Submitted:
16 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
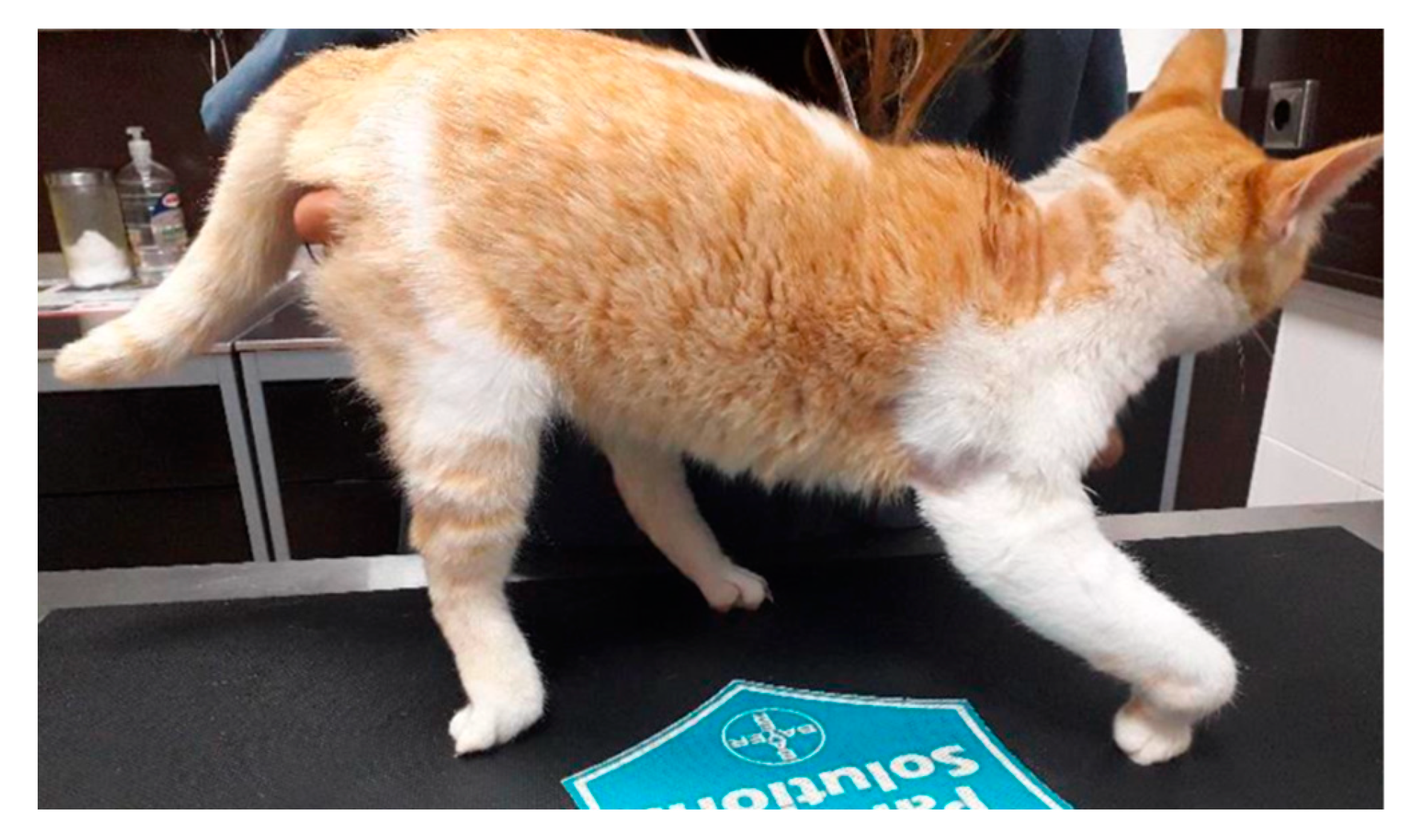
2.1. Participants
2.2. Study Design
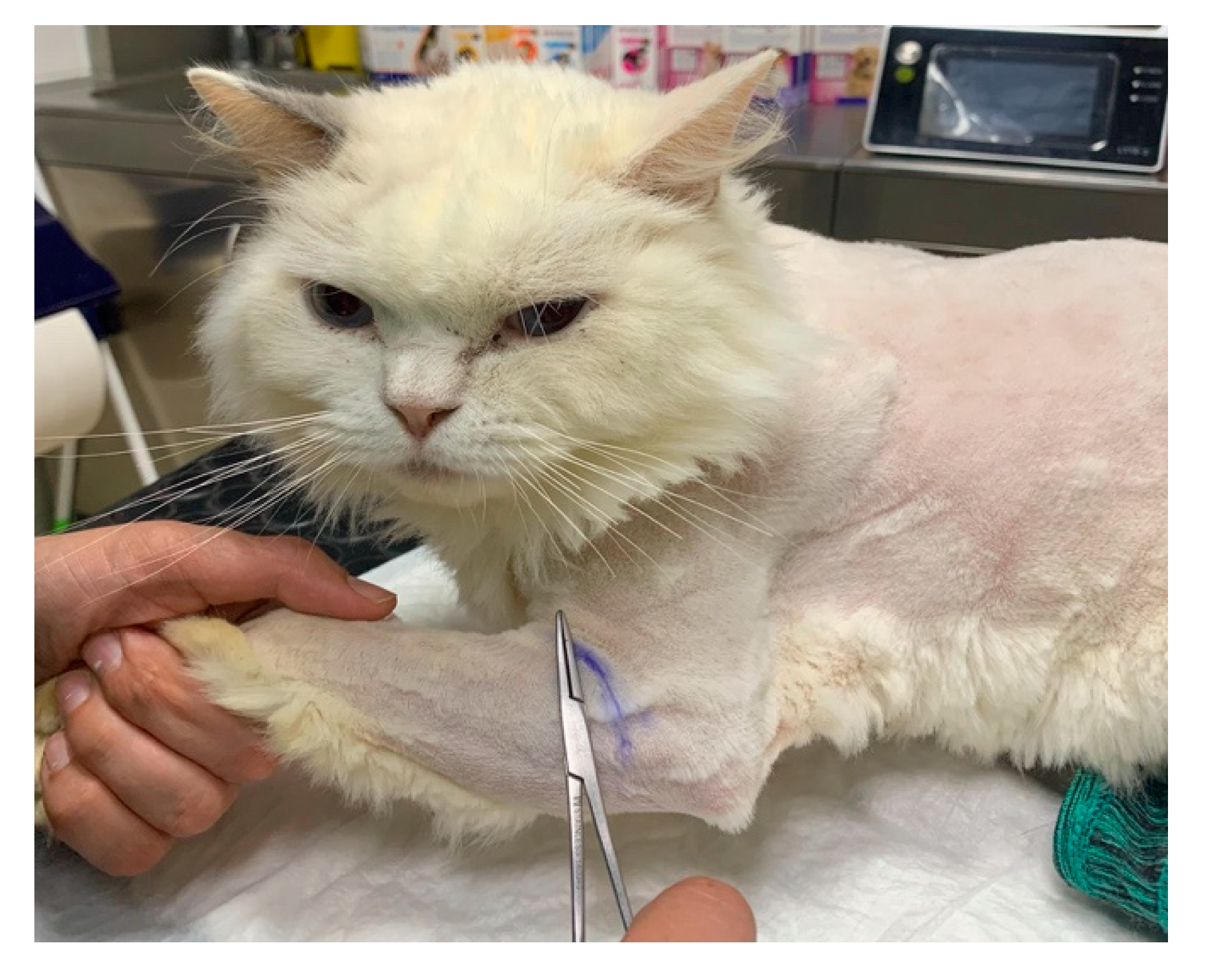
2.3. Procedures
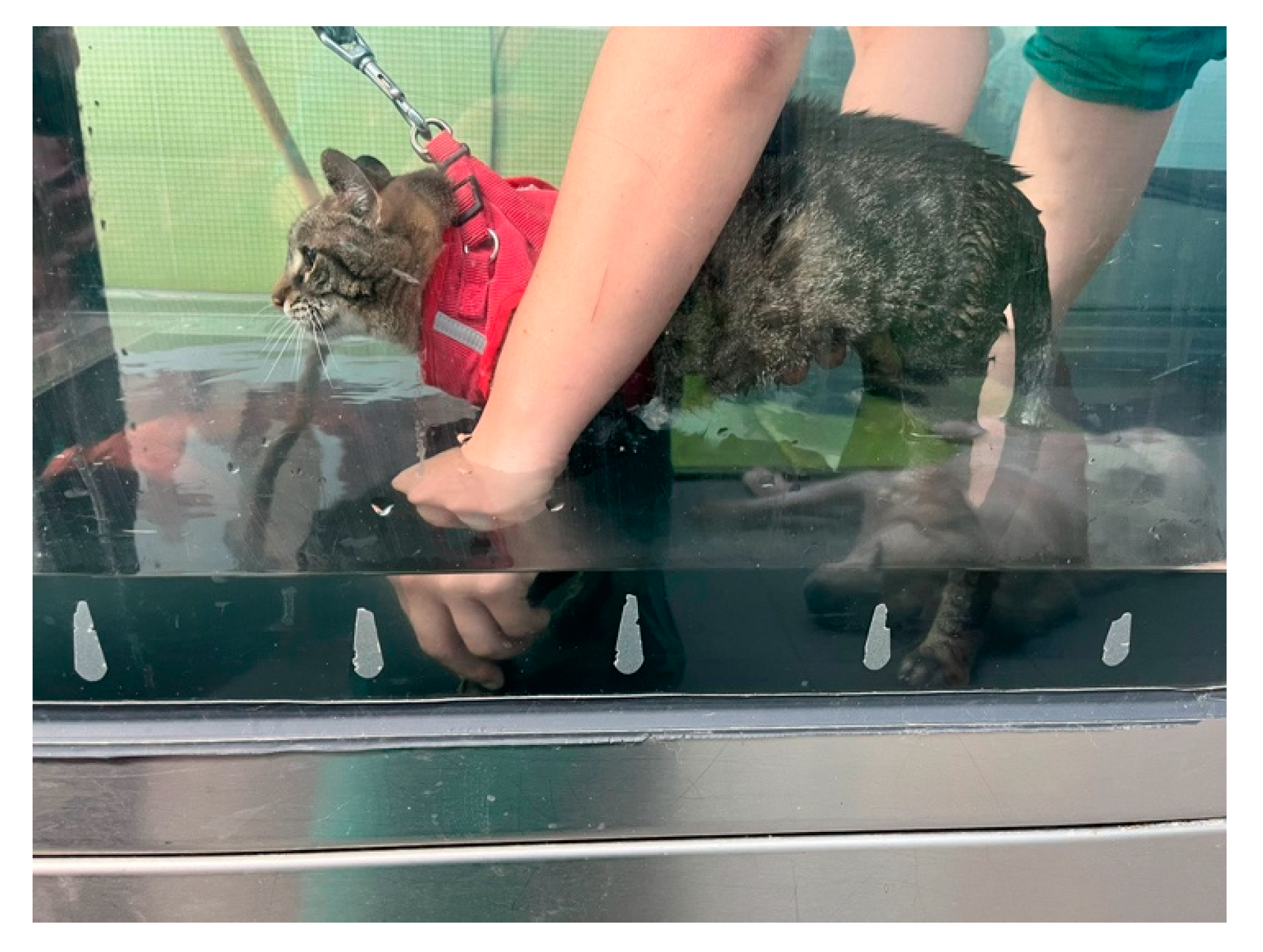
2.3.1. Intensive neurorehabilitation protocol (INRP)
- 1st - 3rd week of INRP
- 4th – 6th week of INRP
- 6th – 8th week of INRP
- Neuropathic pain
2.4. Data collection
2.5. Statistical analyses
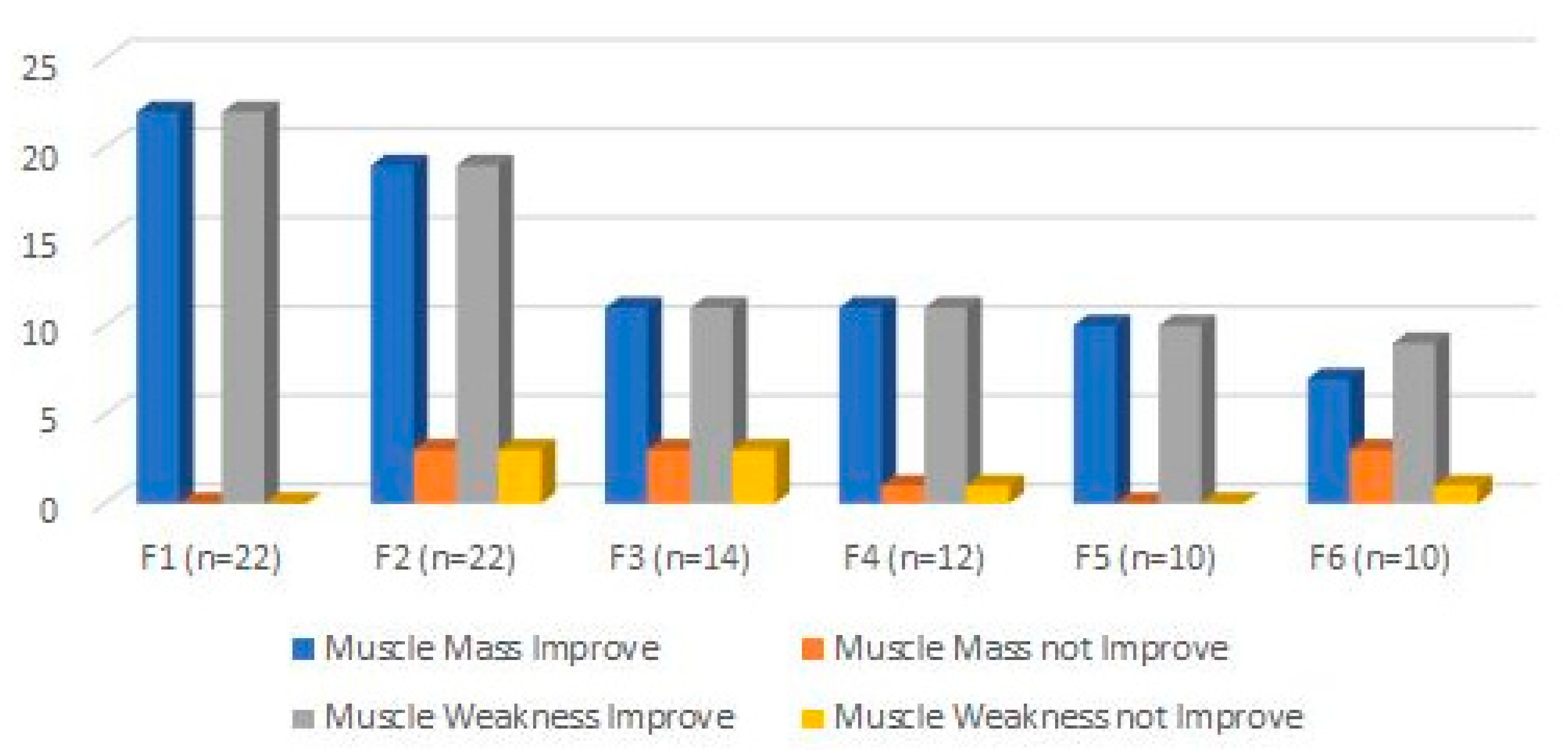
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uemura, E.E. Motor system. In Fundamentals of canine neuroanatomy and neurophysiology. Uemura, E.E., ed. Iowa, USA: John Wiley & Sons, 2015, pp 257‐288.
- Troupel, T.; Caenegem, N.V.; Jeandel, A.; Thibaud, J.; Nicolle, A.; Blot, S. Epidemiological, clinical, and electrophysiological findings in dogs and cats with traumatic brachial plexus injury: a retrospectove study of 226 cases. J Vet Intern Med. 2021, 35, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A.; Glass, E.; Kent, M. Lower motor neuron: spinal nerve, general somatic efferent system. In Veterinary Neuroanatomy and clinical neurology. de Lahunta, A., Glass, E., Klent, M., eds. 4th ed, St. Louis, MO: Elsevier, 2014, pp. 102-161.
- Wheeler, S.J.; Jones, D.G.; Wright, J.A. The diagnosis of brachial plexus disoreders in dogs: a review of twenty-two cases. J Small Anim Pract. 1986, 27(3), 147–157. [Google Scholar] [CrossRef]
- Soens, I.V.; Struys, M.M.; Polis, I.E.; Bhatti, S.F.; Meervenne, S.A.; Martlé, V.A.; Nollet, H.; Tshamala, M.; Vanhaesebrouck, A.E.; Ham, L.M. Magnetic stimulation of the radial nerve in dogs and cats with brachial plexus trauma: a report of 53 cases. Vet J. 2009, 182, 108–113. [Google Scholar] [CrossRef]
- Griffiths, I.R.; Duncan, I.D.; Lawson, D.D. Avulsion of the brachial plexus-2. Clinical aspects. J Small Anim Pract. 1974, 15, 177–183. [Google Scholar] [CrossRef]
- Steinberg, H.S. Brachial plexus injuries and dysfunctions. Vet Clin of North Am: Small Anim Practic 1988, 18, 565–580. [Google Scholar]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Annals of Anatomy 2011, 193, 321–333. [Google Scholar]
- Anson, A.; Gil, F.; Laredo, F.G.; Soler, M.; Belda, E.; Ayala, M.D.; Agut, A. Correlative ultrasound anatomy of the feline brachial plexus and major nerves of the thoracic limb. Vet Radiol Ultrasound 2013, 54, 2, 185–193. [Google Scholar]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Effron, C.R.; Beasley, R.W. Compression neuropathies in the upper limb and electrophysiological studies. In Grabb and Smith’s Plastic Surgery. Thorne, C.H., Bartlett, S.P., Beasley, R.W., Aston, S.J., Gurtner, G.C., Spear, S.L., eds. Philadelphia, USA: Lippincott Williams & Wilkins; 2006, pp. 86.
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve. 2000, 23(6), 863–87. [Google Scholar] [CrossRef]
- Seddon, H.J. Three types of nerve injury. Brain. 1943, 66, 237. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J. C. Peripheral nerve trauma: mechanisms of injury and recovery. Hand Clin. 2013, 29, 3, 317–330. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J. , et al. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. Intern J Bio Sci 2020, 16(1), 116–134. [Google Scholar]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; Schoonhoven, J. The role of current techniques and concepts in peripheral nerve repair. Plastic Surg Intern. 2016, 4175293. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res. 2020, 98(5), 780–795. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: surgical application, atate of the art, and future challenges, Crit Rev Bio Eng. 2011, 39 (2), 81-124.
- De Lahunta, A. Feline neurology. Vet Clin North Am. 1976, 6(3), 433–451. [Google Scholar] [CrossRef] [PubMed]
- Dessal, F. Fundamentos de Neuroanatomia. In Neurologia Felina. Dessal, F., ed. Buenos Aires, Argentina: Editorial Inter-Médica, 2020.
- Drum, M.G.; Bockstahler, B.; Levine, D. : Marcellin-Little, D.J. Feline rehabilitation. Vet Clin North Am Small Anim Pract 2015, 45(1), 185–201. [Google Scholar]
- Willand, M.P. Electrical stimulation enhances reinnervation after nerve injury. Eur J Trans Myol. 2015, 25(4), 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Chen, J.; Hsu, Y.; Bau, D.; Yao, C.; Chen, Y. High-Frequency electrical stimulation can be a complementary therapy to promote nerve regeneration in diabetic rats. Plos One. 2013, 8(11), e79078. [Google Scholar] [CrossRef] [PubMed]
- Foecking, E.M.; Fargo, K.N.; Coughlin, L.M.; Kim, J.T.; Marzo, S.J.; et al. Single session if brief electrical stimulation immeadiately following crush injury enhances functional recovery of rat facial nerve. J Rehabil Res Dev. 2012, 49, 451–458. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Russo, T.L.; Geuna, S.; Domingues, N.; Salvini, T.F.; Parizotto, N.A. Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle & Nerve, 2010; 685–693. [Google Scholar]
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, C. , Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Jari, R.; Verge, V. ; Rohde. C.; Gordon, T. Electrical stimula- tion restores the specificity of sensory axon regeneration. Exp Neurol 2005, 194, 221–229. [Google Scholar]
- Evans, G.R. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001, 263, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Marqueste, T.; Alliez, J.R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation af- ter peripheral nerve injury and repair. J Appl Physiol. 2004, 96, 1988–1995. [Google Scholar] [CrossRef]
- Russo, T.L.; Peviani, S.M.; Durigan, J.L.; Salvini, T.F. Electrical stimulation increases matrix metalloproteinase-2 gene expression but does not change its activity in denervated rat muscle. Muscle Nerve. 2008, 37, 593–600. [Google Scholar] [CrossRef]
- Varejão, A.S.; Cabrita, A.M.; Meek, M.F.; Bulas-Cruz, J.; Melo-Pinto, P.; Raimondo, S.; et al. Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurotrauma. 2004, 21, 1652–1670. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier- Santos, M.A.; Martinez, A.M. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J Peripher Nerv Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Kerns, J.M.; Lucchinetti, C. Electrical field effects on crushed nerve regeneration. Exp Neurol 1992;117:71–80.
- Lundborg, G. Enhancing posttraumatic nerve regeneration. J Peripher Nerv Syst. 2002, 7, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Carvalho, G.A.; Nikkhah, G.; Penkert, G. Surgical reconstruc- tion of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997, 87, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low inten- sity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods. 2003, 129, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dow, D.E.; Cederna, P.S.; Hassett, C.A.; Kostrominova, T.Y.; Faulkner, J.A.; Dennis, R.G. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve. 2004, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Denny, H.R; Butterworth, S.J. Peripheral nerve injury. In A guide to canine and feline orthopaedic surgery, Denny, H.R; Butterworth, S.J, eds. Oxford, UK: Blackwell science Lds, 2000; pp. 201–205. [Google Scholar]
- Añor, S. Monoparesis. In BSAVA Manual of canine and feline neurology. Platt, S.; Olby, N., eds. Cheltenahm, UK: Brithish Small Animal Veterinary Association, 2013; pp. 328–341. [Google Scholar]
- Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R. Therapeutic exercise and manual therapy. In Reabilitação e Fisioterapia na Prática de Pequenos Animais. Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R, eds. São Paulo, Brasil: Roca. 2008, pp. 447-463.
- Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R. Physical therapy for specific diagnoses. In Reabilitação e Fisioterapia na Prática de Pequenos Animais. Levine, D.; Millis, D. L.; Marcellin-Little, D. J.; Taylor, R, eds. São Paulo, Brasil: Roca. 2008, pp. 609-627.
- Shores, A.; Pearce, L. Traumatic and Neoplastic Diseases of the Brachial Plexus. In Mechanisms of disease in Small Animal Surgery. Bojrab, M.J.; Monnet, E., eds. Jackson, WY: Teton New Media Inc. 2010.
- Marcellin-Little DJ, Levine D. Principles and application of range of motion and stretching in companion animals. Vet Clin North Am Small Anim Pract. 2015, 45(1), 57–72. [Google Scholar] [CrossRef] [PubMed]
- Knecht, C. D.; Raffe, M. R. Diseases of the Brachial Plexus. In Textbook of Small Animal Orthopaedics. Newton, C.D.; Nunamaker, D.M., eds. Filadélfia, PA: Lippincott, 1985. [Google Scholar]
- Marcolino, A. M.; Barbosa, R. I.; Fonseca, M. C.; Mazzer, N.; Ellui, V. M. Reabilitação fisioterapêutica na lesão do plexo braquial: relato de caso. Fisioterapia em Movimento 2008, 21, 53–60. [Google Scholar]
- Monte-Raso, V. V.; Barbieri, C. H.; Mazzer, N.; Fazan, V. P. Os efeitos do ultra- som terapêutico nas lesões por esmagamento do nervo ciático de ratos: análise funcional da marcha. Revista Brasileira de Fisioterapia 2006, 10, 113–119. [Google Scholar]
- Millis, D. L.; Levine, D. Basic science of veterinary rheabilitation. In Canine Rehabilitation and Physical Therapy. Millis, D. L.; Levine, D., eds. Filadélfia, PA: W. B. Saunders Co., Ltd, 2014; pp. 79–153. [Google Scholar]
- Bocksthler, B.; Wittek, K. Passive Range of Motion Exercises and Stretching. In Essential facts of physical medicine, rehabilitation and sports medicine in companion animals. Bocksthler, B.; Wittek K, eds. 2019, pp. 110.
- Sawaya SG, Combet D, Chanoit G, Thiebault JJ, Levine D, Marcellin-Little DJ. Assessment of impulse duration thresholds for electrical stimulation of muscles (chronaxy) in dogs. Am J Vet Res 2008, 69(10), 1305–1309.
- Gouveia, D.; Fonseca, S.; Carvalho, C.; Cardoso, A.; Almeida, A.; Gamboa, Ó.; Canejo-Teixeira, R.; Ferreira, A.; Martins, Â. Clinical occurrences in the neurorehabilitation of dogs with severe spinal cord injury. Animals (Basel), 2023, 13(7), 1164. [Google Scholar]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A comparison between body weight-supported treadmill training and conventional over-ground training in dogs with incomplete spinal cord injury. Front Vet Sci. 2021, 8, 597949. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Silva, C.; Coelho, T.; Gamboa, Ó.; Ferreira, A. Functional neurorehabilitation in dogs with an incomplete recovery 3 months following intervertebral disc surgery: a case series. Animals. 2021, 11(2442), 1–21. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J. Ambulation in dogs with absent pain perception after acute thoracolumbar Spinal Cord Injury. Front Vet Sci. 2020, 7, 560–572. [Google Scholar] [CrossRef]
- Menchetti, M.; Gandini, G.; Bravaccini, B.; Dondi, M.; Gagliardo, T.; Bianchi, E. Clinical and electrodiagnostic findings and quality of life of dogs and cats with brachial plexus injury. Vet Sci. 2020, 7(3), 101. [Google Scholar] [CrossRef]
- Mackinnon, S.; Dellon, A.L. Surgery of the peripheral nerve. NY: Thieme; Diagnosis of nerve injury, 1988, pp. 74–8.
- Alvites, R.; Rita Caseiro, A.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Colette Maurício, A.; Spurkland, A. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent. Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon. 2021, 7, e08281. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, L.E.; Shauver, M.J.; Chung, K.C. Patient satisfaction and self-reported outcomes after complete brachial plexus avulsion injury. J. Hand Surg. 2014, 39, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.A. Peripheral nerve injury. Semin. Vet. Med. Surg. (Small Anim.) 1996, 11, 273–284. [Google Scholar]
- Santifort, K.M. Return of function in a feline thoracic limb after suspected traumatic brachial plexus injury with loss of nociception. Vet. Rec. Case Rep. 2016, 4(1), e000334. [Google Scholar] [CrossRef]
- Smith, B.W.; Daunter, A.K.; Yang, L.J.S.; Wilson, T.J. An Update on the Management of Neonatal Brachial Plexus Palsy—Replacing Old Paradigms: A Review. JAMA Pediatr. 2018, 172, 585. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.A.; Newell, A.; Williams, T. Traumatic brachial plexus injury rehabilitation using neuromuscular electrical muscle stimulation in a polytrauma patient. BMJ Case Rep. 2019, 12, e232107. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, K.; Stoneman, P. Shoulder injuries in rugby players: Mechanisms, examination, and rehabilitation. Phys. Ther. Sport. 2014, 15, 218. [Google Scholar] [CrossRef]
- Dijkstra, J.R.; Meek, M.; Robinson, P.H.; Gramsbergen, A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Met. 2000, 96, 89–96. [Google Scholar] [CrossRef]
- Meek, M.F.; Van Der Werff, J.F.A.; Nicolai, J.P.A.; Gramsbergen, A. Biodegradable p (DLLA-e-CL) Nerve guides versus autologous nerve grafts: electromyographic amd video analysis. Muscle & Nerve 2001, 24, 753–759. [Google Scholar]
- Watson, N.C.; Jejurikar, S.; Kalliainen, L.K.; Calderon, M.S.; URbanchek, M.G.; Eguchi, T.; Kuzon, J.R. Range of motion physiotherapy reduces the force deficit in antagonists to denervated rat muscles. J Surg Res. 2001, 99, 156–160. [Google Scholar] [CrossRef]
- Siegel, S.G.; Patton, B.; English, A.W. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol. 2000, 166, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Varejão, A.S.P.; Cabrita, A.M.; Geuna, S.; Melo-Pinto, P.; Filipe, V.M.; Gramsbergen, A.; Meek, M.F. Toe out angle: a functional index for the evaluation of sciatic nerve recovey in the rat model. Exp Neurol. 2003, 183. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016, 17(12). [Google Scholar]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J. , Reid, A.J. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 2015, 82–83, 160–167. [Google Scholar]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int Rev Neurobiol 2009, 87, 295–315. [Google Scholar] [PubMed]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T. ; Wang, J; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front Neurol 2023, 14, 1–13. [Google Scholar]
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial plexus injuries in sport medicine: Clinical evaluation, diagnostic approaches, treatment options and rehabilitative interventions. J Funct Morphol Kinesiol. 2020, 5(2), 22. [Google Scholar] [CrossRef]
- Javeed, S.; Faraji, A.H.; Dy, C.; Ray, W.Z.; MacEwan, M.R. . Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg.: Adv. Tech. Case Manag. 2021, 24, 101117. [Google Scholar]
- Gordon, T.; Udina, E.; Verge; V. M.K.; de Chaves, E.I.P. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009, 13(4), 412–441. [Google Scholar] [CrossRef]
- Aglah, C.; Gordon, T.; de Chaves, E.I.P. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008, 55(1), 8–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Xu, Q.-G.; Franz, C.K.; Zhang, R.; Dalton, C.; Gordon, T.; Verge, V.M.K.; Midha, R.; Zochodne, D.W. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm: Laboratory investigation. J. Neurosurg. 2012, 116(3), 498–512. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.E.; English, A.W. The role of BDNF in peripheral nerve regeneration: activity-dependent treatments and Val66Met. Front. Cell. Neurosci. 2019, 12, 522. [Google Scholar] [CrossRef]
- Hoffman, H. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust. J. Exp. Biol. Med. Sci. 1952, 30, 541–566. [Google Scholar] [CrossRef]
- Nix, W.A.; Hopf, H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Research. 1983, 272(1), 21–25. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013, 38, 3691–701. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; et al. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials. 2021, 275, 120982. [Google Scholar] [CrossRef]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013, 240, 157–67. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Meador, W.; Carrasco, D.I. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005, 21, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Spaich, E.G.; Serrao, M.; Andersen, O.K. Stimulation site and phase modulation of the withdrawal reflex during gait initiaion. Clin Neurophysiol 2015, 126(12), 2282–2289. [Google Scholar] [CrossRef]
- Pilkar, R.B.; Yarossi, M.; Forrest, G. Empirical mode decomposition as a tool to remove the function electrical stimulation artifact from surface electromyograms: preliminary investigation. In: 2012 annual international conference of the IEEE engineering in medicine and biology society. San Diego, CA: IEEE, 2012, pp. 1847–50.
- Martins, A.; Gouveia, D.; Cardoso, A.; Gamboa, Ó.; Millis, D.; Ferreira, A. Nervous system modulation through electrical stimulation in companion animals. Acta Vet Scand. 2021, 63, 22. [Google Scholar] [CrossRef]
- Levine, D.; Bockstahler, B. Electrical stimulation. In Canine rehabilitation and physical therapy; Millis, D., Levine D, Eds.; Elseviers: Philadelphia, 2014; pp. 342–56. [Google Scholar]
- Haastert-Talini, K.; Schmitte, R.; Korte, N.; Klode, D.; Ratzka, A.; Grothe, C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma. 2011, 28, 661–74. [Google Scholar] [CrossRef]
- Pieber, K.; Herceg, M.; Paternostro-Sluga, T.; Schuhfried, O. Optimizing stimulation parameters in functional electrical stimulation of denervated muscles: a cross-sectional study. J Neuroeng Rehabil. 2015, 12, 51. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Pavone, V.; Testa, G.; Pesce, I.; Scaturro, D.; Musumeci, G.; Mauro, G.; Vecchio, M. The role of physical exercise and rehabilitative implication in the process of nerve repair in peripheral neuropathies: a systematic review. Diagnostics (Basel). 2023, 13(3), 364. [Google Scholar] [CrossRef] [PubMed]
- Pachter, B.R.; Eberstein, A. Passive Exercise and Reinnervation of the Rat Denervated Extensor Digitorum Longus Muscle after Nerve Crush. Am. J. Phys. Med. Rehabilitation. 1989, 68, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hartley, R.A.; Kordecki, M.E. Rehabilitation of chronic brachial plexus neuropraxia and loss of cervical extension in a high school football player: A case report. Int. J. Sports Phys Ther. 2018, 13, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Blaha, C.; Moradkhan, R.; Gray, K.S.; Sinoway, L.I. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J. Physiol. 2006, 576, 625. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.J.; da Paz, M.G.D.S.; Bina, M.T.; Santos, S.N.; Raicher, I.; Galhardoni, R.; Fernandes, D.T.; Yeng, L.T.; Baptista, A.F.; de Andrade, D.C. Neuropathic pain after brachial plexus avulsion-central and peripheral mechanisms. BMC Neurol. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.-D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Vannier, J.L.; Belkheyar, Z.; Oberlin, C.; Montravers, P. Management of neuropathic pain after brachial plexus injury in adult patients: A report of 60 cases. Ann. Fr. Anesth. Reanim. 2008, 27, 890–895. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.; Ghaleb, A.H. Cervical Spinal Cord Stimulation for the Management of Pain from Brachial Plexus Avulsion. Pain Med. 2014, 15, 712–714. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D.; et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, P.; Rui, J.; Zhao, X.; Lao, J. The clinical characteristics of neuropathic pain in patients with total brachial plexus avulsion: A 30-case study. Injury. 2016, 47, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Meng, C.; Zhou, Y.; Lao, J.; Zhao, X. A new model for the study of neuropathic pain after brachial plexus injury. Injury. 2017, 48, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lovaglio, A.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India. 2019, 67, 32. [Google Scholar] [CrossRef] [PubMed]
- Emamhadi, M.; Andalib, S. Successful recovery of sensation loss in upper brachial plexus injuries. Acta Neurochir. (Wien). 2018, 160, 2019–2023. [Google Scholar] [CrossRef]
- Sadosky, A.; McDermott, A. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008, 8(1), 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Czerniecki, J.M.; Smith, D.G.; Campbell, K.M.; Edwards, W.T.; Jensen, M.P.; et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000, 81(8), 1039–44. [Google Scholar] [CrossRef]
- Melzack, R. Phantom limbs. Sci Am. 1992, 266(4), 120–6. [Google Scholar] [CrossRef]
- Jensen, T.S.; Krebs, B.; Nielsen, J.; Rasmussen, P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985, 21, 267–78. [Google Scholar] [CrossRef]
- Menchetti, M.; Gandini, G.; Gallucci, A.; Della Rocca, G.; Matiasek, L.; Matiasek, K.; Gentilini, F.; Rosati, M. Approaching phantom complex after limb amputation in the canine species. J. Vet. Behav. 2017, 22, 24–28. [Google Scholar] [CrossRef]
- Probstner, D.; Thuler, L.C.; Ishikawa, N.M.; Alvarenga, R.M. Phantom limb phenomena in cancer amputees. Pain Pract. 2010, 10, 249–256. [Google Scholar] [CrossRef]
- Menchetti, M.; Rocca, G.D.; Tartari, I.; Gandini, G.; Di Salvo, A.; Rosati, M. Approaching phantom complex after limb amputation in cats. J Vet Behav. 2022, 50, 23–29. [Google Scholar] [CrossRef]
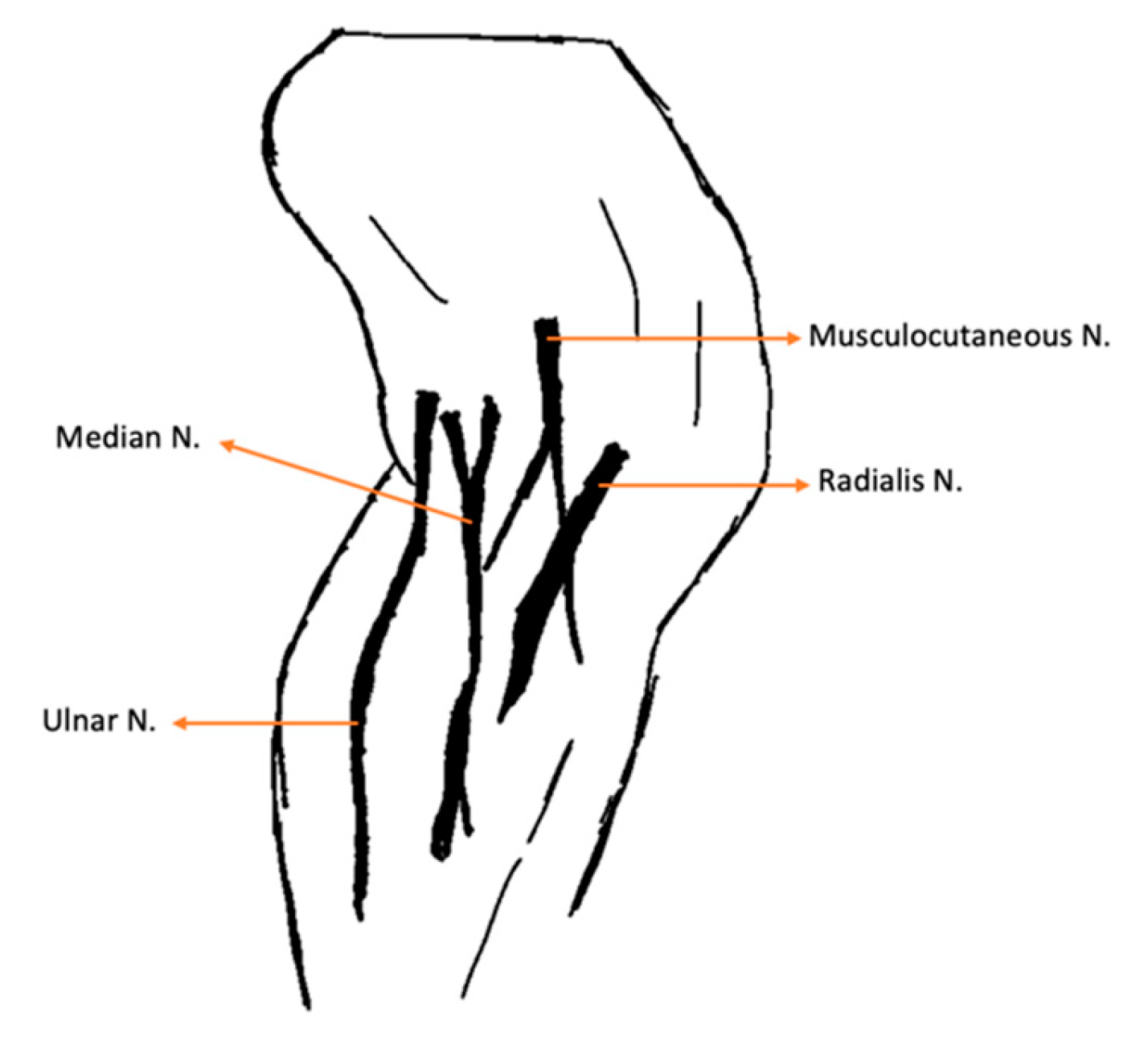
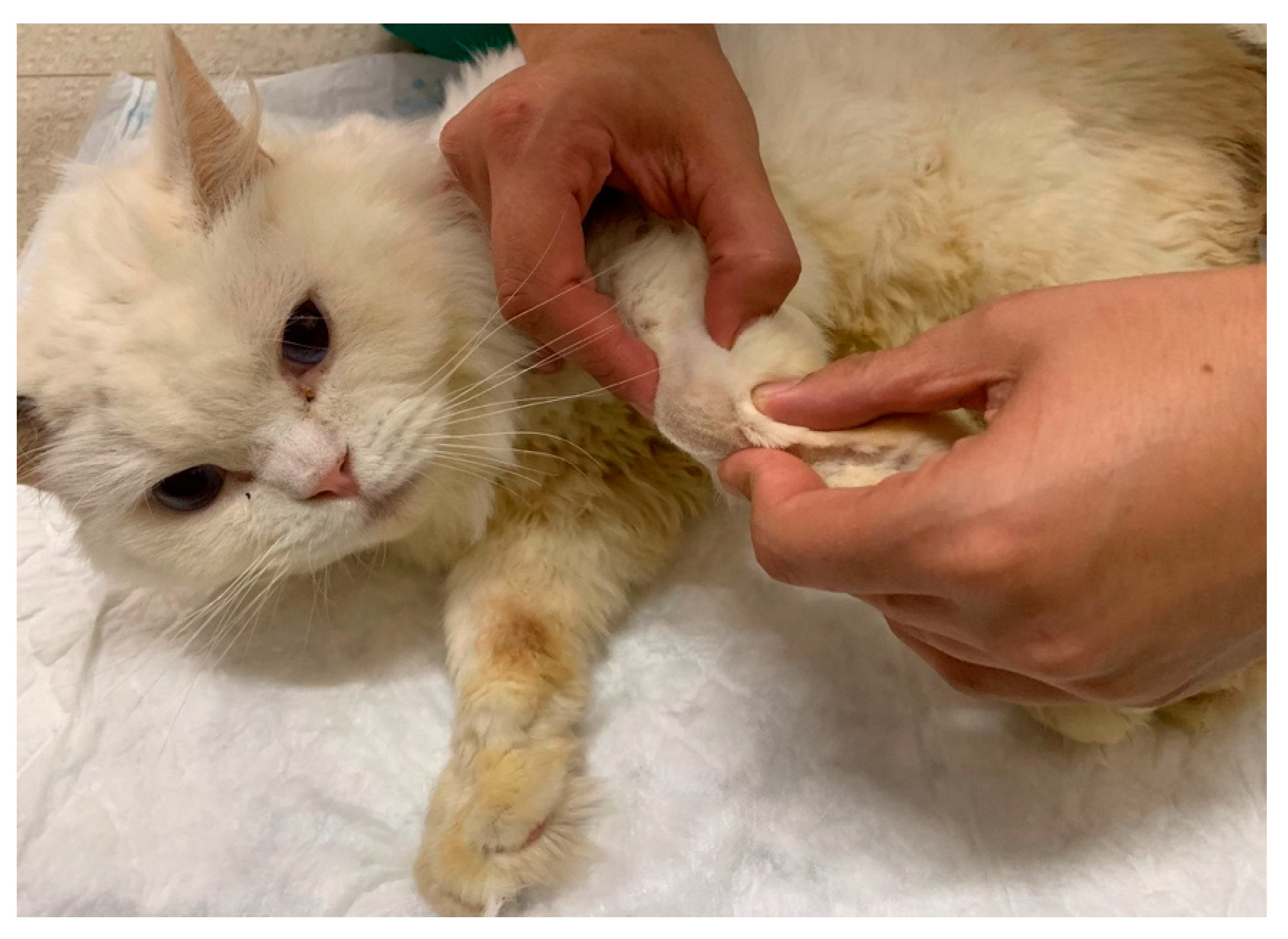
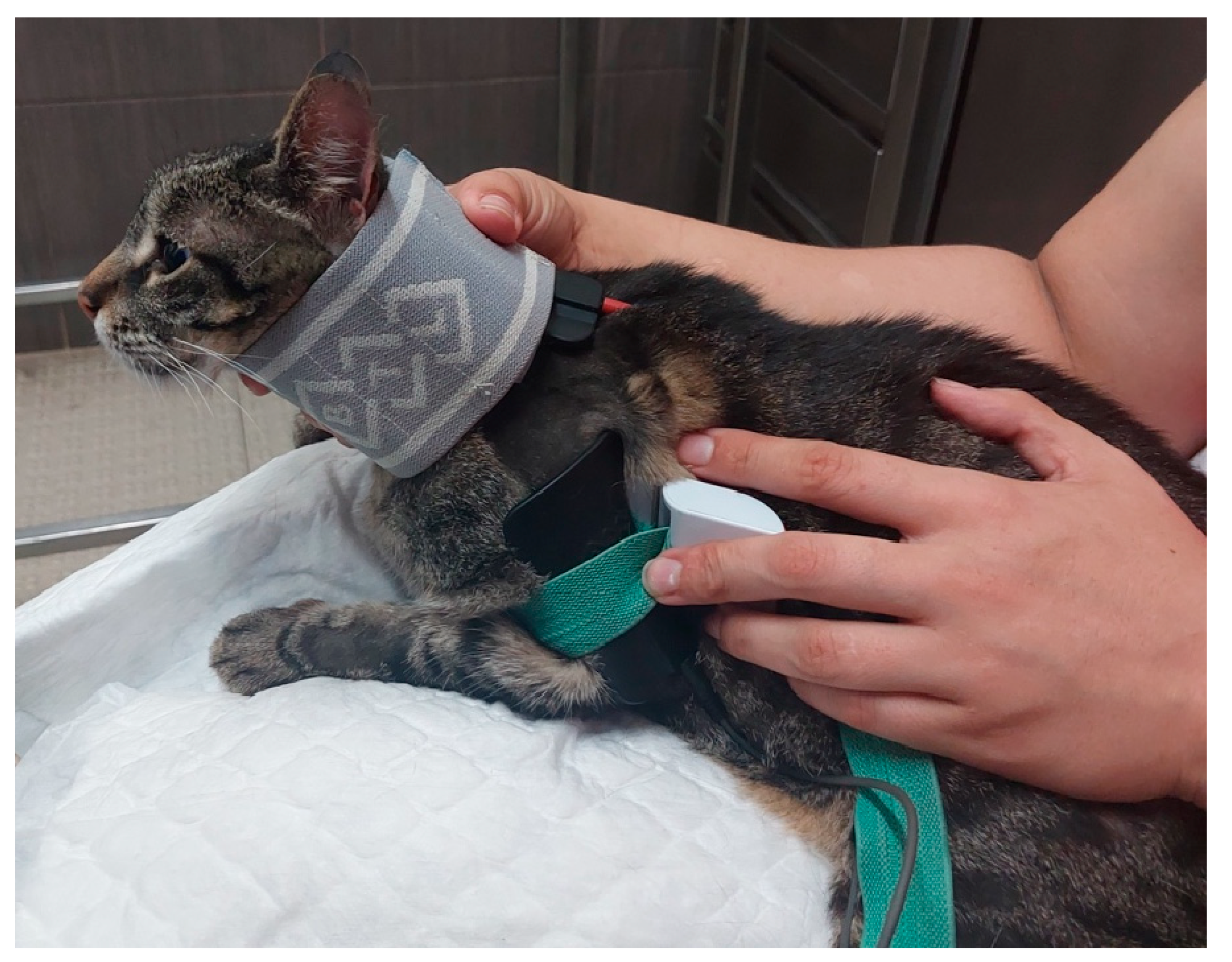

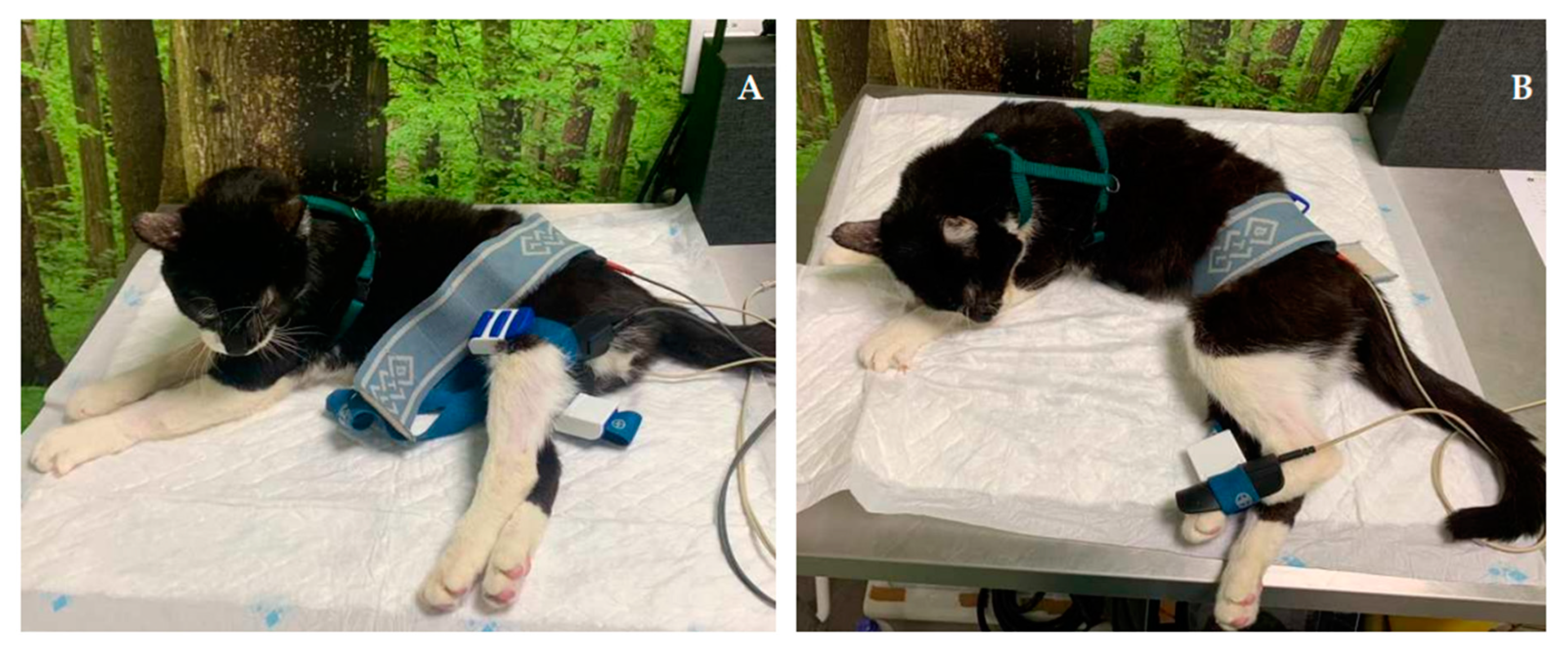
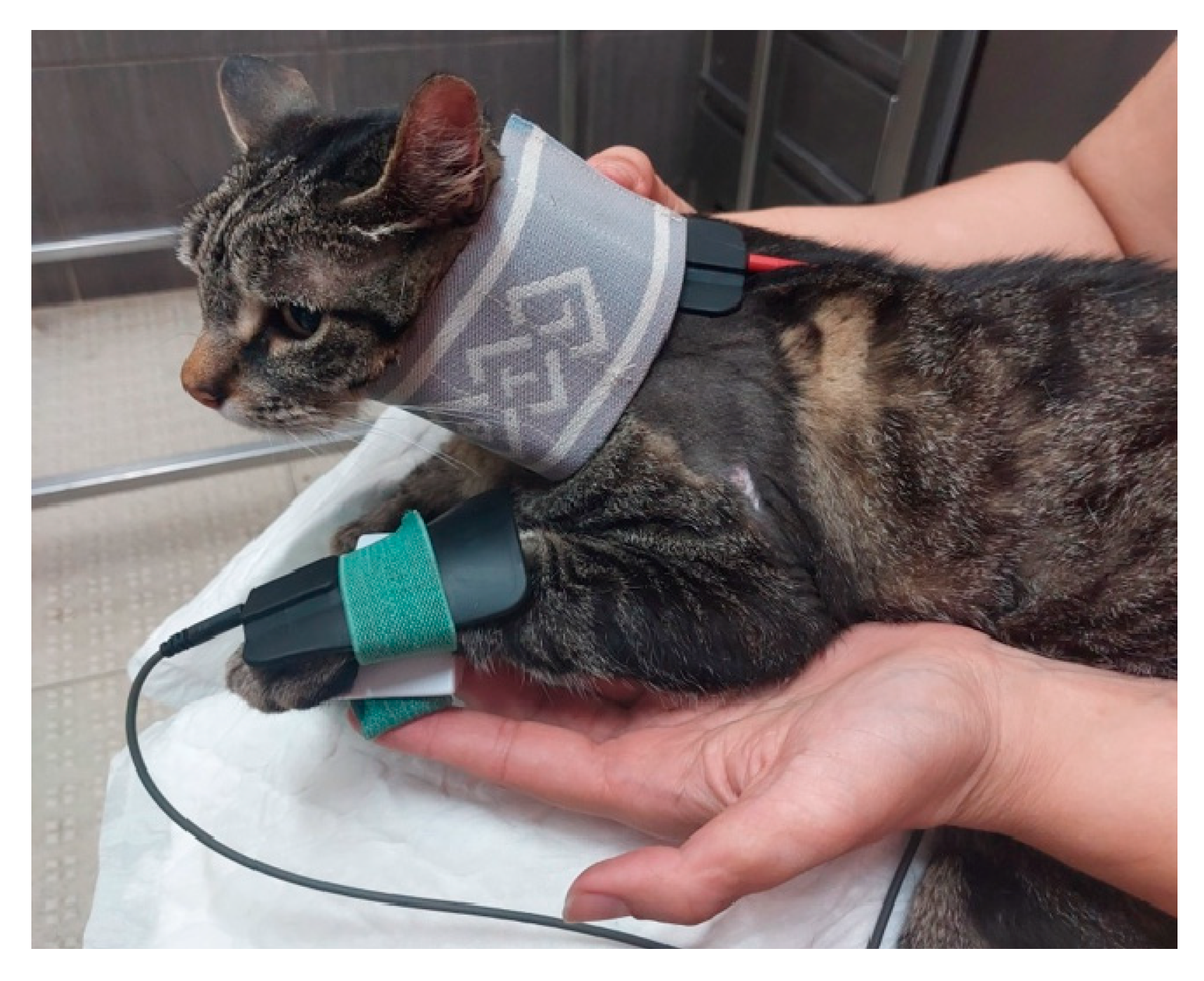
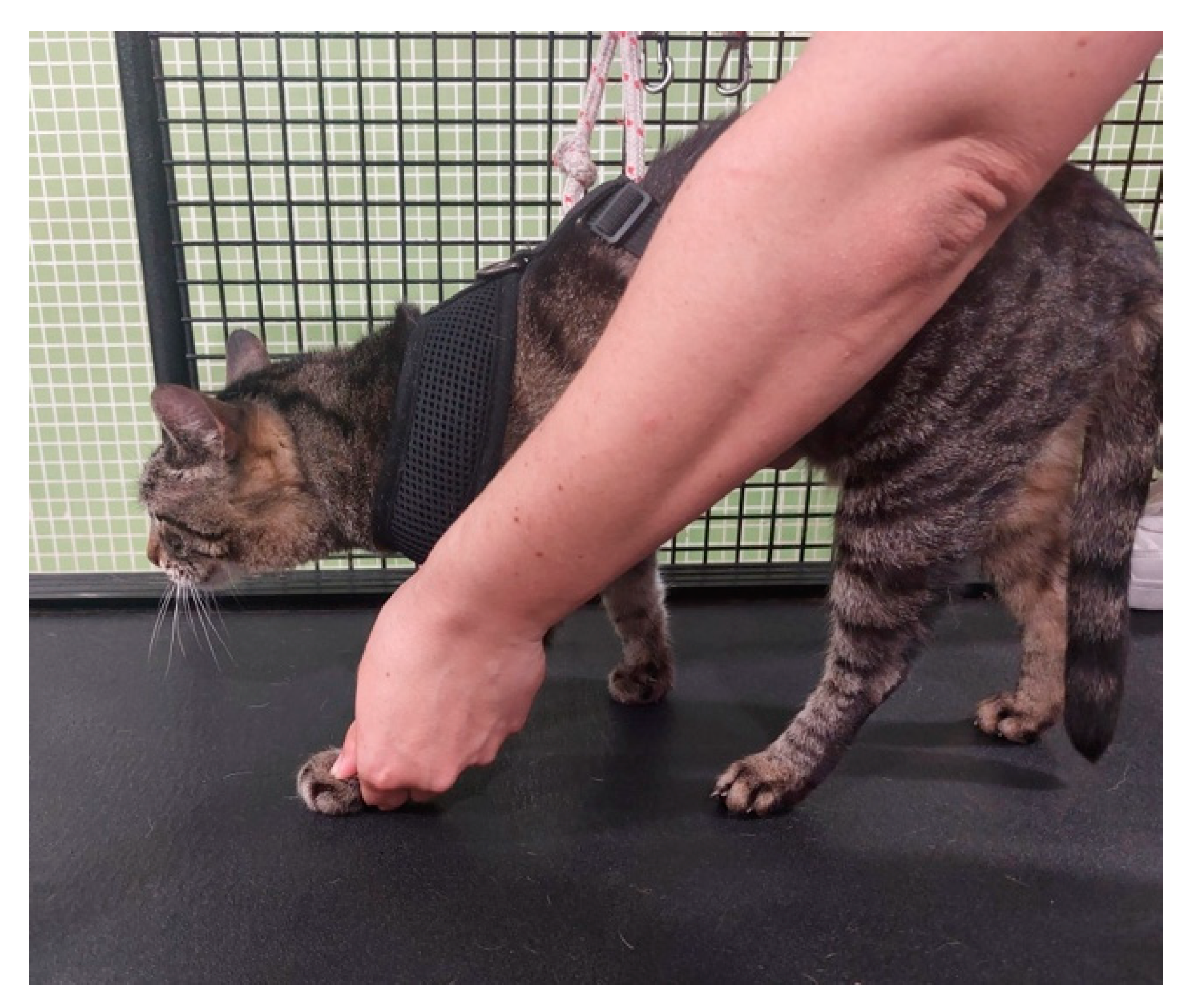
| Nerve | Origin | Innervated muscles |
|---|---|---|
| Radial | C6, C7, C8, T1, T2 | Extensor carpi ulnaris; Triceps brachialis; Extensor carpiradialis; Lateral and common digital extensor |
| Ulnar | C8, T1, T2 | Deep digital flexor; Flexor carpi ulnaris |
| Median | C7, C8, T1 | Superficial digital flexor; flexor carpi radialis |
| Lateral thoracic | C8, T1 | Cutaneous trunci |
| Nerve | ||
|---|---|---|
| Radial |
|
|
| Ulnar |
|
|
| Median |
|
|
| Rehabilitation modality/exercise | Parameters | Implementation |
|---|---|---|
| Laser Therapy (A) |
18-22 J/cm2 Class IV Laser Radial nerve pathway; SID |
Regenerative Role |
| 5-10 J/cm2 Class IV Laser 4-point joint technique; SID (Shoulder, Elbow, Carpus) |
Analgesia, Anti-inflammatory effects | |
| FES of the radial nerve (B) |
30 – 40 Hz; 6-16 mA ; 200µs [49] Trapezoidal modulation 1:4 duty cycle 2-4 s ramp up; 8 s plateau; 1-2 s ramp down; 10 min; TID |
In Deep pain positive |
| 30 – 40 Hz; 6-24 mA; 200µs [49] Trapezoidal modulation 1:4 or 1:5 duty cycle 2-4 s ramp up; 8 s plateau; 1-2 s ramp down; 10 min; TID |
In Deep pain negative | |
| Range of motion exercises (C) |
10-30 sets 4-6 times/day; |
All joints: shoulder, elbow, carpal and digits |
| Postural standing position (D) |
2-3 min 4-6 times/day |
|
| Ultrasound (E) |
1 MHz; 1.5 w/cm2; 10 min Pulsed mode; Duty cycle of 20%; Pulse ratio 1:4; 5 cm transduced head; |
Muscles: Triceps brachialis; Biceps brachialis; Extensor carpi radialis; Lateral and Common digital extensor |
| Total (n=22) | ||
|---|---|---|
| Age | Mean | 4,86 |
| Median | 5 | |
| Mode | 2 | |
| SD | 2,189 | |
| Minimum | 2 | |
| Maximum | 8 | |
| SEM | 0,467 | |
| Shapiro-Wilk Normality Test | 0,021 | |
| Weight | Mean | 4,73 |
| Median | 5 | |
| Mode | 4 | |
| SD | 1,120 | |
| Minimum | 3 | |
| Maximum | 7 | |
| SEM | 0,239 | |
| Shapiro-Wilk Normality Test | 0,071 | |
| Total (n=22) |
||
|---|---|---|
| Age | < 7 years old: 72.7% (16/22) ≥ 7 years old: 27.3% (6/22) |
|
| Weight | < 5 kg: 45.5% (10/22) ≥ 5 kg: 54.5% (12/22) |
|
| Sex | Male: 68.2% (15/22) Female: 31.8% (7/22) |
|
| Breed | Mixed breed: 86.4% (19/22) Persian: 13.6% (3/22) |
|
| Knuckling | Forelimb | Absent: 100% (22/22) |
| Hindlimb | Absent: 90.9% (20/22) Present: 9.1% (2/22) |
|
| DPP | 1-4th Digits | Absent:45.5% (10/22) Doubtful: 54.5% (12/22) |
| 5th Digit | Present:40.9% (9/22) Doubtful: 59.1% (13/22) |
|
| Reflexes | Withdrawl reflex | Absent: 100% (22/22) |
| Extensor carpi radialis reflex | Absent: 100% (22/22) | |
| Cutaneous Trunci reflex | Absent: 27.3% (6/22) Present: 72.7% (16/22) |
|
| Horner Syndrome | Absent: 86.4% (19/22) Present: 13.6% (3/22) |
|
| Dermatomes | Until elbow | Absent: 13.6% (3/22) Present: 86.4% (19/22) |
| Between elbow and carpus | Absent: 100% (22/22) | |
| Between carpus and digits | Absent: 100% (22/22) | |
| Joint motion | Shoulder | Absent: 13.6% (3/22) Present: 86.4% (19/22) |
| Elbow | Absent: 100% (22/22) | |
| Carpus | Absent: 100% (22/22) | |
| Muscle Atrophy | Triceps brachialis | Absent: 86.4% (19/22) Present: 13.6% (3/22) |
| Extensor carpi radialis | Absent: 100% (22/22) | |
| Carpal contracture | Absent: 100% (22/22) | |
| Standing position | Absent: 100% (22/22) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





