Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
Sample Preparation and Storage
2.3. Coatings and Storage Conditions
2.4. Experimental Design
2.5. Physiological Weight Loss
2.6. Firmness
2.7. Color Analysis
2.8. Decay Percentage
2.9. Total Soluble sugar, Titratable Acidity, and pH
2.10. Sugar Analysis
2.11. Carotenoids Analysis
2.12. Statistical Analysis
3. Results
3.1. Texture Analysis
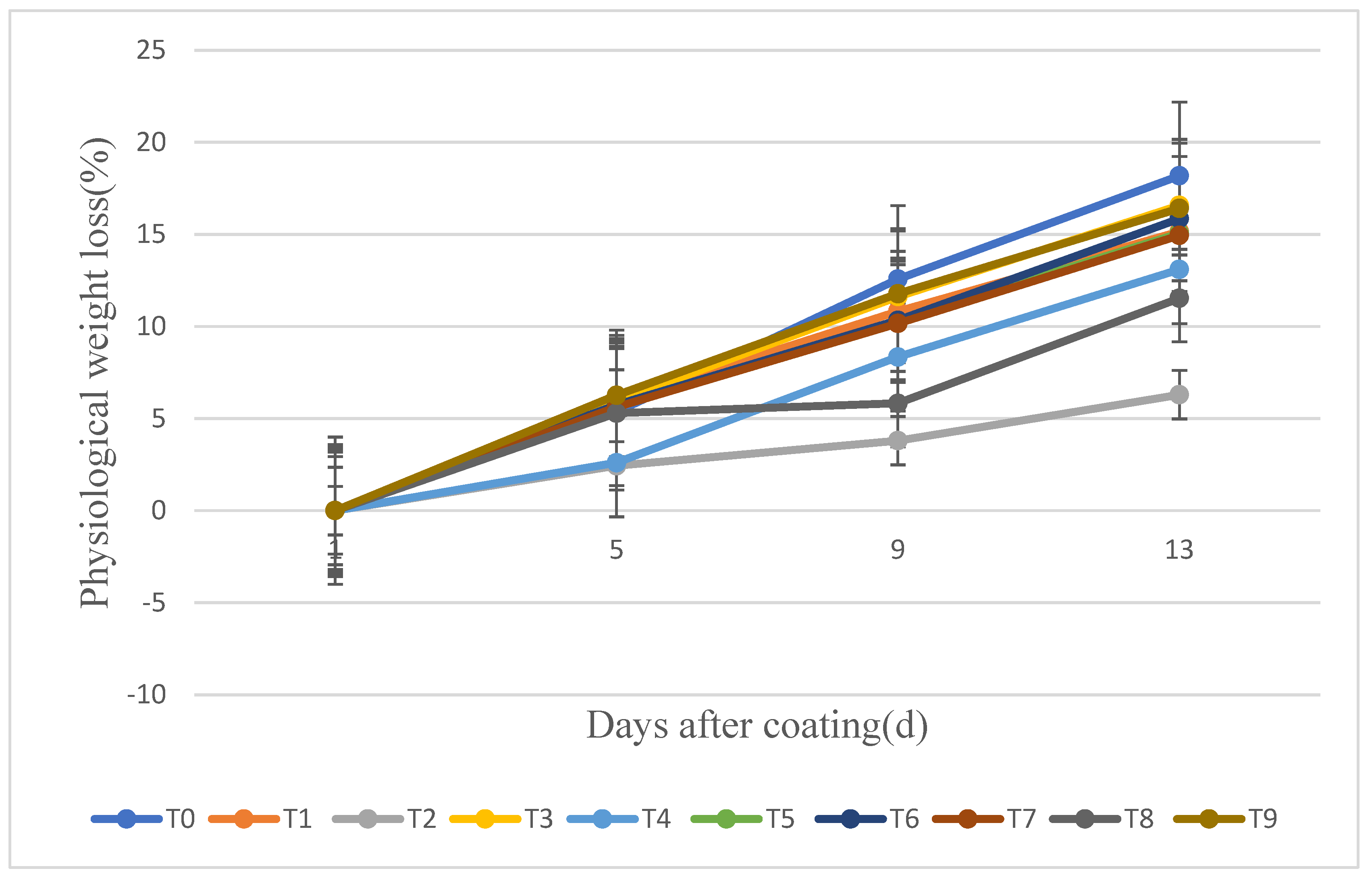
3.2. Physiological Weight Loss
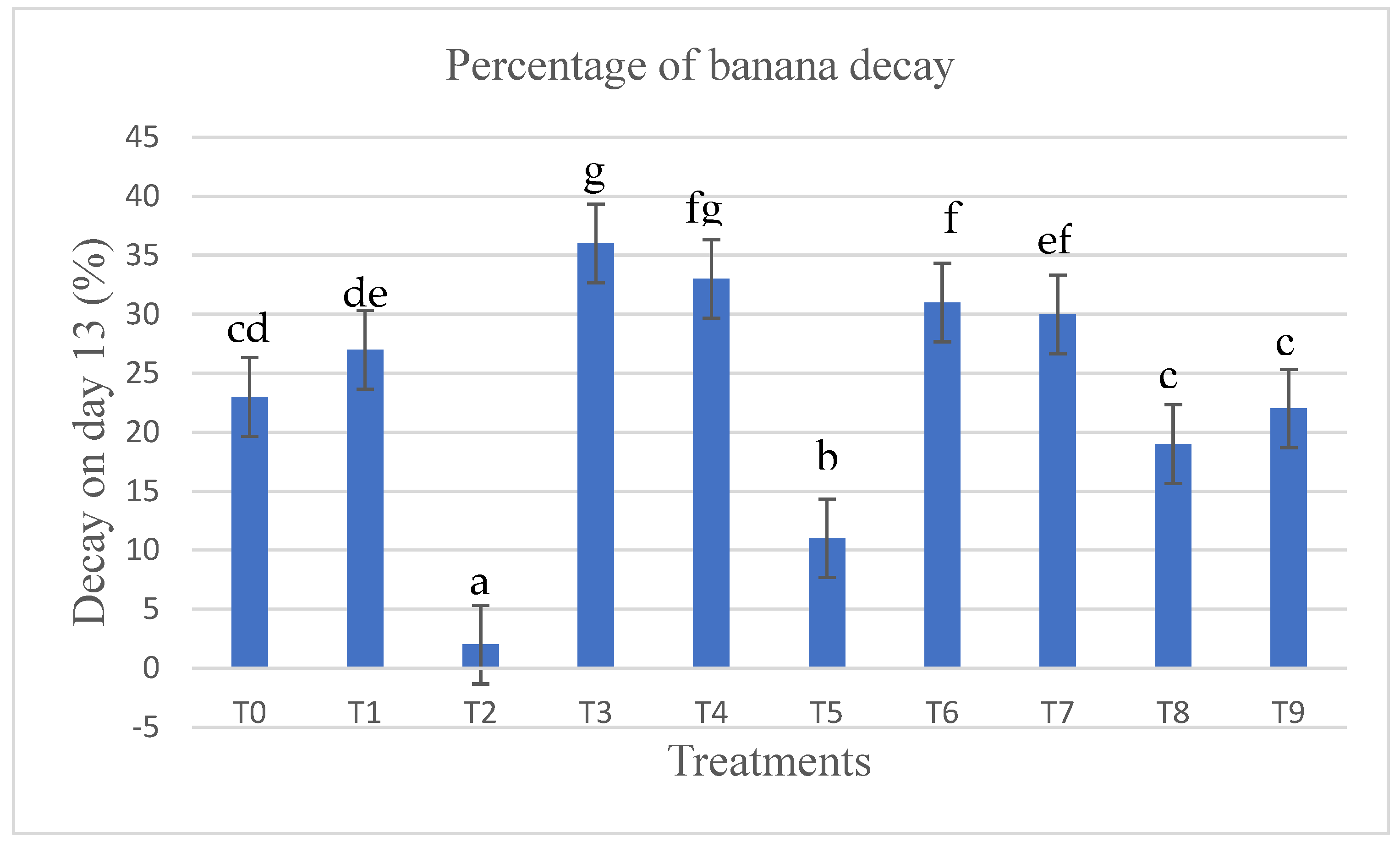
3.3. Decay Percentage
3.4. Total Soluble Sugar
3.5. Color Analysis
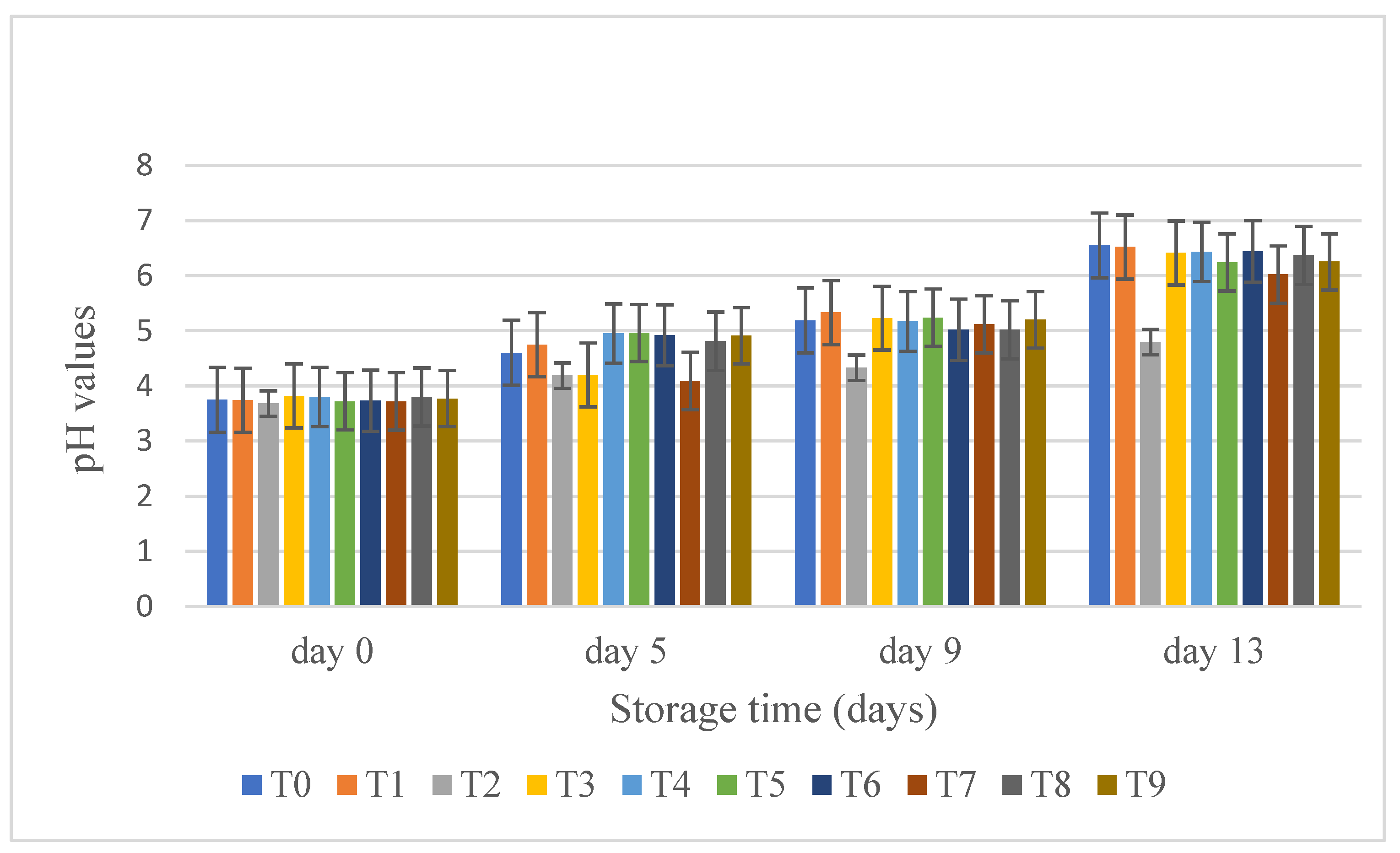
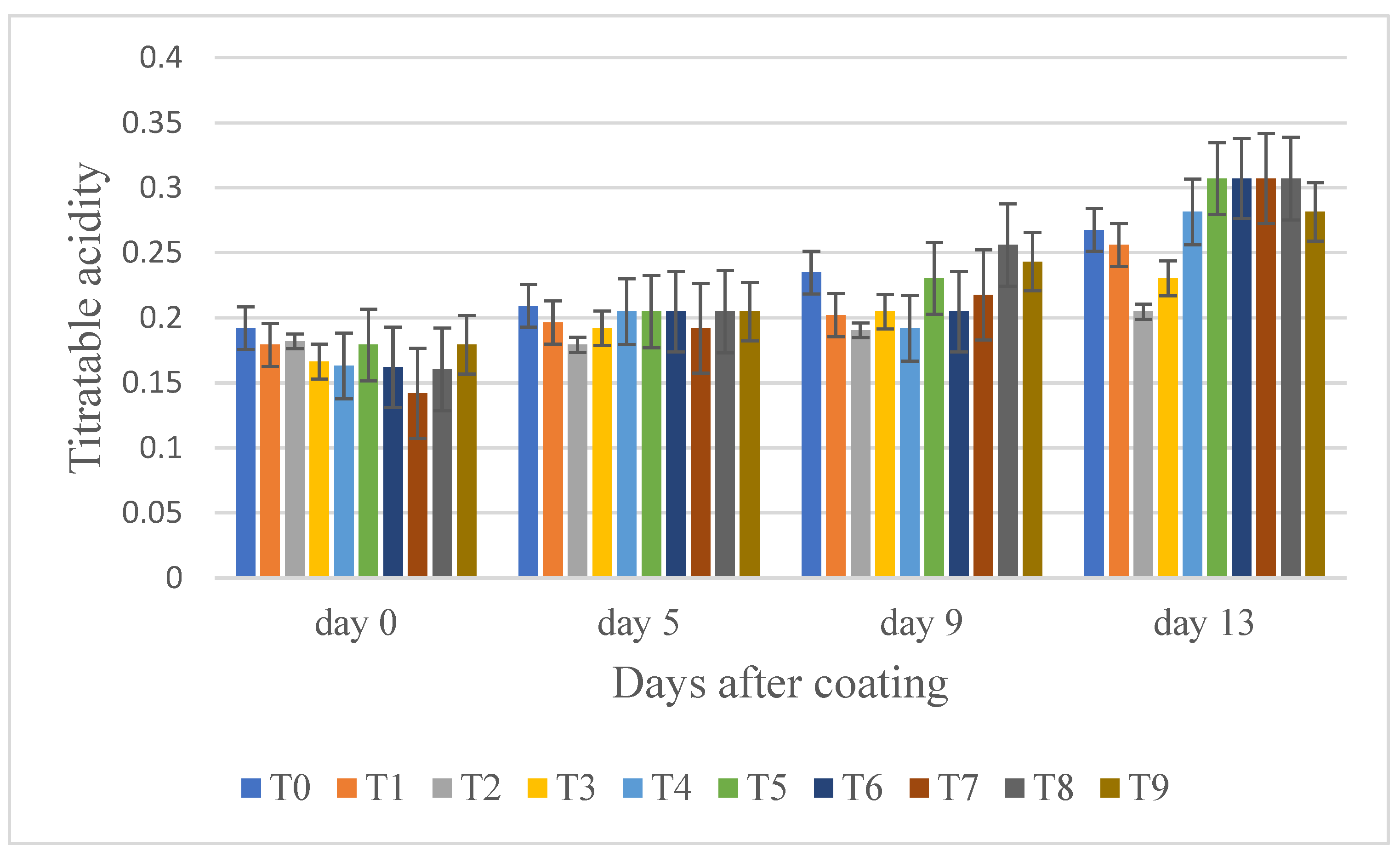
3.6. Titratable Acidity, and pH
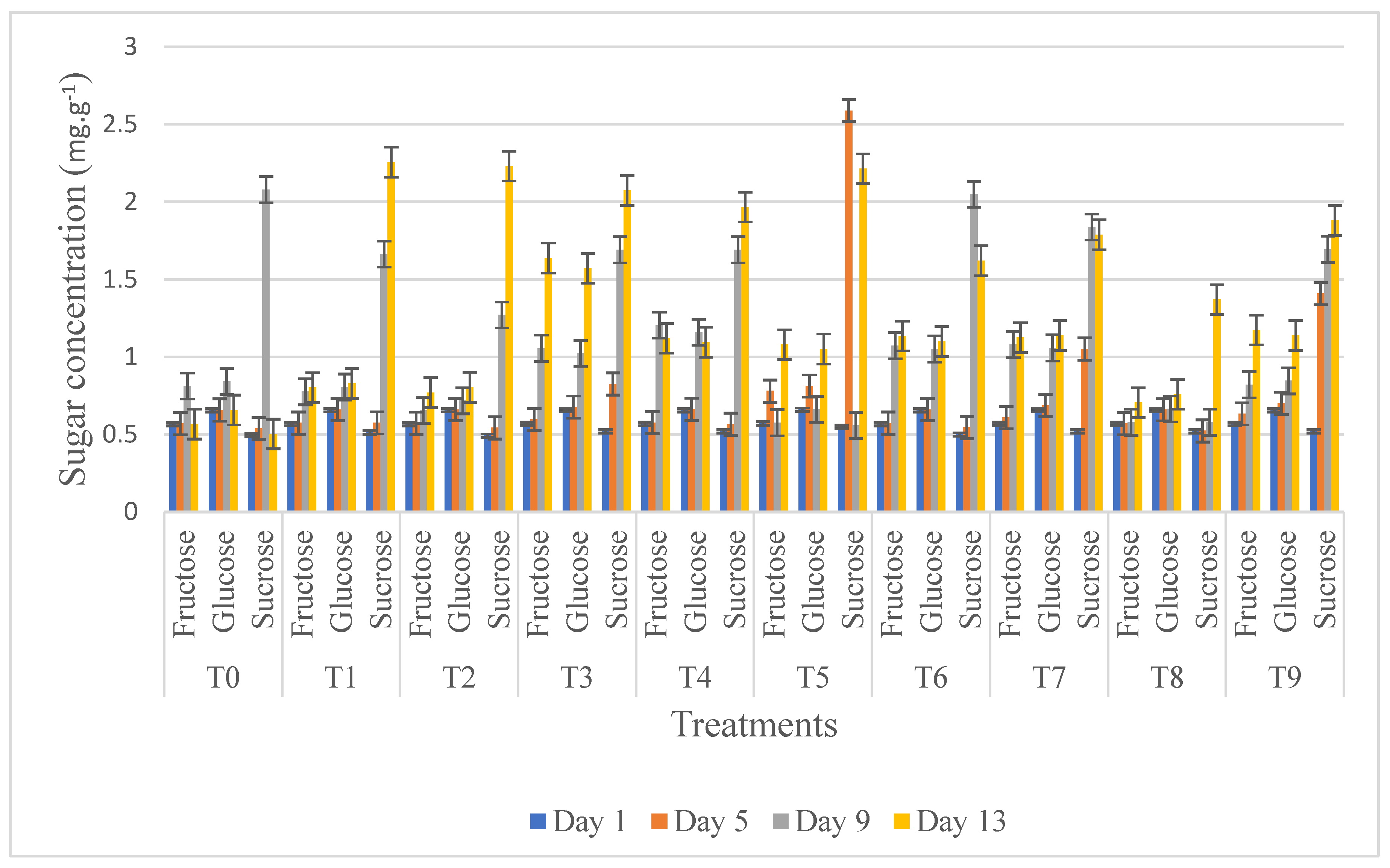
3.7. HPLC Sugar Analysis
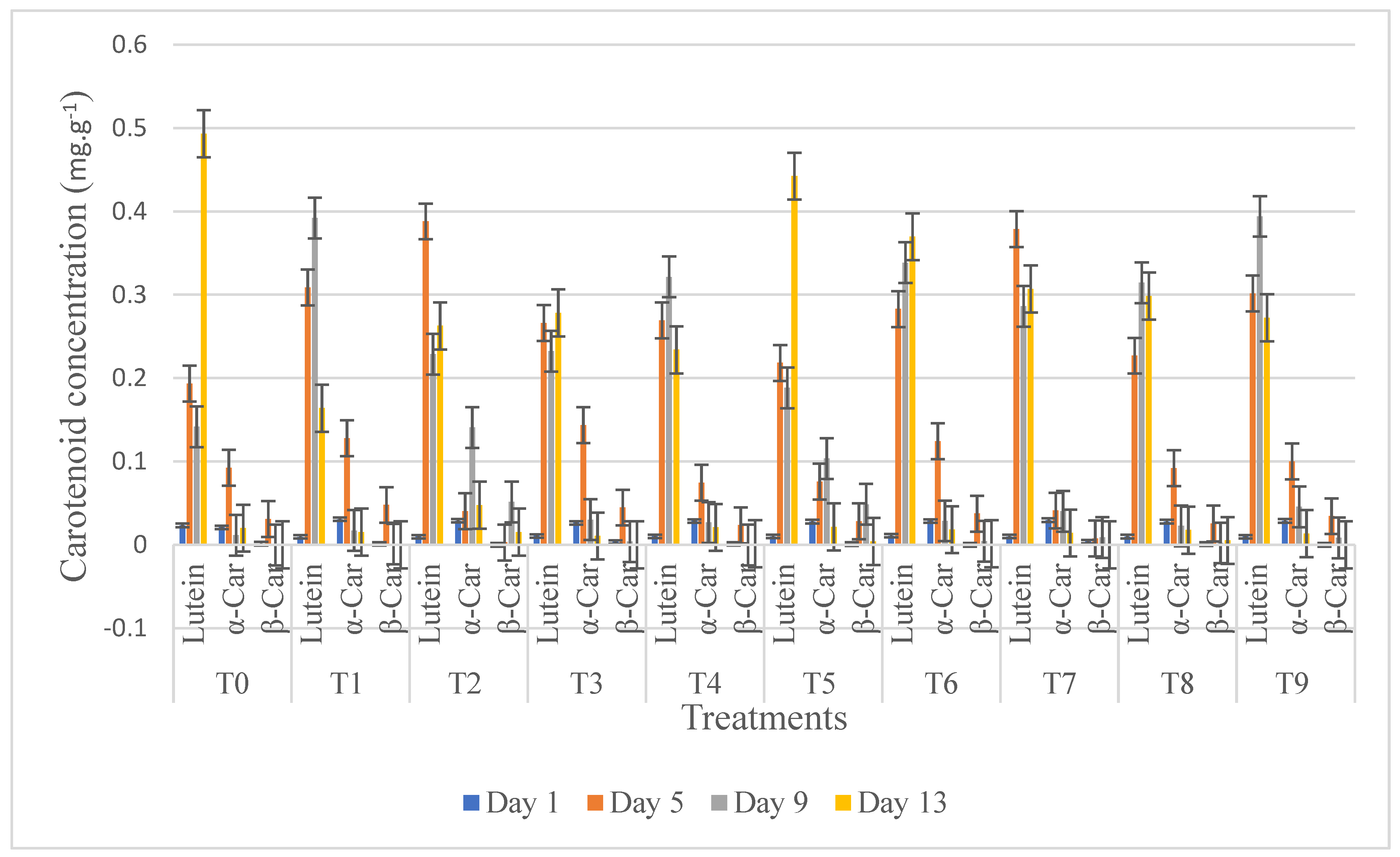
3.8. HPLC Carotenoids Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LPE | Lemon peel extract |
References
- Picq, V.; Ramillon, J.; Balanzat, E. Swift heavy ions on polymers: Hydrocarbon gas release. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 1998, 146, 496–503. [Google Scholar] [CrossRef]
- Karamura, D.; Frison, E.; Karamura, D.A.; Sharrock, S. Banana production systems in eastern and southern Africa. Banan. Food Secur. 1998, 401–412. [Google Scholar]
- In Brief to The State of Food Security and Nutrition in the World 2023; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Dwivany, F.M.; Aprilyandi, A.N.; Suendo, V.; Sukriandi, N. Carrageenan Edible Coating Application Prolongs Cavendish Banana Shelf Life. Int. J. Food Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Palta, J.P. A Postharvest Dip Treatment with of Banana Fruit. HortScience 2015, 50, 1035–1040. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S. Effect of hexanal vapor to control postharvest decay and extend shelf-life of highbush blueberry fruit during controlled atmosphere storage. Can. J. Plant Sci. 2010, 90, 359–366. [Google Scholar] [CrossRef]
- Imahori, Y.; Yamamoto, K.; Tanaka, H.; Bai, J. Residual effects of low oxygen storage of mature green fruit on ripening processes and ester biosynthesis during ripening in bananas. Postharvest Biol. Technol. 2013, 77, 19–27. [Google Scholar] [CrossRef]
- Silva, V.; Arquelau, P.; Silva, M.; Augusti, R.; Melo, J.; Fante, C. Use of Paper Spray-Mass Spectrometry to Determine the Chemical Profile of Ripe Banana Peel Flour and Evaluation of Its Physicochemical and Antioxidant Properties. Quimica Nova 2020, 43, 579–585. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Fuchs, C.; Yeh, S.-Y.; Huang, F.-C.; Hoffmann, T.; Schwab, W. An oxygenase inhibitor study in Solanum lycopersicum combined with metabolite profiling analysis revealed a potent peroxygenase inactivator. J. Exp. Bot. 2010, 62, 1313–1323. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Hoffmann, T.; Schwab, W. Effects of bio-based coatings on the ripening and quality attributes of tomato (Solanum lycopersicum) fruits. J. Sci. Food Agric. 2018, 99, 1842–1849. [Google Scholar] [CrossRef]
- Ghasemzadeh, R.; Karbassi, A.; Ghoddousi, H.B. Application of Edible Coating for Improvement of Quality and Shelf-life of Raisins. World Appl. Sci. J. 2008, 3, 82–87. [Google Scholar]
- Taylor, P. Edible Films and Coatings: Tomorrow’s Packagings: A Review Edible Films and Coatings: Tomorrow ’ s Packagings: A Review. 2010, 37–41. [Google Scholar]
- Kabuki, T.; Nakajima, H.; Arai, M.; Ueda, S.; Kuwabara, Y.; Dosako, S. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000, 71, 61–66. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Xie, H.; Hao, J.; Yang, B.; Qiu, S.; Wei, X.; Chen, F.; Jiang, Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010, 118, 62–66. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2002, 87, 41–44. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N.; Grohmann, K. Biological Properties of Citrus Flavonoids Pertaining to Cancer and Inflammation. Curr. Med. Chem. 2001, 8, 135–153. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11172671. [CrossRef] [PubMed]
- Martín-Diana, A.; Rico, D.; Frías, J.; Barat, J.; Henehan, G.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- Minh, N.P. Effectiveness of CaCl2 treatment on quality attributes of banana fruit during storage. Plant Sci. Today 2021, 9, 206–214. [Google Scholar] [CrossRef]
- Tunde-Akintunde, T. Effect of Pretreatment on Drying Time and Quality of Chilli Pepper. J. Food Process. Preserv. 2010, 34, 595–608. [Google Scholar] [CrossRef]
- Wills, R.; Tirmazi, S.; Scott, K. Effect of postharvest application of calcium on ripening rates of pears and bananas. J. Hortic. Sci. 1982, 57, 431–435. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.; Jamir, A.; Ankalagi, N.; Henny, T.; Devi, O. Edible coatings in fruits and vegetables: A brief review. Pharma Innov. J. 2021, 10, 71–78. Available online: https://www.researchgate.net/profile/Manisha-Ch- Momin/publication/352994139_Edible_coatings_in_fruits_and_vegetables_A_brief_review/links/60e2f066 299bf1ea9ee13a3d/Edible-coatings-in-fruits-and-vegetables-A-brief-review.pdf.
- Gol, N.B.; Rao, T.V.R. Banana Fruit Ripening as Influenced by Edible Coatings. Int. J. Fruit Sci. 2011, 11, 119–135. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Amal, S.H.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving Strawberry Fruit Storability by Edible Coating as a Carrier of Thymol or Calcium Chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. Available online: www.idosi.org/jhsop/2(3)10/2.pdf.
- Rahman, M.; Hossain, T.; Hossain, M.; Sattar, S.; Das, P.C. Effect of banana peel extract on storage stability of banana cv. Sagar. Food Res. 2019, 4, 488–494. [Google Scholar] [CrossRef]
- Özdemir, I.S. Effect of light treatment on the ripening of banana fruit during postharvest handling. Fruits 2016, 71, 115–122. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Amraie, M.; Salehi, M. Color and weight changes of fresh-cut banana slices coated by quince seed gum: Effect of concentration, storage temperature and duration. Iran. Food Sci. Technol. Res. J. 2019, 14, 75–85. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The Starch Is (Not) Just Another Brick in the Wall: The Primary Metabolism of Sugars During Banana Ripening. Front. Plant Sci. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Wathelet, B.; Paquot, M.; Emaga, T.H. Changements texturaux et biochimiques des fruits du bananier au cours de la maturation. Leur influence sur la préservation de la qualité du fruit et la maîtrise de la maturation. Biotechnol. Agron. Soc. Environ. 2008, 12, 1335–1342. [Google Scholar]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Open Archive TOULOUSE Archive Ouverte (OATAO) Mechanism of Fruit Ripening. Dev. Biol. 2010, 1. [Google Scholar]
| Storage period (days) | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 1 | 38.8 ± 0.1 a | 38.2 ± 0.1 a | 38.6 ± 0.5 a | 38.5 ± 0.4 a | 38.8 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.1 a | 38.5 ± 0.1 a | 38.5 ± 0.1 a | 38.3 ± 0.6 a | |
| Firmness (N) | 5 | 32.3 ± 0.8 a | 33.4 ± 1.5 a | 37.3 ± 2.4 a | 34.4 ± 3.3 a | 32.2 ± 2.2 a | 34.7 ± 2.8 a | 34.4 ± 1.7 a | 33.5 ± 0.6 a | 33.1 ± 1.3 a | 33.7 ± 3.3 a |
| 9 | 2.9 ± 0.1 a | 3.5 ± 0.1 a | 32.1 ± 3.2 b | 3.1 ± 0.4 a | 3.4 ± 0.5 a | 5.3 ± 1.4 a | 5.4 ± 2.4 a | 6.4 ± 4.9 a | 15.3 ± 19.4 ab | 2.6 ± 0.3 a | |
| 13 | 2.3 ± 0.4 a | 2.8 ± 0.3 a | 9.6 ± 1.0 a | 2.4 ± 0.4 a | 2.3 ± 0.1 a | 2.9 ± 0.3 a | 2.9 ± 0.2 a | 2.6 ± 0.5 a | 3.4 ± 0.1 a | 2.7 ± 0.4 a | |
| 1 | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | |
| TSS (%) | 5 | 3.0 ± 0.1 d | 3.0 ± 0.1 d | 2.4 ± 0.1 a | 2.7 ± 0.1 b | 3.2 ± 0.1 e | 2.7 ± 0.1 b | 2.9 ± 0.1 c | 2.7 ± 0.1 b | 3.3 ± 0.1 f | 3.4 ± 0.1 f |
| 9 | 16.8 ± 0.1 i | 13.2 ± 0.1 e | 3.3 ± 0.1 a | 16.6 ± 0.1 h | 16.5 ± 0.1 h | 13.5 ± 0.1 f | 12.6 ± 0.1 d | 11.7 ± 0.1 c | 9.6 ± 0.1 b | 15.3 ± 0.1 g | |
| 13 | 17.8 ± 0.1 f | 16.7 ± 0.2 e | 13.5 ± 0.1 a | 16.6 ± 0.1 de | 15.7 ± 0.1 b | 16.3 ± 0.1 cd | 15.9 ± 0.1 bc | 16.4 ± 0.2 de | 15.7 ± 0.2 b | 16.4 ± 0.2 de |
| Treatments | Color (CIELab) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| L1* | L2* | L3* | L4* | a1* | a2* | a3* | a4* | b1* | b2* | b3* | b4* | |
| T0 | 61.8 ± 0.1 | 91.4 ± 0.9a | 91.4 ± 0.9c | 66.4a ± 0.8a | -9.8 ± 0.1 | -243.6 ± 2.9 | 14.5 ± 0.2c | 12.1 ± 0.3a | 12.0± 0.2 | 14.7 ± 0.7 a | 19.7 ± 0.3 d | 12.4±0.9a |
| T1 | 61.1 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5a | 68.1a ± 0.8a | -5.4 ± 0.1 | -240.27 ± 0.71 | 14.6 ± 0.8a | 13.4 ± 0.7a | 6.2 ± 0.1 | 15.2 ± 0.2 a | 18.6 ± 0.2 ad | 11.4±1.5a |
| T2 | 62.3 ± 0.1 | 90.3 ± 1.2a | 90.3 ± 1.2ab | 65.8a ± 0.4a | -8.0 ± 0.1 | -241.8 ± 1.5 | 15.1 ± 0.6ac | 11.8 ± 0.4a | 12.4 ± 0.1 | 15.4 ± 0.5 a | 17.9 ± 0.2 ab | 12.1±0.3a |
| T3 | 61.8 ± 0.1 | 92.1 ± 0.6a | 92.1 ± 0.6bc | 67.5a ± 1.4a | -7.4 ± 0.1 | -237.9 ± 0.6 | 15.3 ± 0.7ac | 12.7 ± 1.5a | 6.8 ± 0.1 | 15.1 ± 0.3 a | 19.1± 0.4 bd | 11.6±1.5a |
| T4 | 61.7 ± 0.1 | 91.4 ± 0.8a | 91.4 ± 0.8ac | 65.3a ± 0.2a | -6.8 ± 0.1 | -240.5 ± 2.6 | 15.9 ± 0.2ac | 11.8 ± 0.4a | 7.5 ± 0.1 | 15.4 ± 0.3 a | 18.2 ± 0.4 abc | 11.3±0.7a |
| T5 | 61.1 ± 0.1 | 91.8 ± 0.8a | 91.8 ± 0.8ab | 67.5a ± 1.2a | -5.4 ± 0.1 | -239.8 ± 3.5 | 15.6 ± 0.6ab | 12.0 ± 1.5a | 6.2 ± 0.1 | 14.6 ± 0.7 a | 17.7 ± 0.3 a | 12.5±1.5a |
| T6 | 61.70 ± 0.1 | 92.0 ± 0.5a | 92.0 ± 0.5ab | 68.3a ± 1.3a | -6.8 ± 0.1 | -239.5 ± 1.2 | 16.0 ± 0.4ab | 12.2 ± 1.3a | 7.5 ± 0.1 | 15.0 ± 0.2 a | 17.8 ± 0.6 a | 11.8±1.2a |
| T7 | 62.2 ± 0.1 | 92.0± 0.6a | 92.0 ± 0.6ac | 66.5a ± 2.2a | -8.0 ± 0.1 | -239.1 ± 1.9 | 16.0 ± 0.4ac | 11.0 ± 3.0a | 12.4 ± 0.1 | 15.2 ± 0.4 a | 18.8 ± 0.5ad | 10.8±0.8a |
| T8 | 61.8 ± 0.1 | 91.5 ± 0.5a | 91.5 ± 0.5ab | 69.8a ± 4.0a | -9.8 ± 0.1 | -240.4 ± 1.5 | 16.4 ± 1.0a | 13.3 ± 3.0a | 12.2 ± 0.1 | 15.3 ± 0.4 a | 18.0 ± 0.4 ab | 11.6±0.8a |
| T9 | 61.9 ± 0.1 | 91.9 ± 1.2a | 91.9 ± 1.2bc | 66.2a ± 2.4a | -5.2 ± 0.1 | -238.4 ± 2.3 | 17.1 ± 0.4bc | 12.1 ± 1.6a | 6.3 ± 0.1 | 14.6 ± 0.9 a | 19.3 ± 0.6 cd | 11.3±0.5a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





