Submitted:
21 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Influence of Gut Microbiota on Hyperglycemia, Insulin Resistance and T2DM
3. Influence of Nutrition in Modulating Gut Microbiota and Markers of T2DM
4. Effects of Macronutrients on T2DM and Gut Microbiota
4.1. Carbohydrates
4.1.1. Dietary Fibers, Gut Microbiota and T2DM
4.1.2. Starch, Gut Microbiota and T2DM
4.2. Proteins, Gut Microbiota and T2DM
4.3. Lipids, Gut Microbiota and T2DM
5. Effects of Micronutrients on T2DM and Gut Microbiota
5.1. Vitamins, Gut Microbiota and T2DM
5.1. Minerals, Gut Microbiota and T2DM
6. Food Groups and Related Effects on Gut Microbiota and T2DM
6.1. Cereals and Cereal Products
6.2. Fruits and Vegetables
6.3. Milk and Dairy Products
6.3. Meat and Meat Products
6.5. Nuts, Oils and Oilseeds
7. Conclusions and Perspective
| Food Group | Study Period | Outcome measured | Results/Implications | Subject type | Reference |
|---|---|---|---|---|---|
| Cereals and Cereal Products | Meta-Analysis | Diabetes risk | 2 servings of whole grains decreased risk of developing T2DM by 21% Refined grain intake increased risk of developing T2DM by 6-14% |
Humans | [272] |
| 3 months | Metabolic Parameters | Improvements in lipid quality, HgbA1c, BMI, adipose distribution, and fasting C-peptide levels | Humans | [263] | |
| 6 weeks | Gut Microbiota and Inflammatory Markers | Whole grains improved effector memory T cell activity and acute innate immune response Increased quantity of SCFA and SCFA-producing genera including Lachnospira Decreased relative abundances of pro-inflammatory bacterial family Enterobacteriaceae |
Humans | [275] | |
| 9 weeks | Gut Microbiota and Gut Hormones | Increased SCFA-producing species Increased GLP-1 secretion |
Mice | [278] | |
| 12 weeks | Gut Microbiota | Increased Bifidobacterium and decreased Bacteroides | Humans | [279] | |
| Fruits and Vegetables | Meta-Analysis | Diabetes risk | Intake of 0.2 servings per day of green leafy vegetables reduced the risk for type 2 diabetes by 13% | Humans | [284] |
| Gut Microbiota and Metabolic Parameters | Increased Akkermansia Reduced Lachnoclostridium, Desulfovibrio, Colidextribacter and Blautia Up-regulation of lipolysis through the FXR, bile acid metabolism pathway |
Mice | [292] | ||
| Inflammatory Markers and Metabolic Parameters | Reduced LPS/TLR-4 activity, TNF-α, and IL-6 Improved IL-10 Improved insulin resistance and HgbA1c Increased GLP-1 secretion |
Mice | [293] | ||
| Glucose Metabolism and Gut Microbiota | Up-regulation of the IRS/AKT signaling pathway to increase GLUT4 translocation and synthesis of glycogen Improved Firmicutes/Bacteroidetes ratio |
Mice | [295] | ||
| Milk and Dairy Products | Diabetes risk | 1 serving of dairy per day has beneficial effects on T2DM risk reduction of 9% in men and 4% in women | Humans | [310,311] | |
| 3 weeks | Gut Microbiota | Increased Bifidobacterium and Lactobacillus spp. Increased serum IgA Decreased Bacteroides fragilis |
Humans | [306,312] | |
| Gut Microbiota and Pancreatic Function |
Lactobacillus isolated from yogurt increased SCFA levels and SCFA receptors, GPR41/43 Increased SCFA-producing genera Inhibited reduction of β-cell mass |
Mice | [315] | ||
| Meat and Meat Products | Meta-Analysis | Diabetes Risk | Risk for T2DM is increased with intake of 100 grams of red meat per day Risk for T2DM is increased with intake of 50 grams of processed meat per day |
Humans | [272,320] |
| Gut Microbiota | Red meat decreases Lactobacillus, Paralactobacillus, Prevotella, while also decreasing SCFA | Dogs | [327] | ||
| 1-4 weeks | Gut Microbiota | Increased Clostridium and Blautia Decreased Bifidobacterium and Akkermansia |
Mice | [328] | |
| 3 months | Gut Microbiota, Inflammatory and Metabolic parameters | Pork meat decreased Blautia, Bifidobacterium, Alistipes Induced low-grade inflammation Induced oxidative stress Up-regulated lipid metabolism genes including PPAR-α and PPAR-γ |
Mice | [332] | |
| Nuts, Oils and Oilseeds | 3 months | Parameters of T2DM | Peanuts or almonds in patients with T2DM improved blood glucose, HgbA1c and inflammatory markers like IL-6 expression | Humans | [335] |
| 6 weeks | Gut Microbiota and Metabolic Parameters | Nut intake increase in the abundance of Faecalibacterium, Clostridium, Dialister and Roseburia and a decrease in the abundance of Ruminococcus, Dorea, Oscillopira and Bifidobacterium Decreased pro-inflammatory bile acid production and LDL cholesterol |
Humans | [337] | |
| 5 weeks | Gut Microbiota and Inflammatory Parameters |
Dietary flaxseed oil decreased severity of T2DM Improved Firmicutes to Bacteroidetes ratio, while increasing Alistipes Reduction in as IL-1β, TNF-α, IL-6, and LPS production |
Rats | [344] |
Author Contributions
Funding
Conflicts of Interest
References
- Salgaço, M.K.; Oliveira, L.G.S.; Costa, G.N.; Bianchi, F.; Sivieri, K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl Microbiol Biotechnol. 2019, 103, 9229–9238. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms 2023, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson's Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Flaig, B.; Garza, R.; Singh, B.; Hamamah, S.; Covasa, M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients 2023, 15, 228. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Tsai, W.-H.; Jheng, Y.-P.; Su, S.-L.; Wang, S.-Y.; Lin, C.-C.; Chen, Y.-H.; Chang, W.-W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Fan, L.; Li, X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res. Clin. Pr. 2020, 169, 108418. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Skantze, V.; Hjorth, T.; Wallman, M.; Brunius, C.; Dicksved, J.; Pelve, E.A.; Esberg, A.; Vitale, M.; Giacco, R.; Costabile, G.; et al. Differential Responders to a Mixed Meal Tolerance Test Associated with Type 2 Diabetes Risk Factors and Gut Microbiota—Data from the MEDGI-Carb Randomized Controlled Trial. Nutrients 2023, 15, 4369. [Google Scholar] [CrossRef] [PubMed]
- Maestri, M.; Santopaolo, F.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front. Nutr. 2023, 10, 1110536. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pal, N.; Sharma, P.; Kumawat, M.; Sarma, D.K.; Nabi, B.; Verma, V.; Tiwari, R.R.; Shubham, S.; Arjmandi, B.; et al. Omega-3 Fatty Acids and Their Interaction with the Gut Microbiome in the Prevention and Amelioration of Type-2 Diabetes. Nutrients 2022, 14, 1723. [Google Scholar] [CrossRef]
- Carranza-Naval, M.J.; Vargas-Soria, M.; Hierro-Bujalance, C.; Baena-Nieto, G.; Garcia-Alloza, M.; Infante-Garcia, C.; del Marco, A. Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models. Biomolecules 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Meng, X.; Xue, Y.; Mao, M.; Liu, Y.; Tian, X.; Sui, B.; Li, X.; Zhang, P. Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front. Pharmacol. 2022, 13, 1027212. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J.; Janssen, A.W.F.; et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef]
- Hamamah, S.; Covasa, M. Gut Microbiota Restores Central Neuropeptide Deficits in Germ-Free Mice. Int. J. Mol. Sci. 2022, 23, 11756. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr. Diabetes Rep. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Monnais, E.; Seboek, D.; Radimerski, T.; Zini, E.; Kaufmann, K.; Lutz, T.; Reusch, C.; Ackermann, M.; Muller, B.; et al. Insulin Resistance and Increased Lipolysis in Bone Marrow Derived Adipocytes Stimulated with Agonists of Toll-like Receptors. Horm. Metab. Res. 2010, 42, 703–709. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Zou, P.; Yang, F.; Ding, Y.; Zhang, D.; Liu, Y.; Zhang, J.; Wu, D.; Wang, Y. Lipopolysaccharide downregulates the expression of ZO-1 protein through the Akt pathway. BMC Infect. Dis. 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.J.; Pallot, G.; Nguyen, M.; Tavernier, A.; Dusuel, A.; Pilot, T.; Deckert, V.; Dugail, I.; Le Guern, N.; De Barros, J.-P.P.; et al. Increased Weight Gain and Insulin Resistance in HF-Fed PLTP Deficient Mice Is Related to Altered Inflammatory Response and Plasma Transport of Gut-Derived LPS. Int. J. Mol. Sci. 2022, 23, 13226. [Google Scholar] [CrossRef] [PubMed]
- Rorato, R.; Borges, B.d.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Elias, C.F.; Elias, L.L.K. LPS-Induced Low-Grade Inflammation Increases Hypothalamic JNK Expression and Causes Central Insulin Resistance Irrespective of Body Weight Changes. Int. J. Mol. Sci. 2017, 18, 1431. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, E.; Kolida, S.; Yaqoob, P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Gao, C.-L.; Guo, H.-L.; Zhang, W.-Q.; Huang, W.; Tang, S.-S.; Gan, W.-J.; Xu, Y.; Zhou, H.; Zhu, Q. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J. Endocrinol. 2018, 238, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2021, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2021, 146, 112563. [Google Scholar] [CrossRef]
- Hu, M.; Alhamwe, B.A.; Santner-Nanan, B.; Miethe, S.; Harb, H.; Renz, H.; Potaczek, D.P.; Nanan, R.K. Short-Chain Fatty Acids Augment Differentiation and Function of Human Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 5740. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Mäurer, J.; Duscha, A.; Lee, D.-H.; Balogh, A.; Gold, R.; Müller, D.N.; Haghikia, A.; Linker, R.A. Propionic Acid Rescues High-Fat Diet Enhanced Immunopathology in Autoimmunity via Effects on Th17 Responses. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y.; Maron, R.; Tukpah, A.-M.; Maioli, T.U.; Murugaiyan, G.; Yang, K.; Wu, H.Y.; Weiner, H.L. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl. Acad. Sci. 2010, 107, 9765–9770. [Google Scholar] [CrossRef]
- Rameshrad, M.; Razavi, B.M.; Lalau, J.-D.; E De Broe, M.; Hosseinzadeh, H. An overview of glucagon-like peptide-1 receptor agonists for the treatment of metabolic syndrome: A drug repositionin. Iran J Basic Med Sci . 2020, 23, 568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lou, X.; Jiang, C.; Ji, X.; Tao, X.; Sun, J.; Bao, Z. Gut microbiota is correlated with gastrointestinal adverse events of metformin in patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 1044030. [Google Scholar] [CrossRef] [PubMed]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Cariou, B.; van Harmelen, K.; Duran-Sandoval, D.; van Dijk, T.H.; Grefhorst, A.; Abdelkarim, M.; Caron, S.; Torpier, G.; Fruchart, J.-C.; Gonzalez, F.J.; et al. The Farnesoid X Receptor Modulates Adiposity and Peripheral Insulin Sensitivity in Mice. J. Biol. Chem. 2006, 281, 11039–11049. [Google Scholar] [CrossRef]
- Bjursell, M.; Wedin, M.; Admyre, T.; Hermansson, M.; Böttcher, G.; Göransson, M.; Lindén, D.; Bamberg, K.; Oscarsson, J.; Bohlooly-Y, M. Ageing Fxr Deficient Mice Develop Increased Energy Expenditure, Improved Glucose Control and Liver Damage Resembling NASH. PLoS ONE 2013, 8, e64721. [Google Scholar] [CrossRef]
- Prawitt, J.; Abdelkarim, M.; Stroeve, J.H.M.; Popescu, I.; Duez, H.; Velagapudi, V.R.; Dumont, J.; Bouchaert, E.; van Dijk, T.H.; Lucas, A.; et al. Farnesoid X Receptor Deficiency Improves Glucose Homeostasis in Mouse Models of Obesity. Diabetes 2011, 60, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Hu, Z.; Zhang, F.; Cui, A.; Chen, X.; Jiang, H.; Gao, J.; Chen, X.; Han, Y.; Liang, Q.; et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. J. Hepatol. 2016, 64, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mo, W.; Feng, J.; Li, J.; Yu, Q.; Li, S.; Zhang, J.; Chen, K.; Ji, J.; Dai, W.; et al. Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br J Pharmacol. 2020, 177, 3760–3777. [Google Scholar] [CrossRef] [PubMed]
- Abaj, F.; Rafiee, M.; Koohdani, F. A personalised diet approach study: Interaction between PPAR-γ Pro12Ala and dietary insulin indices on metabolic markers in diabetic patients. J. Hum. Nutr. Diet. 2022, 35, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2009, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Forouhi, N.G.; Ye, Z.; Buijsse, B.; Arriola, L.; Balkau, B.; Barricarte, A.; Beulens, J.W.; Boeing, H.; Büchner, F.L.; et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012, 66, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Liu, S.; Gao, Y.-T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective Study of Dietary Carbohydrates, Glycemic Index, Glycemic Load, and Incidence of Type 2 Diabetes Mellitus in Middle-aged Chinese Women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; McKeown-Eyssen, G.; Josse, R.G.; Silverberg, J.; Booth, G.L.; Vidgen, E.; Josse, A.R.; Nguyen, T.H.; Corrigan, S.; et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: A randomized trial. Jama 2008, 300, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Kabir, M.; Oppert, J.-M.; Vidal, H.; Bruzzo, F.; Fiquet, C.; Wursch, P.; Slama, G.; Rizkalla, S.W. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002, 51, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cruz, A.; Bacardi-Gascon, M.; Turnbull, W.H.; Rosales-Garay, P.; Severino-Lugo, I. A Flexible, Low-Glycemic Index Mexican-Style Diet in Overweight and Obese Subjects With Type 2 Diabetes Improves Metabolic Parameters During a 6-Week Treatment Period. Diabetes Care 2003, 26, 1967–1970. [Google Scholar] [CrossRef]
- Hur, H.J.; Wu, X.; Yang, H.J.; Kim, M.J.; Lee, K.-H.; Hong, M.; Park, S.; Kim, M.-S. Beneficial Effects of a Low-Glycemic Diet on Serum Metabolites and Gut Microbiota in Obese Women With Prevotella and Bacteriodes Enterotypes: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 861880. [Google Scholar] [CrossRef] [PubMed]
- Bhute, S.S.; Mefferd, C.C.; Phan, J.R.; Ahmed, M.; Fox-King, A.E.; Alarcia, S.; Villarama, J.V.; Abel-Santos, E.; Hedlund, B.P. A High-Carbohydrate Diet Prolongs Dysbiosis and Clostridioides difficile Carriage and Increases Delayed Mortality in a Hamster Model of Infection. Microbiol. Spectr. 2022, 10, e0180421. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Clifton, P.M. C-reactive protein and coronary artery disease: Influence of obesity, caloric restriction and weight loss. J. Nutr. Biochem. 2002, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Björck, I.; Brighenti, F.; Brown, R.; Brynes, A.; et al. Measuring the glycemic index of foods: Interlaboratory study. Am. J. Clin. Nutr. 2008, 87, 247S–257S. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.J.; Yang, H.J.; Kim, M.J.; Lee, K.-H.; Kim, M.-S.; Park, S. Association of Polygenic Variants with Type 2 Diabetes Risk and Their Interaction with Lifestyles in Asians. Nutrients 2022, 14, 3222. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Zhou, X.; Han, D.; Xu, R.; Li, S.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. A Model of Metabolic Syndrome and Related Diseases with Intestinal Endotoxemia in Rats Fed a High Fat and High Sucrose Diet. PLoS ONE 2014, 9, e115148. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Tan, C.K.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. 2016, 113, E5934–E5943. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, Y.; Shi, L.; Li, W.; Chen, K.; Li, M.; Chen, X.; Zhang, H.; Li, T.; Matsuzawa-Ishimoto, Y.; et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest. 2020, 130, 2111–2128. [Google Scholar] [CrossRef]
- Kothari, V.; Luo, Y.; Tornabene, T.; O'Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2016, 6, 174–184. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, M.-F.; Li, Y.-X.; Li, Y.; Gu, T.; Xia, F.-Z.; Yu, J.; Lu, Y.-L. Short reaction of C-peptide, glucagon-like peptide-1, ghrelin and endomorphin-1 for different style diet in type 2 diabetic patients. Chin. Med. J. 2011, 124, 3485–3489. [Google Scholar] [CrossRef] [PubMed]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis. Neurotherapeutics. 2018, 15, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Newman, T.M.; Shively, C.A.; Register, T.C.; Appt, S.E.; Yadav, H.; Colwell, R.R.; Fanelli, B.; Dadlani, M.; Graubics, K.; Nguyen, U.T.; et al. Diet, obesity, and the gut microbiome as determinants modulating metabolic outcomes in a non-human primate model. Microbiome 2021, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Imamura, F.; Davis, C.R.; Sharp, S.J.; Koulman, A.; Hodgson, J.M.; Guevara, M.; Schulze, M.B.; Zheng, J.S.; Agnoli, C.; et al. A nutritional biomarker score of the Mediterranean diet and incident type 2 diabetes: Integrated analysis of data from the MedLey randomised controlled trial and the EPIC-InterAct case-cohort study. PLoS Med. 2023, 20, e1004221. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Tuccinardi, D.; Watanabe, M.; Del Toro, R.; Monte, L.; Giorgino, R.; Rampa, L.; Rossini, G.; Kyanvash, S.; Soare, A.; et al. The Mediterranean diet increases glucagon-like peptide 1 and oxyntomodulin compared with a vegetarian diet in patients with type 2 diabetes: A randomized controlled cross-over trial. Diabetes/Metabolism Res. Rev. 2020, 37, e3406. [Google Scholar] [CrossRef] [PubMed]
- Papamichou, D.; Panagiotakos, D.B.; Holmes, E.; Koutsakis, P.; Katsoulotos, H.; Loo, R.L.; Itsiopoulos, C. The rationale and design of a Mediterranean diet accompanied by time restricted feeding to optimise the management of type 2 diabetes: The MedDietFast randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 32, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Gudban, N.; Yehuda, I.; Nasir, W.; Soboh, S.; Tamir, S.; Blum, A. Effect of Telemedicine Dietary Intervention on Endothelial Function in Patients with Type 2 Diabetes Mellitus on Mediterranean Diet. 2021, 23, 89–93. Isr Med Assoc J . 2021, 23, 89–93. [Google Scholar] [PubMed]
- van de Stolpe, A.; van der Saag, P.T. Intercellular adhesion molecule-1. J Mol Med 1996, 74, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shao, J.; Wu, C.-Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Carbohydrate Metabolism. Cold Spring Harb Perspect Biol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hutchison, A.T.; Heilbronn, L.K. Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lütjens, A.; Verleur, H.; Plooij, M. Glucose and insulin levels on loading with different carbohydrates. Clin. Chim. Acta 1975, 62, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Panagiotakos, D.B. Macronutrient Composition and Management of Non-Insulin-Dependent Diabetes Mellitus (NIDDM): A New Paradigm for Individualized Nutritional Therapy in Diabetes Patients. Rev. Diabet. Stud. 2016, 13, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S. Response to Comment on: Wheeler et al. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes: A Systematic Review of the Literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl S1), S15–S33. [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a Low-Carbohydrate Diet on Appetite, Blood Glucose Levels, and Insulin Resistance in Obese Patients with Type 2 Diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Davis, N.J.; Tomuta, N.; Schechter, C.; Isasi, C.R.; Segal-Isaacson, C.; Stein, D.; Zonszein, J.; Wylie-Rosett, J. Comparative Study of the Effects of a 1-Year Dietary Intervention of a Low-Carbohydrate Diet Versus a Low-Fat Diet on Weight and Glycemic Control in Type 2 Diabetes. Diabetes Care 2009, 32, 1147–1152. [Google Scholar] [CrossRef]
- Yancy, W.S.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef]
- Kokubo, E.; Morita, S.; Nagashima, H.; Oshio, K.; Iwamoto, H.; Miyaji, K. Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial. Nutrients 2022, 14, 2386. [Google Scholar] [CrossRef] [PubMed]
- Gow, M.L.; Garnett, S.P.; Baur, L.A.; Lister, N.B. The Effectiveness of Different Diet Strategies to Reduce Type 2 Diabetes Risk in Youth. Nutrients 2016, 8, 486. [Google Scholar] [CrossRef]
- Alhazmi, A.; Stojanovski, E.; McEvoy, M.; Garg, M.L. Macronutrient Intakes and Development of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. J. Am. Coll. Nutr. 2012, 31, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Maekawa, R.; Ogata, H.; Hayashi, Y. Carbohydrate-induced secretion of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1. J Diabetes Investig. 2016, 1 (Suppl 1), 27–32. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Sun, Y.-S.; Zhao, L.-Q.; Chen, T.-T.; Fan, M.-N.; Jiao, H.-C.; Zhao, J.-P.; Wang, X.-J.; Li, F.-C.; Li, H.-F.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.-F.; He, S.; Chen, X.; Wang, J.; Li, J.; Zhu, Q.; Zhang, Z.; Li, L.; Alam, M.S. Effects of High Carbohydrate Diet-Modulated Microbiota on Gut Health in Chinese Perch. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Chiantera, V.; Laganà, A.S.; Basciani, S.; Nordio, M.; Bizzarri, M. A Critical Perspective on the Supplementation of Akkermansia muciniphila: Benefits and Harms. Life 2023, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-M.; Lu, C.-Y.; Pan, J.; Ye, J.-Z.; Zhu, Q.-H. Alteration of intestinal microbiota is associated with diabetic retinopathy and its severity: Samples collected from southeast coast Chinese. World J. Diabetes 2023, 14, 862–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Yi, J.; Liu, Y.; Yu, Z.; Chen, S.; Liu, X. Increased mucin-degrading bacteria by high protein diet leads to thinner mucus layer and aggravates experimental colitis. J. Gastroenterol. Hepatol. 2021, 36, 2864–2874. [Google Scholar] [CrossRef]
- DeVries, J.W. On defining dietary fibre. Proc. Nutr. Soc. 2003, 62, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Randles, K.M.; Kendall, C.W.C.; Jenkins, D.J.A. Carbohydrate and Fiber Recommendations for Individuals with Diabetes: A Quantitative Assessment and Meta-Analysis of the Evidence. J. Am. Coll. Nutr. 2004, 23, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Steemburgo, T.; Dall'Alba, V.; Almeida, J.C.; Zelmanovitz, T.; Gross, J.L.; de Azevedo, M.J. Intake of soluble fibers has a protective role for the presence of metabolic syndrome in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2007, 63, 127–133. [Google Scholar] [CrossRef]
- Silva, F.M.; Steemburgo, T.; de Mello, V.D.; Tonding, S.F.; Gross, J.L.; Azevedo, M.J. High dietary glycemic index and low fiber content are associated with metabolic syndrome in patients with type 2 diabetes. J. Am. Coll. Nutr. 2011, 30, 141–148. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch Intern Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; van Dam, R.M.; Liu, S.; Franz, M.; Mantzoros, C.; Hu, F.B. Whole-Grain, Bran, and Cereal Fiber Intakes and Markers of Systemic Inflammation in Diabetic Women. Diabetes Care 2006, 29, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Meigs, J.B.; Liu, S.; Manson, J.E.; Mantzoros, C.; Hu, F.B. Dietary Fibers and Glycemic Load, Obesity, and Plasma Adiponectin Levels in Women With Type 2 Diabetes. Diabetes Care 2006, 29, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; E Manson, J.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Ziai, S.A.; Larijani, B.; Akhoondzadeh, S.; Fakhrzadeh, H.; Dastpak, A.; Bandarian, F.; Rezai, A.; Badi, H.N.; Emami, T. Psyllium decreased serum glucose and glycosylated hemoglobin significantly in diabetic outpatients. J. Ethnopharmacol. 2005, 102, 202–207. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Danneskiold-Samsøe, N.B.; Barros, H.D.d.F.Q.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Júnior, M.R.M. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2018, 115, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2017, 23, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic Oligosaccharides: Special Focus on Fructooligosaccharides, Its Biosynthesis and Bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Leshem, A.; Segal, E.; Elinav, E. The Gut Microbiome and Individual-Specific Responses to Diet. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef]
- Liu, D.; Wen, B.; Zhu, K.; Luo, Y.; Li, J.; Li, Y.; Lin, H.; Huang, J.; Liu, Z. Antibiotics-induced perturbations in gut microbial diversity influence metabolic phenotypes in a murine model of high-fat diet-induced obesity. Appl. Microbiol. Biotechnol. 2019, 103, 5269–5283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut Microbiota 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Röytiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch-A Review. Compr Rev Food Sci Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mora-Flores, L.P.; Casildo, R.M.-T.; Fuentes-Cabrera, J.; Pérez-Vicente, H.A.; de Anda-Jáuregui, G.; Neri-Torres, E.E. The Role of Carbohydrate Intake on the Gut Microbiome: A Weight of Evidence Systematic Review. Microorganisms 2023, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Brumer, H. Sticking to starch. J Biol Chem. 2022, 298, 102049. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. Int. Rev. J. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, N.; Zhou, Y.; Zhou, Z.; Bai, Y.; Strappe, P.; Blanchard, C. Manipulations of glucose/lipid metabolism and gut microbiota of resistant starch encapsulated Ganoderma lucidum spores in T2DM rats. Food Sci. Biotechnol. 2021, 30, 755–764. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, S.; Wu, J.; Luo, L.; Qiao, S.; Li, R.; Xu, W.; Wang, N.; Zhao, B.; Wang, X.; et al. A specific gut microbiota and metabolomic profiles shifts related to antidiabetic action: The similar and complementary antidiabetic properties of type 3 resistant starch from Canna edulis and metformin. Pharmacol. Res. 2020, 159, 104985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Song, Y.-H.; Zhao, R.; Xia, L.; Chen, Y.; Cui, Y.-P.; Rao, Z.-Y.; Zhou, Y.; Zhuang, W.; et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: A systematic review and meta-analysis. Nutr. Diabetes 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.E.; Cai, M.; Altieri, N.; Frost, G. A comparison of the effects of resistant starch types on glycemic response in individuals with type 2 diabetes or prediabetes: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1118229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, L.; Wu, J.; Qiao, S.; Xu, W.; Ma, S.; Zhao, B.; Wang, X. Intervention of resistant starch 3 on type 2 diabetes mellitus and its mechanism based on urine metabonomics by liquid chromatography-tandem mass spectrometry. Biomed. Pharmacother. 2020, 128, 110350. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef]
- Collings, P.; Williams, C.; MacDonald, I. Effects of cooking on serum glucose and insulin responses to starch. BMJ 1981, 282, 1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health beneficial effects of resistant starch on diabetes and obesity via regulation of gut microbiota: A review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant Starches Types 2 and 4 Have Differential Effects on the Composition of the Fecal Microbiota in Human Subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2010, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Moraïs, S.; Laverde-Gomez, J.; Sheridan, P.O.; Walker, A.W.; Kelly, W.; Klieve, A.V.; Ouwerkerk, D.; Duncan, S.H.; Louis, P.; et al. Sporulation capability and amylosome conservation among diverse human colonic and rumen isolates of the keystone starch-degrader Ruminococcus bromii. Environ. Microbiol. 2017, 20, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Wylie-Rosett, J. The Role of Dietary Proteins Among Persons with Diabetes. Curr. Atheroscler. Rep. 2013, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, K.M.; Devries, M.C. Nutritional Strategies to Combat Type 2 Diabetes in Aging Adults: The Importance of Protein. Front. Nutr. 2019, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Phillips, S.M. 'Changes in Kidney Function Do Not Differ between Healthy Adults Consuming Higher- Compared with Lower- or Normal-Protein Diets: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef] [PubMed]
- Piatti, P.; Monti, L.; Magni, F.; Fermo, I.; Baruffaldi, L.; Nasser, R.; Santambrogio, G.; Librenti, M.; Galli-Kienle, M.; Pontiroli, A.; et al. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: Comparison to hypocaloric high-carbohydrate diet. Metabolism 1994, 43, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Fujita, Y.; Kieffer, T.J. Glucagon-Like Peptide-1: Glucose Homeostasis and Beyond. Annu. Rev. Physiol. 2014, 76, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a Protein Preload on Gastric Emptying, Glycemia, and Gut Hormones After a Carbohydrate Meal in Diet-Controlled Type 2 Diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women's health study. Diabetes Care. 2004, 27, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; Grobbee, D.E.; van der Schouw, Y.T. Dietary Intake of Total, Animal, and Vegetable Protein and Risk of Type 2 Diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL Study. Diabetes Care 2009, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Scott, D.; Hodge, A.M.; English, D.R.; Giles, G.G.; Ebeling, P.R.; Sanders, K.M. Dietary protein intake and risk of type 2 diabetes: Results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016, 104, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- van Nielen, M.; Feskens, E.J.; Mensink, M.; Sluijs, I.; Molina, E.; Amiano, P.; Ardanaz, E.; Balkau, B.; Beulens, J.W.; Boeing, H.; et al. Dietary protein intake and incidence of type 2 diabetes in Europe: The EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014, 37, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Vila-Altesor, M.; Ameneiros-Rodríguez, E. The differential effect of animal versus vegetable dietary protein on the clinical manifestations of diabetic kidney disease in humans. Clin. Nutr. ESPEN 2022, 48, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Stewart, S.E.; Jayalath, V.H.; Ng, A.P.; Mirrahimi, A.; De Souza, R.J.; Hanley, A.J.; Bazinet, R.P.; Mejia, S.B.; Leiter, L.A.; et al. Effect of Replacing Animal Protein with Plant Protein on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2015, 7, 9804–9824. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Alcalá-Diaz, J.F.; Quintana-Navarro, G.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Cardelo, M.P.; Arenas-Larriva, A.P.; Malagón, M.M.; Romero-Cabrera, J.L.; Ordovás, J.M.; et al. Changes in quantity plant-based protein intake on type 2 diabetes remission in coronary heart disease patients: From the CORDIOPREV study. Eur. J. Nutr. 2023, 62, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Ares, S.; Gutiérrez-Mariscal, F.M.; Alcalá-Díaz, J.F.; Quintana-Navarro, G.M.; Podadera-Herreros, A.; Cardelo, M.P.; Torres-Peña, J.D.; Larriva, A.P.A.-D.; Pérez-Martínez, P.; Delgado-Lista, J.; et al. Quality and Quantity of Protein Intake Influence Incidence of Type 2 Diabetes Mellitus in Coronary Heart Disease Patients: From the CORDIOPREV Study. Nutrients 2021, 13, 1217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2018, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic Administration Increases Amino Acid Absorption from Plant Protein: A Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Zhu, W. Temporal microbiota changes of high-protein diet intake in a rat model. Anaerobe 2017, 47, 218–225. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2016, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Pereira, F.C.; Schintlmeister, A.; Berry, D.; Wagner, M.; Hale, L.P.; Wu, A.; Jiang, S.; Durand, H.K.; Zhou, X.; et al. Microbial nitrogen limitation in the mammalian large intestine. Nat. Microbiol. 2018, 3, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.; Hu, Q.; Baldwin, R.L.; Bequette, B.J. Role of rumen butyrate in regulation of nitrogen utilization and urea nitrogen kinetics in growing sheep1. J. Anim. Sci. 2015, 93, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of Causal Direction Between Gut Microbiota–Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Hentges, D.J.; Maier, B.R.; Burton, G.C.; A Flynn, M.; Tsutakawa, R.K. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977, 37, 568–571. [Google Scholar] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.-. .-D.A. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhuang, X.; Liu, Q.; Sun, B.; Miao, H.; Zhang, X. Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice. Food Sci. Hum. Wellness 2021, 11, 41–48. [Google Scholar] [CrossRef]
- von Frankenberg, A.D.; Silva, F.M.; de Almeida, J.C.; Piccoli, V.; Nascimento, F.V.D.; Sost, M.M.; Leitão, C.B.; Remonti, L.L.R.; Umpierre, D.; Reis, A.F.; et al. Effect of dietary lipids on circulating adiponectin: A systematic review with meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Heal. Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Barbaresko, J.; Pischke, C.R.; Iser, N.; Beckhaus, J.; Schwingshackl, L.; Schlesinger, S. Intake of dietary fats and fatty acids and the incidence of type 2 diabetes: A systematic review and dose-response meta-analysis of prospective observational studies. PLOS Med. 2020, 17, e1003347. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.; Mann, J.; Bennett, P.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Lindström, J.; Louheranta, A. Diet, nutrition and the prevention of type 2 diabetes. PhotoniX 2004, 7, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, Perri MG, Beresford SA, Robinson JG, Rodríguez B; et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: The Women's Health Initiative randomized controlled dietary modification trial. Arch Intern Med. 2008, 168, 1500–1511. [CrossRef]
- Wang, L.-L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.-Y.; Huang, Y.-H.; Wang, X.-H. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. New Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; I Cozma, A.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.-E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding Polyunsaturated and Saturated Fat Causes Distinct Effects on Liver and Visceral Fat Accumulation in Humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Do functional foods have a role in the prevention of cardiovascular disease? Circulation 2011, 124, 538–540. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, J.; Hu, F.B.; E Manson, J.; Stampfer, M.J.; A Colditz, G.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined d-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Mykhalchyshyn, G.; Boccuto, L.; Kononenko, L.; Kyriienko, D.; Komisarenko, I.; Dynnyk, O. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva Medica 2018, 109, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Sekita, A.; Chiji, H.; Kato, N. Consumption of lily bulb modulates fecal ratios of firmicutes and bacteroidetes phyla in rats fed a high-fat diet. Food Sci. Biotechnol. 2016, 25, 153–156. [Google Scholar] [CrossRef]
- de Lartigue, G.; Barbier de la Serre, C.; Espero, E.; Lee, J.; Raybould, H.E. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011, 301, E187–E195. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2013, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2018, 58, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Valdés-Ramos, R.; Guadarrama-López, A.L.; Martínez-Carrillo, B.E.; Benítez-Arciniega, A.D. Vitamins and type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2015, 15, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Stepanova, A.; Bystrova, A.; Jude, E.B. High-Dose Vitamin D Supplementation Improves Microcirculation and Reduces Inflammation in Diabetic Neuropathy Patients. Nutrients 2020, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, R.; Rahim, H.A.; Ahmed, J.K. Decreased inflammatory gene expression accompanies the improvement of liver enzyme and lipid profile following aerobic training and vitamin D supplementation in T2DM patients. BMC Endocr. Disord. 2022, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johny, E.; Jala, A.; Nath, B.; Alam, J.; Kuladhipati, I.; Das, R.; Borkar, R.M.; Adela, R. Vitamin D Supplementation Modulates Platelet-Mediated Inflammation in Subjects With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Immunol. 2022, 13, 869591. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, T.W.; Väisänen, S.; Frank, C.; Molnár, F.; Sinkkonen, L.; Carlberg, C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005, 349, 248–260. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Misra, A.; Pandey, R.M.; Upadhyay, A.D.; Gulati, S.; Singh, N. Vitamin D Supplementation in Overweight/obese Asian Indian Women with Prediabetes Reduces Glycemic Measures and Truncal Subcutaneous Fat: A 78 Weeks Randomized Placebo-Controlled Trial (PREVENT-WIN Trial). Sci Rep. 2020, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Sukik, A.; Alalwani, J.; Ganji, V. Vitamin D, Gut Microbiota, and Cardiometabolic Diseases-A Possible Three-Way Axis. Int J Mol Sci. 2023, 24, 940. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bakke, D.; Chatterjee, I.; Agrawal, A.; Dai, Y.; Sun, J. Regulation of Microbiota by Vitamin D Receptor: A Nuclear Weapon in Metabolic Diseases. Nucl. Recept. Res. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.M.; Elyamany, M.F.; Rashed, L.A.; Sallam, N.A. Vitamin D mitigates diabetes-associated metabolic and cognitive dysfunction by modulating gut microbiota and colonic cannabinoid receptor 1. Eur. J. Pharm. Sci. 2021, 170, 106105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, J.; Zhang, Y.; Tang, J.; Sun, B.; Xu, W.; Wang, X.; Chen, Y.; Sun, Z. Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency. J. Diabetes Res. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Qu, B.; Yan, S.; Ao, Y.; Chen, X.; Zheng, X.; Cui, W. The relationship between vitamin K and T2DM: A systematic review and meta-analysis. Food Funct. 2023, 14, 8951–8963. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Wei, C.; Wang, X.; Li, R.; Xu, X.; Zhang, Y.; Geng, G.; Dang, K.; Ming, Z.; et al. Vitamin K2 supplementation improves impaired glycemic homeostasis and insulin sensitivity for type 2 diabetes through gut microbiome and fecal metabolites. BMC Med. 2023, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate Vitamin C Status in Prediabetes and Type 2 Diabetes Mellitus: Associations with Glycaemic Control, Obesity, and Smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Qin, S.; Zhu, S.; Wu, L. The antidiabetic drug metformin aids bacteria in hijacking vitamin B12 from the environment through RcdA. Commun. Biol. 2023, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kakarlapudi, Y.; Kondabolu, S.K.; Tehseen, Z.; Khemani, V.; J, S.K.; Nousherwani, M.D.; Saleem, F.; Abdelhameed, A.N. Effect of Metformin on Vitamin B12 Deficiency in Patients With Type 2 Diabetes Mellitus and Factors Associated With It: A Meta-Analysis. Cureus 2022, 14, e32277. [Google Scholar] [CrossRef] [PubMed]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Aaseth, J. The Role of Zinc and Copper in Insulin Resistance and Diabetes Mellitus. Curr. Med. Chem. 2020, 27, 6643–6657. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2013, 45, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency During Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Celis, A.I.; Relman, D.A. Competitors versus Collaborators: Micronutrient Processing by Pathogenic and Commensal Human-Associated Gut Bacteria. Mol. Cell 2020, 78, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, H.; Fein, E.; Redecker, P.; Stremmel, W.; Adler, G.; Cetin, Y. Pancreatic beta-cells express hepcidin, an iron-uptake regulatory peptide. J Endocrinol. 2008, 197, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Berthault, C.; Staels, W.; Scharfmann, R. Purification of pancreatic endocrine subsets reveals increased iron metabolism in beta cells. Mol Metab. 2020, 42, 101060. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.-L.; Ktorza, A.; Casteilla, L.; Pénicaud, L. Mitochondrial Reactive Oxygen Species Are Obligatory Signals for Glucose-Induced Insulin Secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative Stress, β-Cell Apoptosis, and Decreased Insulin Secretory Capacity in Mouse Models of Hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [PubMed]
- Salaye, L.; Bychkova, I.; Sink, S.; Kovalic, A.J.; Bharadwaj, M.S.; Lorenzo, F.; Jain, S.; Harrison, A.V.; Davis, A.T.; Turnbull, K.; et al. A Low Iron Diet Protects from Steatohepatitis in a Mouse Model. Nutrients 2019, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Tabas, I. Calcium signalling and ER stress in insulin resistance and atherosclerosis. J. Intern. Med. 2016, 280, 457–464. [Google Scholar] [CrossRef]
- Faysal, M.R.; Akter, T.; Hossain, M.S.; Begum, S.; Banu, M.; Tasnim, J.; Sultana, I.; Krishna, S.P.; Alam, S.; Akter, T.; et al. Study of Serum Calcium and Magnesium Levels in Type 2 Diabetes Mellitus Patients. Mymensingh Med. J. MMJ 2023, 32, 54–60. [Google Scholar] [PubMed]
- Bergillos-Meca, T.; Cabrera-Vique, C.; Artacho, R.; Moreno-Montoro, M.; Navarro-Alarcón, M.; Olalla, M.; Giménez, R.; Seiquer, I.; Ruiz-López, M.D. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats’ milk? Food Chem. 2015, 187, 314–321. [Google Scholar] [CrossRef]
- Kulathunga, J.; Simsek, S. A Review: Cereals on Modulating the Microbiota/Metabolome for Metabolic Health. Curr. Nutr. Rep. 2022, 11, 371–385. [Google Scholar] [CrossRef] [PubMed]
- van Trijp, M.P.H.; Schutte, S.; Esser, D.; Wopereis, S.; Hoevenaars, F.P.M.; Hooiveld, G.J.E.J.; A Afman, L. Minor Changes in the Composition and Function of the Gut Microbiota During a 12-Week Whole Grain Wheat or Refined Wheat Intervention Correlate with Liver Fat in Overweight and Obese Adults. J. Nutr. 2020, 151, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Y.; Wei, D.; Ni, W.; Zhu, N.; Yan, X. Impact of a high dietary fiber cereal meal intervention on body weight, adipose distribution, and cardiovascular risk among individuals with type 2 diabetes. Front. Endocrinol. 2023, 14, 1283626. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.N.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.A.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Ulaszewska, M.M.; Scholz, M.; Stanstrup, J.; Nissen, L.; Mattivi, F.; Vermeiren, J.; Bosscher, D.; Pedrolli, C.; Tuohy, K.M. Impact of wheat aleurone on biomarkers of cardiovascular disease, gut microbiota and metabolites in adults with high body mass index: A double-blind, placebo-controlled, randomized clinical trial. Eur. J. Nutr. 2022, 61, 2651–2671. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Rodriguez, J.; Patrie, J.T.; Whisner, C.M.; Angadi, S.S. Effects of Glycemic Index and Cereal Fiber on Postprandial Endothelial Function, Glycemia, and Insulinemia in Healthy Adults. Nutrients 2019, 11, 2387. [Google Scholar] [CrossRef]
- Williams, P.G. The Benefits of Breakfast Cereal Consumption: A Systematic Review of the Evidence Base. Adv. Nutr. Int. Rev. J. 2014, 5, 636S–673S. [Google Scholar] [CrossRef] [PubMed]
- Harris Jackson, K.; West, S.G.; Vanden Heuvel, J.P.; Jonnalagadda, S.S.; Ross, A.B.; Hill, A.M.; Grieger, J.A.; Lemieux, S.K.; Kris-Etherton, P.M. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: A randomized controlled-feeding trial. Am J Clin Nutr. 2014, 100, 577–586. [Google Scholar] [CrossRef]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Jacobs, D.R., Jr.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; Slavin, J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Oöstman, E.M.; Granfeldt, Y.; Björck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiology 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Lee, H.-B.; Kim, Y.; Park, H.-Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes its Complicat. 2018, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Sun, S.; Liu, S.; Huan, Y.; Li, R.; Liu, Q.; Cao, H.; Zhou, T.; Lei, L.; et al. Effects of a ready-to-eat cereal formula powder on glucose metabolism, inflammation, and gut microbiota in diabetic db/db mice. Food Sci Nutr. 2020, 8, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2017, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Yang, Z.; Hao, Y.; Wang, Z.; Wang, J.; Wang, Z. Wheat Alkylresorcinols Modulate Glucose Homeostasis through Improving GLP-1 Secretion in High-Fat-Diet-Induced Obese Mice. J. Agric. Food Chem. 2023, 71, 16125–16136. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.G.; Licht, T.R.; Kristensen, M.; I Bahl, M. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur. J. Clin. Nutr. 2013, 67, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2007, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Wells, A.L.; Helmolz, K.; Nodet, C.; Molzer, C.; Leonard, C.; McKevith, B.; Thielecke, F.; Jackson, K.G.; Tuohy, K.M. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: A human feeding study. Br J Nutr. 2010, 104, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhu, L.; Gao, M.; Yin, Z.; Zhang, Z.; Zhu, L.; Zhan, X. A Comparative Study of the Effects of Whole Cereals and Refined Cereals on Intestinal Microbiota. Foods 2023, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Basiak-Rasała, A.; Różańska, D.; Zatońska, K. Food groups in dietary prevention of type 2 diabetes. Rocz Panstw Zakl Hig . 2019, 70, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Virtanen, J.K.; Tuomainen, T.-P.; Nurmi, T.; Voutilainen, S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2014, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.P.; Mingione, A.; Pivari, F.; Dogliotti, E.; Brasacchio, C.; Murugesan, S.; Cusi, D.; Lazzaroni, M.; Soldati, L.; Terranegra, A. Modulation of gut microbiota: The effects of a fruits and vegetables supplement. Front. Nutr. 2022, 9, 930883. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, T.-Y.; He, Y.; Gou, W.; Zuo, L.-S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, G.; Abshirini, M.; Bagheri, F.; Siassi, F.; Koohdani, F.; Aslany, Z. Higher dietary total antioxidant capacity is inversely related to prediabetes: A case-control study. Nutrition 2018, 46, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, W.; Xu, W.; Ran, L.; Yan, Y.; Lu, L.; Mi, J.; Zeng, X.; Cao, Y. Modulation of gut microbiota and hypoglycemic/hypolipidemic activity of flavonoids from the fruits of Lycium barbarum on high-fat diet/streptozotocin-induced type 2 diabetic mice. Food Funct. 2022, 13, 11169–11184. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Tang, T.; Tang, W.; Long, Y.; Wang, L.; Liu, M. Astragalus improves intestinal barrier function and immunity by acting on intestinal microbiota to treat T2DM: A research review. Front. Immunol. 2023, 14, 1243834. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Xiong, L.; Bi, C.; Zhang, B. Diosmetin ameliorate type 2 diabetic mellitus by up-regulating Corynebacterium glutamicum to regulate IRS/PI3K/AKT-mediated glucose metabolism disorder in KK-Ay mice. Phytomedicine 2021, 87, 153582. [Google Scholar] [CrossRef]
- Tarwadi, K.; Agte, V. Potential of commonly consumed green leafy vegetables for their antioxidant capacity and its linkage with the micronutrient profile. Int. J. Food Sci. Nutr. 2003, 54, 417–425. [Google Scholar] [CrossRef]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer--Norfolk prospective study. Arch Intern Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Magnesium intake and risk of type 2 diabetes: A meta-analysis. J. Intern. Med. 2007, 262, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Roach, A.K.; Sparks, K.C.; Marquart, L.; D'Agostino, R.B.; Mayer-Davis, E.J. Whole-grain intake and insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Am. J. Clin. Nutr. 2003, 78, 965–971. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Kalle-Uhlmann, T.; Arregui, M.; Buijsse, B.; Boeing, H. Fruit and Vegetable Consumption and Changes in Anthropometric Variables in Adult Populations: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0140846. [Google Scholar] [CrossRef]
- Ramne, S.; Brunkwall, L.; Ericson, U.; Gray, N.; Kuhnle, G.G.C.; Nilsson, P.M.; Orho-Melander, M.; Sonestedt, E. Gut microbiota composition in relation to intake of added sugar, sugar-sweetened beverages and artificially sweetened beverages in the Malmö Offspring Study. Eur J Nutr. 2021, 60, 2087–2097. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Viana, S.D.; Preguiça, I.; Alves, A.; Fernandes, R.; Teodoro, J.S.; Matos, P.; Figueirinha, A.; Salgueiro, L.; André, A.; et al. Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics. Nutrients 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high-glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am J Clin Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V.; Dhaliwal, S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br. J. Nutr. 2010, 104, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Sun, Q.; van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Rosner, B.; Hu, F.B. Adolescent dairy product consumption and risk of type 2 diabetes in middle-aged women. Am. J. Clin. Nutr. 2011, 94, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Dong, J.-Y.; Wu, Z.-W.; Li, W.; Qin, L.-Q. Dairy consumption and risk of type 2 diabetes mellitus: A meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 1027–1031. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.; Hu, F.B. Dairy consumption and risk of type 2 diabetes mellitus in men: A prospective study. Arch Intern Med. 2005, 165, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Choi, H.K.; Ford, E.; Song, Y.; Klevak, A.; Buring, J.E.; Manson, J.E. A Prospective Study of Dairy Intake and the Risk of Type 2 Diabetes in Women. Diabetes Care 2006, 29, 1579–1584. [Google Scholar] [CrossRef]
- Link-Amster, H.; Rochat, F.; Saudan, K.Y.; Mignot, O.; Aeschlimann, J.M. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, I.; Dolar, M.E.; Ozpinar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2020, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, J.; Huang, Y.; Li, X.; Wang, H.; Zhang, Y.; Suo, H. Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct. 2021, 13, 675–687. [Google Scholar] [CrossRef]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. In Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E; et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2023, MDText.com, Inc.; 2000. [Google Scholar]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Follis, J.L.; A Nettleton, J.; Lemaitre, R.N.; Ngwa, J.S.; Wojczynski, M.K.; Kalafati, I.P.; Varga, T.V.; Frazier-Wood, A.C.; Houston, D.K.; et al. Consumption of meat is associated with higher fasting glucose and insulin concentrations regardless of glucose and insulin genetic risk scores: A meta-analysis of 50,345 Caucasians. Am. J. Clin. Nutr. 2015, 102, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.H.; Salehi-Abargouei, A.; Surkan, P.J.; Azadbakht, L. Is there a relationship between red or processed meat intake and obesity? A systematic review and meta-analysis of observational studies. Obes. Rev. 2014, 15, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Tong, M.; Lawton, M.; Longato, L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol. Neurodegener. 2009, 4, 54. [Google Scholar] [CrossRef]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed Red and Processed Meats and Risk of Coronary Artery Disease and Type 2 Diabetes – An Updated Review of the Evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Neusner, A.; Longato, L.; Lawton, M.; Wands, J.R.; De La Monte, S.M. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J. Alzheimer's Dis. 2009, 17, 827–844. [Google Scholar]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2016, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Collinson, A. Red Meat, Dietary Heme Iron, and Risk of Type 2 Diabetes: The Involvement of Advanced Lipoxidation Endproducts. Adv. Nutr. Int. Rev. J. 2013, 4, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.M.; Dong, H.-J.; Li, Z.; Cai, W.; Altomonte, J.; Thung, S.N.; Zeng, F.; Fisher, E.A.; Vlassara, H. Improved Insulin Sensitivity Is Associated With Restricted Intake of Dietary Glycoxidation Products in the db/db Mouse. Diabetes 2002, 51, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Islam, T.; Johnson, P.; Moustaid-Moussa, N. Systematic Review of Beef Protein Effects on Gut Microbiota: Implications for Health. Adv. Nutr. Int. Rev. J. 2020, 12, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Winter, J.M.; Christophersen, C.T.; Young, G.P.; Humphreys, K.J.; Hu, Y.; Gratz, S.W.; Miller, R.B.; Topping, D.L.; Bird, A.R.; et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: A randomised clinical trial. Br. J. Nutr. 2015, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Feng, S.; He, K.; Guo, L.; Chen, W.; Wang, M.; Zhong, L.; Wu, C.; Peng, X.; et al. Differential Effects of Dietary White Meat and Red Meat on NAFLD Progression by Modulating Gut Microbiota and Metabolites in Rats. Oxidative Med. Cell. Longev. 2022, 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Di Zhao, D.; Zhao, F.; Wang, C.; Zamaratskaia, G.; Li, C. Chicken-eaters and pork-eaters have different gut microbiota and tryptophan metabolites. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, X.; Zhao, D.; Zhao, F.; Li, C. Pork Meat Proteins Alter Gut Microbiota and Lipid Metabolism Genes in the Colon of Adaptive Immune-Deficient Mice. Mol Nutr Food Res. 2020, 64, e1901105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, X.; Li, H.; Li, Y.; Shi, X.; Zhao, F.; Xu, X.; Li, C.; Zhou, G. Intake of Meat Proteins Substantially Increased the Relative Abundance of Genus Lactobacillus in Rat Feces. PLoS ONE 2016, 11, e0152678. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X.-H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; A Novotny, J.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Bitsko, E.; Biswas, D. Functional Properties of Peanut Fractions on the Growth of Probiotics and Foodborne Bacterial Pathogens. J. Food Sci. 2015, 80, M635–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Manson, J.E.; Stampfer, M.J.; Liu, S.; Willett, W.C.; Hu, F.B. Nut and Peanut Butter Consumption and Risk of Type 2 Diabetes in Women. JAMA 2002, 288, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Siloto, R.M.; Wickramarathna, A.D.; Mietkiewska, E.; Weselake, R.J. Identification of a Pair of Phospholipid:Diacylglycerol Acyltransferases from Developing Flax (Linum usitatissimum L.) Seed Catalyzing the Selective Production of Trilinolenin. J. Biol. Chem. 2013, 288, 24173–24188. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Kendall, C.W.C.; Mejia, S.B.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Heal. Dis. 2020, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, M.; Bin Oh, S.; Kim, H.-Y.; Kim, C.; Kim, T.-Y.; Park, Y.-H. Superoxide dismutase 3 prevents early stage diabetic retinopathy in streptozotocin-induced diabetic rat model. PLoS ONE 2022, 17, e0262396. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quintero, M.J.; Delgado, J.; Medina-Vera, D.; Becerra-Muñoz, V.M.; Queipo-Ortuño, M.I.; Estévez, M.; Plaza-Andrades, I.; Rodríguez-Capitán, J.; Sánchez, P.L.; Crespo-Leiro, M.G.; et al. Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 4650. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Im, S.-H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef]
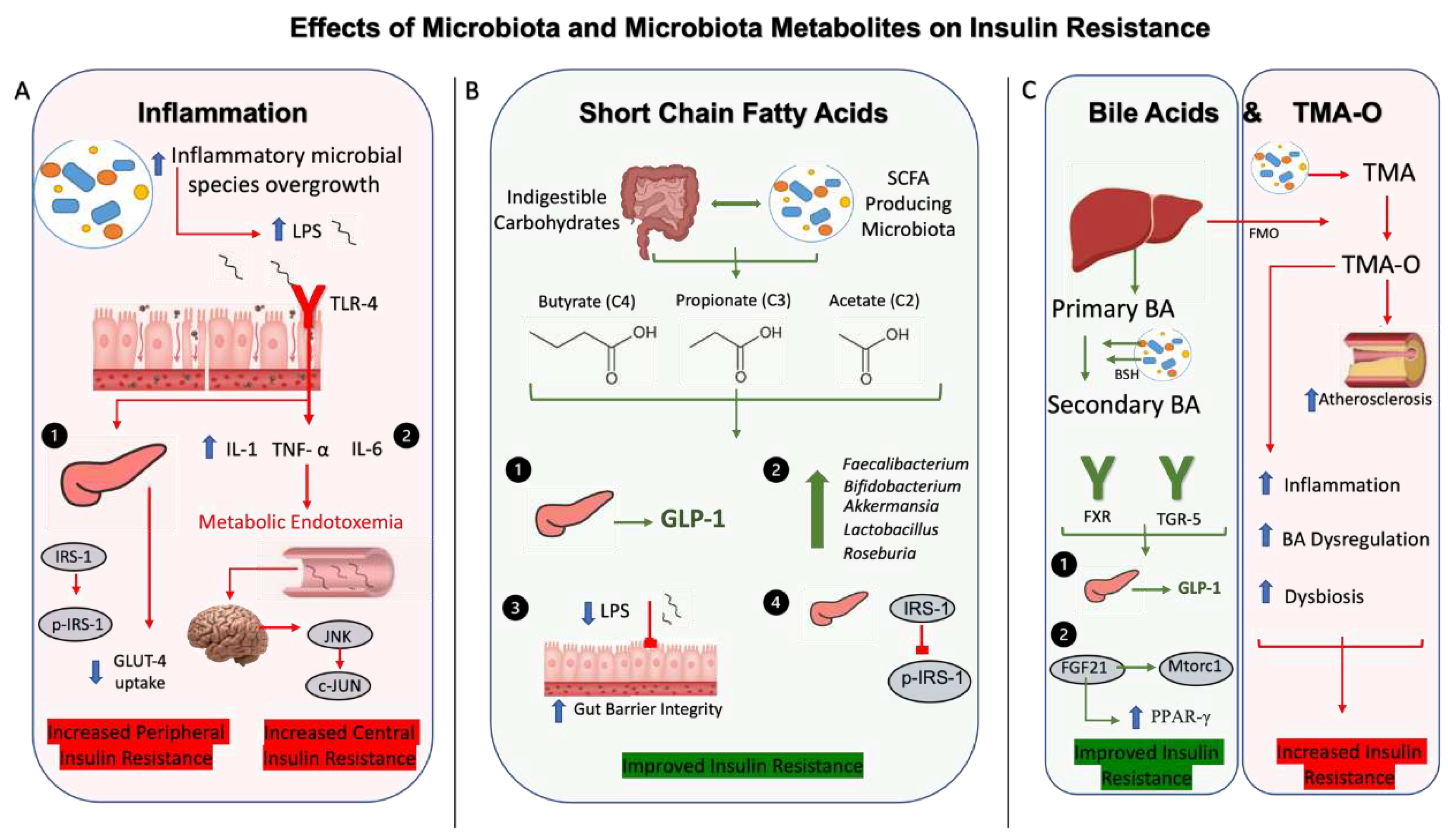
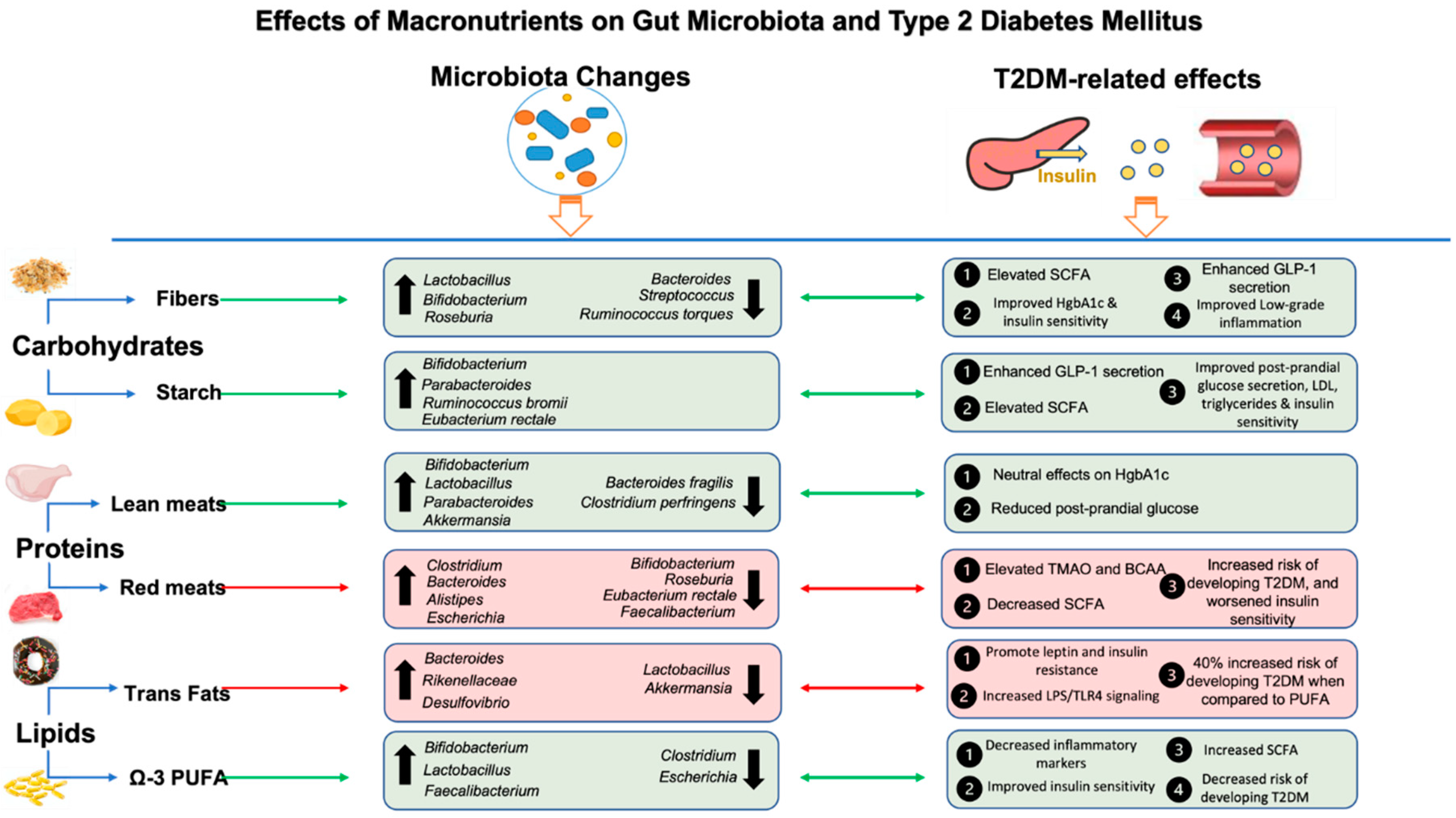
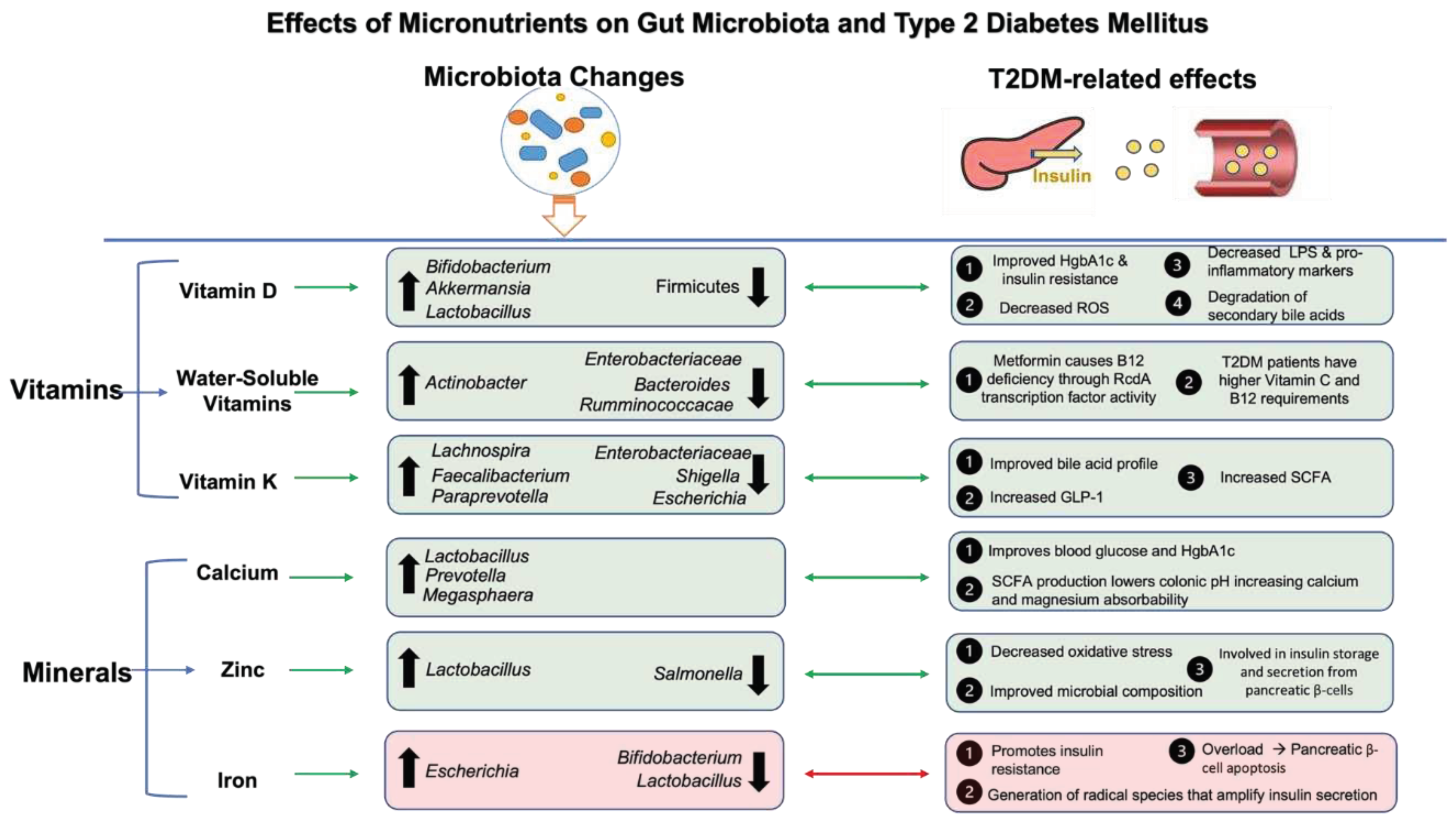
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





