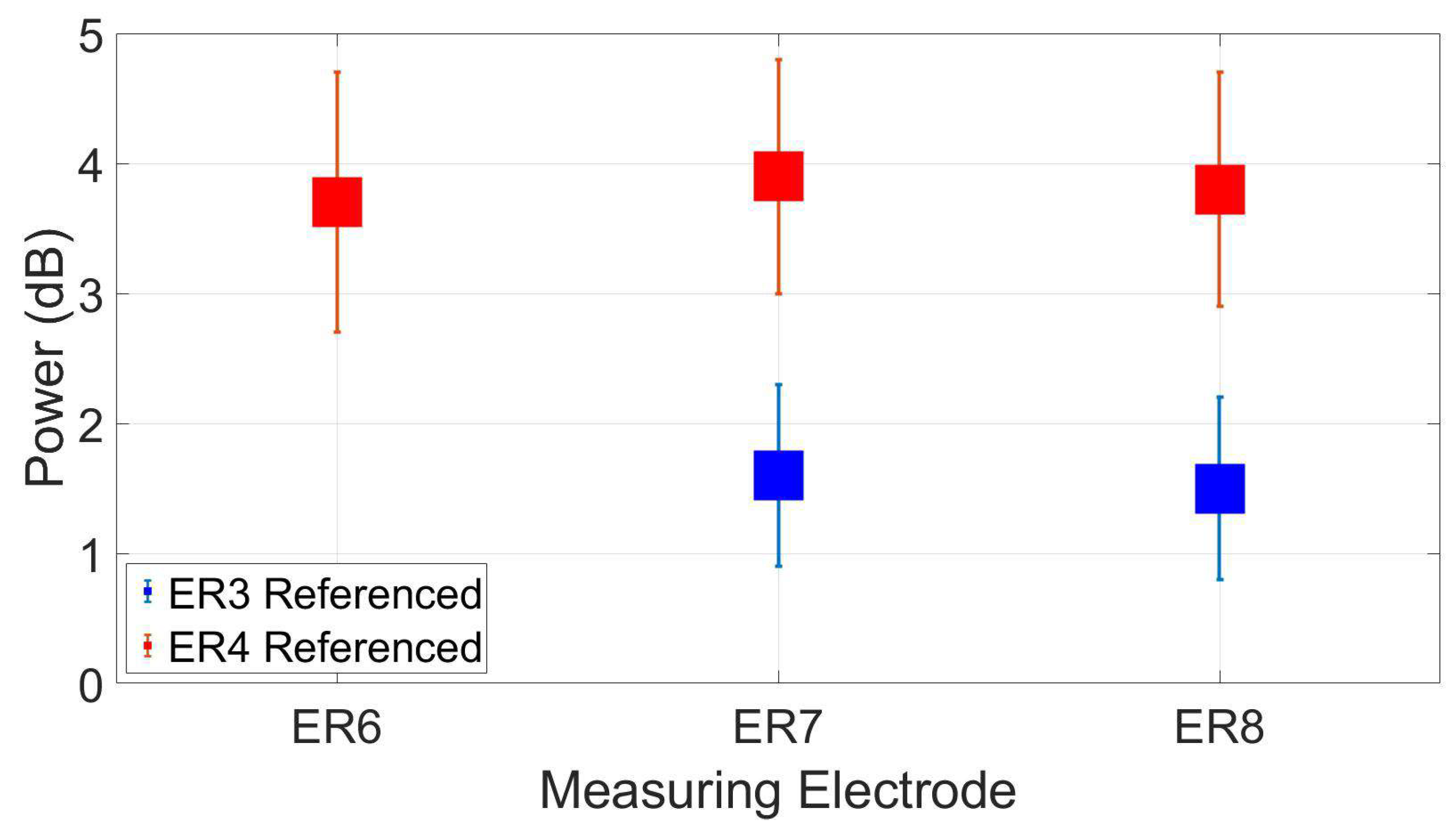
3.3. Reassesment of dry ear-EEG ASSR data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | Electroencephalography |
| BCI | Brain-Computer Interface |
| ERP | Event-Related Potential |
| ASSR | Auditory Steady-State Response |
| SSVEP | Steady-State Visual Evoked Potential |
| AEP | Auditory Evoked Potential |
| VEP | Visual Evoked Potential |
| MMN | Mismatch Negativity |
| EOG | Electro-Oculography |
| ISI | Interstimulus Interval |
| BG | Ballistic Gelatin |
| CF | Carbon Fibers |
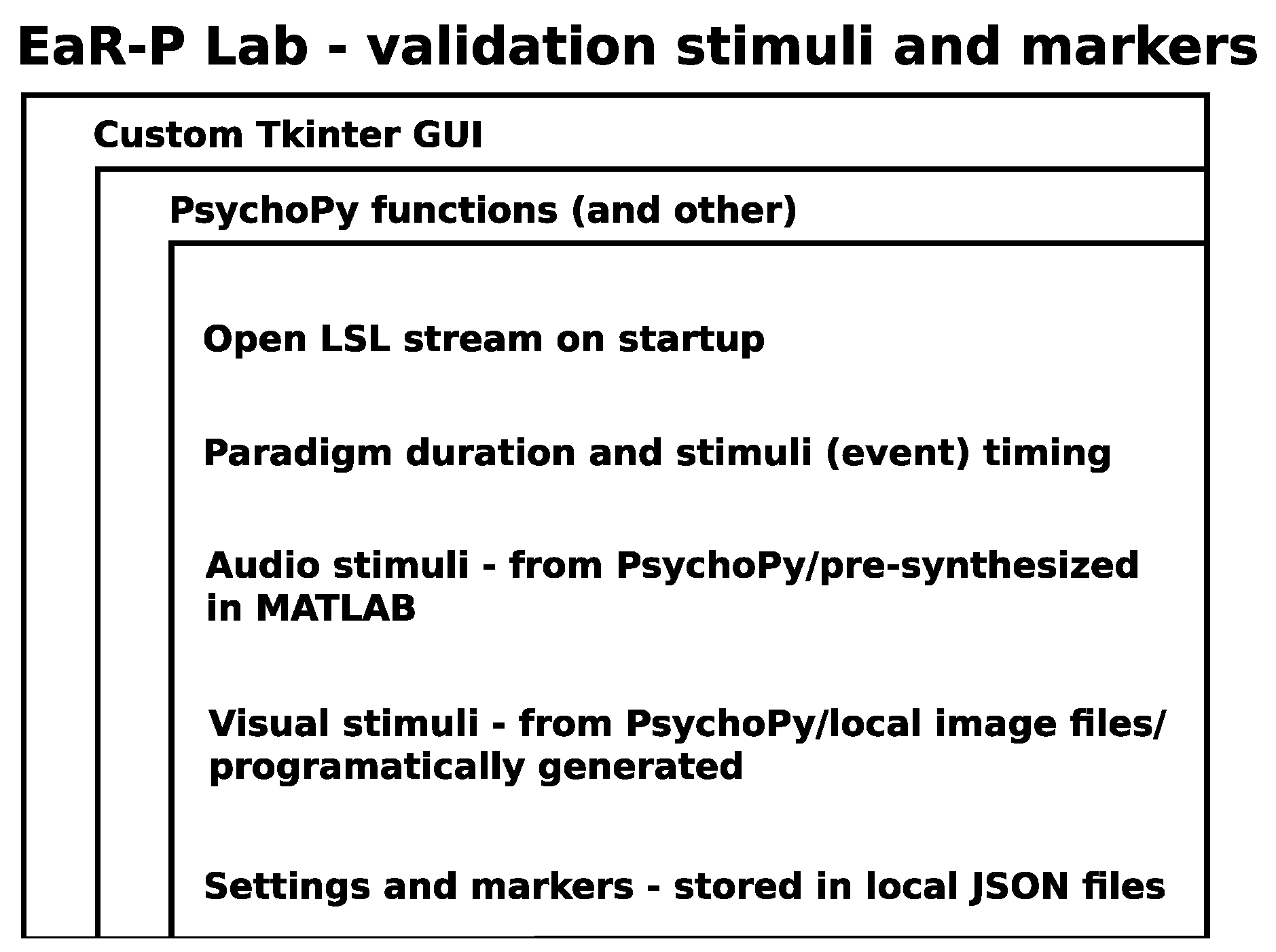
Appendix A. EaR-P Lab Paradigms and Settings
General Recording
Auditory Steady-State Response (ASSR)
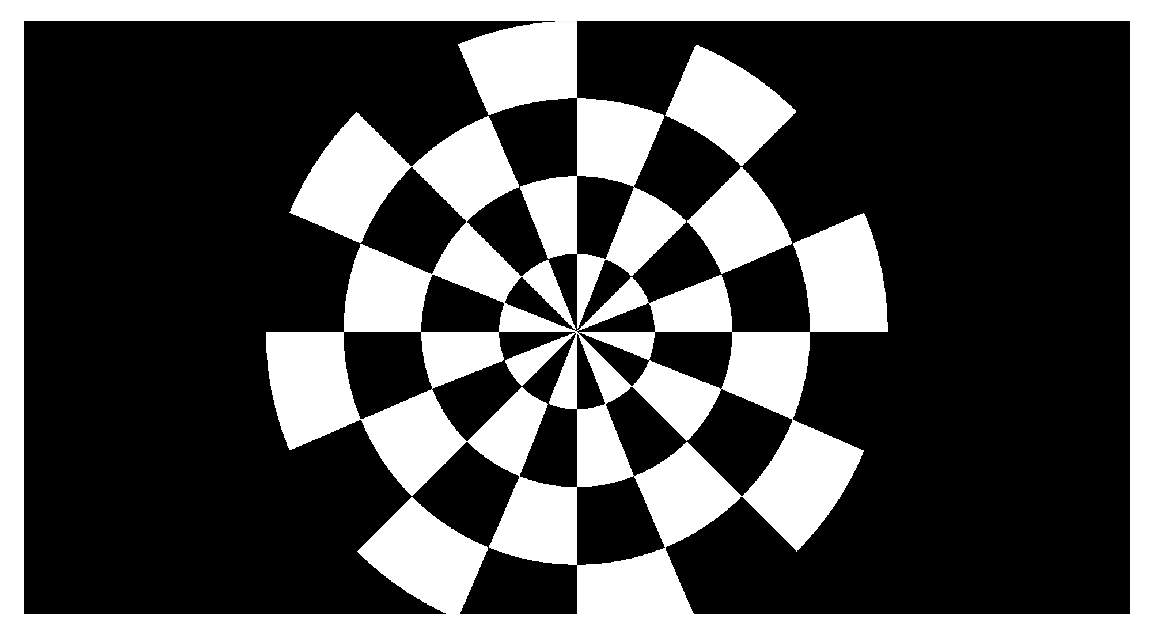
Steady-State Visual Evoked Potential (SSVEP)
Alpha Block
Auditory Evoked Potential (AEP)
Visual Evoked Potential (VEP)
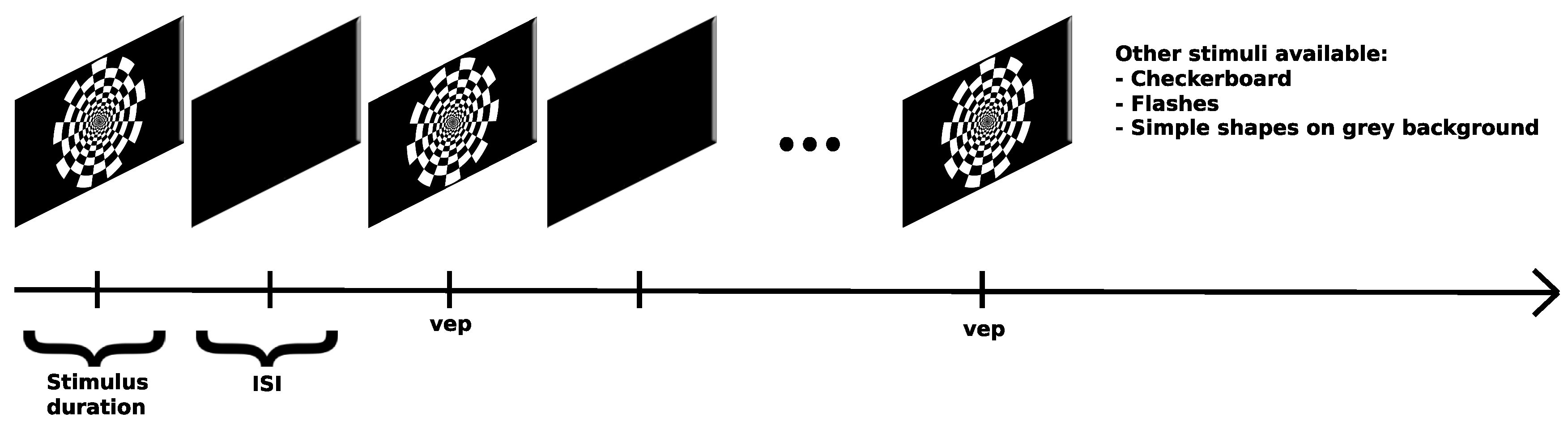
Oddball Paradigms
Electro-Oculography (EOG)
Settings Menu
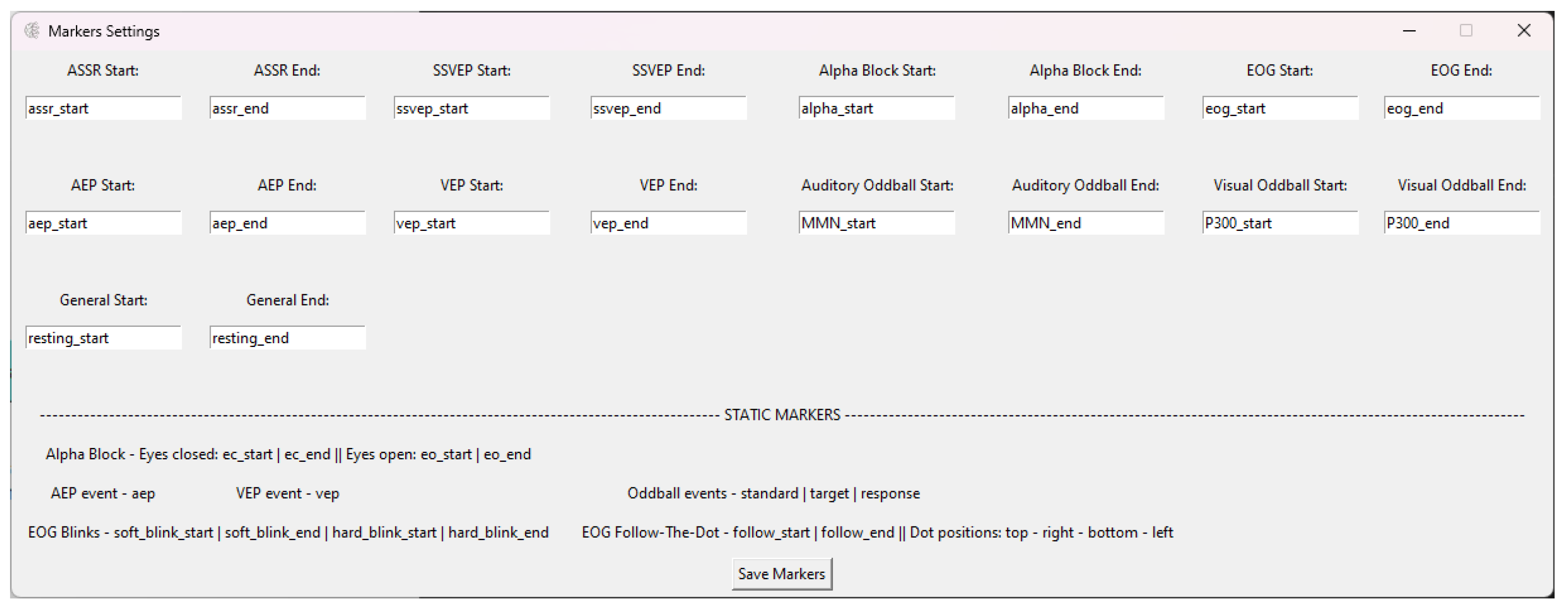
Markers Menu
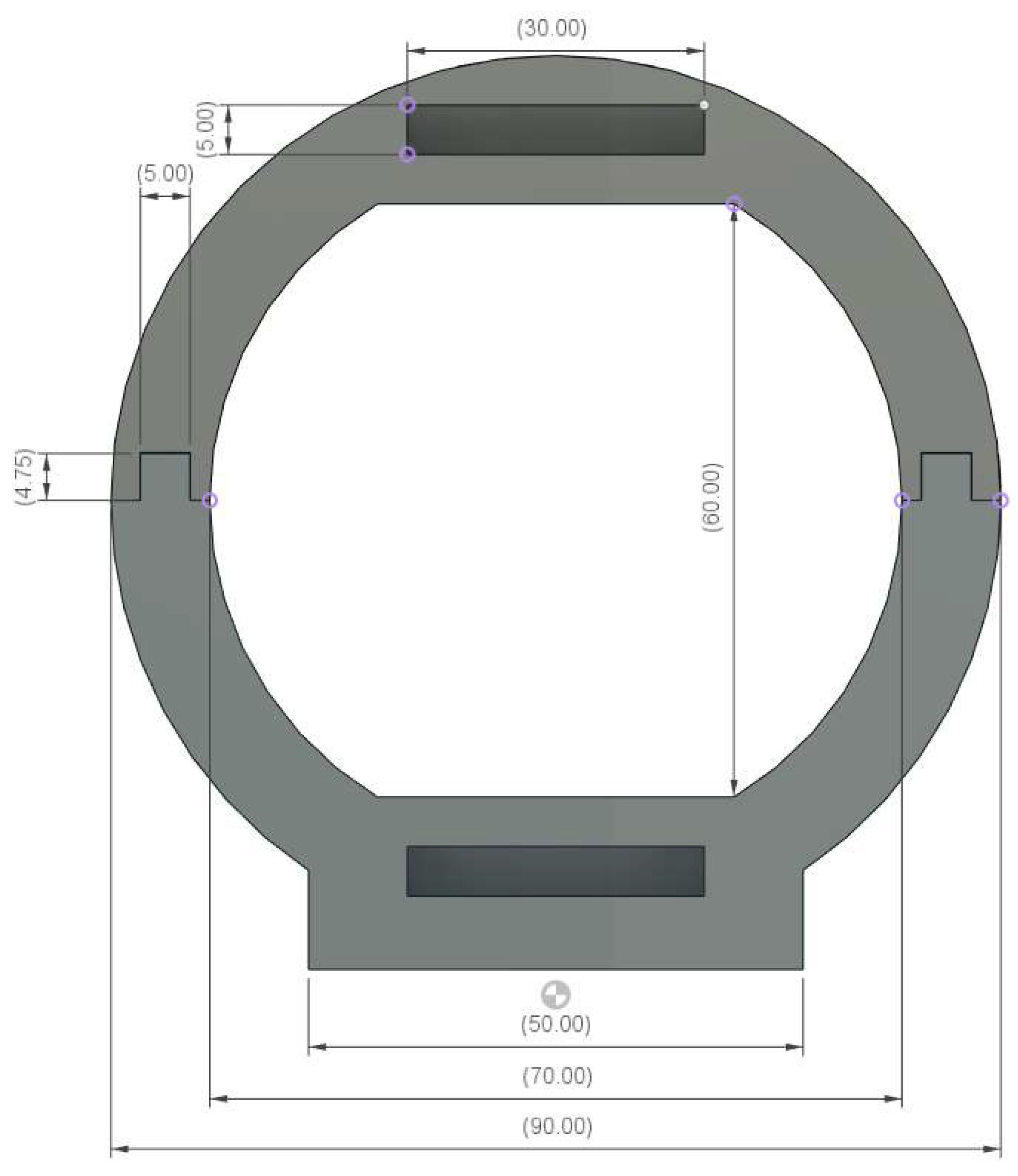
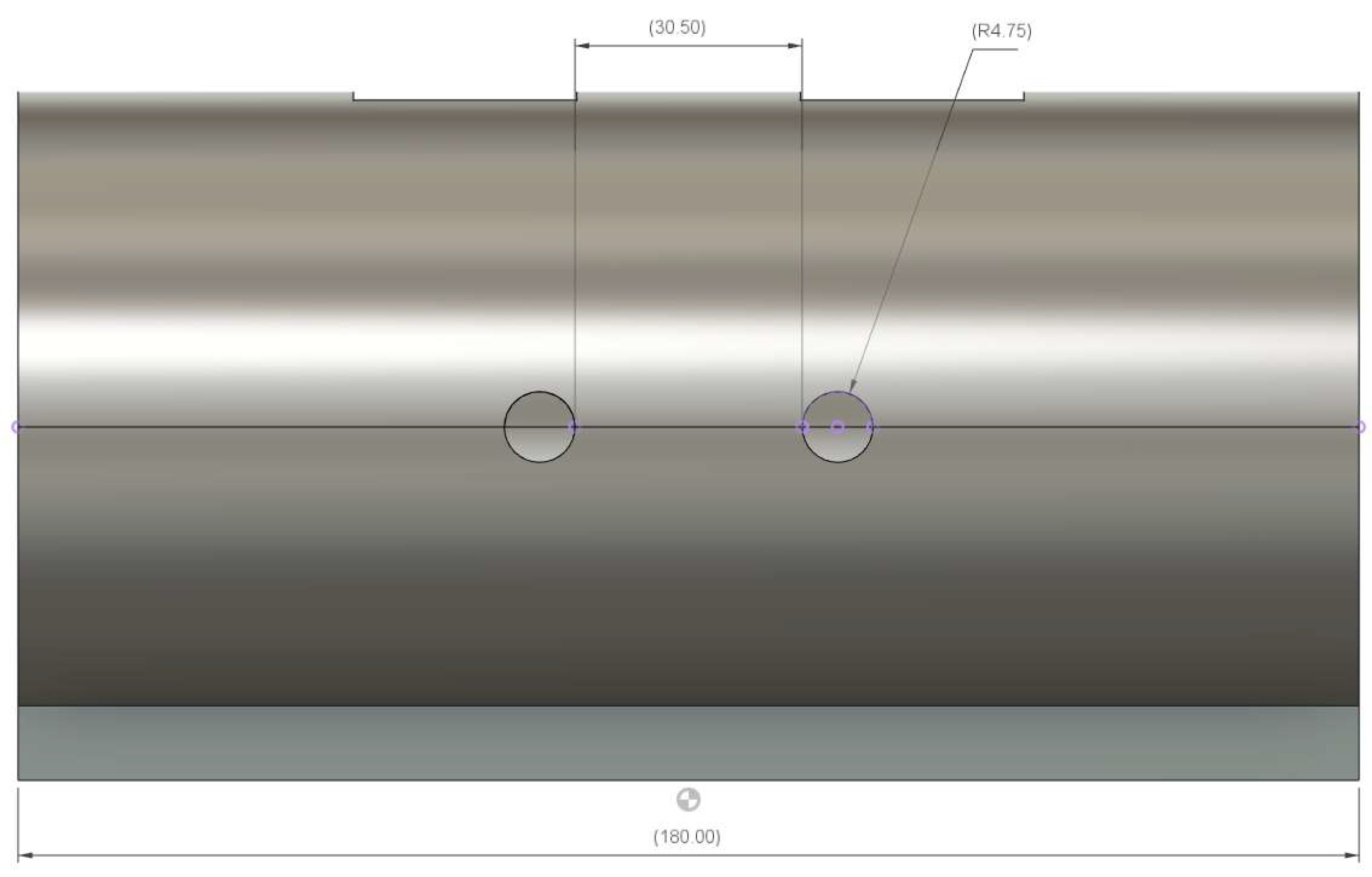
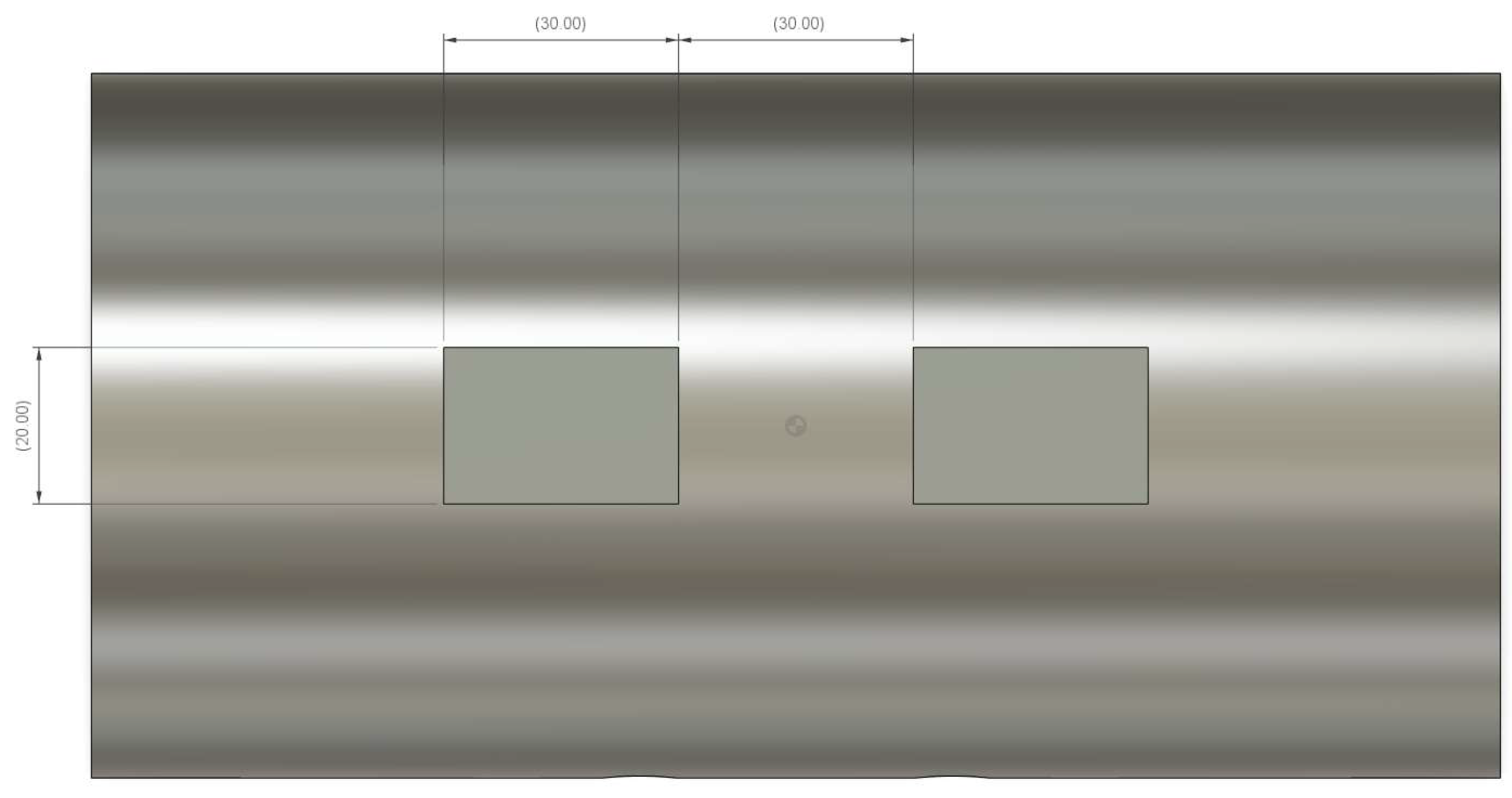
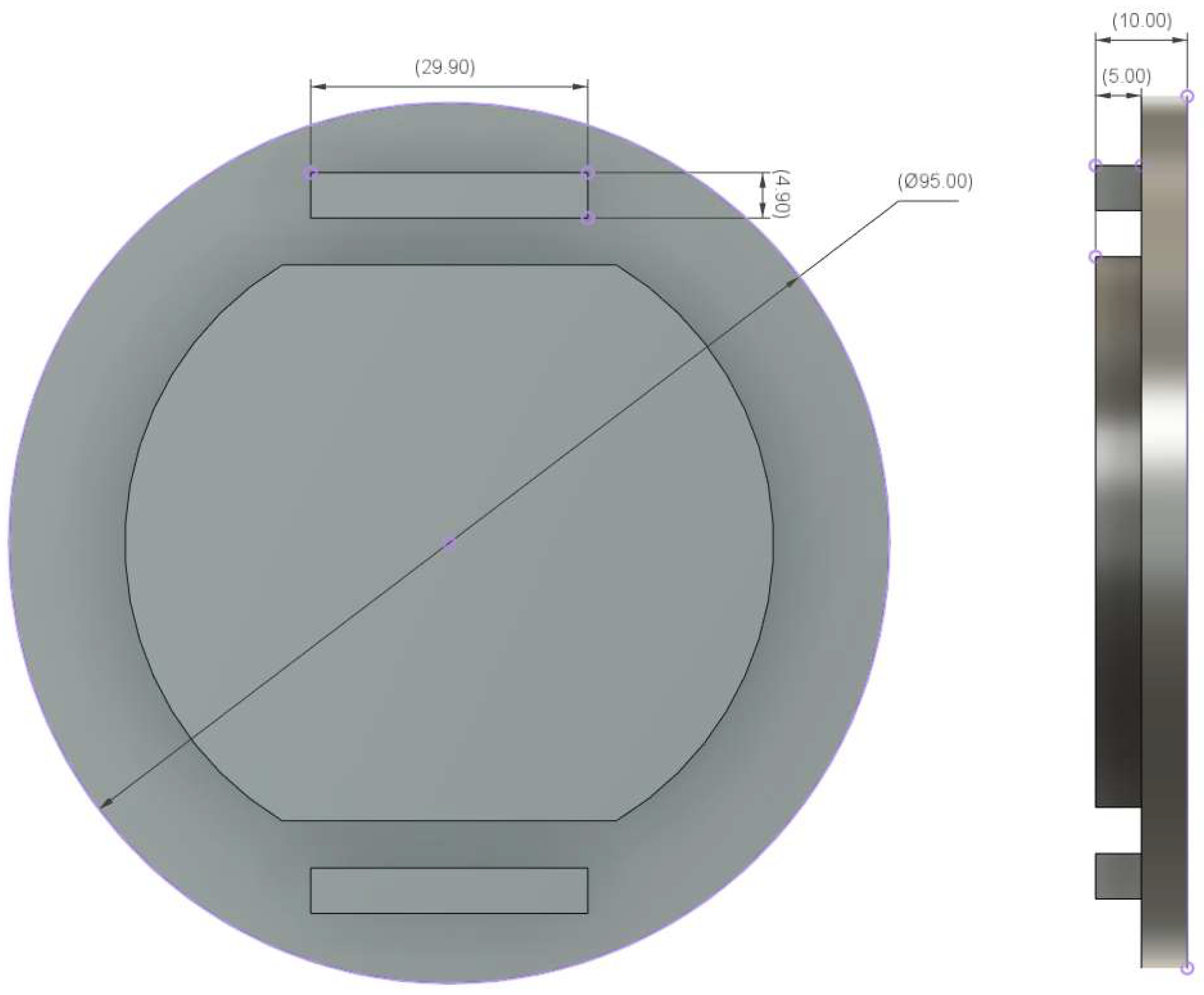
Appendix B. Ear-EEG Phantom Dimensions
References
- Casson, A.; Yates, D.; Smith, S.; Duncan, J.; Rodriguez-Villegas, E. Wearable Electroencephalography. IEEE Engineering in Medicine and Biology Magazine 2010, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.J. Wearable EEG and beyond. Biomedical Engineering Letters 2019, 9, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Soufineyestani, M.; Dowling, D.; Khan, A. Electroencephalography (EEG) Technology Applications and Available Devices. Applied Sciences 2020, 10, 7453. [Google Scholar] [CrossRef]
- Looney, D.; Kidmose, P.; Park, C.; Ungstrup, M.; Rank, M.; Rosenkranz, K.; Mandic, D. The In-the-Ear Recording Concept: User-Centered and Wearable Brain Monitoring. IEEE Pulse 2012, 3, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, S.N.; Sherlin, L.H.; Ford, N.L.; Dalke, D. Validation of a wireless dry electrode system for electroencephalography. Journal of NeuroEngineering and Rehabilitation 2015, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- What are imaging phantoms? https://www.nist.gov/physics/what-are-imaging-phantoms. Available online. Accessed: 11-12-2023.
- Pet-CT phantom. https://www.mirion.com/products/medical/nuclear-medicine-instrumentation/quality-assurance/phantoms/pet-ct-phantom. Available online. Accessed: 11-12-2023.
- Hairston, W.D.; Slipher, G.A.; Yu, A.B. Ballistic gelatin as a putative substrate for EEG phantom devices, 2016. [CrossRef]
- Richer, N.; Downey, R.J.; Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Adding neck muscle activity to a head phantom device to validate mobile EEG muscle and motion artifact removal. 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), 2019, pp. 275–278. [CrossRef]
- Chowdhury, M.E.; Khandakar, A.; Hossain, B.; Alzoubi, K. Effects of the phantom shape on the gradient artefact of electroencephalography (EEG) data in simultaneous EEG-fMRI. Applied Sciences (Switzerland) 2018, 8. [Google Scholar] [CrossRef]
- Audette, W.E.; Allen, L.V. Design and Demonstration of a Head Phantom for Testing of Electroencephalography (EEG) Equipment Hearing Protection View project DPOAE Level and Phase Mapping View project. [CrossRef]
- Wood, S.; Martins, T.; Ibrahim, T.S. How to design and construct a 3D-printed human head phantom. Journal of 3D Printing in Medicine 2019, 3, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Marathe, A.R.; Ries, A.J.; McDowell, K. Sliding HDCA: Single-trial eeg classification to overcome and quantify temporal variability. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2014, 22, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Leahy, R.; Mosher, J.; Spencer, M.; Huang, M.; Lewine, J. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalography and Clinical Neurophysiology 1998, 107, 159–173. [Google Scholar] [CrossRef]
- Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Dual-electrode motion artifact cancellation for mobile electroencephalography. Journal of Neural Engineering 2018, 15. [Google Scholar] [CrossRef]
- Kuratko, D.; Lacik, J.; Koudelka, V.; Vejmola, C.; Wojcik, D.K.; Raida, Z. Forward Model of Rat Electroencephalogram: Comparative Study of Numerical Simulations With Measurements on Rat Head Phantoms. IEEE Access 2022, 10, 92023–92035. [Google Scholar] [CrossRef]
- Owda, A.Y.; Casson, A.J. Investigating Gelatine Based Head Phantoms for Electroencephalography Compared to Electrical and Ex Vivo Porcine Skin Models. IEEE Access 2021, 9, 96722–96738. [Google Scholar] [CrossRef]
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.A.; Jones, A.K.; Casson, A.J. Flexible 3D-printed EEG electrodes. Sensors (Switzerland) 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Doong, J.; Zhou, A.; Schwendeman, C.; Gopalan, K.; Burghardt, F.L.; Arias, A.C.; Maharbiz, M.M.; Muller, R. Wireless User-Generic Ear EEG. IEEE Transactions on Biomedical Circuits and Systems 2020, 14, 727–737. [Google Scholar] [CrossRef]
- Kappel, S.L.; Rank, M.L.; Toft, H.O.; Andersen, M.; Kidmose, P. Dry-Contact Electrode Ear-EEG. IEEE Transactions on Biomedical Engineering 2019, 66, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kaongoen, N.; Choi, J.; Choi, J.W.; Kwon, H.; Hwang, C.; Hwang, G.; Kim, B.H.; Jo, S. The future of wearable EEG: a review of ear-EEG technology and its applications. Journal of Neural Engineering 2023, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Röddiger, T.; Clarke, C.; Breitling, P.; Schneegans, T.; Zhao, H.; Gellersen, H.; Beigl, M. Sensing with Earables: A Systematic Literature Review and Taxonomy of Phenomena. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2022, 6. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; ad Richard Höchenberger, M.M.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in behavior made easy. Behaviour Research Methods 2019, pp. 195–203. [CrossRef] [PubMed]
- Kothe, C.; Medine, D.; Boulay, C.; Grivich, M.; Stenner, T. Lab Streaming Layer open source repository. https://github.com/sccn/labstreaminglayer.
- Razavi, M.; Yamauchi, T.; Janfaza, V.; Leontyev, A.; Longmire-Monford, S.; Orr, J. Multimodal-Multisensory Experiments. Preprints 2020. [Google Scholar] [CrossRef]
- Event triggering and data synchronization with mobile EEG fully mobile EEG devices. https://mbraintrain.com/event-triggering-and-data-synchronization-with-mobile-eeg/. Available online. Accessed: 11-12-2023.
- Setting up precise sound stimulation with psychopy. https://mbraintrain.com/how-to-set-up-precise-sound-stimulation-with-psychopy-and-pylsl. Available online. Accessed: 11-12-2023.
- Bridges, D.; Pitiot, A.; MacAskill, M.R.; Peirce, J.W. The timing mega-study: comparing a range of experiment generators, both lab-based and online. PeerJ 2020, 8, e9414. [Google Scholar] [CrossRef] [PubMed]
- Can PsychoPy deliver millisecond precision. https://www.psychopy.org/general/timing/millisecondPrecision. Available online. Accessed: 11-12-2023.
- Mobile EEG - smarting mobi. https://mbraintrain.com/smarting-mobi/. Available online. Accessed: 11-12-2023.
- Bishop, D.V.M.; Hardiman, M.J. Measurement of mismatch negativity in individuals: A study using single-trial analysis. Psychophysiology 2010, 47, 697–705. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG From Viscoelastic Generic Earpieces: Robust and Unobtrusive 24/7 Monitoring. IEEE Sensors Journal 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Soever, A. Fabrication of extrinsically conductive silicone rubbers with high elasticity and analysis of their mechanical and electrical characteristics. Polymers 2010, 2, 200–210. [Google Scholar] [CrossRef]
- SILC circuits: High performance conductive silicone. https://www.instructables.com/Silc-Circuits-High-Performance-Conductive-Silicone/. Available online. Accessed: 11-12-2023.
- OpenBCI Cyton. https://shop.openbci.com/products/cyton-biosensing-board-8-channel. Available online. Accessed: 11-12-2023.
- OpenBCI EEG Setup. https://docs.openbci.com/GettingStarted/Biosensing-Setups/EEGSetup/. Available online. Accessed: 11-12-2023.
- McCann, H.; Pisano, G.; Beltrachini, L. Variation in reported human head tissue electrical conductivity values. Brain Topography 2019, 32, 825–858. [Google Scholar] [CrossRef] [PubMed]
- Koessler, L.; Colnat-Coulbois, S.; Cecchin, T.; Hofmanis, J.; Dmochowski, J.P.; Norcia, A.M.; Maillard, L.G. In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Human Brain Mapping 2016, 38, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.B.; Kappel, S.L.; Mandic, D.P.; Kidmose, P. EEG Recorded from the Ear: Characterizing the Ear-EEG Method. Frontiers in Neuroscience 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Induction and separation of motion artifacts in EEG data using a mobile phantom head device. Journal of Neural Engineering 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Vanderwal, T.; Kelly, C.; Eilbott, J.; Mayes, L.C.; Castellanos, F.X. Inscapes : A movie paradigm to improve compliance in functional magnetic resonance imaging. NeuroImage 2015, 122, 222–232. [Google Scholar] [CrossRef] [PubMed]


| Conductivity [S/m] | |
|---|---|
| Agar | 0.309 |
| BG | 0.918 |
| CF (1%) | 14.035 |
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| Agar | 855 | 851 | 850 | 845 |
| BG | 963 | 959 | 958 | 956 |
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| Agar | 44 | 40 | 40 | 36 |
| BG | 80 | 52 | 52 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





