1. Introduction
Despite a decrease in the incidence and mortality of lung cancer over the past two decades and even with increasing therapeutic options, the morbidity and mortality remain substantial [
1]. Lung cancer is the second most diagnosed cancer in the United States and is estimated to cause 127,070 deaths in 2023 [
2]. The diagnosis carries a significant impact on patients, their caregivers, and their quality of life [
3]. Additionally, the economic burden of lung cancer was estimated to be 12.12 billion dollars in 2010 and is likely to rise with the advent of newer treatments like immunotherapy [
4]. Thus, optimizing resources with improved diagnostic, prognostic, and therapeutic options are critical.
Lung cancer is broadly characterized into Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (NSCLC), with NSCLC making up 85% of cases. NSCLC contains multiple subtypes with the most common histology being adenocarcinoma, squamous cell carcinoma, and large cell carcinoma in that order [
5]. Morbidity and mortality vary between types of lung cancer and stage of diagnosis. Despite its high initial response rate to chemotherapy, SCLC has an 18- and 9-month median survival time for limited and extensive disease, respectively [
6]. On the other hand, NSCLC has a better prognosis dependent upon stage with a 5-year survival rate of 68.4% for stage I and 5.8% for stage IV [
7].
Galectins are a subfamily of lectin proteins capable of binding β-galactoside glycoconjugates through a conserved carbohydrate recognition domain (CRD) [
8]. Galectins are categorized by structure into three subgroups. Galectin-1, -2, -5, -7, -10, -11, -13, -14, and -15 make up the prototypical galectins that contain a sole CRD and form non-covalently linked dimers [
9]. Galectin-3 is the sole member of the chimeric subgroup of galectins and has profibrotic and proinflammatory modulating macrophage activity in various tissue types [
10]. The final subgroup consists of tandem-repeat galectins that have two distinct CRDs and includes galactin-4, -6, -8, -9 and -12 [
11,
12]. Due to the variety of structures, galectins can bind a wide range of ligands intra- and extracellularly, involving multiple cellular pathways including adhesion, aggregation, angiogenesis, apoptosis, autophagy, growth, and metastasis [
13,
14,
15].
The biochemical functions of galectins are of interest in oncology with galectin-1, -3, -4, -7, -8 and -9 being the most extensively studied in lung cancer [
16]. Galectin-1 has been shown to promote lung cancer metastasis by increasing levels of NOTCH and its ligand, Jagged2, while enhancing AKT activation, promoting tumorigenesis and invasiveness, and is associated with a poor prognosis in lung adenocarcinoma [
17,
18]. Knockdown of galectin-3 has been shown to decrease tumor initiation, aggressiveness and chemoresistance to cisplatin and paclitaxel in lung adenocarcinoma as well as being involved with β-catenin that may contribute to the maintenance of lung cancer stem cells [
19]. Finally, galectin-9 on tumor infiltrating lymphocytes in NSCLC has been shown to correlate with TIM-3, as well as the NSCLC drug targets PD-1 and PD-L1 upregulating IFNβ and γ to decrease lung cancer apoptosis [
20,
21].
Due to the relationship between galectins and their role in lung cancer, there have been multiple studies that examined galectin expression and levels using immunohistochemistry (IHC) and enzyme-linked immunosorbent assays (ELISAs) methodology, respectively. High galectin-1 IHC expression was associated with poor clinical outcome in NSCLC patients [
22]. Additionally, galectin-3 IHC expression is decreased in SCLC when compared to NSCLC, and studies have demonstrated that higher serum and tumor levels of galectin-3 in NSCLC are associated with lymph node metastasis and tumor recurrence [
23,
24,
25]. Lastly, high IHC expression of galectin-9 on tumor-infiltrating lymphocytes in surgically resected SCLC correlated with improved recurrence free survival [
26].
Thus, this study aims to build upon existing research utilizing ELISA to analyze the concentrations of galectin-1, -3, and -9 in patient serum based upon various stages and histologic subtypes of lung cancer. To our knowledge, this is the first study to retrospectively review lung cancer treatments administered prior to the assessment of galectin levels, allowing us to explore the influence of lung cancer treatment on galectin levels. Finally, the study examines galectin levels in relationship to metastasis and probes the potential prognostic ability of galectin-1 in surgically resectable NSCLC irrespective of prior treatment status.
2. Results
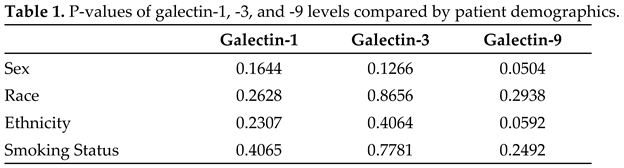
2.1. Patient characteristics do not correlate with galectin-1, -3, and -9 levels
Serum galectin concentrations were compared to the following demographic characteristics: sex, race, ethnicity, and smoking status. Sex was defined as female and male. Race included Black/African American, Hawaiian/Pacific Islander, Multi-racial, and White. Ethnicity included non-Spanish/non-Hispanic and Spanish/Hispanic. Smoking status was described as previous, current, or never. There were no statistically significant differences in galectin-1, -3, -9 concentrations based upon patient demographics with associated
p-values presented in Table 1.
 |
|
1 P-values of serum galectins by patient demographics. Sex includes female and male. Race includes Black/African American, Hawaiian/Pacific Islander, Multi-racial, and White. Ethnicity included non-Spanish/non-Hispanic and Spanish/Hispanic. Smoking status was described by patients themselves and included those who never smoked, current smokers and previous smokers. Two tailed t-test was used for two group comparisons: sex and ethnicity. One-way Anova was utilized for multiple group comparisons: race and smoking status. |
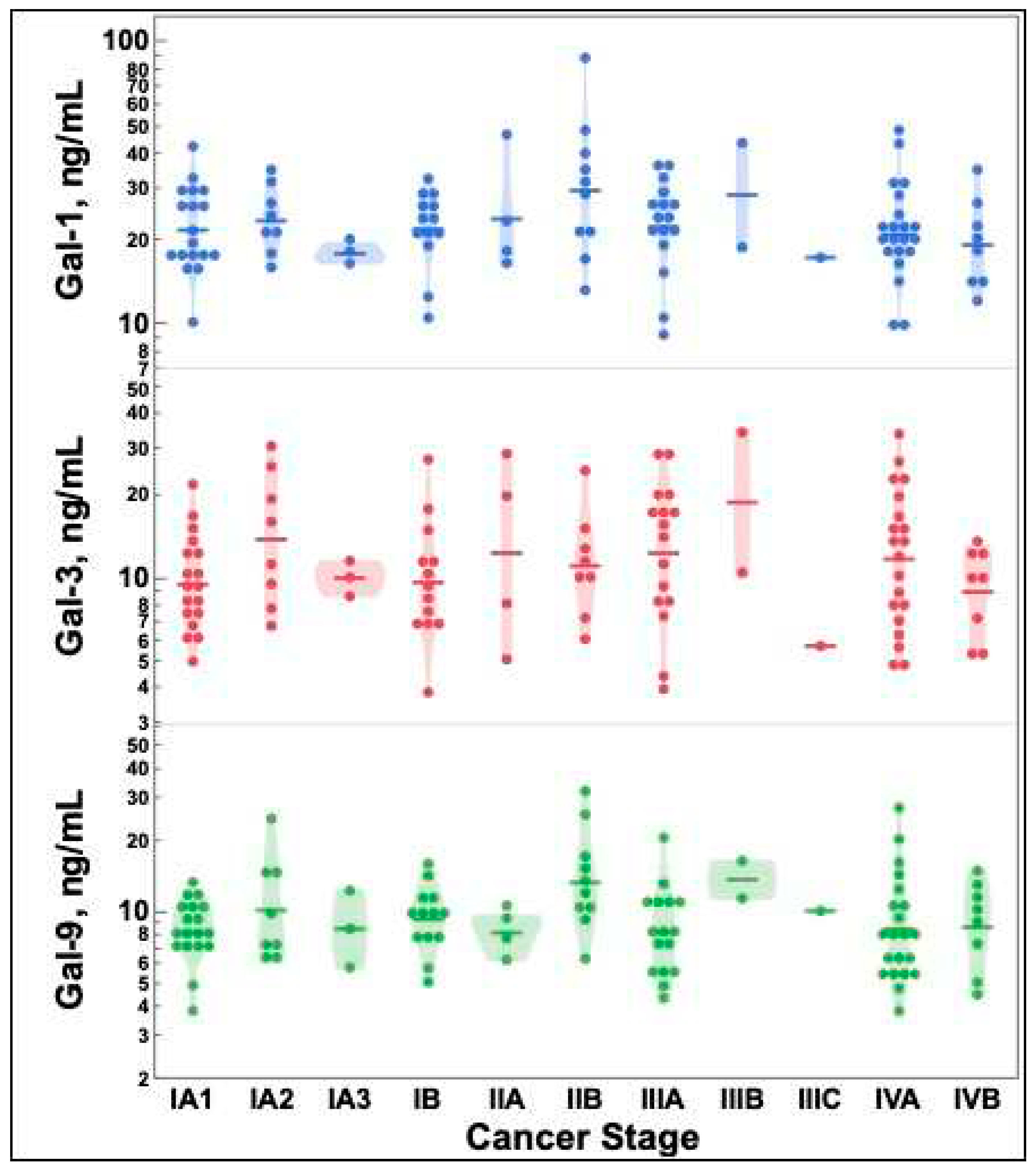
2.2. Galectin-1, -3, and -9 levels do not differ by lung cancer stage
There were no statistically significant differences between galectin-1, -3, and -9 concentrations based on lung cancer stage as depicted in
Figure 1.
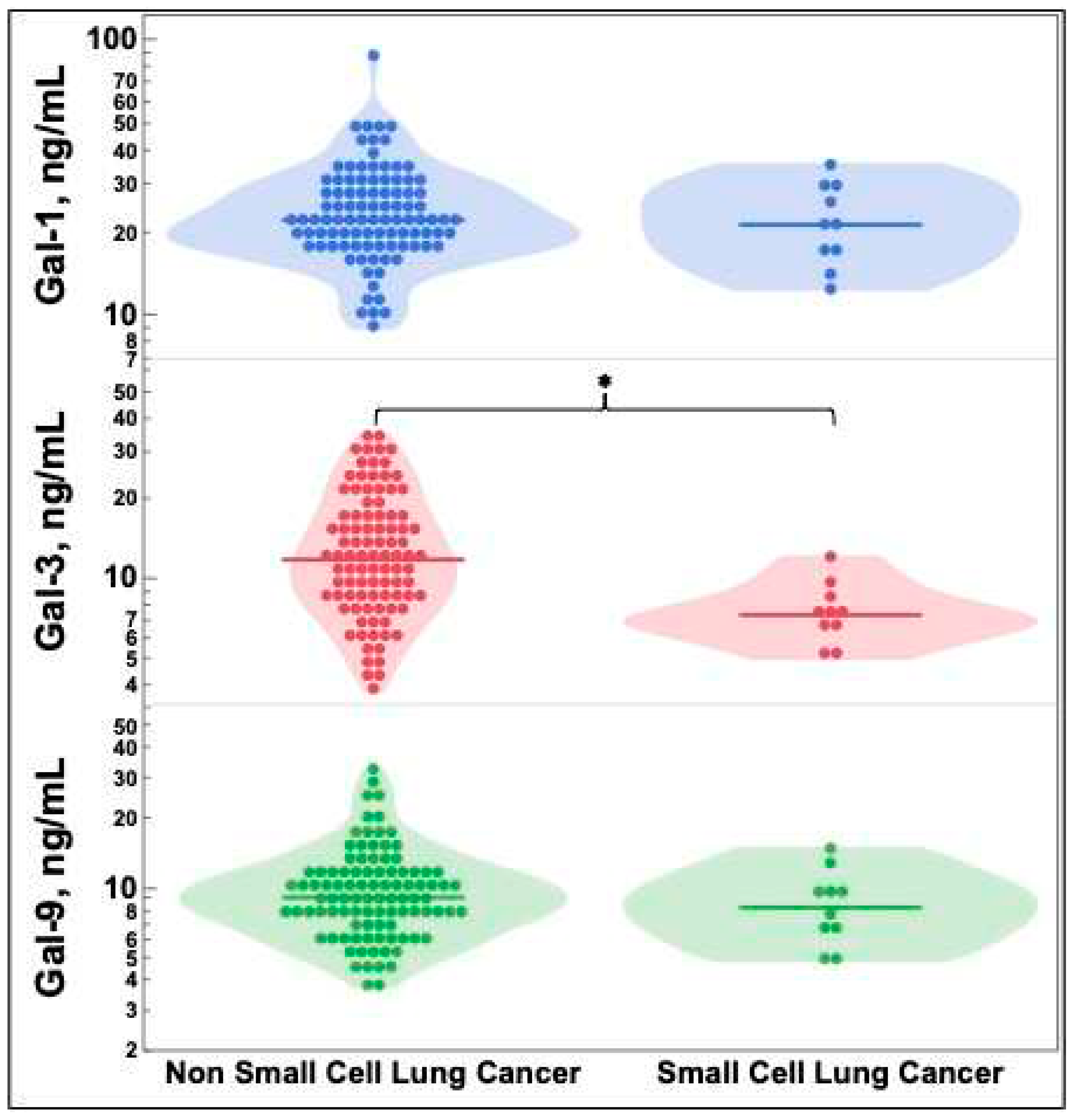
2.3. Galectin-3 levels vary by lung cancer histology
Figure 2 depicts galectin levels by lung cancer histology. SCLC had statistically significantly decreased galectin-3 levels compared to NSCLC. There were no significant differences in galectin-1 or -9.
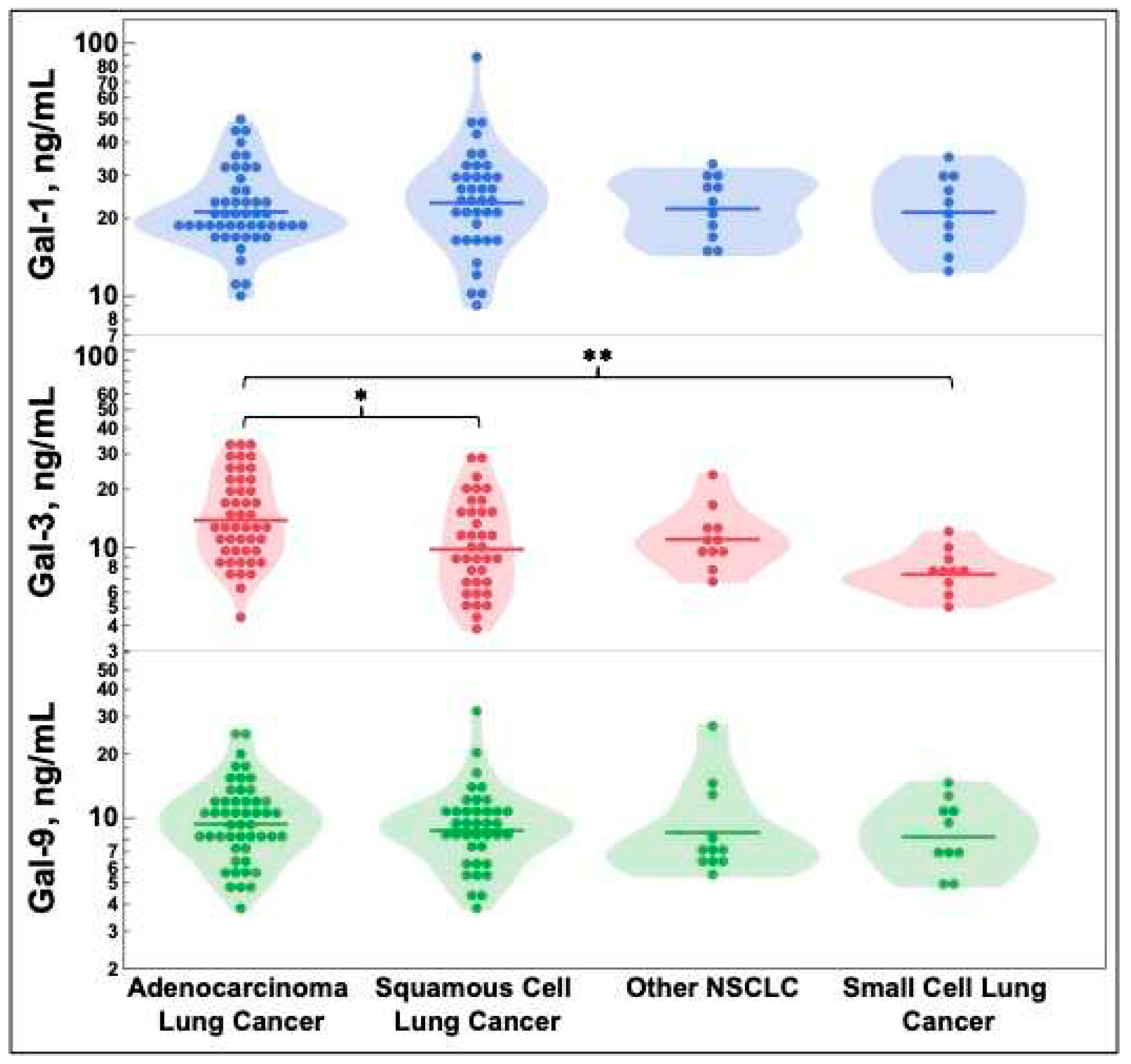
NSCLC was further delineated by histologic subtype of squamous cell carcinoma, adenocarcinoma, and other. Other histology included large cell carcinoma, spindle cell carcinoma, and other rarer histologic subtypes to increase this groups’ sample size. Galectin levels were compared for these three groups and SCLC in
Figure 3. SCLC and squamous cell carcinoma had statistically significantly decreased galectin-3 levels when compared to adenocarcinoma.
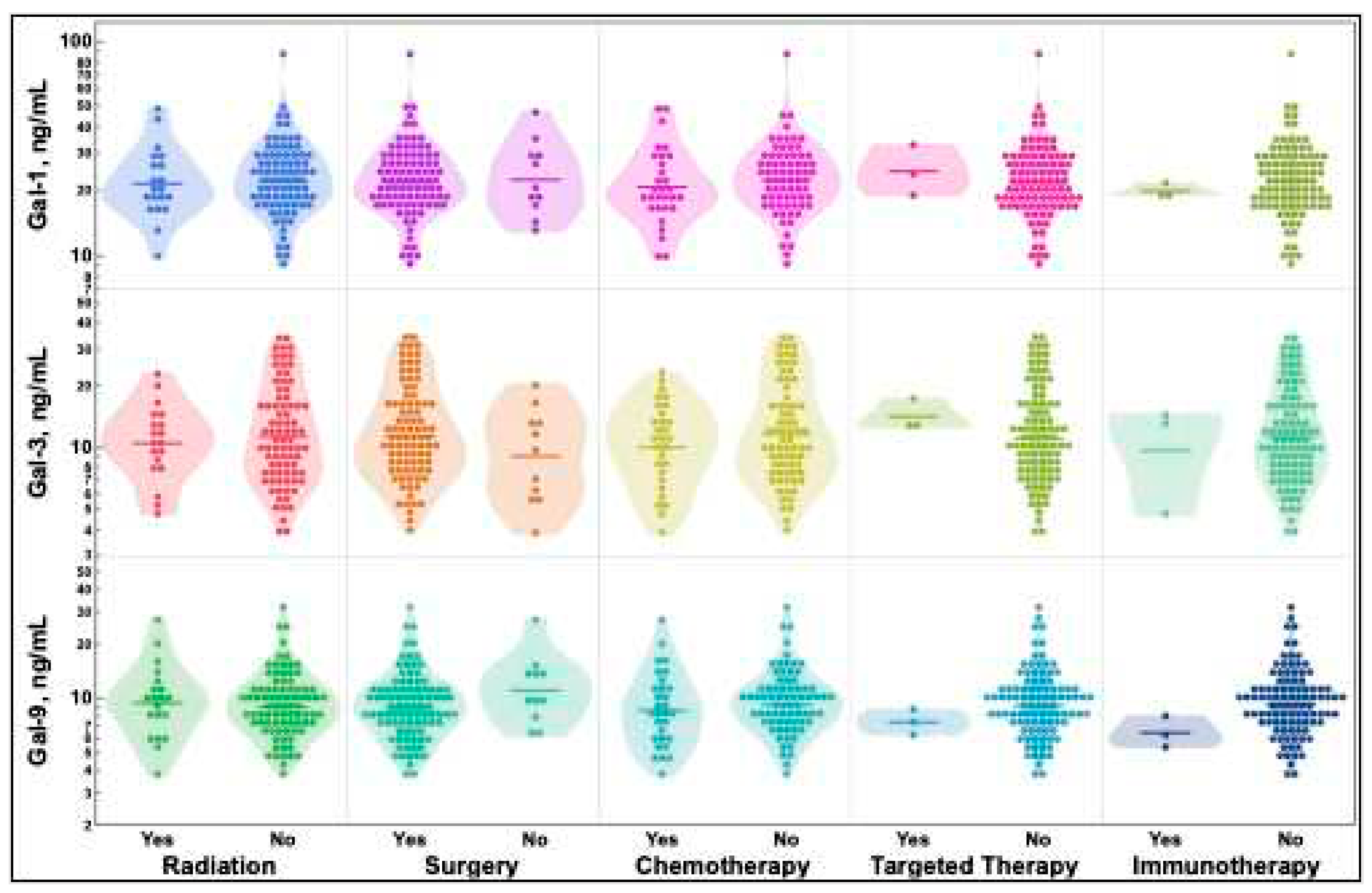
2.4. Previous treatment status does not alter galectin-1, -3, and -9 levels
Treatments patients may have received before sample acquisition were identified and stratified by type as radiation, surgery, chemotherapy, targeted therapy, and immunotherapy. Subsequent treatments that patients received for their lung cancer were recorded and stratified the same way as additional or secondary cancers to identify potential lung cancer exposure to treatment. All treatments that patients received before and after sample acquisition are available in the
supplemental data file. As depicted in
Figure 4, there were no differences in galectin-1, -3, or -9 levels regardless of the treatments received prior to sample acquisition.
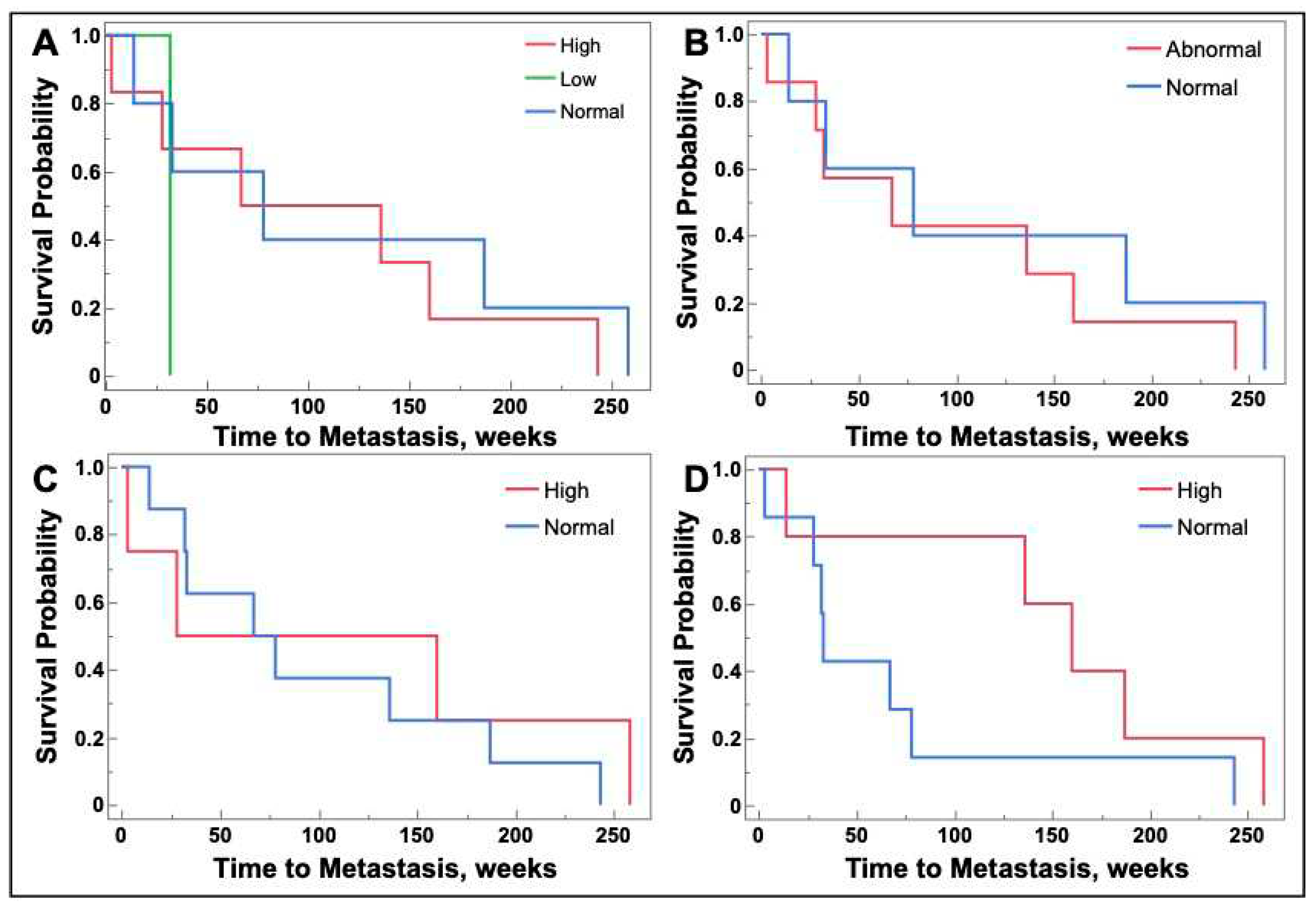
2.5. Galectin-1, -3, and -9 levels are not associated with future metastasis
Patients with stage I, II, or III lung cancer at baseline were monitored until the end of the study to determine if they developed metastatic disease. In patients that developed metastatic disease, time to metastasis was determined in weeks from time of sample procurement. The date of metastasis was determined from radiographic imaging or pathology, if obtained, and subsequent physician documentation. There was no association between serum galectin levels obtained at lung cancer tissue collection and the development of future metastasis (
Figure 5).
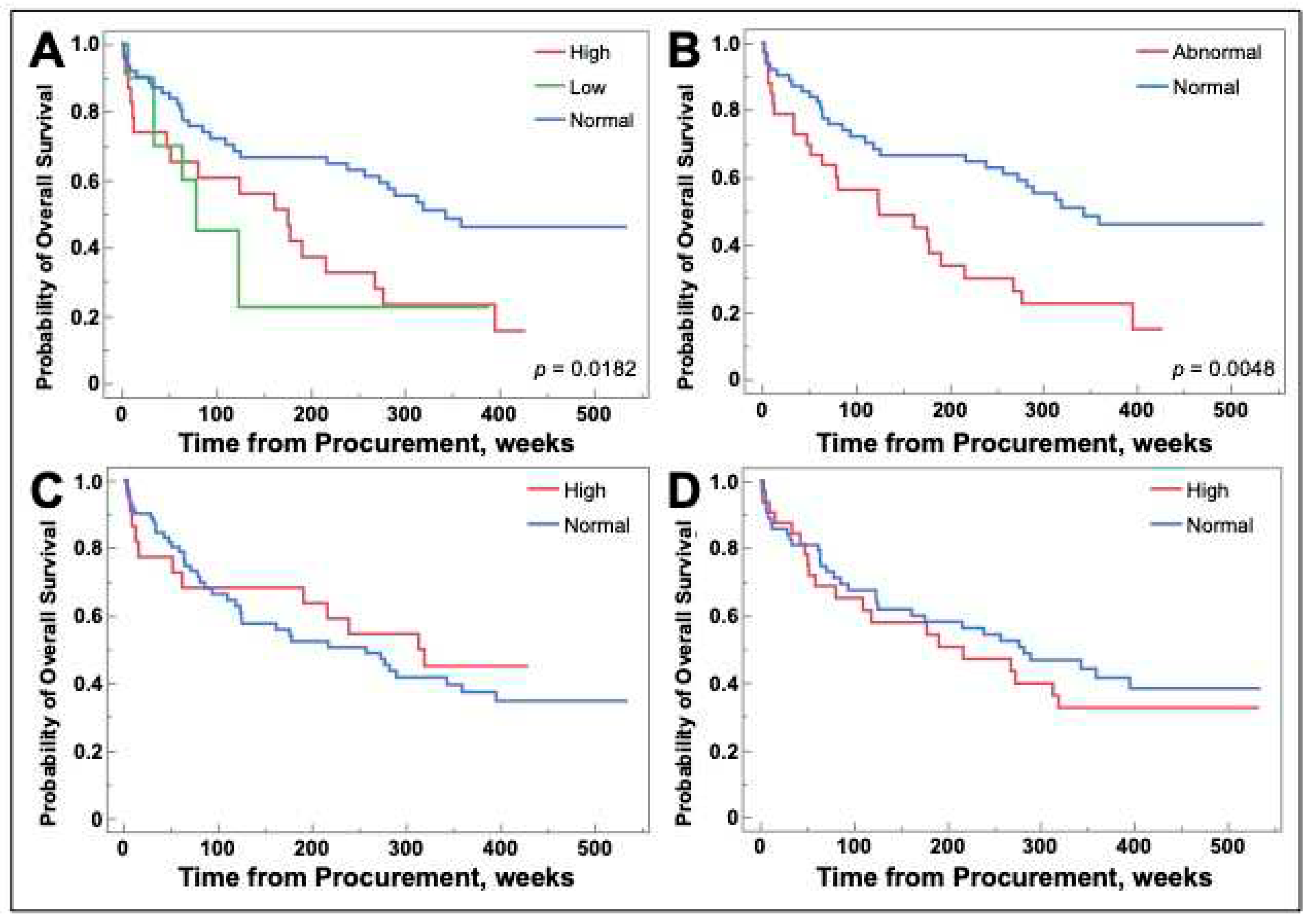
2.6. Galetin-1 may be a prognostic biomarker for select populations of NSCLC
Patient survival was measured in weeks from sample procurement to death or end of study duration. Patients with galectin-1 levels that were high, low, or a combination of those two groups called abnormal had decreased overall survival (
Figure 6A and B). There were no differences in survival for patients with high levels of galectins-3 and -9 (
Figure 6C and D).
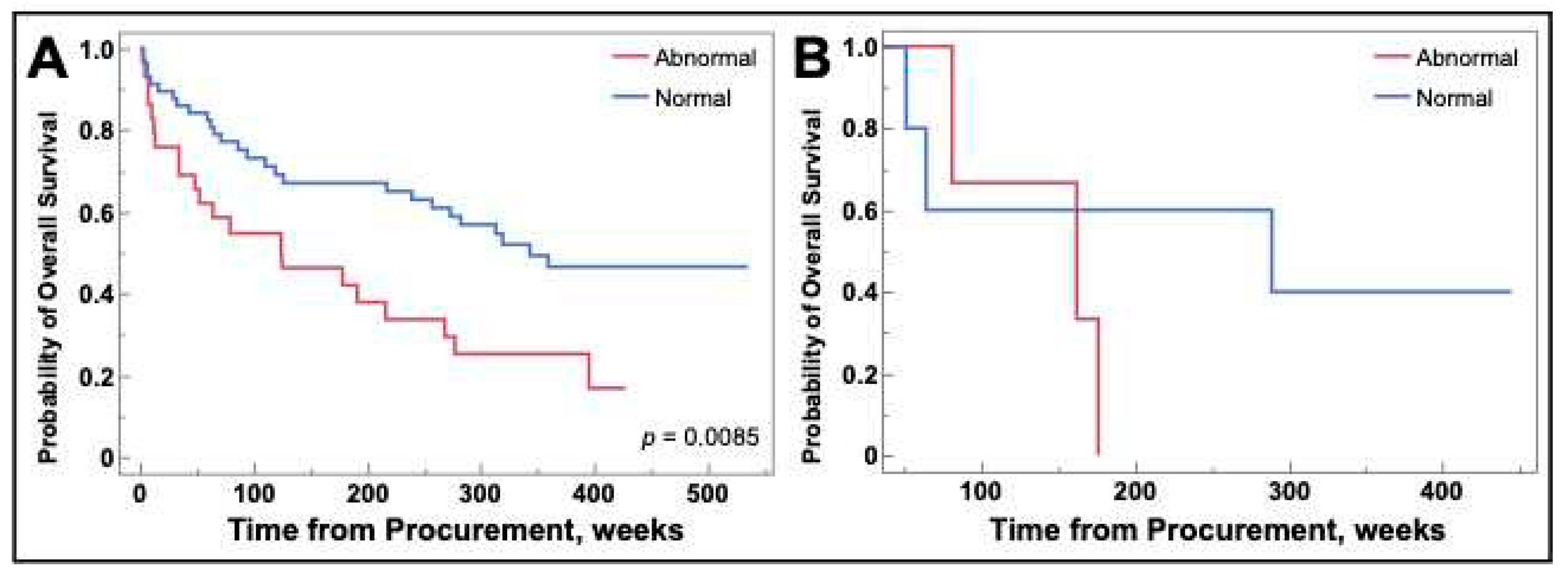
As there was a difference in survival for galectin-1, we performed subsequent analysis grouped by NSCLC and SCLC.
Figure 7 demonstrates that the survival difference remained for NSCLC (
Figure 7A) and was not observed for SCLC (
Figure 7B).
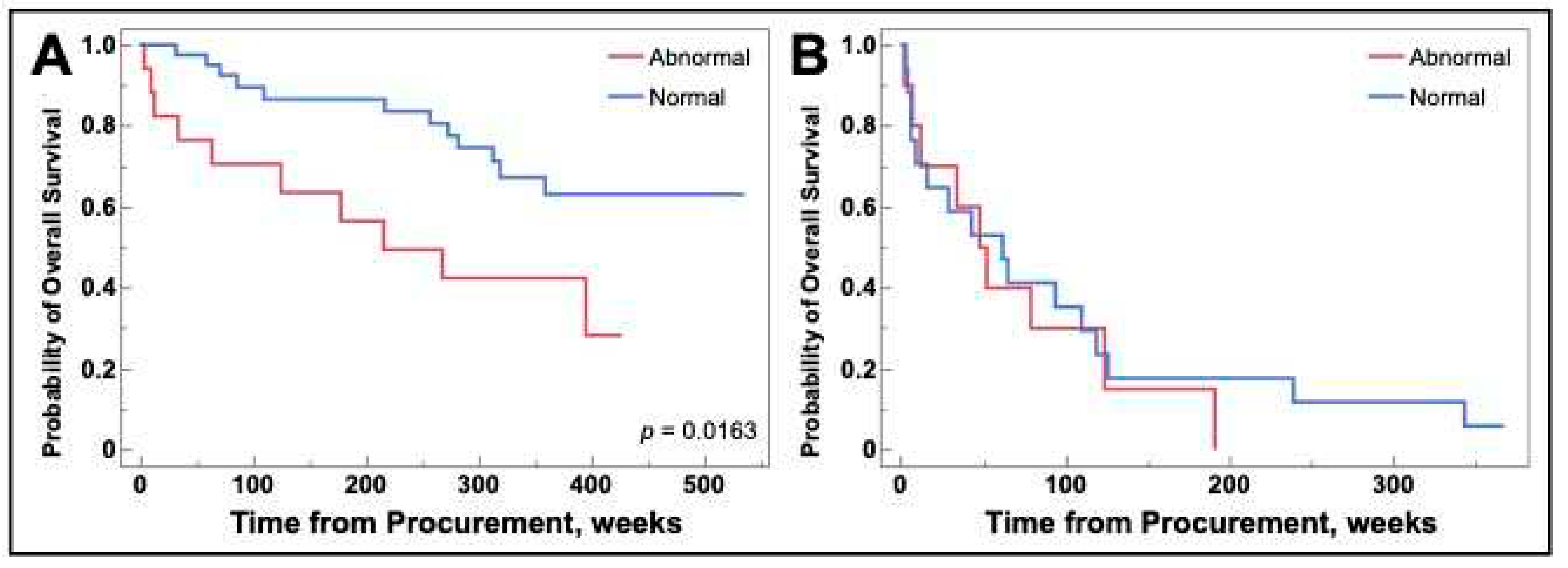
As the difference remained for NSCLC, we performed a subsequent analysis grouped by NSCLC versus SCLC, surgically resectable lung cancer versus non-resectable lung cancer, and curative versus palliative intent. Curative versus palliative intent was added as there was one unresectable patient treated curatively and two resectable patients undergoing palliative therapy. This stratification allows the population to be more generalizable to the overall lung cancer population as surgically resectable disease is most often performed in those treated with curative intent [
33,
34]. Surgically resectable NSCLC patients being treated curatively that had abnormal galectin-1 levels demonstrated decreased overall survival. There were no survival differences for any other group combinations including NSCLC that was unresectable and treated palliatively as demonstrated in
Figure 8.
3. Discussion
3.1. Findings
There was no difference in galectin-1, -3, and -9 levels by lung cancer stage as depicted in
Figure 1 nor by demographic attributes (Table 1). However, a recent study has shown that galectin-3 expression correlated with squamous cell lung cancer stage. This study also found no significant correlation in galectin expression and demographic variables [
35]. Therefore, it is possible that galectin expression may be increased in distinct types of lung cancer, but it does not appear to correlate with quantifiable galectin-1, -3, and -9 tumor levels as seen in our study.
Figure 2 depicts the decreased galectin-3 level measured in SCLC versus NSCLC. Comparable results have been demonstrated utilizing tissue IHC [
23]. However, this is the first example of this finding being replicated using ELISA of patient serum. Furthermore, there is decreased galectin-3 in both squamous cell carcinoma and SCLC when compared to adenocarcinoma as seen in
Figure 3. The differences in galectin-3 levels among squamous cell carcinoma, adenocarcinoma, and SCLC may have future clinical treatment implications as galectin-3 inhibitors are currently in development [
36,
37]. It is possible that response rates to galectin-3 inhibitors may differ dependent on galectin-3 levels with our data suggesting that adenocarcinoma may be the most likely to respond based upon its elevated level. We conjecture that it may be similar to how PD-L1 positivity predicts response to pembrolizumab therapy (albeit imperfectly), as previous trials have utilized different cutoffs in NSCLC [
38,
39,
40]. This may influence future trial design and examination of lung cancer subtypes as squamous cell carcinoma, non-squamous cell carcinoma (including adenocarcinoma), and SCLC are currently treated with different regimens [
41,
42,
43,
44,
45].
Previously, radiation of Lewis lung carcinoma in mice has demonstrated an increase in galectin-1 secretion, and galectin-1 levels in breast cancer have been shown to increase after treatment [
46,
47]. However, there were no differences in galectin levels regardless of treatments given before lung cancer sample collection as seen in
Figure 4. This is of importance as this is the first trial to examine galectin levels in response to all lung cancer treatment modalities in humans.
Our study did not identify an association between galectin-1, -3 and -9 levels at time of sample collection and time to metastatic disease. Earlier studies have demonstrated that elevated levels by ELISA or increasing expression of galectin-3 by IHC correlates with metastasis [
24,
35]. However, this trend was not identified in our data as seen in
Figure 5.
Abnormal galectin-1 level was associated with decreased overall survival as depicted in
Figure 6b with no difference for galectins-3 and -9 in Figures 6c and 6d, respectively. The negative finding with galectin-3 and positive finding with galectin-1 correlates with previous trials [
22,
35,
48]. However, these findings were further expanded upon with subsequent analysis highlighting that the difference stemmed from patients with NSCLC (
Figure 7A) versus SCLC (
Figure 7B). Finally, patients with surgically resected NSCLC treated curatively retained a survival difference (
Figure 8A). This suggests that galectin-1 attained at time of curative surgical resection of NSCLC is prognostic for survival. Validation is warranted as prognostic biomarkers in oncology are crucial for informing treatment decisions and informing patients on prognosis.
Tumor tissue modified viral (TTMV)-HPV DNA is a prognostic biomarker obtained after attempted curative chemotherapy, radiation, and surgical resection for oropharyngeal cancer that can assist in guiding treatment decisions [
49,
50,
51]. Although our trial was preliminary, galectin-1 in surgically resectable NSCLC treated curatively regardless of neoadjuvant treatment was prognostic for overall survival. Further prospective trials could be useful in examining the association of galectin-1 in resectable NSCLC as our preliminary data suggest that galectin-1 may be used similarly to that of TTMV-HPV DNA. It is important to note that TTMV-HPV DNA is a serum test, as was the galectin-1 level obtained in our trial. This allows for galectin-1 levels to be obtained by a non-invasive method, allow for serial assessment of levels to monitor disease activity, and facilitate initiation of chemotherapy, depending upon future trial design, as there is a time window to begin adjuvant chemotherapy in NSCLC [
52].
3.2. Limitations
The total sample size (n=108) is relatively small. This is highlighted when examining lung cancer stage as the Eighth Edition of TNM classification of lung cancer has eleven total groups, translating to small samples in group IIIB (n=2) and group IIIC (n=1). The small sample sizes in these groups may limit the ability to detect a potential difference.
Additionally, within our sample, only 12 patients developed metastatic disease and 29 patients had metastatic disease at time of sample collection. Our sample size included 19 patients with stage III disease, giving a combined total of 48 patients with advanced disease. This does not represent the general population as 75% of lung cancer patients have either stage III or IV disease [
53]. Our study population could be viewed as healthier than the average lung cancer population and may explain the low metastasis rate we observed.
Although we could assess galectin levels regardless of treatments received, serum was only taken once the day of surgery, thus serial galectin levels could not be obtained. This limits the ability to monitor how different lung cancer treatments would impact future galectin levels, or if changes in galectin levels correlated with survival. The relationship between galectin levels after treatment is of interest in oncology, as chemotherapy induced galectin-3 increases had significantly lower rates of recurrence in breast cancer [
54].
4. Materials and Methods
4.1. Patient Samples
Patient tissue and serum was collected from a random and heterogenous sample of 108 patients from 2013-2022 at the Prisma Health Cancer Institute (PHCI) biorepository (Greenville, SC, USA). The standard operating procedures of the PHCI biorepository have been previously described and acknowledged in multiple previous publications [
27,
28,
29,
30]. Among the samples, 98 were from NSCLC patients, and 10 were from SCLC patients. The NSCLC samples consisted of 49 adenocarcinoma, 38 squamous cell, and 11 other NSCLC subtypes, including large cell and spindle cell carcinoma with further details available in the
supplemental data file. 44 samples were categorized as stage I, 16 as stage II, 19 as stage III, and 29 as stage IV lung cancer. The samples were obtained from patients having both resection or biopsy of primary tumors (n=84) and metastatic tissue (n=24). The metastatic samples included 16 brain tissues obtained via craniotomy, 4 lymph node biopsies, 2 chest wall biopsies, and one sample each from spinal cord and mediastinal tissues. Patient serum was obtained in the pre-operative setting regardless of procedure (surgical resection or biopsy).
4.2. Patient information
Patient information was collected from the PHCI database and EPIC®. The PHCI information included demographic (age, race, gender, and smoking status) and tumor data (tissue site, histology, grade, and TNM staging). This was supplemented from information obtained from EPIC® to ensure data concordance. Discrepancies between the PHCI database and EPIC® were rectified through comprehensive chart review in EPIC®. Some samples, procured prior to the transition to EPIC® as the electronic medical record (EMR) in 2015, had missing data.
Treatment, metastasis, and survival data was collected starting on August 1
st, 2023. The data encompassed treatment information including surgery, radiation, chemotherapy, targeted therapy, and immunotherapy exposures before and after tissue sample collection. All treatments, including individual pharmacologic agents and surgery types were recorded if possible and are available in the
supplementary data file.
Consistent with a retrospective sample, some patients had additional primary cancers and were exposed to various treatments before their diagnosis of lung cancer. Their treatment exposure was documented and is available in the
supplementary data file.
In cases where metastasis developed during the trial, time to metastasis was calculated based on the duration in weeks between the date of tissue sample collection and the date of radiographically confirmed metastasis. Subsequent physician documentation of metastasis was verified. Metastases occurring after August 1st, 2023, were not recorded. Similarly, overall survival was calculated in weeks from time of sample procurement to death or survival on August 1st, 2023.
4.3. Galectin Profiling
The galectin levels in the patient’s serum were determined using an ELISA that was described previously and included a subset of the patients in that population [
28]. Galectin-1, -3, and -9 concentrations were obtained using ELISA kits from R&D Systems (Minneapolis, MN, USA) with each sample assayed four times.
4.4. Data Analysis
All statistical analyses were performed using JMP® 17.2.0 software by the SAS Institute (Cary, NC, USA). The distributions of the serum galectin levels were analyzed for normality using q-q plots and histogram visual inspection. Galectin levels are reported in Log
10, ensuring data normality for subsequent parametric analysis. Notably, three samples did not have galectin-3 levels determined, and some tumor, treatment, metastasis, and survival data were missing, as previously described, precluding analysis for those specific tissue samples. Two-group comparisons were conducted using t-test, while multiple comparisons analyzed with one-way ANOVA, followed by Student’s t-test for pairs with ANOVA’s probability > F less than 0.05. Survival analyses were performed for time to metastasis and overall survival by comparing sample galectin levels to controls obtained in previous literature [
28]. Galectin-1 results showed values below, within, and above the normal range (13.90-28.20 ng/mL). Galectin-3 and galectin-9 had values within and above the normal range (2.40-15.70 ng/mL) and (3.10-10.40 ng/mL) respectively. None of the samples evaluated demonstrated low levels of galectin-3 or -9.
For galectin-1, survival analysis was first performed on all three groups and then as a combination of the below and above normal ranges, called abnormal. Survival analysis performed on galectin-3 and -9 included the two groups as described. Data with a p-value less than 0.05 was considered statistically significant and all data are available in the
supplemental data file. Log rank was utilized for time to metastasis and overall survival so that all time points were weighted equally given the availability of ten years of survival data and patients entering this study at different time points.
5. Conclusions
Our study is the first to demonstrate that abnormal galectin-1 is associated with decreased overall survival in NSCLC patients treated by surgical resection with curative intent regardless of neoadjuvant treatment. This demonstrates the potential utility of galectin-1 as a prognostic biomarker and to inform treatment decisions in the NSCLC population.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplemental data file; Investigational Information.
Author Contributions
Conceptualization, Anna Blenda; Data curation, Hayden Shuster; Formal analysis, Hayden Shuster and Avery Funkhouser; Funding acquisition, Julie Martin, William Edenfield and Anna Blenda; Investigation, Hayden Shuster and Anna Blenda; Methodology, Hayden Shuster and Anna Blenda; Project administration, Anna Blenda; Resources, Lorie Allen, Julie Martin, William Edenfield and Anna Blenda; Supervision, Anna Blenda; Validation, Hayden Shuster; Visualization, Hayden Shuster and Avery Funkhouser; Writing – original draft, Hayden Shuster and Avery Funkhouser; Writing – review & editing, Hayden Shuster, Avery Funkhouser, Julie Martin, William Edenfield and Anna Blenda. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Prisma Health Cancer Institute Philanthropy Grant, the Sargent Foundation, and the Transformative Seed Grant Program of the Health Sciences Center at Prisma Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Prisma Health, Committee C (1949871-1 Use of Patient Data from the Prisma Health Center Biorepository for Molecular and Bioinformatics Analysis on 5/30/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data from the Prisma Health Biorepository supporting this study's findings are available in the
supplemental data file of this article. As patient HIPPA protected information, such as dates, were utilized to determine overall survival and time to metastasis, personal identifiable information is not available to individuals not covered by IRB approval.
Acknowledgments
The authors would like to acknowledge the Prisma Health Cancer Biorepository for assistance with sample acquisition, and Alex Kesic and Jonah Shealy for contributing to experimental work. We additionally acknowledge Ginny Simmons for coordinating access to patient information, and Dr. Richard O’Neal for guidance on data analysis and image design.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oliver AL. Lung Cancer: Epidemiology and Screening. Surg Clin North Am. 2022;102(3):335-344. [CrossRef]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. [CrossRef]
- Ellis J. The impact of lung cancer on patients and carers. Chron Respir Dis. 2012;9(1):39-47. [CrossRef]
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. [CrossRef]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. 2019;94(8):1623-1640. [CrossRef]
- Simon GR, Wagner H. Small cell lung cancer. Chest. 2003;123(1):259S−271S. [CrossRef]
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. [CrossRef]
- Cummings RD, Liu FT, Rabinovich GA, Stowell SA, and Vasta GR. Galectins. In: Essentials of Glycobiology [Internet]. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022.
- Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol. 2012;946:21-36. [CrossRef]
- Nangia-Makker P, Hogan V, Balan V, Raz A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J Biol Chem. 2022;298(12):102622. [CrossRef]
- Ko FCF, Yan S, Lee KW, Lam SK, Ho JCM. Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules. 2023;13(6):902. [CrossRef]
- Troncoso MF, Elola MT, Croci DO, Rabinovich GA. Integrating structure and function of 'tandem-repeat' galectins. Front Biosci (Schol Ed). 2012;4(3):864-887. [CrossRef]
- Johannes L, Jacob R, Leffler H. Galectins at a glance. J Cell Sci. 2018;131(9):jcs208884. [CrossRef]
- Newlaczyl AU, Yu LG. Galectin-3--a jack-of-all-trades in cancer. Cancer Lett. 2011;313(2):123-128. [CrossRef]
- Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128(13):2213-2219. [CrossRef]
- Chang WA, Tsai MJ, Kuo PL, Hung JY. Role of galectins in lung cancer. Oncol Lett. 2017;14(5):5077-5084. [CrossRef]
- Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS, Kuo PL. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34(6):1370-1381. [CrossRef]
- Zhou X, Li D, Wang X, Zhang B, Zhu H, Zhao J. Galectin-1 is overexpressed in CD133+ human lung adenocarcinoma cells and promotes their growth and invasiveness. Oncotarget. 2015;6(5):3111-3122. [CrossRef]
- Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY, Sun KH. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with β-catenin. Oncotarget. 2015;6(7):4936-4952. [CrossRef]
- He Y, Jia K, Dziadziuszko R, et al. Galectin-9 in non-small cell lung cancer. Lung Cancer. 2019;136:80-85. [CrossRef]
- Yang R, Sun L, Li CF, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832. [CrossRef]
- Carlini MJ, Roitman P, Nuñez M, et al. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer. 2014;84(1):73-78. [CrossRef]
- Buttery R, Monaghan H, Salter DM, Sethi T. Galectin-3: differential expression between small-cell and non-small-cell lung cancer. Histopathology. 2004;44(4):339-344. [CrossRef]
- Qi D, Zhang Y, Li H, Wang R, Qian K. [Expression and Clinical Significance of Galcetin-3 in the Serum of Non-small Cell Lung Cancer Patients]. Zhongguo Fei Ai Za Zhi. 2020;23(5):333-336. [CrossRef]
- Kataoka Y, Igarashi T, Ohshio Y, Fujita T, Hanaoka J. Predictive importance of galectin-3 for recurrence of non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2019;67(8):704-711. [CrossRef]
- Chen P, Zhang L, Zhang W, et al. Galectin-9-based immune risk score model helps to predict relapse in stage I-III small cell lung cancer. J Immunother Cancer. 2020;8(2):e001391. [CrossRef]
- Funkhouser AT, Strigenz AM, Blair BB, et al. KIT Mutations Correlate with Higher Galectin Levels and Brain Metastasis in Breast and Non-Small Cell Lung Cancer. Cancers. 2022;14:2781. [CrossRef]
- Blair BB, Funkhouser AT, Goodwin JL, et al. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers. 2021;13:4819. [CrossRef]
- Gluck WL, Callahan SP, Brevetta RA, et al. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir. Med. 2020;175:106188. [CrossRef]
- Shuford S, Lipinski L, Abad A, et al. Prospective prediction of clinical drug response in high-grade gliomas using an ex vivo 3D cell culture assay. Neuro-Oncology Adv. 2021;3:vdab065. [CrossRef]
- Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193-203. [CrossRef]
- Lababede O, Meziane MA. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist. 2018;23(7):844-848. [CrossRef]
- Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev. 2013;22(129):382-404. [CrossRef]
- Palade E, Günter J, Gomez JMM, et al. Morbidity, mortality and long-term outcome of lung cancer resections performed in palliative intent. J Thorac Dis. 2019;11(10):4308-4318. [CrossRef]
- Pokhare S, Sharma UC, Attwood K, Mansoor S. Clinical Significance of Galectin-3 Expression in Squamous Cell Carcinoma of Lung. J Cancer Sci Clin Ther. 2022;6(3):322-327. [CrossRef]
- GR-MD-02 Plus Pembrolizumab in Melanoma, Non-small Cell Lung Cancer, and Squamous Cell Head and Neck Cancer Patients. ClinicalTrials.gov Identifier: NCT02575404. Updated June 5, 2023. Accessed October 22, 2023. GR-MD-02 Plus Pembrolizumab in Melanoma, Non-small Cell Lung Cancer, and Squamous Cell Head and Neck Cancer Patients - Full Text View - ClinicalTrials.gov.
- Vuong L, Kouverianou E, Rooney CM, et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019;79(7):1480-1492. [CrossRef]
- Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:1–8. [CrossRef]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823–1833. [CrossRef]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [CrossRef]
- Rodrigues-Pereira J, Kim JH, Magallanes M, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6(11):1907-1914. [CrossRef]
- Kreuter M, Vansteenkiste J, Fischer JR, et al. Three-Year Follow-Up of a Randomized Phase II Trial on Refinement of Early-Stage NSCLC Adjuvant Chemotherapy with Cisplatin and Pemetrexed versus Cisplatin and Vinorelbine (the TREAT Study). J Thorac Oncol. 2016;11(1):85-93. [CrossRef]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21(16):3016-3024. [CrossRef]
- Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30(28):3516-3524. [CrossRef]
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. [CrossRef]
- Kuo P, Bratman SV, Shultz DB, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res. 2014;20(21):5558-5569. [CrossRef]
- Funkhouser A, Shuster H, Martin JC, Edenfield WJ, Blenda AV. Pattern Analysis of Serum Galectins-1, -3, and -9 in Breast Cancer. Cancers (Basel). 2023;15(15):3809. [CrossRef]
- Huang CC, Chuang IC, Su YL, et al. Prognostic Significance of Galectin-1 but Not Galectin-3 in Patients With Lung Adenocarcinoma After Radiation Therapy. Front Oncol. 2022;12:834749. [CrossRef]
- Chera BS, Kumar S, Beaty BT, et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res. 2019;25(15):4682-4690. [CrossRef]
- Chera BS, Kumar S, Shen C, et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol. 2020;38(10):1050-1058. [CrossRef]
- Berger B, Hanna G, Posner M, et al. Detection of occult recurrence using circulating tumor tissue modified viral HPV DNA among patients treated for HPV-driven oropharyngeal carcinoma. Clin Cancer Res. 2022;28(19):4292-4301. [CrossRef]
- Salazar MC, Rosen JE, Wang Z, et al. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol. 2017;3(5):610-619. [CrossRef]
- Nooreldeen R, Bach H. Current and Future Development in Lung Cancer Diagnosis. Int J Mol Sci. 2021;22(16):8661. [CrossRef]
- Shafiq A, Moore J, Suleman A, et al. Elevated Soluble Galectin-3 as a Marker of Chemotherapy Efficacy in Breast Cancer Patients: A Prospective Study. Int J Breast Cancer. 2020;2020:4824813. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).