Submitted:
20 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Procedure
2.3. Characterization
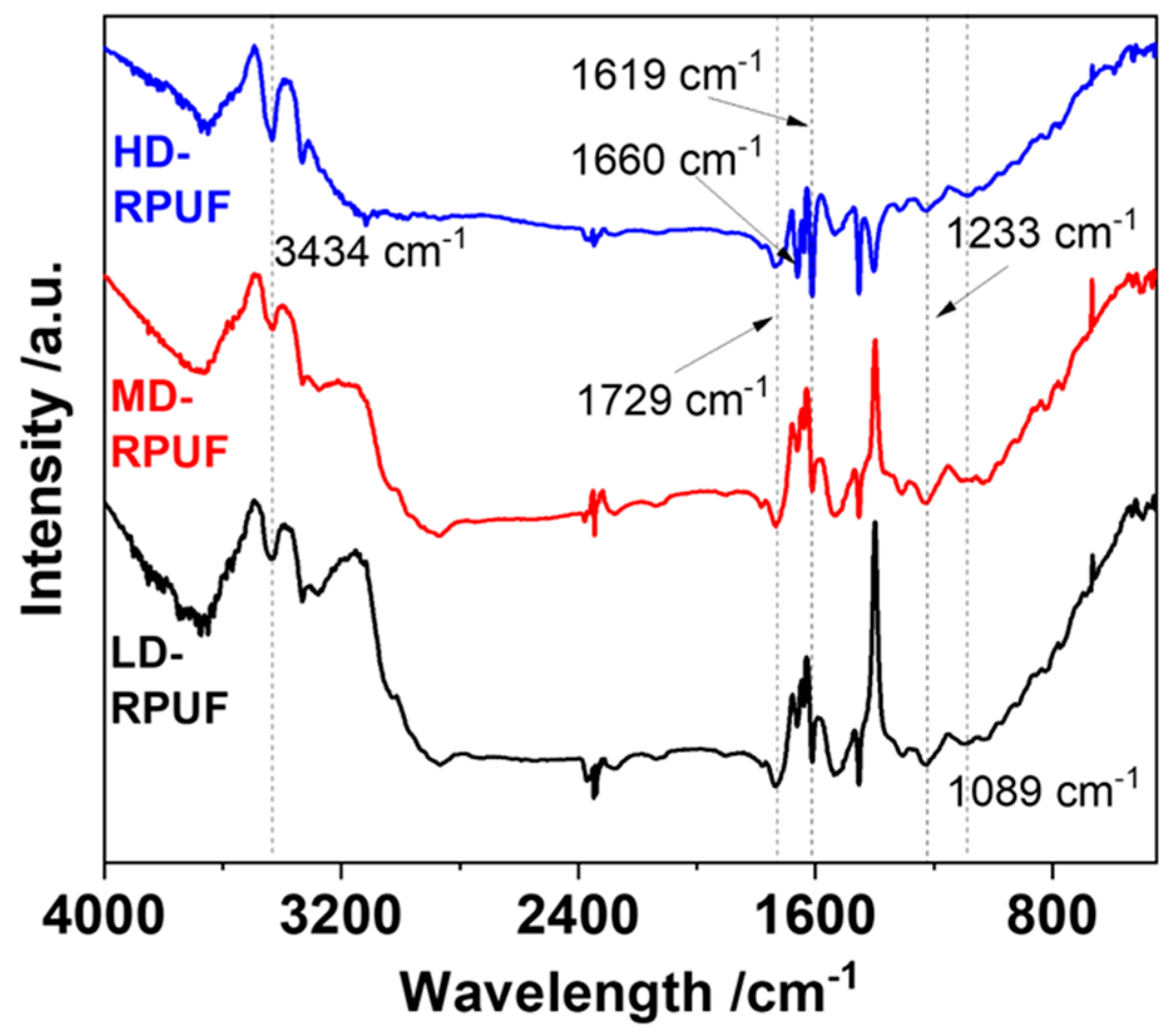
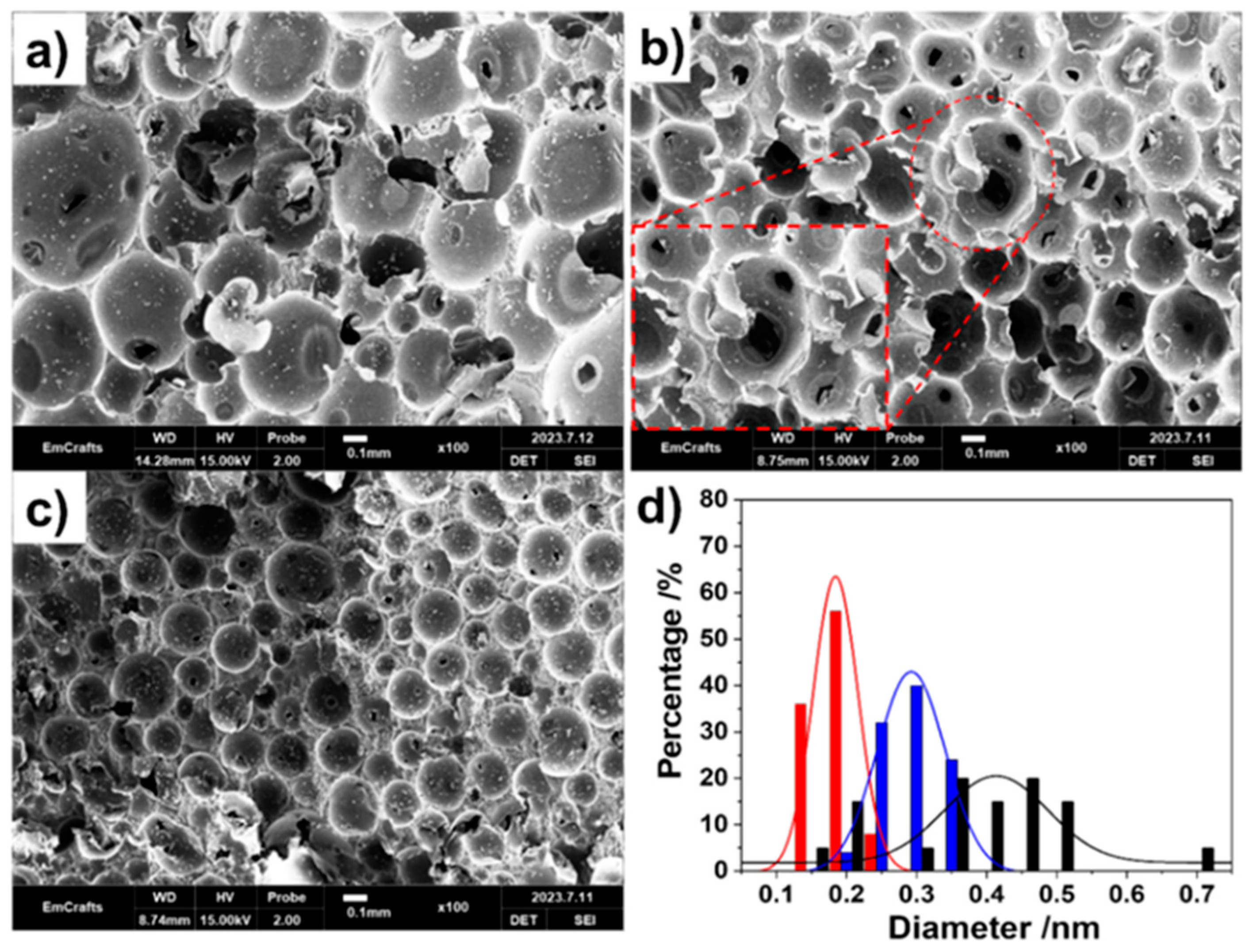
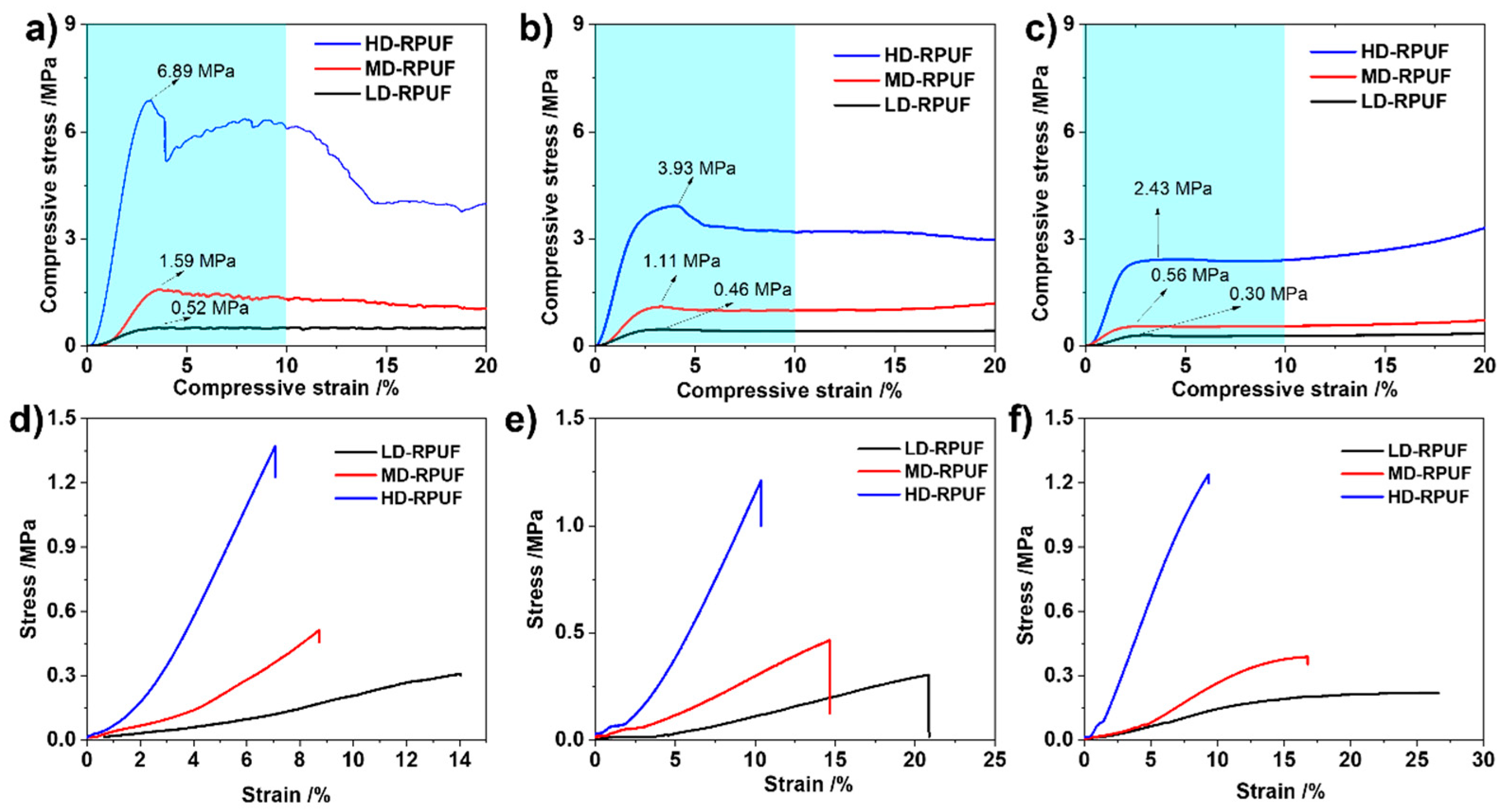
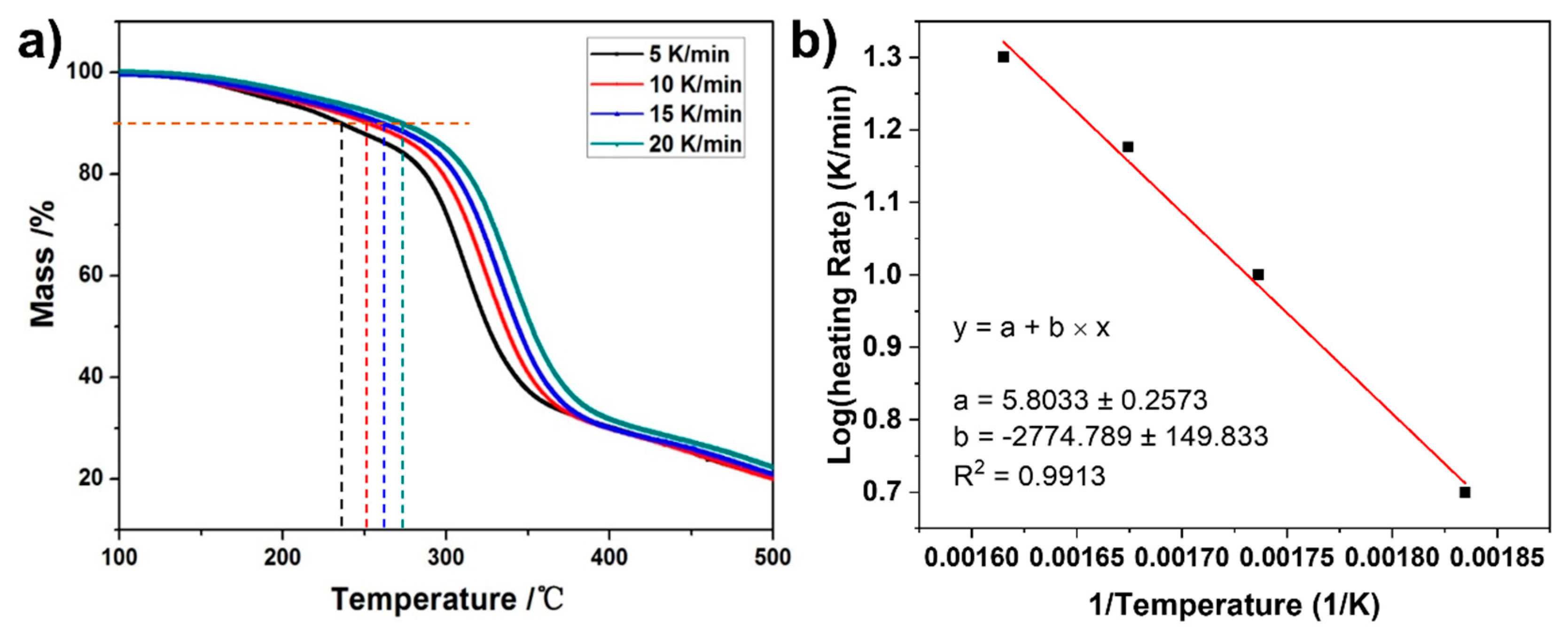
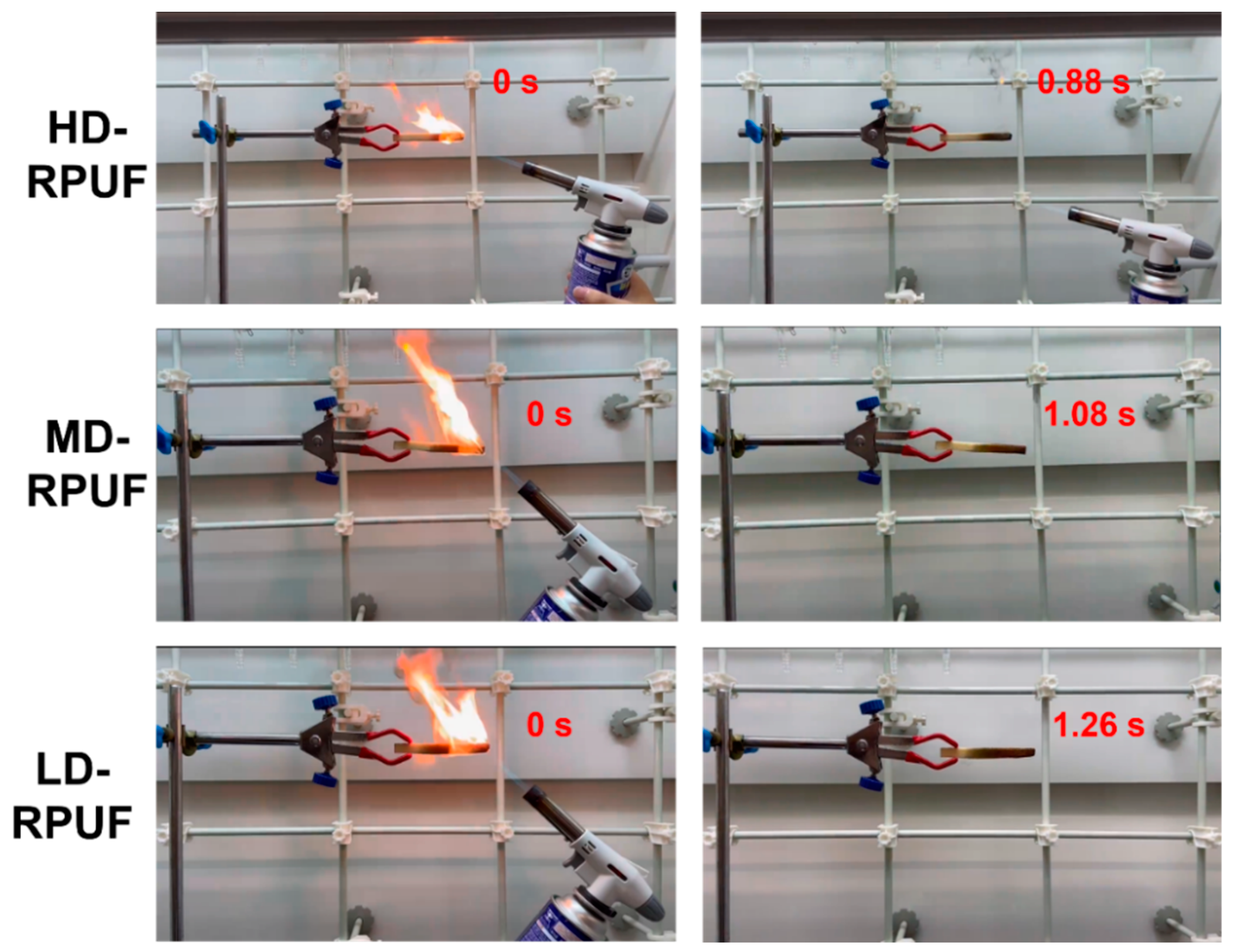
3. Results
| Sample | Contents of elements (wt%) a) | Flame extinction time (s) | Residual carbons (wt%) b) | |||||||
| C | H | N | O | P | Si | Cl | Other | |||
| HD-RPUF | 61.82 | 6.04 | 7.13 | 14.7 | 1.125 | 0.078 | 0.005 | <1 | 0.88 | 96.71±1.70% |
| MD-RPUF | 62.73 | 6.13 | 7.27 | 13.6 | 0.905 | 0.088 | 0.005 | <1 | 1.08 | 92.48±0.77% |
| LD-RPUF | 62.51 | 6.07 | 7.36 | 13.2 | 1,736 | 0.021 | 0.004 | <1 | 1.26 | 90.64±1.44% |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Li, G.; Lim, M.K. China’s power supply chain sustainability: an analysis of performance and technology gap. Ann. Oper. Res. 2020, 1–29. [Google Scholar] [CrossRef]
- Varma, R.; Sushil. Bridging the electricity demand and supply gap using dynamic modeling in the Indian context. Energy Policy 2019, 132, 515–535. [Google Scholar] [CrossRef]
- Shellenberger, M. Nuclear power: Unexpected health benefits. Nat. Energy 2017, 2, 17058. [Google Scholar] [CrossRef]
- Horvath, A.; Rachlew, E. Nuclear power in the 21st century: Challenges and possibilities. Ambio. 2016, 45, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.-A.J. Spent nuclear fuel system dynamic stability under normal conditions of transportation. Nucl. Eng. Des. 2016, 310, 1–14. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, Z.; Li, J.; Guo, Y.; Yang, G.; Yu, Y.; Zhang, J. The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel. Materials 2022, 15, 3255. [Google Scholar] [CrossRef] [PubMed]
- Tae-Man, K.; Ho-Seog, D.; Chun-Hyung, C.; Jae-Hun, K. Preliminary Shielding Analysis of the Concrete Cask for Spent Nuclear Fuel Under Dry Storage Conditions. J. Nucl. Fuel Cycle Waste Technol. 2017, 15, 391–402. [Google Scholar]
- Li, X.; Wu, J.; Tang, C.; He, Z.; Yuan, P.; Sun, Y.; Lau, W.-m.; Zhang, K.; Mei, J.; Huang, Y. High temperature resistant polyimide/boron carbide composites for neutron radiation shielding. Compos. Part B - Eng. 2019, 159, 355–361. [Google Scholar] [CrossRef]
- Kim, K.; Chung, S.; Hong, J. Performance evaluation of METAMIC neutron absorber in spent fuel storage rack. Nucl. Eng. Technol. 2018, 50, 788–793. [Google Scholar] [CrossRef]
- El-Samrah, M.G.; Tawfic, A.F.; Chidiac, S.E. Spent nuclear fuel interim dry storage; Design requirements, most common methods, and evolution: A review. Ann. Nucl. Energy 2021, 160, 108408. [Google Scholar] [CrossRef]
- Weiner, R.F.; Ammerman, D.J. Spent fuel transportation risk assessment: transportation accident analysis. Packag. Transp. Storage Secur. Radioactive Mater. 2013, 24, 147–157. [Google Scholar] [CrossRef]
- Diersch, R.; Weiss, M.; Dreier, G. Investigation of the impact behaviour of wooden impact limiters. Nucl. Eng. Des. 1994, 150, 341–348. [Google Scholar] [CrossRef]
- Aktay, L.; Johnson, A.F.; Kröplin, B.-H. Numerical modelling of honeycomb core crush behaviour. Eng. Fract. Mech. 2008, 75, 2616–2630. [Google Scholar] [CrossRef]
- Peixinho, N.; Carvalho, O.; Areias, C.; Pinto, P.; Silva, F. Compressive properties and energy absorption of metal-polymer hybrid cellular structures. Mater. Sci. Eng. A. 2020, 794, 139921. [Google Scholar] [CrossRef]
- Ryu, J.; Lim, H.; Lee, S.-H.; Lee, J. Polymer filling behaviors and imprinting velocities with pressure variation rates in nanoimprint lithography. Microelectron. Eng. 2015, 140, 67–71. [Google Scholar] [CrossRef]
- Wang, C.; Kilic, K.I.; Koerner, H.; Baur, J.W.; Varshney, V.; Lionti, K.; Dauskardt, R.H. Polyimide Hybrid Nanocomposites with Controlled Polymer Filling and Polymer–Matrix Interaction. ACS Appl. Mater. Interfaces 2022, 14, 28239–28246. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; O’Reilly, R.K. Polymers for Biomedical Applications: The Importance of Hydrophobicity in Directing Biological Interactions and Application Efficacy. Biomacromolecules 2021, 22, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Testing 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications – a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Mane, J.V.; Chandra, S.; Sharma, S.; Ali, H.; Chavan, V.M.; Manjunath, B.S.; Patel, R.J. Mechanical Property Evaluation of Polyurethane Foam under Quasi-static and Dynamic Strain Rates- An Experimental Study. Procedia. Eng. 2017, 173, 726–731. [Google Scholar] [CrossRef]
- Brondi, C.; Di Maio, E.; Bertucelli, L.; Parenti, V.; Mosciatti, T. Competing bubble formation mechanisms in rigid polyurethane foaming. Polymer 2021, 228, 123877. [Google Scholar] [CrossRef]
- Burgaz, E.; Kendirlioglu, C. Thermomechanical behavior and thermal stability of polyurethane rigid nanocomposite foams containing binary nanoparticle mixtures. Polym. Testing 2019, 77, 105930. [Google Scholar] [CrossRef]
- Maia, L.S.; de Bomfim, A.S.C.; de Oliveira, D.M.; Pinhati, F.R.; da Conceição, M.O.T.; Barud, H.S.; Medeiros, S.A.; Rosa, D.S.; Mulinari, D.R. Tuning of renewable sponge-like polyurethane physical-chemical and morphological properties using the pullulan as a reactive filler. J. Appl. Polym. Sci. 2023, 140, e53619. [Google Scholar] [CrossRef]
- Scholz, P.; Wachtendorf, V.; Panne, U.; Weidner, S.M. Degradation of MDI-based polyether and polyester-polyurethanes in various environments - Effects on molecular mass and crosslinking. Polym. Testing 2019, 77, 105881. [Google Scholar] [CrossRef]
- Diaz, T.J.; Cerrutti, P.; Chiacchiarelli, L.M. In-situ thermal aging of biobased and conventional rigid polyurethane foams nanostructured with bacterial nanocellulose. J. Appl. Polym. Sci. 2022, 139, 51824. [Google Scholar] [CrossRef]
- Qiu, F.; Tu, C.; Chen, Y.; Shi, Y.; Song, L.; Wang, R.; Zhu, X.; Zhu, B.; Yan, D.; Han, T. Control of the Optical Properties of a Star Copolymer with a Hyperbranched Conjugated Polymer Core and Poly(ethylene glycol) Arms by Self-Assembly. Chem. Euro. J. 2010, 16, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Olcay, H.; Kocak, E.D. The mechanical, thermal and sound absorption properties of flexible polyurethane foam composites reinforced with artichoke stem waste fibers. J. Ind. Text. 2020, 51, 8738S–8763S. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Ge, C.; Wang, S.; Zheng, W.; Zhai, W. Preparation of microcellular thermoplastic polyurethane (TPU) foam and its tensile property. Polym. Eng. Sci. 2018, 58, E158–E166. [Google Scholar] [CrossRef]
- Son, T.W.; Lee, D.W.; Lim, S.K. Thermal and Phase Behavior of Polyurethane Based on Chain Extender, 2,2-Bis-[4-(2-hydroxyethoxy)phenyl]propane. Polym. J. 1999, 31, 563–568. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Yang, C.; Zhao, J.; Zhang, A. Mechanical and EMI shielding properties of solid and microcellular TPU/nanographite composite membranes. Polym. Testing 2021, 93, 106891. [Google Scholar] [CrossRef]
- Takano, M.; Takamatsu, K.; Saito, H. High-Strength Heat-Elongated Thermoplastic Polyurethane Elastomer Consisting of a Stacked Domain Structure. Polymers 2022, 14, 1470. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.-K.; Gong, Y.; Cheng, F.; Zhang, J.; Li, R.-B.; Pan, J.; Li, N.; Xu, J.-F.; Liu, X.-Q.; Yang, X.-L.; et al. Innovation in lifetime prediction of rigid polyurethane foam through random vibration and comparison with conventional methods. Polym. Testing 2023, 125, 108122. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Sun, B.-N.; Yang, Z.-G.; Shi, X.-Q.; Liu, X.-Q.; Xie, Y.-C.; Xu, X.-L. Comparative study on different methods for determination of activation energies of nuclear cable materials. Polym. Testing 2018, 70, 81–91. [Google Scholar] [CrossRef]
- Zeng, F.; Men, X.; Chen, M.; Liu, B.; Han, Q.; Huang, S.; Zhao, H.; Wang, Y. Molecular-micron multiscale toughening and flame retarding for polyurethane foams. Chem. Eng. J. 2023, 454, 140023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





