Submitted:
22 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SPR Measurements
2.3. Gold Sensing Area Functionalization
2.4. BioFET Set Up and Electrical Measurements
3. Results and Discussion
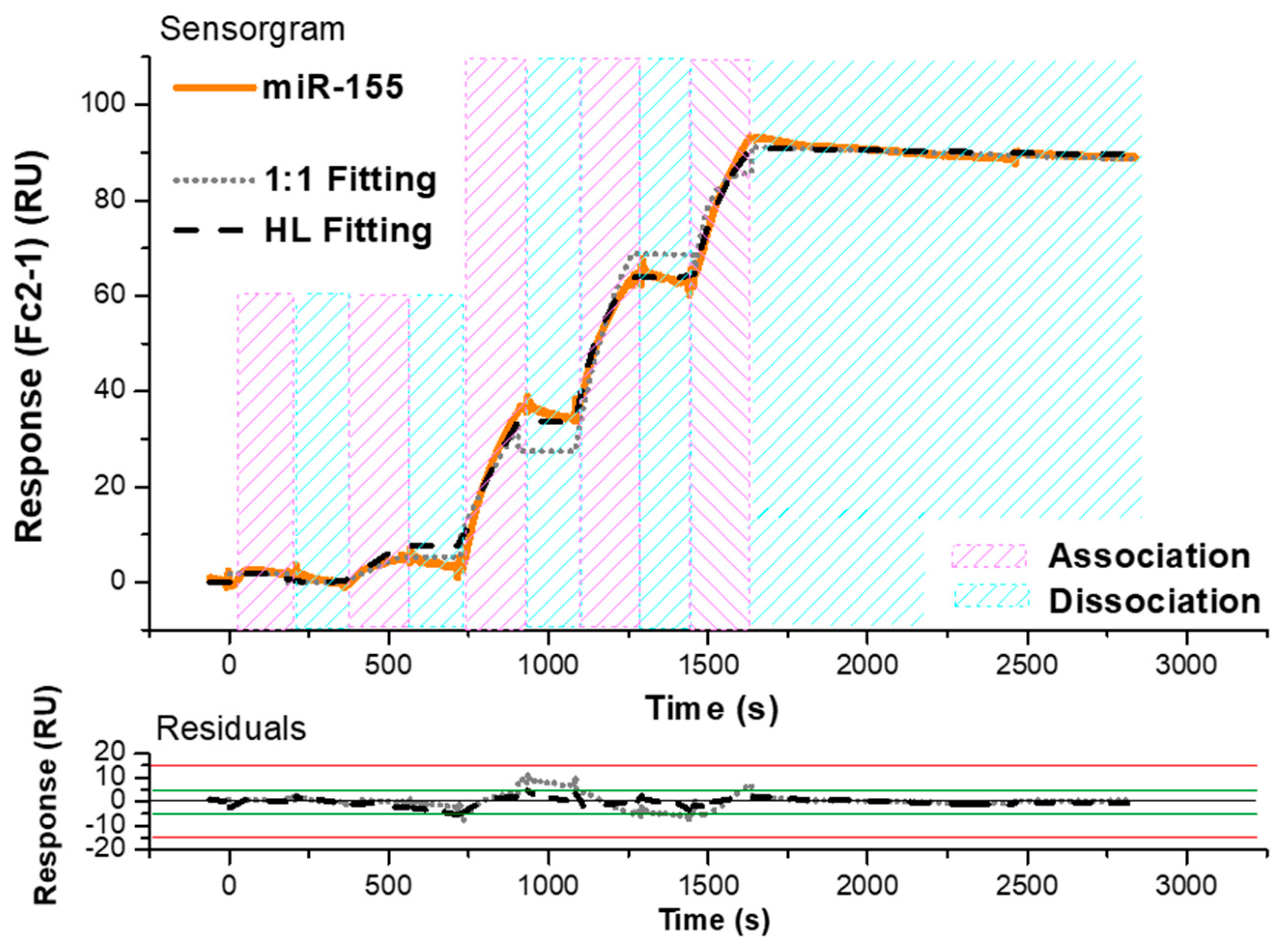
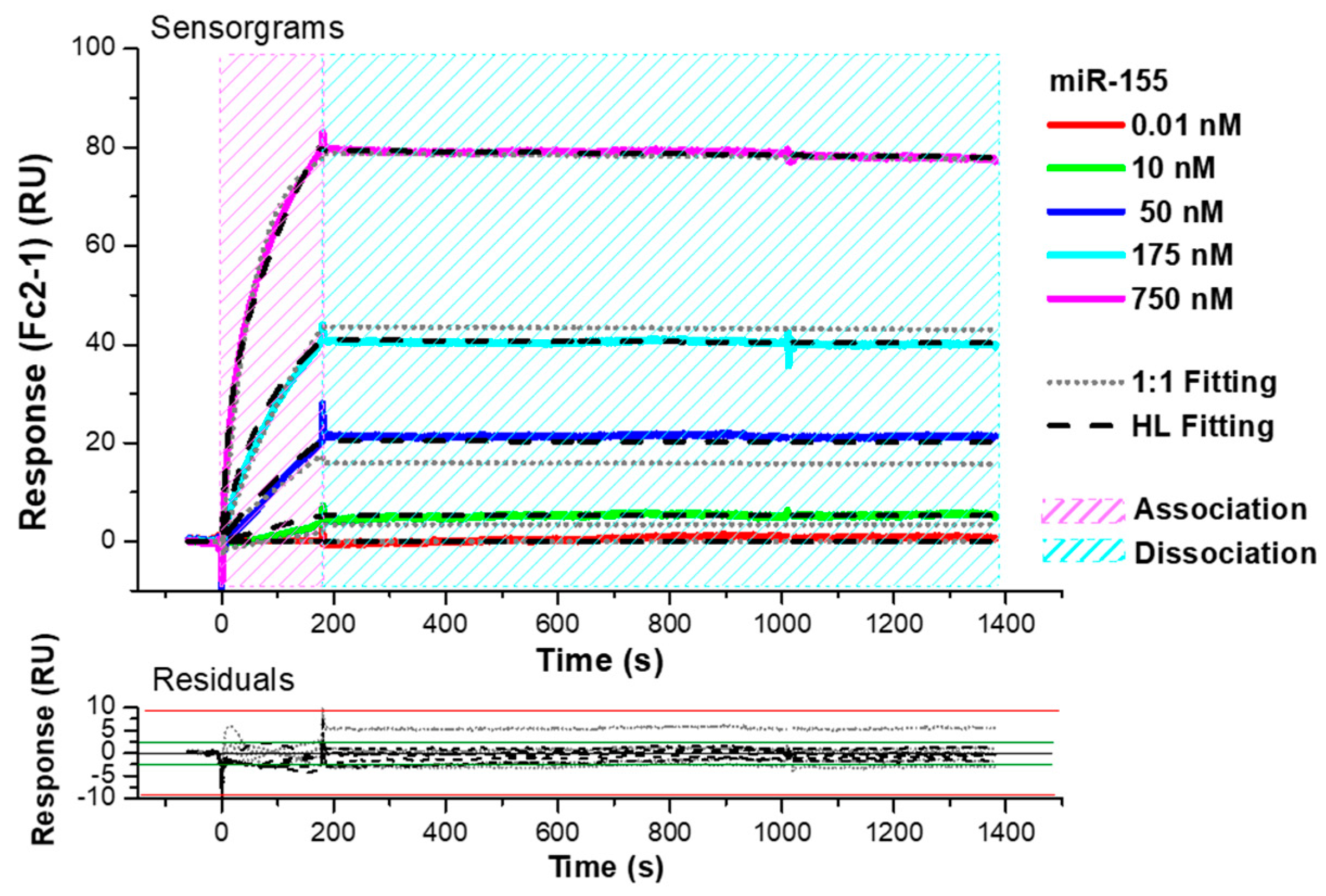
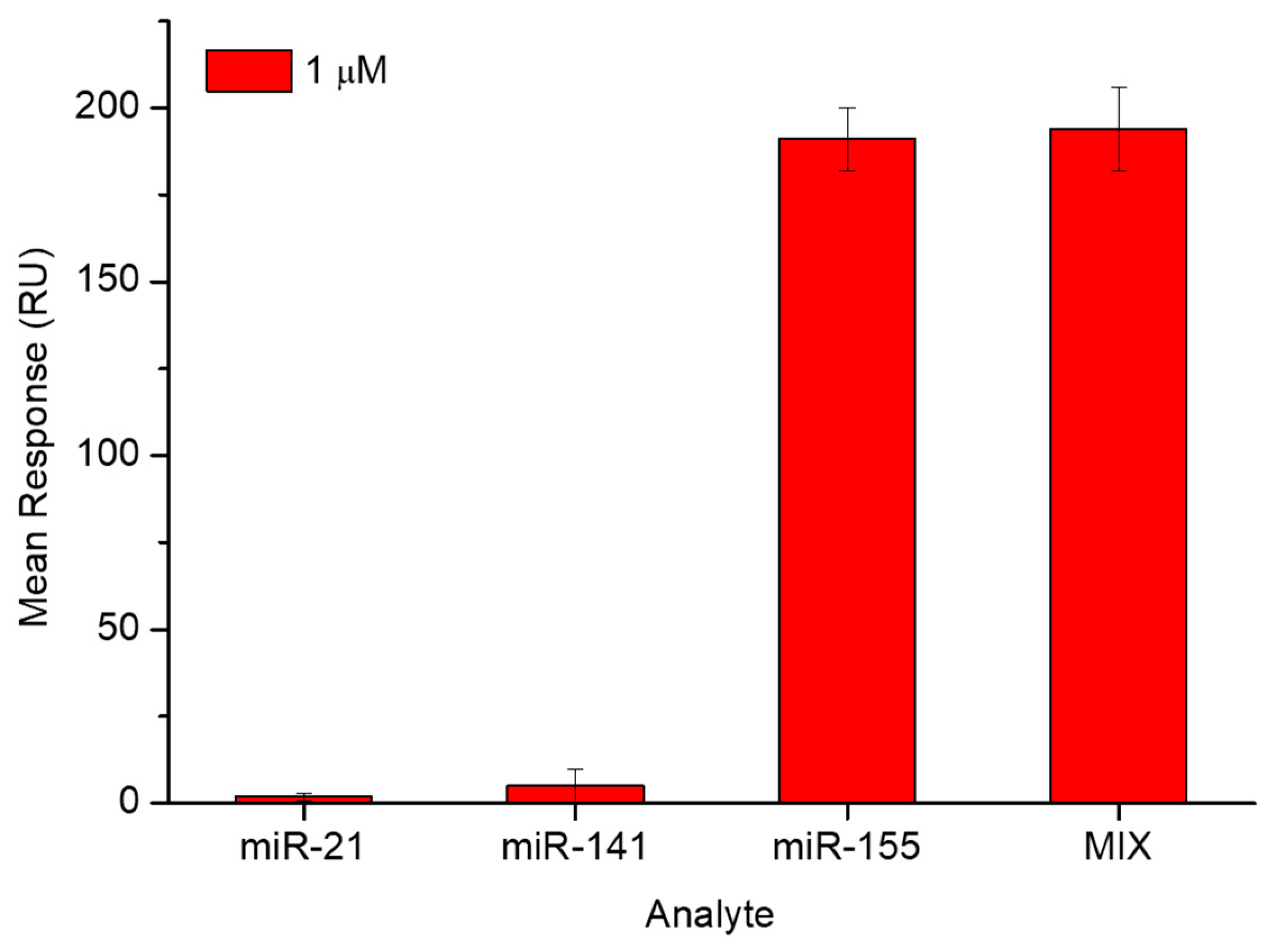
3.1. SPR Investigation
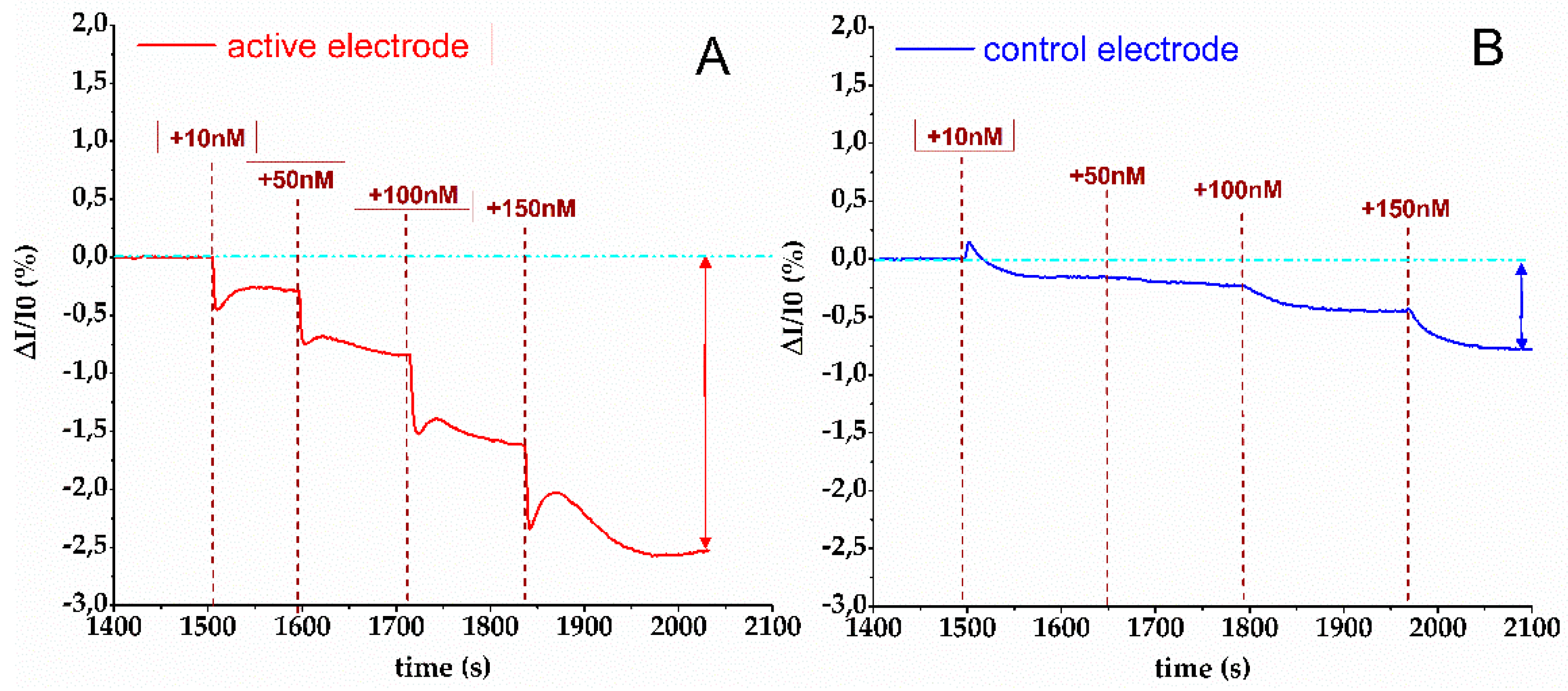
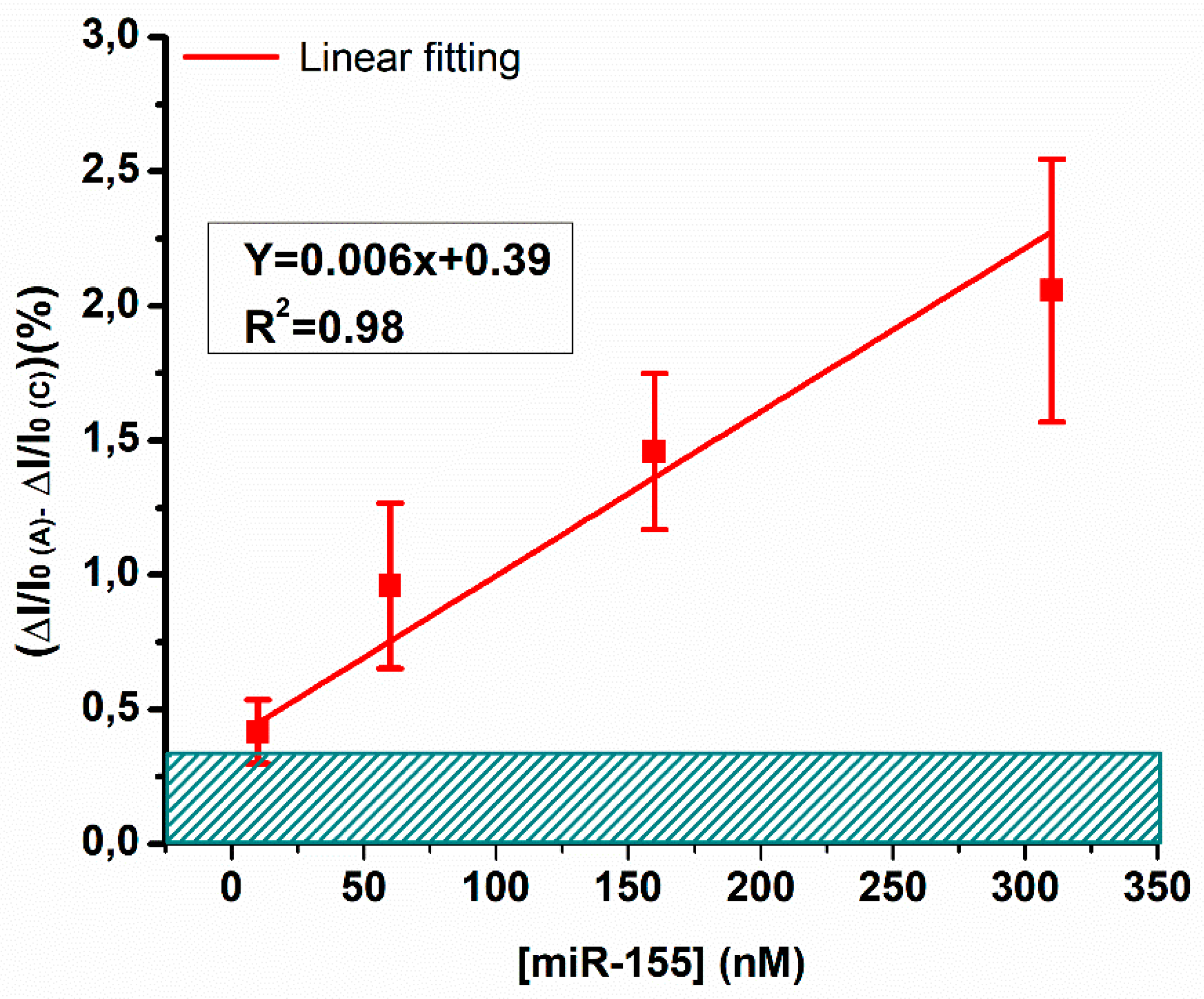
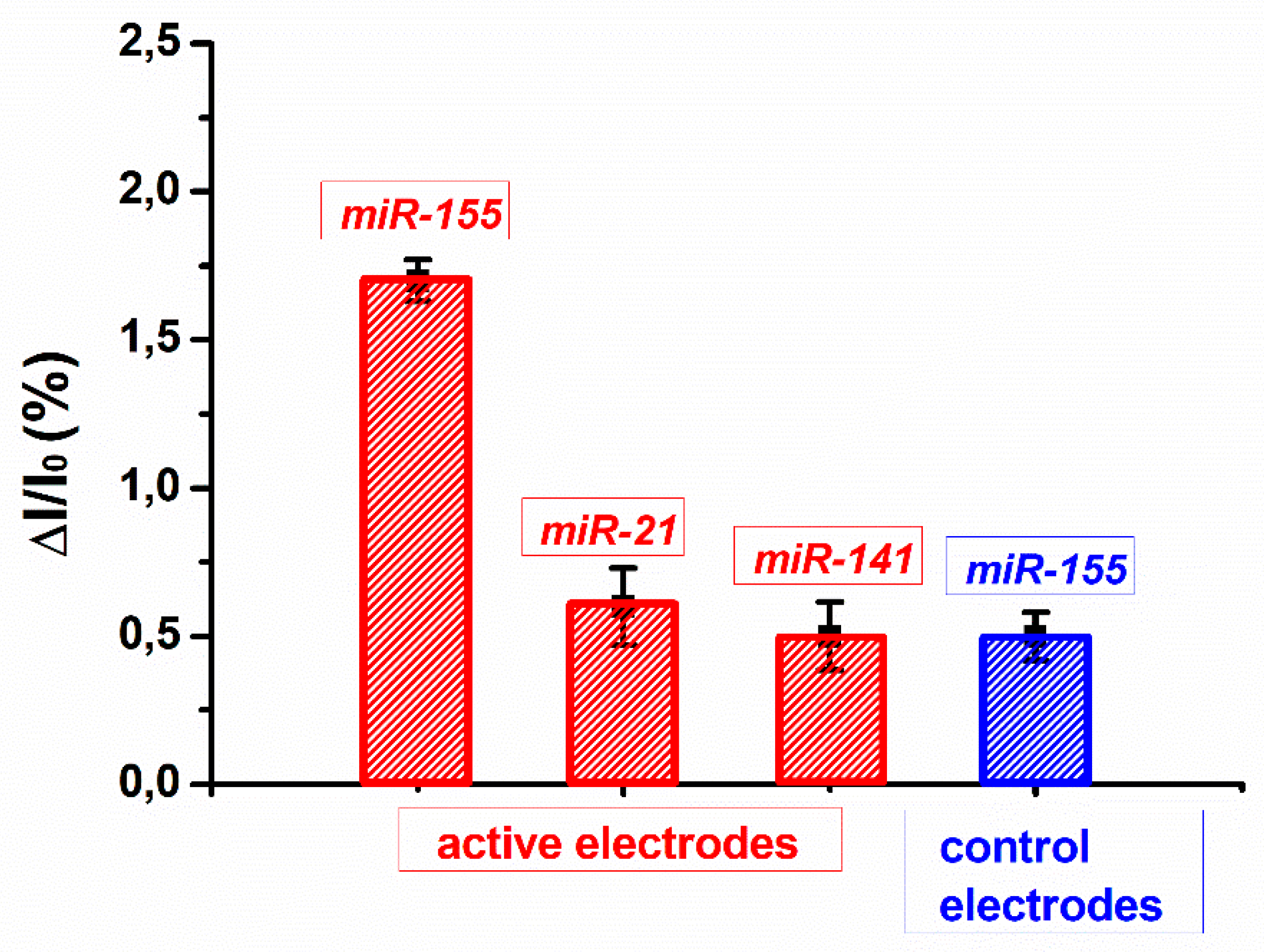
3.2. Biosensing Analyses by a bioFET Setup
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281-97. PMID: 14744438. [CrossRef]
- Ambros V. The functions of animal microRNAs. Nature. 2004 Sep 16;431(7006):350-5. PMID: 15372042. [CrossRef]
- Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015 Jun 29;87:3-14. Epub 2015 May 12. PMID: 25979468; PMCID: PMC4504744. [CrossRef]
- Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010 Nov;11(7):537-61. PMID: 21532838; PMCID: PMC3048316. [CrossRef]
- Vaghf A, Khansarinejad B, Ghaznavi-Rad E, Mondanizadeh M. The role of microRNAs in diseases and related signaling pathways. Mol Biol Rep. 2022 Jul;49(7):6789-6801. Epub 2021 Oct 31. PMID: 34718938. [CrossRef]
- Bertoli G, Cava C, Castiglioni I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Breast Cancer. Theranostics. 2015 Jul 13;5(10):1122-43. [CrossRef]
- Edwards JK, Pasqualini R, Arap W, Calin GA. MicroRNAs and ultraconserved genes as diagnostic markers and therapeutic targets in cancer and cardiovascular diseases. J Cardiovasc Transl Res. 2010 Jun;3(3):271-9. Epub 2010 May 5. [CrossRef]
- Basak I, Patil KS, Alves G, Larsen JP, Møller SG. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell Mol Life Sci. 2016 Feb;73(4):811-27. Epub 2015 Nov 25. PMID: 26608596. [CrossRef]
- Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009 Jun;394(4):1117-24. Epub 2009 Jan 9. [CrossRef]
- Válóczi A, Hornyik C, Varga N, Burgyán J, Kauppinen S, Havelda Z. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004 Dec 14;32(22):e175. [CrossRef]
- Hu Y, Lan W, Miller D. Next-Generation Sequencing for MicroRNA Expression Profile. Methods Mol Biol. 2017;1617:169-177. [CrossRef]
- Zhu CS, Zhu L, Tan DA, Qiu XY, Liu CY, Xie SS, Zhu LY. Avenues Toward microRNA Detection In Vitro: A Review of Technical Advances and Challenges. Comput Struct Biotechnol J. 2019 Jun 20;17:904-916. [CrossRef]
- Johnson BN, Mutharasan R. Biosensor-based microRNA detection: techniques, design, performance, and challenges. Analyst. 2014 Apr 7;139(7):1576-88. [CrossRef]
- Turner AP. Biosensors: sense and sensibility. Chem Soc Rev. 2013 Apr 21;42(8):3184-96. Epub 2013 Feb 19. [CrossRef]
- Bhalla N, Jolly P, Formisano N, Estrela P. Introduction to biosensors. Essays Biochem. 2016 Jun 30;60(1):1-8. [CrossRef]
- Cardoso AR, Moreira FTC, Fernandes R, Sales MGF. Novel and simple electrochemical biosensor monitoring attomolar levels of miRNA-155 in breast cancer. Biosens Bioelectron. 2016 Jun 15;80:621-630. Epub 2016 Feb 14. [CrossRef]
- Mansouri Majd S, Salimi A, Astinchap B. Label-free attomolar detection of lactate based on radio frequency sputtered of nickel oxide thin film field effect transistor. Biosens Bioelectron. 2017 Jun 15;92:733-740. Epub 2016 Sep 28. [CrossRef]
- Gutiérrez-Sanz, Ó.; Andoy, N.M.; Filipiak, M.S.; Haustein, N.; Tarasov, A. Direct, Label-Free, and Rapid Transistor-Based Immunodetection in Whole Serum. ACS Sensors 2017, 2, 1278–1286. [CrossRef]
- Lowe BM, Sun K, Zeimpekis I, Skylaris CK, Green NG. Field-effect sensors - from pH sensing to biosensing: sensitivity enhancement using streptavidin-biotin as a model system. Analyst. 2017 Nov 6;142(22):4173-4200. [CrossRef]
- Sung D, Koo J. A review of BioFET's basic principles and materials for biomedical applications. Biomed Eng Lett. 2021 Apr 9;11(2):85-96. [CrossRef]
- Tadmor, R., Hernández-Zapata, E., Chen, N., Pincus, P., & Israelachvili, J. N. (2002). Debye length and double-layer forces in polyelectrolyte solutions. Macromolecules, 35(6), 2380-2388. [CrossRef]
- Schasfoort, R. B., Bergveld, P., Kooyman, R. P. H., & Greve, J. (1990). Possibilities and limitations of direct detection of protein charges by means of an immunological field-effect transistor. Analytica chimica acta, 238, 323-329. [CrossRef]
- Gong P, Levicky R. DNA surface hybridization regimes. Proc Natl Acad Sci U S A. 2008 Apr 8;105(14):5301-6. Epub 2008 Apr 1. [CrossRef]
- Shakeel, Shabih, Sajjad Karim, and Arif Ali. "Peptide nucleic acid (PNA)—a review." Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology 81.6 (2006): 892-899.
- Saarbach J, Sabale PM, Winssinger N. Peptide nucleic acid (PNA) and its applications in chemical biology, diagnostics, and therapeutics. Curr Opin Chem Biol. 2019 Oct;52:112-124. Epub 2019 Sep 18. [CrossRef]
- Ananthanawat C, Vilaivan T, Hoven VP, Su X. Comparison of DNA, aminoethylglycyl PNA and pyrrolidinyl PNA as probes for detection of DNA hybridization using surface plasmon resonance technique. Biosens Bioelectron. 2010 Jan 15;25(5):1064-9. Epub 2009 Oct 1. [CrossRef]
- Schwarz FP, Robinson S, Butler JM. Thermodynamic comparison of PNA/DNA and DNA/DNA hybridization reactions at ambient temperature. Nucleic Acids Res. 1999 Dec 15;27(24):4792-800. [CrossRef]
- Nakatsuka N, Yang KA, Abendroth JM, Cheung KM, Xu X, Yang H, Zhao C, Zhu B, Rim YS, Yang Y, Weiss PS, Stojanović MN, Andrews AM. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science. 2018 Oct 19;362(6412):319-324. Epub 2018 Sep 6. [CrossRef]
- Park H, Germini A, Sforza S, Corradini R, Marchelli R, Knoll W. Effect of ionic strength on PNA-DNA hybridization on surfaces and in solution. Biointerphases. 2007 Jun;2(2):80-8. [CrossRef]
- Irving D, Gong P, Levicky R. DNA surface hybridization: comparison of theory and experiment. J Phys Chem B. 2010 Jun 10;114(22):7631-40. [CrossRef]
- Kaisti M, Kerko A, Aarikka E, Saviranta P, Boeva Z, Soukka T, Lehmusvuori A. Real-time wash-free detection of unlabeled PNA-DNA hybridization using discrete FET sensor. Sci Rep. 2017 Nov 16;7(1):15734. [CrossRef]
- Papamatthaiou S, Estrela P, Moschou D. Printable graphene BioFETs for DNA quantification in Lab-on-PCB microsystems. Sci Rep. 2021 May 10;11(1):9815. [CrossRef]
- Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009 Jun;1792(6):497-505. Epub 2009 Mar 5. [CrossRef]
- Due H, Svendsen P, Bødker JS, Schmitz A, Bøgsted M, Johnsen HE, El-Galaly TC, Roug AS, Dybkær K. miR-155 as a Biomarker in B-Cell Malignancies. Biomed Res Int. 2016;2016:9513037. Epub 2016 May 16. [CrossRef]
- Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, Chen C. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009 Apr;33(4):698-709. [CrossRef]
- Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012 Aug;21(8):1236-43. Epub 2012 Jun 26. [CrossRef]
- Hou Y, Wang J, Wang X, Shi S, Wang W, Chen Z. Appraising MicroRNA-155 as a Noninvasive Diagnostic Biomarker for Cancer Detection: A Meta-Analysis. Medicine (Baltimore). 2016 Jan;95(2):e2450. [CrossRef]
- Moscetti I, Cannistraro S, Bizzarri AR. Surface Plasmon Resonance Sensing of Biorecognition Interactions within the Tumor Suppressor p53 Network. Sensors (Basel). 2017 Nov 20;17(11):2680. [CrossRef]
- Nguyen, H. H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors. 2015, pp 10481–10510. [CrossRef]
- Marquart, J. A. Surface Plasmon Resonance and Biomolecular Interaction Analysis-Theory and Practice; Pumbo BV, 2013. www.sprpages.nl.
- GE Healthcare. Biacore Assay Handbook; General Electric Company, 2012.
- Blake N. Johnson and Raj MutharasanThe Journal of Physical Chemistry C 2013 117 (3), 1335-1341. [CrossRef]
- Meng X, O'Hare D, Ladame S. Surface immobilization strategies for the development of electrochemical nucleic acid sensors. Biosens Bioelectron. 2023 Oct 1;237:115440. Epub 2023 Jun 16. [CrossRef]
- Rastislav Levicky, Tonya M. Herne, Michael J. Tarlov, and Sushil K. Satija Journal of the American Chemical Society 1998 120 (38), 9787-9792. [CrossRef]
- Movilli J, Rozzi A, Ricciardi R, Corradini R, Huskens J. Control of Probe Density at DNA Biosensor Surfaces Using Poly(l-lysine) with Appended Reactive Groups. Bioconjug Chem. 2018 Dec 19;29(12):4110-4118. Epub 2018 Nov 26. [CrossRef]
- Mateo-Martí E, Briones C, Román E, Briand E, Pradier CM, Martín-Gago JA. Self-assembled monolayers of peptide nucleic acids on gold surfaces: a spectroscopic study. Langmuir. 2005 Oct 11;21(21):9510-7. [CrossRef]
- Chen S., Nyholm L., Jokilaakso N., Karlstrm A.E., Linnros J., Smith U., Zhang S.L. Current instability for silicon nanowire field-effect sensors operating in electrolyte with platinum gate electrodes. Electrochem. Solid-State Lett. 2011;14. [CrossRef]
- O'Shannessy DJ, Brigham-Burke M, Soneson KK, Hensley P, Brooks I. Determination of rate and equilibrium binding constants for macromolecular interactions using surface plasmon resonance: use of nonlinear least squares analysis methods. Anal Biochem. 1993 Aug 1;212(2):457-68. [CrossRef]
- Vanjur L, Carzaniga T, Casiraghi L, Chiari M, Zanchetta G, Buscaglia M. Non-Langmuir Kinetics of DNA Surface Hybridization. Biophys J. 2020 Sep 1;119(5):989-1001. Epub 2020 Jul 29. [CrossRef]
- Botti V, Lavecchia di Tocco F, Cannistraro S, Bizzarri AR. Hybridization Kinetics of miR-155 on Gold Surfaces as Investigated by Surface Plasmon Resonance and Atomic Force Spectroscopy. ACS Omega. 2023 Oct 9;8(42):38941-38949. [CrossRef]
- Jing Z, Qi R, Thibonnier M, Ren P. Molecular Dynamics Study of the Hybridization between RNA and Modified Oligonucleotides. J Chem Theory Comput. 2019 Nov 12;15(11):6422-6432. Epub 2019 Oct 9. [CrossRef]
- Zhang J, Lang HP, Yoshikawa G, Gerber C. Optimization of DNA hybridization efficiency by pH-driven nanomechanical bending. Langmuir. 2012 Apr 17;28(15):6494-501. Epub 2012 Apr 2. [CrossRef]
- Wang X, Dai C, Wu Y, Liu Y, Wei D. Molecular-electromechanical system for unamplified detection of trace analytes in biofluids. Nat Protoc. 2023 Jul;18(7):2313-2348. Epub 2023 May 19. [CrossRef]
- Cai B, Wang S, Huang L, Ning Y, Zhang Z, Zhang GJ. Ultrasensitive label-free detection of PNA-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano. 2014 Mar 25;8(3):2632-8. Epub 2014 Feb 20. [CrossRef]
- Chen Y, Ren R, Pu H, Guo X, Chang J, Zhou G, Mao S, Kron M, Chen J. Field-Effect Transistor Biosensor for Rapid Detection of Ebola Antigen. Sci Rep. 2017 Sep 8;7(1):10974. [CrossRef]
- Jenkins, R., Manne, R., Robin, R., & Senemaud, C. (1991). IUPAC—nomenclature system for x-ray spectroscopy. X-Ray Spectrometry, 20(3), 149-155.
- Minamiki T., Sasaki Y., Tokito S., Minami T. Label-free direct electrical detection of a histidine-rich protein with sub-femtomolar sensitivity using an organic field-effect transistor. ChemistryOpen. 2017;6:472–475. [CrossRef]
- Kaisti M, Kerko A, Aarikka E, Saviranta P, Boeva Z, Soukka T, Lehmusvuori A. Real-time wash-free detection of unlabeled PNA-DNA hybridization using discrete FET sensor. Sci Rep. 2017 Nov 16;7(1):15734. [CrossRef]
- Papamatthaiou, Sotirios, Pedro Estrela, and Despina Moschou. "Printable graphene BioFETs for DNA quantification in Lab-on-PCB microsystems." Scientific Reports 11.1 (2021): 9815. [CrossRef]
- Moccia M, Caratelli V, Cinti S, Pede B, Avitabile C, Saviano M, Imbriani AL, Moscone D, Arduini F. Paper-based electrochemical peptide nucleic acid (PNA) biosensor for detection of miRNA-492: a pancreatic ductal adenocarcinoma biomarker. Biosens Bioelectron. 2020 Oct 1;165:112371. Epub 2020 Jun 8. [CrossRef]
- Roychoudhury A, Dear JW, Bachmann TT. Proximity sensitive detection of microRNAs using electrochemical impedance spectroscopy biosensors. Biosens Bioelectron. 2022 Sep 15;212:114404. Epub 2022 May 20. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




