Submitted:
20 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MIL-88B(Fe)
2.3. Characterization of MIL-88B(Fe)
2.4. Adsorption Experiments
3. Results and Discussion
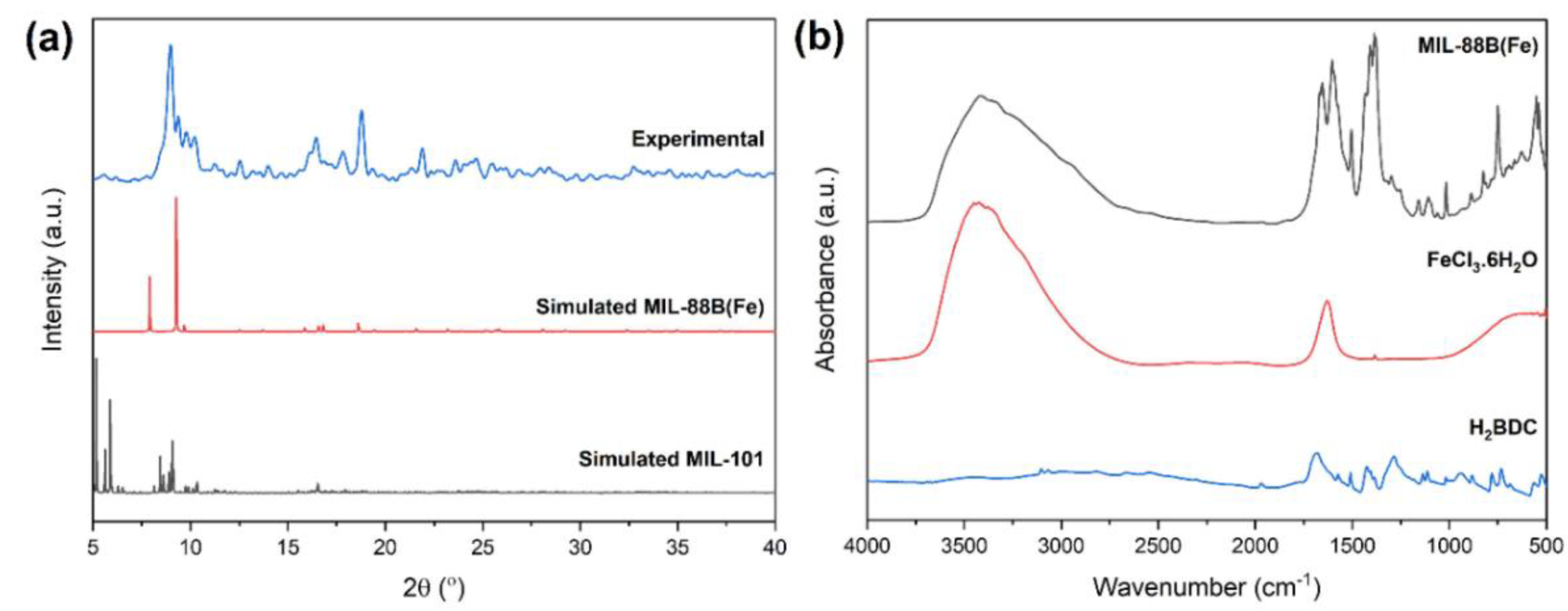
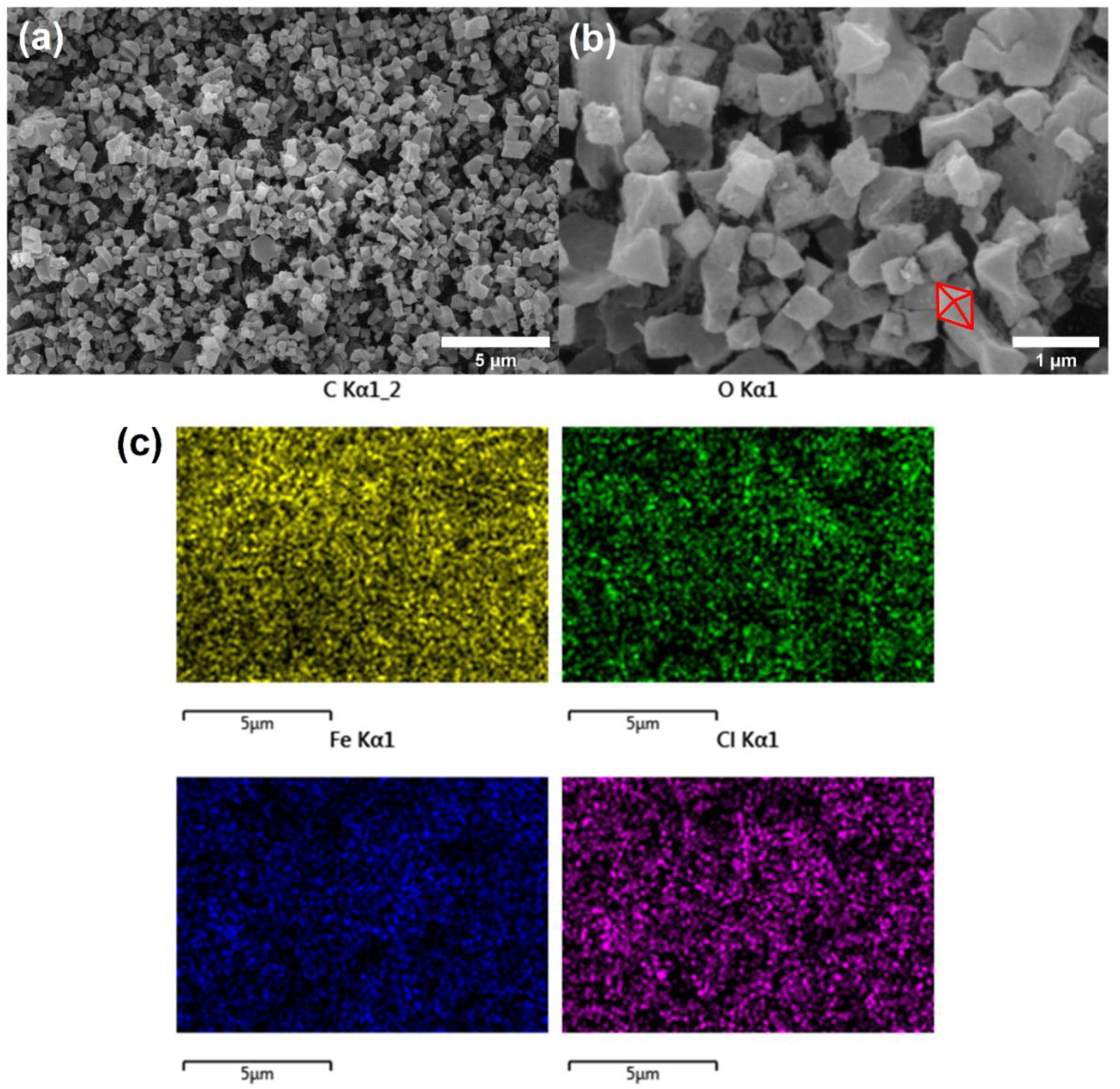
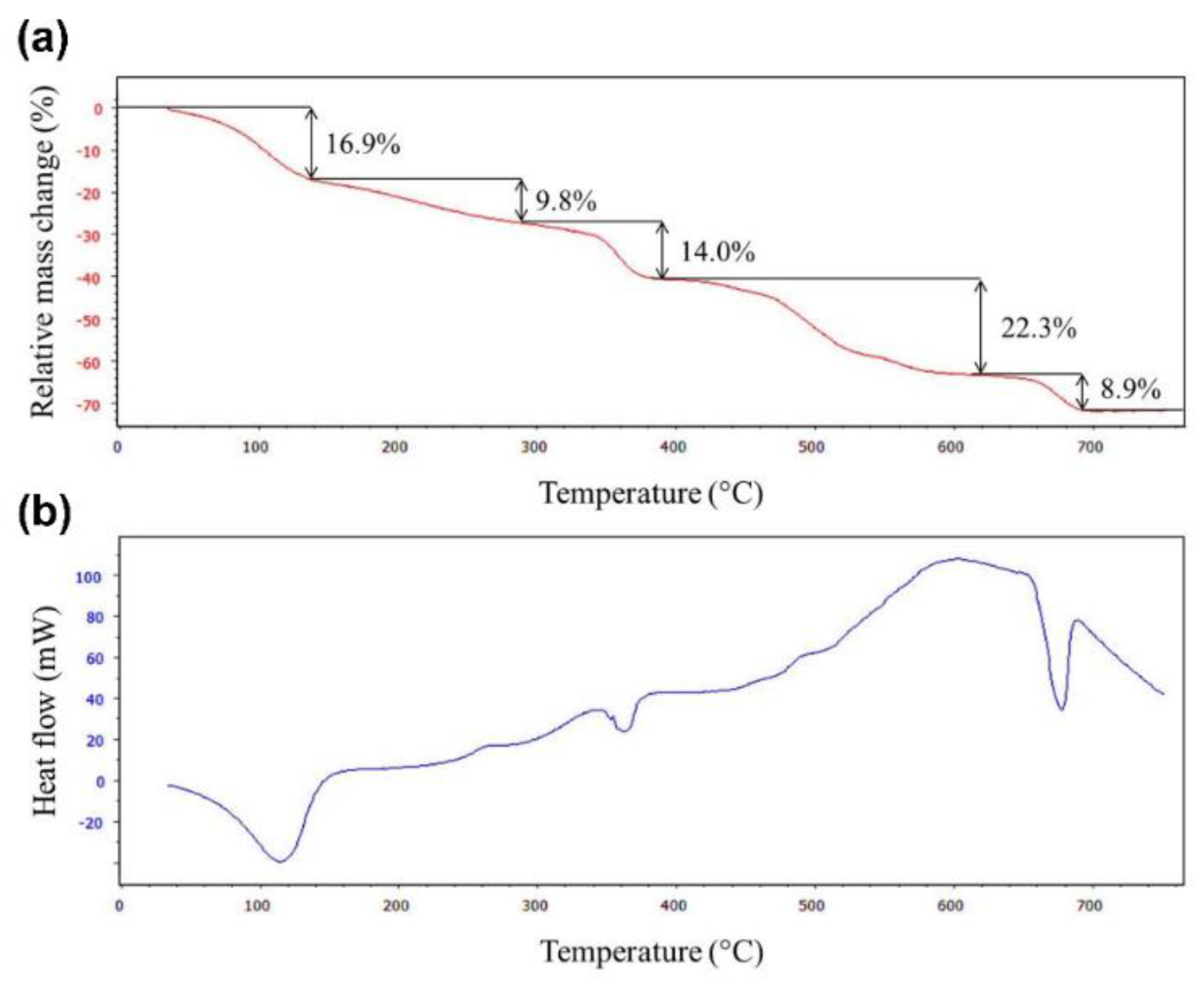
3.1. Synthesis and Characterization of MIL-88B(Fe)
3.2. Adsorption Experiments
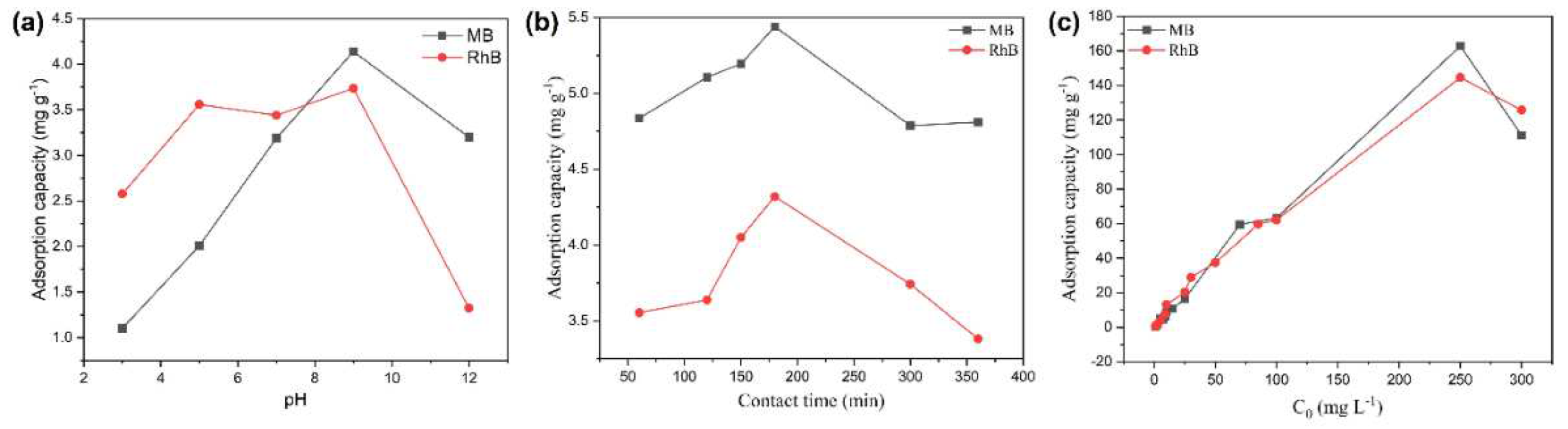
3.2.1. Effect of pH
3.2.2. Effect of Contact Time
3.2.3. Effect of Dyes Initial Concentration
3.2.4. Adsorption Isotherms
3.2.5. Adsorption Kinetics
3.2.6. Adsorption Thermodyamics
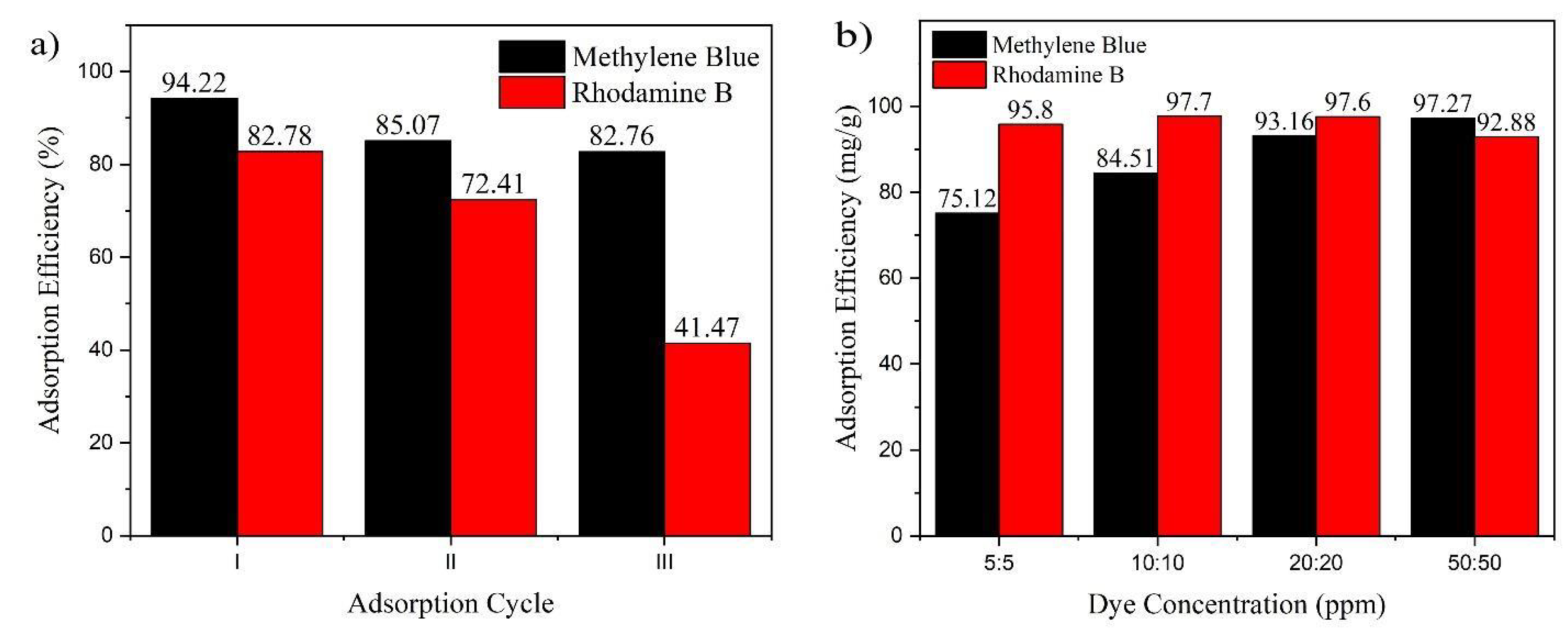
3.2.7. Reusability
3.2.8. Selective Adsorption Capability
3.2.9. Comparison with Other Adsorbents
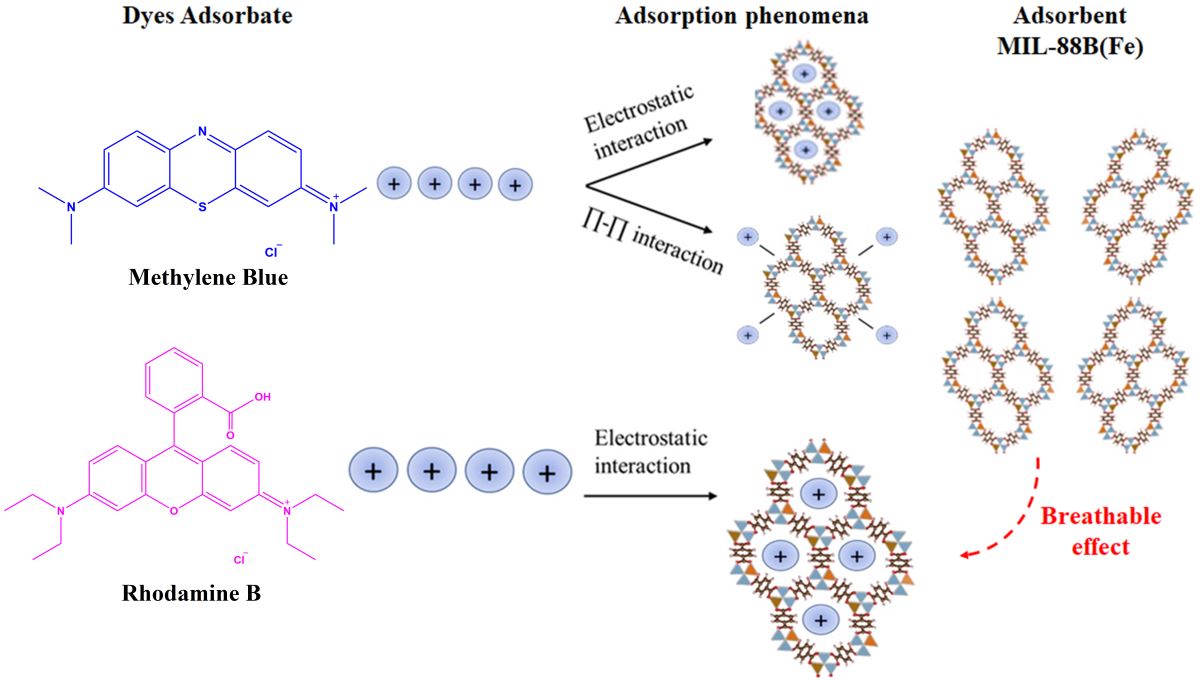
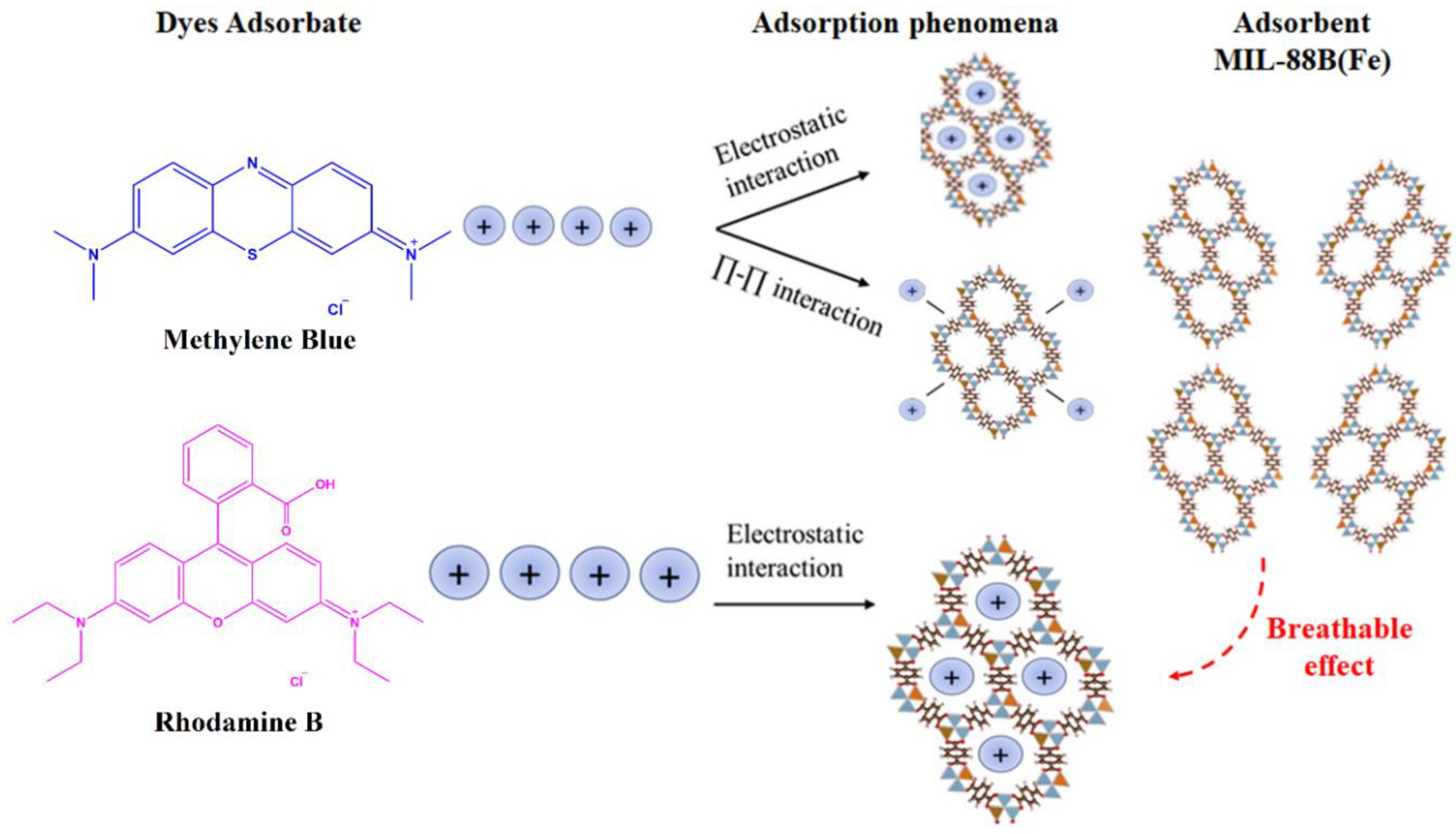
3.2.10. Plausible Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karmakar, S.; Roy, D.; Janiak, C.; De, S. Insights into Multi-Component Adsorption of Reactive Dyes on MIL-101-Cr Metal Organic Framework: Experimental and Modeling Approach. Sep Purif Technol 2019, 215, 259–275. [Google Scholar] [CrossRef]
- Onu, C.E.; Asadu, C.O.; Ohale, P.E.; Ojukwu, E.V.; Ogunaobi, N.C.; Onu, C.P.; Akaeme, F.C. Recent Trends in Textile Wastewater Treatment Using Agricultural Waste. 2022, 89–110. [Google Scholar] [CrossRef]
- Ghasemi, H.; Aghabarari, B.; Alizadeh, M.; Khanlarkhani, A.; Abu-Zahra, N. High Efficiency Decolorization of Wastewater by Fenton Catalyst: Magnetic Iron-Copper Hybrid Oxides. Journal of Water Process Engineering 2020, 37, 101540. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, Vol. 9, Page 430 2019, 9, 430. [Google Scholar] [CrossRef]
- Demissie, H.; An, G.; Jiao, R.; Ritigala, T.; Lu, S.; Wang, D. Modification of High Content Nanocluster-Based Coagulation for Rapid Removal of Dye from Water and the Mechanism. Sep Purif Technol 2021, 259, 117845. [Google Scholar] [CrossRef]
- Zazou, H.; Afanga, H.; Akhouairi, S.; Ouchtak, H.; Addi, A.A.; Akbour, R.A.; Assabbane, A.; Douch, J.; Elmchaouri, A.; Duplay, J.; et al. Treatment of Textile Industry Wastewater by Electrocoagulation Coupled with Electrochemical Advanced Oxidation Process. Journal of Water Process Engineering 2019, 28, 214–221. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhang, Z.M.; Chen, W.L.; Liu, Z.J.; Wang, E.B. Encapsulation of Tungstophosphoric Acid into Harmless MIL-101(Fe) for Effectively Removing Cationic Dye from Aqueous Solution. RSC Adv 2016, 6, 81622–81630. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Metal Organic Frameworks as Adsorbents for Dye Adsorption: Overview, Prospects and Future Challenges. 2012, 94, 1846–1863. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with a Metal-Organic Framework Material, Iron Terephthalate (MOF-235). J Hazard Mater 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption Behavior of Metal–Organic Frameworks for Methylene Blue from Aqueous Solution. Microporous and Mesoporous Materials 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, C.; Wan, H.; Wang, L.; Li, Z.; Li, B.; Guo, Q.; Guan, G. Fabrication of Magnetic NH2-MIL-88B (Fe) Confined Brønsted Ionic Liquid as an Efficient Catalyst in Biodiesel Synthesis. Energy and Fuels 2016, 30, 10739–10746. [Google Scholar] [CrossRef]
- Zhu, G.; Xing, X.; Wang, J.; Zhang, X. Effect of Acid and Hydrothermal Treatments on the Dye Adsorption Properties of Biomass-Derived Activated Carbon. J Mater Sci 2017, 52, 7664–7676. [Google Scholar] [CrossRef]
- Shafqat, S.R.; Bhawani, S.A.; Bakhtiar, S.; Ibrahim, M.N.M. Synthesis of Molecularly Imprinted Polymer for Removal of Congo Red. BMC Chem 2020, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of Efficient Photocatalytic Process Using a Novel BiVO/TiO2-NaY Zeolite Composite for Removal of Acid Orange 10 Dye in Aqueous Solutions: Modeling by Response Surface Methodology (RSM). J Environ Chem Eng 2019, 7, 103253. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Hanif Abu Bakar, N.H.; Fathihah Abdullah, N.A.; Basheer, C.; Saad, B. Removal of Anthracene in Water by MIL-88(Fe), NH 2 -MIL-88(Fe), and Mixed-MIL-88(Fe) Metal–Organic Frameworks. RSC Adv 2019, 9, 41490–41501. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-Organic Frameworks. Chem Soc Rev 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-Organic Frameworks with High Capacity and Selectivity for Harmful Gases. Proc Natl Acad Sci U S A 2008, 105, 11623–11627. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Tang, L.; Luo, L.; Zeng, G. Iron Containing Metal-Organic Frameworks: Structure, Synthesis, and Applications in Environmental Remediation. ACS Appl Mater Interfaces 2017, 9, 20255–20275. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal-Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J Chem Eng Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Fu, Q.; Lou, J.; Zhang, R.; Peng, L.; Zhou, S.; Yan, W.; Mo, C.; Luo, J. Highly Effective and Fast Removal of Congo Red from Wastewater with Metal-Organic Framework Fe-MIL-88NH2. J Solid State Chem 2021, 294, 121836. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Karimi, M.; Daasbjerg, K. Efficient Removal of Crystal Violet and Methylene Blue from Wastewater by Ultrasound Nanoparticles Cu-MOF in Comparison with Mechanosynthesis Method. Ultrason Sonochem 2017, 37, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Sun, Q. Rapid and Selective Adsorption of Cationic Dyes by a Unique Metal-Organic Framework with Decorated Pore Surface. Appl Surf Sci 2018, 440, 1219–1226. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, G. Green Synthesis of Sn(II)-BDC MOF: Preferential and Efficient Adsorption of Anionic Dyes. Microporous and Mesoporous Materials 2020, 297, 110039. [Google Scholar] [CrossRef]
- Agustin, M.; Nurani, D.A.; Zulys, A.; Krisnandi, Y.K. Adsorption of Rhodamine B by Yttrium-Succinate Metal Organic Framework (MOF). AIP Conf Proc 2020, 2243, 030001. [Google Scholar] [CrossRef]
- Nurani, D.A.; Butar, B.C.B.; Krisnandi, Y.K. Synthesis and Characterization of Metal Organic Framework Using Succinic Acid Ligand with Cobalt and Iron Metals as Methylene Blue Dye Adsorbent. IOP Conf Ser Mater Sci Eng 2020, 902, 012055. [Google Scholar] [CrossRef]
- Knyazeva, M.K.; Shkolin, A. V.; Fomkin, A.A.; Tsivadze, A.Y.; Solovtsova, O. V.; Platonova, N.P.; Pulin, A.L.; Men’shchikov, I.E.; Shiryaev, A.A.; Vysotskii, V. V.; et al. Synthesis and Structural-Energy Characteristics of Fe-BDC Metal-Organic Frameworks. Protection of Metals and Physical Chemistry of Surfaces 2018, 54, 1004–1009. [Google Scholar] [CrossRef]
- Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Kashtiban, R.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts 2019, Vol. 9, Page 437 2019, 9, 437. [Google Scholar] [CrossRef]
- Wu, L.; Chaplais, G.; Xue, M.; Qiu, S.; Patarin, J.; Simon-Masseron, A.; Chen, H. New Functionalized MIL-53(In) Solids: Syntheses, Characterization, Sorption, and Structural Flexibility. RSC Adv 2019, 9, 1918–1928. [Google Scholar] [CrossRef]
- Wei, Y.S.; Chen, K.J.; Liao, P.Q.; Zhu, B.Y.; Lin, R.B.; Zhou, H.L.; Wang, B.Y.; Xue, W.; Zhang, J.P.; Chen, X.M. Turning on the Flexibility of Isoreticular Porous Coordination Frameworks for Drastically Tunable Framework Breathing and Thermal Expansion. Chem Sci 2013, 4, 1539–1546. [Google Scholar] [CrossRef]
- Chaplais, G.; Simon-Masseron, A.; Porcher, F.; Lecomte, C.; Bazer-Bachi, D.; Bats, N.; Patarin, J. IM-19: A New Flexible Microporous Gallium Based-MOF Framework with Pressure- and Temperature-Dependent Openings. Physical Chemistry Chemical Physics 2009, 11, 5241–5245. [Google Scholar] [CrossRef] [PubMed]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Férey, G.; Elkaïm, E.; Vimont, A. XRD and IR Structural Investigations of a Particular Breathing Effect in the MOF-Type Gallium Terephthalate MIL-53(Ga). Dalton Transactions 2009, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Férey, G. A New Isoreticular Class of Metal-Organic-Frameworks with the MIL-88 Topology. Chemical Communications 2006, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Carson, F.; Su, J.; Platero-Prats, A.E.; Wan, W.; Yun, Y.; Samain, L.; Zou, X. Framework Isomerism in Vanadium Metal-Organic Frameworks: MIL-88B(V) and MIL-101(V). Cryst Growth Des 2013, 13, 5036–5044. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal-Organic Frameworks for Imaging and Drug Delivery. J Am Chem Soc 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, T.; Alonso-Nocelo, M.; Bouzo, B.L.; Reimondez-Troitiño, S.; Abuin-Redondo, C.; De La Fuente, M.; Horcajada, P. Biocompatible Iron(III) Carboxylate Metal–Organic Frameworks as Promising RNA Nanocarriers. Nanoscale 2020, 12, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean Eng Technol 2020, 1, 100032. [Google Scholar] [CrossRef]
- Zhang, G.; Wo, R.; Sun, Z.; Hao, G.; Liu, G.; Zhang, Y.; Guo, H.; Jiang, W. Effective Magnetic Mofs Adsorbent for the Removal of Bisphenol a, Tetracycline, Congo Red and Methylene Blue Pollutions. Nanomaterials 2021, 11, 1917. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon Composite Lignin-Based Adsorbents for the Adsorption of Dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Yadav, M.; Thakore, S.; Jadeja, R. Removal of Organic Dyes Using Fucus Vesiculosus Seaweed Bioadsorbent an Ecofriendly Approach: Equilibrium, Kinetics and Thermodynamic Studies. Environmental Chemistry and Ecotoxicology 2022, 4, 67–77. [Google Scholar] [CrossRef]
- Kundu, A.; Mondal, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Selective Adsorption and Photocatalysis of Malachite Green from Aqueous Solution Using Layered Na-Intercalated Cu-Doped Titania. Appl Clay Sci 2019, 183, 105323. [Google Scholar] [CrossRef]
- Konicki, W.; Hełminiak, A.; Arabczyk, W.; Mijowska, E. Adsorption of Cationic Dyes onto Fe@graphite Core–Shell Magnetic Nanocomposite: Equilibrium, Kinetics and Thermodynamics. Chemical Engineering Research and Design 2018, 129, 259–270. [Google Scholar] [CrossRef]
- Feng, M.; Yu, S.; Wu, P.; Wang, Z.; Liu, S.; Fu, J. Rapid, High-Efficient and Selective Removal of Cationic Dyes from Wastewater Using Hollow Polydopamine Microcapsules: Isotherm, Kinetics, Thermodynamics and Mechanism. Appl Surf Sci 2021, 542, 148633. [Google Scholar] [CrossRef]
- Liu, H.; Ren, X.; Chen, L. Synthesis and Characterization of Magnetic Metal–Organic Framework for the Adsorptive Removal of Rhodamine B from Aqueous Solution. Journal of Industrial and Engineering Chemistry 2016, 34, 278–285. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Zhang, X.; Huang, Y. Highly Efficient Fenton and Enzyme-Mimetic Activities of NH2-MIL-88B(Fe) Metal Organic Framework for Methylene Blue Degradation. Scientific Reports 2018 8:1 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; Omer, A.M.; Khalifa, R.E.; Abd El-Latif, M.M.; El-Subruiti, G.M.; Omer, A.M. Efficient Removal of Toxic Methylene Blue (MB) Dye from Aqueous Solution Using a Metal-Organic Framework (MOF) MIL-101(Fe): Isotherms, Kinetics, and Thermodynamic Studies. 2020. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of Methylene Blue onto Fe3O4/Activated Montmorillonite Nanocomposite. Appl Clay Sci 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential Candidates in Wastewater Treatment Technology – A Review on the Removal of Methylene Blue Dye. J Magn Magn Mater 2020, 500, 166408. [Google Scholar] [CrossRef]
- Saghanejhad Tehrani, M.; Zare-Dorabei, R. Highly Efficient Simultaneous Ultrasonic-Assisted Adsorption of Methylene Blue and Rhodamine B onto Metal Organic Framework MIL-68(Al): Central Composite Design Optimization. RSC Adv 2016, 6, 27416–27425. [Google Scholar] [CrossRef]
- Guo, H.; Lin, F.; Chen, J.; Li, F.; Weng, W. Metal–Organic Framework MIL-125(Ti) for Efficient Adsorptive Removal of Rhodamine B from Aqueous Solution. Appl Organomet Chem 2015, 29, 12–19. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Su, Y.; Yu, H.; Liu, H.; Qian, S.; Zheng, W.; Zhao, Y. Zr-MOFs Loaded on Polyurethane Foam by Polydopamine for Enhanced Dye Adsorption. Journal of Environmental Sciences 2021, 101, 177–188. [Google Scholar] [CrossRef]
- Arora, C.; Soni, S.; Sahu, S.; Mittal, J.; Kumar, P.; Bajpai, P.K. Iron Based Metal Organic Framework for Efficient Removal of Methylene Blue Dye from Industrial Waste. J Mol Liq 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sert, E.; Atalay, F.S. Synthesis, Characterization of a Metal Organic Framework: MIL-53 (Fe) and Adsorption Mechanisms of Methyl Red onto MIL-53 (Fe). J Taiwan Inst Chem Eng 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Moulin, B.; Heurtaux, D.; Clet, G.; Vimont, A.; Grenéche, J.M.; et al. Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host-Guest Interactions. J Am Chem Soc 2010, 132, 1127–1136. [Google Scholar] [CrossRef]
- Uemura, K.; Matsuda, R.; Kitagawa, S. Flexible Microporous Coordination Polymers. J Solid State Chem 2005, 178, 2420–2429. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Thomas, K.M.; Rosseinsky, M.J. Flexibility in Metal-Organic Framework Materials: Impact on Sorption Properties. J Solid State Chem 2005, 178, 2491–2510. [Google Scholar] [CrossRef]
- Long, J.; Yaghi, O.; Ge´rard, G.; Fe´rey, F.; Serre, C. Large Breathing Effects in Three-Dimensional Porous Hybrid Matter: Facts, Analyses, Rules and Consequences. Chem Soc Rev 2009, 38, 1380–1399. [Google Scholar] [CrossRef]


| SBET (m2 g –1) a | VTotal (cm3 g –1) b | Avg. Pore Diameter c |
% Wt. d | |||
|---|---|---|---|---|---|---|
| C | O | Fe | Cl | |||
| 275.12 | 0.078 | 6.20 | 61.8 | 25.4 | 10.8 | 2.0 |
| Dyes | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qmax (mg g–1) | KL (L mg–1) | R2 | 1/n | KF | R2 | |
| MB | 66.67 | 0.5555 | 0.9965 | 0.4511 | 17.10 | 0.9014 |
| RhB | 86.96 | 0.5928 | 0.8818 | 0.6135 | 29.15 | 0.9877 |
| Dyes | Parameters | Dyes | |
|---|---|---|---|
| MB | RhB | ||
| Pseudo-second-order Model | qcal a (mg g–1) | 4.70 | 3.40 |
| qexp b (mg g–1) | 5.44 | 2.76 | |
| k2 (g mg–1 min–1) | 1.99 x 10-2 | 1.78 x 10-2 | |
| R2 | 0.9951 | 0.9808 | |
| Pseudo-first-order Model | qcal a (mg g–1) | 0.3324 | 44.456 |
| k1 (min–1) | 1.6 x 10-3 | 6.0 x 10-4 | |
| R2 | 0.213 | 0.0971 | |
| Intraparticle Diffusion Models | ki1 (mg g–1 min–1/2) | 0.10 | 0.13 |
| C1 (mg g–1) | 4.04 | 2.44 | |
| R2 | 0.9491 | 0.7908 | |
| ki2 (mg g–1 min–1/2) | 0.12 | 0.17 | |
| C2 (mg g–1) | 7.05 | 6.55 | |
| R2 | 0.8981 | 0.9906 | |
| Dyes | T (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol/K) |
|---|---|---|---|---|
| MB | 298 | –2.42 | 42.98 | 152.30 |
| 308 | –3.95 | |||
| 318 | –5.47 | |||
| RhB | 298 | 1.65 | 28.17 | 88.95 |
| 308 | 0.76 | |||
| 318 | –0.12 |
| No | Adsorbent | BET Surface Area (m2/g) | Adsorbate | Reaction Conditions | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | pH | Time (min) | Temp (°C) | Qmax* (mg/g) | |||||
| 1 | MIL-88B(Fe) | 275.12 | MB | 250 | 9 | 180 | 25 | 162.82 | This work |
| 2 | NH2-MIL-88B(Fe) | 163.9 | MB | 20 | 3-11 | 45 | 35 | 61.46 | [46] |
| 3 | MIL-101(Fe) | 54.71 | MB | 200 | 9 | 500 | 25 | 58.82 | [47] |
| 4 | Fe3O4 activated montmorillonite | 147.92 | MB | 120-1000 | 7.37 | 25 | 20 | 106.38 | [48,49] |
| 5 | MIL-88B(Fe) | 275.12 | RhB | 250 | 9 | 180 | 25 | 144.65 | This work |
| 6 | MIL-68(Al) | 976 | RhB | 15 | 6.45 | 9.9 | 25 | 29.32 | [50] |
| 7 | MIL-125(Ti) | 845 | RhB | 10 | 7 | 180 | 25 | 59.92 | [51] |
| 8 | Zirconium-based MOFs | - | RhB | 10 | 7 | 240 | 25 | 67.73 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





