Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Renin-angiotensin system
2.1. Components of renin-angiotensin system
2.2. Cardiac effects of Angiotensin II
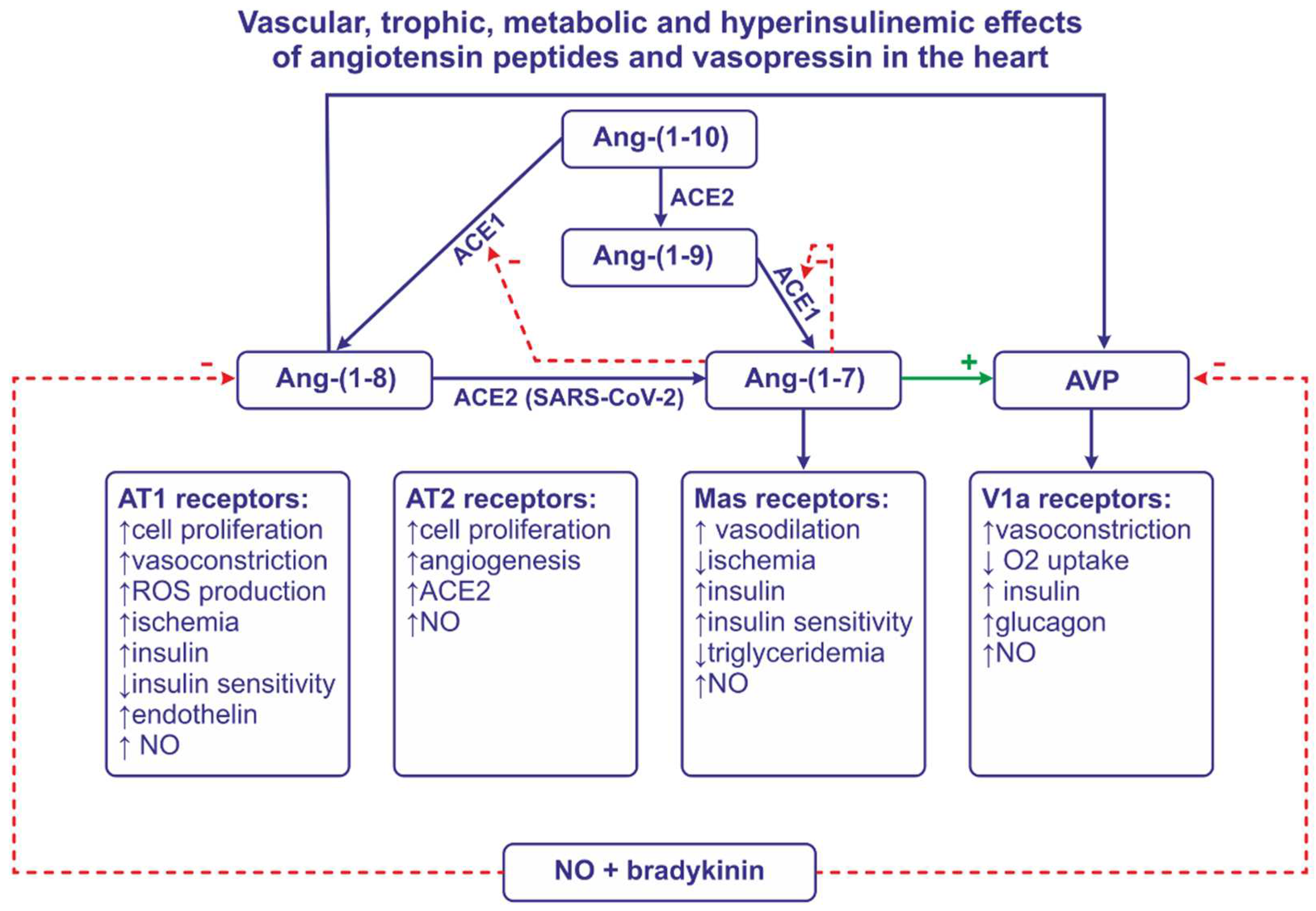
2.3. Cardiac effects of Ang-(1-7)and other angiotensins
2.4. Central effects of angiotensin peptides
2.5. Angiotensin peptides in regulation of the heart in cardiovascular diseases
2.6. Interaction of angiotensin peptides with insulin
2.7. Role of angiotensin peptides in the heart in obesity, diabetes mellitus and hypertension
3. Vasopressin system
3.1. Components of vasopressin system
3.2. Regulation of vasopressin release
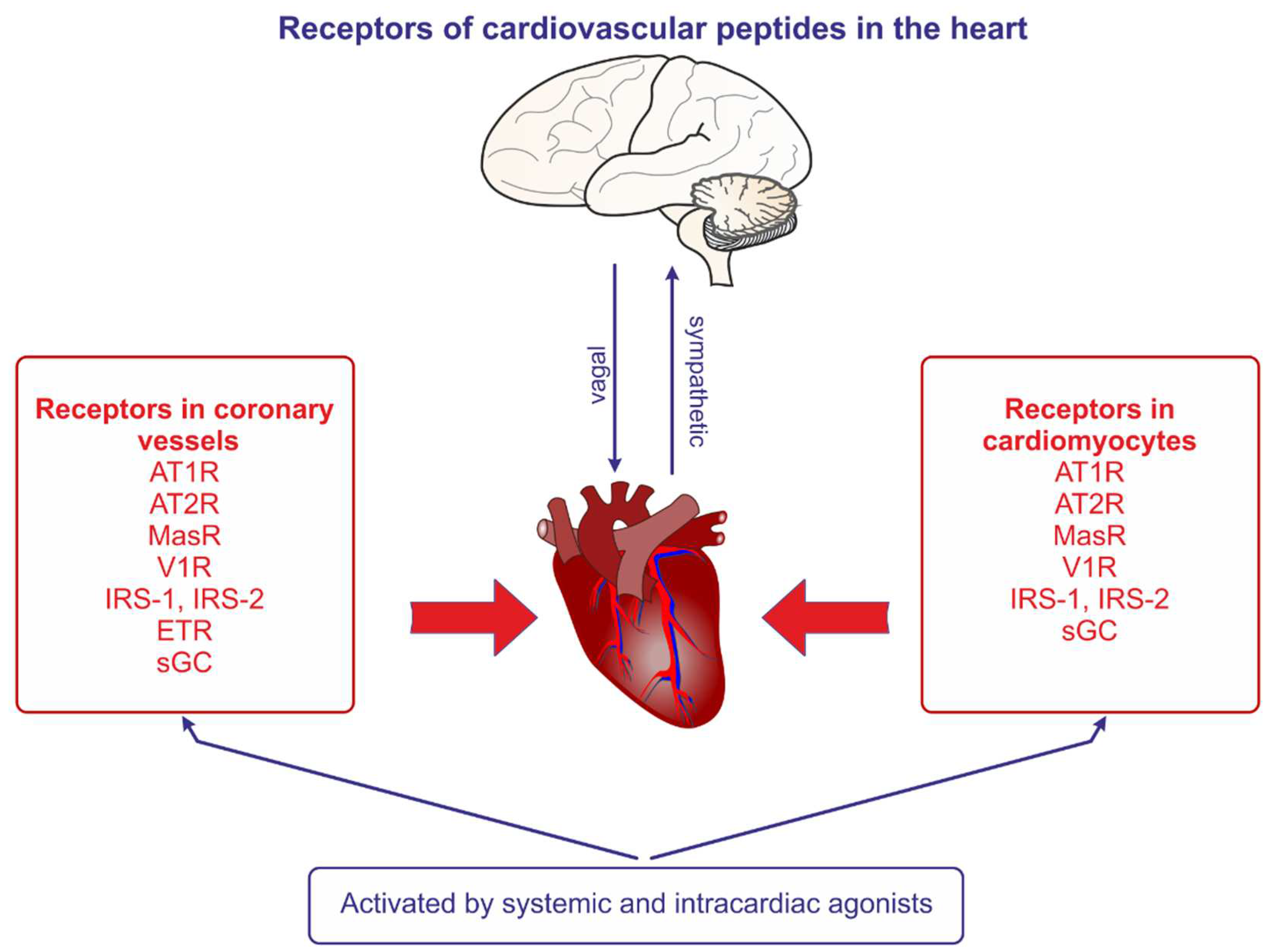
3.3. Cardiac effects of vasopressin
3.4. Vasopressin in the heart in cardiovascular diseases
3.5. Role of vasopressin in obesity and diabetes mellitus
Genotypes of vasopressin receptors as risk factors of coronary diseases
4. Conclusions
Funding
Acknowledgments
Conflict of Interest
Abbreviations
| ACE | Angiotensin converting enzyme 1 |
| ACE2 | Angiotensin converting enzyme 2 |
| Ach- | Acetylcholine |
| ACTH | Adrenocorticotropic hormone |
| AGT | Angiotensinogen |
| Ang | Angiotensin |
| AT1R | Angiotensin receptor of type 1 |
| AT2R | Angiotensin receptor of type 2 |
| AVP | Arginine vasopressin |
| CBF | Coronary blood flow |
| COVID 19 | Coronavirus disease 2019 |
| CRH | Corticotropin-releasing hormone |
| dDAVP | Desmopressin an analog of AVP |
| ERK | Extracellular signal-regulated kinase |
| EAT | Epicardial adipose tissue |
| EDR | Endothelium dependent relaxation |
| ESC | Coronary endothelial cell |
| ET | Endothelin |
| GLUT | Glucose transporter |
| HOMA | Homeostatic model assessment |
| HIF | Hypoxia-inducible factor |
| ICV | Intracerebroventricular |
| IL | Interleukin |
| IRAP | Insulin-regulated aminopeptidase |
| IRS | Insulin receptor substrate |
| ISI | Insulin sensitivity index |
| JNK | Jun N-terminal kinase |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| LV | Left ventricle |
| MAPK | Mitogen-activated protein kinase |
| MasR | MAS receptor for angiotensin-(1-7) |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MPTP | 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine |
| mTOR | Mammalian target of rapamycin |
| NADPH | Dinicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NTS | Nucleus of the solitary tract |
| PLA2 | Phospholipase A2 |
| PLC | Phospholipase C |
| PVN | Paraventricular nucleus |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| RVLM | Rostral ventrolateral medulla |
| SARS-CoV | Severe acute respiratory syndrome associated coronavirus |
| SFO | Subfornical organ |
| SHR | Spontaneously hypertensive rat |
| SON | Supraoptic nucleus |
| SNP | Single nucleotide polymorphism |
| TNFα | Tumor necrosis factor α |
| T1DM1 | Diabetes mellitus of type 1 |
| T1DM2 | Diabetes mellitus of type 2 |
| UCP | Uncoupling protein |
| VSMC | Vascular smooth muscle cell |
| V1aR | Vasopressin receptor of type 1a |
| V1bR | Vasopressin receptor of type 1b |
| V2R | Vasopressin receptor of type 2 |
| VS | Vasopressin system |
| WKY | Wistar Kyoto rat |
| ZDF | Zucker diabetic fatty |
References
- Bakkar, N.Z.; Dwaib, H.S.; Fares, S.; Eid, A.H. Al-Dhaheri, Y.; El-Yazbi, A.F. Cardiac Autonomic Neuropathy: A Progressive Consequence of Chronic Low-Grade Inflammation in Type 2 Diabetes and Related Metabolic Disorders. Int. J. Mol. Sci. 2020, 21, 9005. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Cardiac adiposity and global cardiometabolic risk: New concept and clinical implication. Circ. J. 2009, 73, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Anderson, E.A.; Hoffman, R.P.; Balon, T.W.; Sinkey, C.A. ; Mark, AL Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J. Clin. Invest. 1991, 87, 2246–2252. [Google Scholar] [CrossRef]
- Favre, G.A.; Esnault, V.L.; Van Obberghen, E. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E435–E449. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. Dysregulation of the Renin-Angiotensin System and the Vasopressinergic System Interactions in Cardiovascular Disorders. Curr. Hypertens. Rep. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 14414. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Żera, T., Kowara, M.; Cudnoch-Jedrzejewska, A. The contribution of angiotensin peptides to cardiovascular regulation in health and disease. In: Angiotensin From the Kiney to Coronavirus. In: Molecular Mediators in Health and Disease: How Cells Communicate. Academic Press-Elsevier. 2023; pp 21-76. ISBN 978-0-323-99618-1. [CrossRef]
- Dhalla, N.S.; Shah, A.K.; Tappia, P.S. Role of Oxidative Stress in Metabolic and Subcellular Abnormalities in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, V.; Jafarpour, A.; Pirnia, A.; Pajouhi, N.; Khaksarian, M.; Veiskarami, S.; Nazari, A. The role of vasopressin V1A and oxytocin OTR receptors in protective effects of arginine vasopressin against H2O2-induced oxidative stress in H9C2 cells. Arch. Physiol. Biochem. 2022, 128, 830–835. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Williams, S.M.; Eleftheriadou, A.; Alam, U.; Cuthbertson, D.J.; Wilding, J.P.H. Cardiac Autonomic Neuropathy in Obesity, the Metabolic Syndrome and Prediabetes: A Narrative Review. Diabetes Ther. 2019, 10, 1995–2021. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Lockette,W. ; Sowers, J.R. Mineralocorticoid receptors in the pathogenesis of insulin resistance and related disorders: From basic studies to clinical disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R276–R286. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Blázquez, E.; Hurtado-Carneiro, V.; LeBaut-Ayuso, Y.; Velázquez, E.; García-García, L.; Gómez-Oliver, F.; Ruiz-Albusac, J.M.; Ávila, J.; Pozo, M.Á. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front. Endocrinol. 2022, 13, 873301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Mehta, J.K.; Kaur, G.; Buttar, H.S.; Bagabir, H.A.; Bagabir, R.A.; Bagabir, S.A.; Haque, S.; Tuli, H.S.; Telessy, I.G. Role of the renin-angiotensin system in the pathophysiology of coronary heart disease and heart failure: Diagnostic biomarkers and therapy with drugs and natural products. Front. Physiol. 2023, 14, 1034170. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E; Zera, T. ; Sosnowski, P.; Cudnoch-Jedrzejewska, A.; Puszko A.; Misicka, A. Vasopressin and related peptides; potential value in diagnosis, prognosis and treatment of clinical disorders. Curr. Drug Metab. 2017, 18, 306–345. [Google Scholar] [CrossRef]
- Sadoshima, J.; Xu, Y.; Slayter, H.S.; Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993, 75, 977–984. [Google Scholar] [CrossRef]
- van Esch, J.H.; Gembardt, F.; Sterner-Kock, A.; Heringer-Walther, S.; Le, T.H.; Lassner, D.; Stijnen, T.; Coffman, T.M.; Schultheiss, H.P.; Danser, A.H.; et al. Cardiac phenotype and angiotensin II levels in AT1a, AT1b, and AT2 receptor single, double, and triple knockouts. Cardiovasc. Res. 2010, 86, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Pastukh, V.; Schaffer, S.W. Involvement of the mitochondrial permeability transition pore in angiotensin II-mediated apoptosis. Exp. Clin. Cardiol. 2005, 10, 160–164. [Google Scholar] [PubMed]
- Ide,T. ; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Johnson, S.C.; Villarin, J.J.; Chin, M.T.; Nieves-Cintrón, M.; Chen, T.; Marcinek, D.J.; Dorn, G.W. 2nd; Kang, Y.J.; Prolla, T.A.; et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ. Res. 2011, 108, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.K. , Dhalla, N.S. Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy. Cells. 2022, 11, 3336. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, F.; Anversa, P.; Wiener, J. Effect of angiotensin-induced hypertension on rat coronary arteries and myocardium. Am. J. Pathol. 1976, 84, 111–138. [Google Scholar]
- Bhan, R.D.; Giacomelli, F.; Wiener, J. Adrenoreceptor blockade in angiotensin-induced hypertension: Effect on rat coronary arteries and myocardium. Am. J. Pathol. 1982, 108, 60–71. [Google Scholar]
- Belabbas, H.; Zalvidea, S.; Casellas, D.; Molès, J.P.; Galbes, O.; Mercier, J.; Jover, B. Contrasting effect of exercise and angiotensin II hypertension on in vivo and in vitro cardiac angiogenesis in rats. Am. J. Physiol. Regu. Integr. Comp. Physiol. 2008, 295, R1512–R1518. [Google Scholar] [CrossRef]
- Kohno, M.; Ohmori, K.; Nozaki, S.; Mizushige, K.; Yasunari, K.; Kano, H.; Minam, M.; Yoshikawa, J. Effects of valsartan on angiotensin II-induced migration of human coronary artery smooth muscle cells. Hypertens. Res. 2000, 23, 677–681. [Google Scholar] [CrossRef]
- Hafizi, S.; Wang, X.; Chester, A.H.; Yacoub, M.H.; Proud, C.G. ANG II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1232–H1238. [Google Scholar] [CrossRef]
- Mehta, J.L.; Mercanti, F.; Stone, A.; Wang, X.; Ding, Z.; Romeo, F.; Khaidakov, M. Gene and microRNA transcriptional signatures of angiotensin II in endothelial cells. J. Cardiovasc. Pharmacol. 2015, 65, 123–129. [Google Scholar] [CrossRef]
- Kifor, I.; Dzau. V.J. Endothelial renin-angiotensin pathway: Evidence for intracellular synthesis and secretion of angiotensins. Circ. Res. 1987, 60, 422–428. [Google Scholar] [CrossRef]
- Pinto, J.E.; Viglione, P.; Saavedra, J.M. Autoradiographic localization and quantification of rat heart angiotensin converting enzyme. Am. J. Hypertens. 1991, 4 Pt 1, 321–326. [Google Scholar] [CrossRef]
- Yamada, H.; Fabris, B.; Allen, A.M.; Jackson, B.; Johnston, C.I.; Mendelsohn, A.O. Localization of angiotensin converting enzyme in rat heart. Circ. Res. 1991, 68, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, Y.; Kelly, R.A.; Williams, G.H.; Kifor, I. Coronary microvascular endothelial cells cosecrete angiotensin II and endothelin-1 via a regulated pathway. Am. J. Physiol. Heart Circ, Physiol. 2000, 279, H1087–H1096. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.; Steckelings, U.M.; Paul, M.; Bottari, S.P.; Metzger, R.; Unger, T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J. Clin. Invest. 1995, 95, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.; Saward, L.; Zahradka, P. Both AT₁ and AT₂ receptors mediate proliferation and migration of porcine vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H746–H756. [Google Scholar] [CrossRef] [PubMed]
- Takemoto M, Egashira K, Tomita H, Usui M, Okamoto H, Kitabatake A, Shimokawa H, Sueishi K, Takeshita A. Chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade: Effects on cardiovascular remodeling in rats induced by the long-term blockade of nitric oxide synthesis. Hypertension. 1997, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Egashira, K.; Usui, M.; Numaguchi, K.; Tomita, H.; Tsutsui, H.; Shimokawa, H.; Sueishi, K.; Takeshita, A. Important role of tissue angiotensin-converting enzyme activity in the pathogenesis of coronary vascular and myocardial structural changes induced by long-term blockade of nitric oxide synthesis in rats. J. Clin. Investig. 1997, 99, 278–287. [Google Scholar] [CrossRef]
- Kuga, T.; Mohri, M.; Egashira, K.; Hirakawa, Y.; Tagawa, T.; Shimokawa, H.; Takeshita, A. Bradykinin-induced vasodilation of human coronary arteries in vivo: Role of nitric oxide and angiotensin-converting enzyme. J. Am. Coll. Cardiol. 1997, 30, 108–112. [Google Scholar] [CrossRef]
- Prasad, A.; Husain, S.; Quyyumi, A.A. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: A potential role of bradykinin. J. Am. Coll/ Cardiol. 1999, 33, 796–804. [Google Scholar] [CrossRef]
- Bayraktutan, U.; Ulker, S. Effects of angiotensin II on nitric oxide generation in proliferating and quiescent rat coronary microvascular endothelial cells. Hypertens. Res. 2003, 26, 749–757. [Google Scholar] [CrossRef]
- Munk, V.C.; Sanchez de Miguel, L.; Petrimpol, M.; Butz, N.; Banfi, A.; Eriksson, U.; Hein, L.; Humar, R.; Battegay, E.J. Angiotensin II induces angiogenesis in the hypoxic adult mouse heart in vitro through an AT2-B2 receptor pathway. Hypertension. 2007, 49, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Z.; Wang, B.W.; Yu, Y.J.; Shyu, K.G. Hyperbaric oxygen activates visfatin expression and angiogenesis via angiotensin II and JNK pathway in hypoxic human coronary artery endothelial cells. J. Cell Mol. Med. 2020, 24, 2434–2443. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Polokoff, M.A. Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol. Rev. 1994, 46, 325–415. [Google Scholar]
- Yamaguchi, O.; Kaneshiro, T.; Saitoh, S.; Ishibashi, T.; Maruyama, Y.; Takeishi, Y. Regulation of coronary vascular tone via redox modulation in the alpha1-adrenergic-angiotensin-endothelin axis of the myocardium. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H226–H232. [Google Scholar] [CrossRef]
- de Beer, V.J.; Sorop, O.; Pijnappels, D.A.; Dekkers, D.H.; Boomsma, F.; Lamers, J.M.; Duncker, D.J.; Merkus, D. Integrative control of coronary resistance vessel tone by endothelin and angiotensin II is altered in swine with a recent myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2069–H2077. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A. , Teijeira-Fernández, E.; Fernández, A.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef] [PubMed]
- Roubíček, T.; Dolinková, M.; Bláha, J.; Haluzíková, D.; Bošanská, L.; Mráz, M.; Kremen, J.; Haluzík, M. Increased angiotensinogen production in epicardial adipose tissue during cardiac surgery: Possible role in a postoperative insulin resistance. Physiol Res. 2008, 57, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Couselo-Seijas, M.; Almengló, C.; M Agra-Bermejo, R.; Luis Fernandez, Á. : Alvarez, E.; R González-Juanatey, J.; Eiras, S. Higher ACE2 expression levels in epicardial cells than subcutaneous stromal cells from patients with cardiovascular disease: Diabetes and obesity as possible enhancer. Eur. J. Clin. Invest. 2021, 51, e13463. [Google Scholar] [CrossRef]
- Pörsti, I.; Bara, A.T.; Busse, R.; Hecker, M. Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: Implications for a novel angiotensin receptor. Br. J. Pharmacol. 1994, 111, 652–654. [Google Scholar] [CrossRef]
- Ueda, S.; Masumori-Maemoto, S.; Ashino, K.; Nagahara, T.; Gotoh, E.; Umemura, S.; Ishii, M. Angiotensin-(1-7) attenuates vasoconstriction evoked by angiotensin II but not by noradrenaline in man. Hypertension 2000, 35, 998–1001. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Santos, R.A. Cardiovascular actions of angiotensin-(1-7). Braz. J. Med. Biol. Res. 2005, 38, 499–507. [Google Scholar] [CrossRef]
- Westermeier, F.; Bustamante, M.; Pavez, M.; García, L.; Chiong, M.; Ocaranza, M.P.; Lavandero, S. Novel players in cardioprotection: Insulin like growth factor-1, angiotensin-(1-7) and angiotensin-(1-9). Pharmacol. Res. 2015, 101, 41–55. [Google Scholar] [CrossRef]
- Tom, B.; de Vries, R.; Saxena, P.R.; Danser, A.H. Bradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension 2001, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.; Frábregas, B.C.; Madureira, M.M.; Santos, R.J.; Campagnole-Santos, M.J.; Santos, RA. Angiotensin-(1-7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J. Med. Biol. Res. 2000, 33, 709–713. [Google Scholar] [CrossRef]
- Kozlovski, V.I.; Lomnicka, M.; Fedorowicz, A.; Chlopicki, S. On the mechanism of coronary vasodilation induced by angiotensin-(1-7) in the isolated guinea pig heart. Basic Clin. Pharmacol. Toxicol. 2007, 100, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.L.; Kangussu, L.M.; da Silva, L.G. Jr; Castro, C.H.; Santos, R.A.S.; Ferreira, A.J. Cardiovascular effects of small peptides of the renin angiotensin system. Physiol. Rep. 2017, 5, e13505. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Smith, P.M.; Ferguson, A.V. Subfornical organ neurons projecting to paraventricular nucleus: Whole-cell properties. Brain Res. 2001, 921, 78–85. [Google Scholar] [CrossRef]
- McKinley, M.J.; McAllen, R.M.; Davern, P.; Giles, M.E.; Penschow, J.; Sunn, N.; Uschakov, A.; Oldfield, B.J. The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 2003, 172, 1–122. [Google Scholar] [CrossRef]
- Sunn, N.; McKinley, M.J.; Oldfield, B.J. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J. Neuroendocrinol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Capone, C.; Faraco, G.; Peterson, J.R.; Coleman, C.; Anrather, J.; Milner, T.A.; Pickel, V.M.; Davisson, R.L.; Iadecola, C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J. Neurosci. 2012, 32, 4878–4886. [Google Scholar] [CrossRef]
- Horn, M.; Neubauer, S.; Frantz, S.; Hugel, S.; Hu, K.; Gaudron, P.; Schnackerz, K.; Ertl, G. Preservation of left ventricular mechanical function and energy metabolism in rats after myocardial infarction by the angiotensin-converting enzyme inhibitor quinapril. J. Cardiovasc. Pharmacol. 1996, 27, 201–210. [Google Scholar] [CrossRef]
- Lazar, H.L.; Volpe, C.; Bao, Y.; Rivers, S.; Vita, J.A.; Keaney, J.F. Jr. Beneficial effects of angiotensin-converting enzyme inhibitors during acute revascularization. Ann. Thorac. Surg. 1998, 66, 487–492. [Google Scholar] [CrossRef]
- Danser, A.H.; Tom, B.; de Vries, R.; Saxena, P.R. L-NAME-resistant bradykinin-induced relaxation in porcine coronary arteries is NO-dependent: Effect of ACE inhibition. Br. J. Pharmacol. 2000, 131, 195–202. [Google Scholar] [CrossRef]
- Tom, B.; Dendorfer, A.; de Vries, R.; Saxena, P.R.; Jan Danser, A.H. Bradykinin potentiation by ACE inhibitors: A matter of metabolism. Br. J. Pharmacol. 2002, 137, 276–284. [Google Scholar] [CrossRef]
- Gauthier, K.M.; Cepura, C.J.; Campbell, WB. ACE inhibition enhances bradykinin relaxations through nitric oxide and B1 receptor activation in bovine coronary arteries. Biol. Chem. 2013, 394, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, Y.; Maehara, K.; Saito, T.; Tamagawa, K.; Maruyama, Y. Attenuation of angiotensin II-mediated coronary vasoconstriction and vasodilatory action of angiotensin-converting enzyme inhibitor in pacing-induced heart failure in dogs. J. Am. Coll. Cardiol. 2001, 38, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Asanuma, H.; Funaya, H.; Node, K.; Takashima, S.; Sanada, S.; Asakura, M.; Ogita, H.; Kim, J.; Hori, M. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers synergistically increase coronary blood flow in canine ischemic myocardium: Role of bradykinin. J. Am. Coll. Cardiol. 2002, 40, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Umemoto, S.; Fujii, K.; Itoh, S.; Tanaka, M.; Kawahara, S.; Matsuzaki, M. Effects of angiotensin II type 1 receptor antagonist on smooth muscle cell phenotype in intramyocardial arteries from spontaneously hypertensive rats. Hypertens. Res. 2004, 27, 685–693. [Google Scholar] [CrossRef]
- Babick, A.; Chapman, D.; Zieroth, S.; Elimban, V.; Dhalla, N.S. Reversal of subcellular remodelling by losartan in heart failure due to myocardial infarction. J. Cell Mol. Med. 2012, 16, 2958–2967. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tamura, K.; Koide, Y.; Sakai, M.; Tsurumi, Y.; Noda, Y.; Umemura, M.; Ishigami, T.; Uchino, K.; Kimura, K.; et al. The novel angiotensin II type 1 receptor (AT1R)-associated protein ATRAP downregulates AT1R and ameliorates cardiomyocyte hypertrophy. FEBS Lett. 2005, 579, 1579–1586. [Google Scholar] [CrossRef]
- Wang, J.; He, S.Y. Effect of ischemic postconditioning on cell apoptosis and expression of relevant genes in non-culprit coronary arteries. J. Invest. Med. 2020, 68, 1276–1281. [Google Scholar] [CrossRef]
- Paczwa, P.; Szczepańska-Sadowska, E.; Loń, S.; Ganten, S.L; Ganten, D. Role of central AT1 and V1 receptors in cardiovascular adaptation to hemorrhage in SD and renin TGR rats. Am. J. Physiol. 1999, 276, H1918–H1926. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Gao, L.; Patel, K.P.; Zucker, I.H.; Wang, W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J. Appl. Physiol. (1985). 2004, 97, 1746–1754. [Google Scholar] [CrossRef]
- Hayashi, M.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Tsutsui, T.; Matsui, T.; Fujii, M.; Matsumoto, T.; Yamamoto, T.; et al. Relationship between transcardiac extraction of aldosterone and left ventricular remodeling in patients with first acute myocardial infarction: Extracting aldosterone through the heart promotes ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Halcox, J.P.; Waclawiw, M.A.; Quyyumi, A.A. Angiotensin type 1 receptor antagonism reverses abnormal coronary vasomotion in atherosclerosis. J Am Coll Cardiol. 2001, 38, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Nishikawa, H.; Zhang, B.; Matsuo, Y.; Kawamura, A.; Tsuchiya, Y.; Matsuo, K.; Saku, K. Angiotensin-converting enzyme inhibitor promotes coronary collateral circulation in patients with coronary artery disease. Circ. J. 2003, 67, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Staniloae. C.; Schwab, A.J.; Simard, A.; Gallo, R.; Dyrda, I.; Gosselin, G.; Lesperance, J.; Ryan, J.W.; Dupuis, J. In vivo measurement of coronary circulation angiotensin-converting enzyme activity in humans. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H17–H22. [Google Scholar] [CrossRef] [PubMed]
- Tiefenbacher, C.P.; Friedrich, S.; Bleeke, T.; Vahl, C.; Chen, X.; Niroomand, F. ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1425–H1432. [Google Scholar] [CrossRef]
- Ogino, K.; Kato, M.; Furuse, Y.; Kinugasa, Y.; Kaetsu, Y.; Mizuta, E.; Sugihara, S.; Ishida, K.; Yanagihara, K.; et al. Addition of losartan to angiotensin-converting enzyme inhibitors improves insulin resistance in patients with chronic heart failure treated without β-blockers. Circ. J. 2010, 74, 2346–2352. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Jacoby, B.A.; Araújo, C.A.; Macedo, F.A.; Silva, G.A.; Almeida, A.P.; Caliari, M.V.; Santos, R.A. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1113–H1119. [Google Scholar] [CrossRef]
- Kim, M.A.; Yang, D.; Kida, K.; Molotkova, N.; Yeo, S.J.; Varki, N.; Iwata, M.; Dalton, N.D.; Peterson, K.L.; Siems, W.E. Effects of ACE2 inhibition in the post-myocardial infarction heart. J. Card. Fail. 2010, 16, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Wackenfors, A.; Pantev, E.; Emilson, M.; Edvinsson, L.; Malmsjö, M. Angiotensin II receptor mRNA expression and vasoconstriction in human coronary arteries: Effects of heart failure and age. Basic Clin. Pharmacol. Toxicol. 2004, 95, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Fagerberg, B.; Bergström, G. Angiotensin type 2 receptor is expressed in human atherosclerotic lesions. J. Renin Angiotensin Aldosterone Syst. 2008, 9, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Carretero, O.A.; Xu, J.; Harding, P.; Ramadurai, N.; Gu, X.; Peterson, E.; Yang, X.P. Activation of angiotensin II type 2 receptor suppresses TNF-α-induced ICAM-1 via NF-кB: Possible role of ACE2. Am. J. Physiol. Heart. Circ. Physiol. 2015, 309, H827–H834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, P.; Liang, T.; Chen, Y.; Liu, D.; Yu, H. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS axis and coronary heart disease. Heart Vessels. 2020, 35, 153–161. [Google Scholar] [CrossRef]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, N.; Noval, M.G.; Kaur, R., Sajja, S.; Amadori, L.; Das, D.; Cilhoroz, B.; Stewart, O.; Fernandez, D.M.; Shamailova R et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. bioRxiv. 2023:2023.08.14.553245. Preprint. [CrossRef]
- Xie, L.;Zhang, Z.; Wang, Q.; Chen, Y.; Lu, D.; Wu, W. COVID-19 and Diabetes: A Comprehensive Review of Angiotensin Converting Enzyme 2, Mutual Effects and Pharmacotherapy. Front Endocrinol (Lausanne). 2021;12:772865. [CrossRef]
- Park, H.Y.; Kwon, H.M.; Kim, D.; Jang, Y. : Shim, W.H.; Cho, S.Y.; Kim, H.S. The angiotensin converting enzyme genetic polymorphism in acute coronary syndrome--ACE polymorphism as a risk factor of acute coronary syndrome. J. Korean Med. Sci. 1997, 12, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Narayanan, S.; Waclawiw, M.A.; Epstein, N. : Quyyumi, A. A. The insertion/deletion polymorphism of the angiotensin-converting enzyme gene determines coronary vascular tone and nitric oxide activity. J. Am. Coll. Cardiol. 2000, 36, 1579–1586. [Google Scholar] [CrossRef]
- Guney, A.I.; Ergec, D.; Kirac, D.; Ozturhan, H.; Caner, M.; Koc, G.; Kaspar, C.; Ulucan, K.; Agirbasli, M. Effects of ACE polymorphisms and other risk factors on the severity of coronary artery disease. Genet. Mol. Res. 2013, 12, 6895–6906. [Google Scholar] [CrossRef]
- Ruiz, J.; Blanché, H.; Cohen, N.; Velho, G.; Cambien, F.; Cohen, D.; Passa, P.; Froguel, P. Insertion/deletion polymorphism of the angiotensin-converting enzyme gene is strongly associated with coronary heart disease in non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. U S A. 1994, 91, 3662–3665. [Google Scholar] [CrossRef]
- Mehri, S.; Mahjoub, S.; Farhati, A.; Bousaada, R.; Ben Arab, S.; Baudin, B.; Hammami, M. Angiotensinogen gene polymorphism in acute myocardial infarction patients. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 42–47. [Google Scholar] [CrossRef]
- Amant, C.; Hamon, M.; Bauters, C.; Richard, F.; Helbecque, N.; McFadden, E.P.; Escudero, X.; Lablanche, J.M.; Amouyel, P.; Bertrand, M.E. The angiotensin II type 1 receptor gene polymorphism is associated with coronary artery vasoconstriction. J. Am. Coll. Cardiol. 1997, 29, 486–490. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Bollano, E.; Mobini, R.; Larsson, B.M.; Omerovic, E.; Fu, M.; Waagstein, F.; Holmäng, A. Hyperinsulinemia: Effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H787–H796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. The renin-angiotensin system and insulin resistance. Curr. Diab. Rep. 2007, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Ura, N.; Takizawa, H.; Yoshida, D.; Moniwa, N.; Murakami, H.; Higashiura, K.; Shimamoto, K. Blockade of the renin-angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J. Hypertens. 2004, 22, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Andersson, I.J.; Alexanderson, C.; Skøtt, O.; Holmäng, A.; Bergström, G. Hyperinsulinemic rats are normotensive but sensitized to angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1240–R1247. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Shefer, G.; Sasson, K.; Kohen, F.; Limor, R.; Pappo, O.; Nevo, N.; Biton, I.; Bach, M.; Berkutzki, T. Angiotensin 1-7 as means to prevent the metabolic syndrome: Lessons from the fructose-fed rat model. Diabetes. 2013, 62, 1121–1130. [Google Scholar] [CrossRef]
- Williams, I.M.; Otero, Y.F.; Bracy, D.P.; Wasserman, D.H.; Biaggioni, I.; Arnold, A.C. Chronic Angiotensin-(1-7) Improves Insulin Sensitivity in High-Fat Fed Mice Independent of Blood Pressure. Hypertension. 2016, 67, 983–991. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, W.; Jiang, P. Role of the ACE2/Ang-(1-7)/Mas axis in glucose metabolism. Rev. Cardiovasc. Med. 2021, 22, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, M.E.; McCarthy, J.J.; Stocker, S.D. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010, 55, 284–290. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Lee, C.C.; Francis, J. Age-dependent alterations to paraventricular nucleus insulin-like growth factor 1 receptor as a possible link between sympathoexcitation and inflammation. J.Neurochem. 2016, 139, 706–721. [Google Scholar] [CrossRef]
- Feher, A.; Cassuto, J.; Szabo, A.; Patel, V.; Vinayak Kamath, M.; Bagi. Z. Increased tissue angiotensin-converting enzyme activity impairs bradykinin-induced dilation of coronary arterioles in obesity. Circ. J. 2013, 77, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Shiuchi, T.; Iwai, M.; Li, H.S.; Wu, L.; Min, L.J.; Li, J.M.; Okumura, M.; Cui, T.X.; Horiuchi, M. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension. 2004, 43, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Higaki, J.; Horiuchi, M. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension. 2012, 59, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.S.; Trask, A.J. : Souza-Smith, F.M.; Hutchinson, K.R.; Galantowicz, M.L.; Lord, K.C.; Stewart, J.A. Jr; Cismowski, M.J.; Varner. K.J.; Lucchesi, P.A. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res. Cardiol. 2011, 106, 1123–1134. [Google Scholar] [CrossRef]
- Husarek, K.E.; Katz, P.S.; Trask, A.J.; Galantowicz, M.L.; Cismowski, M.J.; Lucchesi, P.A. The angiotensin receptor blocker losartan reduces coronary arteriole remodeling in type 2 diabetic mice. Vascul. Pharmacol. 2016;76:28-36. Vascul. Pharmacol. [CrossRef]
- Zhou, M.S.; Schulman, I.H.; Raij, L. Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H833–H839. [Google Scholar] [CrossRef]
- Estato, V.; Obadia, N.; Carvalho-Tavares, J.; Freitas, F.S.; Reis, P.; Castro-Faria Neto, H.; Lessa, M.A.; Tibiriçá, E. Blockade of the renin-angiotensin system improves cerebral microcirculatory perfusion in diabetic hypertensive rats. Microvasc. Res. 2013;87:41-49. [CrossRef]
- Oliveira-Junior, S.A.; Martinez, P.F.; Guizoni, D.M.; Campos, D.H.; Fernandes, T.; Oliveira, E.M.; Okoshi, M.P. : Okoshi, K.; Padovani, C.R.; Cicogna, A.C. AT1 receptor blockade attenuates insulin resistance and myocardial remodeling in rats with diet-induced obesity. PLoS ONE. 2014, 9, e86447. [Google Scholar] [CrossRef]
- Luther, J.M. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54-60. [CrossRef]
- Ren, J.; Sowers, J.R.; Zhang, Y. Metabolic Stress, Autophagy, and Cardiovascular Aging: From Pathophysiology to Therapeutics. Trends Endocrinol. Metab. 2018, 29, 699–711. [Google Scholar] [CrossRef]
- Igbekele, A.E.; Jia, G.; Hill, M.A.; Sowers, J.R.; Jia, G. Mineralocorticoid Receptor Activation in Vascular Insulin Resistance and Dysfunction. Int. J. Mol. Sci. 2022, 23, 8954. [Google Scholar] [CrossRef]
- Patel, V.B.; Basu, R.; Oudit, G.Y. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016, 5, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, H.; Mori, J.; Nakajima, H.; Kawabe, Y.; Tsuma, Y.; Fukuhara, S.; Kodo, K.; Ikoma, K.; Matoba, S.; Oudit, G.Y.; et al. Angiotensin 1-7 stimulates brown adipose tissue and reduces diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E131–E138. [Google Scholar] [CrossRef] [PubMed]
- Pscherer, S.; Heemann, U.; Frank, H. Effect of Renin-Angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care. 2010, 33, 914–919. [Google Scholar] [CrossRef]
- Yang, F.S.; Yun, C.H.; Wu, T.H. Hsieh, Y.C. Bezerra, H.G.; Liu, C.C.; Wu, Y.J.; Kuo, J.Y.; Hung, C.L.; Hou, C.J. et al. High pericardial and peri-aortic adipose tissue burden in pre-diabetic and diabetic subjects. BMC Cardiovasc. Disord. 2013; 11;13:98. [CrossRef]
- Nelson, K.K.; Schlöndorff, J.; Blobel, C.P. Evidence for an interaction of the metalloprotease-disintegrin tumour necrosis factor alpha convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease-disintegrin MDC9 with a novel MAD2-related protein, MAD2beta. Biochemical J, 1999; 343: 673–680. [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Buijs, R.M.; De Vries, G.J.; Van Leeuwen, F.W.; Swaab, D.F. Vasopressin and oxytocin: Distribution and putative functions in the brain. Prog. Brain Res. 1983;60:115-122. [CrossRef]
- Hupf, H.; Grimm, D.; Riegger, G.A.; Schunkert, H. Evidence for a vasopressin system in the rat heart. Circ. Res. 1999, 84, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, G.; Tang, K.; Li, Q.; Gao, X. The secretion patterns and roles of cardiac and circulating arginine vasopressin during the development of heart failure. Neuropeptides 2015, 51, 63–73. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C. CardioPulse: ’Ten Commandments’ of 2015 European Society of Cardiology Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation (NSTE-ACS). Eur. Heart J. 2016, 37, 208. [Google Scholar] [CrossRef]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, N.L; Lolait, S.J.; Bradley, D.J.; O’Carroll, A.M.; Brownstein, M.J. , Young, W.S. 3rd. Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992, 131, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Folny, V.; Raufaste, D.; Lukovic, L.; Pouzet, B.; Rochard, P.; Pascal, M.; Serradeil-Le Gal, C. Pancreatic vasopressin V1b receptors: Characterization in In-R1-G9 cells and localization in human pancreas. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E566–E576. [Google Scholar] [CrossRef] [PubMed]
- Monstein, H.J.; Truedsson, M.; Ryberg, A.; Ohlsson, B. Vasopressin receptor mRNA expression in the human gastrointestinal tract. Eur. Surg. Res. 2008, 40, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Corbani, M.; Marir, R.; Trueba, M.; Chafai, M.; Vincent, A.; Borie, A.M. Desarménien, M.G.; Ueta, Y.; Tomboly, C.; Olma, A.; et al. Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. Gen. Comp. Endocrinol. 2018, 258, 15–32. [Google Scholar] [CrossRef]
- Góźdź, A.; Szczepańska-Sadowska, E.; Szczepańska, K.; Maśliński, W.; Luszczyk, B. Vasopressin V1a, V1b and V2 receptors mRNA in the kidney and heart of the renin transgenic TGR(mRen2)27 and Sprague Dawley rats. J. Physiol. Pharmacol. 2002, 53, 349–357. [Google Scholar]
- Morel, A.; Lolait, S.J.; Brownstein, M.J. Molecular cloning and expression of rat V1a and V2 arginine vasopressin receptors. Regul. Pept. 1993, 45, 53–59. [Google Scholar] [CrossRef]
- Gutkowska, J.; Miszkurka, M.; Danalache, B.; Gassanov, N.; Wang, D.; Jankowski, M. Functional arginine vasopressin system in early heart maturation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2262–H2270. [Google Scholar] [CrossRef]
- Nishimura, M.; Takahashi, H.; Matsusawa, M.; Ikegaki, I.; Nakanishi, T.; Hirabayashi, M.; Yoshimura, M. Intracerebroventricular infusions of insulin increase vasopressin release in rats. Clin. Exp. Pharmacol. Physiol. 1990, 17, 763–768. [Google Scholar] [CrossRef]
- Yarkov, A.; Montero, S.; Lemus, M.; Roces de Alvarez-Buylla. E.; Alvarez-Buylla, R. Arginine-vasopressin in nucleus of the tractus solitarius induces hyperglycemia and brain glucose retention. Brain Res. 2001, 902, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Montero, S.A.; Yarkov, A.; Lemus, M.; Mendoza, H.; Valles, V.; de Alvarez-Buylla, E.R.; Alvarez-Buylla, R. Enhancing effect of vasopressin on the hyperglycemic response to carotid body chemoreceptor stimulation. Role of metabolic variables. Adv. Exp. Med. Biol. 2003, 536, 95–107. [Google Scholar] [CrossRef]
- Montero, S.; Mendoza, H.; Valles, V.; Lemus, M.; Alvarez-Buylla, R.; de Alvarez-Buylla, E.R. Arginine-vasopressin mediates central and peripheral glucose regulation in response to carotid body receptor stimulation with Na-cyanide. J. Appl. Physiol. (1985). 2006, 100, 1902–1909. [Google Scholar] [CrossRef]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997, 138, 4445–4455. [CrossRef]
- Zueco, J.A.; Esquifino, A.I.; Chowen, J.A.; Alvarez, E.; Castrillón, P.O.; Blázquez, E. Coexpression of glucagon-like peptide-1 (GLP-1) receptor, vasopressin, and oxytocin mRNAs in neurons of the rat hypothalamic supraoptic and paraventricular nuclei: Effect of GLP-1(7-36)amide on vasopressin and oxytocin release. J. Neurochem. 1999, 72, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Scharrer, E. Vasopressin increases blood glucose in pygmy goats (Capra hircus) by an alpha-adrenergic mechanism. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 100, 863–866. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, M.; Xiao, M.; Li, L.; Ding, J. Nitric oxide mediates feedback inhibition in angiotensin II-induced upregulation of vasopressin mRNA. Peptides. 2009, 30, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.A.; Fernández, N.; García-Villalón, A.L.; Monge, L.; Diéguez, G. Comparison of the in vivo coronary action of endothelin-1 and vasopressin role of nitric oxide and prostanoids. Vascul. Pharmacol. 2003, 40, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Giusti-Paiva, A.; Elias, L.L.; Antunes-Rodrigues, J. Inhibitory effect of gaseous neuromodulators in vasopressin and oxytocin release induced by endotoxin in rats. Neurosci. Lett. 2005, 381, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, P.B.; Pancoto, J.A.; de Oliveira-Pelegrin, G.R.; Cárnio, E.C.; Rocha, M.J. Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J. Neuroimmunol. 2007, 183, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Coletti, R.; de Lima, J.B.M.; Vechiato, F.M.V.; de Oliveira, F.L.; Debarba, L.K.; Almeida-Pereira, G.; Elias, L.L.K.; Antunes-Rodrigues, J. Nitric oxide acutely modulates hypothalamic and neurohypophyseal carbon monoxide and hydrogen sulphide production to control vasopressin, oxytocin and atrial natriuretic peptide release in rats. J. Neuroendocrinol. 2019, 31, e12686. [Google Scholar] [CrossRef]
- Peters, K.G.; Marcus, M.L.; Harrison, D.G. Vasopressin and the mature coronary collateral circulation. Circulation. 1989, 79, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.L.; Chilian, W.M.; Kanatsuka, H.; Dellsperger, K.C.; Eastham, C.L.; Lamping, K.G. Understanding the coronary circulation through studies at the microvascular level. Circulation. 1990, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.F.; Martin, S.E.; Markle, D.; Maxwell, M.; Burruss, C.R.; Speir, E.; Greene, R. , Ro, Y.M.; Vitale, D.; Green, M.V. et al. Coronary vasoconstriction induced by vasopressin. Production of myocardial ischemia in dogs by constriction of nondiseased small vessels. Circulation. 1991, 83, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.A 3rd; Segel, L.D. Attenuation of vasopressin-mediated coronary constriction and myocardial depression in the hypoxic heart. Circ. Res. 1990, 66, 710–721. [Google Scholar] [CrossRef]
- Kam, P.C.; Williams, S.; Yoong, F.F. Vasopressin and terlipressin: Pharmacology and its clinical relevance. Anaesthesia. 2004, 59, 993–1001. [Google Scholar] [CrossRef]
- Adachi, Y.; Sakakura, K.; Akashi, N.; Wada, H.; Momomura, S.I.; Fujita, H. Coronary Spastic Angina Induced after Oral Desmopressin (DDAVP) Administration. Intern. Med. 2016, 55, 3603–3606. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ikeda, U.; Okada, K.; Saito, T.; Kawahara, Y.; Okuda, M.; Yokoyama, M.; Shimada, K. Arginine vasopressin increases nitric oxide synthesis in cytokine-stimulated rat cardiac myocytes. Hypertension. 1997, 30, 1112–1120. [Google Scholar] [CrossRef]
- Okamura, T.; Ayajiki, K.; Fujioka, H.; Toda, N. Mechanisms underlying arginine vasopressin-induced relaxation in monkey isolated coronary arteries. J. Hypertens. 1999, 17, 673–678. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhao, L.Y.; Zheng, Q.S.; Xue, Y.S.; Yang, X.D.; Tian, J.W.; Xu, L. Arginine vasopressin-induced nitric oxide content changes in cultured cardiac fibroblasts and its relation to nuclear factor-kappaB. Sheng Li Xue Bao. 2003, 55, 417–421. [Google Scholar]
- Li, X.; Chan, T.O.; Myers, V.; Chowdhury, I.; Zhang, X.Q.; Song, J.; Zhang, J.; Andrel, J.; Funakoshi, H.; Robbins, J.; et al. Controlled and cardiac-restricted overexpression of the arginine vasopressin V1A receptor causes reversible left ventricular dysfunction through Gαq-mediated cell signaling. Circulation. 2011, 124, 572–581. [Google Scholar] [CrossRef]
- Wagner,J. A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef]
- Foreman, B.W.; Dai, X.Z.; Bache, R.J. Vasoconstriction of canine coronary collateral vessels with vasopressin limits blood flow to collateral-dependent myocardium during exercise. Circ. Res. 1991, 69, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Sadr, S.S.; Faghihi, M.; Azizi, Y. ; Hosseini. M.J.; Mobarra,N.; Tavakoli, A.; Imani, A. Vasopressin attenuates ischemia-reperfusion injury via reduction of oxidative stress and inhibition of mitochondrial permeability transition pore opening in rat hearts. Eur. J. Pharmacol. 2015, 760, 96–102. [Google Scholar] [CrossRef]
- Patel, B.M.; Chittock, D.R.; Russell, J.A.; Walley, K.R. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002, 96, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Dünser, M.W.; Mayr, A.J.; Ulmer, H.; Knotzer, H.; Sumann, G.; Pajk, W.; Friesenecker, B.; Hasibeder, W.R. Arginine vasopressin in advanced vasodilatory shock: A prospective, randomized, controlled study. Circulation. 2003, 107, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Milles, J.J.; Baylis, P.H.; Wright, A.D. Plasma vasopressin during insulin withdrawal in insulin-dependent diabetes. Diabetologia. 1981, 20, 607–611. [Google Scholar] [CrossRef]
- Baylis, P.H.; Zerbe, R.L.; Robertson, G.L. Arginine vasopressin response to insulin-induced hypoglycemia in man. J. Clin. Endocrinol. Metab. 1981, 53, 935–940. [Google Scholar] [CrossRef]
- Chiodera, P.; Volpi, R.; Capretti, L.; Speroni, G.; Marcato, A.; Rossi, G.; Coiro, V. . Hypoglycemia-induced arginine vasopressin and oxytocin release is mediated by glucoreceptors located inside the blood-brain barrier. Neuroendocrinology. 1992, 55, 655–659. [Google Scholar] [CrossRef]
- Nagai, N.; Nagai, K.; Takezawa, K.; Chun, S.J.; Nakagawa, H. Suppressive effect of vasopressin on the hyperglycemic response to intracranial injection of 2-deoxy-D-glucose. Neurosci. Lett. 1995, 190, 187–190. [Google Scholar] [CrossRef]
- Amico, J.A.; Finn, F.M.; Haldar. J. Oxytocin and vasopressin are present in human and rat pancreas. Am. J. Med. Sci. 1988, 296, 303–307. [Google Scholar] [CrossRef]
- Mineo, H.; Ito, M.; Muto, H.; Kamita, H.; Hyun, H.S.; Onaga, T.; Yanaihara, N. Effects of oxytocin, arginine-vasopressin and lysine-vasopressin on insulin and glucagon secretion in sheep. Res. Vet. Sci. 1997, 62, 105–110. [Google Scholar] [CrossRef]
- Mohan, S.; Moffett, R.C.; Thomas, K.G.; Irwin, N.; Flatt, P.R. Vasopressin receptors in islets enhance glucose tolerance, pancreatic beta-cell secretory function, proliferation and survival. Biochimie 2019, 158, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Abu-Basha, E.A.; Yibchok-Anun, S.; Hsu, W.H. Glucose dependency of arginine vasopressin-induced insulin and glucagon release from the perfused rat pancreas. Metabolism. 2002, 51, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Knudsen, J.G.; Madara, J.C.; Benrick, A.; Hill, T.G.; Abdul Kadir, L.; Kellard, J. A.; Mellander, L.; Miranda, C.; Lin, H.; et al. Arginine-vasopressin mediates counter-regulatory glucagon release and is diminished in type 1 diabetes. Elife 2021, 10, e72919. [Google Scholar] [CrossRef]
- Engler, D.; Pham, T.; Fullerton, M.J.; Ooi, G.; Funder, J.W.; Clarke, I.J. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989, 49, 367–381. [Google Scholar] [CrossRef]
- Ellis, M.J.; Schmidli, R.S.; Donald, R.A.; Livesey, J.H.; Espiner, EA. Plasma corticotrophin-releasing factor and vasopressin responses to hypoglycaemia in normal man. Clin. Endocrinol (Oxf). 1990, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tallroth, G.; Ryding, E.; Ekman, R.; Agardh, C.D. The response of regulatory peptides to moderate hypoglycaemia of short duration in type 1 (insulin-dependent) diabetes mellitus and in normal man. Diabetes Res. 1992, 20, 73–85. [Google Scholar] [PubMed]
- Watabe, T.; Tanaka, K.; Kumagae, M.; Itoh, S.; Takeda, F.; Morio, K.; Hasegawa, M.; Horiuchi, T.; Miyabe, S.; Shimizu, N. Hormonal responses to insulin-induced hypoglycemia in man. J. Clin. Endocrinol. Metab. 1987, 65, 1187–1191. [Google Scholar] [CrossRef]
- Muret, L.; Priou, A.; Oliver, C.; Grino, M. Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992, 130, 2725–2732. [Google Scholar] [CrossRef]
- Raff, H.; Papanek, P.E.; Liard, J.F.; Cowley, A.W. Jr. The effect of intracarotid vasopressin infusion on ACTH release in neurohypophysectomized, conscious dogs. Am. J. Physiol. 1994, 267(3 Pt 2), R653–R658. [Google Scholar] [CrossRef]
- Zelena, D.; Domokos, A.; Jain, S.K.; Jankord, R.; Filaretova, L. The stimuli-specific role of vasopressin in the hypothalamus-pituitary-adrenal axis response to stress. J. Endocrinol. 2009, 202, 263–278. [Google Scholar] [CrossRef]
- Fisher, B.M.; Baylis, P.H.; Thornton, S.; Frier, B.M. Arginine vasopressin and oxytocin responses to insulin-induced hypoglycemia in type 1 (insulin-dependent) diabetes. J. Clin. Endocrinol. Metab. 1989, 68, 688–692. [Google Scholar] [CrossRef]
- Saravia, F.E.; Gonzalez, S.L.; Roig, P.; Alves, V.; Homo-Delarche, F.; De Nicola, A.F. Diabetes increases the expression of hypothalamic neuropeptides in a spontaneous model of type I diabetes, the nonobese diabetic (NOD) mouse. Cell Mol. Neurobiol. 2001, 21, 15–27. [Google Scholar] [CrossRef]
- García-Villalón, A.L.; Sanz, E.; Monge, L.; Fernández, N.; Martínez, M.A.; Climent, B.; Diéguez, G. Vascular reactivity to vasopressin during diabetes: Gender and regional differences. Eur. J. Pharmacol. 2003, 459, 247–254. [Google Scholar] [CrossRef]
- Aoyagi, T.; Birumachi, J.; Hiroyama, M.; Fujiwara, Y.; Sanbe, A.; Yamauchi, J. : Tanoue, A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology. 2007, 148, 2075–2084. [Google Scholar] [CrossRef]
- Nakamura, K.; Aoyagi, T.; Hiroyama, M.; Kusakawa, S.; Mizutani, R.; Sanbe, A.; Yamauchi, J.; Kamohara, M.; Momose, K.; Tanoue, A. Both V(1A) and V(1B) vasopressin receptors deficiency result in impaired glucose tolerance. Eur. J. Pharmacol. 2009, 613, 182–188. [Google Scholar] [CrossRef]
- Nakamura, K.; Velho, G.; Bouby, N. Vasopressin and metabolic disorders: Translation from experimental models to clinical use. J. Intern. Med. 2017, 282, 298–309. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Kim, Y.N.; Kim, I.Y.; Pak, S.I.; Lee, I.S.; Seong, J.K.; Yoon, Y.S. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem. Res. 2008, 33, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Struck, J.; Wirfält, E.; Hedblad, B.; Morgenthaler, N.G.; Melander, O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1065–E1072. [Google Scholar] [CrossRef] [PubMed]
- Asferg, C.L.; Andersen, U.B.; Linneberg, A.; Goetze, J.P.; Jeppesen, J.L. Copeptin, a surrogate marker for arginine vasopressin secretion, is associated with higher glucose and insulin concentrations but not higher blood pressure in obese men. Diabet. Med. 2014, 31, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Magkos, F. Oxytocin and Vasopressin Systems in Obesity and Metabolic Health: Mechanisms and Perspectives. Curr. Obes. Rep. 2019, 8, 301–316. [Google Scholar] [CrossRef]
- Wolf, J.P.; Massol, J.; Nguyen, N.U.; Berthelay, S. Arginine vasopressin response to insulin-induced hypoglycemia in insulin-dependent diabetics with asymptomatic hypoglycemia. Horm. Metab. Res. 1990, 22, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.A.; Risvanis, J.; Hutchins, A.M.; Burrell, L.M.; MacGregor, D.; Gundlach, A.L.; Johnston, C.I. Down-regulation of vasopressin V1a receptor mRNA in diabetes mellitus in the rat. Clin. Sci (Lond). 1995, 88, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Leosdottir, M.; Wallström, P.; Gullberg, B.; Berglund, G.; Wirfält, E.; Melander, O. Relation between human vasopressin 1a gene variance, fat intake, and diabetes. Am. J. Clin. Nutr. 2009, 89, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Sjögren, M.; Hedblad, B.; Nilsson, P.M.; Struck, J.; Melander, O. Genetic vasopressin 1b receptor variance in overweight and diabetes mellitus. Eur J Endocrinol. 2016, 174, 69–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




