Submitted:
19 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
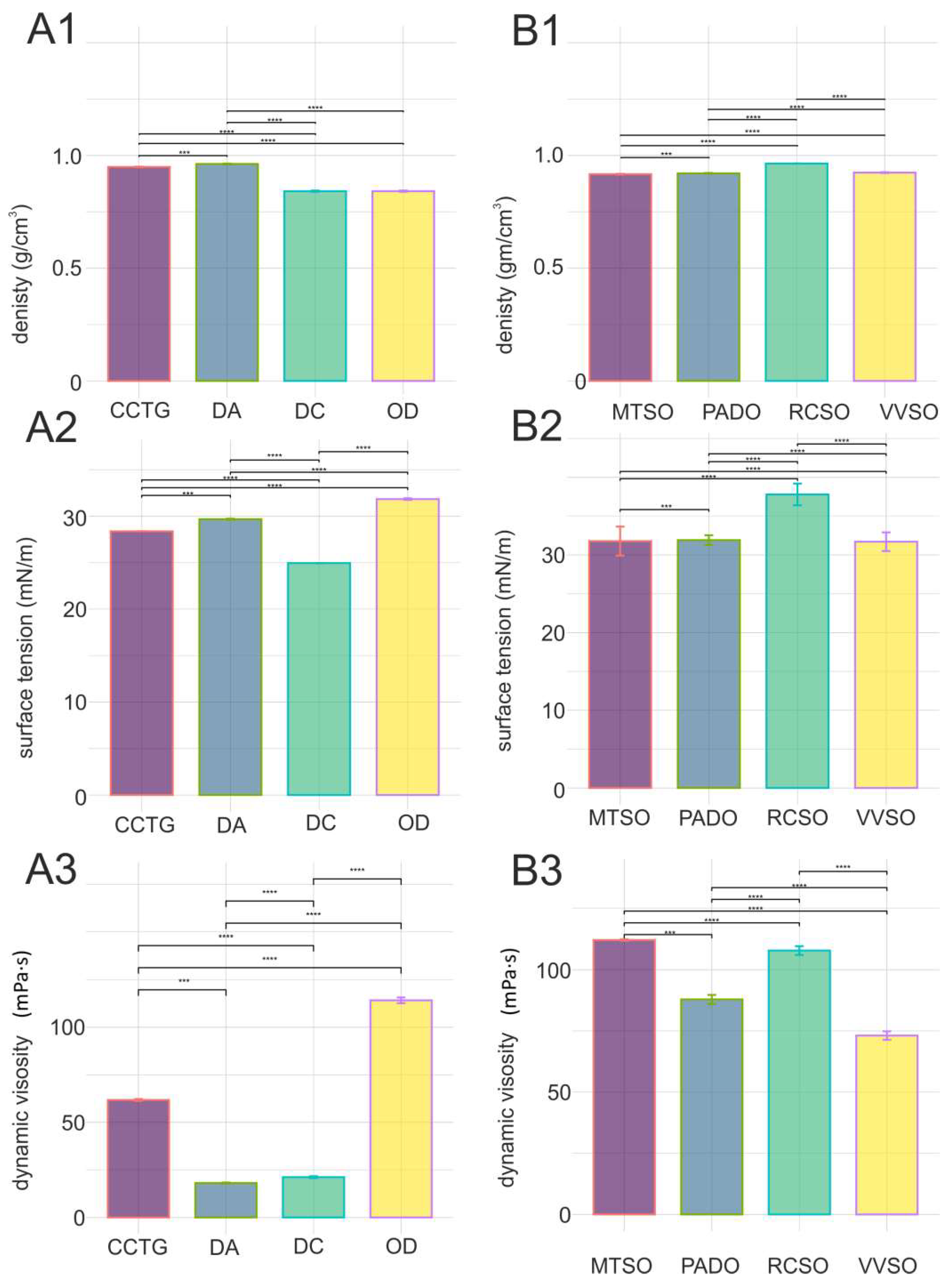
2.1. Physicochemical properties of natural and synthetic cosmetic emollients
- Spreading properties of cosmetic emollients
2.2. Physicochemical properties of cosmetic oils
- Spreading properties of body oils
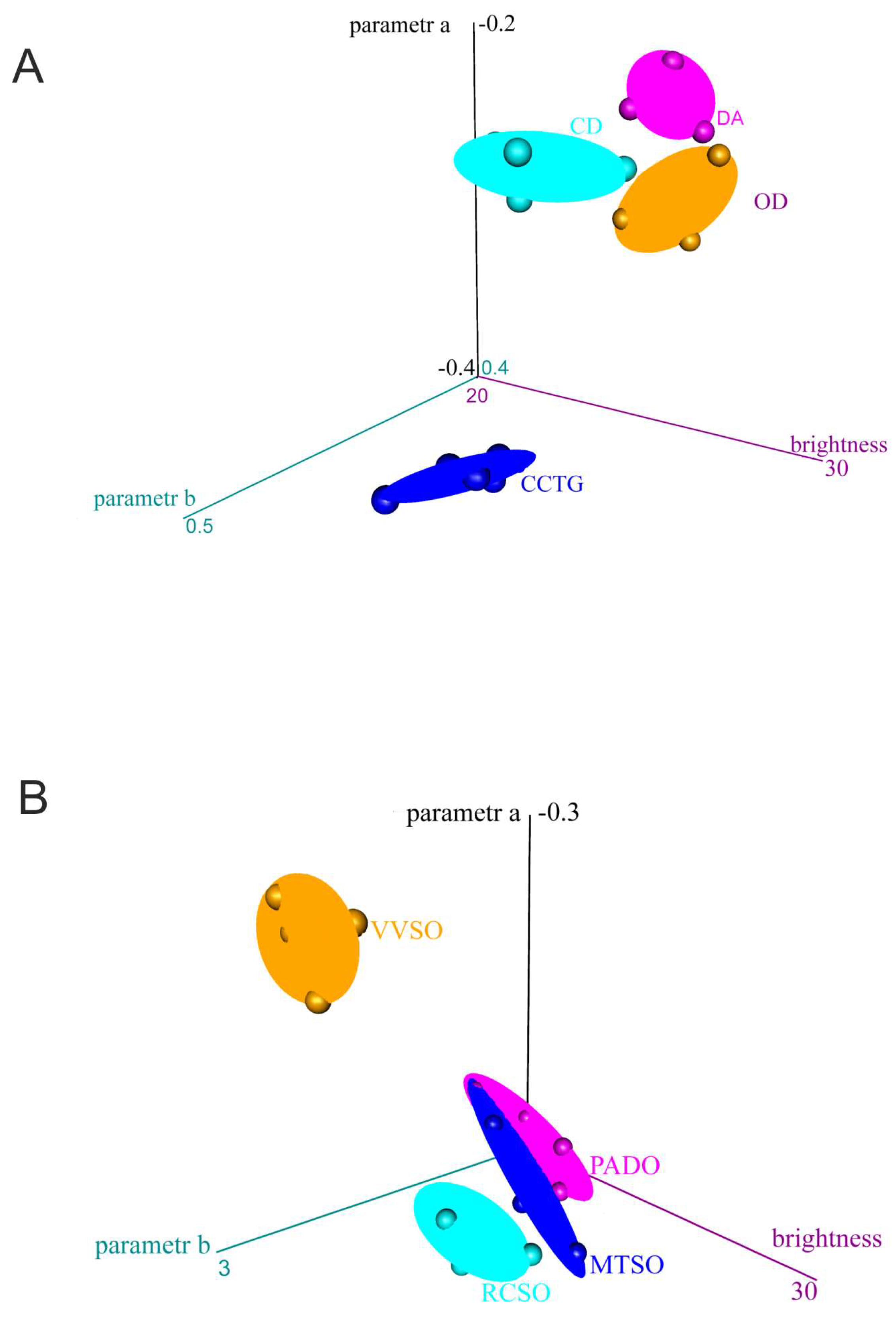
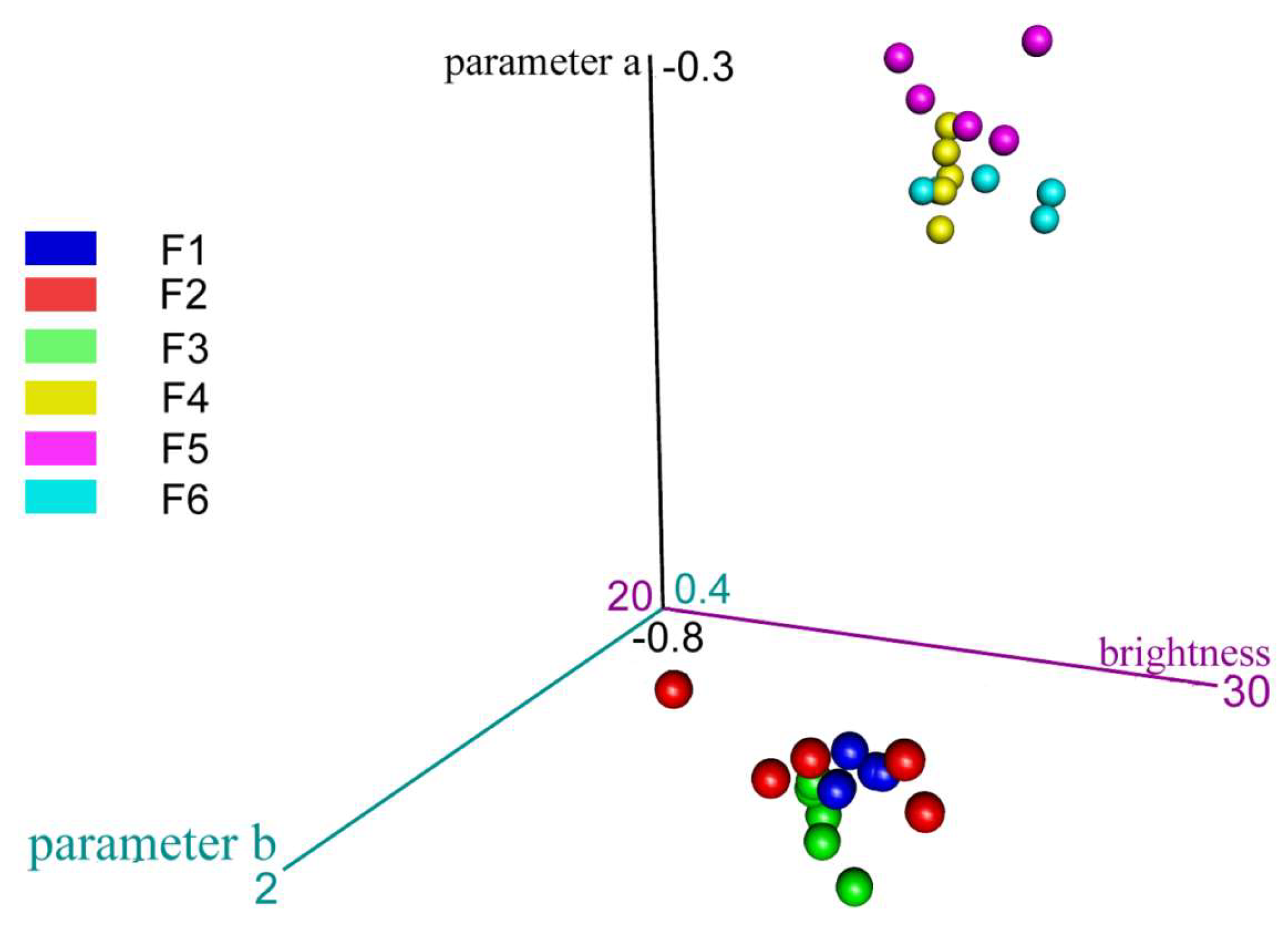
- Color evaluation of body oils
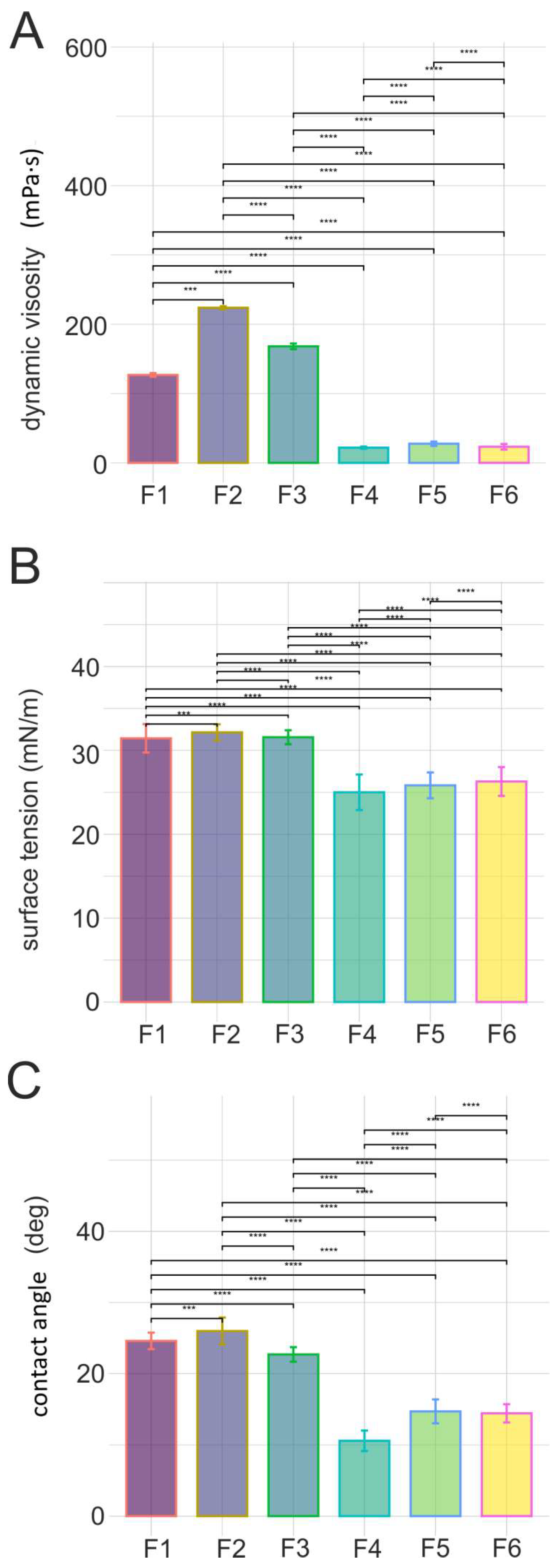
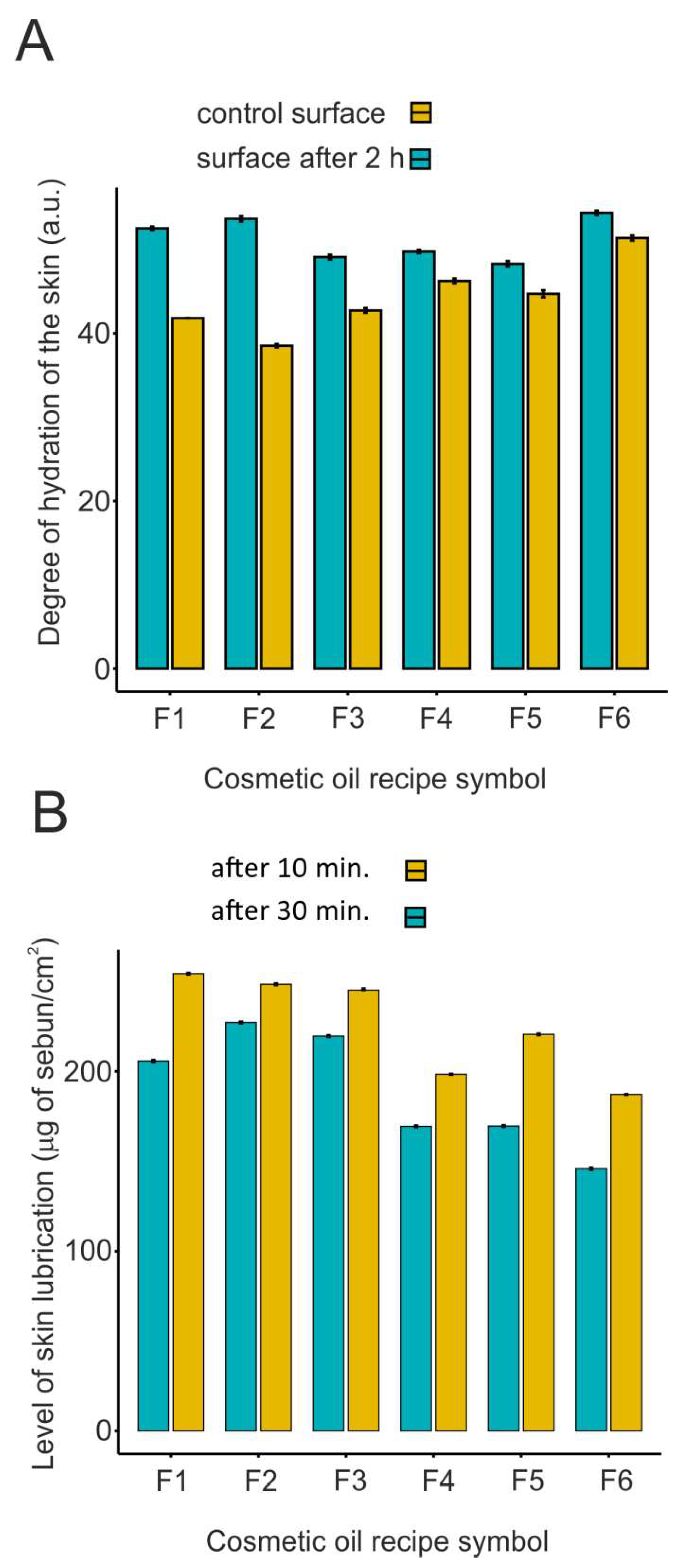
2.3. Selected parameters of epidermal barrier function after application of developed cosmetic oils
3. Discussion
4. Materials and methods
4.1. Chemicals
4.2. Formulations
1.1. Methods
- Density
- Viscosity
- Wetting angle
- Surface tension
- Color
- Selected parameters of epidermal barrier function
- Statistical analyses
2. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gore, E.; Picard, C.; Savary, G. Spreading behavior of cosmetic emulsions: Impact of the oil phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Terescenco, D.; Picard, C.; Clemenceau, F.; Grisel, M.; Savary, G. Colloids Surf. A Physicochem. Eng. Asp. 2018, 10–19. [CrossRef]
- Wenninger, J.A.; Canterbery, R.C., McEwan, G.N. T. and F.A. Cosmetic, International Cosmetic Ingredient Dictionary and Handbook, Cosmetic, Toiletry, and Fragrance Association,, Ninth ed. CTFA, Washington, DC, 2002.
- Douguet, M.; Picard, C.; Savary, G.; Merlaud, F.; Loubat-Bouleuc, N.; Grisel, M. Spreading properties of cosmetic emollients: Use of synthetic skin surface to elucidate structural effect. Colloids Surf. B. 2017, 154, 307–314. [Google Scholar] [CrossRef] [PubMed]
- de Clermont-Gallerande, H.; Abidh, S.; Lauer, A.; Navarro, S.; Cuvelier, G.; Delarue, J. Relations between the sensory properties and fat ingredients of lipstics. OCL 2018, 25, 5–1. [Google Scholar] [CrossRef]
- Lombardi, S.A.; Ratti, A.M. Emotional effects induced by lip balms containing different emollients: neuroscientific approach to studying the tactual experience. H&PC Today 2017, 12, 52–57. [Google Scholar]
- Fernandes, A.R.; Dario, M.F.; Pinto, C.A.S.O.; Kaneko, T.M.; Baby, A.R.; Velasco, M.V.R. Stability evaluation of organic Lip Balm. BJPS 2013, 49, 293–299. [Google Scholar] [CrossRef]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Van Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A: Physicochem. Eng. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Pincelli, C.; Sparavigna, A.; Luger, T. The Role of a Novel Generation of Emollients, ‘Emollients Plus’, in Atopic Dermatitis. Clin Cosmet Investig Dermatol. 2022, 14, 15–2705. [Google Scholar] [CrossRef]
- Ahmad, A.; Haseeb, A. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. dermatol. 2020, 4, 12–10. [Google Scholar] [CrossRef]
- Danby, S.; Gordon, W.; Duff, Cork, M.J. Current and future trends: skin diseases and treatment. Transdermal and Topical Drug Delivery: Principles and Practice. Hoboken: John Wiley & Sons, Inc 2011, 367- 407. [CrossRef]
- Arct, J.; Pytkowska, K. Kosmetyki do pielęgnacji skóry suchej. Cosmetol Today 2009, 3, 34–37. [Google Scholar]
- Schmitt, M.; Jiang, Y.J.; Elias, P.M. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res 2008, 49, 3–499. [Google Scholar] [CrossRef]
- Welz-Kubiak, K.; Reich, A. The role of emollients in the daily skin care. Forum Derm. 2016, 2, 1–20. [Google Scholar]
- Penzer, R.; Maguire, S.; Nicol, N.; Peters, J. Best practice statement for emollient therapy. Dermatol. Nurs. 2012, 11, 4. [Google Scholar]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schmuth, M. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Pope, S.M.; Jaboori, K.A. Diagnosis and Treatment of Seborrheic Dermatitis. Am Fam Physician. 2015, 91, 185–19. [Google Scholar] [PubMed]
- Maroto-Morales, D.; Montero-Vilchez, T.; Arias-Santiago, S. Study of skin barrier function in psoriasis: The impact of emollients. Life 2021, 11, 7–651. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.; Irondelle, M.; Duteil, L.; Cardot-Leccia, N.; Rocchi, S.; Passeron, T.; Tulic, M.K. Impact of topical emollient, steroids alone or combined with calcipotriol, on the immune infiltrate and clinical outcome in psoriasis. Exp. Dermatol. 2022, 11, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Proksh, E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol Physiol 2008, 21, 2–75. [Google Scholar] [CrossRef]
- Kamińska, E. Rola emolientów w atopowym zapaleniu skóry u dzieci. J. mother child, 2021, 22, 4–396. [Google Scholar] [CrossRef]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs Context. 2018, 17, 7–212530. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Suf. B. 2013, 102, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Comfort, D.; Hillier, D. Common ground: The sustainable development goals and the marketing and advertising industry. J. Public Aff. 2018, 18, e1619. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A sustainable life cycle for cosmetics: From design and development to post-use phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Gagliardini, A.; Lohani, A. From cosmetics to innovative cosmeceuticals—non-woven tissues as new biodegradable carriers. Cosmetics 2021, 8, 65. [Google Scholar] [CrossRef]
- Morea, D.; Fortunati, S.; Martiniello, L. Circular economy and corporate social responsibility: Towards an integrated strategic approach in the multinational cosmetics industry. J. Clean. Prod. 2021, 315, 128232. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.H. The significant transformation of life into health and beauty in metaverse era. J. Cosmet. Dermatol. 2022, 21, 6575–6583. [Google Scholar] [CrossRef]
- Natural cosmetics market analysis and review of natural cosmetic market by products type skin care, hair care, color cosmetics, fragrance,oral care, toilettries for 2019-2027, Future Market Insights 2019.
- Pinto, J.R.; Monteiro e Silva, S.A.; Holsback, V.D.S.S.; Leonardi, G.R. Skin occlusive performance: Sustainable alternatives for petrolatum in skincare formulations. Journal of Cosmetic Dermatology, 2022; 21, 4775–4780. [Google Scholar] [CrossRef]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing synthetic ingredients by sustainable natural alternatives: a case study using topical O/W emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.; Radzi, S.M.; Rehan, M.M.; Amin, N.A.M. A Review on Cosmetic Formulations and Physicochemical Characteristics of Emollient and Day Cream Using Vegetable Based-Wax Ester. Malaysian J. Sci. 2022; 8, 38–45. [Google Scholar] [CrossRef]
- Gorcea, M.; Laura, D. Evaluating the physiochemical properties of emollient esters for cosmetic use. Cosmetics and toiletries 2010, 125, 12. [Google Scholar]
- Parente, M.E.; Gambaro, A.; Ares, G. Sensory characterization of emollients. J. Sens. Stud. 2008, 23, 149–161. [Google Scholar] [CrossRef]
- Oram, P.; Strine, J. Color measurement of a solid active pharmaceutical ingredient as an aid to identifying key process parameters. J. Pharm. And Biomed. Sci., 2006, 40, 1021–1024. [Google Scholar] [CrossRef]
- Ivens, U.I.; Steinkjer, B.; Serup, J.; Tetens, V. Oimnent is evenly spread on the skin in contrast to creams and soloutions. Br. J. Dermatol. 2001, 145, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Lan, S.; Zhao, Y.; Xiong, Y.; Yang, W.; Du, Y. Characterization of Skin Moisture and Evaluation of Cosmetic Moisturizing Properties Using Miniature Near-infrared Spectrometer. Infrared Phys Technol. 2023, 104759. [Google Scholar] [CrossRef]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: what dermatologists and cosmeticians should know. Int. J. Women’s Dermatology 2018, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856–863. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Klimaszewska, E.; Ogorzałek, M.; Ruman, J.; Rożnawska, K. Effectiveness of protective preparations: Impact of vegetable oil additives to recipes. Eur. J. Lipid Sci. Technol. 2020, 122, 2000130. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute viscosities of vegetable oils at different temperatures and shear rate range of 64.5 to 4835 s− 1. J. Food Process. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.H.; Kwon, S.B.; An, I.S.; An, S.; Ahn, K.J. Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp Ther Med. 2016, 12, 1171–1176. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Zieba, M.; Gregorczyk, K.; Markuszewski, L. Application of blue honeysuckle powder obtained by an innovative method of low-temperature drying in skincare face masks. Molecules, 2021, 26, 7184. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wasilewski, T.; Hordyjewicz-Baran, Z. Antioxidant and Cytoprotective Properties of Plant Extract from Dry Flowers as Functional Dyes for Cosmetic Products. Molecules 2021, 26, 2809. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin–Theory and practice. Advances in colloid and interface science 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Bishbishy, M.H.B.; El Kersh, D.M.; Zayed, A.; Salem, M.A.; Salama, M.M.; Chapter 7 - Herbal cosmeticology pp. 129–168 in. Preparation of Phytopharmaceuticals for the Management of Disorders The Development of Nutraceuticals and Traditional Medicine, Accademic Press 2021. [CrossRef]
- Man, V.; Polonska, T. Use of natural oils as bioactive ingredients of cosmetic. Ukrainian Food Journal 2016, 5, 281–289. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetics industry. Chemik, 2014; 68, 103–110. [Google Scholar]
- Nasrollahi, S.A.; Ayatollahi, A.; Yazdanparast, T.; Samadi, A.; Hosseini, H.; Shamsipour, M.; Akhlaghi, A.A.; Yadangi, S.; Abels, C.; Firooz, A. Comparison of linoleic acid-containing water-in-oil emulsion with urea-containing water-in-oil emulsion in the treatment of atopic dermatitis: a randomized clinical trial. Clin Cosmet Investig Dermatol. 2018, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, E.; Ogorzałek, M.; Zięba, M.; Małysa, A.; Barańska, S. Effect of emollient type on physicochemical and functional properties of baby oils. Polish Journal of Commodity Science 2017, 2, 122–131. [Google Scholar] [CrossRef]
- Kunik, O.; Saribekova, D.; Lazzara, G.; Cavallaro, G. Emulsions based on fatty acid from vegetable oils for cosmetics. Ind Crops Prod. 2022, 189, 115776. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative study on the extraction of macadamia (Macadamia integrifolia) oil using different processing methods. LWT, 2022, 154, 112614. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahmad, M.; Madni, A.; Bakhsh, S. Evaluation of basic properties of macadamia nut oil. Gomal Univ. J. Res. 2006, 22, 21–27. [Google Scholar] [CrossRef]
- Čolić, S.; Zec, G.; Natić, M.; Fotirić-Akšić, M. Almond (Prunus dulcis) oil. Fruit oils: chemistry and functionality, 2019; 149–180. [Google Scholar] [CrossRef]
- Guici El Kouacheur, K.; Cherif, H.S.; Saidi, F.; Bensouici, C.; Fauconnier, M.L. Prunus amygdalus var. amara (bitter almond) seed oil: fatty acid composition, physicochemical parameters, enzyme inhibitory activity, antioxidant and anti-inflammatory potential. J. Food Meas. Charact. 2023, 17, 371–384. [Google Scholar] [CrossRef]
- Salimon, J.; Noor, D.A.M.; Nazrizawati, A.; Noraishah, A. Fatty acid composition and physicochemical properties of Malaysian castor bean Ricinus communis L. seed oil. Sains Malays., 2010, 39, 761–764. [Google Scholar]
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Yin, X. Castor oil (Ricinus communis): a review on the chemical composition and physicochemical properties. J. Food Sci. Technol. 2020, 41, 399–413. [Google Scholar] [CrossRef]
- Yusuf, A.K.; Mamza, P.A.P.; Ahmed, A.S.; Agunwa, U. Extraction and characterization of castor seed oil from wild Ricinus communis Linn. Int. J. Environ. Sci. 2015, 4, 1392–1404. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Lakatos, E.H.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Characterization of fatty acid, antioxidant, and polyphenol content of grape seed oil from different Vitis vinifera L. varieties. OCL 2021, 28, 30. [Google Scholar] [CrossRef]
- Levent, O.A. Detailed Comparative Study on Some Physicochemical Properties, Volatile Composition, Fatty Acid, and Mineral Profile of Different Almond (Prunus dulcis L.) Varieties. Hortic. 2022, 8, 488. [Google Scholar] [CrossRef]
- Team, R.C.R. A language and environment for statistical computing. In: Vienna, Austria 2013.
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. The Annals of Statistics 1979, 7, 1–26. [Google Scholar] [CrossRef]
| Designation | INCI name/ Trade name/ Manufacturer | Chemical Family/Name | Molecular Weight [g/mol] |
Appearance: | Spreading value mm2/10 min |
|---|---|---|---|---|---|
| CCTG | Caprylic/Capric Triglyceride/Crodamol GTCC/Croda Poland | fatty acid esters, esters/ Mix of tri-esters with carbon chains of C8 and C10 derived from coconut oil and glycerin; | 408.58 | Liquid |
550 |
| DA | Dibutyl Adipate/Cetiol B/Basf Poland | esters/adypinian dibutylu; diester of butyl alcohol and adipic acid | 258.35 | Liquid |
1000 |
| OD | Octyldodecanol/ Eutanol G/Basf Poland | primary branched-chain alkohol/ 2-Octyl-1-dodecanol; | 298.50 | Liquid |
600 |
| DC | Dicaprylyl Carbonate/ Cetiol CC/Basf Poland | Esters/dioctyl carbonate; diester of carbonic acid and octanol |
286.50 | Liquid |
1600 |
| Designation | INCI name/ Common name / Manufacturer | Fatty acids composition of vegetable oils [54,55,56,57,58,59,60,61,62] | Appearance: |
|---|---|---|---|
| MTSO | Macadamia Ternifolia Seed Oil/ Macadamia nut oil/Ecospa, Poland | Oleic acid 54-68 % Palmitoleic acid 16-23 % Palmitic acid 7-10 % Stearic acid 2-5.5 % Arachidic acid 1.5-3 % Linoleic acid 1-3 % Eicosenoic acid 1-3 % |
Liquid |
| PADO | Prunus Amygdalus Dulcis (Sweet Almond) Oil/ Sweet almond oil/ Ecospa, Poland | Oleic acid 50-77.63% Linoleic acid 13.38 – 27.69% Palmitic acid 5.59 -8.15% Stearic acid 1.36-3.37 % Palmitoleic acid 0.41-0.76 % |
Liquid |
| RCSO | Ricinus Communis (Castor) Seed Oil/ Castor oil/ Ecospa, Poland | Ricinoleic acid 74 - 84% Linoleic acid 7.3 - 10.32% Oleic acid 5.5 - 7.55% Stearic acid 1.2 - 2.81% Palmitic acid 1.3 -2.59% Erucic acid 1.70% Eicosadienoic acid 0.93% linolenic acid 0.5% |
Liquid |
| VVSO | Vitis Vinifera (Grape) Seed Oil/ Grapeseed oil/ Ecospa, Poland | Stearic acid 3.42–9.93% Palmitic acid 7.81–10.66% Oleic acid 14.29–19.92% Linoleic acid 66.85–72.47% |
Liquid |
| Ingredients [INCI] | Symbol of cosmetic oils | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Macadamia Ternifolia Seed Oil | 50 | 30 | 15 | 1.5 | 1.5 | 1.5 |
| Ricinus Communis (Castor) Seed Oil | 15 | 50 | 30 | 1.5 | 1.5 | 1.5 |
| Prunus Amygdalus Dulcis (Sweet Almond) Oil | 30 | 15 | 50 | 1.5 | 1.5 | 1.5 |
| Dicaprylyl Carbonate | 1.5 | 1.5 | 1.5 | 50 | 30 | 15 |
| Caprylic/Capric Triglyceride | 1.5 | 1.5 | 1.5 | 15 | 50 | 30 |
| Diputyl Adipate | 1.5 | 1.5 | 1.5 | 30 | 15 | 50 |
| Tocpheryl Acetate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





