Submitted:
20 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Taxonomic Aspects and Occurrence
3. Entomopathogenicity
3.1. Interactions with Biocontrol Agents
3.2. Plant-mediated Interactions
4. Other Ecological Relationships
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Braun, U.; Dijksterhuis, J.; de Jesús Yáñez-Morales, M.; Crous, P.W. Common but different: The expanding realm of Cladosporium. Stud. Mycol. 2015, 82, 23–74. [Google Scholar] [CrossRef]
- Liu, W.; Yu, S.H.; Zhang, H.P.; Fu, Z.Y.; An, J.Q.; Zhang, J.Y.; Yang, P. Two Cladosporium fungi with opposite functions to the Chinese white wax scale insect have different genome characters. J. Fungi 2022, 8, 286. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Qi, Y.; Chu, S.; Ma, Y.; Xu, L.; Lv, S.; Zhang, H.; Yang, D.; Zhu, Y.; Mans, D.R.A.; Liang, Z. Endophytic fungus Cladosporium tenuissimum DF11, an efficient inducer of tanshinone biosynthesis in Salvia miltiorrhiza roots. Phytochemistry 2022, 194, 113021. [Google Scholar] [CrossRef]
- Kishore Varma, P.; Chandra Sekhar, V.; Bhavani, B.; Upendhar, S. Cladosporium cladosporioides: A new report of parasitism on sugarcane woolly aphid, Ceratovacuna lanigera Zehntner. J. Entomol. Zool. Stud. 2019, 7, 1122–1126. [Google Scholar]
- Becchimanzi, A.; Zimowska, B.; Nicoletti, R. Cryptic diversity in Cladosporium cladosporioides resulting from sequence-based species delimitation analyses. Pathogens 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Morales-Rodriguez, C.; Sferrazza, I.; Aleandri, M.; Dalla Valle, M.; Mazzetto, T.; Speranza, S.; Contarini, M.; Vannini, A. Fungal community associated with adults of the chestnut gall wasp Dryocosmus kuriphilus after emergence from galls: Taxonomy and functional ecology. Fungal Biol. 2019, 123, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Morales-Rodríguez, C.; Sferrazza, I.; Aleandri, M.P.; Dalla Valle, M.; Speranza, S.; Contarini, M.; Vannini, A. The fungal community associated with the ambrosia beetle Xylosandrus compactus invading the mediterranean maquis in central Italy reveals high biodiversity and suggests environmental acquisitions. Fungal Biol. 2021, 125, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Swett, C.L.; Hamby, K.A.; Hellman, E.M.; Carignan, C.; Bourret, T.B.; Koivunen, E.E. Characterizing members of the Cladosporium cladosporioides species complex as fruit rot pathogens of red raspberries in the mid-Atlantic and co-occurrence with Drosophila suzukii (spotted wing drosophila). Phytoparasitica 2019, 47, 415–428. [Google Scholar] [CrossRef]
- Abdel-Galil, F.A.K.; Amro, M.A.R.; Mahmoud, M.B. Species composition of phytophagous, entomophagous insects and prevalent aphid fungi species inhabiting cabbage plantations. Egypt. Acad. J. Biol. Sci. A, Entomol. 2021, 14, 11–18. [Google Scholar] [CrossRef]
- Luz, C.; Netto, M.C.B.; Rocha, L.F.N. In vitro susceptibility to fungicides by invertebrate-pathogenic and saprobic fungi. Mycopathologia 2007, 164, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; May, G.; Bushley, K.E. Sources of fungal symbionts in the microbiome of a mobile insect host, Spodoptera frugiperda. Microb. Ecol. 2023, 86, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Shaker, N.O.; Ahmed, G.M.M.; El-Sayed Ibrahim, H.Y.; El-Sawy, M.M.; El-Hoseiny Mostafa, M.; Abd El-Rahman Ismail, H.N. Secondary metabolites of the entomopathogenic fungus, Cladosporium cladosporioides and its relation to toxicity of cotton aphid, Aphis gossypii Glov.). Int. J. Entomol. Nematol. 5, 115–120.
- Yamoah, E.; Jones, E.E.; Weld, R.J.; Suckling, D.M.; Waipara, N.; Bourdôt, G.W.; Hee, A.K.W.; Stewart, A. Microbial population and diversity on the exoskeletons of four insect species associated with gorse (Ulex europaeus L.). Aus. J. Entomol. 2008, 47, 370–379. [Google Scholar] [CrossRef]
- Moubasher, A.H.; Abdel-Sater, M.A.; Soliman, Z. Yeasts and filamentous fungi inhabiting guts of three insect species in Assiut, Egypt. Mycosphere 2017, 8, 1297–1316. [Google Scholar] [CrossRef]
- Zimowska, B.; Becchimanzi, A.; Krol, E.D.; Furmanczyk, A.; Bensch, K.; Nicoletti, R. New Cladosporium species from normal and galled flowers of Lamiaceae. Pathogens 2021, 10, 369. [Google Scholar] [CrossRef]
- Pagnocca, F.C.; Rodrigues, A.; Nagamoto, N.S.; Bacci, M. Yeasts and filamentous fungi carried by the gynes of leaf-cutting ants. Antonie van Leeuwenhoek 2008, 94, 517–526. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Arafat, N.S.; Abdel-Salam, A.H. Three Cladosporium spp. as promising biological control candidates for controlling whiteflies (Bemisia spp.) in Egypt. Pakistan. J. Biol. Sci. 1998, 1, 188–195. [Google Scholar] [CrossRef]
- Cline, A.R.; Skelley, P.E.; Kinnee, S.A.; Rooney-Latham, S.; Winterton, S.L.; Borkent, C.J.; Audisio, P. Interactions between a sap beetle, sabal palm, scale insect, filamentous fungi and yeast, with discovery of potential antifungal compounds. PLoS ONE 2014, 9, e89295. [Google Scholar] [CrossRef]
- Ibrahim, H.Y. Biodiversity of entomopathogenic fungi naturally infecting cabbage aphid, Brevicoryne brassicae. L. J. Plant Prot. Pathol. Mansoura Univ. 2017, 8, 631–634. [Google Scholar] [CrossRef]
- Monk, K.A.; Samuels, G.J. Mycophagy in grasshoppers (Orthoptera: Acrididae) in Indo-Malayan rain forests. Biotropica 1990, 16–21. [Google Scholar] [CrossRef]
- Ezz, N. Entomopathogenic fungi associated with certain scale insects (Hemiptera: Coccoidea) in Egypt. Egypt. Acad. J. Biol. Sci. 2012, 5, 211–221. [Google Scholar]
- Singh, S.; Poornesha, B.; Sandhu, R.K.; Ramanujam, B. Natural occurrence of entomopathogenic fungus, Cladosporium cladosporioides on blow fly, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) on ber in Punjab, India. J. Biol. Control 2020, 34, 140–143. [Google Scholar] [CrossRef]
- Woolfolk, S.W.; Inglis, G.D. Microorganisms associated with field-collected Chrysoperla rufilabris (Neuroptera: Chrysopidae) adults with emphasis on yeast symbionts. Biol. Control 2004, 29, 155–168. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Kauserud, H.; Sverdrup-Thygeson, A.; Bjorbækmo, M.M.; Birkemoe, T. Wood-inhabiting insects can function as targeted vectors for decomposer fungi. Fungal Ecol. 2017, 29, 76–84. [Google Scholar] [CrossRef]
- Jayasimha, P.; Henderson, G. Fungi isolated from integument and guts of Coptotermes formosanus and their antagonistic effect on Gleophyllum trabeum. Ann. Entomol. Soc. America 2007, 100, 703–710. [Google Scholar] [CrossRef]
- Badran, R.A.; Aly, M.Z.Y. Studies on the mycotic inhabitants of Culex pipiens collected from fresh water ponds in Egypt. Mycopathologia 1995, 132, 105–110. [Google Scholar] [CrossRef]
- Turky, W.; Hassoon, A.; Al-Taee, M. Isolation and diagnosis of fungi associated with the larvae of the mosquitoes Culex quinquefasciatus and its biological control. J. Multidiscip. Engin. Sci. Technol. 2018, 5, 9028–9035. [Google Scholar]
- Pherson, D.A.; Beattie, A.J. Fungal loads of invertebrates in beech leaf litter. Rev. Ecol. Biol. Sol 1979, 16, 325–335. [Google Scholar]
- Meyer, J.M.; Hoy, M.A. Removal of fungal contaminants and their DNA from the surface of Diaphorina citri (Hemiptera: Psyllidae) prior to a molecular survey of endosymbionts. Florida Entomol. 2008, 91, 702–705. [Google Scholar]
- Trovão, J.; Mesquita, N.; Paiva, D.S.; de Carvalho, H. P.; Avelar, L.; Portugal, A. Can arthropods act as vectors of fungal dispersion in heritage collections? A case study on the archive of the University of Coimbra, Portugal. Int. Biodeter. Biodegr. 2013, 79, 49–55. [Google Scholar] [CrossRef]
- Bing, X.L.; Winkler, J.; Gerlach, J.; Loeb, G.; Buchon, N. Identification of natural pathogens from wild Drosophila suzukii. Pest Manag. Sci. 2021, 77, 1594–1606. [Google Scholar] [CrossRef]
- Özdal, M.; İncekara, Ü.; Polat, A.; Gür, Ö.; Kurbanoğlu, E.; Taşar, G. Isolatıon of fılamentous fungı assocıated wıth two common edıble aquatıc ınsects Hydrophılus pıceus and Dytıscus margınalıs. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 95–105. [Google Scholar]
- Sun, T.; Wang, X.Q.; Zhao, Z.L.; Yu, S.H.; Yang, P.; Chen, X.M. A lethal fungus infects the Chinese white wax scale insect and causes dramatic changes in the host microbiota. Sci. Rep. 2018, 8, 5324. [Google Scholar] [CrossRef]
- Marques, E.J.; França, I.W.B.; Menezes, M. Ocorrência de Cladosporium cladosporioides (Fres.) De Vries em Euphalerus clitoriae Burckhardt & Guajará, 2000 (Hemiptera: Psyllidae) em Clitoria fairchildiana no Estado de Pernambuco. In: Anais do XIX Congresso Brasileiro de Entomologia, Manaus, Brazil, 16-21 June 2002, p.78.
- Jiang, Z.R.; Masuya, H.; Kajimura, H. Novel symbiotic association between Euwallacea ambrosia beetle and Fusarium fungus on fig trees in Japan. Front. Microbiol. 2021, 12, 725210. [Google Scholar] [CrossRef]
- Sun, B.D.; Liu, X.Z. Occurrence and diversity of insect-associated fungi in natural soils in China. Appl. Soil Ecol. 2008, 39, 100–108. [Google Scholar] [CrossRef]
- Sun, B.D.; Yu, H.Y.; Chen, A.J.; Liu, X.Z. Insect-associated fungi in soils of field crops and orchards. Crop Prot. 2008, 27, 1421–1426. [Google Scholar] [CrossRef]
- Yoder, J.A.; Glenn, B.D.; Benoit, J.B.; Zettler, L.W. The giant Madagascar hissing-cockroach (Gromphadorhina portentosa) as a source of antagonistic moulds: concerns arising from its use in a public setting. Mycoses 2008, 51, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeier, D.; Hurka, S.; Klüber, P.; Brinkrolf, K.; Heise, P.; Vilcinskas, A. Cottonseed press cake as a potential diet for industrially farmed black soldier fly larvae triggers adaptations of their bacterial and fungal gut microbiota. Front. Microbiol, 2021, 12, 634503. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.; Parkinson, D.; Hassall, M. Fungi associated with Onychiurus subtenuis (Collembola) in an aspen woodland. Can. J. Bot. 1987, 65, 635–642. [Google Scholar] [CrossRef]
- Ettinger, C.L.; Wu-Woods, J.; Kurbessoian, T.; Brown, D.J.; de Souza Pacheco, I.; Vindiola, B.G.; Walling, L.L.; Atkinson, P.W.; Byrne, F.J.; Redak, R.; Stajich, J.E. Geographical survey of the mycobiome and microbiome of Southern California glassy-winged sharpshooters. Msphere 2023, 8, e00267-23. [Google Scholar] [CrossRef]
- Wrigley, S.K.; Ainsworth, A.M.; Kau, D.A.; Martin, S.M.; Bahl, S.; Tang, J.S.; Hardick, D.J.; Rawlins, P.; Sadheghi, R.; Moore, M. Novel reduced benzo [j] fluoranthen-3-ones from Cladosporium cf. cladosporioides with cytokine production and tyrosine kinase inhibitory properties. J. Antibiot. 2001, 54, 479–488. [Google Scholar] [CrossRef]
- Davydenko, K.; Vasaitis, R.; Elfstrand, M.; Baturkin, D.; Meshkova, V.; Menkis, A. Fungal communities vectored by Ips sexdentatus in declining Pinus sylvestris in Ukraine: focus on occurrence and pathogenicity of ophiostomatoid species. Insects 2021, 12, 1119. [Google Scholar] [CrossRef]
- Pan, W.Y.; Chen, S.L.; Lian, J.H.; Qiu, H.Z.; Lan, G. A preliminary report on control of Hemiberlesia pitysophila using Cladosporium cladosporioides. Forest Pest Dis. 1989, (3), 22–23. [Google Scholar]
- Adam, J.; Overton, B. Forgotten fungi that could be used to control the spread of the spotted lanternfly (Hemiptera: Fulgoridae). Fungi 2023, 15(5), 41–50. [Google Scholar]
- Ceriani-Nakamurakare, E.; Mc Cargo, P.; Gonzalez-Audino, P.; Ramos, S.; Carmarán, C. New insights into fungal diversity associated with Megaplatypus mutatus: gut mycobiota. Symbiosis 2020, 81, 127–137. [Google Scholar] [CrossRef]
- Al-Shindah, R.S.; Hassan, A.A.; Mansour, M.S. Isolation and identification of entomopathogenic fungi from of green peach aphid Myzus persicae and evaluation of their activity for insect control. In IOP Conference Series: Earth and Environmental Science. IOP Publishing: Bristol, United Kingdom, 2022; Volume 1060, 012093.
- Islam, T.; Gupta, D.R.; Surovy, M.Z.; Mahmud, N.U.; Mazlan, N.; Islam, T. Identification and application of a fungal biocontrol agent Cladosporium cladosporioides against Bemisia tabaci. Biotechnol. Biotechnol. Equipment 2019, 33, 1698–1705. [Google Scholar] [CrossRef]
- Xu, X.; Shao, M.; Yin, C.; Mao, Z.; Shi, J.; Yu, X.; Wang, Y.; Sun, F.; Zhang, Y. Diversity, bacterial symbionts, and antimicrobial potential of termite-associated fungi. Front. Microbiol. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.W.; Lu, Y.H.; Miao, S.; Zhang, Y.; Chen, T.T.; Zhang, Y.L. Diversity, bacterial symbionts and antibacterial potential of gut-associated fungi isolated from the Pantala flavescens larvae in China. PLoS ONE 2015, 10, e0134542. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Rossa, R. Associations between Pityogenes bidentatus and fungi in young managed Scots pine stands in Poland. Forest Pathol. 2008, 38, 169–177. [Google Scholar] [CrossRef]
- Oliveira, I.; Pereira, J.A.; Quesada-Moraga, E.; Lino-Neto, T.; Bento, A.; Baptista, P. Effect of soil tillage on natural occurrence of fungal entomopathogens associated to Prays oleae Bern. Scientia Horticult. 2013, 159, 190–196. [Google Scholar] [CrossRef]
- Jaber, S.; Mercier, A.; Knio, K.; Brun, S.; Kambris, Z. Isolation of fungi from dead arthropods and identification of a new mosquito natural pathogen. Parasit. Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Masuya, H.; Kajimura, H.; Tomisawa, N.; Yamaoka, Y. Fungi associated with Scolytogenes birosimensis (Coleoptera: Curculionidae) infesting Pittosporum tobira. Environ. Entomol. 2012, 41, 255–264. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Khan, T. Efficacy of entomopathogenic fungi against three major stored insect pests, Rhyzopertha dominica, Sitophilus zeamais and Trogoderma granarium. J. Stored Prod. Res. 2023, 104, 102188. [Google Scholar] [CrossRef]
- Jankowiak, R.; Bilański, P. Fungal flora associated with Tomicus piniperda L. in an area close to a timber yard in southern Poland. J. Appl. Entomol. 2007, 131, 579–584. [Google Scholar] [CrossRef]
- Moraes, A.M.L.; Junqueira, A.C.V.; Costa, G.L.; Celano, V.; Oliveira, P.C.; Coura, J.R. Fungal flora of the digestive tract of 5 species of triatomines vectors of Trypanosoma cruzi, Chagas 1909. Mycopathologia 2001, 151, 41–48. [Google Scholar] [CrossRef]
- Marti, G.A.; García, J.J.; Cazau, M.C.; López Lastra, C.C. Flora fúngica de tractos digestivos en Triatoma infestans (Hemiptera: Reduviidae) en Argentina. Bol. Soc. Argentina Bot. 2007, 42, 175–179. [Google Scholar]
- Gunde-Cimerman, N.; Zalar, P.; Jeram, S. Mycoflora of cave cricket Troglophilus neglectus cadavers. Mycopathologia 1998, 141, 111–114. [Google Scholar] [CrossRef]
- Yun, T.S.; Park, S.Y.; Yu, J.; Hwang, Y.; Hong, K.J. Isolation and identification of fungal species from the insect pest Tribolium castaneum in rice processing complexes in Korea. Plant Pathol. J. 2018, 34, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Amirmijani, A.R.; Khodaparast, S.A.; Zare, R. Additions to the knowledge of the genus Cladosporium in Iran. Mycol. Iranica 2015, 2, 11–21. [Google Scholar]
- Souza, C.R.; Teixeira, M.F.N.P.; Morais, P.B. Diversity of cellulolytic and xylanolytic fungi associated with the digestive tract of aquatic insect larvae in streams of the Amazon Forest and Cerrado in Brazil. Brazil. J. Biol. 2022, 82, e265681. [Google Scholar] [CrossRef]
- Větrovský, T.; Soukup, P.; Stiblik, P.; Votýpková, K.; Chakraborty, A.; Odriozola Larranaga, I.; Sillam-Dussés, D.; Lo, N.; Bourguignon, T.; Baldrian, P.; et al. Termites host specific fungal communities that differ from those in their ambient environments. Fungal Ecol. 2020, 48, 100991. [Google Scholar] [CrossRef]
- Di Napoli, M.; Silvestri, B.; Castagliuolo, G.; Carpentieri, A.; Luciani, G.; Di Maro, A.; Sorbo, S.; Pezzella, A.; Zanfardino, A.; Varcamonti, M. High density polyethylene (HDPE) biodegradation by the fungus Cladosporium halotolerans. FEMS Microbiol. Ecol. 2023, 99, fiac148. [Google Scholar] [CrossRef]
- Wu, H.; Rao, Z.C.; Cao, L.; De Clercq, P.; Han, R.C. Infection of Ophiocordyceps sinensis fungus causes dramatic changes in the microbiota of its Thitarodes host. Front. Microbiol. 2020, 11, 577268. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.B.; de Aquino, M.L.N.; de Oliveria, M.H.C.C. Considerations on the biological control of Aleurodicus cocois (Curtis) (Homoptera: Aleyrodidae), Cashew whitefly. Anais Inst. Ciencias Biol. 1972, 2, 25–30. [Google Scholar]
- Rita, S.; Valentina, P.; Maija, J.; Liga, J. Studies on ecological and physiological host range of entomopathogenic Hyphomycetes. IOBC/wprs Bull. 2008, 31, 198–203. [Google Scholar]
- Sales, M.D.S.N.; Costa, G.L.D.; Bittencourt, V.R.E.P. Isolation of fungi in Musca domestica Linnaeus, 1758 (Diptera: Muscidae) captured at two natural breeding grounds in the municipality of Seropédica, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2002, 97, 1107–1110. [Google Scholar] [CrossRef]
- Phoku, J.; Barnard, T.; Potgieter, N.; Dutton, M. Fungal dissemination by housefly (Musca domestica L.) and contamination of food commodities in rural areas of South Africa. Int. J. Food Microbiol. 2016, 217, 177–181. 217 2016, 217, 177–181. [Google Scholar]
- Klimaszewski, J.; Morency, M.-J.; Labrie, P.; Seguin, A.; Langor, D.; Work, T.; Bourdon, C.; Thiffault, E.; Pare, D.; Newton, A.F.; et al. Molecular and microscopic analysis of the gut contents of abundant rove beetle species (Coleoptera, Staphylinidae) in the boreal balsam fir forest of Quebec, Canada. Zookeys 2013, 353, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.; Berggreen, I.E.; Tedeschi, F.; Ntrallou, K.; Gika, H.; Corredig, M. Gut microbiome and degradation product formation during biodegradation of expanded polystyrene by mealworm larvae under different feeding strategies. Molecules 2021, 26, 7568. [Google Scholar] [CrossRef]
- Teixeira, M.F.N.P.; Souza, C.R.; Morais, P.B. Diversity and enzymatic capabilities of fungi associated with the digestive tract of larval stages of a shredder insect in Cerrado and Amazon Forest, Brazil. Brazil. J. Biol. 2022, 82, e260039. [Google Scholar] [CrossRef]
- Samways, M.J.; Grech, N.M. Assessment of the fungus Cladosporium oxysporum (Berk. and Curt.) as a potential biocontrol agent against certain Homoptera. Agric. Ecosyst. Environ. 1986, 15, 231–239. [Google Scholar] [CrossRef]
- Samways, M.J. Interrelationship between an entomogenous fungus and two ant-homopteran (Hymenoptera: Formicidae-Hemiptera: Pseudococcidae & Aphididae) mutualisms on guava trees. Bull. Entomol. Res. 1983, 73, 321–331. [Google Scholar]
- Carrión, G.; Bonet, A. Mycobiota associated with the coffee berry borer (Coleoptera: Scolytidae) and its galleries in fruit. Ann. Entomol. Soc. America 2004, 97, 492–499. [Google Scholar] [CrossRef]
- Vitale, S.; Toccafondi, P.; Luongo, L.; Binazzi, F.; Petrucci, M.; Francardi, V.; Landi, S.; Giovannini, L.; Simoni, S.; Roversi, P.F.; Pennacchio, F. Fungi obtained from olive twig dieback and adults of the alien pest Xylosandrus compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae). Redia 2022, 105, 197–204. [Google Scholar] [CrossRef]
- Park, J.M.; You, Y.H.; Back, C.G.; Kim, H.H.; Ghim, S.Y.; Park, J.H. Fungal load in Bradysia agrestis, a phytopathogen-transmitting insect vector. Symbiosis 2018, 74, 145–158. [Google Scholar] [CrossRef]
- Simpanya, M.F.; Allotey, J.; Mpuchane, S.F. A mycological investigation of phane, an edible caterpillar of an emperor moth, Imbrasia belina. J. Food Prot. 2000, 63, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Nagam, V.; Aluru, R.; Shoaib, M.; Dong, G.R.; Li, Z.; Pallaval, V.B.; Ni, J.F. Diversity of fungal isolates from fungus-growing termite Macrotermes barneyi and characterization of bioactive compound from Xylaria escharoidea. Insect Sci. 2021, 28, 392–402. [Google Scholar] [CrossRef]
- Erdoğdu, M.; Öğütçü, H.; Koçak, Y.; IÇağlar, Ü. solation of microfungi associated with the gut and the surface of Acmaeodera flavolineata (Coleoptera: Buprestidae). In Abstract Book of the 2nd International Congress on The World of Technology and Advanced Materials, Kırşehir, Turkey, 28 September-2 October 2016, PP94.
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L. ; Shao, Y Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, W.A. Fungus diseases affecting Adelges piceae in the fir forest of the Gaspe Peninsula, Quebec. Can. Entomol. 1970, 102, 799–805. [Google Scholar] [CrossRef]
- Gouli, V.; Gouli, S.; Marcelino, J.A.; Skinner, M.; Parker, B.L. Entomopathogenic fungi associated with exotic invasive insect pests in Northeastern forests of the USA. Insects 2013, 4, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Luis, P.; Vallon, L.; Tran, F.H.; Hugoni, M.; Tran-Van, V.; Mavingui, P.; Minard, G.; Valiente Moro, C. Aedes albopictus mosquitoes host a locally structured mycobiota with evidence of reduced fungal diversity in invasive populations. Fungal Ecol. 2019, 39, 257–266. [Google Scholar] [CrossRef]
- Guégan, M.; Tran Van, V.; Martin, E.; Minard, G.; Tran, F.H.; Fel, B.; Hay, A.E.; Simon, L.; Barakat, M.; Poitier, P.; et al. Who is eating fructose within the Aedes albopictus gut microbiota? Environ. Microbiol. 2020, 22, 1193–1206. [Google Scholar] [CrossRef]
- Edwards, C.C.; McConnel, G.; Ramos, D.; Gurrola-Mares, Y.; Arole, K.D.; Green, M.J.; Cañas-Carrell, J.E.; Brelsfoard, C.L. Microplastic ingestion perturbs the microbiome of Aedes albopictus (Diptera: Culicidae) and Aedes aegypti. J. Med. Entomol. 2023, 60, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Bara, J.J.; Rooney, A.P.; Hansen, A.K. Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol. Ecol. 2016, 25, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.X.; Li, D.Z.; Bernhardt, P.; Wang, H. Flowers of Cypripedium fargesii (Orchidaceae) fool flat-footed flies (Platypezidae) by faking fungus-infected foliage. Proc. Nat. Acad. Sci. 2011, 108, 7478–7480. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, S.; Li, X.; Li, M. Bacterial and fungal gut communities of Agrilus mali at different developmental stages and fed different diets. Sci. Rep. 2018, 8, 15634. [Google Scholar] [CrossRef]
- Reddersen, J. Feeding biology of fungivorous insects from Danish cereal fields. Pedobiologia 1995, 39, 370–384. [Google Scholar] [CrossRef]
- Baoyu, H.; Zengzhi, L. Growing characteristics on Czapek medium of 8 kinds of entomogenous fungi from Aleurocanthus spiniferus and their infection ratios. Entomol. J. East China 2001, 10, 39–43. [Google Scholar]
- Nunes Farias, A.R.; Santos Filho, H.P. Ocorrencia de Cladosporium spp. infectando a masca branca Aleurothrixus aepim (Goeldi, 1886) em mandioca no Estado da Bahia. Rev. Bras. Mandioca 1987, 6, 79–80. [Google Scholar]
- Lee, G.Y.; Wertman, D.L.; Carroll, A.L.; Hamelin, R.C. Filamentous fungal associates of the alder bark beetle, Alniphagus aspericollis, including an undescribed species of Neonectria. PLoS ONE 2023, 18, e0284393. [Google Scholar] [CrossRef] [PubMed]
- Machota, Jr.R.; Bortoli, L.C.; Botton, M.; Grutzmacher, A.D. Fungi that cause rot in bunches of grape identified in adult fruit flies (Anastrepha fraterculus) (Diptera: Tephritidae). Chilean J. Agr. Res. 2013, 73, 196–201. [Google Scholar] [CrossRef]
- Krajacich, B.J.; Huestis, D.L.; Dao, A.; Yaro, A.S.; Diallo, M.; Krishna, A.; Xu, J.; Lehmann, T. Investigation of the seasonal microbiome of Anopheles coluzzii mosquitoes in Mali. PLoS ONE 2018, 13, e0194899. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, Y.; Degawa, Y. Novel Metschnikowia yeasts from the gut of green lacewing in Japan. Antonie van Leeuwenhoek 2023, 116, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Baky, N.F.; Abdel-Salam, A.H. Natural incidence of Cladosporium spp. as a bio-control agent against whiteflies and aphids in Egypt. J. Appl. Entomol. 2003, 127, 228–235. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Latoch, P.; Hurd, P.J.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Gancarz, M.; et al. Amplicon sequencing of variable 16S rRNA from bacteria and ITS2 regions from fungi and plants, reveals honeybee susceptibility to diseases results from their forage availability under anthropogenic landscapes. Pathogens 2021, 10, 381. [Google Scholar] [CrossRef]
- Gilliam, M.; Prest, D.B. Fungi isolated from the intestinal contents of foraging worker honey bees, Apis mellifera. J. Invertebr. Pathol. 1972, 20, 101–103. [Google Scholar] [CrossRef]
- Callegari, M.; Crotti, E.; Fusi, M.; Marasco, R.; Gonella, E.; De Noni, I.; Romano, D.; Borin, S.; Tsiamis, G.; Cherif, A.; et al. Compartmentalization of bacterial and fungal microbiomes in the gut of adult honeybees. npj Biofilms Microbiomes 2021, 7, 42. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Singavarapu, B.; Paxton, R.J.; Wubet, T. Bees under interactive stressors: the novel insecticides flupyradifurone and sulfoxaflor along with the fungicide azoxystrobin disrupt the gut microbiota of honey bees and increase opportunistic bacterial pathogens. Sci. Total Environ. 2022, 849, 157941. [Google Scholar] [CrossRef]
- Adair, R.J.; Burgess, T.; Serdani, M.; Barber, P. Fungal associations in Asphondylia (Diptera: Cecidomyiidae) galls from Australia and South Africa: Implications for biological control of invasive acacias. Fungal Ecol. 2009, 2, 121–134. [Google Scholar] [CrossRef]
- Bernardo, U.; Nugnes, F.; Gualtieri, L.; Nicoletti, R.; Varricchio, P.; Sasso, R.; Viggiani, G. A new gall midge species of Asphondylia (Diptera: Cecidomyiidae) inducing flower galls on Clinopodium nepeta (Lamiaceae) from Europe, its phenology, and associated fungi. Environ. Entomol. 2018, 47, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Zimowska, B.; Viggiani, G.; Nicoletti, R.; Furmanczyk, A.; Becchimanzi, A.; Kot, I. First report of the gall midge Asphondylia serpylli on thyme (Thymus vulgaris), and identification of the associated fungal symbiont. Ann. Appl. Biol. 2017, 171, 89–94. [Google Scholar] [CrossRef]
- Duarte, A.P.M.; Ferro, M.; Rodrigues, A.; Bacci, M.; Nagamoto, N.S.; Forti, L.C.; Pagnocca, F.C. Prevalence of the genus Cladosporium on the integument of leaf-cutting ants characterized by 454 pyrosequencing. Antonie Van Leeuwenhoek 2016, 109, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Palmeri, V. Molecular analysis of the fungal microbiome associated with the olive fruit fly Bactrocera oleae. Fungal Ecol. 2015, 18, 67–74. [Google Scholar] [CrossRef]
- Majumder, R.; Sutcliffe, B.; Taylor, P.W.; Chapman, T.A. Microbiome of the Queensland fruit fly through metamorphosis. Microorganisms 2020, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.H.; Li, W.L.; Wang, J.P.; Li, X.J.; Li, D.; Cao, Z.; Huang, Q.; Li, J.L.; Zhang, J.; Wang, Z.W.; et al. Effects of spinetoram and glyphosate on physiological biomarkers and gut microbes in Bombus terrestris. Front. Physiol. 2023, 13, 1054742. [Google Scholar] [CrossRef]
- Park, J.M.; You, Y.H.; Park, J.H.; Kim, H.H.; Ghim, S.Y.; Back, C.G. Cutaneous microflora from geographically isolated groups of Bradysia agrestis, an insect vector of diverse plant pathogens. Mycobiology 2017, 45, 160–171. [Google Scholar] [CrossRef]
- Yoder, J.A.; Rausch, B.A.; Griffith, L.M.; Rosselot, A.E.; Jajack, A.J.; Gribbins, K.M.; Benoit, J.B.; Keeney, G.D. Rearing and storage in mung beans reduce medically significant molds by the seed beetle, Callosobruchus maculatus (Coleoptera: Chrysomelidae), utilized in science classrooms. Mycology 2013, 4, 169–177. [Google Scholar] [CrossRef]
- Heitmann, N.; Glemnitz, M.; Lentzsch, P.; Platen, R.; Müller, M.E. Quantifying the role of ground beetles for the dispersal of Fusarium and Alternaria fungi in agricultural landscapes. J. Fungi 2021, 7, 863. [Google Scholar] [CrossRef]
- Galíndez-Chicaíza, E.; Lagos-Mora, L.E.; Castillo-Belalcázar, G.; Salazar-González, C.; Betancourth-García, C. Fungi detected in insects associated to Espeletia pycnophylla. Rev. U.D.C.A. Actualidad Divulg. Científica 2020, 23, e1497. [Google Scholar]
- Gomez-Polo, P.; Ballinger, M.J.; Lalzar, M.; Malik, A.; Ben-Dov, Y.; Mozes-Daube, N.; Perlman, S.J.; Iasur-Kruh, L.; Chiel, E. An exceptional family: Ophiocordyceps-allied fungus dominates the microbiome of soft scale insects (Hemiptera: Sternorrhyncha: Coccidae). Mol. Ecol. 2017, 26, 5855–5868. [Google Scholar] [CrossRef] [PubMed]
- Shabana, Y.M.; Ragab, M.E. Alternaria infectoria, a promising biological control agent for the fig wax scale, Ceroplastes rusci (Homoptera: Coccidae), in Egypt. Biocontrol Sci. Technol. 1997, 7, 553–564. [Google Scholar] [CrossRef]
- Çağlar, Ü.; Koçak, Y.; Öğütçü, H.; Erdoğdu, M. Fungal associates of the flatheaded wood borer beetle, Chalcophora detrita (Buprestidae: Chalcophorinae). In Abstract Book of the 2nd International Congress on The World of Technology and Advanced Materials, Kırşehir, Turkey, 28 September-2 October 2016, PP93. 2 October.
- Hettiarachchi, A.; Cnockaert, M.; Joossens, M.; Gekière, A.; Meeus, I.; Vereecken, N.J.; Michez, D.; Smagghe, G.; Vandamme, P. The wild solitary bees Andrena vaga, Anthophora plumipes, Colletes cunicularius, and Osmia cornuta microbiota are host specific and dominated by endosymbionts and environmental microorganisms. Microbial Ecol. 2023, 86, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Liu, L.; Huang, Z.; Shi, S.; Kong, K.; Zhang, Y. Diversity, antibacterial activity and chemical analyses of gut-associated fungi isolated from the Crocothemis servilia. Front. Microbiol. 2022, 13, 970990. [Google Scholar] [CrossRef] [PubMed]
- Querner, P.; Sterflinger, K. Evidence of fungal spreading by the grey silverfish (Ctenolepisma longicaudatum) in Austrian museums. Restaurator. Int. J. Preserv. Library Archiv. Mater. 2021, 42, 57–65. [Google Scholar] [CrossRef]
- Chandler, J.A.; Liu, R.M.; Bennett, S.N. RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front. Microbiol. 2015, 6, 185. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 2018, 8, 1352–1368. [Google Scholar] [CrossRef]
- Flores, G.A.; Lopez, R.P.; Cerrudo, C.S.; Consolo, V.F.; Berón, C.M. Culex quinquefasciatus holobiont: A fungal metagenomic approach. Front. Fungal Biol. 2022, 3, 918052. [Google Scholar] [CrossRef]
- Kleespies, R.G.; Huger, A.M.; Zimmermann, G. Microbial antagonists of the codling moth, Cydia pomonella L., diagnosed from 1955 to 2008. IOBC/wprs Bull. 2009, 45, 509–512. [Google Scholar]
- Pyszko, P.; Višňovská, D.; Drgová, M.; Šigut, M.; Drozd, P. Effect of bacterial and fungal microbiota removal on the survival and development of bryophagous beetles. Environ. Entomol. 2020, 49, 902–911. [Google Scholar] [CrossRef]
- Molnár, O.; Wuczkowski, M.; Prillinger, H. Yeast biodiversity in the guts of several pests on maize; comparison of three methods: classical isolation, cloning and DGGE. Mycol. Progr. 2008, 7, 111–123. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Olesen, J.M. The fruit-wasp route to toxic nectar in Epipactis orchids? Flora 1997, 192, 223–229. [Google Scholar] [CrossRef]
- Lewis, M.T.; Koivunen, E.E.; Swett, C.L.; Hamby, K.A. Associations between Drosophila suzukii (Diptera: Drosophilidae) and fungi in raspberries. Environ. Entomol. 2019, 48, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Krishnamoorthy, R.; Gandhi, P.I.; Senthilkumar, M.; Janahiraman, V.; Kumutha, K.; Choudhury, A.R.; Samaddar, S.; Anandham, R.; Sa, T. Endomicrobial community profiles of two different mealybugs: Paracoccus marginatus and Ferrisia virgata. J. Microbiol. Biotechnol. 2020, 30, 1013–1017. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, L.; Lv, J.; Pu, Z.; Zhang, L.; Chen, G.; Hu, X.; Zhang, Z.; Zhang, H. Dietary effects on biological parameters and gut microbiota of Harmonia axyridis. Front. Microbiol. 2021, 12, 818787. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.H.; Backhouse, D.; Gregg, P.C.; Mensah, R. Efficacy of a Cladosporium sp. fungus against Helicoverpa armigera (Lepidoptera: Noctuidae), other insect pests and beneficial insects of cotton. Biocontrol Sci. Technol. 2011, 21, 1387–1397. [Google Scholar] [CrossRef]
- Pérez, J.; Infante, F.; Vega, F.E.; Holguín, F.; Macías, J.; Valle, J.; Nieto, G.; Peterson, S.W.; Kurtzman, C.P.; O'Donnell, K. Mycobiota associated with the coffee berry borer (Hypothenemus hampei) in Mexico. Mycol. Res. 2003, 107, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kobune, S.; Kajimura, H.; Masuya, H.; Kubono, T. Symbiotic fungal flora in leaf galls induced by Illiciomyia yukawai (Diptera: Cecidomyiidae) and in its mycangia. Microbial Ecol. 2012, 63, 619–627. [Google Scholar] [CrossRef]
- Chakraborty, A.; Modlinger, R.; Ashraf, M.Z.; Synek, J.; Schlyter, F.; Roy, A. Core mycobiome and their ecological relevance in the gut of five Ips bark beetles (Coleoptera: Curculionidae: Scolytinae). Front. Microbiol. 2020, 11, 568853. [Google Scholar] [CrossRef]
- Benvenuti, C.; Strangi, A.; Iovinella, I.; Barzanti, G.P.; Simoni, S.; Vitale, S.; Luongo, L.; Francardi, V.; Roversi, P.F. Xylosandrus compactus and Liparthrum colchicum (Coleoptera Scolytinae) in Tuscany: A preliminary screening of associated fungi. Redia 2021, 104, 139–146. [Google Scholar] [CrossRef]
- Van Zandt, P.A.; Townsend Jr, V.R.; Carlton, C.E.; Blackwell, M.; Mopper, S. Loberus impressus (LeConte) (Coleoptera: Erotylidae) fungal associations and presence in the seed capsules of Iris hexagona. Coleopterists' Bull. 2003, 57, 281–288. [Google Scholar] [CrossRef]
- Lim, Y.Z.; Poh, Y.H.; Lee, K.C.; Pointing, S.B.; Wainwright, B.J.; Tan, E.J. Influence of native and exotic plant diet on the gut microbiome of the Gray's Malayan stick insect, Lonchodes brevipes. Front. Microbiol. 2023, 14, 1199187. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Soto, S.U.; Bernal, T.D.V. Identification of pathogenic fungi for Lutzomyia sp. (Diptera: Psychodidae), vectors of leishmaniasis. Rev. Colomb. Entomol. 1996, 22, 13–17. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Shi, J.H.; Shi, Z.B.; Guo, J.X.; Zhang, G.C.; Bi, B. Avermectin stress varied structure and function of gut microbial community in Lymantria dispar asiatica (Lepidoptera: Lymantriidae) larvae. Pesticide Biochem. Physiol. 2020, 164, 196–202. [Google Scholar] [CrossRef]
- Fodor, E.; Hâruţa, O. Niche partition of two invasive insect species, Parectopa robiniella (Lepidoptera; Gracillariidae) and Phyllonorycter robiniella (Clem.) (Lepidoptera: Gracillariidae). Res. J. Agr. Sci. 2009, 41, 261–269. [Google Scholar]
- Zhang, Y.; Liu, S.; Huang, X.Y.; Zi, H.B.; Gao, T.; Ji, R.J.; Sheng, J.; Zhi, D.; Zhang, Y.L.; Gong, C.M.; Yang, Y.Q. Altitude as a key environmental factor shaping microbial communities of tea green leafhoppers (Matsumurasca onukii). Microbiol. Spectrum 2023, e01009-23. [Google Scholar] [CrossRef]
- Minard, G.; Kahilainen, A.; Biere, A.; Pakkanen, H.; Mappes, J.; Saastamoinen, M. Complex plant quality—microbiota–population interactions modulate the response of a specialist herbivore to the defence of its host plant. Funct. Ecol. 2022, 36, 2873–2888. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, M.; Vazirianzadeh, B.; Solary, S.S.; Mahmoudabadi, A.Z.; Rahdar, M. Isolation of fungi from housefly (Musca domestica) in Ahwaz, Iran. Pakistan J. Med. Sci. 2007, 23, 917–919. [Google Scholar]
- Park, R.; Dzialo, M.C.; Spaepen, S.; Nsabimana, D.; Gielens, K.; Devriese, H.; Crauwels, S.; Tito, R.Y.; Raes, J.; Lievens, B.; Verstrepen, K.J. Microbial communities of the house fly Musca domestica vary with geographical location and habitat. Microbiome 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Latella, L.; Castioni, A.; Bignotto, L.; Salvetti, E.; Torriani, S.; Felis, G.E. Exploring gut microbiota composition of the cave beetles Neobathyscia pasai Ruffo, 1950 and Neobathyscia mancinii Jeannel, 1924 (Leiodidae; Cholevinae). Boll. Mus. Civico Storia Nat. Verona 2017, 41, 3–24. [Google Scholar]
- Wang, Z.L.; Wang, T.Z.; Zhu, H.F.; Pan, H.B.; Yu, X.P. Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. 2020, 27, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Muratore, M.; Sun, Y.; Prather, C. Environmental nutrients alter bacterial and fungal gut microbiomes in the common meadow katydid, Orchelimum vulgare. Front. Microbiol. 2020, 11, 557980. [Google Scholar] [CrossRef] [PubMed]
- Malacrinò, A.; Rassati, D.; Schena, L.; Mehzabin, R.; Battisti, A.; Palmeri, V. Fungal communities associated with bark and ambrosia beetles trapped at international harbours. Fungal Ecol. 2017, 28, 44–52. [Google Scholar] [CrossRef]
- Maleki-Ravasan, N.; Oshaghi, M.A.; Hajikhani, S.; Saeidi, Z.; Akhavan, A.A.; Gerami-Shoar, M.; Shirazi, M.H.; Yakhchali, B.; Rassi, Y.; Afshar, D. Aerobic microbial community of insectary population of Phlebotomus papatasi. J. Arthropod-Borne Dis. 2014, 8, 69–81. 8 2014, 8, 69–81. [Google Scholar]
- Layouni, S.; Remadi, L.; Chaâbane-Banaoues, R.; Haouas, N.; Babba, H. Identification of cuticle and midgut fungal microflora of phlebotomine sandflies collected in Tunisia. Arch. Microbiol. 2023, 205, 64. [Google Scholar] [CrossRef]
- Belmont-Montefusco, E.L.; Nacif-Marcal, L.; Nogueira de Assunção, E.; Hamada, N.; Nunes-Silva, C.G. Cultivable cellulolytic fungi isolated from the gut of Amazonian aquatic insects. Acta Amazon. 2020, 50, 346–354. [Google Scholar] [CrossRef]
- Gloder, G.; Bourne, M.E.; Verreth, C.; Wilberts, L.; Bossaert, S.; Crauwels, S.; Dicke, M.; Poelman, E.H.; Jacquemyn, H.; Lievens, B. Parasitism by endoparasitoid wasps alters the internal but not the external microbiome in host caterpillars. Anim. Microbiome 2021, 3, 73. [Google Scholar] [CrossRef]
- Iasur-Kruh, L.; Taha-Salaime, L.; Robinson, W.E.; Sharon, R.; Droby, S.; Perlman, S.J.; Zchori-Fein, E. Microbial associates of the vine mealybug Planococcus ficus (Hemiptera: Pseudococcidae) under different rearing conditions. Microbial Ecol. 2015, 69, 204–214. [Google Scholar] [CrossRef]
- Devarajan, P.T.; Suryanarayanan, T.S. Evidence for the role of phytophagous insects in dispersal of non-grass fungal endophytes. Fungal Divers. 2006, 23, 111–119. [Google Scholar]
- Martinez, A.J.; Onchuru, T.O.; Ingham, C.S.; Sandoval-Calderón, M.; Salem, H.; Deckert, J.; Kaltenpoth, M. Angiosperm to Gymnosperm host-plant switch entails shifts in microbiota of the Welwitschia bug, Probergrothius angolensis (Distant, 1902). Mol. Ecol. 2019, 28, 5172–5187. [Google Scholar] [CrossRef]
- Martin Jr, W.R.; Grisham, M.P.; Kenerley, C.M.; Sterling, W.L.; Morgan, P.W. Microorganisms associated with cotton fleahopper, Pseudatomoscelis seriatus (Heteroptera: Miridae). Ann. Entomol. Soc. America 1987, 80, 251–255. [Google Scholar] [CrossRef]
- Guo, L.; Tang, C.; Gao, C.; Li, Z.; Cheng, Y.; Chen, J.; Wang, T.; Xu, J. Bacterial and fungal communities within and among geographic samples of the hemp pest Psylliodes attenuata from China. Front. Microbiol. 2022, 13, 964735. [Google Scholar] [CrossRef]
- Mousavi, K.; Rajabpour, A.; Parizipour, M.H.G.; Yarahmadi, F. Biological and molecular characterization of Cladosporium sp. and Acremonium zeylanicum as biocontrol agents of Aphis fabae in a tri-trophic system. Entomol. Experim. Appl. 2022, 170, 877–886. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Abdel-Salarn, A.H.; EI Fadaly, H.A. Pulvinaria tenuivalvata (Newstead) and its natural enemies on ratoon sugarcane in Dakahlia Governorate. J. Agr. Sci. Mansoura Univ. 2003, 28, 5699–5712. [Google Scholar]
- Harris, R.J.; Harcourt, S.J.; Glare, T.R.; Rose, E.A.F.; Nelson, T.J. Susceptibility of Vespula vulgaris (Hymenoptera: Vespidae) to generalist entomopathogenic fungi and their potential for wasp control. J. Invertebr. Pathol. 2000, 75, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Cai, X.; Yang, X.; Lin, J.; Shu, B. The bacterial and fungal communities of the larval midgut of Spodoptera frugiperda (Lepidoptera: Noctuidae) varied by feeding on two cruciferous vegetables. Sci. Rep. 2022, 12, 13063. [Google Scholar]
- Wang, Y.; Zhao, X.; Wang, J.; Weng, Y.; Wang, Y.; Li, X.; Han, X. Ingestion preference and efficiencies of different polymerization types foam plastics by Tenebrio molitor larvae, associated with changes of both core gut bacterial and fungal microbiomes. J. Environ. Chem. Engin. 2023, 11, 110801. [Google Scholar] [CrossRef]
- Corallo, B.; Simeto, S.; Martínez, G.; Gómez, D.; Abreo, E.; Altier, N.; Lupo, S. Entomopathogenic fungi naturally infecting the eucalypt bronze bug, Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae), in Uruguay. J. Appl. Entomol. 2019, 143, 542–555. [Google Scholar] [CrossRef]
- Russo, E.; Becchimanzi, A.; Garonna, A.P.; Abate, G.; Nicoletti, R. Fungi associated with the pine tortoise scale Toumeyella parvicornis. In Book of abstracts of the XII International Congress of Plant Pathology, Lyon, France, 20-25 August 2023, 309.
- Rassati, D.; Marini, L.; Malacrinò, A. Acquisition of fungi from the environment modifies ambrosia beetle mycobiome during invasion. PeerJ 2019, 7, e8103. [Google Scholar] [CrossRef]
- Cruz, L.F.; Menocal, O.; Kendra, P.E.; Carrillo, D. Phoretic and internal transport of Raffaelea lauricola by different species of ambrosia beetle associated with avocado trees. Symbiosis 2021, 84, 151–161. [Google Scholar] [CrossRef]
- Bateman, C.; Šigut, M.; Skelton, J.; Smith, K.E.; Hulcr, J. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ. Entomol. 2016, 45, 883–890. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, S.; Li, Y.J.; Song, Y.Q.; Lei, C.Y.; Peng, Y.Y.; Wang, J.J.; Lou, B.H.; Jiang, H.B. Novel isolate of Cladosporium subuliforme and its potential to control Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae). Egypt. J. Biol. Pest Control 2023, 33, 37. [Google Scholar] [CrossRef]
- Pereira, M.L.; Carvalho, J.L.; Lima, J.M.; Barbier, E.; Bernard, E.; Bezerra, J.D.; Souza-Motta, C.M. Richness of Cladosporium in a tropical bat cave with the description of two new species. Mycol. Progr. 2022, 21, 345–357. [Google Scholar] [CrossRef]
- Idrees, A.; Afzal, A.; Qadir, Z.A.; Li, J. Virulence of entomopathogenic fungi against fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. Front. Physiol. 2023, 14, 1107434. [Google Scholar] [CrossRef]
- Ragab, M.; Abdel-Baky, N.F. Cladosporium uredinicola Speg. and Alternaria infectoria EG Simmons, as promising biocontrol agents for Bemisia argentifolii Bellows & Perring and Aphis gossypii Glov. on tomatoes. J. Agr. Sci. Mansoura Univ. 2004, 29, 5897–5906. [Google Scholar]
- Braun, U.; Crous, P.W.; Dugan, F.; Groenewald, J.Z.; De Hoog, G.S. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycol. Progr. 2003, 2, 3–18. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F.; Hamed, K.E.; Al-Otaibi, N.D.; Aldeghairi, M.A. Bioassay of some indigenous entomopathogens for controlling Rhynchophorus ferrugineus, Olivier in Saudi Arabia. Pakistan J. Biol. Sci. 2021, 24, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Aldeghairi, M.A.; El-Meleigi, M.A.; Baky, N.F.A.; Ibrahim, G.H. The entomopathogenic fungus Cladosporium sp. as a candidate biocontrol agent against the sweetpotato whitefly, Bemisia tabaci in Saudi Arabia. In Recent Advances in Biofertilizers and Biofungicides (PGPR) for Sustainable Agriculture. Reddy, M.S., Ilao, R.I., Faylon, P.S., et al. Eds.; Cambridge Scholars Publishing: Newcastle, United Kingdom, 2013; pp. 532–563. [Google Scholar]
- Braun, U.; Crous, P.W.; Schubert, K. Taxonomic revision of the genus Cladosporium s. lat. 8. Reintroduction of Graphiopsis (= Dichocladosporium) with further reassessments of cladosporioid hyphomycetes. Mycotaxon 2008, 103, 207–216. [Google Scholar]
- Lu, Y.H.; Shuai, L.I.; Shao, M.W.; Xiao, X.H.; Kong, L.C.; Jiang, D.H.; Zhang, Y.L. Isolation, identification, derivatization and phytotoxic activity of secondary metabolites produced by Cladosporium oxysporum DH14, a locust-associated fungus. J. Integrative Agr. 2016, 15, 832–839. [Google Scholar] [CrossRef]
- Bartnik, C.; Michalcewicz, J.; Ledwich, D.; Ciach, M. Mycobiota of dead Ulmus glabra wood as breeding material for the endangered Rosalia alpina (Coleoptera: Cerambycidae). Polish J. Ecol. 2020, 68, 13–22. [Google Scholar] [CrossRef]
- Sinpoo, C.; Williams, G.R.; Chantawannakul, P. Dynamics of fungal communities in corbicular pollen and bee bread. Chiang Mai J. Sci. 2017, 44, 1244–1256. [Google Scholar]
- Bell, J.V.; King, E.G.; Hamalle, R.J. Some microbial contaminants and control agents in a diet and larvae of Heliothis spp. J. Invertebr. Pathol. 1981, 37, 243–248. [Google Scholar] [CrossRef]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Mosca, S.; Giunti, G.; Strano, C.P.; Palmeri, V. A metabarcoding survey on the fungal microbiota associated to the olive fruit fly. Microbial Ecol. 2017, 73, 677–684. [Google Scholar] [CrossRef]
- Gharsallah, H.; Ksentini, I.; Frikha-Gargouri, O.; Hadj Taieb, K.; Ben Gharsa, H.; Schuster, C.; Chatti-kolsi, A.; Triki, M.A.; Ksantini, M.; Leclerque, A. Interactions Exploring bacterial and fungal biodiversity in eight Mediterranean olive orchards (Olea europaea L.) in Tunisia. Microorganisms 2023, 11, 10–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Luo, J.Y.; Men, Y.; Liu, Z.H.; Zheng, Z.K.; Wang, Y.H.; Xie, Q. Different roles of host and habitat in determining the microbial communities of plant-feeding true bugs. Microbiome 2023, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Grief, M.D.; Currah, R.S. Patterns in the occurrence of saprophytic fungi carried by arthropods caught in traps baited with rotted wood and dung. Mycologia 2007, 99, 7–19. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Stone, L.B.; Bidochka, M.J. The multifunctional lifestyles of Metarhizium: Evolution and applications. Appl. Microbiol. Biotechnol. 2020, 104, 9935–9945. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Becchimanzi, A. Endophytism of Lecanicillium and Akanthomyces. Agriculture 2020, 10, 205. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Nicoletti, R.; Becchimanzi, A. Talaromyces–insect relationships. Microorganisms 2021, 10, 45. [Google Scholar] [CrossRef]
- Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.; Dalzoto, P.; Zawadeneak, M.A.; Pimentel, I.C. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller) (Lepidoptera: Crambidae). Braz. J. Biol. 2017, 78, 429–435. [Google Scholar] [CrossRef]
- Ouidad, A.; Senoussi, M.M.; Oufroukh, A.; Birgücü, A.K.; Karaca, İ.; Kouadri, F.; Bensegueni, A. Pathogenicity of three entomopathogenic fungi, to the aphid species, Metopolophium dirhodum (Walker) (Hemiptera: Aphididae), and their alkaline protease activities. Egypt. J. Biol. Pest Control 2018, 28, 1–5. [Google Scholar]
- Bensaci, O.A.; Daoud, H.; Lombarkia, N.; Rouabah, K. Formulation of the endophytic fungus Cladosporium oxysporum Berk. and MA Curtis, isolated from Euphorbia bupleuroides subsp. luteola, as a new biocontrol tool against the black bean aphid (Aphis fabae Scop.). J. Plant Prot. Res. 2015, 55, 80–87. [Google Scholar] [CrossRef]
- Renuka, S.; Ramanujam, B. Fungal endophytes from maize (Zea mays L.): isolation, identification and screening against maize stem borer, Chilo partellus (Swinhoe). J. Pure Appl. Microbiol 2016, 10, 523–529. [Google Scholar]
- Saranya, S.; Ushakumari, R.; Jacob, S.; Philip, B.M. Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J. Biopesticides 2010, 3, 138–142. [Google Scholar]
- Abdel-Baky, N.F.; Fadaly, H.A.; EI-Nagar, M.E.; Arafat, N.S.; Abd-Allah, R.H. Virulence and enzymatic activities of some entomopathogenic fungi against whiteflies and aphids. J. Agr. Sci. Mansoura Univ. 2005, 30, 1153–1167. [Google Scholar] [CrossRef]
- Idrees, A.; Qadir, Z.A.; Akutse, K.S.; Afzal, A.; Hussain, M.; Islam, W.; Waqas, M.S.; Bamisile, B.S.; Li, J. Effectiveness of entomopathogenic fungi on immature stages and feeding performance of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Insects 2021, 12, 1044. [Google Scholar] [CrossRef]
- Abbas, H.K.; Mulrooney, J.E. Effect of some phytopathogenic fungi and their metabolites on growth of Heliothis virescens (F.) and its host plants. Biocontrol Sci. Technol. 1994, 4, 77–87. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, V.; Kaur, A.; Kaur, S. Insecticidal potential of an endophytic fungus, Cladosporium uredinicola, against Spodoptera litura. Phytoparasitica 2013, 41, 373–382. [Google Scholar] [CrossRef]
- Shaker, N.O.; Ahmed, G.M.M.; El-Sawy, M.M.; El-Sayed Ibrahim, H.Y.; Abd El-Rahman Ismail, H.N. Isolation, characterization and insecticidal activity of methylene chloride extract of Cladosporium cladosporioides secondary metabolites against Aphis gossypii (Glov.). J. Plant Prot. Pathol. 2019, 10, 115–119. [Google Scholar] [CrossRef]
- Bensaci, O.A.; Rouabah, K.; Aliat, T.; Lombarkia, N.; Plushikov, V.G.; Kucher, D.E.; Dokukin, P.A.; Temirbekova, S.K.; Rebouh, N.Y. Biological pests management for sustainable agriculture: understanding the influence of Cladosporium-bioformulated endophytic fungi application to control Myzus persicae (Sulzer, 1776) in potato (Solanum tuberosum L.). Plants 2022, 11, 2055. [Google Scholar] [CrossRef] [PubMed]
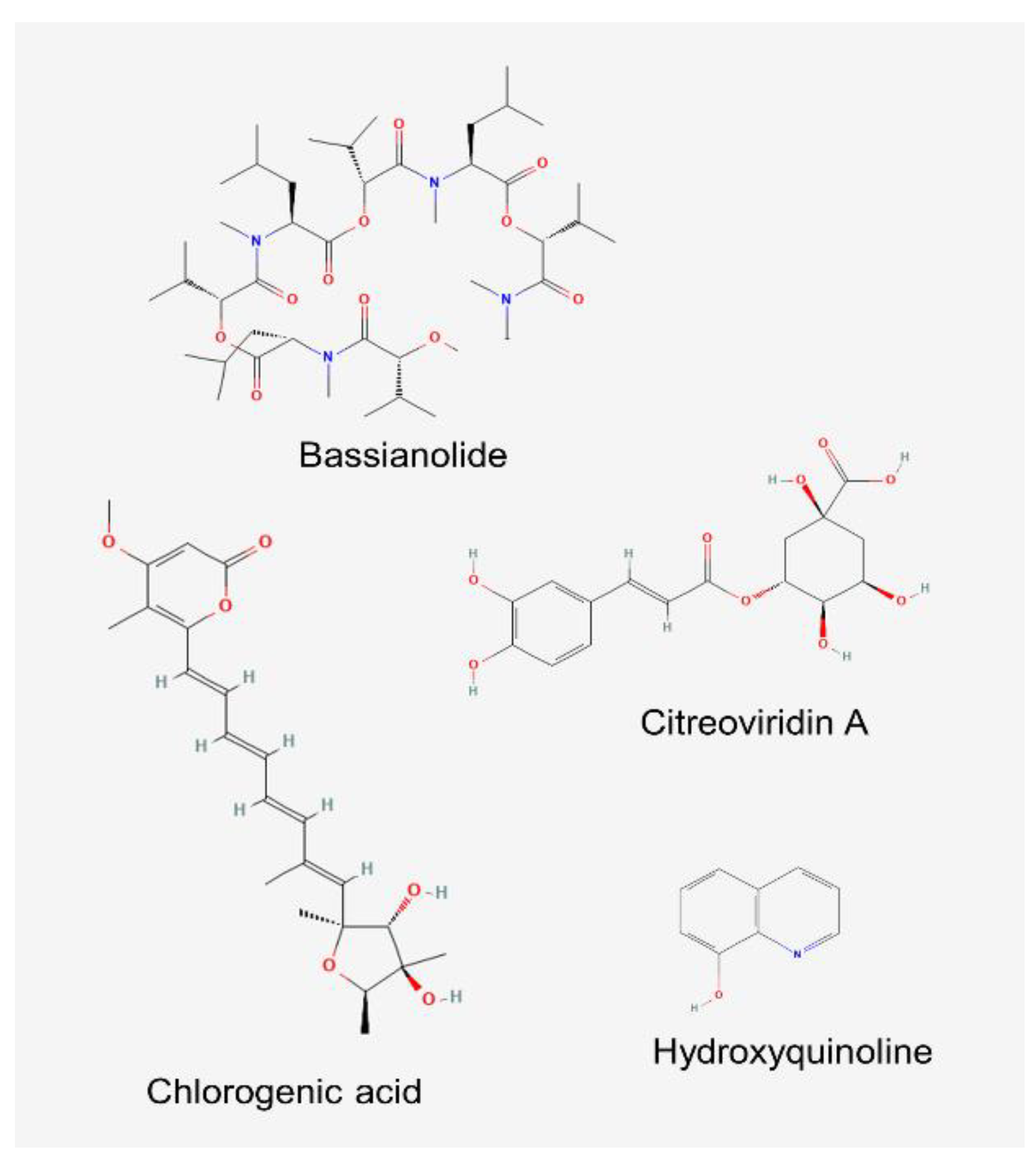
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, K.; Rajabpour, A.; Parizipour, M.H.G.; Yarahmadi, F. Insecticidal bioactive compounds derived from Cladosporium cladosporioides (Fresen.) GA de Vries and Acremonium zeylanicum (Petch) W. Gams & HC Evans. J. Plant Dis. Prot. 2023, 130, 543–549. [Google Scholar]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V. ; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): potential mode of action for phenolic compoundsin plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, T.; Kaur, S.; Manhas, R.K.; Kaur, A. An alpha-glucosidase inhibitor from an endophytic Cladosporium sp. with potential as a biocontrol agent. Appl. Biochem. Biotechnol. 2015, 175, 2020–2034. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, T.; Kaur, S.; Manhas, R.K.; Kaur, A. Insecticidal potential of an endophytic Cladosporium velox against Spodoptera litura mediated through inhibition of alpha glycosidases. Pesticide Biochem. Physiol. 2016, 131, 46–52. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F. among the entomopathogenic fungus, Cladosporium uridenicolla Speg., and two whiteflies beneficial insects, Eretmocerus mundus Mercert and Coccienlla undecimpunctata L.: from an IPM prospective. J. Agr. Sci. Mansoura Univ. 2005, 30, 4217–4236. [Google Scholar] [CrossRef]
- Shankar Naik, B. Functional roles of fungal endophytes in host fitness during stress conditions. Symbiosis 2019, 79, 99–115. [Google Scholar] [CrossRef]
- Nicoletti, R.; Beccaro, G.L.; Sekara, A.; Cirillo, C.; Di Vaio, C. Endophytic fungi and ecological fitness of chestnuts. Plants 2021, 10, 542. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Afolabi, O.G.; Siddiqui, J.A.; Xu, Y. Endophytic insect pathogenic fungi-host plant-herbivore mutualism: elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 2023, 39, 326. [Google Scholar] [CrossRef]
- Picciotti, U.; Araujo Dalbon, V.; Ciancio, A.; Colagiero, M.; Cozzi, G.; De Bellis, L.; Finetti-Sialer, M.M.; Greco, D.; Ippolito, A.; Lahbib, N.; et al. “Ectomosphere”: Insects and microorganism interactions. Microorganisms 2023, 11, 440. [Google Scholar] [CrossRef]
- Thakur, A.; Kaur, S.; Kaur, A.; Singh, V. Enhanced resistance to Spodoptera litura in endophyte infected cauliflower plants. Environ. Entomol. 2013, 42, 240–246. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, V.; Kaur, A.; Kaur, S. Suppression of cellular immune response in Spodoptera litura (Lepidoptera: Noctuidae) larvae by endophytic fungi Nigrospora oryzae and Cladosporium uredinicola. Ann. Entomol. Soc. America 2014, 107, 674–679. [Google Scholar] [CrossRef]
- Gange, A.C.; Eschen, R.; Wearn, J.A.; Thawer, A.; Sutton, B.C. Differential effects of foliar endophytic fungi on insect herbivores attacking a herbaceous plant. Oecologia 2012, 168, 1023–1031. [Google Scholar] [CrossRef]
- Santamaria, B.; Verbeken, A.; Haelewaters, D. Mycophagy: A global review of interactions between invertebrates and fungi. J. Fungi 2023, 9, 163. [Google Scholar] [CrossRef]
- Mills, J.T.; Sinha, R.N. Interactions between a springtail, Hypogastrura tullbergi, and soil-borne fungi. J. Econ. Entomol. 1971, 64, 398–401. [Google Scholar] [CrossRef]
- Walsh, M.I.; Bolger, T. Effects of diet on growth and reproduction of some Collembola in laboratory cultures. Pedobiologia 1990, 34, 161–171. [Google Scholar] [CrossRef]
- Harasymek, L.; Sinha, R.N. Survival of springtails Hypogastrura tullbergi and Proisotoma minuta on fungal and bacterial diets. Environ. Entomol. 1974, 3, 965–968. [Google Scholar] [CrossRef]
- Imai, T.; Miyamoto, Y. The developmental parameters of the minute brown scavenger beetle Dienerella argus (Coleoptera: Latridiidae). Appl. Entomol. Zool. 2019, 54, 75–78. [Google Scholar] [CrossRef]
- Sinha, R.N. Fungus as food for some stored product insects. J. Econ. Ent. 1971, 64, 3–6. [Google Scholar] [CrossRef]
- Kao, S.S.; Dunkel, F.V.; Harein, P.K. Behavioral response of the larger black flour beetle (Coleoptera: Tenebrionidae) to olfactory cues from food sources. J. Econ. Entomol. 1984, 77, 110–112. [Google Scholar] [CrossRef]
- David, M.H.; Mills, R.B.; Sauer, D.B. Development and oviposition of Ahasverus advena (Waltl) (Coleoptera, Silvanidae) on seven species of fungi. J. Stored Prod. Res. 1974, 10, 17–22. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef]
- Kostovcik, M.; Bateman, C.; Kolarik, M.; Stelinski, L.; Jordal, B.; Hulcr, J. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J. 2015, 9, 126–138. [Google Scholar] [CrossRef]
- Joseph, R.; Keyhani, N.O. Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 2021, 105, 3393–3410. [Google Scholar] [CrossRef]
- Brown, E.S. Xyleborus morstatti Hag. (Coleoptera, Scolytidae) a shot-hole borer attacking avocado pear in the Seychelles. Bull. Entomol. Res. 1954, 45, 707–710. [Google Scholar] [CrossRef]
- Kobayashi, C.; Fukasawa, Y.; Hirose, D.; Kato, M. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol. Ecol. 2008, 22, 711–722. [Google Scholar] [CrossRef]
- Li, X.; Wheeler, G.S.; Ding, J. A leaf-rolling weevil benefits from general saprophytic fungi in polysaccharide degradation. Arthropod-Plant Interact. 2012, 6, 417–424. [Google Scholar] [CrossRef]
- Lawson, S. P.; Christian, N.; Abbot, P. Comparative analysis of the biodiversity of fungal endophytes in insect-induced galls and surrounding foliar tissue. Fungal Divers. 2014, 66, 89–97. [Google Scholar] [CrossRef]
- Lasota, J.A.; Waldvogel, M.G.; Shetlar, D.J. Fungus found in galls of Adelges abietis (L.) (Homoptera: Adelgidae): identification, within-tree distribution, and possible impact on insect survival. Environ. Entomol. 1983, 12, 245–246. [Google Scholar] [CrossRef]
- Lebel, T.; Peele, C.; Veenstra, A. Fungi associated with Asphondylia (Diptera: Cecidomyiidae) galls on Sarcocornia quinqueflora and Tecticornia arbuscula (Chenopodiaceae). Fungal Divers. 2012, 55, 143–154. [Google Scholar] [CrossRef]
- Munoz-Benavent, M.; Perez-Cobas, A.E.; Garcia-Ferris, C.; Moya, A.; Latorre, A. Insects’ potential: understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Peng, C.; Liu, H.; Hu, M.; Zhong, G. Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS ONE 2012, 7, e47205. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Hu, M.; Hu, Q.; Luo, J.; Li, Y. Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS One 2012, 7, e38137. [Google Scholar] [CrossRef]
- Bisht, J.; Harsh, N.S.K.; Palni, L.M.S.; Agnihotri, V.; Kumar, A. Bioaugmentation of endosulfan contaminated soil in artificial bed treatment using selected fungal species. Bioremed. J. 2019, 23, 196–214. [Google Scholar] [CrossRef]
- Malassigné, S.; Valiente Moro, C.; Luis, P. Mosquito mycobiota: an overview of non-entomopathogenic fungal interactions. Pathogens 2020, 9, 564. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Lu, X. Diversity and functional roles of the gut microbiota in Lepidopteran insects. Microorganisms 2022, 10, 1234. [Google Scholar] [CrossRef]
- Berasategui, A.; Jagdale, S.; Salem, H. Fusarium phytopathogens as insect mutualists. PLoS Pathogens 2023, 19, e1011497. [Google Scholar] [CrossRef]
- Nicoletti, R.; Becchimanzi, A. Ecological and molecular interactions between insects and fungi. Microorganisms 2022, 10, 96. [Google Scholar] [CrossRef]
- Eken, C.; Hayat, R. Preliminary evaluation of Cladosporium cladosporioides (Fresen.) de Vries in laboratory conditions, as a potential candidate for biocontrol of Tetranychus urticae Koch. World J. Microbiol. Biotechnol. 2009, 25, 489–492. [Google Scholar] [CrossRef]
- Saranya, S.; Ramaraju, K.; Jeyarani, S.; Roseleen, S.S.J. Natural epizootics of Cladosporium cladosporioides on Tetranychus urticae Koch. (Acari: Tetranychidae) in Coimbatore. J. Biol. Control 2013, 27, 95–98. [Google Scholar]
- Torres, D.E.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Guevara-Fefer, P.; Márquez-Guzmán, G.J.; Pérez-Martínez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, e0170782. [Google Scholar] [CrossRef]
- Barge, E.G.; Leopold, D.R.; Rojas, A.; Vilgalys, R.; Busby, P.E. Phylogenetic conservatism of mycoparasitism and its contribution to pathogen antagonism. Mol. Ecol. 2022, 31, 3018–3030. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- Okyere, A.A. Food safety management of insect-based foods. In Food Safety Management, 2nd ed.; Andersen, V., Lelieveld, H., Motarjemi, Y., Eds.; Academic Press, 2023; pp. 223–233. [Google Scholar]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current knowledge on the microbiota of edible insects intended for human consumption: A state-of-the-art review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef]
- Evans, N.M.; Shao, S. Mycotoxin metabolism by edible insects. Toxins 2022, 14, 217. [Google Scholar] [CrossRef]
- Bisconsin-Junior, A.; Fonseca Feitosa, B.; Lopes Silva, F.; Barros Mariutti, L.R. Mycotoxins on edible insects: Should we be worried? Food Chem. Toxicol. 2023, 177, 113845. [Google Scholar] [CrossRef]
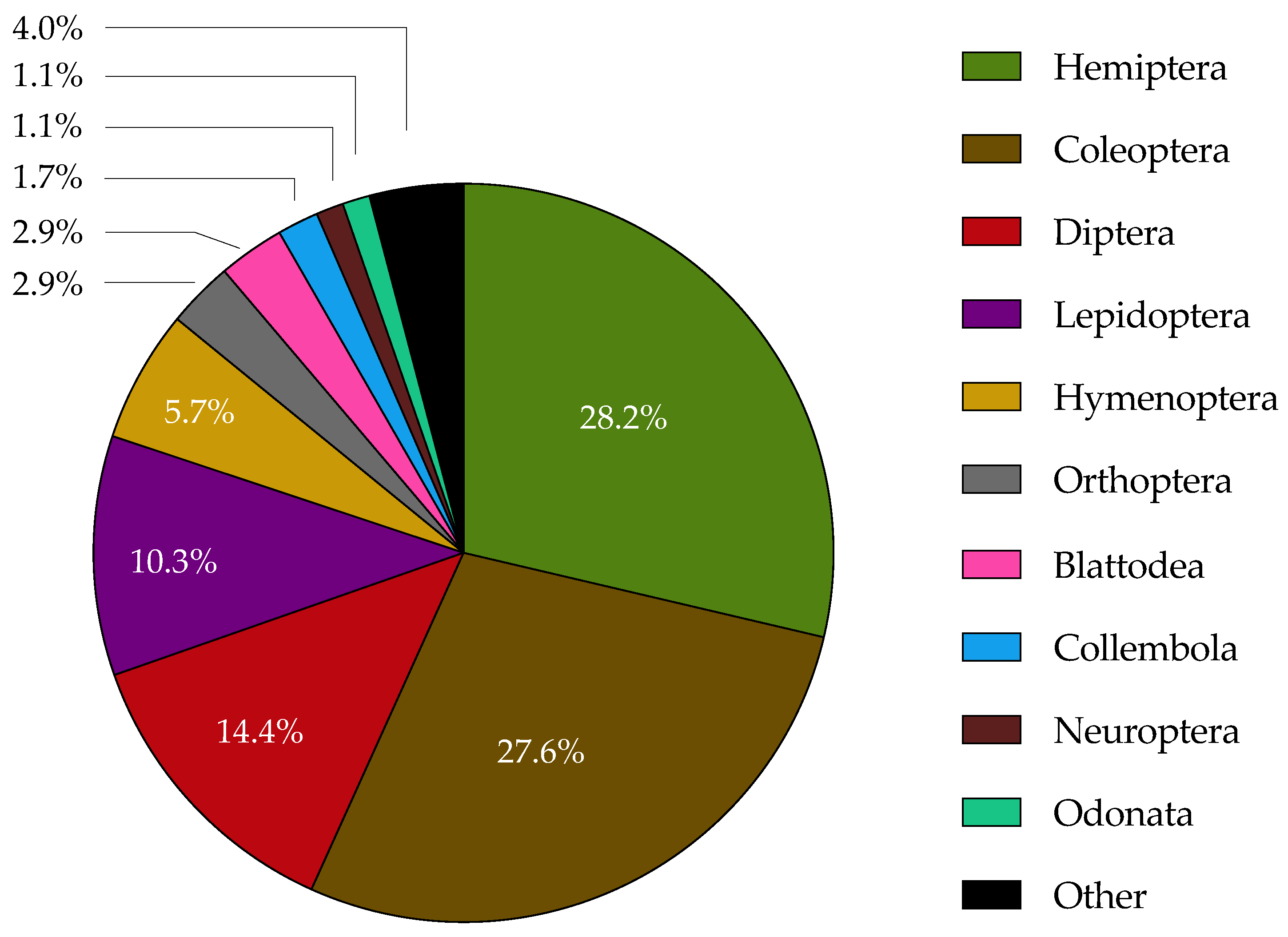
| Cladosporium species | Insect species* | Location | Reference |
|---|---|---|---|
| C. aggregatocicatricatum | Dryocosmus kuriphilus | Monti Cimini (Italy) | [7] |
| Xylosandrus compactus | Circeo promontory (Italy) | [8] | |
| C. anthropophilum | Drosophila suzukii | Maryland (USA) | [9] |
| C. aphidis | unidentified aphid | Piracicaba (São Paulo, Brazil) Berlin; Brandenburg (Germany) Parma (Italy) Wilmington (Delaware, USA) |
[1] |
| Aphis gossypii | Puerto Rico | ||
| Aphis sp. | Berlin (Germany) | ||
| Aphis symphyti | Klosterneuburg (Austria) | ||
| Brevicoryne brassicae | Assiut (Egypt) | [10] | |
| Rhopalosiphum maidis | Honolulu (Hawaii, USA) | [1] | |
| unidentified scale | Essen (Germany) | ||
| C. austrohemisphaericum | X. compactus | Circeo promontory (Italy) | [8] |
| C. chasmanthicola | Spodoptera frugiperda | Kansas (USA) | [11] |
| C. cladosporioides | Aleurothrixus aepim | Brazil | [12] |
| Aphis craccivora | Egypt | [13] | |
| Apion ulicis | Christchurch area (New Zealand) | [14] | |
| Apis mellifera | Assiut governatorate (Egypt) | [15] | |
|
Asphondylia micromeriae
Asphondylia nepetae |
Isle of Vivara (Italy) Averno; Matera (Italy) |
[16] | |
|
Atta capiguara Atta laevigata |
Botucatu (São Paulo, Brazil) | [17] | |
| Bemisia spp. | Dakahlia governorate (Egypt) | [18] | |
| Brachypeplus glaber | Florida (USA) | [19] | |
| B. brassicae | Dakahlia governorate (Egypt) Assiut (Egypt) |
[20] [10] |
|
| Ceratovacuna lanigera | Anakapalle (Andhra Pradesh, India) | [5] | |
| Chitaura brachyptera | Dumoga-Bone (Sulawesi, Indonesia) | [21] | |
| Chrysomphalus aonidum | Qualubia (Egypt) | [22] | |
| Chrysomya megacephala | Ludhiana (Punjab, India) | [23] | |
| Chrysoperla rufilabris | Monroe; Oktibbeha (Mississippi, USA) | [24] | |
| unidentified lignicolous Coleoptera | Ås (Norway) | [25] | |
| Coptotermes formosanus | New Orleans (Louisiana, USA) | [26] | |
| Culex pipiens | Qena governatorate (Egypt) | [27] | |
| Culex quinquefasciatus | Basrah (Iraq) | [28] | |
| Cydia ulicetana | Christchurch area (New Zealand) | [14] | |
| Desoria albella | Warren Woods (Michigan, USA) | [29] | |
| Diaphorina citri | Florida (USA) | [30] | |
| unidentified Diptera | Coimbra (Portugal) | [31] | |
| D. suzukii | Maryland (USA) Geneva (New York, USA) |
[9] [32] |
|
| Dytiscus marginalis | East Anatolia (Turkey) | [33] | |
| Epiphyas postvittana | Christchurch area (New Zealand) | [14] | |
| Ericerus pela | Kunming (China) | [34] | |
| Euphalerus clitoriae | Pernambuco (Brazil) | [35] | |
| Euwallacea interjectus | Hiroshima prefecture (Japan) | [36] | |
| Galleria mellonella | Northern China Beijing (China) |
[37] [38] |
|
| Gromphadorhina portentosa | Columbus (Ohio, USA) | [39] | |
| unspecified grasshoppers | Ulu-Endau (Malaysia) | [21] | |
| Hermetia illucens | Giessen (Germany) | [40] | |
| Heteraphorura subtenuis | Alberta (Canada) | [41] | |
| Homalodisca vitripennis | Southern California (USA) | [42] | |
| Hydrophilus piceus | East Anatolia (Turkey) | [33] | |
| unidentified dead insect | Thailand | [43] | |
| Ips sexdentatus | Northeastern Ukraine | [44] | |
| Kermes sp. | Guangdong (China) | [45] | |
| Lycorma delicatula | Berks county (Pennsylvania, USA) | [46] | |
| Megaplatypus mutatus | Bragado (Argentina) | [47] | |
| Mesambria maculipes | Dumoga-Bone (Sulawesi, Indonesia) | [21] | |
| Myzus persicae | Salah El-Din governatorate (Iraq) | [48] | |
| Nilaparvata lugens | Gazipur (Bangladesh) | [49] | |
| Odontotermes formosanus | Jinhua (China) | [50] | |
| Pantala flavescens | Jinhua (China) | [51] | |
| Pityogenes bidentatus | Babimost; Mielec; Opole (Poland) | [52] | |
| Prays oleae | Mirandela-Bragança region (Portugal) | [53] | |
| Pterostichus melanarius | Assiut governatorate (Egypt) | [15] | |
| unidentified Pyrrhocoridae | Lebanon | [54] | |
| Rhynchophorus ferrugineus | Assiut governatorate (Egypt) | [15] | |
| Scolytogenes birosimensis | Central and Western Japan | [55] | |
| Sericothrips staphylinus | Christchurch area (New Zealand) | [14] | |
| Sitophilus oryzae | Multan (Pakistan) | [56] | |
| unidentified Thysanura | Coimbra (Portugal) | [31] | |
| Tomicus piniperda | Mościska forest (Poland) | [57] | |
|
Triatoma brasiliensis Triatoma pseudomaculata |
Rio de Janeiro (Brazil) | [58] | |
| Triatoma infestans | Mendoza; Salta; Santa Fe (Argentina) | [59] | |
| Troglophilus neglectus | Tolmin (Slovenia) | [60] | |
| C. cucumerinum | unidentified Diptera unidentified Thysanura |
Coimbra (Portugal) | [31] |
| C. cycadicola | Tribolium castaneum | Iksan (South Korea) | [61] |
| C. delicatulum | Thrips sp. | Hamedan (Iran) | [62] |
| C. dominicanum | X. compactus | Circeo promontory (Italy) | [8] |
| C. endophyticum | Stenochironomus sp. | Adolpho Ducke reserve (Amazonas); Lajeado state park (Tocantins, Brazil) | [63] |
| C. exasperatum | Nasutitermes octopilis | Nouragues nature reserve (French Guiana) | [64] |
| C. exile | Aphis sp. | Somesara (Iran) | [62] |
| C. halotolerans | G. mellonella | Napoli area (Italy) | [65] |
| Stenochironomus sp. | Lajeado state park (Tocantins, Brazil) | [63] | |
| Thitarodes xiaojinensis | Xiaojin (China) | [66] | |
| T. castaneum | Sangju (South Korea) | [61] | |
| C. herbarum | Aleurodicus cocois | Recife (Brazil) | [67] |
| A. gossypii | Latvia | [68] | |
| A. ulicis | Christchurch area (New Zealand) | [14] | |
| A. mellifera | Assiut governatorate (Egypt) | [15] | |
| B. brassicae | Assiut (Egypt) | [10] | |
| C. ulicetana | Christchurch area (New Zealand) | [14] | |
| D. marginalis | East Anatolia (Turkey) | [33] | |
| E. postvittana | Christchurch area (New Zealand) | [14] | |
| H. subtenuis | Alberta (Canada) | [41] | |
| H. piceus | East Anatolia (Turkey) | [33] | |
| Musca domestica | Seropedica (Rio de Janeiro, Brazil) | [69] | |
| Gauteng province (South Africa) | [70] | ||
| P. bidentatus | Babimost (Poland) | [52] | |
| Pseudopsis subulata | Montmorency forest (Canada) | [71] | |
| S. staphylinus | Christchurch area (New Zealand) | [14] | |
| Tenebrio molitor | Aarhus (Denmark) | [72] | |
| T. piniperda | Mościska forest (Poland) | [57] | |
|
T. brasiliensis T. pseudomaculata |
Rio de Janeiro (Brazil) | [58] | |
| C. iranicum | unidentified scale | Iran | [1] |
| C. kenpeggii | Stenochironomus sp. | Adolpho Ducke reserve (Amazonas); Lajeado state park (Tocantins, Brazil) | [63] |
| Triplectides sp. | Lajeado state park (Tocantins, Brazil) | [73] | |
| C. langeronii | D. kuriphilus | Monti Cimini (Italy) | [7] |
| E. pela | Kunming (China) | [34] | |
| C. macrocarpum | H. subtenuis | Alberta (Canada) | [41] |
| C. oxysporum |
Anoplolepis custodiens Aonidiella aurantii |
Eastern Transvaal (South Africa) | [74] |
| A. gossypii | Mataffin (South Africa) | [75] | |
| A. mellifera | Assiut governatorate (Egypt) | [15] | |
| C. lanigera | Anakapalle (Andhra Pradesh, India) | [5] | |
| C. aonidum | Eastern Transvaal (South Africa) | [74] | |
| Hypothenemus hampei | El Tizal (Mexico) | [76] | |
| unidentified Muscidae | Eastern Transvaal (South Africa) | [74] | |
| Planococcus citri | Mataffin (South Africa) | [75] | |
| P. oleae | Mirandela–Bragança region (Portugal) | [53] | |
| Pseudococcus longispinus | Eastern Transvaal (South Africa) | [74] | |
| P. melanarius | Assiut governatorate (Egypt) | [15] | |
|
Pulvinaria aethiopica Toxoptera citricidus Trioza erytreae |
Eastern Transvaal (South Africa) | [74] | |
| C. perangustum | X. compactus | Grottammare (Italy) | [77] |
| C. pini-ponderosae | unidentified Diptera | Coimbra (Portugal) | [31] |
| C. pseudocladosporioides | Eulophid parasitoid | Averno; Napoli (Italy) | [16] |
| D. suzukii | Maryland (USA) | [9] | |
| C. ramotenellum | A. nepetae | Napoli (Italy) | [16] |
| C. sphaerospermum | Bradysia impatiens | South Korea | [78] |
| B. brassicae | Assiut (Egypt) | [10] | |
| D. kuriphilus | Monti Cimini (Italy) | [7] | |
| E. pela | Kunming (China) | [34] | |
| unidentified grasshoppers | Ulu-Endau (Malaysia) | [21] | |
| H. vitripennis | Southern California (USA) | [42] | |
| H. subtenuis | Alberta (Canada) | [41] | |
| Imbrasia belina | Francistown (Botswana) | [79] | |
| M. maculipes | Dumoga-Bone (Sulawesi, Indonesia) | [21] | |
| Macrotermes barneyi | Guangdong (China) | [80] | |
| Onychiurus pseudofimetarius | Auckland (New Zealand) | [1] | |
|
P. melanarius R. ferrugineus |
Assiut governatorate (Egypt) | [15] | |
| S. birosimensis | Central and Western Japan | [55] | |
| T. piniperda | Mościska forest (Poland) | [57] | |
| T. infestans | Mendoza; Santa Fe (Argentina) | [59] | |
| T. castaneum | Goseong; Sangju (South Korea) | [61] | |
| Cladosporium sp. | Acmaedora flavolineata | Turkey | [81] |
| Acronicta major | Hangzhou (China) | [82] | |
| Adelges piceae | Gaspé peninsula (Canada) | [83] | |
| Adelges tsugae | Northeastern USA | [84] | |
| Aedes albopictus | Nice; Portes-lès-Valence; Saint Priest (France) Mananjary; Toamasina; Tsimbazaza (Madagascar) Bình Dương; Hồ Chí Minh; Vũng Tàu (Vietnam) Villeurbanne (France) Lubbock (Texas, USA) |
[85] [86] [87] |
|
| Aedes aegypti | Lubbock (Texas, USA) | [87] | |
| Aedes japonicus | Urbana (Illinois, USA) | [88] | |
| Aedes triseriatus | |||
| Agathomyia sp. | Yaoshan Mountain (China) | [89] | |
| Agrilus mali | Yining (China) | [90] | |
| Aleocharinae spp. | Denmark | [91] | |
| Aleurocanthus spiniferus | Southern Anhui (China) | [92] | |
| A. aepim | Bahia (Brazil) | [93] | |
| Alniphagus aspericollis | Greater Vancouver region (Canada) | [94] | |
| Anastrepha fraterculus | Caxias do Sul (Rio Grande do Sul, Brazil) | [95] | |
| Anopheles coluzzii | Koulikoro (Mali) | [96] | |
| Apertochrysa formosanus | Sugadaira-kogen (Japan) | [97] | |
|
A. craccivora Aphis durantae A. gossypii |
Dakahlia governorate (Egypt) | [98] | |
| Apis cerana | Chiang Mai (Thailand) | [99] | |
| A. mellifera | Tucson (Arizona, USA) Assiut governatorate (Egypt) Grugliasco (Italy) |
[100] [15] [101] |
|
| Halle (Germany) Athens (Greece) Lublin (Poland) Chiang Mai (Thailand) London (United Kingdom) |
[102] [99] |
||
| Asphondylia glabrigerminis | Mittagong; Melbourne area (Australia) | [103] | |
| A. micromeriae | Astroni nature reserve (Italy) | [104] | |
| Asphondylia serpylli | Lublin area (Poland) | [105] | |
|
A. capiguara
A. laevigata |
Botucatu (São Paulo, Brazil) | [17,106] | |
| Atomaria spp. | Denmark | [91] | |
| Bactrocera oleae | Calabria (Italy) | [107] | |
| Bactrocera tryoni | New South Wales; Victoria (Australia) | [108] | |
| Bemisia tabaci | Dakahlia governorate (Egypt) | [98] | |
| Bombus terrestris | Beijing (China) | [109] | |
| Bombyx mori | Hangzhou (China) | [82] | |
| B. impatiens | South Korea | [110] | |
| Calliopum aeneum | Denmark | [91] | |
| Callosobruchus maculatus | Columbus; Miami county (Ohio); Erie county (Pennsylvania, USA) | [111] | |
| Carabidae spp. | Uckermark (Germany) | [112] | |
| Carpophilus sp. | Gualmatán (Colombia) | [113] | |
| Ceroplastes floridensis | Mansoura (Egypt) Kiryat Tivon (Israel) |
[22] [114] |
|
| Ceroplastes rusci | Mansoura (Egypt) Larnaca (Cyprus) |
[115] [114] |
|
| Ceroplastes sp. | Malaga (Spain) | [114] | |
| Chalcophora detrita | Turkey | [116] | |
| Coccus hesperidum | Ramat HaShofet (Israel) | [114] | |
| unspecified Coleoptera | Ås (Norway) | [25] | |
| unidentified Collembola | Warren Woods (Michigan, USA) | [29] | |
| Colletes cunicularius | Ave-et-Auffe (Belgium) | [117] | |
| Cortinicara gibbosa | Denmark | [91] | |
| Crocothemis servilia | Hefei (China) | [118] | |
| Ctenolepisma longicaudatum | Wien (Austria) | [119] | |
| C. pipiens | San Francisco; San Rafael (California, USA) | [120] | |
| C. quinquefasciatus | Nakhon Nayok (Thailand) Mar del Plata (Argentina) |
[121] [122] |
|
| Cydia pomonella | Austria | [123] | |
| Cytilus sericeus | Ostrava (Czechia) | [124] | |
| Diabrotica sp. | Gualmatán (Colombia) | [113] | |
| Diabrotica virgifera | Deutsch-Jahrndorf (Germany) | [125] | |
| Diaphania pyloalis | Hangzhou (China) | [82] | |
| unidentified Diptera | Warren Woods (Michigan, USA) | [29] | |
| Dolichovespula sylvestris | Havreballe forests (Denmark) | [126] | |
| D. suzukii | Maryland (USA) | [127] | |
| Drosophilidae spp. Enicmus transversus |
Denmark | [91] | |
| E. pela | Kunming (China) | [3] | |
| Ferrisia virgata | Madurai (India) | [128] | |
| H. vitripennis | Southern California (USA) | [42] | |
| Harmonia axyridis | Hubei (China) | [129] | |
| Helicoverpa armigera | Narrabri; New South Wales (Australia) | [130] | |
| H. hampei | Chiapas (Mexico) El Tizal (Mexico) |
[131] [76] |
|
| Illiciomyia yukawai | Mie prefecture (Japan) | [132] | |
| Ips acuminatus | Libavá (Czechia) | [133] | |
|
Ips cembrae Ips duplicatus |
Rouchovany (Czechia) | ||
| I. sexdentatus | Rouchovany (Czechia) Northeastern Ukraine |
[133] [44] |
|
| Ips typographus | Rouchovany (Czechia) | [133] | |
| unidentified Lepidoptera | Coimbra (Portugal) | [31] | |
| Liparthrum colchicum | Migliarino natural park (Italy) | [134] | |
| Loberus impressus | Iberia Parish (Louisiana, USA) | [135] | |
| Lonchodes brevipes | Singapore | [136] | |
| Lonchoptera spp. | Denmark | [91] | |
| Lutzomyia sp. | Antioquia department (Colombia) | [137] | |
| Lymantria dispar asiatica | Harbin (China) | [138] | |
| Macrosaccus robiniella | Transylvania (Romania) | [139] | |
| Marchalina hellenica | Ischia (Italy) | † | |
| Matsumurasca onukii | various regions in China | [140] | |
| Meiltaea cinxia | Eckerö; Sund (Finland) | [141] | |
| Milviscutulus mangiferae | Upper Galilee (Israel) | [114] | |
|
Minettia fasciata Minettia longipennis Minettia plumicornis |
Denmark | [91] | |
| M. domestica | Ahwaz (Iran) | [142] | |
| Gauteng province (South Africa) | [70] | ||
| Bruxelles (Belgium) Butare (Rwanda) |
[143] | ||
|
Neobathyscia mancinii Neobathyscia pasai |
Damati cave (Italy) Tana delle Sponde cave (Italy) |
[144] | |
| N. lugens | Hangzhou (China) | [145] | |
| Orchelimum vulgare | Houston (Texas, USA) | [146] | |
| Orthoperus brunnipes | Denmark | [91] | |
| Orthotomicus erosus | Italian harbors, on imported wood | [147] | |
| Ostrinia nubilalis | Andau (Germany) | [125] | |
| Paracoccus marginatus | Madurai (India) | [128] | |
| Parasaissetia nigra | Kiryat Tivon (Israel) | [114] | |
| Paravespula vulgaris | Havreballe forests (Denmark) | [126] | |
| Parectopa robiniella | Transylvania (Romania) | [139] | |
| Phlebotomus papatasi | Tehran (Iran) | [148] | |
| Phlebotomus spp. | Tunisia | [149] | |
| Phylloicus sp. | Adolpho Ducke reserve (Amazonas, Brazil) | [150] | |
| Pieris brassicae | Bornem (Belgium) Randwijk; Wageningen (Netherlands) |
[151] | |
| Planococcus ficus | Gholan heights (Israel) | [152] | |
| Poecilocerus pictus | Chennai (India) | [153] | |
| Polygraphus polygraphus | Rouchovany (Czechia) | [133] | |
| Probergrothius angolensis | Namibia | [154] | |
| Pseudatomoscelis seriatus | College Station (Texas, USA) | [155] | |
| unidentified Psocoptera | Warren Woods (Michigan, USA) | [29] | |
| Psylliodes attenuata | Daqing, Harbin, Changchun, Qujing (China) | [156] | |
| P. oleae | Mirandela–Bragança region (Portugal) | [53] | |
| P. melanarius | Assiut governatorate (Egypt) | [15] | |
| Pulvinaria aurantii | Northern Iran | [157] | |
| Pulvinaria psidii | Mansoura (Egypt) | [22] | |
| Pulvinaria tenuivalvata | Dakahlia governorate (Egypt) | [158] | |
| R. ferrugineus | Assiut governatorate (Egypt) | [15] | |
| Saissetia sp. | Larnaca (Cyprus) | [114] | |
| Sapromyza quadripunctata | Denmark | [91] | |
| Scolypopa australis | Nelson (New Zealand) | [159] | |
| S. birosimensis | Central and Western Japan | [55] | |
| S. frugiperda | Guangzhou (China) Kansas (USA) |
[160] [12] |
|
| Stenochironomus sp. | Adolpho Ducke reserve (Amazonas); Lajeado state park (Tocantins, Brazil) | [63,150] | |
|
Stephostethus lardarius Stilbus testaceus |
Denmark | [91] | |
| Taeniothrips inconsequens | Northeastern USA | [84] | |
| T. molitor | Aarhus (Denmark) Shenyang (China) |
[72] [161] |
|
| Thaumastocoris peregrinus | South-east Uruguay | [162] | |
| unidentified Thysanura | Coimbra (Portugal) | [31] | |
| Toumeyella parvicornis | Campania (Italy) | [163] | |
| Trialeurodes ricini | Dakahlia governorate (Egypt) | [98] | |
|
T. brasiliensis T. pseudomaculata |
Rio de Janeiro (Brazil) | [58] | |
| T. castaneum | South Korea | [61] | |
| Triplectides sp. | Lajeado state park (Tocantins, Brazil) | [73] | |
| Xyleborinus saxesenii | Italian harbors, on imported wood Northeastern Italy South Florida (USA) |
[147] [164] [165] |
|
|
Xyleborus bispinatus Xyleborus volvulus |
South Florida (USA) | [165] | |
| X. compactus | Central Florida (USA) Migliarino natural park (Italy) |
[166] [134] |
|
| Xylosandrus crassiusculus | South Florida (USA) | [165] | |
| Xylosandrus germanus | Northeastern Italy | [164] | |
| C. subtilissimum | A. capiguara | Botucatu (São Paulo, Brazil) | [17] |
| C. subuliforme | D. citri | Guilin (China) | [167] |
| unidentified Streblidae | Furna do Morcego (Pernambuco, Brazil) | [168] | |
| Triplectides sp. | Lajeado state park (Tocantins, Brazil) | [73] | |
| C. tenuissimum | M. mutatus | Bragado (Argentina) | [47] |
| M. persicae | Salah El-Din governatorate (Iraq) | [48] | |
| unidentified Thysanura | Coimbra (Portugal) | [31] | |
| Trachymela sloanei | Guangdong (China) | [169] | |
| C. uredinicola | A. gossypii | Dakahlia governorate (Egypt) | [170] |
| Bemisia spp. | Dakahlia governorate (Egypt) | [18] | |
| B. tabaci | Dakahlia governorate (Egypt) | [170] | |
| C. velox | B. oleae | Calabria (Italy) | [107] |
| T. castaneum | Mungyeong; Sangju (South Korea) | [61] | |
| C. verrucocladosporioides | Triplectides sp. | Lajeado state park (Tocantins, Brazil) | [73] |
| C. xanthocromaticum | M. persicae | Salah El-Din governatorate (Iraq) | [48] |
| Cladosporium species | Source | Insect targets | Country | Reference |
|---|---|---|---|---|
| C. cladosporioides | Bemisia sp. | Bemisia sp. | Egypt | [18] |
| Brevicoryne brassicae | B. brassicae | Egypt | [20] | |
| Culex quinquefasciatus | C. quinquefasciatus | Iraq | [28] | |
| endophytic | Duponchelia fovealis | Brazil | [188] | |
| Kermes sp. | Hemiberlesia pitysophila | China | [45] | |
| Lycorma delicatula | Tenebrio molitor | United States | [46] | |
| Myzus persicae | M. persicae | Iraq | [48] | |
| Nilaparvata lugens | Bemisia tabaci | Bangladesh | [49] | |
| Pulvinaria aurantii | Aphis fabae | Iran | [157] | |
| Sitophilus oryzae |
Rhyzopertha dominica Sitophilus zeamais Trogoderma granarium |
Pakistan | [56] | |
| soil | Metopolophium dirhodum | Egypt | [189] | |
| C. oxysporum | endophytic | A. fabae | Algeria | [190] |
| endophytic | Chilo partellus | India | [191] | |
| Planococcus citri |
Pseudococcus longispinus Pulvinaria aethiopica Toxoptera citricida Trioza erytreae |
South Africa | [75] | |
| unknown | Aphis craccivora | India | [192] | |
| C. sphaerospermum | endophytic | D. fovealis | Brazil | [188] |
| Cladosporium sp. | Helicoverpa armigera |
Aphis gossypii B. tabaci H. armigera |
Australia | [130] |
| Spodoptera frugiperda | S. frugiperda | China | [169] | |
| Cladosporium spp. | several species of sap-sucking Hemiptera |
A. craccivora A. gossypii B. tabaci |
Egypt | [98,193] |
| C. subuliforme | Diaphorina citri | D. citri | China | [167] |
| C. tenuissimum | M. persicae | M. persicae | Iraq | [48] |
| Trachymela sloanei | S. frugiperda | China | [194] | |
| C. uredinicola |
A. gossypii B. tabaci |
A. gossypii B. tabaci |
Egypt | [170] |
| Bemisia sp. | Bemisia sp. | Egypt | [18] | |
| C. xanthocromaticum | M. persicae | M. persicae | Iraq | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





