Submitted:
19 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biological material
2.2. Experimental phase 1: disinfection procedure for the establishment of biological material
2.2.1. In vitro base culture medium and general maintenance conditions
2.2.2. In vitro multiplication
2.3. Experimental phase 2: callogenesis induction
Prevention of oxidation in calli
2.4. Feasibility of the callogenesis protocol
2.5. Statistical analysis
3. Results
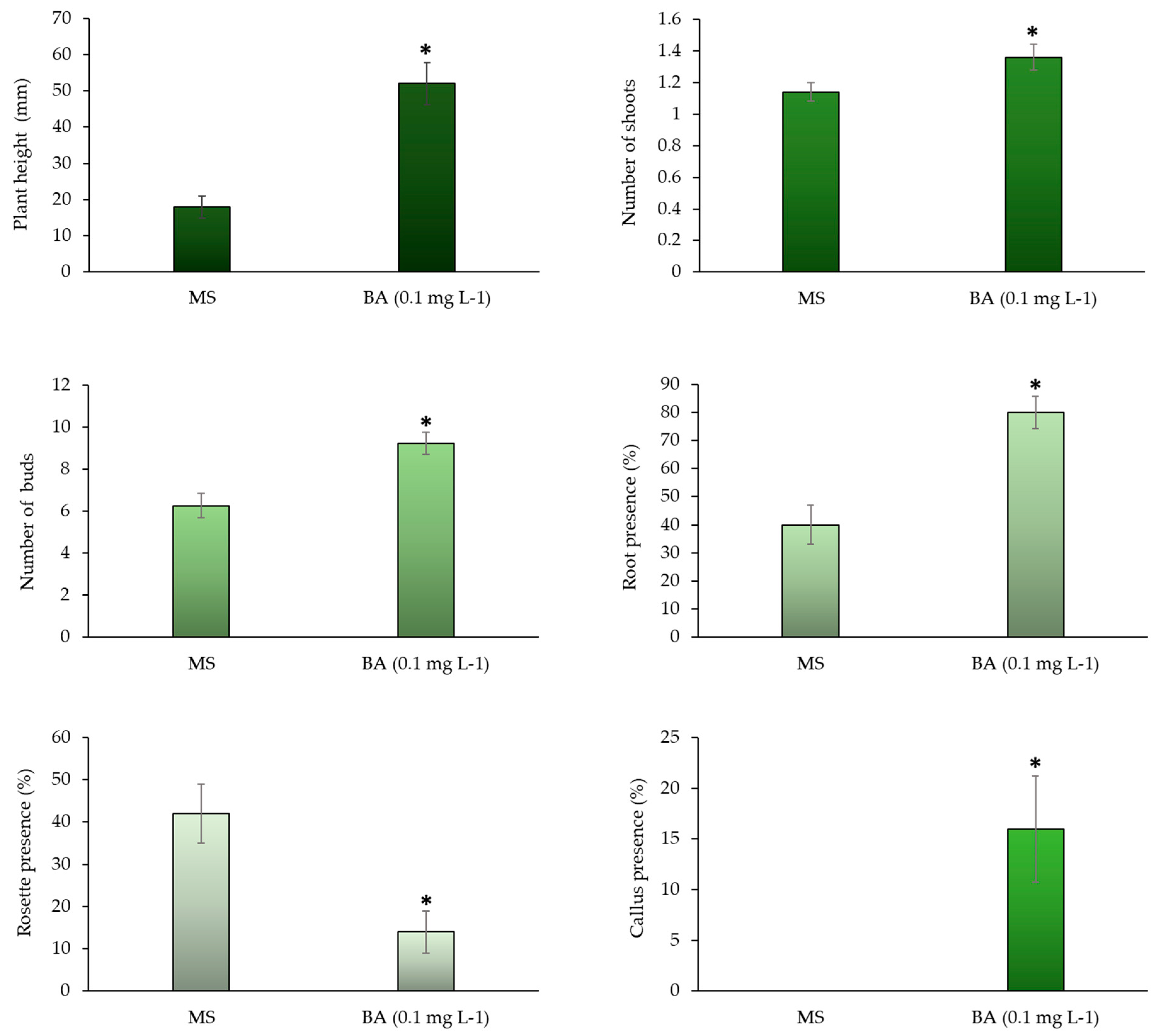
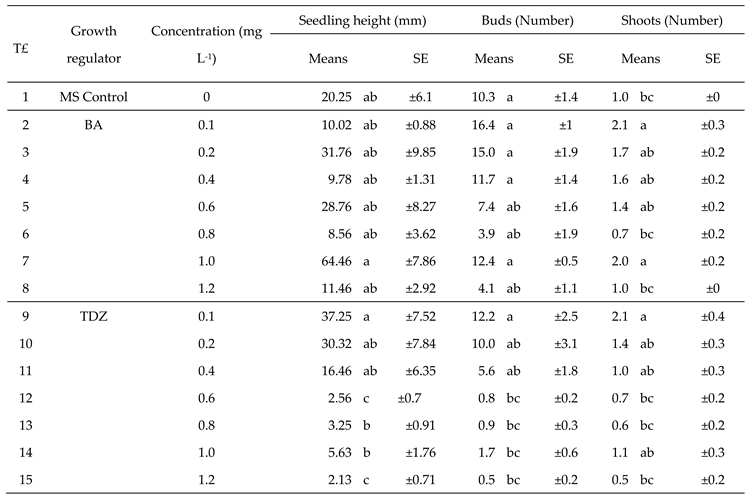
3.1. Experimental phase 1: in vitro multiplication
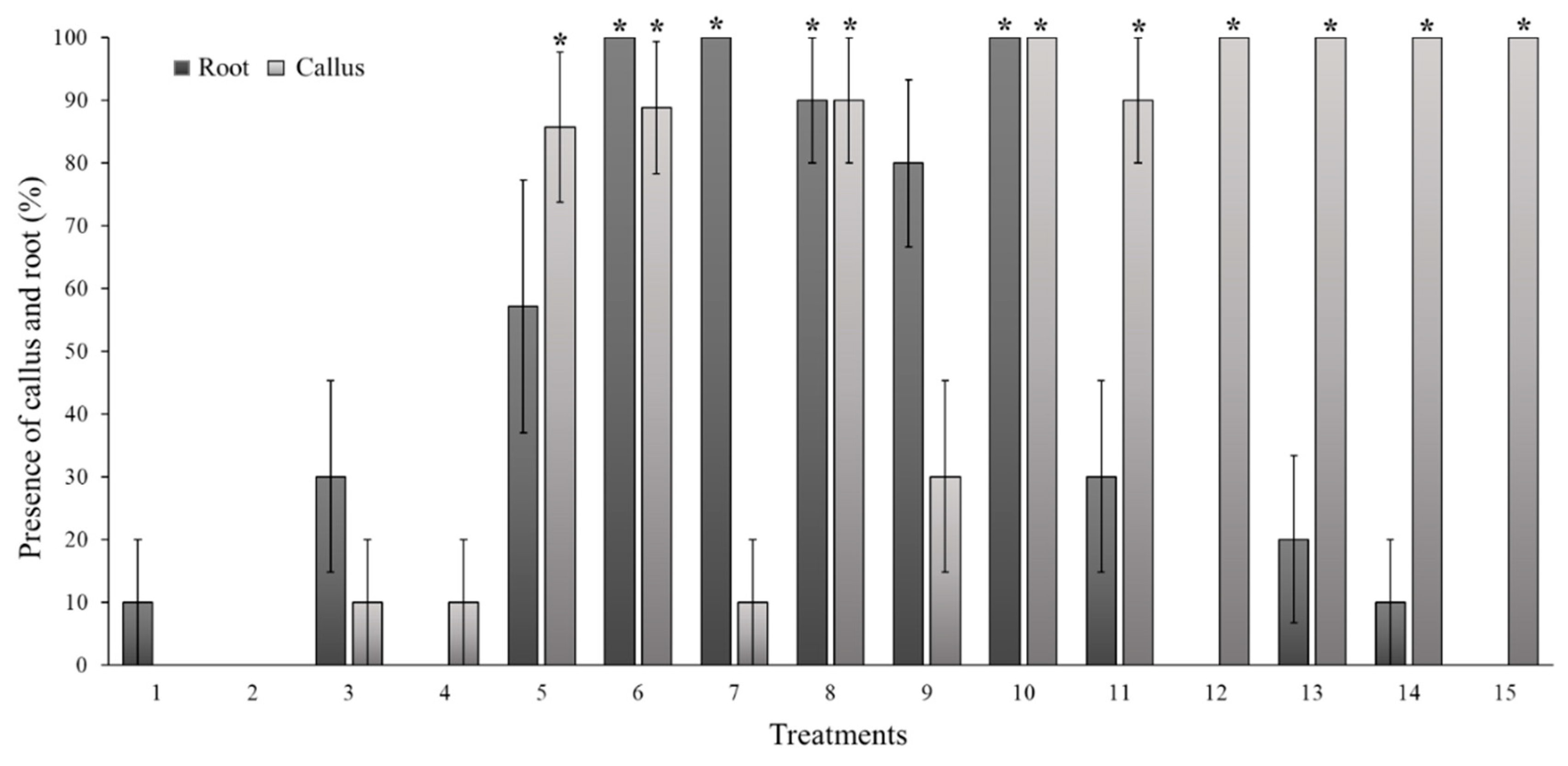
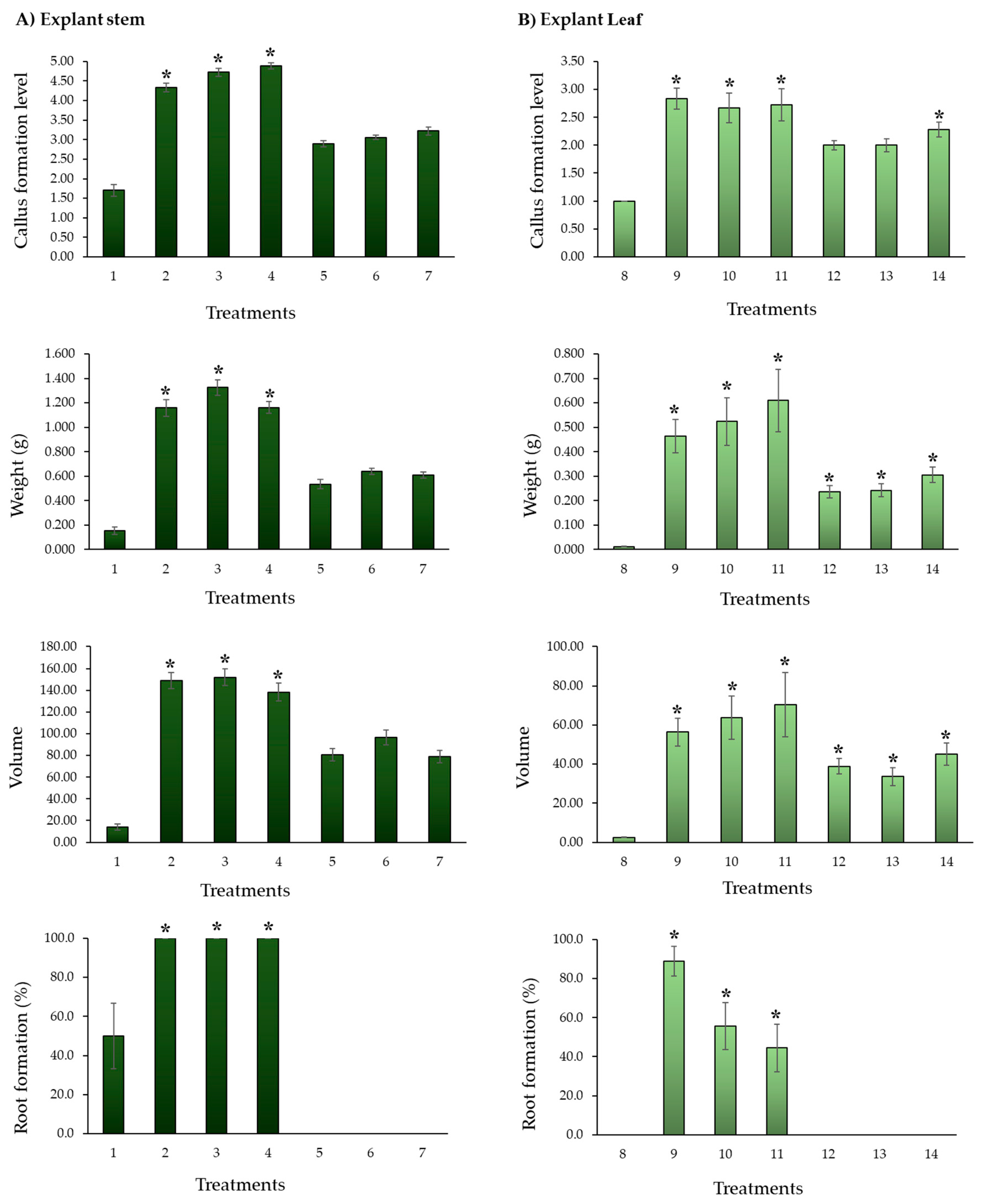
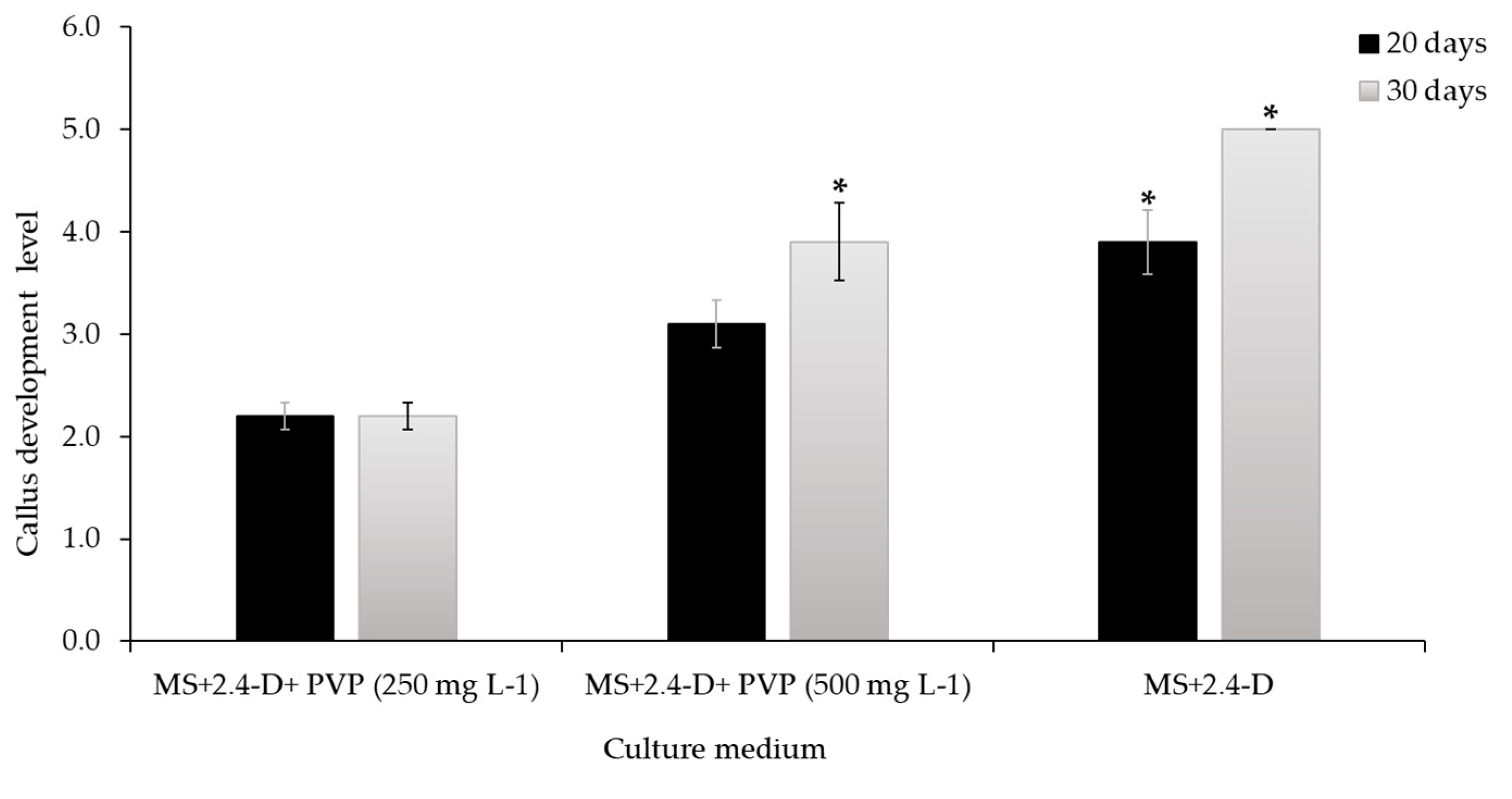
3.2. Experimental phase 2: callogenesis induction
Control of callus oxidation
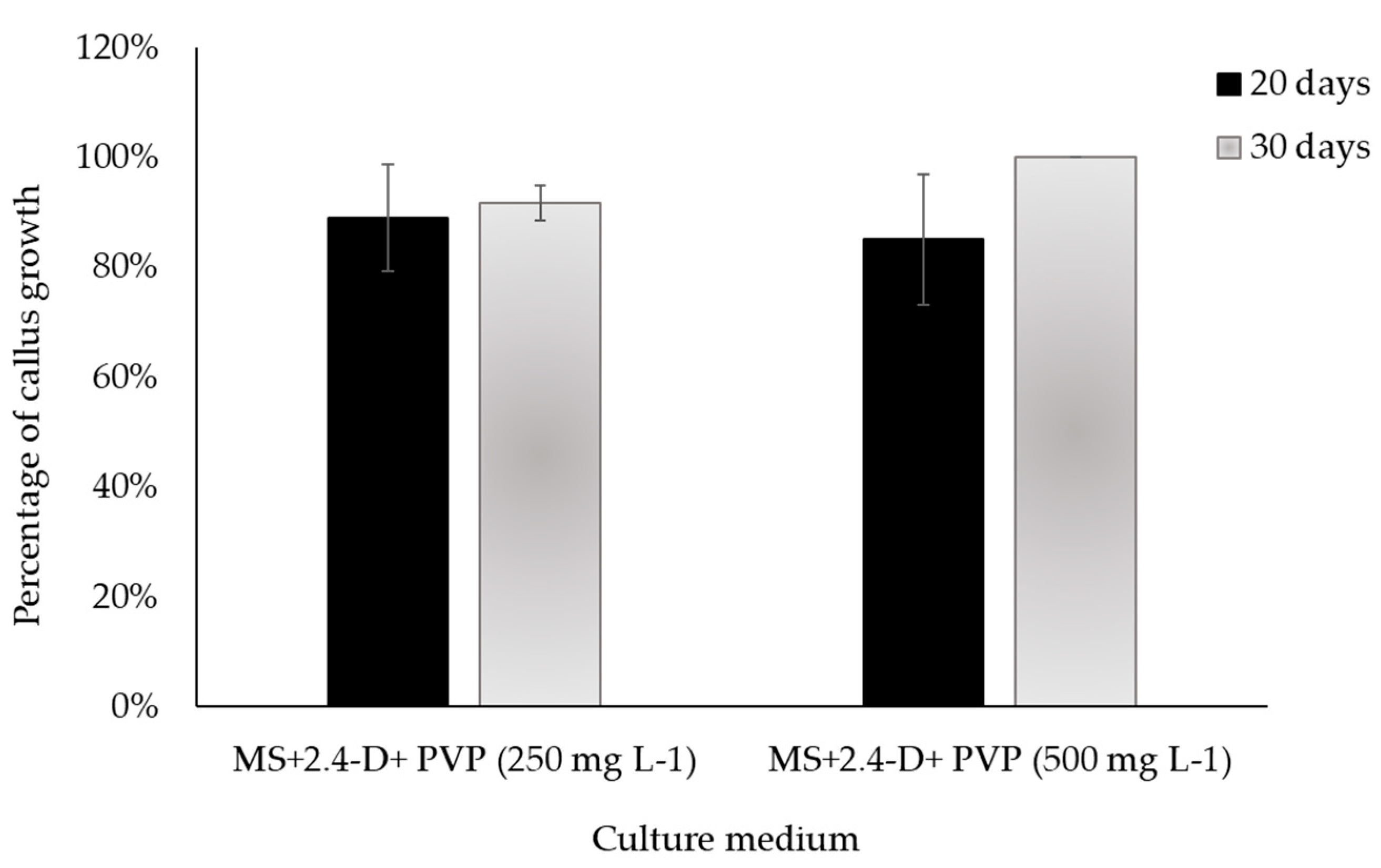
3.3. Validation of the callogenesis protocol
4. Discussion
4.1. Experimental phase 1: in vitro multiplication
4.2. Experimental phase 2: Callogenesis induction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casas, A., & Vallejo, M. Agroecología y agrobiodiversidad. Crisis ambiental en México, 2019; 103.
- CDB. Convenio de Diversidad Biológica. Available online: https://www.cbd.int/convention/text/default.shtml.
- Lobo, M., Medina, C.I. Conservación de recursos genéticos de la agrobiodiversidad como apoyo al desarrollo de sistemas de producción sostenibles. Revista Corpoica. Ciencia y Tecnología Agropecuaria, 2009; 10(1): 33-42.
- Cadena-Íñiguez, J., Trejo-Téllez, B. I., Morales-Flores, F. J., & Ruíz-Vera, V. M. Análisis de tratados internacionales relacionados con recursos genéticos y su congruencia con el marco jurídico mexicano. In Proceedings from the 22th International Congress on Project Engineering. Comunicaciones presentadas al XXII Congreso Internacional de Ingeniería de Proyectos, celebrado en Madrid del 11 al 13 de julio de 2018. (p. 97). Asociación española de ingeniería de proyectos (AEIPRO).
- Cadena-Iñiguez, J. El chayote (Sechium edule (Jacq.) Sw., importante recurso fitogenético mesoamericano. Agro Productividad, 2010; 3(2).
- Rodas, Y. C. R., Galarza, M. L. A., Hernández, M. S., Íñiguez, J. C., & Solano, V. M. C. Gestión de un recurso fitogenético para producción de metabolitos secundarios en proyectos de diversificación económica rural. In Comunicaciones presentadas al XXV Congreso Internacional de Ingeniería de Proyectos: celebrado en Alcoy del 6 al 9 de julio de 2021 (p. 101). Asociación española de ingeniería de proyectos (AEIPRO).
- Salas, S. M. P., Aguilar-Galván, F., & Sandoval, L. H. Plantas silvestres comestibles de la Barreta, Querétaro, México y su papel en la cultura alimentaria local. Revista Etnobiología. 2021; Vol, 19(1), 41-62.
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. AGP- Conservación de los Recursos Fitogenéticos. Available online: https://www.fao.org/agriculture/crops/core-themes/theme/seeds-pgr/conservation/en (accessed on 2023).
- Cadena Iñiguez, J., González Santos, R., Cuevas Sánchez, J., Riviello Flores, M. D. L. L., & Ruiz Posadas, L. D. M. La Conservación in situ de la Biodiversidad Agrícola, y la generación de proyectos en ejidos y comunidades de México. In Proceedings from the 22th International Congress on Project Engineering. Comunicaciones presentadas al XXIV Congreso Internacional de Ingeniería de Proyectos, celebrado en Madrid del 07 al 09 de julio de 2020. (p. x). Asociación española de ingeniería de proyectos (AEIPRO).
- González-Santos, R., Cadena-Iñiguez, J., Morales-Flores, F. J., Ruiz-Vera, V. M., Pimentel-López, J., & Peña-Lomelí, A. Model for the conservation and sustainable use of plant genetic resources in México. Wulfenia J, 2015; 22, 333-353.
- Beeching JR, Marmey P, Hughes MA, Charrier A. Evaluation of molecular approaches for determining genetic diversity in Cassava germplasm. Proc. 2nd Internat. Scient. Meet. The Cassava Biotechnology Network. Bogor, Indonesia. 1994; pp. 22-26.
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. El segundo informe sobre el estado de los recursos fitogenéticos para la alimentación y la agricultura en el mundo. Available online: https://www.fao.org/documents/card/es?details=22fabd61-4b41-5bb3-b6be-d38e1516dccc/ (accessed on 23 november 2023 ).
- Vargas-Bermúdez J. D. Conservación In situ de plantas nativas herbáceas, medicinales o bioplaguicidas, como reservorio de diversidad genética y cultural, mariscal sucre-guayas. Doctoral Dissertation, Universidad Agraria Del Ecuador, 2020.
- Barrera-Guzmán, L. A., Cadena-Iñiguez, J., Legaria-Solano, J. P., & Sahagún-Castellanos, J. Phylogenetics of the genus Sechium P. Brown: A review. Spanish Journal of Agricultural Research, 2021; 19(1), e07R01-e07R01.
- Avendaño-Arrazate, C. H., Cadena Iiguez, J., Arevalo Galarza, M. L. C., Campos Rojas, E., Cisneros Solano, V. M., Aguirre Medina, J.F. Las variedades del chayote mexicano, recurso ancestral con potencial de comercialización (No. Libro 635.62 A8). Grupo Interdisciplinario de Investigación en Sechium edule en México (GISeM), 2010.
- GISeM. Rescatando y aprovechando los recursos fitogenéticos de Mesoamérica. Volumen 2: Chayote. Grupo Interdisciplinario de Investigación en Sechium edule en México. Colegio de Postgraduados. Texcoco, Estado de México, 2011; 24p.
- Iñiguez-Luna, M. I., Cadena-Iñiguez, J., Soto-Hernández, R. M., Morales-Flores, F. J., Cortes-Cruz, M., & Watanabe, K. N. Natural bioactive compounds of Sechium spp. for therapeutic and nutraceutical supplements. Frontiers in Plant Science, 2021;12, 772389.
- Iñiguez-Luna, M. I., Cadena-Iñiguez, J., Soto-Hernández, R. M., Morales-Flores, F. J., Cortes-Cruz, M., Watanabe, K. N., ... & Cadena-Zamudio, J. D. Bioprospecting of Sechium spp. varieties for the selection of characters with pharmacological activity. Scientific Reports, 2021; 11(1), 1-12.
- Rivera-Ponce, E. A., Cadena-Iñiguez, J., Cisneros-Solano, V. M., Soto-Hernández, R. M., San Miguel-Chávez, R., García-Osorio, C., & Arévalo-Galarza, M. D. L. Composición fitoquímica y uso potencial del jugo de Sechium compositum (Donn. Sm.) C. Jeffrey. Agro-Divulgación, 2022; 2(4).
- Aguiñiga Sánchez, I. Potencial antileucémico in vitro de extractos de cuatro genotipos de Sechium spp (Cucurbitaceae) (Master's thesis), 2013.
- Gordillo Salinas, L. S. Actividad antifúngica de Sechium compositum contra Botrytis cinerea y Colletotrichum gloeosporioides en condiciones in vitro (Master's thesis), 2019.
- Cruz-Martínez, V., Castellanos-Hernández, O. A., Acevedo-Hernández, G. J., Torres-Morán, M. I., Gutiérrez-Lomelí, M., Ruvalcaba-Ruiz, D., ... & Rodríguez-Sahagún, A. Genetic fidelity assessment in plants of Sechium edule regenerated via organogenesis. South African Journal of Botany, 2017; 112, 118-122.
- Castillo-Martínez C, Cisneros-Solano VM, Hernández-Marini R, Cadena-Iñiguez J, Avendaño-Arrazate CH. Conservación y multiplicación de una colección de Sechium spp. Colegio de Postgraduados y Grupo Interdisciplinario de Investigación Sechium edule en México A.C, México, 2013.
- Cruz-Martínez, V. O., Acevedo-Hernández, G. J., Castellanos-Hernández, O. A., & Rodríguez-Sahagún, A. Regeneración de Sechium edule mediante organogénesis y uso de marcadores ramp para evaluar la fidelidad genética. Biotecnología y Sustentabilidad, 2016; 1(1).
- Wei, Y., Li, Z., Lv, L. et al. Overexpression of MbICE3 increased the tolerance to cold and drought in lettuce (Lactuca sativa L.). In Vitro Cell.Dev.Biol.-Plant. 2023. [CrossRef]
- Romo-Paz, F., Orozco-Flores, J.D., Delgado-Aceves, L. et al. Micropropagation of Physalis angulata L. and P. chenopodifolia Lam. (Solanaceae) via indirect organogenesis. In Vitro Cell.Dev.Biol.-Plant, 2023; 59, 497–506. [CrossRef]
- Alihodzic, A., Davis, J., Roberts, C. et al. Production of secondary metabolites in regenerated Southern wormwood (Artemisia abrotanum L.) under various experimental conditions. In Vitro Cell.Dev.Biol.-Plant, 2023. [CrossRef]
- Behera, S., Kar, S.K., Monalisa, K. et al. Assessment of genetic, biochemical fidelity, and therapeutic activity of in vitro regenerated Hedychium coronarium. In vitro Cell.Dev.Biol.-Plant, 2023. [CrossRef]
- Zhang, W., Dai, W. In vitro plant regeneration of ‘Prelude’ red raspberry (Rubus idaeus L.). In Vitro Cell.Dev.Biol.-Plant, 2023; 59, 461–466. [CrossRef]
- Gaspar, T., Kevers, C., Penel, C., Greppin, H., Reid, D. M., & Thorpe, T. A. Plant hormones and plant growth regulators in plant tissue culture. In vitro Cellular & Developmental Biology-Plant, 1996; 32, 272-289.
- Kaur, K., Dolker, D., Behera, S., & Pati, P. K. Critical factors influencing in vitro propagation and modulation of important secondary metabolites in Withania somnifera (L.) dunal. Plant Cell, Tissue and Organ Culture (PCTOC), 2022; 149(1-2), 41-60.
- Alvarenga, S., & Morera, J. In vitro micropropagation of the chayote (Sechium edule Jacq. Sw.). Tecnologia en Marcha (Costa Rica), 1992; vol. 11, no 3.
- Abdelnour, A., Ramírez, C., & Engelmann, F. Micropropagación de chayote (Sechium edule Jacq. SW.) a partir de brotes vegetativos. Agronomía mesoamericana, 2002; 13(2), 147-151.
- Abdelnour-Esquivel, A., Bermudez, L. C., Rivera, C., & Alvarenga-Venutolo, S. Cultivo de meristemas, termo y quimioterapia en chayote (Sechium edule Jacq. Sw.) para la erradicación del virus del mosaico del chayote (ChMV). Manejo Integrado de Plagas y Agroecología Número 77 (Abril 2006), 2006.
- Del Ángel, O., Vela, G., Rodríguez, A., Gómez, M., & García, H. (2014). Desinfección y regeneración eficiente de chayote in vitro (Sechium edule Jacq. Sw.). In Ciencias Agropecuarias Handbook T-II: Congreso Interdisciplinario de Cuerpos Académicos, ECORFAN, 2014; p. 23.
- Mora, D. F., Esquivel, A. A., & Venutolo, S. A. Micropropagación de fenotipos seleccionados de chayote. Tecnología en marcha, 1999; 13(3), 9-15.
- Abdelnour-Esquivel, A., Brenes-Madriz, J., & Alvarenga-Venutolo, S. Guía Técnica Semilla de Chayote. 2015; [PDF] Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj. Available online: https://repositoriotec.tec.ac.cr/bitstream/handle/2238/6768/gu%C3%ADa-tecnica-semillachayote-14%20SET-15.pdf?sequence=1.
- García, J. G., Alvarado, E. S., & Bolaños, J. A. Efecto del AIA y el AIB sobre el enraizamiento in vitro de brotes de Sechium edule (Jacq.) Sw. Biotecnología Vegetal, 2015; 15(1).
- Soto-Contreras, A., Núñez-Pastrana, R., Rodríguez-Deméneghi, M. V., Aguilar-Rivera, N., Galindo-Tovar, M. E., & Ramírez-Mosqueda, M. A. Indirect organogenesis of Sechium edule (Jacq.) Swartz. In Vitro Cellular & Developmental Biology-Plant, 2022; 58(6), 903-910.
- Da Silva, R. F. Sistema de regeneración in vitro por embriogénesis somática indirecta en variedades venezolanas de arroz (Oryza sativa L.). Revista Científica UDO Agrícola, 2012; 12(1), 55-68.
- Riviello-Cogco, E., Robledo-Paz, A., Gutiérrez-Espinosa, M. A., Suárez-Espinosa, J., & Mascorro-Gallardo, J. O. Maduración y germinación de embriones somáticos de Coffea arabica cv. colombia. Revista Fitotecnia Mexicana, 2021; 44(2), 161-161.
- Classic Murashige, T., & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 1962; 15, 473-497.
- Cadena Iñiguez, J., Soto Hernández, M., Arévalo Galarza, M., Avendaño Arrazate, C. H., Aguirre Medina, J. F., & Ruiz Posadas, L. D. M. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Revista Chapingo. Serie Horticultura, 2011; 17(SPE2), 45-55.
- Ramos-Parra, M., Ulín-Montejo, F., Aguilar-Nieto, J. A., Solis-Trapala, I. L., & Fierro-Carbajal, J. B. Modelación y estimación del volumen de tejido vegetal in vitro de Strombocactus disciformis basada en mediciones no intrusivas. Universidad y ciencia, 2010; 26(2), 195-203.
- Dwass, C. Some k-sample rank-order test. Contributions to probability and Statistics. 1960; Pp 198-202.
- Steel, R. A rank sum test for comparing all pairs of treatments. Technometrics, 1960; Vol. 2, No. 2 pp 197-207.
- Critchlow, D. y Fligner, M. On distribution-free multiple comparisons in the one-way analysis of variance. Commun. Statist.- Theory meth., 1991; Vol. 20, No. 1 pp 127-139.
- Thilagam, D., Kumudini, B. S., & Manohar, S. H. Regeneration of Sechium edule (jacq) sw. From sterile in vitro nodal explants and assessment of clonalfidelity using issr and rapd markers. International Journal of Agricultural, 2016; Vol. 6, pag. 285-292.
- Larson, C. G., Hasbún, R., Jofré, M. P., Sánchez-Olate, M., & Ríos, D. Efecto del genotipo y fuente de citoquinina en la etapa de iniciación de cultivo in vitro de tejido adulto de Castanea sativa Mill. Gayana. Botánica, 2017; 74(1), 30-40.
- Meier-Dinkel, A., Becker, B., Duckstein, D. Micropropagation and ex vitro rooting of several clones of late-flushing Quercus robur L. Annales des Sciences Forestières, 1993; 50(1): 319-322.
- Castillo-Martínez, C. R., Gutiérrez-Espinosa, M., Buenrostro-Nava, M. T., Cetina Alcalá, V. M., & Cadena Iñiguez, J. Regeneración de plantas de Paulownia elongata Steud: Por organogénesis directa. Revista mexicana de ciencias forestales, 2012; 3(10), 41-49.
- Gil C, A. I., Ariza C, C. A., Castillo T, L. M., Salgado D, L. E., Banda Sánchez, L., & Vanegas M, L. E. Induction of organogenesis in vitro with 6-benzylaminopurine in Cattleya trianae Linden & Rchb. f. Revista UDCA Actualidad & Divulgación Científica, 2019; 22(2).
- Mora-Cruz, Y., López-Peralta, M. C. G., Hernández-Meneses, E., & Cruz-Huerta, N. Regeneración in vitro de plantas de prosthechea vitellina (lindley) we higging por organogenesis directa. Revista Fitotecnia Mexicana, 2023; 46(1), 33-33.
- Hussain, A., Ahmed Qarshi, I., Nazir, H. & Ullah, I. Plant tissue culture: current status and opportunities. Recent advances in plant in vitro culture, 2012, vol. 6, no 10, p. 1-28. [CrossRef]
- Chen, X., Ye, C., Yang, H., Ji, W., Xu, Z., Ye, S., ... & Zhu, X. Callogenesis and plant regeneration in peony (Paeonia× suffruticosa) using flower petal explants. Horticulturae, 2022; 8(5), 357.
- Hernández-Amasifuen, A. D., Cortez-Lázaro, A. A., Argüelles-Curaca, A., & Díaz-Pillasca, H. B. Callogénesis in vitro de durazno (Prunus persica L.) var. Huayco rojo a partir de explantes foliares. Ciencia y Tecnología Agropecuaria, 2022; 23(1), e2032-e2032.
- Sidek, N., Nulit, R., Kong, Y. C., Yien, C. Y. S., Sekeli, R., & EL-Barghathi, M. F. (2022). Callogenesis and somatic embryogenesis of Oryza sativa L.(cv. MARDI Siraj 297) under the influence of 2, 4-dichlorophenoxyacetic acid and kinetin. AIMS Agriculture and Food, 2022.
- Zayova, E., Nedev, T., & Stancheva, I. Callus via shoot organogenesis and plant regeneration of Stevia rebaudiana Bertoni. In Proceedings of the Bulgarian Academy of Sciences, 2022; Vol. 75, No. 4, pp. 620-628.
- Da Silva, R. F. Sistema de regeneración in vitro por embriogénesis somática indirecta en variedades venezolanas de arroz (Oryza sativa L.). Revista Científica UDO Agrícola, 2012; 12(1), 55-68.
- Phillips, G. C., & Garda, M. Plant tissue culture media and practices: an overview. In Vitro Cellular & Developmental Biology-Plant, 2019; 55, 242-257.
- Domínguez-Perales, L. A., Domínguez-Álvarez, J. L., Cruz-Izquierdo, S., Santacruz-Varela, A., Barrientos-Priego, A., Padilla-Ramírez, J. S., & Gutiérrez-Espinosa, M. A. Propagación in vitro de selecciones de guayabo (Psidium guajava L.). Revista fitotecnia mexicana, 2016; 39(3), 285-295.
- Cureño, H. B., Hernadez, R. S., valdivia, A. R., Télle, L. T., Martínez, M., Vazquez, M. E., ... & Uptón, J. L. Extracción y cuantificación de taxoides por HPLC en hojas in situ y en callos inducidos in vitro de Taxus globosa Schlecht. III Jornadas Internacionales sobre el Tejo (Taxus baccata L.), 2011.
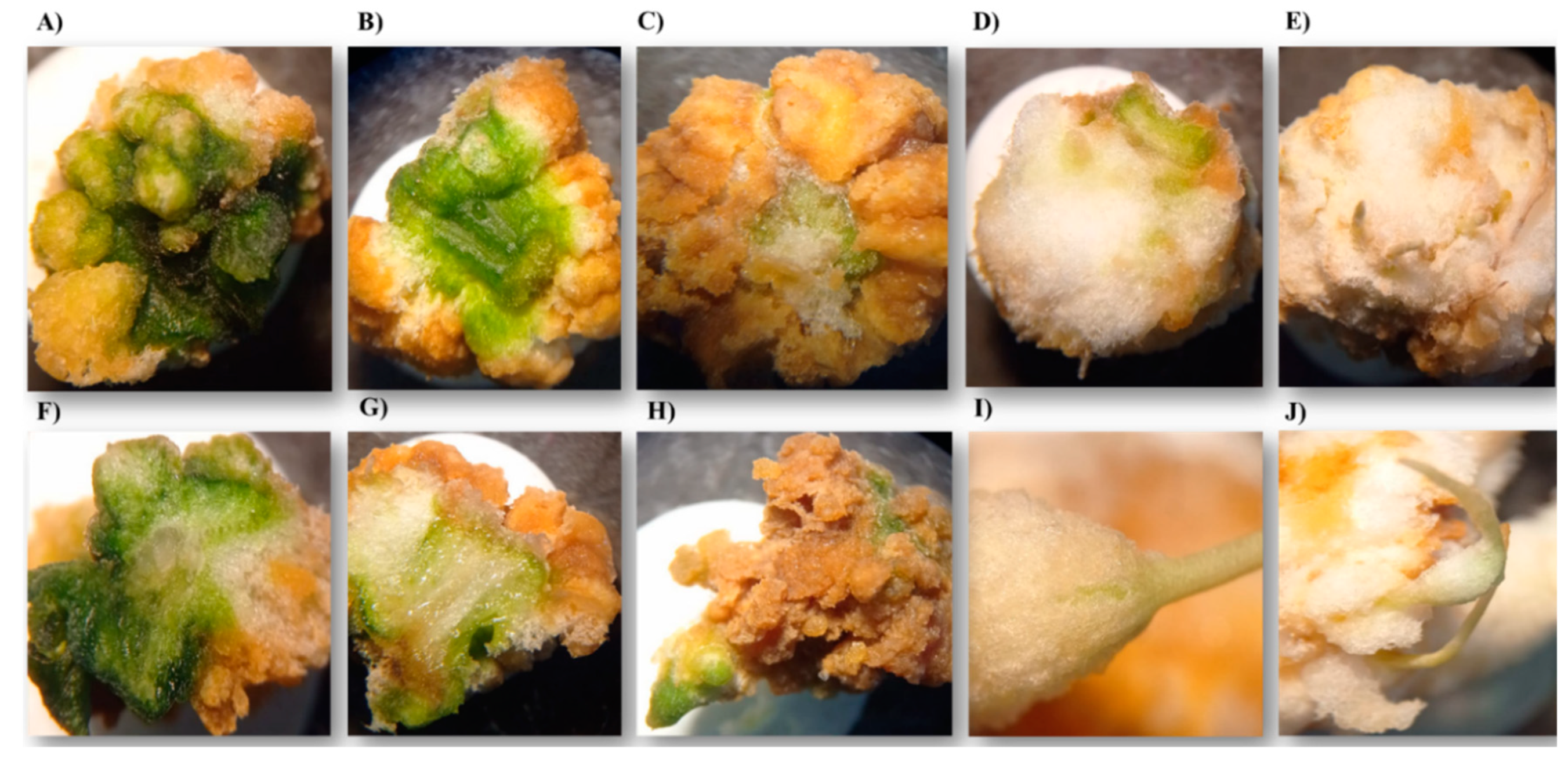

| Level | Callus formation scale (%) | Description |
| 1 | 0 | There is no tissue response. |
| 2 | 1-25 | The tissue swells (turgor) and begins to form a light-yellow callus at the ends. |
| 3 | 26-50 | The ends surrounding tissue areas show a greater amount of white callus. |
| 4 | 52-75 | A green tissue portion is observed at the top. The rest of the callus is white. |
| 5 | 76-100 | The callus has completely covered the tissue and there is an increase in the white mass, with a slight brown tone in small areas. |
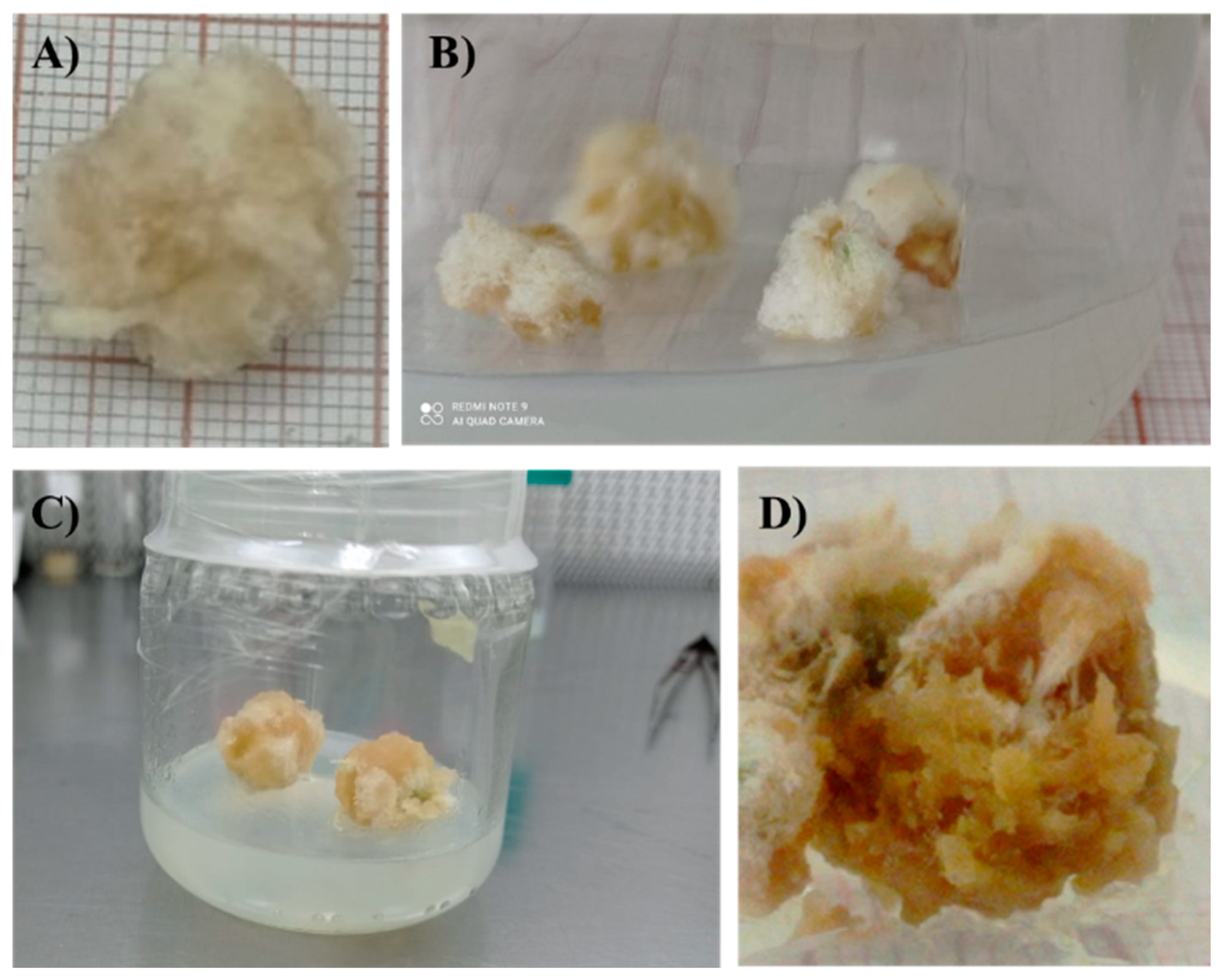
| Callus development (%) | Description |
| 0 | Brown callus and yellow medium are observed. |
| 25 | The callus maintains a greater number of brown areas and the mass does not increase. The medium looks slightly yellow. |
| 50 | A greater percentage of potentially active callus is observed, and the medium turns a light yellow. |
| 75 | A considerable decrease in brown areas is observed, along with has a greater number of active areas in the callus, and a transparent medium. |
| 100 | The callus presents mostly or all active zones, its mass increases, and root formation is observed. The medium is transparent. |
 |
| T | E | GR |
[GRC] (mg L-1) |
Callus Formation level | Weight (g) | Ø 1 | Ø 2 | Height | Volume | Root formation (%) | ||||||||||||||
| X̅ | SE | X̅ | SE | X̅ | SE | X̅ | SE | X̅ | SE | X̅ | SE | X̅ | SE | |||||||||||
| 1 | Stem | MS | 1.70 | e | ±0.15 | 0.153 | c | ±0.029 | 7.15 | de | ±0.44 | 5.70 | cd | ±0.51 | 4.75 | c | ±0.51 | 14.06 | ce | ±2.75 | 50.0 | abc | ±16.7 | |
| 2 | 2,4-D | 0.5 | 4.33 | b | ±0.11 | 1.160 | a | ±0.068 | 16.26 | a | ±0.30 | 13.48 | a | ±0.51 | 11.73 | a | ±0.31 | 149.11 | a | ±7.31 | 100.0 | a | ±0.0 | |
| 3 | 1.0 | 4.72 | ab | ±0.11 | 1.326 | a | ±0.064 | 16.51 | a | ±0.30 | 12.95 | a | ±0.31 | 11.69 | a | ±0.28 | 152.05 | a | ±7.66 | 100.0 | a | ±0.0 | ||
| 4 | 2.0 | 4.89 | a | ±0.08 | 1.161 | a | ±0.048 | 16.08 | a | ±0.40 | 12.55 | a | ±0.25 | 11.12 | a | ±0.26 | 138.28 | a | ±8.14 | 100.0 | a | ±0.0 | ||
| 5 | TDZ | 0.5 | 2.89 | c | ±0.08 | 0.534 | b | ±0.039 | 13.07 | b | ±0.43 | 10.68 | b | ±0.40 | 9.64 | b | ±0.31 | 80.82 | b | ±5.68 | 0.0 | ce | ±0.0 | |
| 6 | 1.0 | 3.06 | c | ±0.06 | 0.638 | b | ±0.026 | 13.79 | b | ±0.30 | 10.08 | b | ±0.28 | 10.54 | b | ±0.37 | 96.43 | b | ±6.69 | 0.0 | ce | ±0.0 | ||
| 7 | 2.0 | 3.22 | c | ±0.10 | 0.607 | b | ±0.026 | 13.12 | b | ±0.36 | 10.40 | b | ±0.41 | 9.41 | b | ±0.31 | 79.01 | b | ±5.61 | 0.0 | ce | ±0.0 | ||
| 8 | Sheet | MS | 1.00 | e | ±0.00 | 0.012 | d | ±0.001 | 5.72 | e | ±0.05 | 4.61 | d | ±0.39 | 0.92 | d | ±0.11 | 2.45 | f | ±0.17 | 0.0 | cde | ±0.0 | |
| 9 | 2,4-D | 0.5 | 2.83 | cd | ±0.19 | 0.464 | bc | ±0.068 | 11.48 | bc | ±0.48 | 9.04 | b | ±0.51 | 8.12 | bc | ±0.55 | 56.37 | bcd | ±7.05 | 88.9 | ab | ±7.6 | |
| 10 | 1.0 | 2.67 | cde | ±0.27 | 0.523 | bc | ±0.098 | 12.07 | b | ±0.96 | 8.93 | b | ±0.74 | 6.78 | bc | ±0.78 | 63.73 | bc | ±11.11 | 55.6 | ab | ±12.1 | ||
| 11 | 2.0 | 2.72 | cde | ±0.29 | 0.610 | bc | ±0.128 | 11.61 | b | ±1.00 | 8.81 | b | ±0.91 | 7.52 | bc | ±1.02 | 70.47 | bc | ±16.39 | 44.4 | bcd | ±12.1 | ||
| 12 | TDZ | 0.5 | 2.00 | e | ±0.08 | 0.236 | c | ±0.024 | 10.17 | cd | ±0.51 | 7.83 | c | ±0.40 | 7.21 | c | ±0.37 | 38.82 | cde | ±3.93 | 0.0 | ce | ±0.0 | |
| 13 | 1.0 | 2.00 | de | ±0.11 | 0.241 | c | ±0.027 | 9.77 | cd | ±0.59 | 6.99 | c | ±0.39 | 6.42 | c | ±0.47 | 33.58 | cde | ±4.65 | 0.0 | ce | ±0.0 | ||
| 14 | 2.0 | 2.28 | de | ±0.14 | 0.305 | c | ±0.032 | 11.39 | c | ±0.54 | 8.06 | c | ±0.29 | 6.64 | c | ±0.45 | 45.15 | cd | ±5.73 | 0.0 | ce | ±0.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





