1. Introduction
Colorectal cancer (CRC) is the third most common neoplasm diagnosed worldwide in men and women and it is the second in terms of cancer related mortality according to GLOBOCAN [
1]. In Brazil, CRC incidence varies according to the region, oscillating from the second to the fourth most frequent diagnosed malignancy, according to data published from the Brazilian National Cancer Institute (INCA) [
2]. The incidence of early onset CRC (EOCRC), which occurs in persons < 50 years old, has been rising in recent years, especially in high-income countries. This increase can be explained by the exposure to risk factors 33 since childhood and adolescence, such as sedentarism, industrialized diet and intestinal microbiota alterations [
3,
4,
5]. Nonetheless, according to GLOBOCAN estimates, low and 35 middle income countries will also have an increment in CRC and EOCRC incidence in the 36 next 20 years because of the increasing availability of diagnostic tools in these regions, like 37 colonoscopy and imaging [
6].
Some medical societies have suggested a reduction in the age of initiating CRC screening from 50 to 45 years [
7]. Interestingly, a meta-analysis including 51,811 individuals who underwent colonoscopy in 4 continents showed that the average-risk population aged 45 to 49 years had similar rates of CRC in comparison to individuals aged 50 to 59 years, suggesting that expanding screening to those aged 45 to 49 years could be beneficial [
8].
Precision Oncology is a field in constant evolution focusing on tailoring cancer therapies according to specific genetic alterations from the patient’s tumor with the aim of obtaining better clinical outcomes [
9]. Although fluoropyrimidine-based chemotherapy remains as the backbone of systemic therapy of mCRC, an adequate characterization of the tumor molecular profile in addition to tumor sidedness has an important role in refining the adequate combination and sequence of therapies, such as the EGFR inhibitors or the antiangiogenic agents [
10].
Likewise, several studies have shown that
BRAF V600 mutation is a powerful negative prognostic marker in mCRC, leading to the investigation of BRAF inhibitors combinations to improve the clinical outcomes [
11]. Furthermore, microsatellite instability (MSI-high) or defective mismatch repair (dMMR) has become a meaningful prognostic and predictive biomarker, since these tumors tend to portend better prognosis in early stages and to be highly sensitive to immune checkpoint inhibition in the metastatic setting [
12].
According to various consensus, an adequate pathology report should contain data of
RAS and
BRAF mutation status as well as MSI/MMR [
13]. In recent years, with the development of specific KRAS-G12C inhibitors, this information is also being used for treatment decision-making [
14].
This fact is reflected by the discrepancies in incidence of CRC, which is higher in high income countries in comparison to low and middle income countries [
4,
5]. Nonetheless, according to GLOBOCAN estimates, low and middle income countries will also have an increment in CRC and EOCRC incidence in the next 20 years because of the increasing availability of diagnostic tools in these regions, like colonoscopy and imaging (6].
The access to oncology consultations and medical facilities is not equal throughout the Brazilian territory. Laboratories with capacity to run molecular profiling in solid tumors are concentrated in large cities from the Southeast region [
15]. Coordination of a network of oncology units from diverse regions of the country to a centralized molecular pathology laboratory has increased access to reliable results [
16]. Our study is the effort of our network to analyze the molecular profile of mCRC patients with standard-of-care biomarkers and integrate with patient demographics and tumor characteristics.
2. Materials and Methods
Population: Retrospective and multicenter study evaluating adult (>18 years-old) mCRC patients who were treated at Oncoclinicas units in Brazil from January 2019 to July 2023 and who were tested for KRAS/NRAS and BRAF mutations in a centralized genomics lab (Oncoclinicas Precision Medicine). Demographic and clinical data (gender, age, region of origin and sidedness of the tumor) were retrieved from electronic medical records, together with MSI/MMR status if not performed in the central laboratory.
Analysis and Genotyping: Specimens came to our central laboratory review from several Brazilian centers within the Oncoclinicas network, from January 2021 to July 2023. The cases were reviewed by a group of expert pathologists, all of them were tissue-based analysis. No liquid biopsies were included. DNA was extracted from tissue samples (FFPE) using QIASymphony extractions kits and appropriate tissue area was defined as 100-300 µ2 with >10% tumor cells. An input of 100 ng was required. The NGS library was prepared using QIAseq Targeted DNA Custom Panel (QIAGEN). Sequencing was performed in the Illumina platform (MiSeq) in paired-end 2 x 150 cycles. The test covered 23 cancer genes, with oncogenic mutations in
KRAS (exons 2 to 5),
NRAS (exons 2 to 4) and
BRAF (exons 7, 11, 12, 15, 16) analyzed as part of a patient support program sponsored by Amgen (RAStrear). Analytical sensitivity was defined at 1% variant allele frequency [
17,
18]. MSI or MMR status was examined with standard PCR-based techniques or immunohistochemistry (IHC) of MLH1, MSH2, MSH6 and PMS2 proteins, respectively [
19].
Statistical analysis: Descriptive statistics were used to characterize demographic and clinical data. Frequencies and percentage were used for the categorical variables and mean, median, standard deviation for numerical variables. The correlation among variables was done by chi-square and fisher exact test when applicable. Age groups were categorized in three strata: <50 years (as the age of recommendation for screening colonoscopy), 50-80 years and >80 years. Right-sided tumors encompassed cecum to transverse colon, and left-sided tumors from splenic flexure to rectum. Statistical significance for all results was established as p value < 0.05. The statistical software used was SPSS 24. The study was approved by the Institutional Review Board at July 12th, 2023, protocol code 70914323.0.0000.0070.
3. Results
A total of 858 patients were included in our database (
Table 1). Regarding demographic data, male patients represented 50.3%, the most frequent region of origin of the samples was the Southeast region (76.1%), and the most frequent states were Rio de Janeiro (32.1%) and Minas Gerais (31.7%). The median age was 63.7 years (range 22 - 95 years). The age groups distribution of participants was: 149 (17.4%) <50 years, 602 (70.1%) from 50 to 80 years and 107 (12.5%) >80 years. Right-sided tumors were present in 316 (36.8%), left-sided tumors in 508 (59.2%), and 34 (4%) cases were not specified.
From the 858 cases, 401 (46.7%) were RAS wild-type, the proportion of
KRAS and
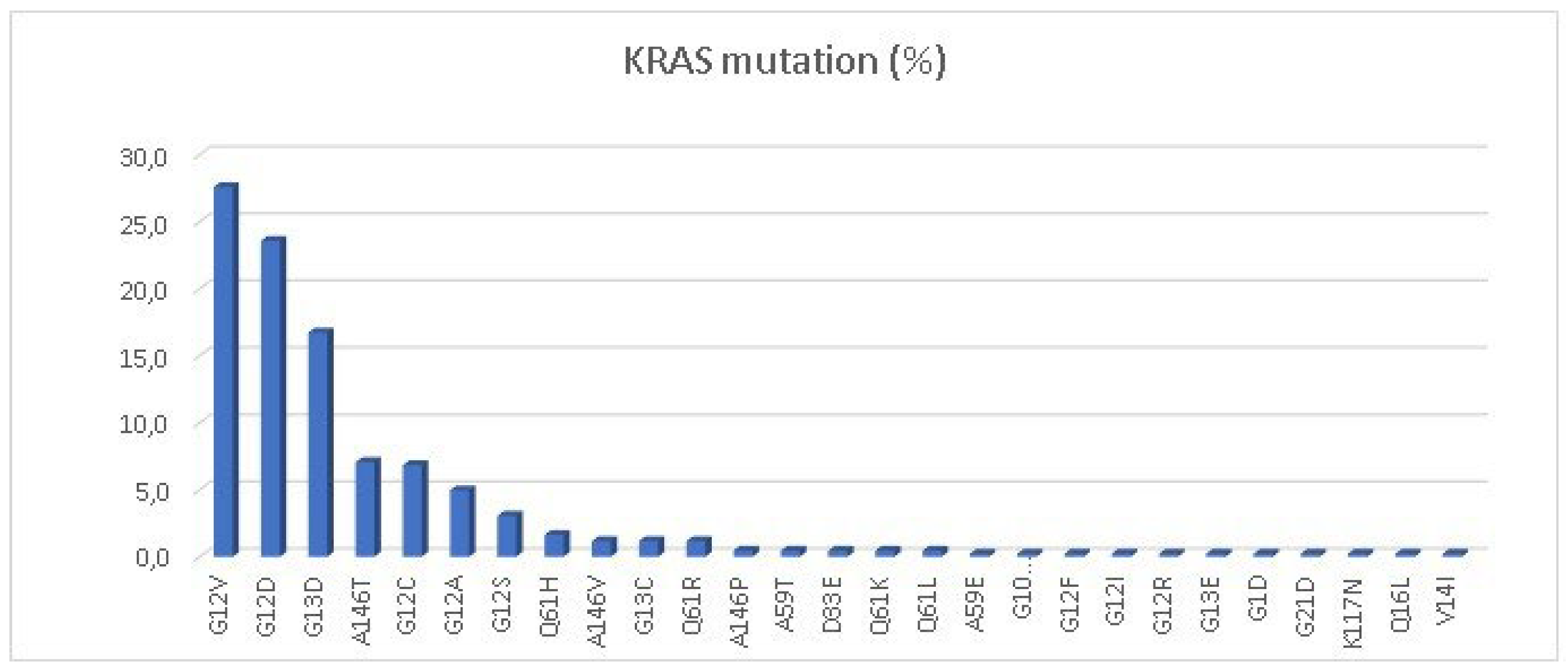
NRAS mutations was: 424 (49.4%) and 33 (3.9%) respectively. The most common
KRAS mutations were G12V (27.6%), G12D (23.5%) and G13D (16.7%) (
Figure 1). Of special interest, the specific
KRAS G12C mutation was present in 27 cases, representing 6.8% of the total
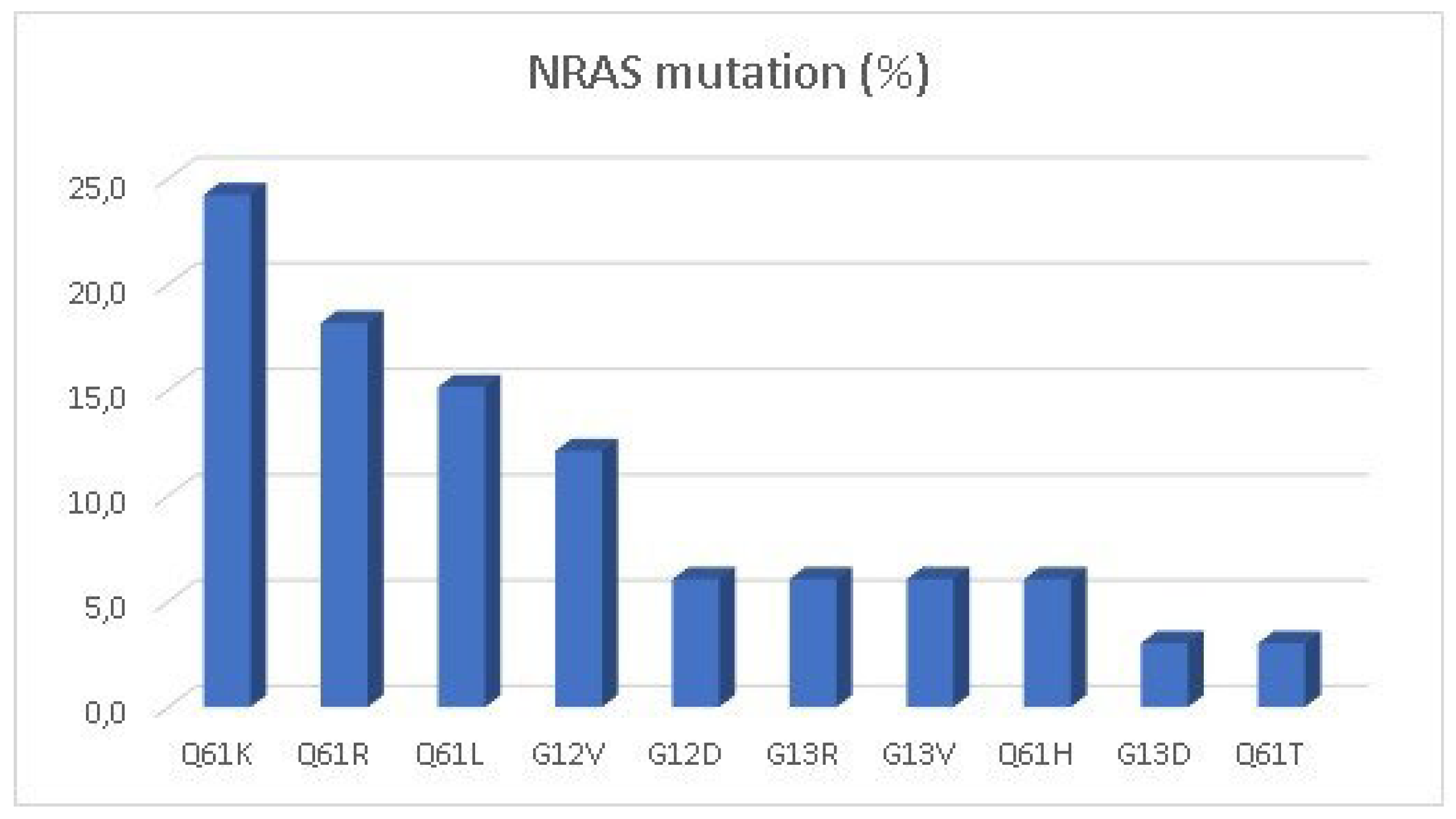
KRAS-mutated patients and 3.1% of the total population. The most common
NRAS mutation (
Figure 2) was the Q61K (24.2%). Only 63 cases (7.3%) were identified as BRAF mutant, most of them with the classical V600E, but there were 5 cases of non-canonical V600E mutation (D201N, D549N, D594G, G466V and V600K). MSI-High/dMMR was present in 14 cases (1.6%).
We performed two correlation analyses (using the chi-square method) stratifying by age (
Table 2) and sidedness (
Table 3). In the age-stratified analysis, the variables with statistical significance were sidedness (p<0.001) and
KRAS G12C mutation (p=0.046 by chi-square method and p=0.064 by fisher exact test). There was a higher incidence of left-sided tumors in patients <50 years, while right-sided tumors were more frequent in the elderly population (>80 years). Likewise, in the sidedness-stratified analysis, age also resulted in a significant correlation (p<0.001) (
Table 3). Other variables that demonstrated statistical significance were
BRAF-mutant (p=0.001) and MSI-high/dMMR status (p=0.009), which were more commonly found in right-sided tumors.
4. Discussion
To our knowledge, this is the first work to assess the mutational status (
KRAS, NRAS,
BRAF) together with the MSI/MMR status of metastatic CRC in the Brazilian population and the largest analysis in Latin America. Most previous studies have been unicentric or focused only on one molecular characteristic [
20,
21,
22]. We considered young patients less than 50 years old according to the recommendation for CRC screening. In our data, 17.4% were <50 years old, with a predominance of left-sided tumors (76.8%). A study. in Belo Horizonte/Brazil with 388 patients found that 20% of patients were younger than 50 years and 74% of the participants had left-sided tumors, but no correlation with any mutational status was encountered [
20]
Our distribution of RAS mutant status in total population (
KRAS 49.4% and
NRAS 3.9%) is similar to the 50.3%
KRAS and 3.8%
NRAS-mutant patients reported in a large Brazilian cohort of 2067 tissue samples, including both metastatic and localized disease. In the same study, the prevalence of
BRAF-mutant status was 6.6% as compared to 7.3% in our cohort. This study found a significant correlation between
NRAS and
BRAF-mutant status with older age (>75 years old) [
21].
In our data, there was no significant association between KRAS, NRAS or BRAF status and age at onset of CRC. But we did find a statistically significant correlation (p=0.046) between the
KRAS G12C mutation and patients younger than 50 years. The prevalence of this specific mutation in our whole population was 3.1% (n=27), which is less than what was found in a large Japanese database, which reported a prevalence of 6.5% (n=45), with most of them older than 65 years. However, this study did not find a significant association of age at onset with age (p=0.879) or sidedness (p=0.776) [
23]. Similarly, in the phase I/II trial of adagrasib plus cetuximab in pre-treated
KRAS G12C mutated mCRC patients, most patients were older than 60 years [
24].
Another Brazilian multicentric study included 989 patients and focused on their
KRAS status. The authors found a 38% prevalence of
KRAS-mutant tumors, of whom G12D was the most common [
22]. Numerically, the largest multicentric study in the Brazilian population evaluated the prevalence of
KRAS mutations in 8,234 CRC samples .and reported a prevalence of 31.9%, of which
KRAS G12V was the most frequent and most samples came from the Southeast region [
25]. A weakness of our study is that we had no representation from the North region of Brazil.
Similar to our data, an Argentinian study with 9,150 CRC patients reported a frequency of KRAS and NRAS mutations of 43.4% and 3.6%, respectively. However, their BRAF mutation rate was 12.1%, slightly higher than in our data [
26].
A large American database with 13,336 advanced CRC patients, reported a prevalence of 51.9% (n=6,926) of
KRAS mutation, 4.5% (n=594) of
NRAS mutation, and 3.3% (n=455) of MSI-H [
27]. Our MSI-H frequency was smaller when compared to this American study.
Unfortunately, only scarce data exist on the prevalence of advanced MSI-H/dMMR CRC in Brazil. A small unicentric study from Barretos/Brazil comprising both localized and metastatic CRC with only 95 patients, whose samples were analyzed with PCR techniques, found a prevalence of 13.3% (n=12) of MSI-high patients [
28]. However, these results can be biased since most of the patients had localized disease when the prevalence of MSI-H tumors is higher.
The Keynote-177 trial (n=307) reported an association of MSI-high/dMMR with sidedness (67% right vs 30% left side) [
29]. Our results showed a similar proportion; we found 14 patients with MSI-high status with a significant association (p=0.019) to right-sided tumors.
The prevalence of
BRAF status in our data was 7.3% with a significant association (p=0.001) with right-sided mCRC (11%) in comparison to left-sided tumors (3%). Our finding compares similarly to an international meta-analysis including 15,981 patients with mCRC that reported a significant difference (p=0.0001) in the
BRAF mutant status between right (16.3%) and left-sided (4.3%) tumors [
30].
Finally, we must emphasize the importance of a structured pathology network that provides access to molecular testing to a large population [
31]. The cooperation between various oncology-dedicated centers has a key-role in the development of research, prevention and treatment strategies in CRC [
16]. This is a one of the challenges in a large and underdeveloped country such as Brazil, as most of the medical facilities and technologies are concentrated in the South-East region from where more than three quarters of our samples were derived [
32].
Adequate information about the molecular status of mCRC patients has an impact on the clinical decision-making process for oncologists, as appointed by a nation-wide survey conducted by the Brazilian Group of Gastrointestinal Tumors [
33]. In addition, the knowledge generated by our study could help health-care authorities to develop a more efficient administration of resources in Brazil, as demonstrated in a cost-comparison analysis of sequential therapies in the Brazilian population [
34].
5. Conclusions
In our Brazilian cohort of mCRC patients, frequencies of RAS and BRAF mutations were in line with worldwide data. However, we found lower than expected frequency of MSI-high/dMMR tumors. The KRAS G12C mutation was associated with early-onset mCRC, an emergent population in which KRAS G12C inhibitors might be useful. To the best of our knowledge, this is the largest cohort in the Brazilian population with mCRC reporting RAS, BRAF and MSI-High status. Larger studies are needed to confirm these findings.
Author Contributions
Conceptualization, R.D. and R.P.; methodology, R.D.; software, M.A.; Formal analysis, R.D.; Investigation, M.A.; F.C. and V.L.; resources, R.D.; data curation, R.D.; writing original draft preparation, M.A.; writing—review and editing, A.J.; R.P.; F.C. and V.L.; visualization, R.P.; supervision, R.P.; project administration, R.D.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Hospital Alemão Oswaldo Cruz, the board responsible for research projects proposed by the Centro Paulista de Oncologia. Protocol code 70914323.0.0000.0070, date July 12th, 2023).
Informed Consent Statement
Patient consent was waived due to the retrospective and anonymized nature of the present study, All the patients that realize molecular analysis in the OC Precision Medicine lab sign a consent form in accordance with Brazilian Data Protection Act, which details that the data generated from the results of tests can be anonymized and integrated in a central database for their use in observational studies approved by the Ethics Committee.
Data Availability Statement
Our database is not publicly available due to institutional privacy restrictions.
Acknowledgments
Administrative and technical support from the OC Precision Medicine laboratory staff.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [CrossRef]
- INCA - Instituto Nacional de Câncer [Internet]. 2023 [cited 2023 May 15]. Estimativa 2023: incidência de câncer no Brasil. Available from: https://www.inca.gov.br/publicacoes/livros/estimativa-2023-incidencia-de-cancer-no-brasil.
- Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer — a call to action. Nat Rev Clin Oncol. 2021 Apr;18(4):230–43. [CrossRef]
- Araghi M, Arnold M, Rutherford MJ, Guren MG, Cabasag CJ, Bardot A, et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut. 2021 Jan 1;70(1):114–26. [CrossRef]
- Safiri S, Sepanlou SG, Ikuta KS, Bisignano C, Salimzadeh H, Delavari A, et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019 Dec 1;4(12):913–33. [CrossRef]
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023 Feb 1;72(2):338–44. [CrossRef]
- US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 May 18;325(19):1965–77. [CrossRef]
- Kolb JM, Hu J, DeSanto K, Gao D, Singh S, Imperiale T, et al. Early-Age Onset Colorectal Neoplasia in Average-Risk Individuals Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology. 2021 Oct 1;161(4):1145-1155.e12. [CrossRef]
- Normanno N, Rachiglio AM, Roma C, Fenizia F, Esposito C, Pasquale R, et al. Molecular diagnostics and personalized medicine in oncology: challenges and opportunities. J Cell Biochem. 2013 Mar;114(3):514–24. [CrossRef]
- Armstrong SA, Malley R, Weinberg BA. Molecular Profiling in Metastatic Colorectal Cancer. Oncol Williston Park N. 2020 Sep 15;34(9):352–5. [CrossRef]
- Guerrero RM, Labajos VA, Ballena SL, Macha CA, Lezama MS, Roman CP, et al. Targeting BRAF V600E in metastatic colorectal cancer: where are we today? Ecancermedicalscience. 2022;16:1489. [CrossRef]
- Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer. 2022 Nov 1;175:136–57. [CrossRef]
- Harada S, Morlote D. Molecular Pathology of Colorectal Cancer. Adv Anat Pathol. 2020 Jan 6;27(1):20–6. [CrossRef]
- Zhao MH, Wu AW. Targeting KRAS G12C mutations in colorectal cancer. Gastroenterol Rep. 2023 Jan 1;11:goac083. [CrossRef]
- Barbosa EC, Cookson R. Multiple inequity in health care: An example from Brazil. Soc Sci Med. 2019 May 1;228:1–8. [CrossRef]
- Broeders M, Elfström KM. Importance of International Networking and Comparative Research in Screening to Meet the Global Challenge of Cancer Control. JCO Glob Oncol. 2020 Jan 31;6:JGO.19.00388. [CrossRef]
- RAS Extension Pyro Kit [Internet]. [cited 2023 Nov 23]. Available from: https://www.qiagen.com/us/products/diagnosti2c83s- and-clinical-research/oncology/therascreen-solid-tumor/ras-ext-pyro-kit-row.
- BRAF Pyro Kit [Internet]. [cited 2023 Nov 23]. Available from: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/oncology/therascreen-solid-tumor/braf-pyro-kit.
- Dedeurwaerdere F, Claes KB, Van Dorpe J, Rottiers I, Van der Meulen J, Breyne J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep. 2021 Jun 18;11(1):12880. [CrossRef]
- Kazzi AIM, Passarini TDM, Duarte FA, Paes FR, Ferrari BL, Jacome A a. DA. 509P A different epidemiological trend in Brazilian early-onset colorectal cancer (EOCRC): Real-world data from a reference cancer center. Ann Oncol. 2020 Sep 1;31:S456. [CrossRef]
- Pereira AAL, Fernandes GDS, Braga GTP, Marchetti KR, Mascarenhas CDC, Gumz B, et al. Differences in Pathology and Mutation Status Among Colorectal Cancer Patients Younger Than, Older Than, and of Screening Age. Clin Colorectal Cancer. 2020 Dec;19(4):e264–71. [CrossRef]
- Zalis MG, Vieira FM, Zalcberg-Renault I, Bonamino MH, Ferreira CG, Oliveira S. KRAS mutation profile in colorectal cancer patients in Brazil: A cohort of 989 individuals. J Clin Oncol. 2009 May 20;27(15_suppl):e15017–e15017. [CrossRef]
- Chida K, Kotani D, Masuishi T, Kawakami T, Kawamoto Y, Kato K, et al. The Prognostic Impact of KRAS G12C Mutation in Patients with Metastatic Colorectal Cancer: A Multicenter Retrospective Observational Study. The Oncologist. 2021 Oct;26(10):845–53. [CrossRef]
- Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SHI, et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023 Jan 5;388(1):44–54. [CrossRef]
- Gil Ferreira C, Aran V, Zalcberg-Renault I, Victorino AP, Salem JH, Bonamino MH, et al. KRAS mutations: variable incidences in a Brazilian cohort of 8,234 metastatic colorectal cancer patients. BMC Gastroenterol. 2014 Apr 10;14(1):73. [CrossRef]
- Mendez G, Garino N, Dibarbora D, Salanova R, Bertelli AM, Ruiz A, et al. Frequency of KRAS, NRAS, and BRAF mutations in colorectal cancer in an Argentinian population. J Clin Oncol. 2023. [CrossRef]
- Serebriiskii IG, Connelly C, Frampton G, Newberg J, Cooke M, Miller V, et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun. 2019 Aug 19;10(1):3722. [CrossRef]
- dos Santos W, Sobanski T, de Carvalho AC, Evangelista AF, Matsushita M, Berardinelli GN, et al. Mutation profiling of cancer drivers in Brazilian colorectal cancer. Sci Rep. 2019 Sep 23;9(1):13687. [CrossRef]
- Diaz LA, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022 May 1;23(5):659–70. [CrossRef]
- Bylsma LC, Gillezeau C, Garawin TA, Kelsh MA, Fryzek JP, Sangaré L, et al. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020; 9(3):1044–57. [CrossRef]
- Araujo D, Merchan-Hamann E, Lima F, Laguardia J, Gutierrez MM. Avaliação de desempenho das redes de atenção à saúde: uma proposta de indicadores. Rev Eletronica Comun Inf Em Saude. 2016 Jul 1;10(3):1–16. [CrossRef]
- Gaspar RS, Rossi L, Hone T, Dornelles AZ. Income inequality and non-communicable disease mortality and morbidity in Brazil States: a longitudinal analysis 2002-2017. Lancet Reg Health – Am. 2021 Oct 1;2:1–9. [CrossRef]
- Peixoto RD, Riechelmann RP, Prolla G, Weschenfelder RF, Fernandes G dos S, Pereira GS, et al. Treatment choices in metastatic colorectal cancer according to sidedness and RAS/BRAF status: a national survey by the Brazilian Gastrointestinal Tumors Group (GTG). Braz J Oncol. 2019;15(0):1–6. [CrossRef]
- Weschenfelder RF, Tsuchiya CT, Kim HSJ, Simões JA, La Scala CSK. Sequential biological therapies in metastatic colorectal cancer (mCRC): a cost comparison analysis for wildtype RAS mCRC patients in Brazil. J Bras Econ Saúde. 2016;8(1):24–38. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).