1. Introduction
Ultrafine bubble water (UFW), also known as nanobubble or microbubble water, contains small molecules less than 100 nm in diameter, which can carry gases on their surfaces [
1]. UFW water penetrates the soil quickly and can be taken up by roots more effectively, enhancing plant growth and development. It has been widely used in crop production [
2,
3]. It has been reported that plants irrigated with UFW have increased seed germination [
4,
5,
6], show significantly enhanced rooting and adventitious root development [
7], and enhanced root nutrient uptake and increased nutrient use efficiency [
8]. Many reports have shown UFW irrigation increased crops yield and quality, such as in rice [
9], lettuce [
10], tomatoes [
11,
12], cucumbers [
13], lettuce [
10], muskmelon [
14], and strawberries [
15].
Ultrafine water enriched with hydrogen prolongs the vase life and the quality of cut flowers [
16,
17]. Also, it extends the shelf life of kiwifruit [
18] and strawberries [
19]. It also plays an essential role in plant tolerance to abiotic stresses. Hydrogen pretreatment can improve the salt stress resistance of rice and
Arabidopsis [
20]. It has been reported that molecular hydrogen (H
2) has antioxidant activity, removing reactive oxygen species (ROS) and reactive nitrogen species (RNS) and reducing free radical toxicity [
21,
22].
Melon or muskmelon (
Cucumis melo L.) is a popular fruit consumed worldwide, and it has significant economic value in the global market. Melon crops are susceptible to infestation by a variety of insects, such as aphids, thrips, whiteflies, cucumber beetles, and spider mites [
23]. Aphids are tiny insects that suck the sap from the plant and can cause stunted growth, curled leaves, transmit viruses, and decrease crop yield. Pathogens such as aphid-transmitted melon cucumber mosaic virus (CMV) and watermelon mosaic virus-2 (WMV-2) [
24] cause severe damage to melon plants and lead to reduced yields and decreased fruit quality. Consequently, farmers spray pesticides frequently, which causes a food safety issue. Therefore, how to develop a new cropping system for the
Sustainability
Assessment of
Farming and the
Environment (
SAFE) [
25] in melon production is very important.
Trichomes are hair-like outgrowth on the surface of plant organs such as leaves, stems, and flowers. Trichomes act as a physical barrier against herbivorous insects by deterring their ability to feed on the plant and reducing insect movement. Plants with higher trichome density are known to be more resistant to insects [
26] and have a strong positive correlation between trichome density and insect resistance [
27]. Moreover, glandular trichomes can also produce volatile compounds that are toxic or repellent to insects [
28]. Jasmonic acid (JA) is a herbivory-induced hormone that participates in terpene biosynthesis [
29]. Methyl jasmonate (Me-JA) treatment significantly enhanced the expression of several monoterpene and sesquiterpene synthases. Research showed that the knockout of an HD-ZIP IV transcription factor (TF), woolly (wo), led to a significant defect in trichomes and a reduction of terpene levels and is associated with insect resistance in tomatoes [
30]. Me-JA induced type VI glandular trichome formation on the newly expanding tomato leaves, thus decreasing herbivore insect populations [
31]. The gene regulation network controlling trichome development is complex [
32]. It is regulated by GLABRA1 (GL1), GLABRA2 (GL2), GLABRA3 (GL3), and TRANSPARENT TESTA GLABRA1 (TTG). Loss of function of these TFs showed glabrous phenotypes [
33,
34,
35,
36,
37,
38,
39]. GLABRA3 (GL3) is a wound-induced trichome formation acting downstream of the JA signaling pathway [
35]. TRIPTYCHON (TRY) is a negative regulator of trichome and root hair development [
40]. It has been reported that JAZ is required for jasmonate-meditated glandular trichome development in
Nicotiana benthamiana [
41] and rice [
42].
The application of hydrogen in agriculture has attracted much attention over the last decade and has several prospects [
43]. However, to the best of our knowledge, there is no report on UFW-induced JA and increasing trichome development. The aims of the present work were as follows: (i) to observe whether UFW improves melon seedling growth and fruit production; (ii) to understand whether UFW enhances pest resistance; and (iii) to clarify whether UFW regulates the JA-pathway and induces trichome development in melon.
2. Results
2.1. UFW treatment improved the growth of melon seedlings
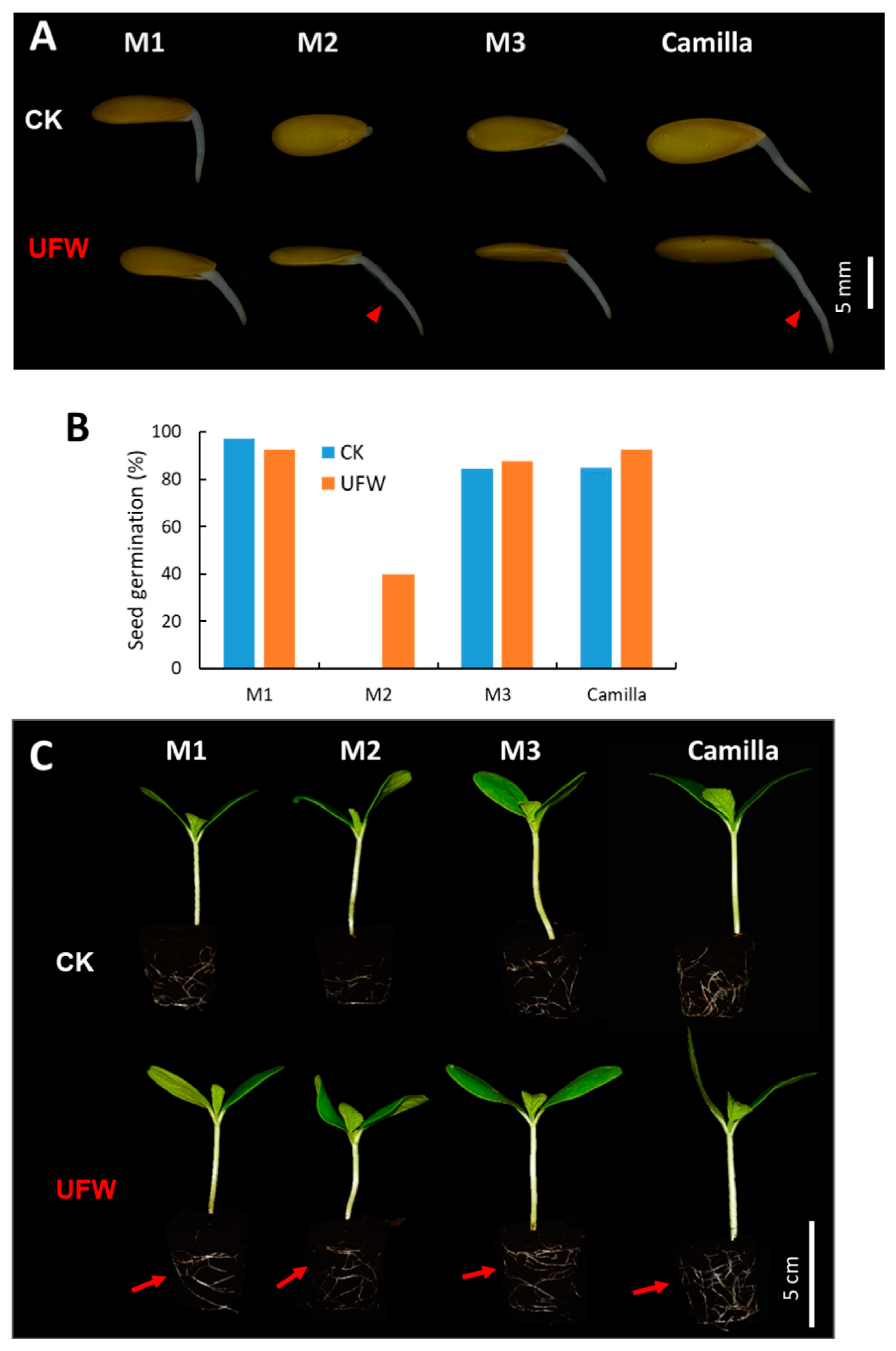
To understand the effects of ultrafine water (UFW) on seed germination and seedling growth, we tested four lines of melon seeds: M1, M2, M3, and Camilla. Forty seeds per line were imbibed in UFW and RO water overnight, and then placed in square Petri dishes containing UFW and reverse osmosis (RO) water as a control (CK), respectively. The dishes were then placed in a growth chamber in the dark and set to a constant temperature of 28 °C. After germination for one day, the melon seeds in UFW produced longer and more root hairs than CK (
Figure 1A). The germination rates of UFW-treated seeds of M2, M3, and Camilla were higher than those of CK (
Figure 1B). We transplanted the germinated melon seeds into a #104 plug tray filled with peat moss and raised the seedlings in the greenhouse. Treatment with UFW produced more vigorous roots and seedlings than CK at 7 days after transplantation (
Figure 1C).
2.2. UFW reduced aphid infestation of seedlings
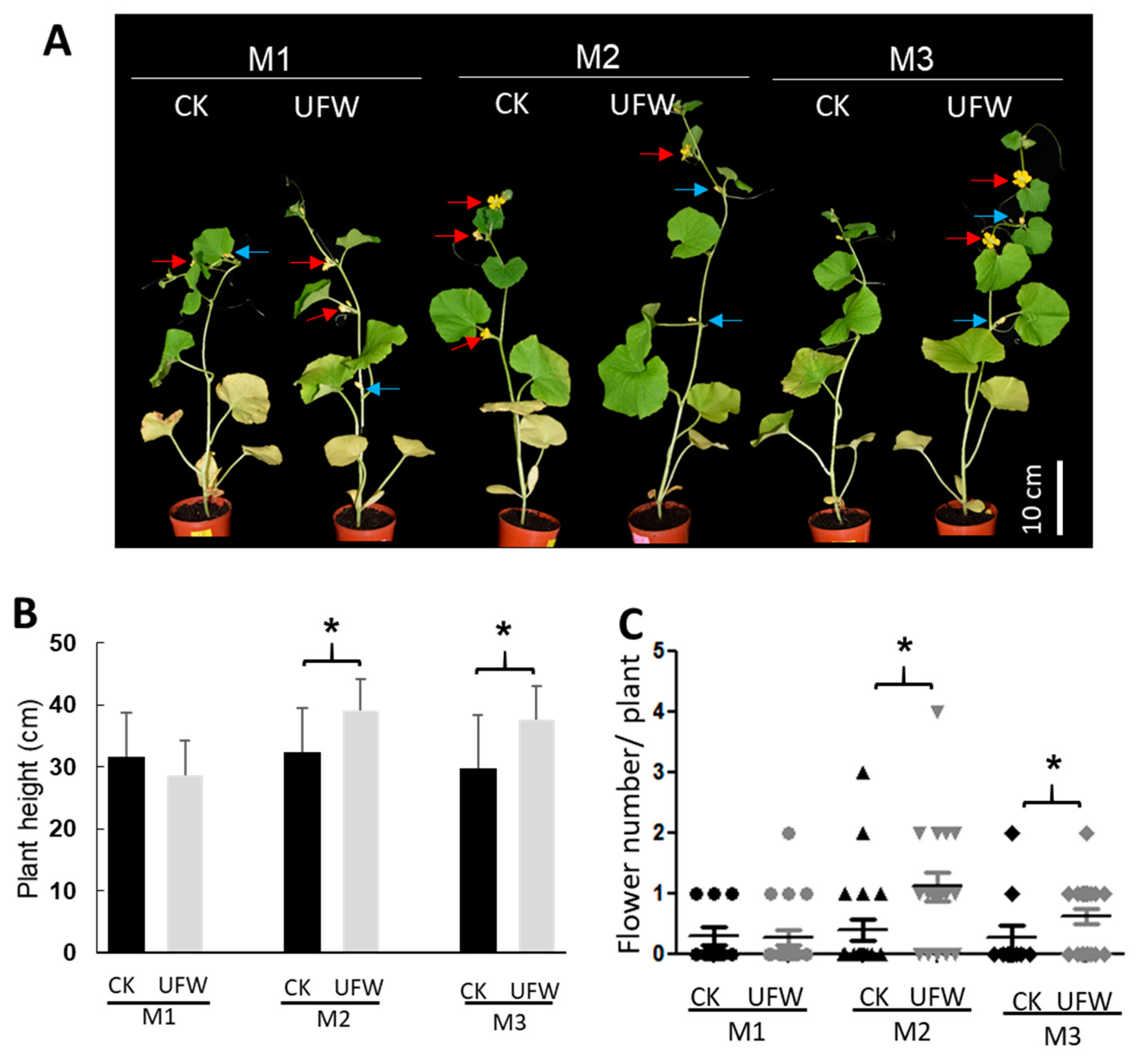
In order to understand the effect of UFW on the growth of melon seedlings, we transplanted melon seedlings from plugs into pots and placed them in the same growth chamber to grow, but irrigated with RO water and UFW. At 14 days after transplanting, the plant heights of the UFW-irrigated melon lines M2 and M3 were higher and produced more flowers than those of CK (
Figure 2).
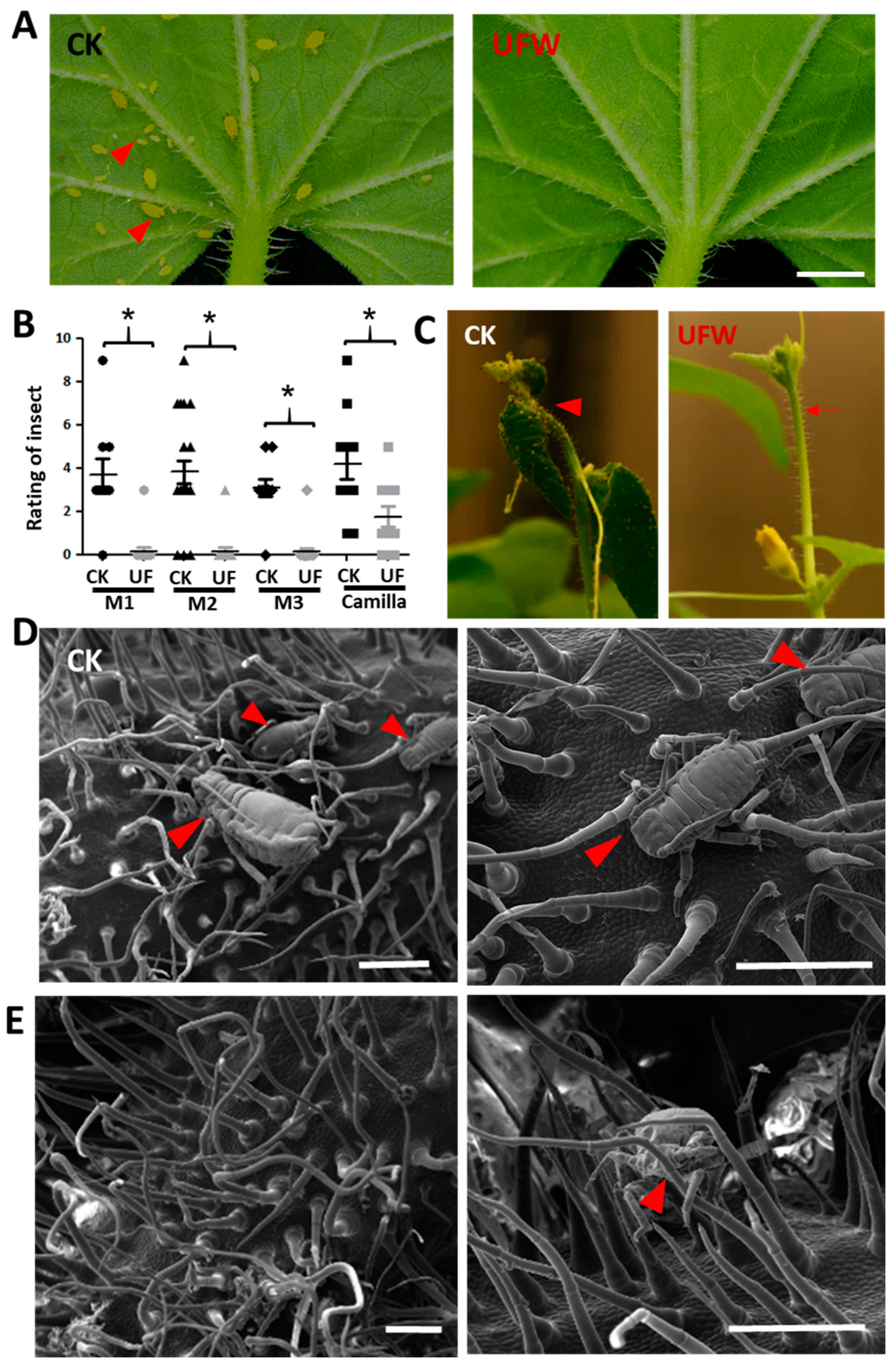
We found that aphids attacked melon seedlings in the growth chamber at 14 days after transplantation (DAT). Surprisingly, it was found that the aphid density in the leaves and flower buds of melons irrigated with UFW was lower than that of the control group (
Figure 3A-C). We performed cryo-SEM and observed that the trichomes of CK were fewer and drooping, and the mouthparts of aphids could easily reach the leaf surface (
Figure 3D). However, the UFW-irrigated melon plants have upright and dense trichomes that interfered with aphid movement and feeding (
Figure 3E).
2.3. Effect of hydrogen-rich (UF+H2) or oxygen-rich (UF+O2) ultrafine water on trichome development
Our previous experiments showed that UFW containing both H
2 and O
2 significantly increased trichome density and deterred aphid infestation (
Figure 3). We were, thus, interested to know whether this phenomenon was due to the effect of H
2 or O
2 molecules. Hence, we prepared UF water enrichment of pure hydrogen (UF+H
2), pure oxygen (UF+O
2), and RO water (CK), and irrigated melon cultivar “Camilla”, respectively. Melon plants irrigated with UF+H
2 produced taller and denser trichomes on the petioles, leaf veins, leaves, and leaf tip compared with CK. Meanwhile, UF+O
2 irrigated plants had longer trichomes than CK (
Figure S1). Under a dissecting microscope, we could observe that melon irrigated with UF+H
2 produced longer and denser trichomes on the mid-rib than those irrigated with UF+O
2 or CK. We took pictures and measured trichome density in petioles and found that UF+H
2 and UF+O
2 irrigation significantly increased trichome density (
Figure 4A, D).
Under a cryo-SEM microscope, we observed that long unicellular trichomes and granular trichomes were produced in the midrib of melon leaves after UF+H
2 or UF+O
2 irrigation. However, at the study stage, there were no glandular trichomes in the midrib of CK (
Figure 4B). Compared with CK, the morphology of midrib glandular trichomes in melon leaves irrigated with UF+H
2 or UF+O
2 were multicellular with medium and long stalks and small globular secretory heads (
Figure 4B, blue arrows). The abaxial leaves had denser trichome density than CK after UF+H
2 and UF+O
2 irrigation (
Figure 4C, D). Consistently, we performed qRT-PCR and found that the positive regulator of the trichome development marker gene, GLABRA2 (GL2), was significantly increased in the premature young leaf after irrigation with UF+H
2 (
Figure 4E).
2.4. Enrichment of hydrogen-induced jasmonic acid accumulation
We detected JA and MeJA contents in melon leaves and found that JA was significantly increased (6.9-fold) under UF+H
2 treatment compared to the CK (
Figure 5A). Although UF+H
2 and UF+O
2 slightly increased MeJA content, there was no statistically significant difference at p = 0.05 (
Figure 5B). Our qRT-PCR showed that JASMONATE ZIM DOMAIN PROTEIN (JAZ) and JA carboxyl methyltransferase (JMT) were upregulated after UF+H
2 treatment but there was no statistically significant difference at p = 0.05 (
Figure 5C).
2.5. Effect of UF water on photosynthesis parameters, fruit yield and quality
We grew melons in a greenhouse to understand the effect of UF+H
2 and UF+O
2 irrigation on melon fruit production. All crop management practices were similar except for irrigation water use. We measured chlorophyll content and photosynthesis parameters using a SPAD meter and Li-600 Porometer/Fluorometers meter, respectively. The results showed that H
2 and O
2 enrichment significantly increased SPAD values (representing chlorophyll content) and stomatal conductance (gsw). UF+O
2 increased the quantum yield of PSII calculated from fluorescence (ΦPSII) and electron transport rate (ETR) (
Figure 6).
During the growth process of melons in the late harvest stage, melon plants irrigated with UF+H
2 or UF+O
2 retained more green leaves (
Figure 7A, arrows) than CK irrigated with tap water. After irrigation with UFW, the root system of the melon plants developed more vigorously, and the fresh weight and dry weight of the roots were significantly higher than the control (
Figure 7B-D). UF+H
2 irrigation increased melon fruit size and weight (
Figure 7E, F). Furthermore, UF+H
2 and UF+O
2 irrigation were both able to increase melon fruit sweetness (
Figure 7G).
3. Discussion
3.1. UFW enhances root and plant growth
This study showed that UFW irrigation significantly improved melon seed germination, seedling growth, and enhanced root development. Our greenhouse experiment also showed that UFW enrichment of hydrogen (UF+H
2) or oxygen (UF+O
2) produced higher root biomass than the control without UFW treatment. The robust root system contributed to plant growth and development. Previous studies have demonstrated that hydrogen-rich water increased auxin and GA3 biosynthesis and enhanced root development [
44]. It regulated heme oxygenase-1/carbon monoxide pathways and increased root development [
13]. Some researchers have suggested that hydrogen has antioxidant properties, which can help to reduce oxidative stress in plants, improve nutrient uptake by plants, and improve overall plant growth and development [
6,
8,
10,
13,
22]. Hydrogen molecules are not easy to apply. Nonetheless, water electrolysis produces hydrogen gas, which is easily fused into ultrafine water and provides a good solution for agricultural applications.
Our data indicated that UFW positively affects crop production compared to the previous reports on cucumber [
45] and maize [
46]. This study showed that hydrogen enrichment water is better than oxygen. Compared to H
2-rich water, studies of O
2-rich water on plant growth are rare. Recently, a report highlighted that nanobubble water enrichment of O
2 improves soil structure and microbial diversity, thereby increasing tomato yield [
47]. UFW enrichment of O
2 could enhance oxygen delivery to soil and promote aerobic respiration [
48]. Some reports indicated that high O
2 content in UFW does not necessarily lead to better crop performance. In a previous study on maize treated with dissolved oxygen (DO) concentrations of 10, 20, and 30 mg/L, moderate DO of 20 mg/L had the highest root growth and yield [
46]. In this study, we grew melon in a well-ventilated soilless medium of peat moss, which may reduce the positive effects of UFW. A more significant impact would be expected if the UFW irrigated a high-density clay field with poor aeration. It is hypothesized that UF+O
2 may benefit plant survival under flooding-induced hypoxic conditions.
3.2. UF+H2 irrigation improves melon fruit yield and quality
We observed that melons irrigated with UF+H
2 or UF+O
2 retained green leaves, and in the later stages of melon development, the leaves contained higher chlorophyll. This is a beneficial trait that can increase the rate of photosynthesis and produce more assimilates for fruit development, thereby increasing the fruit weight and sweetness of the melon (
Figure 7). As reported previously, hydrogen enrichment water increased strawberry fruit flavor and quality [
15].
3.3. UFW induces glandular trichome density
Non-glandular trichomes have been reported to play a role in mechanical defense against insects, while glandular trichomes secrete metabolites such as terpene [
49]. In this study, glandular trichomes were found in the midrib of melon leaves after irrigation with UFW (UF+H
2 and UF+O
2) but not in the control (
Figure 4). To our knowledge, this is the first report showing that ultrafine water can increase trichome density and induce glandular trichome development. We discovered that UF+H
2 can induce JA-biosynthesis genes and enhance root and trichome development. Trichomes deter herbivores and reduce insect damage. In the future, it will be worth investigating what secondary metabolites were induced after UFW treatment.
3.4. UFW treatment increased genes related to JA biosynthesis
JA is known to be involved in trichome development [
30,
31]. In this study, we found that a trichome initiation marker gene
GL2 was significantly upregulated in young leaves after UF+H
2 treatment (
Figure 4E), further supporting the notion that hydrogen may induce JA and enhance trichome initiation to prevent herbivory infestation and improve plant growth. Our data show that irrigating melon with UFW (H
2 and O
2) improved the resistance of three melon lines to aphid infestation (
Figure 3). Furthermore, we found that UFW enrichment of H
2 plays an important role in trichome development due to the upregulation of JA biosynthesis genes and increased JA accumulation in the plants irrigated with UFW enrichment of H
2 (
Figure 5). This enhanced trichome formation and deterred insects or interfered with their feeding and growth, making the plants less susceptible to damage. Furthermore, reducing pest infestation will reduce systemic viral infection problems. Overall, these data indicate that hydrogen-rich water upregulates JA-pathway marker genes and increases melon trichome development, supported by the upregulation of
GL2. A high density of globular trichomes may help to resist aphids. In the future, it will be worth conducting more extensive research on the underlying mechanisms through which UFW induces JA, enhances trichome development, and confers insect resistance.
3.5. Application of UFW to achieve safe and sustainable agricultural production
We demonstrated that UFW increased trichome density and prevented aphid feeding (
Figure 3 and
Figure 4), induced more flower development, increased fruit weight, and increased sweetness of melon fruits (
Figure 7). All these beneficial effects contribute to melon crop production. These results demonstrate that hydrogen-rich water management has excellent potential as a natural, non-toxic pest and disease control treatment in crop plants. This will reduce pesticide spraying and improve food security. The UFW-induced JA response facilitates the establishment of a natural defense system in crop plants against insect attacks. Therefore, agriculture is safe without relying on pesticides. It is an environmentally friendly agricultural practice that increases crop yields and fruit quality and reduces pest damage. In addition, UFW has hydrophobic and surface charge properties that enhance the release and absorption of soil nutrients, thereby reducing fertilizer demand [
9]. This will reduce the carbon footprint in crop production and enable sustainable agricultural production. Some studies emphasize enhancing insect resistance through genetic engineering trichome genes, but due to consumer concern about the biosafety of the genetically modified organisms (GMO), here we proposed a hydrogen-rich UFW through irrigation which would be more acceptable to consumers as it does not include GMO. Furthermore, hydrogen-rich water is safe and easy to use [
50].
4. Materials and Methods
4.1. Ultrafine water preparation
We used a Hydrogen-Oxygen Ultrafine bubble system model HOU-3 (Season Agricultural Technology Co., Ltd., Taiwan) to prepare ultrafine bubble water (UFW) enriched with hydrogen or oxygen. Hydrogen-rich water (UF+H2) was prepared using reverse osmosis (RO) water to obtain 1000 ppb H2 molecules, and oxygen-rich water (UF+O2) water was prepared using RO water to contain 10 mg/L O2. Hydrogen concentration was determined with a portable Dissolved Hydrogen Meter (Trustlex Co., Ltd., ENH-1000, Japan). Dissolved oxygen (DO) content was determined using a Dissolved Oxygen Meter (Lutron Co., Ltd., PDO-519, Taiwan).
4.2. Plant materials and growth conditions
Melon (Cucumis melo L.) seeds were provided by Known-you Seed Co. Ltd. (Taiwan). Melon lines 6792T-744 (M1), 6792T-LD (M2), 6792T-LQ (M3), and a popular and high-quality melon cultivar “Camilla” were used in this study. To test the effect of UFW (containing H2 and O2) on seed germination, a total of 40 melon seeds per line were imbibed in RO water and UFW overnight and sown on a wetted tissue paper in a 125×125×20 mm square petri dish (SPL, Korea). Seed germination rates were recorded one day after sowing. Germination was defined as when the root length was over half the seed length. The germinated seeds were transferred into a #104 plug tray containing peat moss (Known-you Seed). Then, the plug seedling was transplanted into a plastic pot (7.5 cm width × 7.5 cm height) containing 140 mL peat moss. The melon seedlings were raised in a greenhouse at a temperature of 24 ± 4°C.
The commercial melon cultivar “Camilla” was used to evaluate the effect of UF+H2, UF+O2, and a tap water control on the growth and fruit production in a plastic greenhouse of the Biotechnology Center in Southern Taiwan (AS-BCST) (23°06′14.4″ N 120°17′31.2″ E). We transplanted two melon seedlings at the four-leaf stage into a package of 80 L peat moss (Known-you Seed). A total of 22 seedlings were planted for each treatment. In the plastic greenhouse, we used the tap water to prepare hydrogen-rich water (UF+H2) and oxygen-rich water (UF+O2), and the control was ordinary tap water. Water was supplied using a drip irrigation system. In this study, melons were grown vertically to keep the fruit cleaner and healthier. Flowers were pollinated at 13 to 16 nodes, retaining one fruit per plant. When the number of nodes on the mother vine reached 26, we removed the top growth point. According to the weather and soil moisture conditions, we supplied the appropriate water amount (500 mL ~ 1000 mL per day per plant) during planting to ensure good plant growth and avoid fruit cracking in the later stages of fruit maturity, which may reduce fruit quality.
4.3. Observation of trichome density
To quantify trichome number, we collected 4-6 fully developed leaves of melon ‘Camilla’, excised the middle part of the petioles, and obtained images of trichomes under a dissecting microscope (Leica S9D, Germany) at 10× magnification. We counted the number of trichomes and calculated the average number of trichomes per square centimeter.
4.4. Cryo-scanning electron microscopy
The first newly developed melon leaves (L1) were used to observe trichome development and aphid infestation on melon seedlings. The abaxial sides of the leaves were observed using a cryogenic scanning electron microscopy (cryo-SEM), a FEI Quanta 200/Quorum PP2000TR FEI, 2007 high-resolution SEM at the Plant Cell Biology Core Laboratory in the Institute of Plant and Microbial Biology, Academia Sinica, Taiwan. Briefly, leaf section samples containing insect tarsals were loaded onto frozen specimen holders and cryo-fixed in slush nitrogen (-210°C), then rapidly transferred to a cryo-unit in the frozen state. Samples were imaged by cryo-SEM at an accelerating voltage of 20 kV.
4.5. Total RNA isolation and real-time PCR
Total RNAs of melon leaf tissues were isolated using the TRIzol Plus RNA purification kit (Thermo Fisher Scientific, San Jose, USA), treated with DNase (Promega), and the first-strand cDNA was synthesized using an M-MLV Reverse Transcriptase cDNA synthesis kit (Promega, Madison, WI, United States). Quantitative real-time PCR (qRT–PCR) reactions were performed using 2× KAPA SYBR FAST master mix (KAPA Biosystems, USA) as described previously (Ko et al., 2021). β-
Actin (MELO3C023264) and
ADP ribosylation factor 1 (
ADP, MELO3C023630) were used as reference genes for normalization. The primers used in this study are listed in
Supplementary Table S1. Each sample had three biological replicates.
4.6. Detected JA and methyl-JA content
Melon leaf samples were snap-frozen in liquid nitrogen and ground to fine powder with a pestle and mortar. The powder (500 mg) was suspended in 2.5 mL of ice-cold 50% MeOH (-20°C). The extracts were vortexed for 5 min and then centrifuged at 3°C at 4,000 rpm for 15 min. The supernatants were collected and the pellets were re-extracted using 500 µL ice-cold 50% MeOH (-20°C). The supernatants were combined and applied to Sep-Pak Vac 3 mL C18 200 mg cartridges (Waters, Milford, USA) for sample clean-up and concentration. The cartridges were conditioned with 2 mL of MeOH and equilibrated with 2 mL of water, then 3 mL of sample was applied to the C18 cartridge. The Solid Phase Extraction (SPE) cartridges were eluted with 1 mL of 100% acetonitrile to release the MeJA, followed by a 1 mL clean-up with MeOH. The eluates from the cartridges were filtered through 0.22 μm filters, transferred into chromatography vials, and detected using an ultra-performance liquid chromatography- high resolution tandem mass spectrometry (UPLC-HRMS/MS) (Thermo Fisher Scientific). UPLC separation was carried out on a BEH C18 column (2.5 × 100 mm, 1.7 µm, Waters) at a 0.3 mL/min flow rate. Column oven temperature was 40°C. The gradient program was applied using 0.1% formic acid (FA) in water (phase A), and 0.1% FA in ACN (phase B). The sample injection volume was 20 µL.
4.7. SPAD value and photosynthesis rate of melon
A non-destructive portable chlorophyll (Chl) meter SPAD-502 Plus (Konica Minolta Optics, Japan) was used to measure the chlorophyll content of the 4th newly established melon leaf (L4) at 45 days after pollination. To determine the photosynthesis rate, we used a Li-600 Porometer/ Fluorometers meter (Li-COR, Lincoln, NE, USA) to measure stomatal conductance (gsw), electron transport rate (ETR), and the quantum yield of PSII calculated from fluorescence parameters (ΦPSII) of the L1 leaves at 23 days after pollination.
4.8. Statistical analysis
Student’s t-test was used to compare the difference between CK and UF+H2 or UF+O2 treatment. P values of less than 5% were considered statistically significant.
5. Conclusions
This study has demonstrated that ultrafine hydrogen-rich water can significantly increase JA accumulation, induce trichome development, reduce insect infestation, promote root development, and increase melon fruit yield and sweetness. We showed that ultrafine water significantly reduces insect infestation and can effectively reduce pesticide use, increasing crop yields and reducing environmental impact (
Figure 8). By incorporating UFW into irrigation system, farmers can work towards a safer and sustainable melon production system.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure S1. Morphology of trichomes in different tissues of melon after ultrafine water irrigation; Supplementary Table S1. List of primers used in this study.
Author Contributions
S.S. Ko and C.C. Yang designed and supervised the study; J.C. Hung, N.J. Lee, and C.Y. Peng performed the experiments; J.C. Hung analyzed and prepared the data. S-S. Ko and J.C. Hung wrote the article. .
Funding
This study was supported by a cooperative research program between Academia Sinica Agricultural Biotechnology Research Center and Season Agricultural Technology Co. Ltd. (14T-1090311-1C). .
Data Availability Statement
Data supporting the findings of this study are available within this paper and its supplementary data published online. .
Acknowledgments
We thank Known-you Seed Co. Ltd. for providing melon seeds for this study; the genetically modified greenhouse of the Biotechnology Center of Southern Taiwan (AS-BCST) for providing greenhouse and technical support; the Mass Analysis Core facility at AS-BCST for the determination of JA and Me-JA contents; Dr. Chyi-Chuann Chen for taking cryo-SEM pictures; and Miranda Loney for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloid Surface A 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Li, L.N.; Zeng, Y.; Cheng, X.; Shen, W.B. The Applications of Molecular Hydrogen in Horticulture. Horticulturae 2021, 7, 513. [Google Scholar] [CrossRef]
- Takahata, J.; Takaki, K.; Satta, N.; Takahashi, K.; Fujio, T.; Sasaki, Y. Improvement of growth rate of plants by bubble discharge in water. Japanese Journal of Applied Physics 2014, 54, 01AG07. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, S.; Cho, A.R.; Shim, M.S.; Chung, Y.K.; Kim, Y.J. Germination and seedling growth response of sprouts and leafy vegetables after applying oxygen nanobubble water. J People Plants Environ 2021, 24, 607–617. [Google Scholar] [CrossRef]
- Oshita, S.; Boerzhijin, S.; Kameya, H.; Yoshimura, M.; Sotome, I. Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination. Nanomaterials (Basel) 2023, 13, 1677. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustainable Chemistry & Engineering 2015, 4, 1347–1353. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W. A positive role for hydrogen gas in adventitious root development. Plant Signal Behav 2016, 11, e1187359. [Google Scholar] [CrossRef]
- Xue, S.; Marhaba, T.; Zhang, W. Nanobubble Watering Affects Nutrient Release and Soil Characteristics. Acs Agricultural Science & Technology 2022, 2, 453–461. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci Total Environ 2021, 800, 149627. [Google Scholar] [CrossRef]
- Park, J.S.; Kurata, K. Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth. Horttechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Xue, S.; Gao, J.; Liu, C.; Marhaba, T.; Zhang, W. Unveiling the potential of nanobubbles in water: Impacts on tomato’s early growth and soil properties. Sci Total Environ 2023, 903, 166499. [Google Scholar] [CrossRef]
- Marcelino, K.R.; Ling, L.; Wongkiew, S.; Nhan, H.T.; Surendra, K.; Shitanaka, T.; Lu, H.; Khanal, S.K. Nanobubble technology applications in environmental and agricultural systems: Opportunities and challenges. Critical Reviews in Environmental Science and Technology 2023, 53, 1378–1403. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Qi, F.; Cui, W.; Xie, Y.; Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol 2014, 171, 1–8. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.Z.; Wang, T.Z.; Chen, W.J.; Liu, B.; Zhou, Y.P.; Li, Y.K. Effects of nanobubble in subsurface drip irrigation on the yield, quality, irrigation water use efficiency and nitrogen partial productivity of watermelon and muskmelon. International Agrophysics 2022, 36, 163–171. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Jiang, K.; Kuang, Y.; Zeng, Y.; Cheng, X.; Liu, Y.; Wang, S.; Shen, W. Preharvest application of hydrogen nanobubble water enhances strawberry flavor and consumer preferences. Food Chem 2022, 377, 131953. [Google Scholar] [CrossRef]
- Li, L.; Yin, Q.; Zhang, T.; Cheng, P.; Xu, S.; Shen, W. Hydrogen Nanobubble Water Delays Petal Senescence and Prolongs the Vase Life of Cut Carnation (Dianthus caryophyllus L.) Flowers. Plants (Basel, Switzerland) 2021, 10, 1662. [Google Scholar] [CrossRef]
- Ren, P.J.; Jin, X.; Liao, W.B.; Wang, M.; Niu, L.J.; Li, X.P.; Xu, X.T.; Zhu, Y.C. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic Environ Biote 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Wang, Y.; Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem 2014, 156, 100–109. [Google Scholar] [CrossRef]
- Jin, Z.W.; Liu, Z.Y.; Chen, G.M.; Li, L.N.; Zeng, Y.; Cheng, X.; Pathier, D.; Xu, G.Y.; Shen, W.B. Molecular hydrogen-based irrigation extends strawberry shelf life by improving the synthesis of cell wall components in fruit. Postharvest Biology and Technology 2023, 206, 112551. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H(2) enhances arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 2012, 7, e49800. [Google Scholar] [CrossRef]
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the Role of Molecular Hydrogen in Plants. Plants (Basel, Switzerland) 2020, 9, 1136. [Google Scholar] [CrossRef]
- Iijima, M.; Yamashita, K.; Hirooka, Y.; Ueda, Y.; Yamane, K.; Kamimura, C. Ultrafine bubbles alleviated osmotic stress in soybean seedlings. Plant Production Science 2022, 25, 218–223. [Google Scholar] [CrossRef]
- Webb, S.E. Insect Management for Cucurbits (Cucumber, Squash, Cantaloupe, and Watermelon): ENY-460/IN168, rev. 9/2005. EDIS 2005, 2005.
- Luis Alonso-Prados, J.; Luis-Arteaga, M.; Alvarez, J.M.; Moriones, E.; Batlle, A.; Laviña, A.; García-Arenal, F.; Fraile, A. Epidemics of aphid-transmitted viruses in melon crops in Spain. European Journal of Plant Pathology 2003, 109, 129–138. [Google Scholar] [CrossRef]
- Van Cauwenbergh, N.; Biala, K.; Bielders, C.; Brouckaert, V.; Franchois, L.; Cidad, V.G.; Hermy, M.; Mathijs, E.; Muys, B.; Reijnders, J. SAFE—A hierarchical framework for assessing the sustainability of agricultural systems. Agriculture, ecosystems & environment 2007, 120, 229–242. [Google Scholar]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced plant resistance to herbivory, Springer: 2008; pp. 89–105.
- Handley, R.; Ekbom, B.; Ågren, J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of. Ecological Entomology 2005, 30, 284–292. [Google Scholar] [CrossRef]
- Lin, S.Y.; Trumble, J.T.; Kumamoto, J. Activity of volatile compounds in glandular trichomes of Lycopersicon species against two insect herbivores. Journal of chemical ecology 1987, 13, 837–850. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Hua, B.; Chang, J.; Wu, M.; Xu, Z.; Zhang, F.; Yang, M.; Xu, H.; Wang, L.J.; Chen, X.Y.; Wu, S. Mediation of JA signalling in glandular trichomes by the woolly/SlMYC1 regulatory module improves pest resistance in tomato. Plant Biotechnol J 2021, 19, 375–393. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol 2005, 31, 2211–2216. [Google Scholar] [CrossRef]
- Pattanaik, S.; Patra, B.; Singh, S.K.; Yuan, L. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front Plant Sci 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Oka, A.; Rodrigues-Pousada, R.; Possenti, M.; Ruberti, I.; Morelli, G.; Aoyama, T. Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 2003, 300, 1427–1430. [Google Scholar] [CrossRef]
- Oppenheimer, D.G.; Herman, P.L.; Sivakumaran, S.; Esch, J.; Marks, M.D. A Myb Gene Required for Leaf Trichome Differentiation in Arabidopsis Is Expressed in Stipules. Cell 1991, 67, 483–493. [Google Scholar] [CrossRef]
- Yoshida, Y.; Sano, R.; Wada, T.; Takabayashi, J.; Okada, K. Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 2009, 136, 1039–1048. [Google Scholar] [CrossRef]
- Zhao, M.; Morohashi, K.; Hatlestad, G.; Grotewold, E.; Lloyd, A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 2008, 135, 1991–1999. [Google Scholar] [CrossRef]
- Rerie, W.G.; Feldmann, K.A.; Marks, M.D. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev 1994, 8, 1388–1399. [Google Scholar] [CrossRef]
- Morohashi, K.; Zhao, M.; Yang, M.; Read, B.; Lloyd, A.; Lamb, R.; Grotewold, E. Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol 2007, 145, 736–746. [Google Scholar] [CrossRef]
- Bernhardt, C.; Lee, M.M.; Gonzalez, A.; Zhang, F.; Lloyd, A.; Schiefelbein, J. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 2003, 130, 6431–6439. [Google Scholar] [CrossRef]
- Schellmann, S.; Schnittger, A.; Kirik, V.; Wada, T.; Okada, K.; Beermann, A.; Thumfahrt, J.; Jurgens, G.; Hulskamp, M. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 2002, 21, 5036–5046. [Google Scholar] [CrossRef]
- Yan, X.; Cui, L.; Liu, X.; Cui, Y.; Wang, Z.; Zhang, H.; Chen, L.; Cui, H. NbJAZ3 is required for jasmonate-meditated glandular trichome development in Nicotiana benthamiana. Physiol Plant 2022, 174, e13666. [Google Scholar] [CrossRef]
- Sun, B.; Shang, L.; Li, Y.; Zhang, Q.; Chu, Z.; He, S.; Yang, W.; Ding, X. Ectopic Expression of OsJAZs Alters Plant Defense and Development. Int J Mol Sci 2022, 23, 4581. [Google Scholar] [CrossRef]
- English, N.J. Environmental Exploration of Ultra-Dense Nanobubbles: Rethinking Sustainability. Environments 2022, 9, 33. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.N.; Huang, X.; Ling, X.P.; Yu, M.; Cui, J.; Shabala, S. Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Functional Plant Biology 2020, 47, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J Plant Physiol 2016, 195, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Liu, X.; Wang, K.; Muhammad, T. Synergistic improvement in spring maize yield and quality with micro/nanobubbles water oxygation. Sci Rep 2019, 9, 5226. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Bastida, F.; Liu, Y.Z.; Zhou, Y.P.; He, J.; Song, P.; Kuang, N.K.; Li, Y.K. Nanobubble oxygenated increases crop production via soil structure improvement: The perspective of microbially mediated effects. Agricultural Water Management 2023, 282, 108263. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lyu, T.; Pan, G.; Li, P. Aquatic macrophytes in morphological and physiological responses to the nanobubble technology application for water restoration. ACS ES&T Water 2020, 1, 376–387. [Google Scholar]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef]
- Hancock, J.T.; LeBaron, T.W.; May, J.; Thomas, A.; Russell, G. Molecular hydrogen: is this a viable new treatment for plants in the UK? Plants 2021, 10, 2270. [Google Scholar] [CrossRef]
Figure 1.
Ultrafine water affected seed germination and rooting of melons. (A) Effects of ultrafine water (UFW) on melon seed germination. Four melon varieties, each with 40 seeds, were germinated in petri dishes containing RO water and UFW. Arrows show the presence of root hairs on the root at 1 day after seed germination. (B) Germination rate of melon seeds at 7 days after germination. (C) Melon seedlings grown in plug trays containing peat moss at 7 days after sowing (DAS). Arrows show vigorous root development.
Figure 1.
Ultrafine water affected seed germination and rooting of melons. (A) Effects of ultrafine water (UFW) on melon seed germination. Four melon varieties, each with 40 seeds, were germinated in petri dishes containing RO water and UFW. Arrows show the presence of root hairs on the root at 1 day after seed germination. (B) Germination rate of melon seeds at 7 days after germination. (C) Melon seedlings grown in plug trays containing peat moss at 7 days after sowing (DAS). Arrows show vigorous root development.
Figure 2.
UFW irrigation affected the growth of melon seedlings. (A) The phenotype of melon potted plants at 14 days after transplantation (DAT). Bars, 10 cm. (B) Plant height of melons (n=10-21). (C) Flower number per plant at 14 DAT.
Figure 2.
UFW irrigation affected the growth of melon seedlings. (A) The phenotype of melon potted plants at 14 days after transplantation (DAT). Bars, 10 cm. (B) Plant height of melons (n=10-21). (C) Flower number per plant at 14 DAT.
Figure 3.
UFW irrigation affected aphid infestation on melon seedlings. (A) Phenotype of melon leaves attacked by aphids at 14 days after transplantation. Scale bars, 2 mm. (B) Rating of melon leaf aphid infestation. A rating of 0 indicates no aphids were observed, and 9 indicates a high aphid density. (C) Aphids attacked the young flower buds of melon. (D) Cryo-SEM showed aphid infestation on flower buds of CK (D) and UFW (E). The arrowheads point to the aphids. Scale bars, 500 µm (D, E).
Figure 3.
UFW irrigation affected aphid infestation on melon seedlings. (A) Phenotype of melon leaves attacked by aphids at 14 days after transplantation. Scale bars, 2 mm. (B) Rating of melon leaf aphid infestation. A rating of 0 indicates no aphids were observed, and 9 indicates a high aphid density. (C) Aphids attacked the young flower buds of melon. (D) Cryo-SEM showed aphid infestation on flower buds of CK (D) and UFW (E). The arrowheads point to the aphids. Scale bars, 500 µm (D, E).
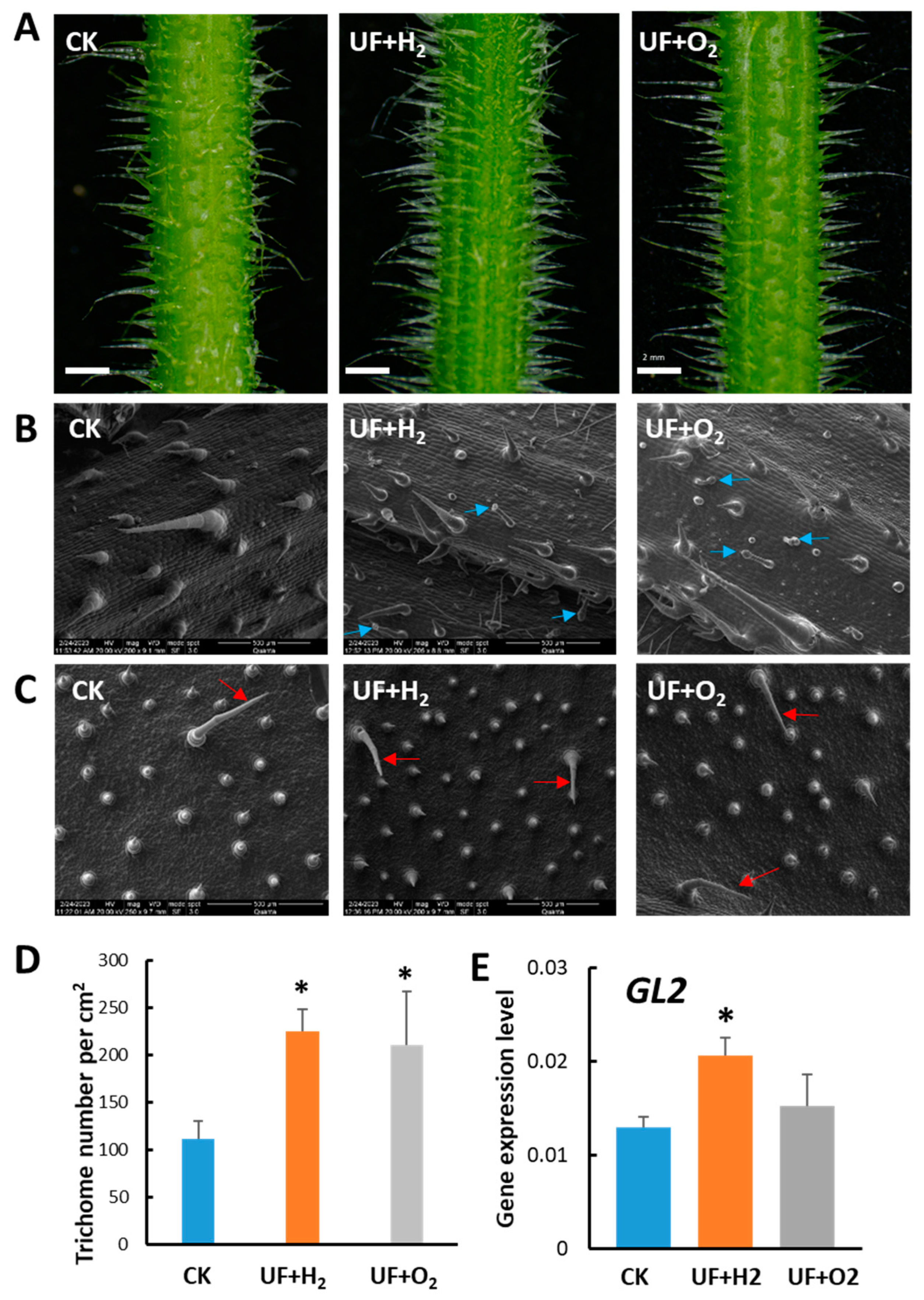
Figure 4.
Hydrogen-rich or oxygen-rich ultrafine water irrigation affected the development of trichomes in melon cv. “Camilla”. (A) Dissecting microscope observation of the development of trichomes in melon petioles after irrigation with ultrafine water enrichment of hydrogen (UF+H2), oxygen (UF+O2), and RO water (Ck), respectively. Bars, 2mm. (B) Cryo-scanning electron microscope (cryo-SEM) showing trichomes on the midribs of the melons. (C) Cryo-SEM showed the development of trichomes on the abaxial of newly established young leaves of melon. (D) Trichome density in melon petioles irrigated with RO water, UF+H2, and UF+O2, n = 3 to 6. Arrows show trichomes. Blue arrows indicate the presence of granular trichomes. (E) RT-qPCR showed GLABRA2 (GL2) gene expression patterns in young melon leaves irrigated with UF+H2, UF+O2, and RO water control (CK).
Figure 4.
Hydrogen-rich or oxygen-rich ultrafine water irrigation affected the development of trichomes in melon cv. “Camilla”. (A) Dissecting microscope observation of the development of trichomes in melon petioles after irrigation with ultrafine water enrichment of hydrogen (UF+H2), oxygen (UF+O2), and RO water (Ck), respectively. Bars, 2mm. (B) Cryo-scanning electron microscope (cryo-SEM) showing trichomes on the midribs of the melons. (C) Cryo-SEM showed the development of trichomes on the abaxial of newly established young leaves of melon. (D) Trichome density in melon petioles irrigated with RO water, UF+H2, and UF+O2, n = 3 to 6. Arrows show trichomes. Blue arrows indicate the presence of granular trichomes. (E) RT-qPCR showed GLABRA2 (GL2) gene expression patterns in young melon leaves irrigated with UF+H2, UF+O2, and RO water control (CK).
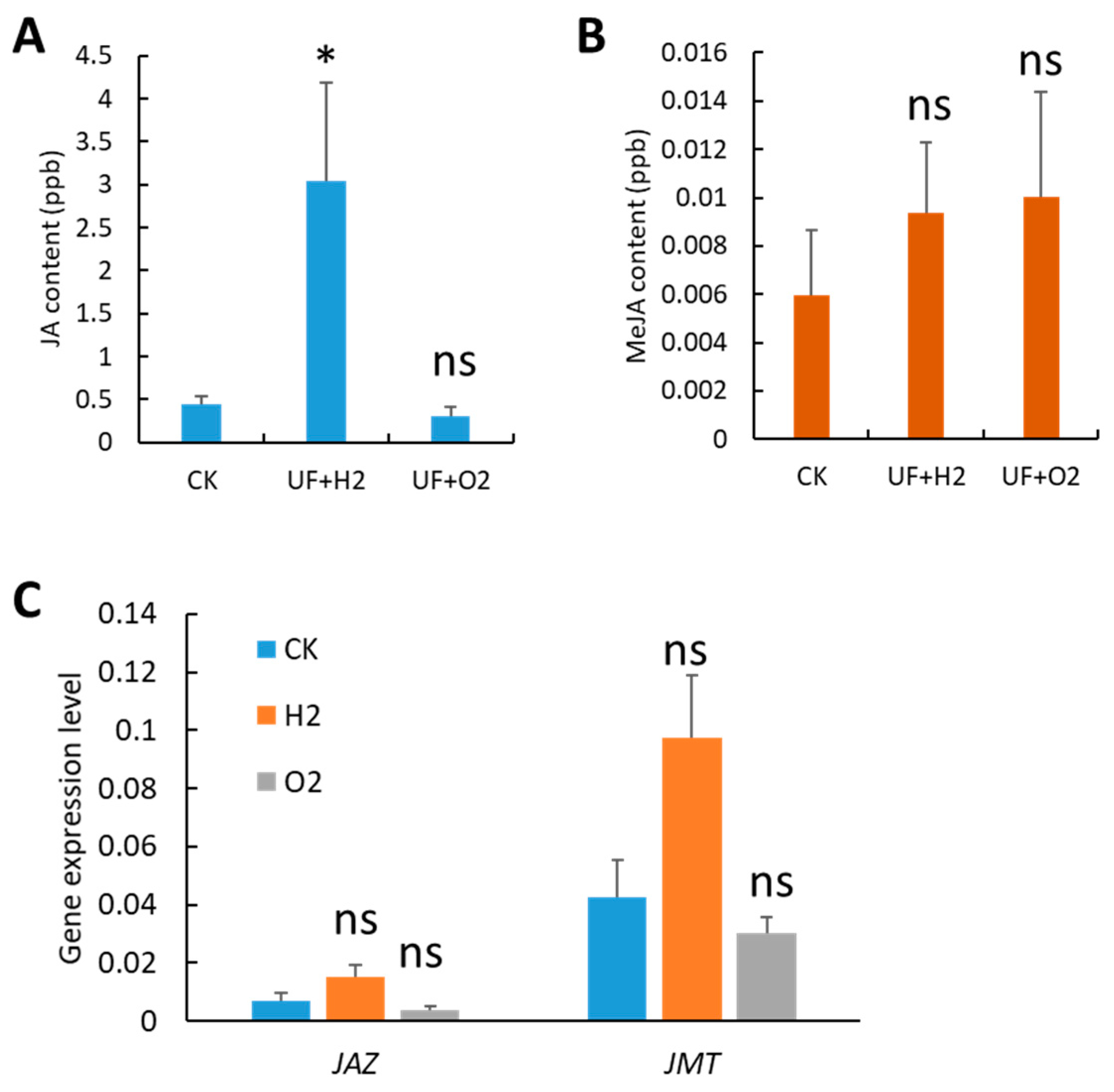
Figure 5.
Melon irrigated with hydrogen- and oxygen- rich ultrafine water altered jasmonic acid (JA) and methyl-JA (MeJA) contents, and gene expression patterns. (A) JA content. (B) MeJA content. (C) The gene expression level of JASMONATE ZIM DOMAIN PROTEIN (JAZ) and JA carboxyl methyltransferase (JMT). The gene expression level was normalized to two housekeeping genes: Actin (MELO3C023264) and ADP ribosylation factor 1 (ADP, MELO3C023630). Error bars represent the standard error of the mean (n= 3). Student’s t-test was used to find the significant difference between CK and UF+H2 or UF+O2 treatment. *, p < 0.05; n.s., not significant.
Figure 5.
Melon irrigated with hydrogen- and oxygen- rich ultrafine water altered jasmonic acid (JA) and methyl-JA (MeJA) contents, and gene expression patterns. (A) JA content. (B) MeJA content. (C) The gene expression level of JASMONATE ZIM DOMAIN PROTEIN (JAZ) and JA carboxyl methyltransferase (JMT). The gene expression level was normalized to two housekeeping genes: Actin (MELO3C023264) and ADP ribosylation factor 1 (ADP, MELO3C023630). Error bars represent the standard error of the mean (n= 3). Student’s t-test was used to find the significant difference between CK and UF+H2 or UF+O2 treatment. *, p < 0.05; n.s., not significant.
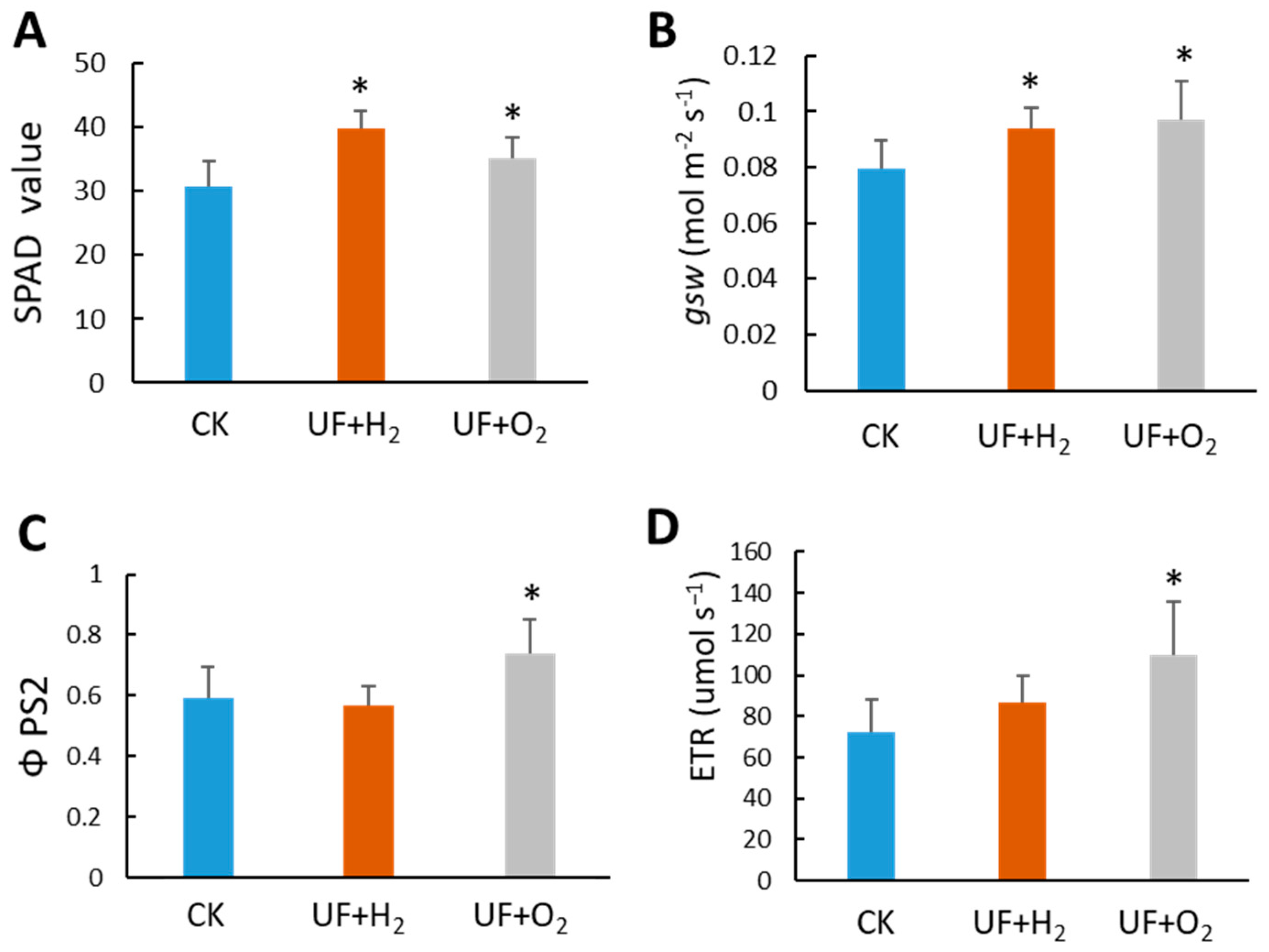
Figure 6.
Ultrafine water irrigation affected the photosynthesis capacity of melons. (A) Chlorophyll content in melons. The SPAD value was measured on the 4th leaf at the late stage of fruit maturity. n = 4 plants. The Li600 Porometer/Fluorometers meter detected the photosynthesis parameters of (B) stomatal conductance (gsw); (C) ΦPSII, the quantum yield of PSII calculated from fluorescence, and (D) the electron transport rate (ETR) of L1 melon leaves. Student’s t-test was used to find the significant difference between UFW and the regular tap water (CK). *, p < 0.05; n.s., no significant difference. Error bars represent the standard error of the mean (n= 4).
Figure 6.
Ultrafine water irrigation affected the photosynthesis capacity of melons. (A) Chlorophyll content in melons. The SPAD value was measured on the 4th leaf at the late stage of fruit maturity. n = 4 plants. The Li600 Porometer/Fluorometers meter detected the photosynthesis parameters of (B) stomatal conductance (gsw); (C) ΦPSII, the quantum yield of PSII calculated from fluorescence, and (D) the electron transport rate (ETR) of L1 melon leaves. Student’s t-test was used to find the significant difference between UFW and the regular tap water (CK). *, p < 0.05; n.s., no significant difference. Error bars represent the standard error of the mean (n= 4).
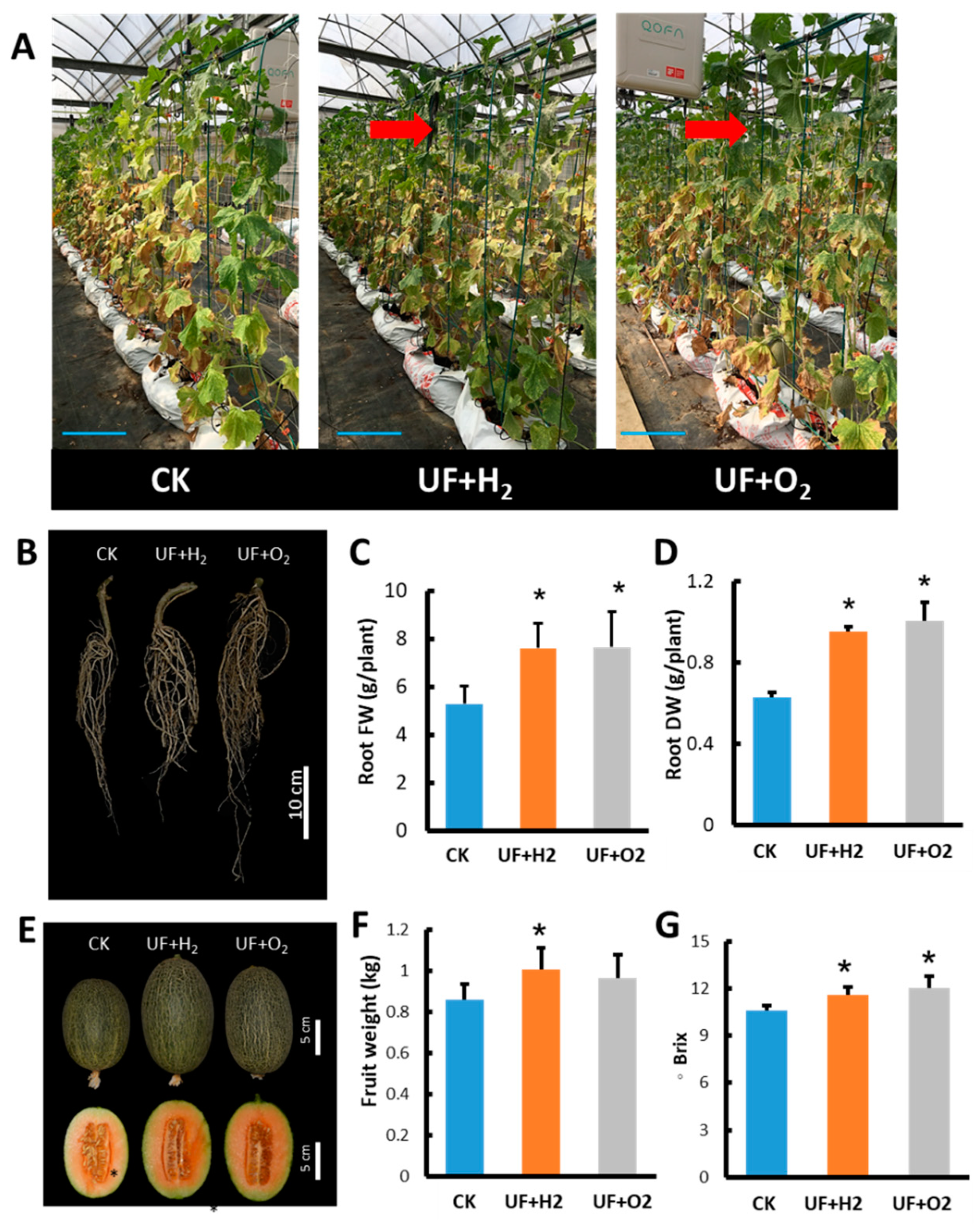
Figure 7.
UFW irrigation affected fruit weight and sweetness of melon cv. “Camilla”. (A) Melons were planted in a greenhouse. Photo taken at 42 days after pollination. Bars, 20 cm. (B) Root morphology at harvest stage. (C) Root fresh weight of each plant. (D) Root dry weight per plant. (E) Melon fruits at 5 days after harvest. (F) Average fruit weight of melon. (G) The sweetness of melon fruits. UF+H2, hydrogen-rich ultrafine water irrigation. UF+O2, oxygen-rich ultrafine water irrigation. CK, irrigated with tap water. Bars, standard deviation of 22 plants. Student’s t-test was used to find significant difference between CK and UF+H2 or UF+O2 treatment. *, p < 0.05; **, p < 0.01; n.s., not significant.
Figure 7.
UFW irrigation affected fruit weight and sweetness of melon cv. “Camilla”. (A) Melons were planted in a greenhouse. Photo taken at 42 days after pollination. Bars, 20 cm. (B) Root morphology at harvest stage. (C) Root fresh weight of each plant. (D) Root dry weight per plant. (E) Melon fruits at 5 days after harvest. (F) Average fruit weight of melon. (G) The sweetness of melon fruits. UF+H2, hydrogen-rich ultrafine water irrigation. UF+O2, oxygen-rich ultrafine water irrigation. CK, irrigated with tap water. Bars, standard deviation of 22 plants. Student’s t-test was used to find significant difference between CK and UF+H2 or UF+O2 treatment. *, p < 0.05; **, p < 0.01; n.s., not significant.
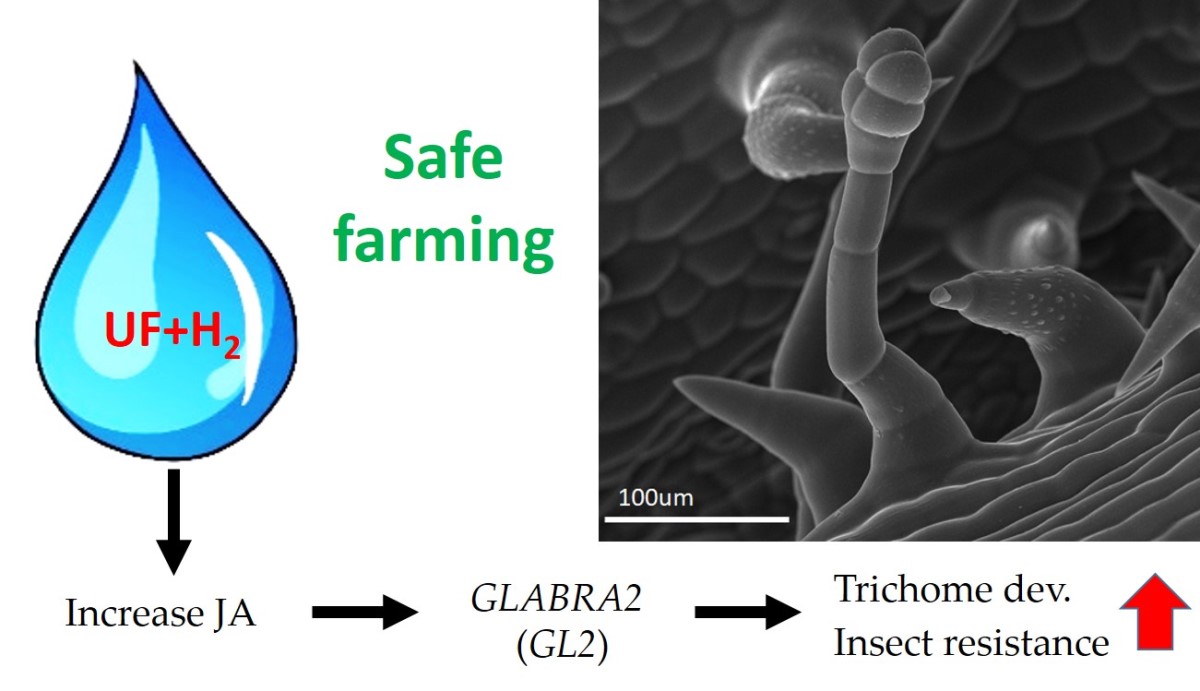
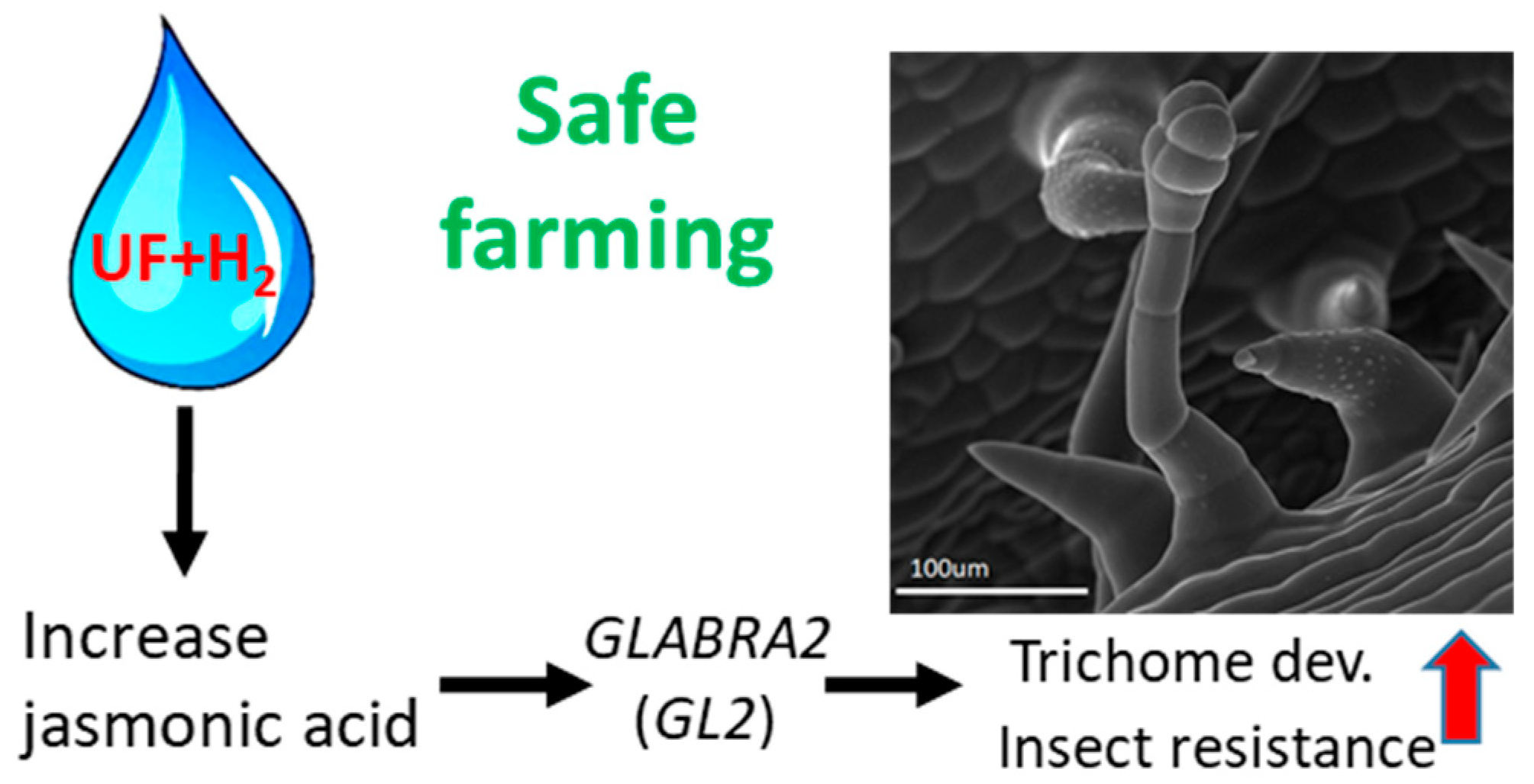
Figure 8.
Working model of this study. Hydrogen-rich ultrafine bubble water can increase the content of jasmonic acid, increase the gene expression of GLABRA2, promote the development of trichomes, prevent melon insect damage, and achieve the ultimate goal of safe farming.
Figure 8.
Working model of this study. Hydrogen-rich ultrafine bubble water can increase the content of jasmonic acid, increase the gene expression of GLABRA2, promote the development of trichomes, prevent melon insect damage, and achieve the ultimate goal of safe farming.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).