1. Introduction
The sensorimotor control system of the human masticatory apparatus has evolved to mix all food items into a saliva-mixed slurry that the lower gut can process further. Human mastication is principally powered by unilateral activities of jaw adductor muscles. Automatic masticatory jaw movements are modulated by the composition of the food items, which can be hard or soft, crunchy or sticky, or an unpredictable composition of all that at the same time. Bite force and other masticatory muscle activities are modulated by peripheral sensory innervation and by intentional or semi-conscious adjustment according to the composition of food [[
1,
2]]. During the initial chewing cycles of mastication, the working side teeth are first cushioned by the resilient, or compacted food particles. After a few or more repeated cycles of food grinding, the piece of food is sufficiently triturated into a slurry, so that teeth of the upper and lower dental arches from the anterior and the non-working side also start to contact.
Intentional, natural jaw closures automatically and repeatedly result in stable maxillomandibular relationships [[
3]], for which interocclusal tooth contacts are more or less evenly distributed to the anterior and both lateral parts of the dental arch. To close one’s mouth and achieve the natural intercuspal position (IP) is a snap, it takes about 0,15-0,4 seconds. An experienced dental professional can manually achieve a stable match between the plaster casts of the mandibular and maxillary dentitions of her patient within a few seconds. Yet, without the natural tendon, joint, and muscle attachments between mandible and maxilla this task is a very arbitrary imitation of the natural course of the closing path of mandible in jaw closure. A dentist might try to imitate the real dynamic path of mandible to the jaw-closed position by gently manipulating the lower jaw of the live patient to make a match between the teeth of mandible and maxilla. This could possibly be executed in a few seconds, but very often the resistance of the jaw muscles takes over, and the patient unconsciously starts to resist every slightest manipulative effort of the dentist, no matter how gentle-handed the clinician is, or by whatever of the methods of jaw-manipulation recommended in the textbooks of prosthetic dentistry are being used. Manipulating the lower jaw stably together with the upper may sometimes succeed in seconds, but at times an afternoon is not enough for the (futile) efforts to mimic the real kinetic moves of mandible undergoing natural jaw closure. Trying to make a match between the complicated forms of the cusps and fossae of mandibular and maxillary dentitions by external manipulation can be extremely difficult. The question raises: why the natural jaw closure is such an effortless task when operated by the patient herself? A successful match between the teeth of the upper and lower dental arches requires a complex set of automatic neuromuscular operations.
Jaw closing muscle activity is the pre-requisition to start the natural “mandible-meets-maxilla (MMM) -process”. The muscle activity begins before the first maxillomandibular tooth contact. It is not quite clear which muscles are activated to raise mandible and which muscles are inhibited in the process. In a brisk closure movement, the kinetic energy of mandible is subjected to the first tooth contact between mandible and maxilla. It is often very difficult to find out which part of the dental arch does this first collision happen. The kinematic consequences of the first collision can only be imagined. The first tooth contact certainly is registered by the periodontal mechanoreceptors (PMR) of the tooth itself and by the stretching of the spindles of the jaw muscles. The jaw closing tooth contacts have been shown to cause sensorimotor reflexes [
1], but it is not clearly understood which sensory nerves and ganglia are the synaptic messengers of these reflexes [[
4]]. We only have ambiguous and variable experimental data of how many milliseconds (ms) would it take for the sensory inputs of a tooth contact to be transformed into reflex actions of jaw muscles.
The MMM-process continues its course for the second tooth contact of the process. If the first tooth contact already elicited a meaningful jaw muscle reflex, then would the second tooth contact, just a few ms later, cause another reflex reaction? Would it be possible that the second reflex reaction could be in conflict with the reflex activity elicited by the first contact of MMM? We don’ know how the sensory input data of the successive tooth contacts of MMM are processed and prioritized.
More questions arise as we go on contemplating the kinematic consequences of the successive tooth contacts of the MMM. The first tooth contact is a very unstable point of a fulcrum enabling an unlimited number of possible directions for the tilting of mandible in relation to maxilla. The second tooth contact probably creates, together with the first contact, a cross-the-dental-arch-axis limiting the number of directions for the consequent unavoidable kinetic tilts of mandible. Only two directions of tilts are theoretically possible in a situation where there are only two points of contact between the mandibular and maxillary arches. The third tooth contact of MMM may create a maxillo-mandibular tripod. Three distinct tooth contacts, each in a separate location of the dental arch may create a triangle of support, that completely prevents further, adjusting tilts of mandible. Yet, a mere triangle of support comprising of three tooth-contacts can’t be regarded a stable mandible-maxilla relation. The interocclusal space of the tripod-phase of the developing occlusion must be compressed for the proper, food-crushing functions of IP. I am not aware of any actual measurements of how much tipping and intrusion of an individual tooth can occur in the process to create the IP. These are some of the questions that can’t be answered by just comparing jaw muscle activities of the “mouth open” and the static “jaw closed” -positions. The MMM is a dynamic and interactive process.
Dental professionals and researchers have been divided by a century-old schism between the proponents of two schools of philosophy of dental occlusion. The “tooth-contacts matter”-school of jaw-relations is opposed by the “they don’t”-school [[
5,
6,
7,
8,
9,
10,
11,
12]]. Looking at the tooth-contact dependent, but interconnected causalities of the mandible-meets-maxilla phenomenon, it becomes clear that without an analysis of tangible, recorded data of the force, location, and timing of each of the accumulating tooth contacts in the dental occlusion process, both schools of dental occlusion philosophy are doomed to remain proponents of opinions only. The two conflicting schools polemically derogate each other for maintaining “occult”—instead of proper “occlusion” philosophies.
The description of the dynamic continuum of the MMM as presented here is based on clinical T-Scan-recorded data from dental patients. The present study aims to illustrate the sequence of kinematic events of mandible undergoing MMM. The importance of tooth contact -related afferent neural inputs in provoking the jaw muscle activities of each successive step of the kinematic events of MMM is hypothesized and discussed.
2. Method
2.1. The T-Scan
Recent decades have introduced a technological innovation to measure the timing, location, and force of the tooth contacts that develop in the occluding process of the upper and lower jaw dental arches. The T-Scan occlusal contact sensor consists of a mesh of pressure sensing wires embedded in a horseshoe -shaped mylar sheet. The test subject bites her mandibular teeth against the sensor sheet placed against maxillary teeth. The bite force of each of the accumulating maxillo-mandibular tooth contact location is registered by the pressure sensor mesh to be located and shown on a schematic view of the dental arch on the computer screen.
2.2. The T-Scan sensor
The T-Scan system comprises of a mylar foil sensor embedded with a matrix-grid of columns and rows of pressure-sensitive electronic ink. The horseshoe-shaped area of the pressure-sensels of the hand-held sensor apparatus are placed to cover the occlusal-contacting areas of dental arches. For each sensel the location, timing and voltage (the force of a tooth contact) is recorded by the software to be presented graphically on the computer display. A T-Scan Novus, version 9 sensor foil (Tekscan Inc., South Boston, MA 02127, United States), large or small, was selected to match each patient’s dental arches. For the recording session of each patient, the software-wizard-guide was used to adjust the fitting of the sensor and voltage for individual occlusal anatomy and bite force, according to manufacturer’s instructions.
2.3. The force parameters of the T-Scan recordings
The T-Scan displays the time elapsed and the bite force at the given timepoint, expressed as the calculated percentage of the maximum force of each particular recording (the “Force” -parameter as shown in
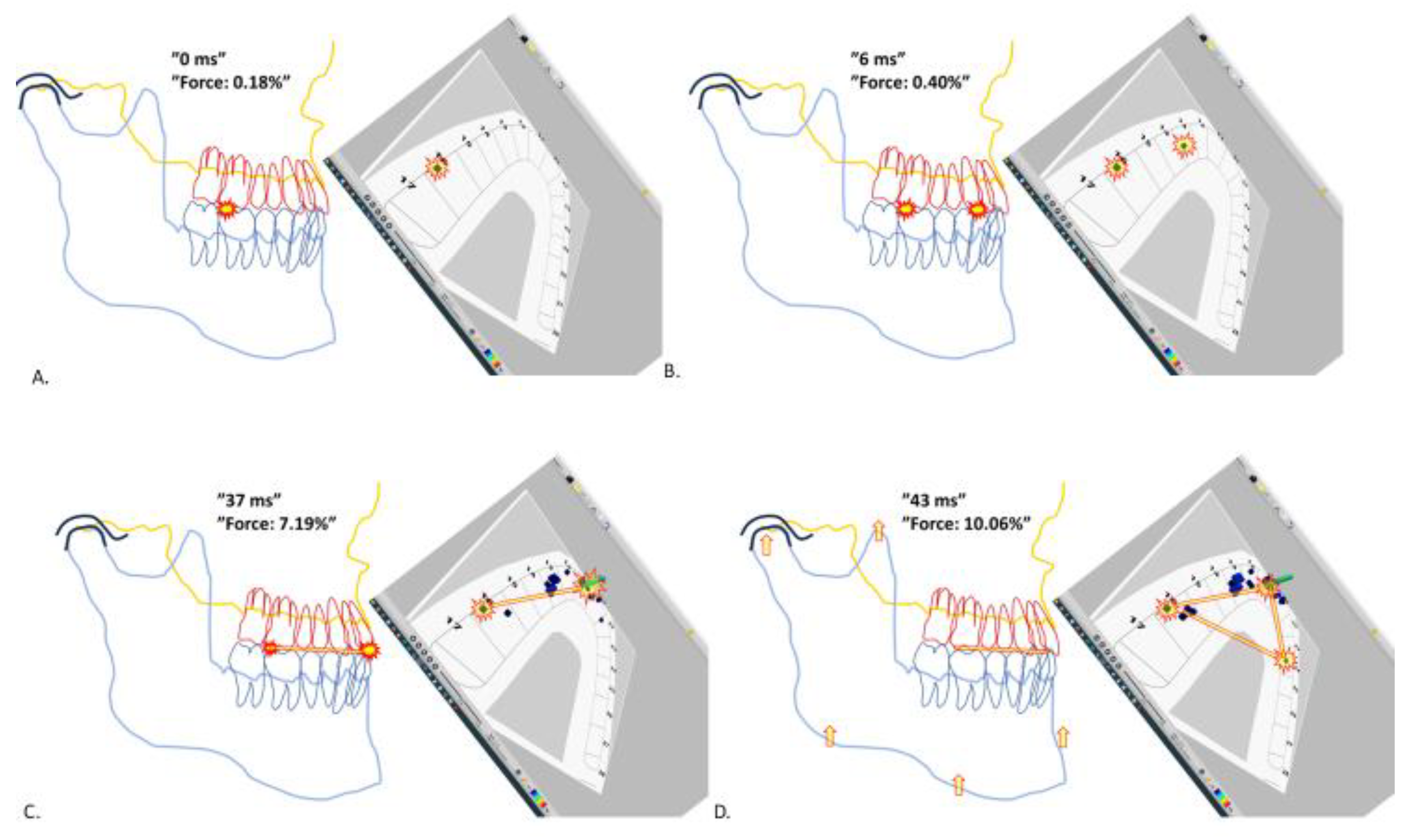
Figure 1 and
Figure 2). The “T-Scan-3-D-view” display visualizes the force subjected to each depicted contact point. The taller the colored pillar of force is, the greater is the bite force subjected to this specific contact point, to give the observer an intuitively comprehensible impression of the instantaneously changing static bite forces fluctuating at different parts of the dental arch during the MMM.
2.4. The recording procedure
The recordings were taken from subjects sitting upright in a simple table chair, without a headrest. They were instructed to imitate a slightly forward inclined upright head position, that they would feel comfortable for chewing and swallowing normally, as at a dinner table. I placed the sensor against the maxillary teeth with the pointed tip of the sensor-holder between the upper incisors, according to the manufacturers’ instructions. With the sensor sheet between their teeth, the subjects were instructed to close their mouth normally, until they felt their teeth comfortably seated in a stable closed position. For the present study the “MMM” -parts from the total of 144 clinical T-Scan recordings were scrutinized frame-by frame. Typical exemplars of natural jaw closure events, as judged by the author, were chosen for discussion and illustrative purposes.
2.5. Interpretation of the T-Scan data
The T-Scan software converts the location and force of each successively accumulating tooth contact points into a slow-motion movie-presentation with the picture frames propagating every 3-6 ms, from the first tooth contact of MMM to the IP (
Figure 1 and
Figure 2).
2.6. Subjects of this study
T-Scan data were obtained from patients of an earlier study [[
13]] approved by the ethical committee of the Northern Ostrobothnia Central Hospital. T-Scan recordings were scrutinized from a pool of 48 subjects from 30 women and 18 men. The age of participants varied from 22 to 72 years. The patients were individuals without crossbites or any known medical disturbances that could be assumed to limit the coherence of their normal jaw muscle function. Most patients had variable signs and symptoms of possible TMJ-related problems. When examined in the maximum intercuspal position, all subjects had at least one natural occluding tooth and no more than one dental implant per each upper and lower, left and right set of molars, premolars and incisors. None of the implants were canines, or without an adjacent natural tooth of the same set. I made no attempts to categorise the study subjects according to their dental, or TMJ health status.
3. Results and Discussion
3.1. The first contact of dental occlusion is not a forceful collision
The graphically displayed tooth contact signals, as recorded with the T-Scan system, repeatedly appeared in the same order, and the same locations of the dental arch, following a typical pattern for each individual patient. At the start of almost every T-Scan recording of this study, the very first signals from different locations of the dental arch were very low-force signals (
Figure 1, A). It is reasonable to assume these initial, feather-light signals were genuine tooth contacts, with bite force well below 1 % (the “Force” -parameter at the eventual maximum bite force of each recording has the value of 100%). Some unreported, off-the-record pilot experiments conducted with some of the patients of this study also attest the bite force of the first tooth contact remained below the 1% threshold despite the patient was instructed to deliberately “hit ones’ teeth together (against the T-Scan sensor sheet) as fast and forcefully as possible”. Previous T-Scan studies have demonstrated that the bite force of the MMM always develops incrementally following the first, second, third, and so forth contacts, of the MMM-process [[
14,
15]]. The initial, low force tooth contact events seen at the start of the T-Scan recordings attest the first tooth contact of the MMM is not a high energy collision between the upper and lower jaw, but a controlled, gentle freeze of the upward movement of mandible. Considering the velocity and the kinetic energy of the upward surging mandible – a half kilos’ lump of bone and flesh – charging against a single, minimum area enamel-to-enamel contact-point, it is reasonable to assume that a subconscious, protective muscle engram mechanism prevents excess velocity of mandible just in time, before the collision with maxilla.
3.2. Volitional, empty mouth jaw closures are probably operated by swallowing pattern generators
For awake subjects, jaw closures with tooth contacts – or jaw closures with “teeth almost contacting” happen thousands of times a day as saliva-swallowing is automatically repeated about once a minute. The swallowing involves a stereotyped pattern of orchestrated muscle movement commands from the central nervous system (CNS) to the motoneurons of the trigeminal, facial, glossopharyngeal, vagal, and hypoglossal motor nuclei, that are located in the brainstem [[
16]]. Biting one’s teeth intentionally together most likely involves similar non-volitional neuromuscular mechanisms that control the initial, jaw-closing phase of the swallowing of minute amounts of liquids.
The jaw closure, at the initial phase of swallowing can be regarded the first in the series of the stereotyped swallowing activity events. The neural commands for the step-by-step events of the swallowing cascade emanate from the CNS, but they are triggered and modulated by peripheral sensory feedback from proprioceptive receptors of the oral cavity. As described by Sherrington [[
17]] the jaw-closing reflex can be elicited by putting a little fluid into the mouth, or
“…by stroking with a feather the tongue dorsum near its tip. Tongue tip is curved upward and somewhat retracted and at the same time mandible is raised and the mouth deliberately closed and in the decerebrate preparation remain so.” (A “decerebrate preparation” is a euphemism for vivisection, and here Sherrington refers to an anesthetized cat with its forebrain neural connections guillotined and severed apart from the brainstem).
Jaw-closing, volitional or not, is the essential, initial part of swallowing. What seems like a simple “mandible-raise” neural command probably involves invariant neural processes and commands for a wide array of craniofacial and neck-muscles, that remain to be fully elucidated [[
18]]. Swallowing begins with raising the hyoid bone by the activation of the mylohyoid muscle, which is followed by bilateral activity of medial pterygoid and anterior digastric muscles. The activity of anterior digastric muscles is probably essential to resist the backward pull of the hyoid bone by tongue retracting muscles concomitant to the raising of mandible by medial pterygoid activities. The creation of the oropharyngeal vacuum is necessary for the continuation of the swallowing cascade. The oropharynx is sealed by the raise of the hyoid bone and the mandible to thrust the tongue against palatal aspect of incisors, canines and premolars. The tongue-thrusting against teeth must be felt by tooth periodontal mechanoreceptors (PMR). Thereafter, bilateral activity of masseter muscles finalizes the jaw closure phase of swallowing [
16,
19,
20].
At the end of swallowing-associated jaw-closure, mandible is statically stalled against maxilla, assisted by masseteric force. Perhaps, tongue-thrusting against teeth is the essential proprioceptive signal for the continuation of the swallowing cascade, which in turn triggers the offset of the anterior digastric activity and the tongue thrusting against anterior part of dental arches.
3.3. The automatic, CNS-orchestrated neural commands decussate and cause bilateral muscle activation patterns
The stereotyped muscle activity patterns of the swallowing process are the result of bilateral and simultaneous activity of many of the muscles of the head and neck. Once these automatic patterns of muscle activity get going their voluntary modulation is difficult. The reader can try to swallow while intentionally trying to keep the tip of tongue low, and resisting the urge to thrust ones’ tongue against the anterior palate and teeth. It is hard. Proprioceptive feedback from tooth mechanoreceptors is essential for triggering the smooth continuation of the successive phases of muscle activities of the swallowing cascade. The demand of a bilateral, simultaneous sensory feedback for the continuity of swallowing cascade can be demonstrated for the reader. Positing an obstacle, a pen tip, unilaterally between the either side canine teeth prevent the normal, bilaterally distributed tooth contacts of the closing mouth. Smooth propagation of the swallowing cascade becomes almost impossible. The normal subconscious task of swallowing turns into an alarming challenge that requires intentional attention and several seconds of problem solving by the entire brain. The do-it-yourself experimentation may be continued in front of a mirror to observe the thrust of one’s tongue against the palatal aspects of teeth. Should the tip of pen now be positioned in the midline of dentition, between the upper and lower central incisor teeth, swallowing is once again relatively easy, probably because both left- and right-side premolars and canine teeth (and their sensor organs, the PMR) are simultaneously being pushed and slightly dislocated by tongue muscles. During the jaw-closing phase of the swallowing cycle there may or may not be maxillomandibular tooth contacts, but the forceful tongue thrusting against the anterior palate and bilaterally against the teeth certainly must be a signal to be monitored and dealt with by the swallowing pattern generator (sCPG) of the CNS. Bilateral, simultaneous tooth contacts are essential for the successful offset of masseteric and anterior digastric activities, and to mark the end of the jaw-closure phase of the swallowing cascade.
3.4. The curiously logical antagonism of the masseter and medial pterygoid muscles
The force vector projections of a major part of the masseter muscle motor units are almost identical with those of the pterygoid medialis muscles. The directional force vectors of the masseter muscle motor units might be ideally oriented for raising the mandible upward from the jaw open position. Yet, it seems these two sets of bilateral, jaw-closing-oriented muscles, the medial pterygoids and the masseters, can’t be simultaneously active. Masseter muscles don’t get completely activated without simultaneous feedback from tooth contact proprioception delivered by the PMR of contacting teeth [[
21,
22]]. As stated above in the subsection 3.3, at the initiation of the swallowing cascade, the medial pterygoid is activated synchronously to the anterior digastric muscle, probably to raise the mandible and assist in developing tongue-thrusting against the anterior palate [
16,
19,
20]. Apparently, this task does not require great muscle force. Interestingly, the onset of activity of the medial pterygoid muscles is not dependent on the direct proprioceptive inputs from tooth contacts [[
23]]. In the initial, dynamic, and low-force part of the jaw closing movement, before there are any tooth contacts yet, the bilateral activity of medial pterygoid muscles appears to be the active set of muscles, while the masseters are not.
Vice versa, in the swallowing cascade, as the tongue-thrusting dislocates the teeth in their sockets the medial pterygoid muscles are inactivated.
For masticatory jaw closing movements, the activities of the medial pterygoid muscles and masseter muscles appears to be innervated for being temporally reciprocal. The masseter is active in the power-close-phase, whereas medial pterygoid is active in the initial raise of mandible during the masticatory stroke [[
24]]. This probably explains why in the EMG-recordings of cyclically repeated masticatory jaw-closures, the masseteric activity remains at low levels until the dynamic upward surge of mandible becomes hindered by the piece of food compressed between the teeth. The rhythmically repeated sudden onsets of masseteric activity of mastication can be observed as peaks of EMG activity only at the most static (and most forceful) part of the chewing cycle, at the end of the closing path of mandible movement [[
25]].
The paralleling direction of force vectors of masseter and medial pterygoid muscles, and the curious antagonism between these two sets of jaw adductors is logical. The masseter activity is facilitated by tooth-contact reflexes, while the activity of medial pterygoids is not. During the dynamic, upward charge of mandible there isn’t any feedback from masseteric spindle-stretching yet, whereas the more static food-crushing-part of jaw-closures causes heavy stretching of masseteric spindles. The bilateral activity of medial pterygoid muscles in the dynamic, initial part of jaw-closing is not a reflex response. It is a CNS-driven movement. The tooth-contact-induced, reflex force of the masseter muscles is reserved for the static, power-clench part of jaw closure.
3.5. The peak force of masseter muscle is preceded by the “silent period” phenomenon
Following the instant of the occluding tooth-contacts of deliberate, empty mouth jaw-closures (as verified by a microphone collecting the sound of collision) the EMG-activity of the masseter muscles goes first inactive, and then resumes the activity again [[
26,
27,
28]]. A purposedly brisk jaw closure starting from the maximal opening position to the “median occlusal position” causes a stall of 20-30 ms duration in the masseteric activity [[
29,
30]]. The “silent period” of the masseter muscle is defined as a pause in muscle activity following functional tooth contact. The silent period (and the following peak of masseteric force) is naturally induced by tooth contact stimuli and stretching of the masseter muscles. The masseteric silent periods are manifest in chewing, swallowing, or in reaction to a tap on the chin of a subject whose teeth are clenched together.
3.6. The synergy of spindle, and tooth mechanoreceptor-induced action potentials induces unilateral masseteric activity
At the instant of the first tooth contact of MMM, the masseter muscles are not fully active yet. The activation of masseter motor efferent neurons requires excitatory synaptic transmission from the proprioceptive neurons of the sensor organs that register the tooth contact and its repercussions. The proprioceptive PMR-stimuli from the molar tooth, as well as the proprioceptive spindle-stimuli from the tooth-contact-caused stretching of the same side masseter muscle are conveyed by afferent neurons that have their cell bodies in the mesencephalic ganglion (VMes) of the trigeminal nerve [
1,
4]. The VMes-housed proprioceptive neurons synapse with the efferent motoneurons of masseter muscle, in the trigeminal motor nucleus (VMot), which is located in close vicinity to the caudal part of Vmes. Both the molar-contacts and spindle-stretching proprioceptive sensations cause excitatory synaptic potentials for the VMes-housed sensory neurons [
1,
4]. The summation of the PMR- and the spindle-sensations of the molar tooth contact enables the precise targeting of the unilateral reflex bite force. The neural process for the precise timing and pin-point-targeting the masticatory bite force can be termed the unilateral food-crushing reflex (UFCR), [[
31]].
At the time-point 0 ms of the MMM, the simultaneous molar contact and the masseter-stretching (
Figure 1, A) cause PMR- and spindle-generated action potentials, respectively, for the VMes-housed neurons. These two types of neurons synapse with the ipsilateral motor efferent neurons of the ipsilateral masseter muscle. For rabbits, it takes 8 ms to put the masseter muscle firing, after an ipsilateral molar tooth contact [[
32]]. Evidence of a similar, rapid excitatory response of masseter muscle to mechanical molar tooth stimulation has also been demonstrated for humans [[
33]].
The UFCR-hypothesis postulates that the two simultaneous and excitatory proprioceptive inputs, the PMR and the spindles, are summated. Apparently, the unilateral reflex overrides the pre-existing action potentials of the CNS-guided jaw movement patterns. The first molar contact of MMM can be hypothesized as a sensory switch, that changes the initial “light-force-dynamic-mode” of jaw closure to the “heavy-gear-crushing-mode” involving the full-fledged power from the masseter muscle.
3.7. The first tooth contact of jaw closure is a kinematic revolution
The mechanistic stall of mandible by the first collision of MMM is rapidly followed by an entirely new movement. The first contact of occlusion acts as its pivot. The mandible is transformed into a see-saw, a class 1 lever system, for which the first tooth contact acts as the fulcrum. For the case verified by the T-Scan recording depicted in Fig, 1, panels A and B, the movement in question, is a minuscule tilt of mandible to restart the successive move of mandible toward the second point of contact of the MMM. For the T-Scan recording discussed here, the latency of the reflex activation of the muscle force starting the first kinematic tilt of mandible, lasted something between 3-6 ms. The most likely agonist of the muscle force for this tilt was the unilateral, right side masseter muscle.
Since the start of the recorded MMM, new tooth contacts started to accumulate exclusively on the right side of the dental arch only.
Figure 1, panel D, 43 ms after the start of MMM there is already a considerable amount of bite force, practically all of which is subjected to the right side of the jaw. Except for one, weak and tiny tooth contact on the left premolar.
The relatively low-force first molar contact caused a rapid reflex activation of the right-side masseter muscle, that caused a revolution of mandible kinematics. The consequences of a revolution are - as always - difficult to predict.
3.8. The inclination of tooth-cusps may effect a jaw-closing tilt, or a jaw-opening tilt
The first pin-point contact between mandible and maxilla is the veritable turning-point of mandible undergoing MMM. Sagittal aspect of the maxillary occlusal plane, the curve of Spee [[
34]] is an imaginary, slightly curved plane following the tips of incisors and the canine tooth, and the cusp tips of premolars and molars. Its curvature ideally follows the perpendiculars of the roots of the maxillary teeth. The kinematic consequences following the collision of the tip of a single mandibular molar cusp with a flat plane, the imaginary maxillary plane of occlusion, should be fairly predictable. Following the short latency of the silent period, the stretched, ipsilateral masseter muscle would be firing. The synovial spaces of the temporomandibular joint ipsilateral to the point of first tooth contact, as well as the ipsilateral masseter muscle would be distracted in reaction to the rapidly increasing reflex force of the masseter muscle. Which in turn, would tilt the mandible for the next tooth contact located on the contralateral side (
Figure 1D).
For the natural teeth, however, with the complicated anatomy of the cusps and fossae, the direction of the tilt is not always easy to predict. In the real life, the occlusal plane is never a flat surface, except perhaps for individuals with extremely abraded dentitions. The first tooth contact of occlusion may be considered a tiny pin-point-sized flat plane that can possibly exist at any location of the many-faceted cusp-inclines of any maxillary tooth. Each individually inclined slope of the cusps and fossae presents a miniature plane of diversion from the imaginary occlusal plane of Spee.
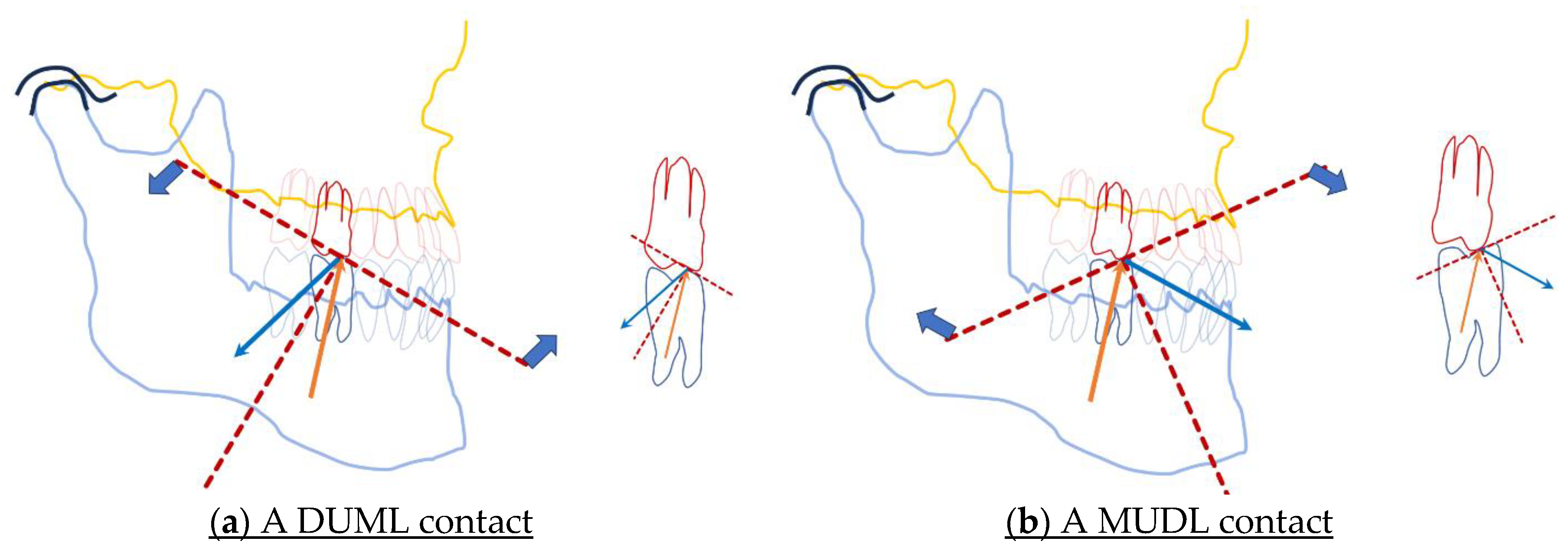
The impact of the collision of the first tooth contact of occlusion probably is very weak, but its reaction, the firing of masseter muscles that follows, is a very powerful manifesto of the UFCR, the leverage of rapidly increasing, pin-point-targeted incidence of unilateral muscle force. The rapidly increasing static bite force is directed against the cusp-slope of the opposing maxillary tooth. Similar to the bounce of a ball hitting against a flat wall, the energy from the rapid activation of the masseter muscle determines the direction for the forthcoming bounce of mandible. Anyone of the inclines of the pyramid-shaped cusp, against which the first tooth contact is subjected to, is a miniature flat wall that determines the angle of reflection of the first tilt of mandible. A minuscule change of tooth anatomy changes the location of the incidence of impact. A slight alteration of the anatomy of a pair of occluding molars may potentially transform a jaw-closure oriented tilt of mandible into a jaw-opening tilt (
Figure 3).
By definition, any prosthetic restoration of teeth does the same thing – the slight alteration of tooth anatomy. A tiny alteration of the slope of a filling might cause a revolution for the direction of the tilt of mandible. A tiny change in the location of the first contact of occlusion, could change the incidence of a tooth contact from MUDL-situation to a DUML-situation. Rotations of mandible around the see-saw fulcrum are difficult to predict. However, the rotational movements around the first tooth contact fulcrum are minuscule. A DUML situation would very soon cause a second tooth contact in the anterior dentition area, whereas a MUDL situation would cause the reaction force of the rotational tilt to be subjected to the ipsilateral temporomandibular joint.
3.9. An anterior-area tooth contact causes a silent period of masseteric activity
The first anterior-tooth (ANT)-contact is inhibitory for the masseter muscle [[
35,
36]]. The silent period is the protective withdrawal reflex, that causes a temporary inhibition of masticatory tooth contacts to prevent excessive, non-axial bite force being subjected to the upper incisor. The sensory afferent neurons conveying the back-tooth (BAT)-PMR are equipped with excitatory synaptic messenger molecules for the VMot motoneurons. However, there is circumstantial evidence that the synaptic transmission of the axonal endings of anterior tooth (ANT)-PMR-conveying neurons could be inhibitory. Canine tooth contacts of gliding, lateral jaw movements, akin to incisor contacts, inhibit and switch off the temporalis and masseter activity [[
37,
38]]. Overall, the ANT-contacts may be regarded inhibitory for the masseteric motoneurons, while the BAT-contacts are excitatory. The functionally reciprocal innervation of ANT and BAT, can be termed the hypothetical “ANT is not BAT” -principle [
13,
31]. The neuronal infrastructure underlying the “ANT is not BAT”-principle probably also explains the silent periods of masticatory and volitional jaw closures.
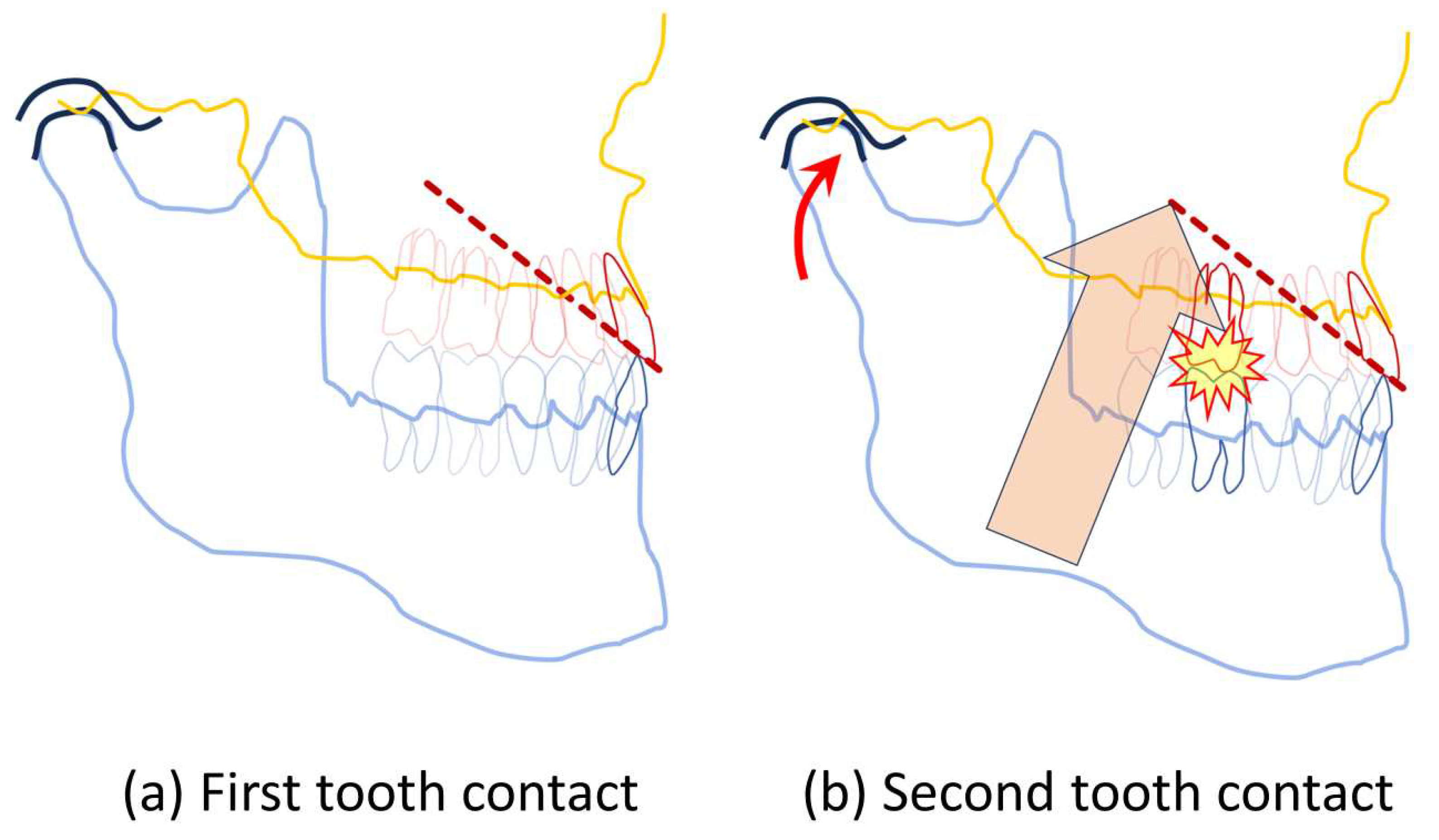
Figure 4 illustrates the kinematic consequences of a case with an alternative beginning of the MMM-narrative.
For the T-Scan recording of
Figure 4, the first contact of natural jaw closure is located in the ANT-area. For most individuals, most of the time, the first tooth contact of MMM typically happens in the incisor tooth area [
13]. Instead of the class 1 mode of force leverage of mandible, as explained earlier for the first BAT-contact, the first ANT-contact is a fulcrum of a class 2 lever. The class 2 -levered masticatory forces are subjected either to the second tooth contact in the BAT-area, or a piece of food in the BAT-area, or to the synovia of the temporomandibular joints. The two-chamber synovial pump of the temporomandibular joint is an ingenious evolutionary innovation to resist the heavy leverage of bite force compressing the mandibular condyles against the roof of the glenoid fossa [[
39,
40]]
3.10. The initial toothcontacts and their two consequent tilts create a tripod
Theoretically, to create a stable jaw-relation, only three contact points are required, one for each of the three sides of the mandible triangle.
Figure 1, panel D depicts the first, left-side tooth contact of the MMM of a live patient. The imaginary axis of rotation formed by the previously accumulated right-side tooth contacts has dictated a tilt of mandible for left-side contact to induce a rapid UFCR reflex of the left side masseter muscle. Tooth contacts of the ANT and bilateral BAT create a triangle of support preventing the future tilting movements of mandible.
3.11. The compressive phase of MMM
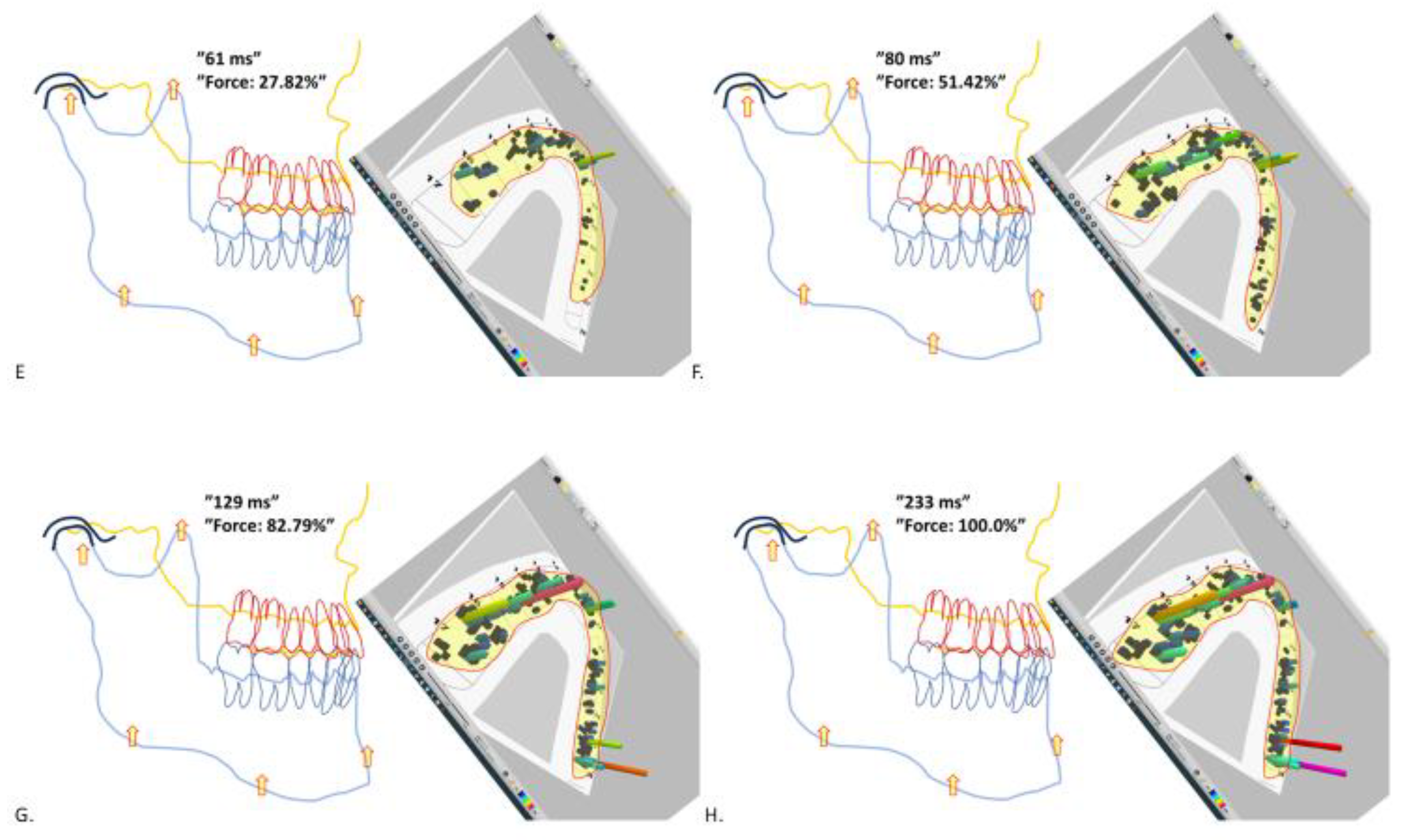
The rapidly increasing, semi-static bite force compresses mandible against the maxilla. A multitude of new tooth contacts accumulate in all parts of the dental arch.
A study shows that the power stroke of mastication expanded the span between left and right maxillary molars of young pigs by quite significant 192+/-95 μm, on the average. The displacement was thought to be due to tipping and intrusion of teeth and the bending of the alveolar bone under masticatory loading [[
41]]. Cast models in a dental articulator cannot mimic the displacement of an individual tooth under the static loading, but the images (
Figure 2, panels E,F,G, and H) generated on the data of the shifts of static loading of individual teeth, as shown by T-Scan suggests the bending and intrusion of individual teeth is an essential element of the semi-static kinematics of the final phase of jaw-closing
3.12. During the forceful, compressive phase of MMM the inhibitory ANT-contacts compete with the excitatory BAT-contacts
Most of our understanding of the reflex activation of the masseter muscle is based on observations of its inhibition. The masseteric silent period is extensively studied [
1]. The set-ups for the jaw-jerk studies examining the duration of the masseteric spindle -induced silent period necessitates the pre-existing clenching-activation of jaw muscles prior to the chin tapping, which is the actual stretch-stimulus [[
42]]. Studies examining the duration of masseteric silent period employ pre-existing masseteric activity before the controlled tap- or push-stimulus [
35,
36] produced consistent and repeatable measurements of the duration of the silent period. In contrast, more variable durations of the silent period have been obtained in studies employing natural jaw-closing-stimuli (almost all teeth are each sending the contact-stimulus almost simultaneously) [
26,
27,
28,
29,
30]. The relatively short duration of masseteric silent periods in an experiment setting mimicking a natural jaw-closure stimulus (20-30 ms, for study [
30]) could possibly be explained by the natural abundance of excitatory BAT-PMR neurons firing in the jaw-closed situation, as compared to the experiments where the inhibitory silent periods are elicited by the firing of ANT-PMRs of a single incisor tooth (44 ms, for study [
36]). The sheer number of the VMot synapses conveyed by the BAT-PMR connected neurons from several teeth, may just outnumber the ANT-PMR connections from a single tooth [[
43]].
Towards the end of MMM (
Figure 2, panels G and H), the relative proportion of bite force increases in the BAT area, as compared to the bite force subjected to the ANT area [
13,
15]. It could be speculated that the lesser bite force subjected to the ANT-PMR, would elicit less intense action potentials to be conveyed to inhibit the VMot motoneuron synapses. The excitatory BAT-PMR-neurons not only outnumber the inhibitory ANT-PMR connections to the VMot synapses [
43], but in the case of forceful jaw closure the BAT-PMR probably also “make their case clear by shouting louder”. Approaching the IP phase of the MMM, it is likely the excitatory BAT-PMR inputs are more victorious than the inhibitory feedback from ANT-contacts.
3.13. The final thrust of reflex activity
Overall, in the case of
Figure 2, panels E to H, the last 172 ms before the 100% of bite force, the MMM may be considered a war of attrition where the more forceful, more numerous, and excitatory BAT contacts gradually push the mandible into an ever more intimate connection against its counterpart, the maxilla. The inhibitory ANT-contacts are overwhelmed, despite constant increase of masseteric strain and pressure on the PMRs of their non-axially aligned roots. There is no interocclusal space left for any tooth to tip, bend, or intrude any more. Suddenly, the war is over. A few dozens of ms after the echo of the last tooth percussion has faded, silence prevails. All teeth, their periodontal ligaments, joints and bones are maximally twisted, and compressed motionless under masseteric force. The mandible can’t tilt anywhere. Not a single unstrained spindle left to be stretched any more. The most forceful event of the MMM, the
grande finale, is a static freeze of all movement. The excitatory signals of the massive number of bilateral BAT contacts, as well as the inhibitory signals from ANT-contacts have died out.
3.14. The end of the reflex cycle signals the restart of the central pattern generators
The MMM narrative discussed in the present study is about an individual with normal functional connections between the CNS and the peripheral sensory nervous system. The reader can be assured the test subject of
Figure 1 and 2 did not continue clenching her teeth together forever. In contrast, the brainstem-guillotined cat of Sherrington was less fortunate. At the end of the feather tip-stimulated jaw closure experiment, the mandible of the cat remained clenching against her maxilla. “…
the mandible is raised and the mouth rather deliberately closed and in the decerebrate preparation remain so.” [
17]. Decorticate rigidity is a sign of a severe lesion of the brainstem neural connections [[
44]]. If the brainstem-cerebral-cortex connection is mutilated, vital and automatic functions, such as swallowing can be disturbed. For the normal, healthy individual of this study, the simultaneous “nothing moves” -signal from all three sides of mandible (the ANT, and bilateral BAT -areas of dental arches) probably was the message recognized by the CNS pattern generators. The tooth-contact elicited reflex action coordination of jaw movements is null and void if nothing moves. Without UFCR-commands the masseteric force quickly wears off, and the CNS-orchestrated pulsations of stereotyped, bilateral muscle movement patterns of mandible are enabled to resume once again.
4. Limitations of this study
The T-Scan recording system enables a perspective to visualize the kinematic events of mandible during the jaw-closing process. There were not two MMMs alike in the 144 recordings scrutinized for this study. The step-by-step description of the illustrated sequence of kinematic events of the MMM is based on only one selected recording. An additional case, for which the MMM starts from an ANT-contact served to produce the illustration
Figure 4.
The anatomical dimensions of the sagittal view image-objects of mandible and maxilla are generic, not based on real data. The purpose of the images is to give an idea of the minute tilting movements and rotations of mandible in the course of MMM.
The jaw-closure associated activities of the temporalis muscle is not covered in this article. Plenty of evidence is available of the mutual synergy of masseter and temporalis muscles, but I have limited the discussions of jaw adductors to the actions of the masseter and medial pterygoid muscles. Most of the statements of this article concerning the masseter muscle activation should also comply with the temporalis muscle.
5. Conclusions
This study shows the neurophysiology of the MMM-kinematics can’t be fully explained by the dichotomous “jaw-open-jaw-closed” paradigm. The data presented here suggests jaw-closure is a kinematic process for which every single tooth contact of the process is an on-, or off-stimulus, a single tooth contact can be excitatory or inhibitory. The proprioceptive afferent neurons of incisors and canines can cause not excitatory, but inhibitory synaptic transmission to the VMot motoneurons of the principal jaw-closing muscles. The individual variation in the location of the ANT- and BAT-contacts during MMM determines the durations of the masseteric silent periods, and the directions of the tilts of mandible in a typical manner for each individual and bite.
The co-existence of a matching pair of mandible and maxilla is constantly consolidated by repeated, harmonious jaw-closures, in which the least possible number of unfavorable tooth-contact-elicited masseteric reflexes occur. The everyday life of a non-match mandible-maxilla-pair is strained by unavoidable strains of ipsi- and contralateral masseteric reflexes, which are being caused by an unfavorable tooth contact location during swallowing and mastication.
The MMM-narrative can be regarded the kinematic reflection of two, temporally alternating modes of control of jaw muscles, the UFCR- and CNS-driven neurophysiologies. Tooth-contacts matter for the excitatory and inhibitory jaw muscle actions that are executed by proprioceptive, VMes-mediated UFCR pathways. Tooth-contacts also matter to signal the start of some of the CNS-controlled automatic muscle-action patterns. Once the bilateral, stereotyped movements get going, tooth-contacts will interrupt the automatic jaw movement to be replaced by the UFCR control. Furthermore, the absence UFCR-activity, the absence of inhibitory or excitatory proprioceptive feedback from jaw muscles and tooth-contacts may also be considered a signal to start the CNS-controlled phase of mandible kinematic cycles.
Future studies should aim to clarify what kind of interventional modification of tooth and dental arch anatomies could be beneficial for the promotion of health of the masticatory apparatus, and the prevention of its diseases.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Central Hospital of Northern Ostrobothnia.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The author declares no conflict of interest.
References
- Türker, K.S. Reflex control of human jaw muscles. Crit Rev Oral Biol Med. 2002, 13, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.S.; Pruszynski, J.A.; Edin, B.B.; Westberg, K.G. Biting intentions modulate digastric reflex responses to sudden unloading of the jaw. J Neurophysiol. 2014, 112, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Jaschouz, S.; Mehl, A. Reproducibility of habitual intercuspation in vivo. J Dent. 2014, 42, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Linden, R.W.; Scott, B.J. Distribution of mesencephalic nucleus and trigeminal ganglion mechanoreceptors in the periodontal ligament of the cat. J Physiol. 1989, 410, 35–44. [Google Scholar] [CrossRef] [PubMed]
- de Kanter, R.J.; Battistuzzi, P.G.; Truin, G.J. Temporomandibular Disorders: “Occlusion” Matters! Pain Res Manag. 2018, 2018, 8746858. [Google Scholar] [CrossRef] [PubMed]
- Türp, J.C.; Greene, C.S.; Strub, J.R. Dental occlusion: a critical reflection on past, present and future concepts. J Oral Rehabil. 2008, 35, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Hahnel, S.; Faltermeier, A.; Bürgers, R.; Kolbeck, C.; Handel, G.; Proff, P. The two main theories on dental bruxism. Ann Anat. 2012, 194, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Manfredini, D.; Winocur, E. Are bruxism and the bite causally related? J. Oral Rehabil. 2012, 39, 489–501. [Google Scholar] [CrossRef]
- Fukushima, S. A controversy with respect to occlusion. Jpn Dent Sci Rev. 2016, 52, 49–53. [Google Scholar] [CrossRef]
- Zonnenberg, A.J.; Türp, J.C.; Greene, C.S. Centric relation critically revisited-What are the clinical implications? J Oral Rehabil. 2021, 48, 1050–1055. [Google Scholar] [CrossRef]
- Zonnenberg, A.J.; Türp, J.C.; Greene, C.S. RE: Paper by Fornai C, Tester I, Parlett K, Basili C, Costa HN. J Oral Rehabil. 2022, 49, 935–936. [Google Scholar] [CrossRef]
- Fornai, C.; Tester, I.; Parlett, K.; Basili, C.; Costa, H.N. Centric relation: A matter of form and substance. J Oral Rehabil. 2022, 49, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Vaahtoniemi, L. The reciprocal jaw-muscle reflexes elicited by anterior- and back-tooth-contacts-a perspective to explain the control of the masticatory muscles. BDJ Open, 2020; 6, 27. [Google Scholar]
- Chapman, R.J.; Maness, W.L.; Osorio, J. Occlusal contact variation with changes in head position. Int J Prosthodont. 1991, 4, 377–381. [Google Scholar] [PubMed]
- Koos, B.; Höller, J.; Schille, C.; Godt, A. Time-dependent analysis and representation of force distribution and occlusion contact in the masticatory cycle. J Orofac Orthop. 2012, 73, 204–214. [Google Scholar] [CrossRef]
- Jean, A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef]
- Sherrington, C.S. Reflexes elicitable in the cat from pinna vibrissae and jaws. J Physiol. 1917, 51, 404–431. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Nordh, E.; Eriksson, P.O. Spatiotemporal consistency of human mandibular and head-neck movement trajectories during jaw opening-closing tasks. Exp Brain Res. 2002, 146, 70–76. [Google Scholar] [CrossRef]
- Doty, R.W.; Bosma, J.F. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956, 19, 44–60. [Google Scholar] [CrossRef]
- Ono, T.; Iwata, H.; Hori, K.; Tamine, K.; Kondoh, J.; Hamanaka, S.; Maeda, Y. Evaluation of tongue-, jaw-, and swallowing-related muscle coordination during voluntarily triggered swallowing. Int J Prosthodont. 2009, 22, 493–498. [Google Scholar] [PubMed]
- Homsi, G.; Kumar, A.; Almotairy, N.; Wester, E.; Trulsson, M.; Grigoriadis, A. Assessment of masticatory function in older individuals with bimaxillary implant-supported fixed prostheses or with a natural dentition: A case-control study. J Prosthet Dent. 2023, 129, 871–877. [Google Scholar] [CrossRef]
- Grigoriadis, A.; Trulsson, M. Excitatory drive of masseter muscle during mastication with dental implants. Sci Rep. 2018, 8, 8597. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Whittle, T.; Gal, J.A.; Murray, G.M.; Klineberg, I.J. The medial pterygoid muscle: a stabiliser of horizontal jaw movement. J Oral Rehabil. 2017, 44, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hannam, A.G.; Wood, W.W. Medial pterygoid muscle activity during the closing and compressive phases of human mastication. Am J Phys Anthropol. 1981, 55, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, M.F.; Dias, F.; Farfán-Beltrán, N.; Manzanares-Céspedes, M.C.; Cerda, C.; Fuentes, R. Synchronization of Surface Electromyography and 3D Electromagnetic Articulography Applied to Study the Biomechanics of the Mandible: Proof of Concept. Appl. Sci. 2023, 13, 5851. [Google Scholar] [CrossRef]
- Griffin, C.J.; Munro, R.R. Electromyography of the jaw-closing muscles in the open-close-clench cycle in man. Arch Oral Biol. 1969, 14, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hannam, A.G.; Matthews, B.; Yemm, R. Changes in the activity of the masseter muscle following tooth contact in man. Arch Oral Biol. 1969, 14, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Ainine, S.; Mason, A.G.; Cadden, S.W. Quantification of jaw reflexes evoked by natural tooth contact in human subjects. Arch Oral Biol. 2011, 56, 855–863. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.C. Inhibitory effects in the masticatory neuromusculature of human subjects at median occlusal position. Arch Oral Biol. 1976, 21, 329–331. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.C. Occlusal adjustment for a physiologically balanced occlusion. J Prosthet Dent. 1977, 38, 284–293. [Google Scholar] [CrossRef]
- Vaahtoniemi, L.H. The Food-Crushing Reflex and Its Inhibition. Appl. Biosci. 2023, 2, 550–564. [Google Scholar] [CrossRef]
- Lavigne, G.; Kim, J.S.; Valiquette, C.; Lund, J.P. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J Neurophysiol. 1987, 58, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, R.S.; Male, C.; Türker, K.S. Response of human jaw muscles to axial stimulation of a molar tooth. Exp Brain Res. 2004, 159, 214–224. [Google Scholar] [CrossRef] [PubMed]
- The Glossary of Prosthodontic Terms: Ninth Edition. J Prosthet Dent. 2017, 117, e1–e105. [CrossRef] [PubMed]
- Louca, C.; Cadden, S.W.; Linden, R.W. The roles of periodontal ligament mechanoreceptors in the reflex control of human jaw-closing muscles. Brain Res. 1996, 731, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, R.S.; Türker, K.S.; Savundra, A.W. Response of human jaw muscles to axial stimulation of the incisor. J Physiol. 2003, 547, 233–245. [Google Scholar] [CrossRef]
- Manns, A.; Chan, C.; Miralles, R. Influence of group function and canine guidance on electromyographic activity of elevator muscles. J. Prosthet. Dent. 1987, 57, 494–501. [Google Scholar] [CrossRef]
- Williamson, E.H.; Lundquist, D.O. Anterior guidance: its effect on electromyographic activity of the temporal and masseter muscles. J. Prosthet. Dent. 1983, 49, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Kubein-Meesenburg, D.; Nägerl, H.; Fialka-Fricke, J.; Hahn, W.; Weber, S.; Hönig, J.; Hansen, C.; Fanghänel, J.; Thieme, K.M.; Ihlow, D; Functional states of mandibular movements and synovial pumps of the temporomandibular joint. Is it possible to provide a biomechanically correct replacement for the TMJ? Ann Anat. 2012, 194, 200–207. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, J.M.; Zheng, Y.H.; Han, Y.; Zhang, Z.G.; Xi, C. Computational synovial dynamics of a normal temporomandibular joint during jaw opening. J Formos Med Assoc. 2013, 112, 346–351. [Google Scholar] [CrossRef]
- Salamati, A.; Chen, J.; Herring, S.W.; Liu, Z.J. Functional tooth mobility in young pigs. J Biomech. 2020, 104, 109716. [Google Scholar] [CrossRef]
- Lewis, G.R.; Pilcher, R.; Yemm, R. The effect of stimulus strength on the jaw-jerk response in man. J Neurol Neurosurg Psychiatry. 1980, 43, 699–704. [Google Scholar] [CrossRef]
- Hassanali, J. Quantitative and somatotopic mapping of neurones in the trigeminal mesencephalic nucleus and ganglion innervating teeth in monkey and baboon. Arch Oral Biol. 1997, 42, 673–682. [Google Scholar] [CrossRef]
- Kumar, A.; Tummala, P.; Feen, E.S.; Dhar, R. Spinal Decerebrate-Like Posturing After Brain Death: A Case Report and Review of the Literature. J Intensive Care Med. 2016, 31, 622–624. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).