Submitted:
24 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
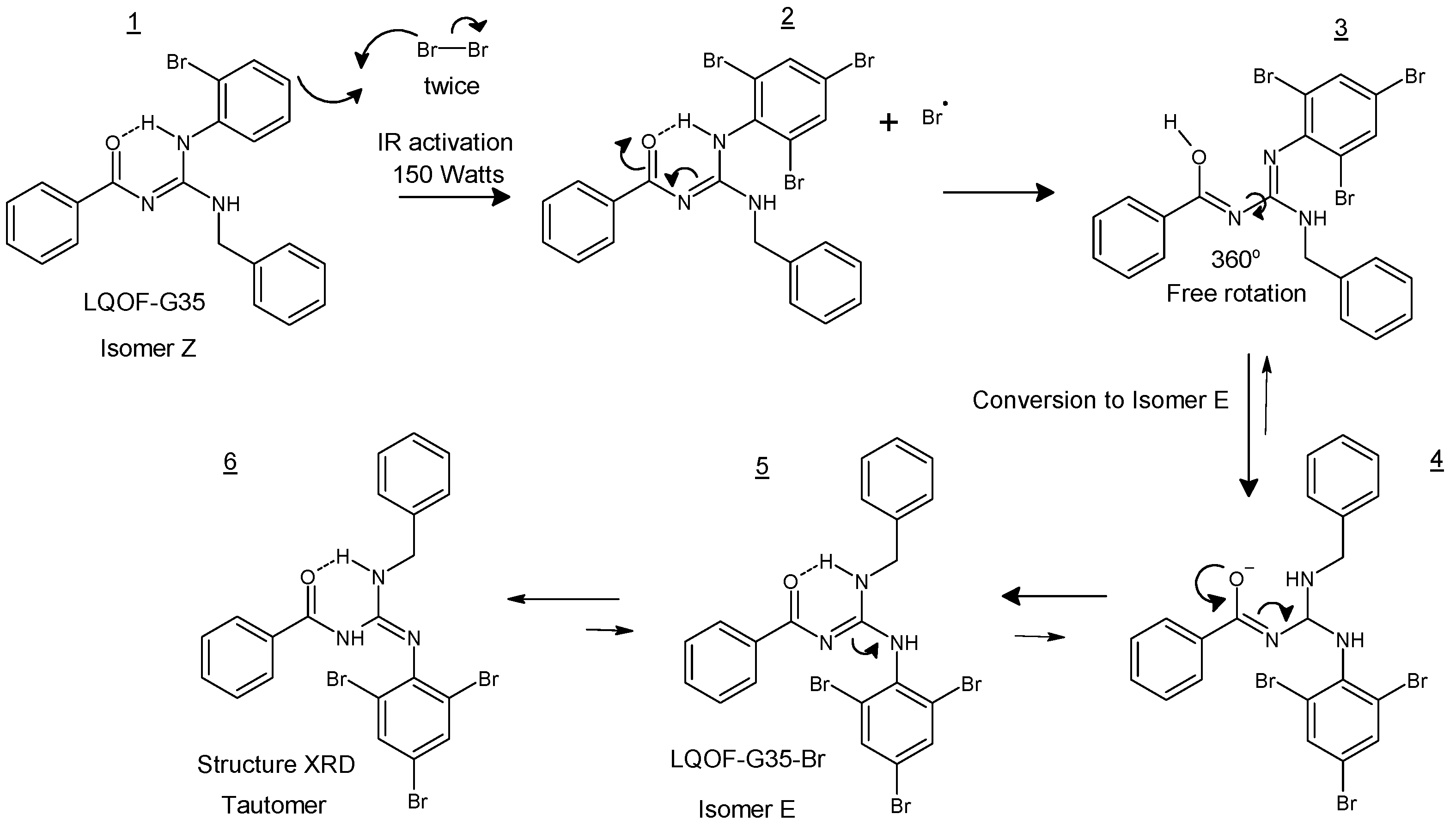
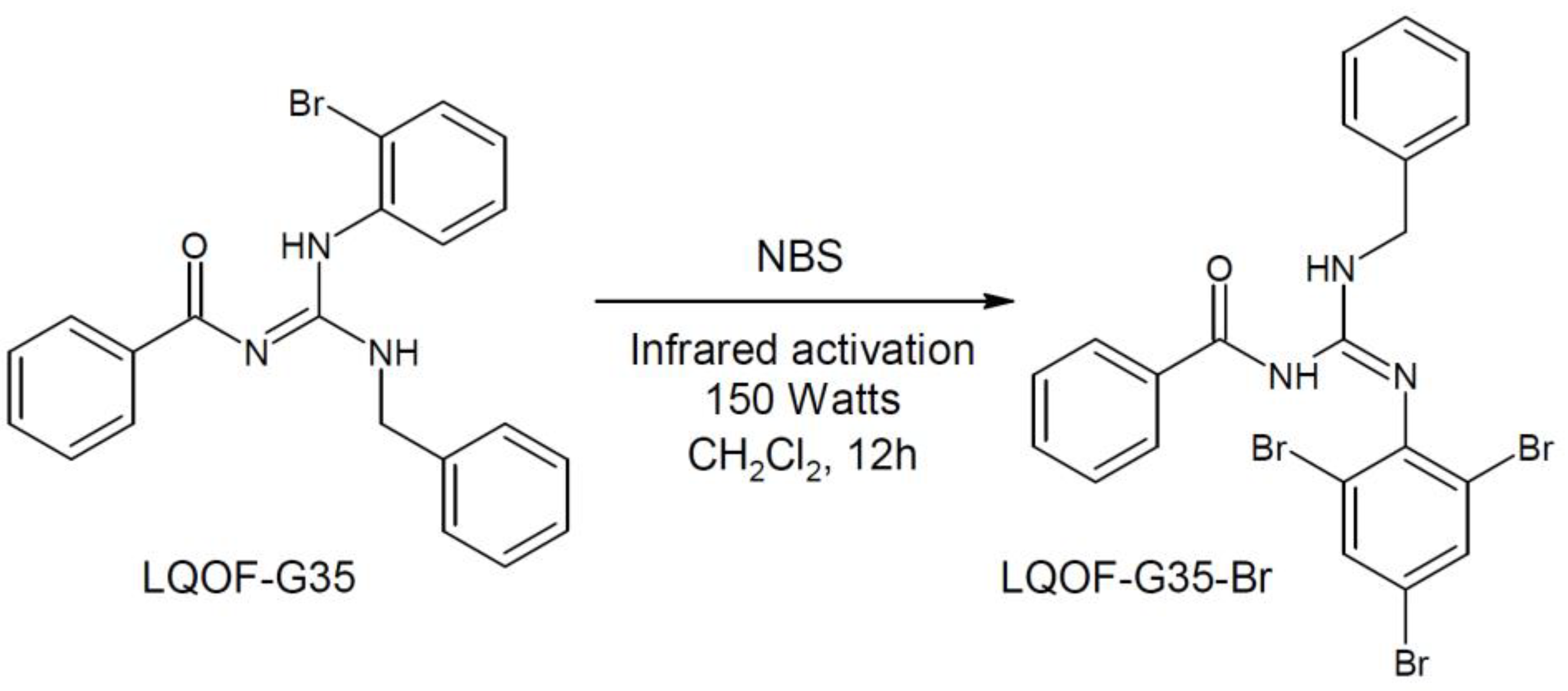
2.1. Guanidine bromination
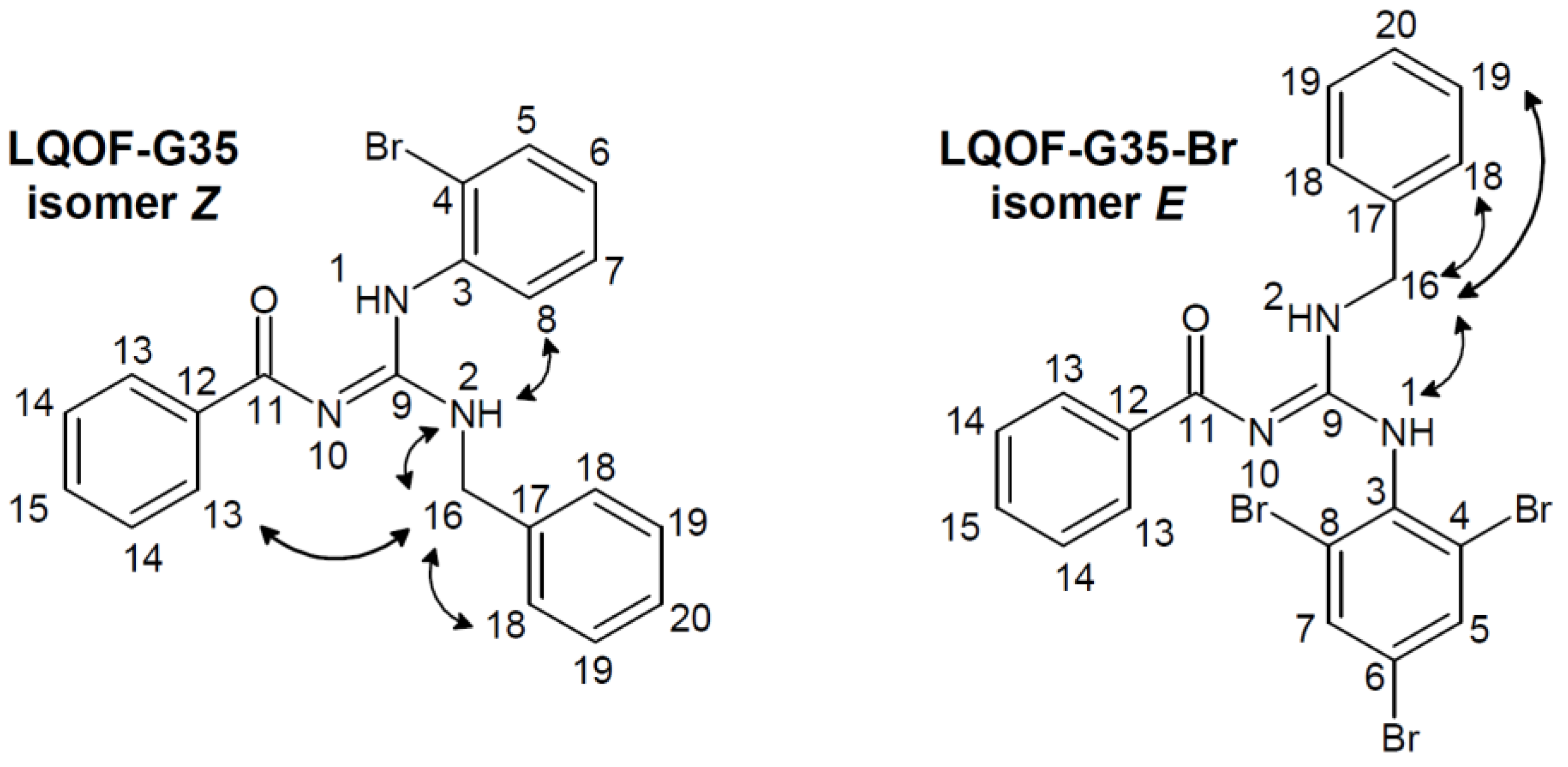
2.2. Compounds characterization
2.3. Conformacional study
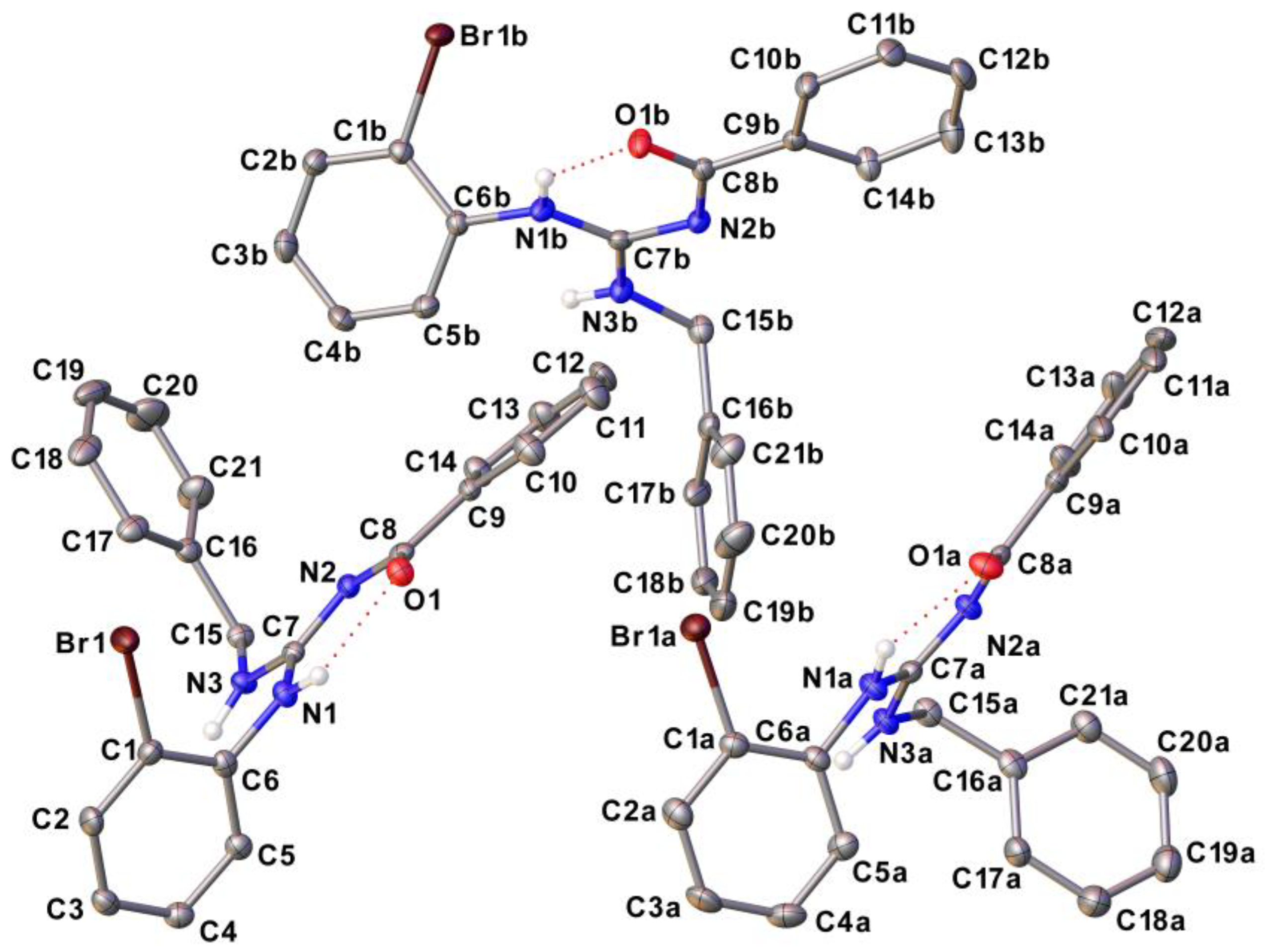
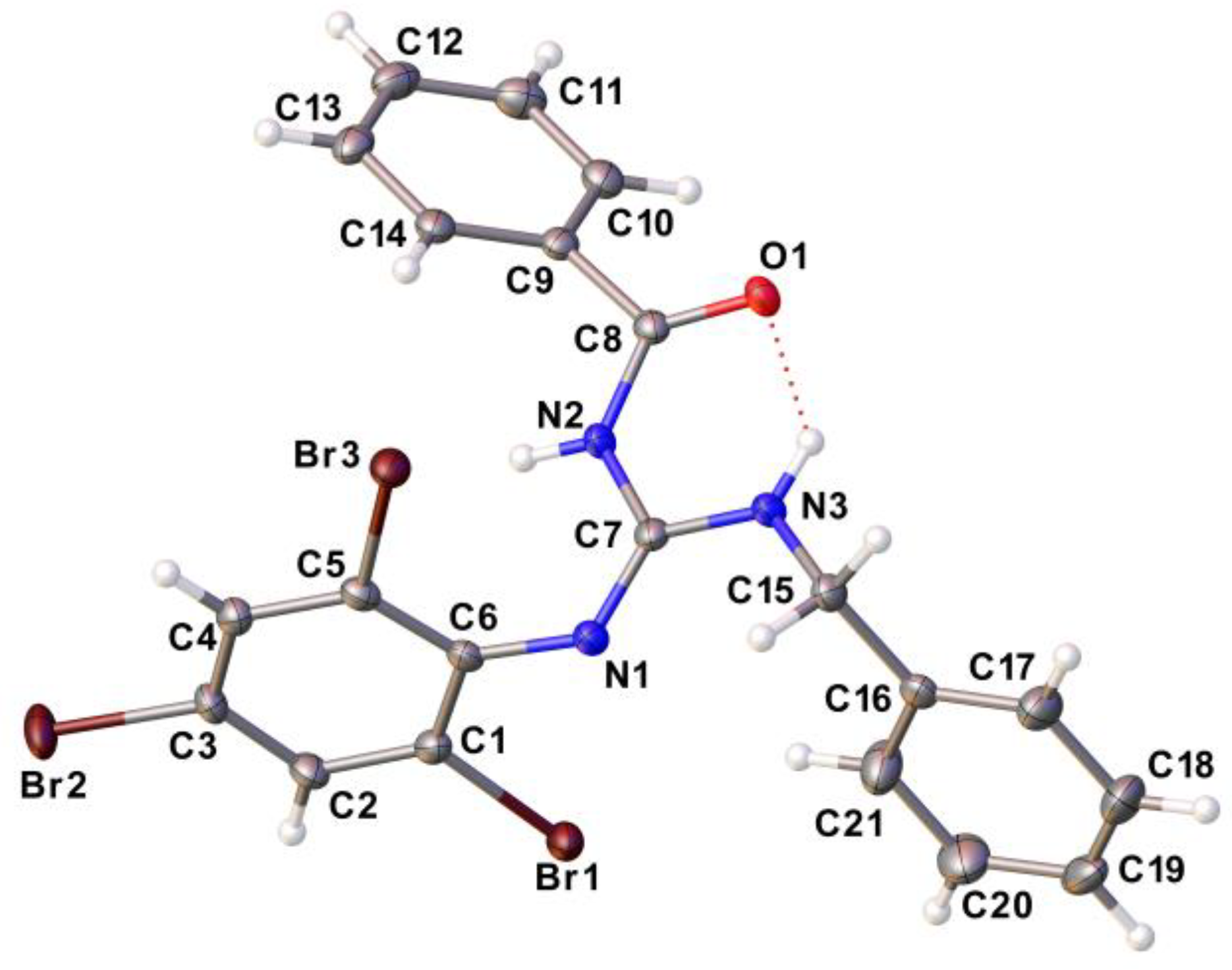
2.4. Structural data of compounds
3. Materials and Methods
3.1. Guanidine bromination using NBS and IR irradiation
3.2. Melting point
3.3. Electronic ionization mass spectrometry
3.4. NMR
3.5. HRESIMS
3.6. Single crystal X-ray diffraction (SCXRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 02 December 2023).
- Abadías-Granado, I.; Diago,4. ; Cerro, P. A.; Palma-Ruiz, A. M.; Gilaberte, Y. Cutaneous and Mucocutaneous Leishmaniasis. Actas Dermo-Sifiliográficas (English Edition) 2021, 112, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Ikeogu, N.M.; Akaluka, G.N.; Edechi, C.A.; Salako, E.S.; Onyilagha, C.; Barazandeh, A.F.; Uzonna, J.E. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms 2020, 8, 1201. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Schwartz, R. A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. CED 2022, 47, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Basmaciyan, L.; Casanova, M. Cell death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.; Ozerov, A.; Kosolapov, V.; Gurova, N.; Kucheryavenko, A.; Naumenko, L.; Babkov, D.; Sirotenko, V.; Taran, A.; Borisov, A.; et al. Guanidine Derivatives of Quinazoline-2,4(1H,3H)-Dione as NHE-1 Inhibitors and Anti-Inflammatory Agents. Life 2022, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Morrill, C.; Friesen, W. J.; Babu, S.; Baiazitov, R. Y; Du, W. et al. Guanidino quinazolines and pyrimidines promote readthrough of premature termination codons in cells with native nonsense mutations. Bioorganic & Medicinal Chemistry Letters 2022, 76, 128989. [Google Scholar]
- Yoshida, Y.; Endo, T. Synthesis of multifunctional 4-hydroxymethyl 2-oxazolidinones from glycidyl carbamate derivatives catalyzed by bicyclic guanidine. Tetrahedron Letters 2021, 72, 153086. [Google Scholar] [CrossRef]
- Shakaroun, R. M.; Jéhan, P.; Alaaeddine, A.; Carpentier, J.; Guillaume, S. M. Organocatalyzed ring-opening polymerization (ROP) of functional β-lactones: new insights into the ROP mechanism and poly(hydroxyalkanoate)s (PHAs) macromolecular structure. Polym. Chem. 2020, 11, 2640–2652. [Google Scholar] [CrossRef]
- Mesías-Salazar, Á.; Martínez, J.; Rojas, R. S.; Carrillo-Hermosilla, F.; Ramos, A.; Fernández-Galánb, R.; Antiñolo, A. Aromatic guanidines as highly active binary catalytic systems for the fixation of CO2 into cyclic carbonates under mild conditions. Catal. Sci. Technol. 2019, 9, 3879–3886. [Google Scholar] [CrossRef]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.-S.; Lee, Y.-J.; Kozlov, S. Marine Cyclic Guanidine Alkaloids Monanchomycalin B and Urupocidin A Act as Inhibitors of TRPV1, TRPV2 and TRPV3, but not TRPA1 Receptors. Mar. Drugs 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Staszewski, M.; Iwan, M.; Werner, T.; Bajda, M.; Godyń, J.; Latacz, G.; Korga-Plewko, A.; Kubik, J.; Szałaj, N.; Stark, H.; et al. Guanidines: Synthesis of Novel Histamine H3R Antagonists with Additional Breast Anticancer Activity and Cholinesterases Inhibitory Effect. Pharmaceuticals 2023, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M. A.; Nagargoje, A. A.; Shaikh, M. H.; Siddiqui, R. A.; Pund, A. A.; Khedkar, V. M. et al. Design, Synthesis and Bioevaluation of Highly Functionalized 1,2,3-Triazole-Guanidine Conjugates as Anti-Inflammatory and Antioxidant Agents. Polycyclic Aromatic Compounds 2023, 43, 5567–5581. [Google Scholar] [CrossRef]
- Zubair, S.; Badshah, A.; Patujo, J.; Khan, M.; Raheel, A.; Asghar, F.; Imtiaz, S. New ferrocene integrated amphiphilic guanidines: Synthesis, spectroscopic elucidation, DFT calculation and in vitro α-amylase and α-glucosidase inhibition combined with molecular docking approach. Heliyon 2023, 9, e14919. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, J.; Wang, S. et al. Guanidine-functionalized cotton fabrics for achieving permanent antibacterial activity without compromising their physicochemical properties and cytocompatibility. Cellulose 2020, 27, 6027–6036. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Pei, P.; Hao, J.; Lu, Y. et al. In vitro Antibacterial Activity of Isopropoxy Benzene Guanidine Against Multidrug-Resistant Enterococci. Infection and Drug Resistance 2019, 12, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Baugh, S.D.P. Guanidine-Containing Antifungal Agents against Human-Relevant Fungal Pathogens (2004–2022)—A Review. J. Fungi 2022, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A. R.; Varela, C. L.; Pires, A. S.; Tavares-da-Silva, E. J.; Roleira, F. M. F. Synthetic and natural guanidine derivatives as antitumor and antimicrobial agents: A review. Bioorganic Chemistry 2023, 138, 106600. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, R. D.; Velásquez, Á. M. A.; Passianoto, L. V. G.; Sepulveda, A. A. L.; Clementino, L. C. et al. N, N`, N``-trisubstituted guanidines: Synthesis, characterization and evaluation of their leishmanicidal activity. European Journal of Medicinal Chemistry 2019, 171, 116e128. [Google Scholar] [CrossRef]
- Costa, N. C. S.; Anjos, L. R.; Souza, J. V. M.; Brasil, M. C. O. A.; Moreira, V. P. et al. Development of New Leishmanicidal Compounds via Bioconjugation of Antimicrobial Peptides and Antileishmanial Guanidines. ACS Omega 2023, 8, 34008–34016. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.P.; da Silva Mela, M.F.; Anjos, L.R.; Saraiva, L.F.; Arenas Velásquez, A.M.; Kalaba, P.; Fabisiková, A.; Clementino, L.d.C.; Aufy, M.; Studenik, C.; et al. Novel Selective and Low-Toxic Inhibitor of LmCPB2.8ΔCTE (CPB) One Important Cysteine Protease for Leishmania Virulence. Biomolecules 2022, 12, 1903. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.S.; Moreira, V.P.; Silva, E.d.S.; Cardoso, L.L.; de Sousa Palmeira, P.H.; Cavalcante-Silva, L.H.A.; Araújo, D.A.M.d.; Amaral, I.P.G.d.; González, E.R.P.; Keesen, T.S.L. Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Trop. Med. Infect. Dis. 2023, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Anjos, L. R.; de Souza, V. M. R.; Machado, Y. A. A.; Partite, V. M.; Aufy, M.; Dias-Lopes, G.; studenik, C.; Alves, C. R.; Lubec, G.; González, E. R. P.; Rodrigues, K. A. F. Evidence of guanidines potential against Leishmania (Viannia) braziliensis: Exploring in vitro effectiveness, toxicities and of innate immunity response effects. Biomolecules 2023, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, R. D.; Simas, R. C.; Magalhães, A. et al. Experimental NMR and MS study of benzoylguanidines. Investigation of E/Z isomerism. Journal of Physical Organic Chemistry 2013, 26, 315–321. [Google Scholar] [CrossRef]
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; Martínez-García, H. Experimental Organic Chemistry: Laboratory Manual., 1st ed.; Academic Press: USA, 2015; pp. 371–408. [Google Scholar]
- Ram, V. J.; Sethi, A.; Nath, M.; Pratap, R. The Chemistry of Heterocycles., 1st ed.; Elsevier: Cambridge, USA, 2019; pp. 149–478. [Google Scholar]
- Barnes, R. A. N-Bromosuccinimide as a Dehydrogenating Agent1. Journal of the American Chemical Society 1948, 70, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Sivakamasundari, S.; Ganesan, R. Kinetics and mechanism of the bromination of aromatic compounds byN-bromosuccinimide in solution. International Journal of Chemical Kinetics 1980, 12, 837–850. [Google Scholar] [CrossRef]
- Appa, R.M.; Naidu, B.R.; Lakshmidevi, J. et al. Added catalyst-free, versatile and environment beneficial bromination of (hetero)aromatics using NBS in WEPA. SN Appl. Sci. 2019, 1, 1281. [Google Scholar] [CrossRef]
- Lee, S.; Ra, C. S. Benzylic Brominations with N-Bromosuccinimide in 1,2-Dichlorobenzene: Effective Preparation of (2-Bromomethyl-phenyl)-Methoxyiminoacetic Acid Methyl Ester. Clean Technol. 2016, 22, 269–273. [Google Scholar] [CrossRef]
- Oxford Diffraction/Agilent Technologies UK Ltd CrysAlisPRO.
- Sheldrick, G.M. SHELXT – Integrated space-group and crystalstructure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
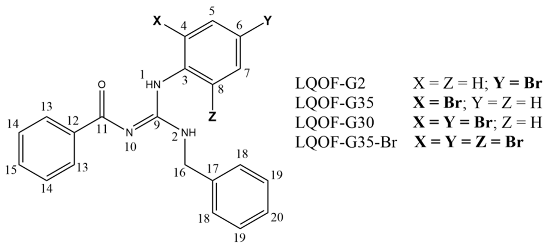 |
|||||||||||||||||||||||
| HYDROGENS | |||||||||||||||||||||||
| H1 | H2 | H1’ | H2’ | H5 | H8 | H13 | H14 | H16 | H18 | ||||||||||||||
| LQOF-G2 | 12.21 | 5.23 | - | 11.25 | 7.55 | 7.15 | 8.28 | 7.44 | 4.82 | 7.38 | |||||||||||||
| LQOF-G35 | 12.33 | 5.19 | - | 11.28 | 7.68 | 7.31 | 8.30 | 7.43 | 4.76 | 7.38 | |||||||||||||
| LQOF-G30 | 12.34 | 5.10 | - | 11.37 | 7.84 | - | 8.31 | 7.45 | 4.83 | 7.45 | |||||||||||||
| LQOF-G35-Br | 12.26 | 5.31 | 8.61 | 9.64 | 7.71 | - | 8.28 | 7.48 | 4.82 | 7.56 | |||||||||||||
| CARBONS | |||||||||||||||||||||||
| C3 | C4 | C6 | C8 | C9 | C11 | C12 | C13 | C14 | C16 | C17 | C18 | ||||||||||||
| LQOF-G2 | 134.6 | 127.3 | 120.6 | - | 158.2 | 177.8 | 138.0 | 129.0 | 127.9 | 45.1 | 138.2 | 127.6 | |||||||||||
| LQOF-G35 | 134.8 | 121.5 | - | - | 158.1 | 177.7 | 138.1 | 129.3 | 128.1 | 45.0 | 138.4 | 127.8 | |||||||||||
| LQOF-G30 | 134.0 | 122.1 | 121.0 | - | 157.9 | 177.9 | 137.7 | 127.9 | 129.1 | 45.2 | 138.0 | 127.7 | |||||||||||
| LQOF-G35-Br | 119.3 | 115.4 | 143.3 | 115.4 | 144.4 | 166.8 | 132.6 | 127.0 | 127.6 | 45.0 | 138.0 | 129.1 | |||||||||||
| Compound | Isomer E % | Isomer Z % |
| LQOF-G2 | 7.02 | 92.98 |
| LQOF-G35 | 17.07 | 82.93 |
| LQOF-G30 | 36.14 | 63.86 |
| LQOF-G35-Br | 53.54 | 46.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





