1. Introduction
In recent years, depression has become a serious global health problem. The World Health Organization predicts that by 2030, depression will rise from the third highest global burden of diseases to the first (WHO, 2008). The biological mechanisms of depression are complex and mianly related to the multiple different systems and targets (Malhi & Mann, 2018), which poses great difficulties to the development of depression drugs. Frontline antidepressants such as selective serotonin reuptake inhibitors (SSRIs) not only take a long time to produce therapeutic effects, but are often accompanied by serious side effects such as sexual dysfunction, weight gain, nausea, and headache (Moret, Isaac, & Briley, 2009). Therefore, it is urgent to discover new methods and targets for the treatment of depression.
Patients with depression usually experience an increase in systemic inflammation and neuroinflammation, and the inflammatory process is related to the pathophysiology of depression (Beurel, Toups, & Nemeroff, 2020). Inflammatory response is the result of immune system activation. Increasing evidence suggests that there are different types of immune cell behavior imbalances in mental illnesses, including depression. Immune cells release cytokines, chemokines, and secondary inflammatory mediators such as prostaglandins in response to inflammatory stimuli (Leonard, 2018). Microglia plays an important role in various physiological processes of the central nervous system, including immunity, inflammation, synaptic transmission, neural plasticity, maintenance of neural networks, and tissue damage repairments (Tremblay et al., 2011). Behavioral imbalance of microglia is considered as an important trigger for inflammatory response in depression. However, studies have shown that by regulating the polarization phenotype of microglia, which is usually simplified as the M1/M2 polarization phenotype, it is possible to antagonize behavioral imbalance of microglia, counteract inflammatory damage, and promote the recovery and remodeling of neural tissue (Guo, Wang, & Yin, 2022). Therefore, inhibition of overactivated inflammatory M1 microglia by switching to the protective M2 phenotype is considered as one of the potential therapeutic targets of depression.
Polygonum sibiricum is a Chinese traditional food and medicine homologues with multiple active ingredients and extensive pharmacological effects. Polygonum sibiricum polysaccharides (PSP) are one of the main active ingredients with the antidepressant activity. PSP could reverse the depressive-like behavior in lipopolysaccharides (LPS)- induced mice by inhibiting oxidative stress, hypothalamic-pituitary-adrenal (HPA) axis hyperfunction, and inflammatory response (Shen et al., 2021). PSP also exerted the antidepressant effects by regulating the oxidative stress-calpain-1-NLRP3 signaling axis (Shen et al., 2022). PSP also could improve the performance of acute despair mouse in forced swimming and tail suspension tests, which may be related to reduce the monoamine neurotransmitter levels, inhibit the release of inflammatory cytokines, and regulate tryptophan metabolism (Wei et al., 2022). PSP could restore beneficial gut microbiota, improve intestinal barrier integrity, and exert antidepressant effects through the microbiota gut brain axis (Zhang et al., 2023; Zhang et al., 2023). However, it is unclear whether PSP could inhibit the activation of microglia and promote M2 phenotype polarization, and its related molecular mechanisms still need further research.
Therefore, in order to provide a new perspective for the potential of PSP in the prevention and treatment of depression, the current study was conducted to evaluate the potential effects of PSP on M1/M2 polarization of microglia and its molecular mechanisms on the LPS-induced BV2 cell activation model.
2. Materials and Methods
2.1. Materials
BV2 mouse microglial line was purchased from Wuhan sunncell Bio-Technology Co., Ltd (Wuhan, China); fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) from Gibco; PSP from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China); lipopolysaccharides (LPS) from Sigma; enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor (TNF-α), interleukin (IL)-6, IL-1β and IL-10 from Jianglai Biological Technology Co., Ltd (Shanghai, China); Nitric Oxide (NO) assay kit, CCK-8 assay kit and DCFH-DA Reactive Oxygen (ROS) Fluorescent Probe from Solarbio Science & Technology Co., Ltd (Beijing, China). Antibodies targeting iNOS, BDNF, ERK, Phospho-ERK, CREB, Phospho-CREB, TrkB and Phospho-TrkB from Abcam; antibody targeting Arginase-1 from Cell Signaling Technology; antibodies targeting Iba-1, Notch1 and Hes1 from Santacruz; goat anti-rabbit AlexFluor 488® secondary antibodies from Servicebio Co., Ltd (Shanghai, China); CD16/CD32 monoclonal antibody PE and CD206 (MMR) monoclonal antibody PE from Ebioscience.
2.2. Cell Culture and Treatment
The BV2 mouse microglial cell lines were cultured in DMEM medium containing 10% FBS, 5% CO2, at 37°C. Cells were passaged every 24 h. After 24 h, the experiment was performed with control group, LPS group (1 μg/mL), and PSP groups (400, 800 and 1000 μg/mL). PSP was added to pre-activate for 24 h, and then 1 μg/mL of LPS was added to co-stimulate for 12 h.
2.3. Cell Viability Assay
BV2 cells were seeded in a 96-well plate at a density of 5 × 103 cells/well. After 12 h, cells were pretreated with different concentrations of PSP for 24 h, and incubated with or without LPS for 12 h. 10 μL of CCK-8 solution was added to each well. After incubation for 1 h at 37 °C, the absorbance at 450 nm was read on a microplate reader.
2.4. NO Assay
Seeding BV2 cells in the logarithmic growth phase into a 24-well culture plate at a density of 2.5 × 104 cells/well. After 12 h, cells were pretreated with different concentrations of PSP for 24 h, and incubated with or without LPS for 24 h. The NO contents in the supernatant were determined following NO assay kit protocol (Beyotime Biotechnology, Shanghai, China).
2.5. ROS Assay
BV2 cells were cultured overnight in a 24-well culture plate at a density of 5 × 104 cells/well, then exposed to different concentrations of PSP for 24 h, and incubated with or without LPS for 6 h. The cells were stained with 4 μM DCFH-DA fluorescent probe (Solarbio Science & Technology, China) for 10 min at 37℃ and washed with PBS. All images were observed with fluorescence microscopy (Olympus, Japan) and analyzed by Image J software (National Institutes of Health, USA).
2.6. Quantitative PCR (qPCR) Assay
Seeding BV2 cells into a 6-well culture plate at a density of 1 × 10
5 cells/well. After 12 h, cells were pretreated with different concentrations of PSP for 24 h, and incubated with or without LPS for 12 h. RNA was isolated according to the EASYspinPlus rapid tissue/cellular RNA extraction kit (Aidlab Biotechnologies, China). GoScript Reverse Transcription System (Promega Biotech, China) was used for synthesis of cDNA. qRT-PCR was performed in Applied Biosystems QuantStudio 7 Flex system (Thermo FisherScientific, United States) using NovoStart SYBR qPCR SuperMix Plus kit (Novoprotein, China). The amplification parameters were 95°C for 1 min, followed by 40 cycles of 95°C for 2 s and 60°C for 1 min. Relative gene expression was calculated using the 2
-ΔΔCT method and normalized with control gene β-actin. Primers information is shown in
Table 1.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
Seeding BV2 cells into a 6-well culture plate at a density of 1 × 105 cells/well. After 12 h, cells were pretreated with different concentrations of PSP for 24 h, and incubated with or without LPS for 12 h. The supernatant was collected to measure the levels of TNF-α, IL-1β, IL-6 and IL-10 using commercial ELISA kits (Jianglai Biological Technology, Shanghai, China) based on the manufacturer's instructions.
2.8. Immunofluorescence Staining
BV2 cells were seeded in 6-well culture plate at a density of 1 × 105 cells/well, and cultured and treated as before mentioned. BV2 cells were fixed with 4% paraformaldehyde for 10 min at room temperature, then permeabilized using 0.5% Triton X-100 for 30 min on the ice. The cells were blocked with 5% BSA for 1 h. The cells were incubated with iNOS (1:50, ab283655) and Arg-1 (1:50, #93668) primary antibodies at 4°C overnight. On the following day, cells were washed with PBS and incubated with AlexFluor 488® (1:500) secondary antibodies at 37°C for 1 h. The cells were treated with an anti-fade mounting medium with DAPI for 10 min at 37°C. All images were observed with fluorescence microscopy (Olympus, Japan) and analyzed by Image J software (National Institutes of Health, USA).
2.9. Flow Cytometry
BV2 cells in 6-well culture plate were cultured and treated as indicated. Then, the cells were washed and re-suspended in cold PBS at a density of 1 × 106 cells/mL. Added 0.125 μg of anti-CD16/CD32 PE (93), and anti-CD206 PE (MR6F3), separately. Incubated at room temperature in dark for 30 min. The cells were washed twice with PBS and re-suspended in 400 μL PBS. The samples were analyzed using flow cytometry (CytoFLEX, USA).
2.10. Western Blot Analysis
BV2 cells were seeded in 6-well culture plate at a density of 1 × 105 cells/well, cultured and treated as mentioned above. BV-2 microglia were washed with pre-cooled PBS. The samples were collected by centrifugation (1000 rpm, 5 min). Then added the RIPA buffer (containing PMSF) and incubated on ice for 10 min, followed by centrifugation (12,000g, 10 min, 4℃) to collect the supernatant. The protein concentration was determined by BCA Kit (Beyotime, Nanjing, China). According to the protein quantitative results, the protein concentrations were unified. Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes for 120min at 300mA. The membranes were sequentially incubated with primary (BDNF, ab108319; ERK, ab32537; CREB, ab32515; Phospho-CREB, ab32096; TrkB, ab187041; Phospho-TrkB, ab229908; Notch1, sc-376403-1; Hes1, sc-166410-1) and secondary antibodies and enhanced chemiluminescence (ECL) solutions, followed by autoradiography. The intensity of the blot was analyzed using Image pro plus 6.0.
2.11. Statistical Analysis
Statistical analysis was performed using SPSS 23.0, GraphPad Prism 8.0 and ImageJ software, and the results were expressed as mean ± standard error (Mean ± SEM). Multiple group comparisons were performed using one-way ANOVA, while pairwise comparisons between groups were performed using the Least Significant Difference (LSD) method. P<0.05 was considered statistically significant.
3. Results
3.1. Effects of PSP on the Viability of BV2 Cells and Microglial Activation
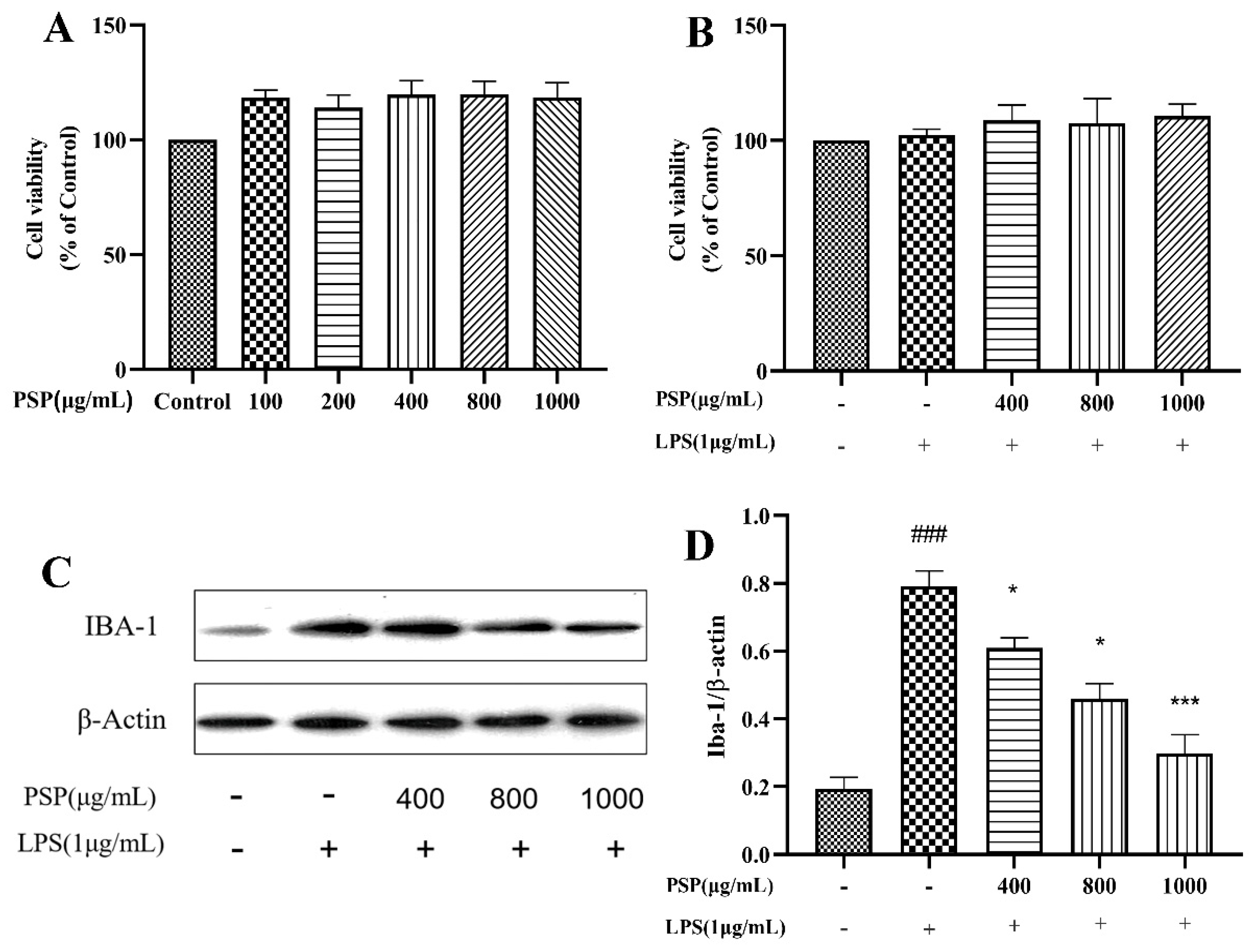
The effects of different concentrations of PSP on BV2 cell viability was shown in
Figure 1A. PSP concentrations ranging from 100 to 1000 μg/mL did not decrease the viability of BV2 cells after 24 h treatment. PSP did not induce any detectable cytotoxicity compared with the control group. The combined effects of PSP and LPS on BV2 cell viability was shown in
Figure 1B. The CCK-8 results showed that PSP at different concentrations (400,800,1000 μg/mL) with or without LPS (1 μg/mL) had no significant toxic effects on cell viability. Thus, 400,800 and1000 μg/mL of PSP were used in subsequent experiments.
Iba-1 is a marker protein of microglia. To investigate the effects of PSP on LPS-induced microglial activation, the protein expression levels of Iba-1 in BV2 cells were evaluated. As shown in
Figure 1C and D, LPS induced microglial activation. The protein expression level of Iba-1 was significantly increased after LPS stimulation (
P<0.001). However, PSP intervention significantly reduced the protein expression level of Iba-1 (
P<0.05,
P<0.001). These results indicated that PSP pretreatment could reverse LPS induced microglial activation.
3.2. Effects of PSP on Intracellular Reactive Oxygen Species in LPS-Induced BV2 Cells
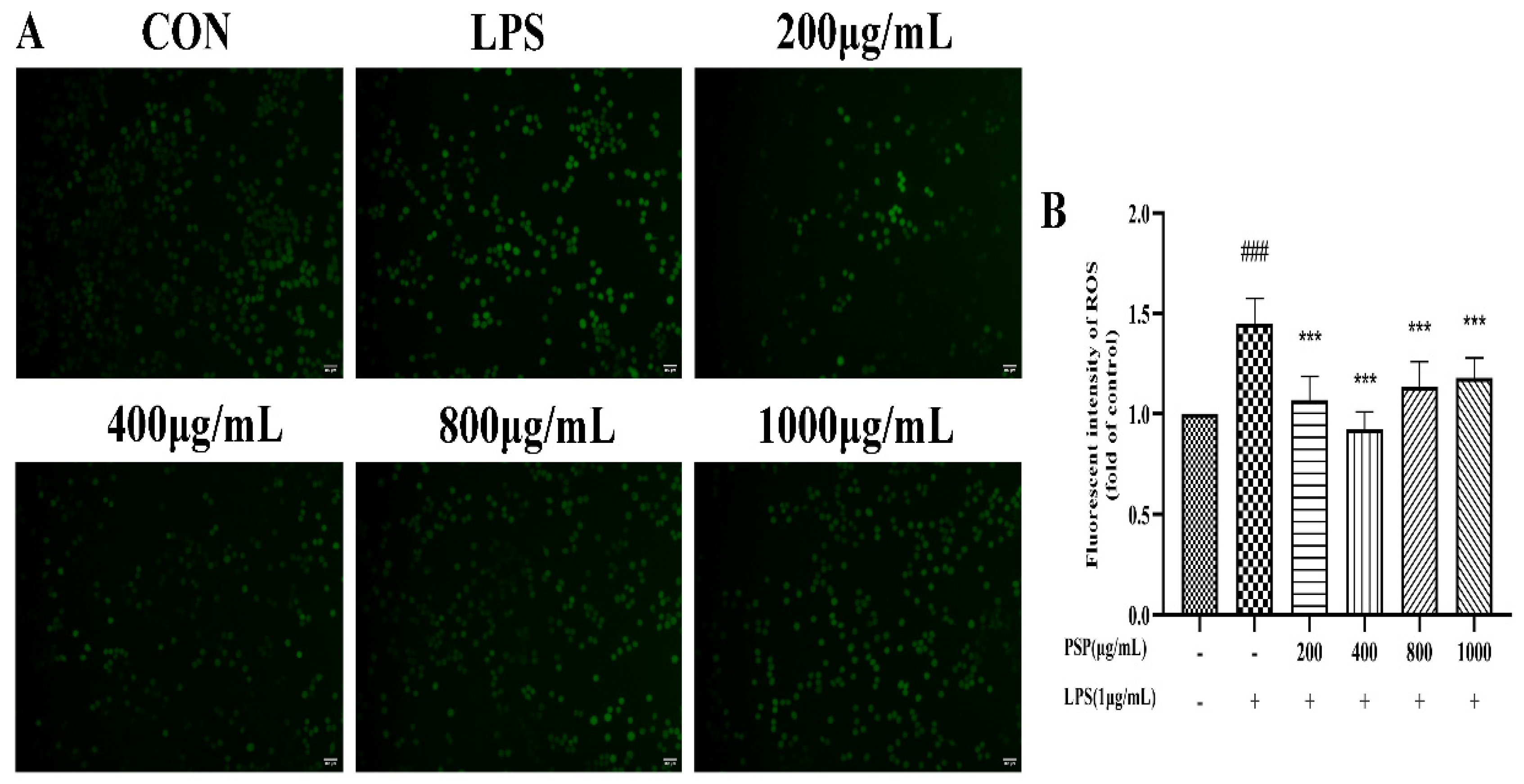
The fluorescence intensity of LPS group was substantially higher than the control group (
Figure 2,
P<0.001). LPS stimulation increased ROS production in BV2 cells. Compared with the LPS group, PSP (200, 400, 800, 1000 μg/mL) pretreatment significantly reduced fluorescence intensity (
P<0.01,
P<0.001), indicating that PSP could inhibit LPS induced oxidative damage in BV2 cells to a certain extent.
3.3. Effects of PSP on the Production of NO and Inflammatory Cytokine in LPS-Induced BV2 Cells
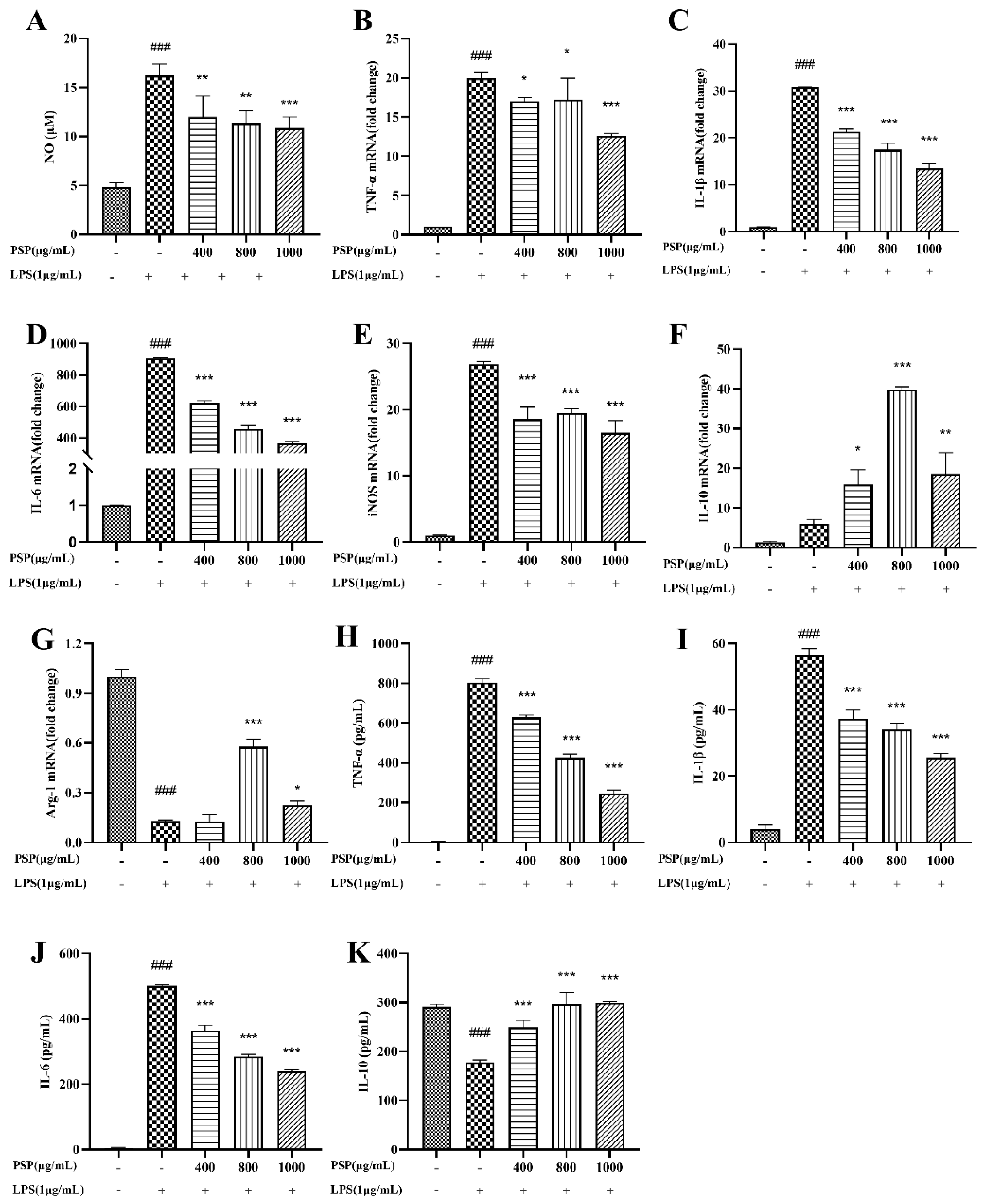
Results showed (
Figure 3A) that NO level was significantly increased after LPS treatment compared with the control group. PSP (400, 800, 1000 μg/mL) reduced NO levels in a dose-dependent manner. Both qRT-PCR and ELISA were used to determine the effects of PSP on the expression and release of inflammatory factors in LPS induced BV2 cells. The qRT-PCR results (
Figure 3B-G) showed that LPS stimulation significantly increased the expression level of M1 pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and iNOS) and decreased M2 anti-inflammatory cytokines (Arg-1) (
P<0.001). PSP intervention significantly decreased the mRNA levels of M1 markers (TNF-α, IL-1β, IL-6 and iNOS) and increased M2 marker (IL-10, Arg-1) (
P<0.05,
P<0.01,
P<0.001). Consistent with the results of q-PCR, ELISA (
Figure 3H-K) showed that treatment with LPS significantly increased the level of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) while the anti-inflammatory cytokine (IL-10) level was markedly decreased (
P<0.001). These changes were reversed in the presence of PSP (
P<0.001). These results indicated that PSP could promote the polarization of BV2 cells from the M1 to M2 phenotype, inhibit the expression and release of pro-inflammatory cytokines, exerting the anti-inflammatory effects.
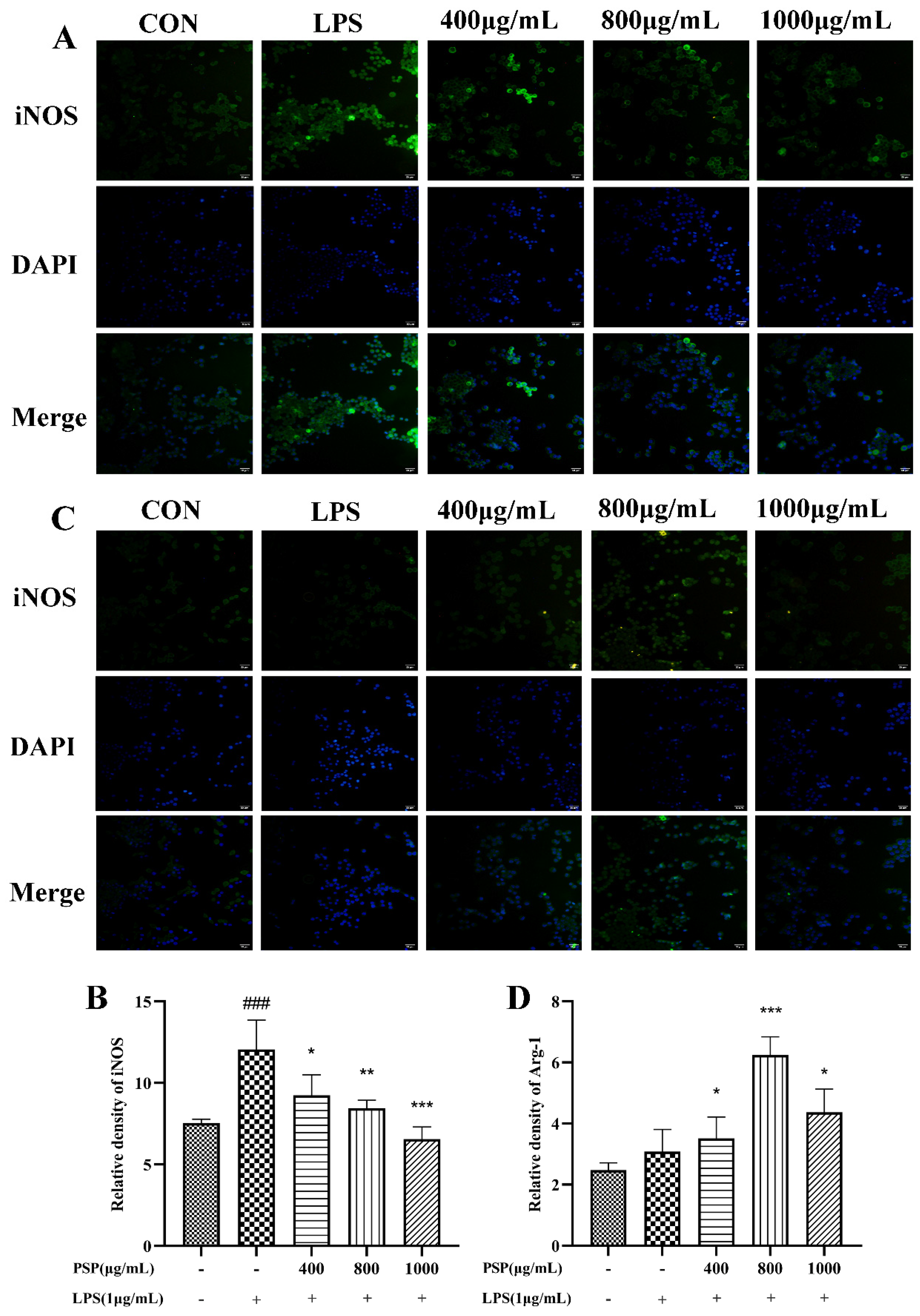
3.4. PSP Promoted Microglial Polarization to the M2 Phenotype in LPS-Induced BV2 Cells
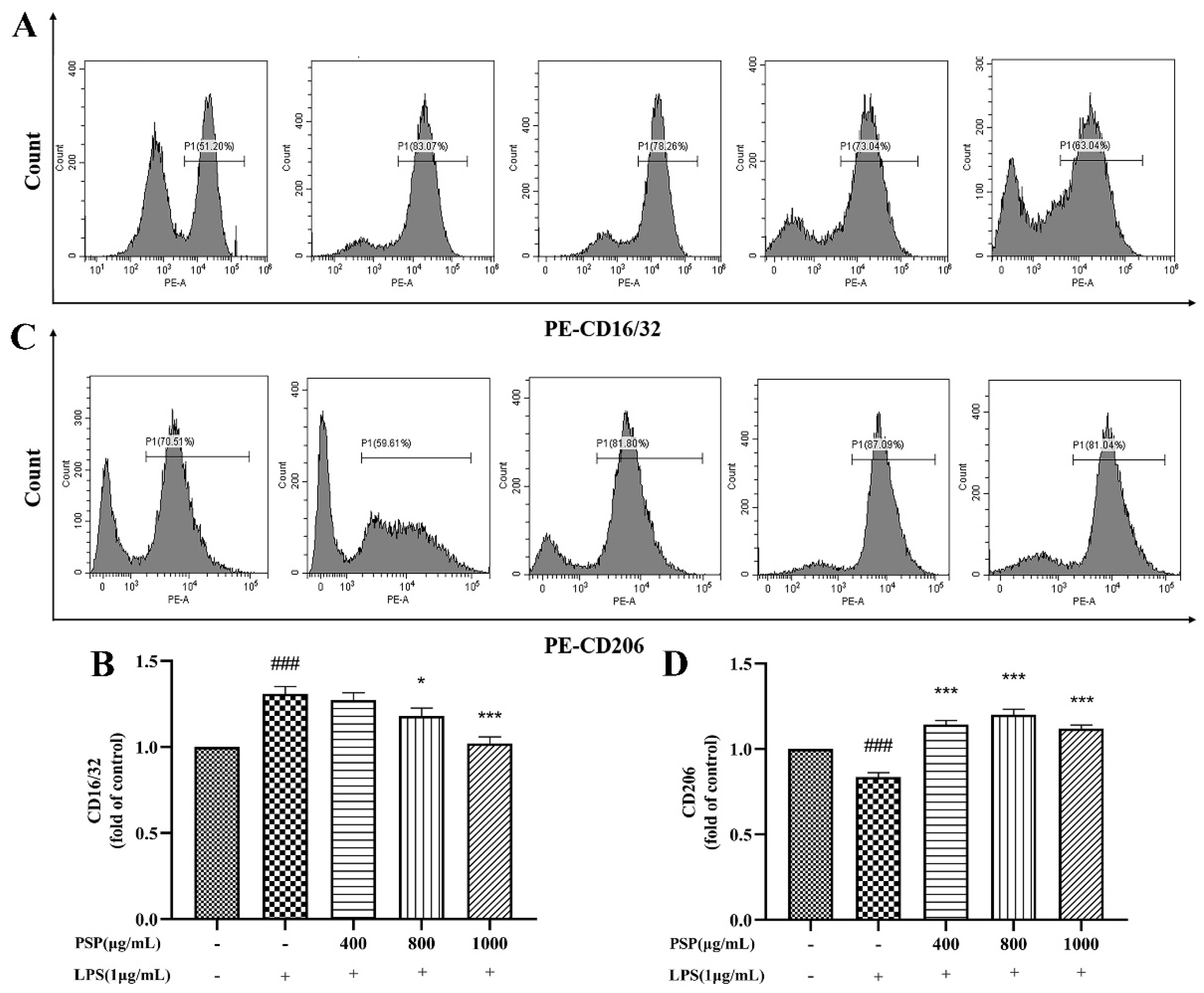
CD16/32 and iNOS were markers of M1 polarization in microglia, while CD206 and Arg-1 are markers of M2 polarization. The effects of PSP on polarization phenotype of BV2 cells was analyzed by flow cytometry and immunofluorescence staining. Firstly, the protein expression levels of CD16/32 and CD206 were measured by flow cytometry. As shown in
Figure 4, LPS treatment significantly increased the protein expression of CD16/32 and reduced CD206 (
P<0.001). The protein expression of CD16/32 was markedly downregulated and CD206 was upregulated by PSP pretreatment compared with LPS group (
P<0.05,
P<0.001). Then, the expression of iNOS and Arg-1 were further measured by immunofluorescence staining. As shown in
Figure 5, the fluorescence intensity of iNOS in the LPS group was significantly increased (
P<0.001), and PSP intervention significantly reduced the fluorescence intensity of iNOS (
P<0.05,
P<0.01,
P<0.001). Although the fluorescence intensity of Arg-1 in the LPS group did not significantly change compared with the control group, PSP pretreatment obviously enhanced the immunoreactivity of Arg-1 (
P<0.05,
P<0.001). These results suggested that PSP inhibited microglial polarization to the M1 phenotype and promoted microglial polarization to the M2 phenotype.
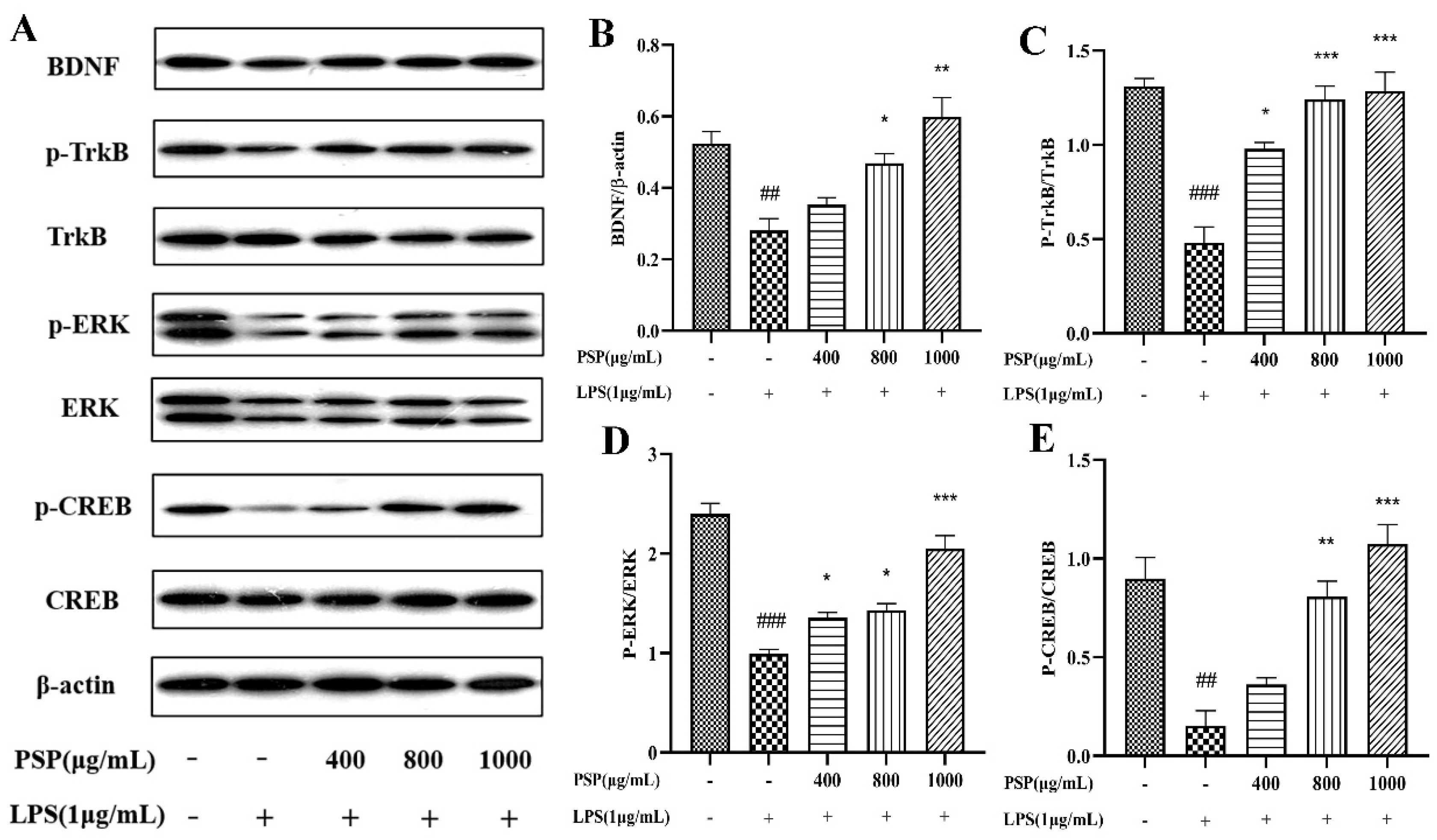
3.5. Effects of PSP on the BDNF/TrkB/CREB Signaling Pathway in LPS-Induced BV2 Cells
As shown in
Figure 6, the results indicated that the expressions of BDNF, p-TrkB/TrkB and p-CREB/CREB were remarkably decreased in the LPS group when compared with that of the control group (
P < 0.01,
P<0.001). Meanwhile, PSP pretreatment significantly reversed these changes (
P<0.05,
P < 0.01,
P <0.001). The results indicated that PSP could regulate the BDNF/TrkB/CREB pathway, which may probably be contribute to the regulation of microglial polarization.
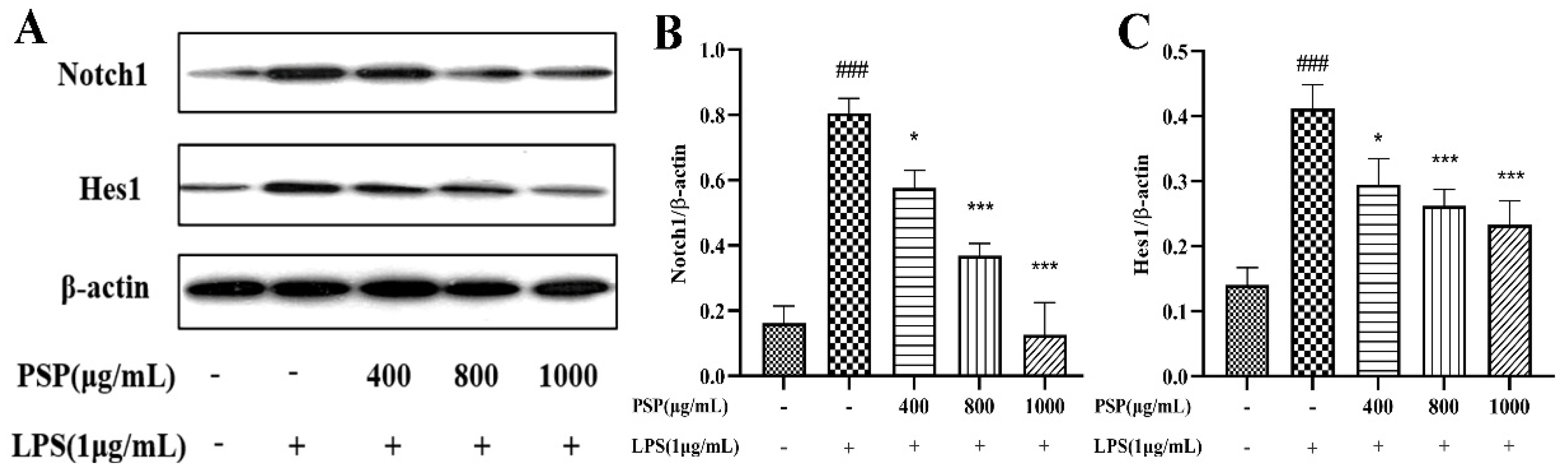
3.6. Effects of PSP on the Notch Signaling Pathway in LPS-Induced BV2 Cells
As shown in
Figure 7, we measured the expressions of Notch1 and Hes1. The results indicated that the expressions of Notch1 and Hes1 were remarkably increased in the LPS group when compared with that of the control group (
P < 0.001). Meanwhile, PSP pretreatment significantly reversed these changes (
P <0.05,
P <0.001). The results indicated that PSP could regulate the Notch pathway, which may be related to the regulation of microglial polarization.
4. Discussion
In recent years, a growing number of studies have shown that neuroinflammation plays a crucial role in the function of the central nervous system(CNS), such as controlling stress response, emotion and cognitive activities (Kip & Parr-Brownlie, 2023), and inflammation has a dual effect on CNS. Acute inflammatory reactions help maintain the body balance, eliminate necrotic and damaged cells, initiate tissue repair, and exert protective effects. However, excessive inflammatory reactions often release the multiple inflammatory and toxic factors, resulting in toxic damage and various diseases, including depression. Among them, the imbalance of M1/M2 polarization of microglia is considered to be the key factor affecting the severity of neuroinflammation. M1 phenotype leads to chronic neuroinflammatory reaction, induce the release of proinflammatory cytokines such as TNF-α and IL-1β, exacerbate tissue damage, and further aggravate the pathological process of depression. M2 phenotype promotes damage repair and functional recovery by releasing the anti-inflammatory factors such as IL-4 and IL-10 and some neurotrophic factors (Yang et al., 2023). Therefore, current research believes that in the treatment of neuroinflammatory-related diseases, inhibiting M1 phenotype while activating M2 phenotype polarization may bring more significant overall effects than inhibiting M1-microglia alone.
The M1/M2 phenotype of Microglia represents two extremes of the neuroinflammatory phenotype. Studies show that different phenotypes can also be divided into different subtypes, for example, M2 phenotype can also be divided into M2a, M2b, M2c, and M2d subtypes (Franco & Fernandez-Suarez, 2015), however, these are not completely independent activation states. Therefore, Simple classification of microglia into classic activated-M1 phenotype and alternative activated-M2 phenotype is the most common method to clarify the role of microglia in the CNS. At present, the identification of M1/M2 phenotype mainly focuses on three aspects, cell surface specific molecule expression, cell secretion of inflammatory and anti-inflammatory factors and arginine pathway metabolites (Jaguin, Houlbert, Fardel, & Lecureur, 2013; Kittan et al., 2013; Mills, Kincaid, Alt, Heilman, & Hill, 2000; Orecchioni, Ghosheh, Pramod, & Ley, 2019). Based on these reports and combined with the previous relevant researches, our study selected iNOS, TNF-α, IL-1β, IL-6, CD16/32 as M1 phenotype markers and Arg-1, IL-10, CD206 as M2 phenotype markers.
LPS is the inflammatory response inducer and a classic activator of microglia (Bernath, Murray, Shirley Yang, Gibon, & Klegeris, 2023). Immortality mouse BV2 cell line retains many morphological, phenotypic and functional characteristics of microglia (Li et al., 2021). LPS-induced BV2 cell activation model is the most commonly used model for studying the M1/M2 polarization regulation of active ingredients in vitro. In rodent models, intraperitoneal injection of LPS is a classic depression model based on the neuroinflammation hypothesis. It can induce the depressive-like behaviors such as lack of pleasure, reduced social and exploratory activities in rodents, and also result in the excessive activation of microglia (Shi et al., 2023). Consistent with the previous research (Kim et al., 2022), NO and ROS levels and the expression level of microglial marker protein Iba-1 significantly were increased after exposed to LPS, indicating that BV2 cells were activated. The mRNA levels and supernatant contents of M1 phenotype markers (iNOS, TNF-α, IL-1β, IL-6) and protein expression of CD16/32 were markedly increased. However, the mRNA levels and supernatant contents of M2 phenotype markers (IL-10, Arg-1) and protein expression of CD206 were significantly decreased. These results indicated that LPS induced the transition of BV2 cells to M1 phenotype, and neuroinflammation model in vitro was successfully constructed.
Based on that the antidepressant and anti-inflammation effects of PSP have been reported, our study mainly focused on the role of PSP in the modulation of microglial polarization.Treatment with PSP prior to LPS stimulation reversed the M1 polarization and strongly increased the expression of the M2 microglial markers, IL-10, CD206 and Arg-1. Collectively, these results indicated that PSP switched the polarization of LPS activated BV2 microglia from M1 to a predominantly M2 phenotype, which was correlated with a significant decrease in neurotoxicity and enhanced anti-inflammatory cytokine production. However, the molecular mechanisms about that how PSP induces changes in microglial polarization remains unclear.
BDNF (Brain-derived neurotrophic factor), widely expressed in CNS, is an important regulator of synaptic formation and synaptic plasticity, and also a key point for the brain to regulate emotional behaviors. The neurotrophic hypothesis of depression makes BDNF an important biomarker of depression. In the LPS-induced depression model of rats, the reduced level of BDNF and the related changes of synaptic plasticity in the hippocampus were observed (Fang et al., 2019). During brain development, microglia plays an important role in synaptic plasticity by trimming synapses and refining neural circuit through phagocytosis (Schafer & Stevens, 2013). BDNF secreted by Microglia is an important material basis for maintaining the formation of learning dependent synapse (Parkhurst et al., 2013). BDNF is also an important medium for information transmission between microglia and neurons. Promoting M2 phenotype polarization of microglia can directly enhance the synthesis of BDNF and the expression of neurotrophic receptors, thus protecting neuron survival or preventing neuron apoptosis (Song, Zhang, & Dong, 2013). TrkB is a specific receptor for BDNF, which binds to cause receptor dimerization and self-phosphorylation, activating intracellular signaling pathways (Fayard, Loeffler, Weis, Vogelin, & Kruttgen, 2005). ERK pathway (including ERK1 and ERK2) is one of the activated downstream signal pathways. Activation of ERK can induce phosphorylation of CREB serine 133 residue, produce activated transcriptional complexes, and trigger activation of target genes (Lesiak et al., 2013). CREB is a transcription factor that plays an important role in neuronal plasticity and neurogenesis (Tao, Finkbeiner, Arnold, Shaywitz, & Greenberg, 1998). CREB and BDNF/TrkB signaling pathways form the positive feedback loop, and phosphorylated CREB promotes BDNF transcription and translation and enhances the TrkB signaling pathway, exerting the antidepressant-like effects. In the present study, LPS stimulation significantly reduced the protein expression level of BDNF and phosphorylation levels of TrkB, ERK, CREB in BV2 cells, while PSP intervention reversed these changes. Thus, M1/M2 polarization regulation and inhibition of PSP in LPS-induced neuroinflammatory responses might be related to activate the BDNF/TrkB/CREB signaling pathway.
The Notch signaling pathway is mainly composed of receptors, ligands, transcription factors, regulatory molecules, and downstream effector molecules. When the Notch signaling pathway is activated, intracellular domains are released into the cytoplasm and translocated to the nucleus, promoting the production of transcription activating factors and inducing the expression of downstream target genes such as Hes1 and Hes5 (Iso, Kedes, & Hamamori, 2003; Oswald, Liptay, Adler, & Schmid, 1998). It has been found that the Notch signaling pathway can regulate the polarization of monocyte macrophages. Activation of the Notch signaling pathway leads to an increase in M1 type macrophages and large release of inflammatory cytokines (Tao, Chen, Lin, & Dong, 2020; Xu et al., 2012). Ganoderma lucidum polysaccharides can regulate M1/M2 polarization of macrophage, and its regulatory mechanism is related to the Notch signaling pathway (Li et al., 2022). Several studies have shown that Notch signaling pathway is also closely related to the activation and polarization of Microglia. The expressions of Notch1, Jagged1, Hes1 and TNF-α, IL-1β is increased in LPS stimulated BV2 cells. However, the expression level of Hes1 is reduced and the translocation of NF-κB/p65 was inhibited when blocking the Notch signaling pathway (Cao et al., 2010). Lipoxygenase A4 may regulate the microglial polarization after cerebral ischemia-reperfusion injury through Notch signaling pathway (Li, Ding, Wang, Sun, & Wu, 2021). Notch pathway may be an important target for regulating the activation, polarization and inflammatory response of Microglia. In the present study, LPS stimulation significantly reduced the protein expression level of Notch1 and Hes1, while PSP intervention reversed these changes. Thus, M1/M2 polarization regulation and inhibition of PSP in LPS-induced neuroinflammatory responses might be associated with modulating the Notch signaling pathway.
5. Conclusions
In summary, the present study demonstrates that PSP facilitates microglial polarization from the pro-inflammatory phenotype towards the anti-inflammatory phenotype, which may be related to the regulate of BDNF/TrkB/CREB and Notch/Hes1 signaling pathways. This study provides novel insights into fully elucidating the molecular mechanisms of PSP treatment in depression.
Author Contributions
CL and FZW designed the study. YYZ conducted the experiments and collected the data. DYW, JML, JS,XML,BF helped with data analysis. YYZ wrote this paper. CL and FZW revised the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No.2023YFD2201300), China Agriculture Research System (CARS-04-PS29), and Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2023-IFST).
Data availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bernath, A. K., Murray, T. E., Shirley Yang, S., Gibon, J., & Klegeris, A. (2023). Microglia secrete distinct sets of neurotoxins in a stimulus-dependent manner. Brain Res, 1807, 148315. [CrossRef]
- Beurel, E., Toups, M., & Nemeroff, C. B. (2020). The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron, 107(2), 234-256. [CrossRef]
- Cao, Q., Li, P., Lu, J., Dheen, S. T., Kaur, C., & Ling, E. A. (2010). Nuclear factor-kappaB/p65 responds to changes in the Notch signaling pathway in murine BV-2 cells and in amoeboid microglia in postnatal rats treated with the gamma-secretase complex blocker DAPT. J Neurosci Res, 88(12), 2701-2714. [CrossRef]
- Fang, K., Li, H. R., Chen, X. X., Gao, X. R., Huang, L. L., Du, A. Q., Jiang, C., Li, H., & Ge, J. F. (2019). Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of Copine 6 and TREM1/2 in the hippocampus and PFC. Front Pharmacol, 10, 1544. [CrossRef]
- Fayard, B., Loeffler, S., Weis, J., Vogelin, E., & Kruttgen, A. (2005). The secreted brain-derived neurotrophic factor precursor pro-BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J Neurosci Res, 80(1), 18-28. [CrossRef]
- Franco, R., & Fernandez-Suarez, D. (2015). Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol, 131, 65-86. [CrossRef]
- Guo, S., Wang, H., & Yin, Y. (2022). Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci, 14, 815347. [CrossRef]
- Iso, T., Kedes, L., & Hamamori, Y. (2003). HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol, 194(3), 237-255. [CrossRef]
- Jaguin, M., Houlbert, N., Fardel, O., & Lecureur, V. (2013). Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol, 281(1), 51-61. [CrossRef]
- Kim, J. H., Ju, I. G., Kim, N., Huh, E., Son, S. R., Hong, J. P., Choi, Y. J., Jang, D. S., & Oh, M. S. (2022). Yomogin, isolated from artemisia iwayomogi, inhibits neuroinflammation stimulated by lipopolysaccharide via regulating MAPK pathway. Antioxidants (Basel), 12(1). [CrossRef]
- Kip, E., & Parr-Brownlie, L. C. (2023). Healthy lifestyles and wellbeing reduce neuroinflammation and prevent neurodegenerative and psychiatric disorders. Front Neurosci, 17, 1092537. [CrossRef]
- Kittan, N. A., Allen, R. M., Dhaliwal, A., Cavassani, K. A., Schaller, M., Gallagher, K. A., Carson, W. F., Mukherjee, S., Grembecka, J., Cierpicki, T., Garai, J., Westwick, J., Kunkel, S. L., & Hogaboam, C. M. (2013). Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One, 8(10), e78045. [CrossRef]
- Leonard, B. E. (2018). Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr, 30(1), 1-16. [CrossRef]
- Lesiak, A., Pelz, C., Ando, H., Zhu, M., Davare, M., Lambert, T. J., Hansen, K. F., Obrietan, K., Appleyard, S. M., Impey, S., & Wayman, G. A. (2013). A genome-wide screen of CREB occupancy identifies the RhoA inhibitors Par6C and Rnd3 as regulators of BDNF-induced synaptogenesis. PLoS One, 8(6), e64658. [CrossRef]
- Li, H., Xiao, Y., Han, L., Jia, Y., Luo, S., Zhang, D., Zhang, L., Wu, P., Xiao, C. J., Kan, W. J., Du, J., & Bao, H. (2021). Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system. Brain Res Bull, 171, 16-24. [CrossRef]
- Li, Q. Q., Ding, D. H., Wang, X. Y., Sun, Y. Y., & Wu, J. (2021). Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway. Exp Neurol, 339, 113645. [CrossRef]
- Li, T., Zhang, Y. S., Wan, M., Wu, W., Yao, Y. F., & Li, W. J. (2022). Ganoderma atrum polysaccharide modulates the M1/M2 polarization of macrophages linked to the Notch signaling pathway. Food Funct, 13(7), 4216-4228. [CrossRef]
- Malhi, G. S., & Mann, J. J. (2018). Depression. Lancet, 392(10161), 2299-2312. [CrossRef]
- Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J., & Hill, A. M. (2000). M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol, 164(12), 6166-6173. [CrossRef]
- Moret, C., Isaac, M., & Briley, M. (2009). Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J Psychopharmacol, 23(8), 967-974. [CrossRef]
- Orecchioni, M., Ghosheh, Y., Pramod, A. B., & Ley, K. (2019). Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol, 10, 1084. [CrossRef]
- Oswald, F., Liptay, S., Adler, G., & Schmid, R. M. (1998). NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol, 18(4), 2077-2088. [CrossRef]
- Parkhurst, C. N., Yang, G., Ninan, I., Savas, J. N., Yates, J. R., 3rd, Lafaille, J. J., Hempstead, B. L., Littman, D. R., & Gan, W. B. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155(7), 1596-1609. [CrossRef]
- Schafer, D. P., & Stevens, B. (2013). Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol, 23(6), 1034-1040. [CrossRef]
- Shen, F., Song, Z., Xie, P., Li, L., Wang, B., Peng, D., & Zhu, G. (2021). Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J Ethnopharmacol, 275, 114164. [CrossRef]
- Shen, F., Xie, P., Li, C., Bian, Z., Wang, X., Peng, D., & Zhu, G. (2022). Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-calpain-1-NLRP3 signaling axis. Oxid Med Cell Longev, 2022, 2566917. [CrossRef]
- Shi, L., Xia, Z., Guo, J., Wang, L., Peng, Z., Qiu, D., Zhou, Y., Zhou, D. D., Kuang, L., & Qiu, T. (2023). Maresin-1 improves LPS-induced depressive-like behavior by inhibiting hippocampal microglial activation. J Affect Disord, 328, 261-272. [CrossRef]
- Song, C., Zhang, Y., & Dong, Y. (2013). Acute and subacute IL-1beta administrations differentially modulate neuroimmune and neurotrophic systems: possible implications for neuroprotection and neurodegeneration. J Neuroinflammation, 10, 59. [CrossRef]
- Tao, S., Chen, Q., Lin, C., & Dong, H. (2020). Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res, 39(1), 191. [CrossRef]
- Tao, X., Finkbeiner, S., Arnold, D. B., Shaywitz, A. J., & Greenberg, M. E. (1998). Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron, 20(4), 709-726. [CrossRef]
- Tremblay, M. E., Stevens, B., Sierra, A., Wake, H., Bessis, A., & Nimmerjahn, A. (2011). The role of microglia in the healthy brain. J Neurosci, 31(45), 16064-16069. [CrossRef]
- Wei, Z., Song, H. B., An, F. P., Sun, J., Li, S. Y., Jiang, N., Liu, X. M., Wang, F. Z., & Lu, C. (2022). Protective effects and mechanism of polysaccharide from Polygonati rhizoma on behavioral despair mice. J Food Sci Technol, 43(6), 351-357. [CrossRef]
- Xu, H., Zhu, J., Smith, S., Foldi, J., Zhao, B., Chung, A. Y., .Outtz, H., Kitajewski, J., Shi, C., Weber, S., Saftig, P., Li, Y. M., Ozato, K., Blobel, C P., Ivashkiv, L. B., & Hu, X. Y. (2012). Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol, 13(7), 642-650. [CrossRef]
- Yang, Y., Ding, H., Yang, C., Wu, J., Bao, Y., Lan, S. Zhou, L., Zhou, L., Liu, B. L., Hong, T., Wan, X. C., & Wu, X. (2023). Sestrin2 provides cerebral protection through activation of Nrf2 signaling in microglia following subarachnoid hemorrhage. Front Immunol, 14, 1089576. [CrossRef]
- Zhang, Y. Y., Sun, Y., Liu, Y. P., Liu, J. M., Sun, J., Liu, X. M., Fan, B., Lu, C., & Wang, F. Z. (2023). Polygonum sibiricum polysaccharides exert the antidepressant-like effects in chronic unpredictable mild stress-induced depressive mice by modulating microbiota-gut-brain axis. Phytotherapy Research, 37(8), 3408-3423. [CrossRef]
- Zhang, Y. Y., Sun, Y., Liu, Y. P., Liu, J. M., Sun, J., Bai, Y. J., Fan, B., Lu, C., & Wang, F. Z. (2023). Polygonum sibiricum polysaccharides alleviate chronic unpredictable mild stress-induced depressive-like behaviors by regulating the gut microbiota composition and SCFAs levels. Journal of Functional Foods, 101, 105411. [CrossRef]
Figure 1.
Effects of PSP on the viability and activation of BV2 cells. (A) Effects of different concentrations of PSP on cell viability. (B) Effects of PSP on cell viability with LPS stimulation. (C) Protein expression of Iba-1, representative protein bands. (D) The ratio of Iba-1/β-actin. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Figure 1.
Effects of PSP on the viability and activation of BV2 cells. (A) Effects of different concentrations of PSP on cell viability. (B) Effects of PSP on cell viability with LPS stimulation. (C) Protein expression of Iba-1, representative protein bands. (D) The ratio of Iba-1/β-actin. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Figure 2.
Effects of PSP on ROS release in LPS-induced BV2 cells. (A) Representative microscopic images. (B) Fluorescence intensity of ROS analysed with Image J. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; ***P < 0.001, versus the LPS-treated group.
Figure 2.
Effects of PSP on ROS release in LPS-induced BV2 cells. (A) Representative microscopic images. (B) Fluorescence intensity of ROS analysed with Image J. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; ***P < 0.001, versus the LPS-treated group.
Figure 3.
Effects of PSP on LPS-induced NO production and inflammatory cytokine production in BV2 cells. (A) Effects of PSP on NO release in LPS-induced BV2 cells. (B-E) The mRNA expression levels of M1 phenotype genes (TNF-α, IL-1β, IL-6, iNOS). (F, G) The mRNA expression levels of M2 phenotype genes (IL-10, Arg-1). (H-K) TNF-α、IL-1β、IL-6、IL-10 levels of cell supernatant detected by ELISA. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01,***P < 0.001, versus the LPS-treated group.
Figure 3.
Effects of PSP on LPS-induced NO production and inflammatory cytokine production in BV2 cells. (A) Effects of PSP on NO release in LPS-induced BV2 cells. (B-E) The mRNA expression levels of M1 phenotype genes (TNF-α, IL-1β, IL-6, iNOS). (F, G) The mRNA expression levels of M2 phenotype genes (IL-10, Arg-1). (H-K) TNF-α、IL-1β、IL-6、IL-10 levels of cell supernatant detected by ELISA. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01,***P < 0.001, versus the LPS-treated group.
Figure 4.
Effects of PSP on LPS-induced protein expression of CD16/32 and CD206 in BV2 cells. (A, B) CD16/32 (M1) and (C, D) CD206 (M2) protein expression measured by flow cytometry. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Figure 4.
Effects of PSP on LPS-induced protein expression of CD16/32 and CD206 in BV2 cells. (A, B) CD16/32 (M1) and (C, D) CD206 (M2) protein expression measured by flow cytometry. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Figure 5.
The relative fluorescence intensity of iNOS and Arg-1 in the different groups of BV-2 cells. Representative immunofluorescence picture of (A) iNOS (M1) and (C) Arg-1 (M2), Fluorescence intensity of (B) iNOS (M1) and (D) Arg-1 (M2) analysed with Image J. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001, versus the LPS-treated group.
Figure 5.
The relative fluorescence intensity of iNOS and Arg-1 in the different groups of BV-2 cells. Representative immunofluorescence picture of (A) iNOS (M1) and (C) Arg-1 (M2), Fluorescence intensity of (B) iNOS (M1) and (D) Arg-1 (M2) analysed with Image J. The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001, versus the LPS-treated group.
Figure 6.
Effect of PSP on the BDNF/TrkB/CREB signaling pathway in LPS-induced BV2 cell. (A) Representative protein bands, (B) protein expression of BDNF. Phosphorylation of (C) TrkB (D) ERK (E) CREB. The data are expressed as the means ± SEM. ##P < 0.01, ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001, versus the LPS-treated group.
Figure 6.
Effect of PSP on the BDNF/TrkB/CREB signaling pathway in LPS-induced BV2 cell. (A) Representative protein bands, (B) protein expression of BDNF. Phosphorylation of (C) TrkB (D) ERK (E) CREB. The data are expressed as the means ± SEM. ##P < 0.01, ###P < 0.001 versus the control group; *P < 0.05, **P < 0.01, ***P < 0.001, versus the LPS-treated group.
Figure 7.
Effect of PSP on the Notch signaling pathway in LPS-induced BV2 cell. (A) Representative protein bands. Protein expression of Notch1 (B), and Hes1 (C). The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Figure 7.
Effect of PSP on the Notch signaling pathway in LPS-induced BV2 cell. (A) Representative protein bands. Protein expression of Notch1 (B), and Hes1 (C). The data are expressed as the means ± SEM. ###P < 0.001 versus the control group; *P < 0.05, ***P < 0.001, versus the LPS-treated group.
Table 1.
Primers for qPCR.
Table 1.
Primers for qPCR.
| Gene |
Sense |
Anti-sense |
| TNF-α |
CACCACCATCAAGGACTCAA |
AGGCAACCTGACCACTCTCC |
| IL-1β |
AAATACCTGTGGCCTTGGGC |
CTTGGGATCCACACTCTCCAG |
| IL-6 |
CCAGAGATACAAAGAAAT |
ACTCCAGAAGACCAGAGGAAAT |
| IL-10 |
GTGGAGCAGGTGAAGAGTGA |
TCGGAGAGAGGTACAAACGAG |
| iNOS |
GAGGCCCAGGAGGAGAGAGATCCG |
TCCATGCAGACAACCTTGGTGTTG |
| CD206 |
CTTCGGGCCTTTGGAATAAT |
TAGAAGAGCCCTTGGGTTGA |
| Arg-1 |
GTGAAGAACCCACGGTCTGT |
CTGGTTGTCAGGGGAGTGTT |
| β-actin |
GGCTGTATTCCCCTCCATCG |
CCAGTTGGTAACAATGCCATGT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).