Submitted:
21 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Tested bacterial isolates which used in antimicrobial test
2.2. Sampling and fungal isolation
2.3. Preparation of cell-free extract
2.4. Green synthesis of Ag-NPs
2.5. Characterizations of Ag-NPs
2.6. Genotypic characterization of a potent isolate
2.7. Anti-fungal test activity of Ag-NPs
2.8. Determination of antioxidant activity
2.9. Cytotoxic Activity
2.10. Wound Healing Assay
2.11. Statistical analysis
3. Results
3.1. Fungal isolation and screening for silver nanoparticle synthesis
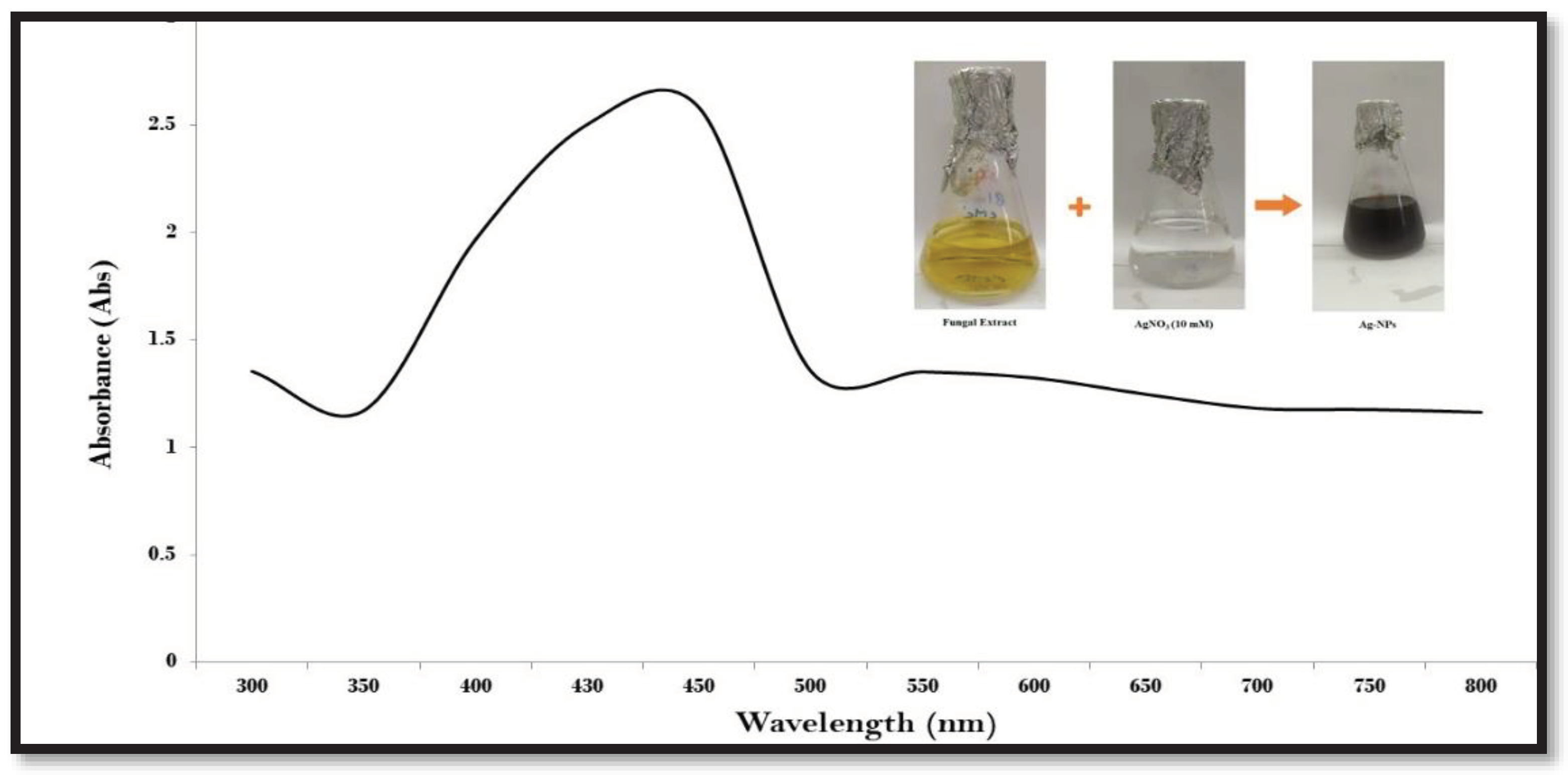
3.2. Analysis by UV-Visible spectroscopy
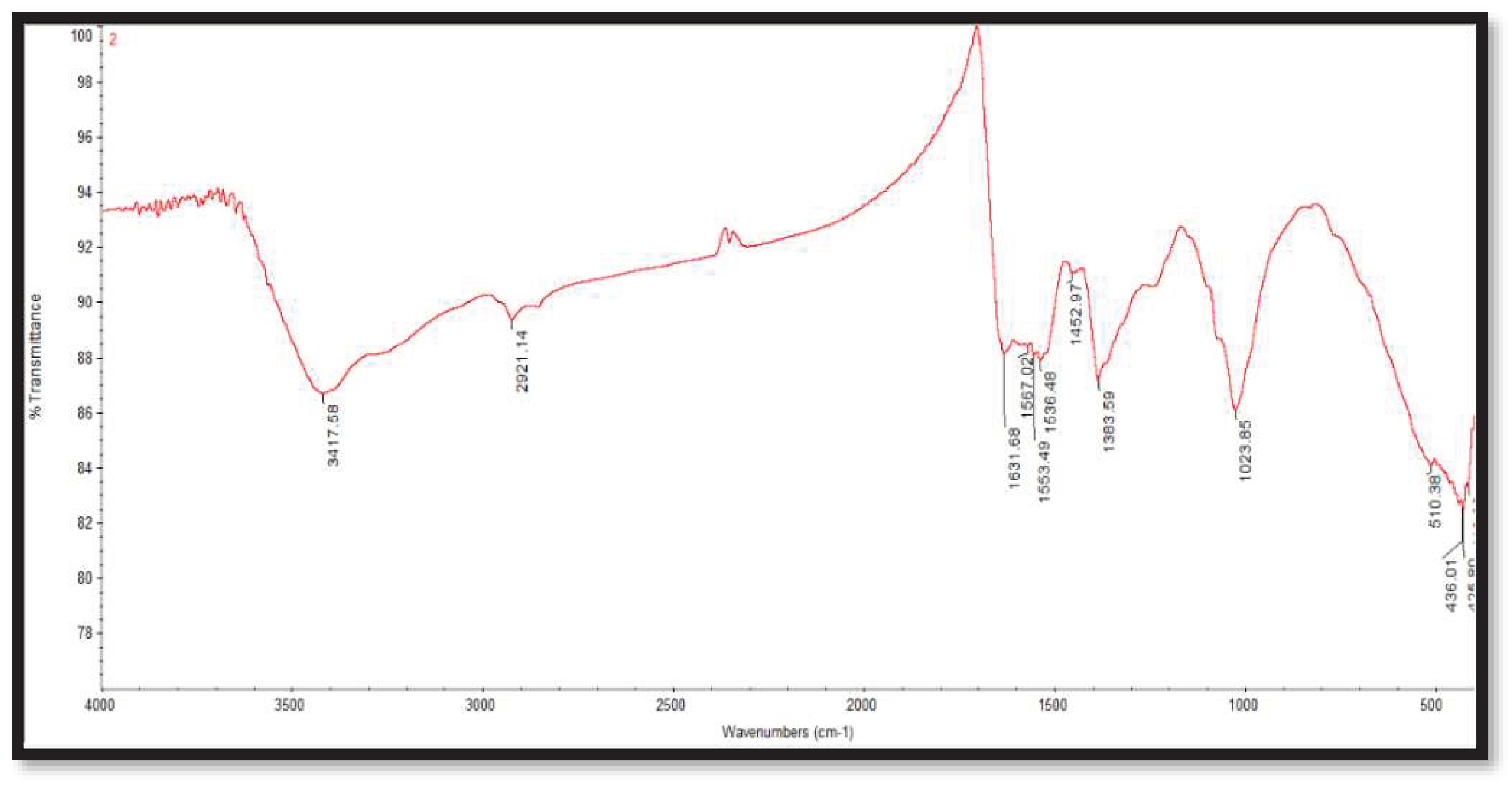
3.3. Fourier transform infrared spectroscopy
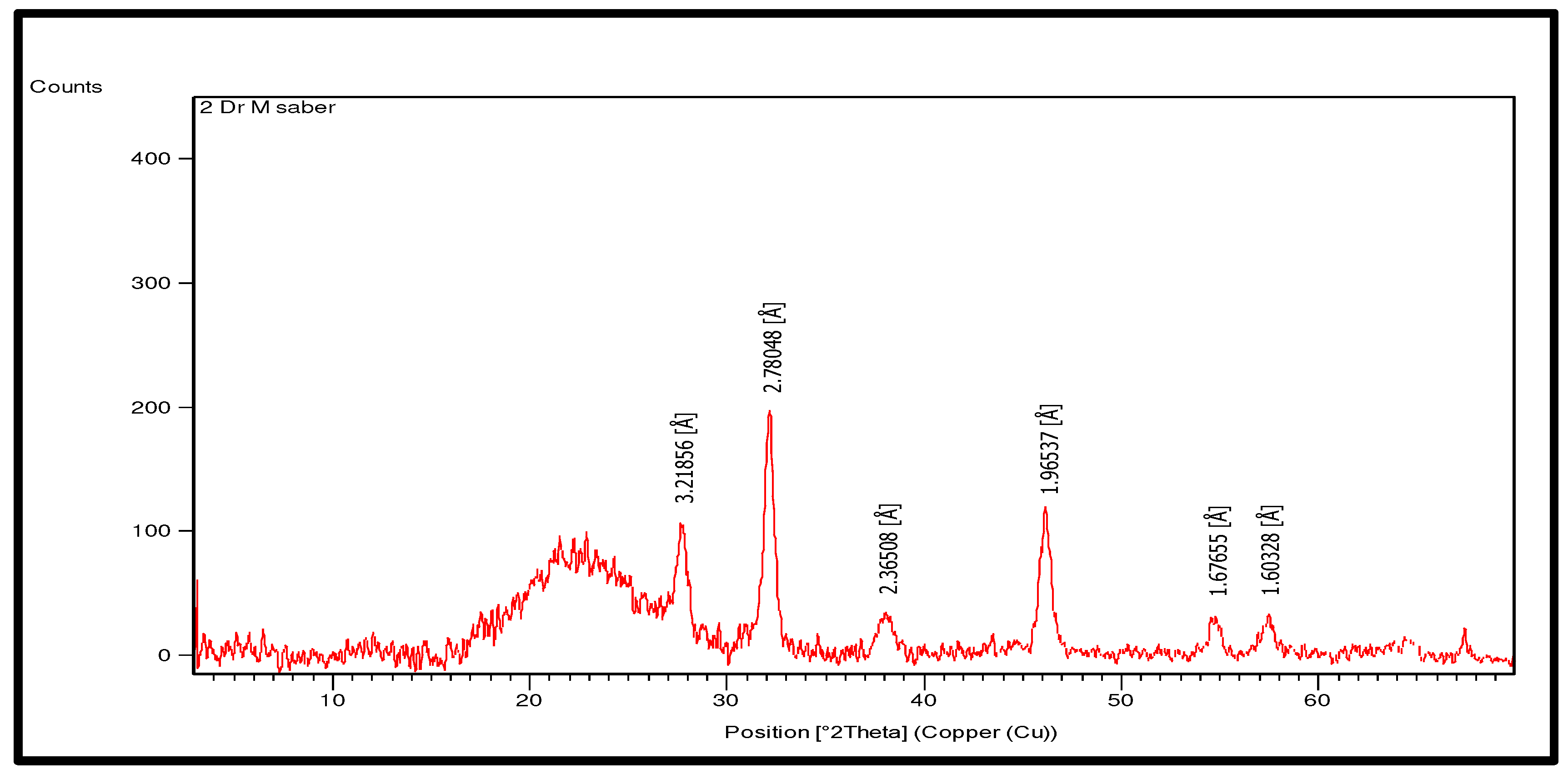
3.4. X-ray diffraction analysis
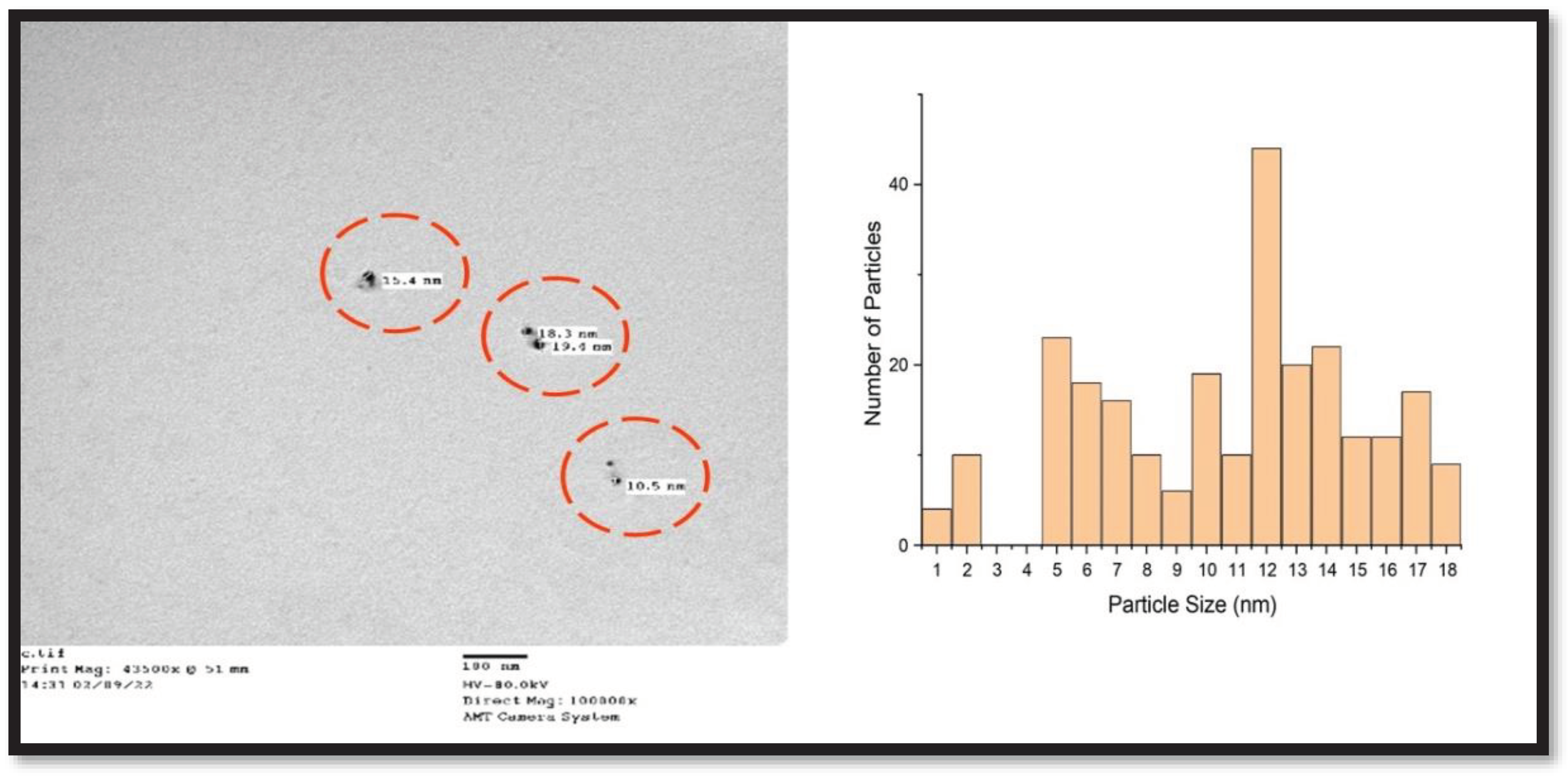
3.5. Analysis via Transmission Electron Microscopy (TEM)
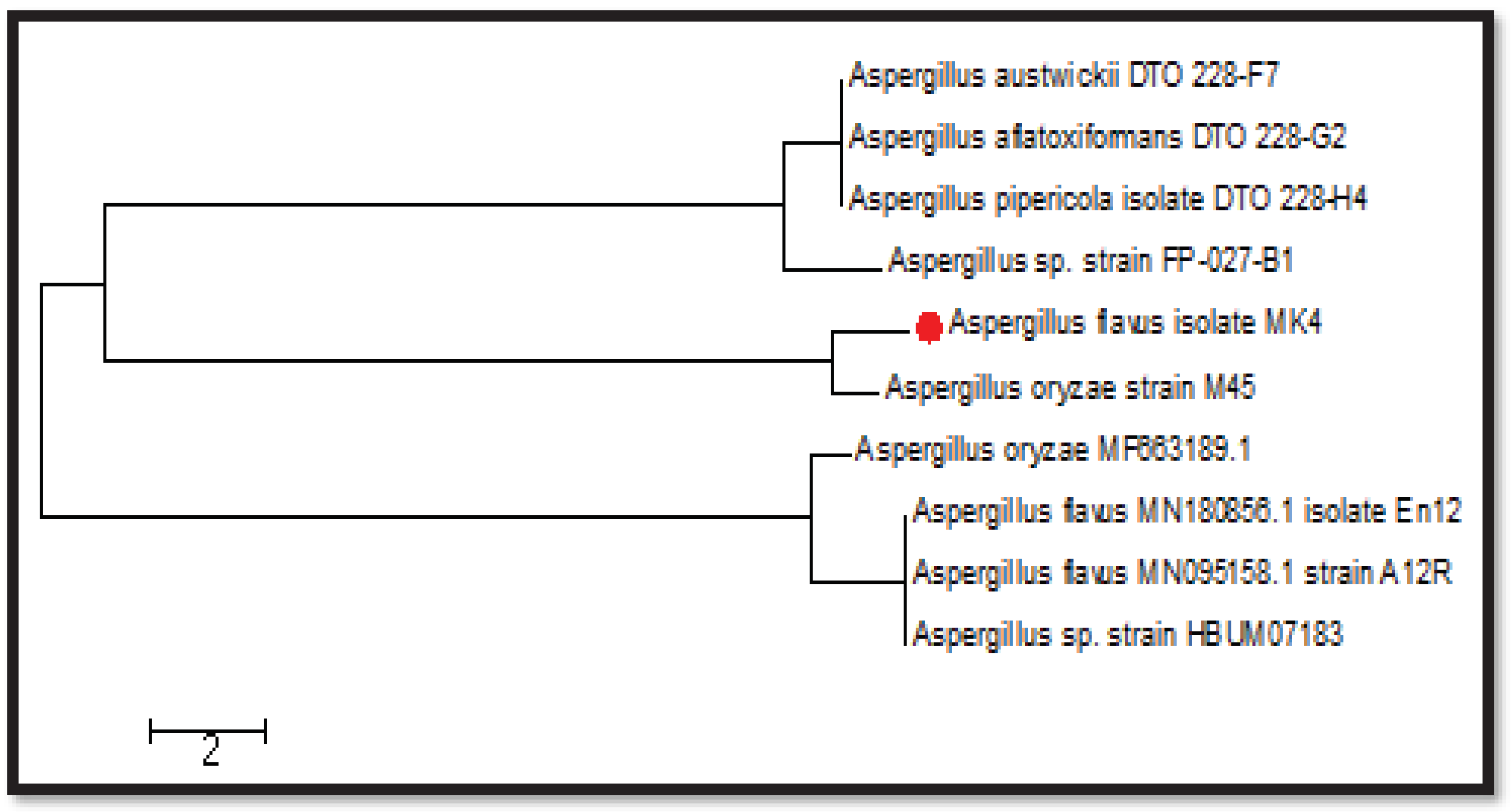
3.6. Sequencing the most effective fungal isolate (MK4)
3.7. Antibacterial activity
3.8. Anti-Fungal activity
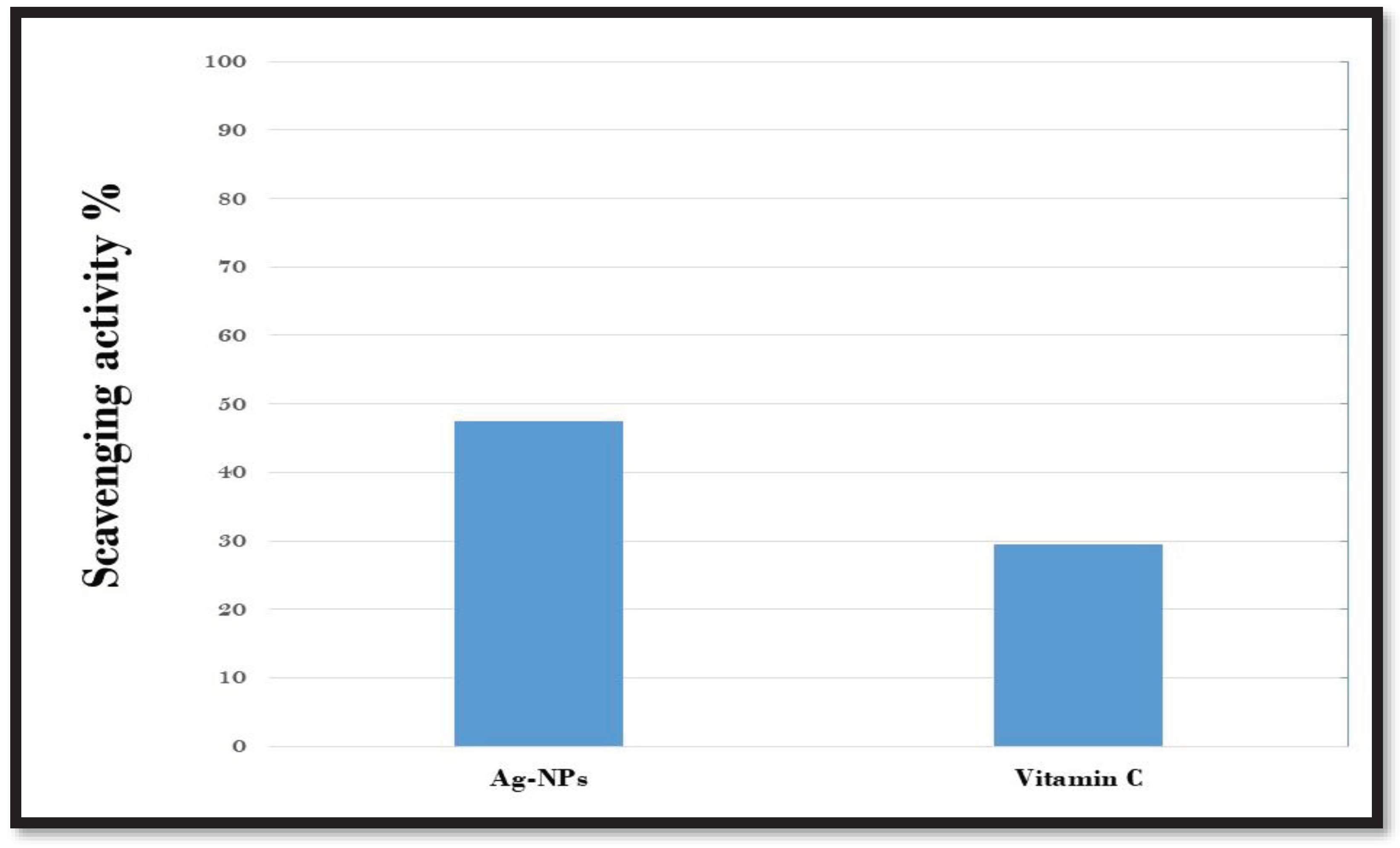
3.9. Antioxidant activity
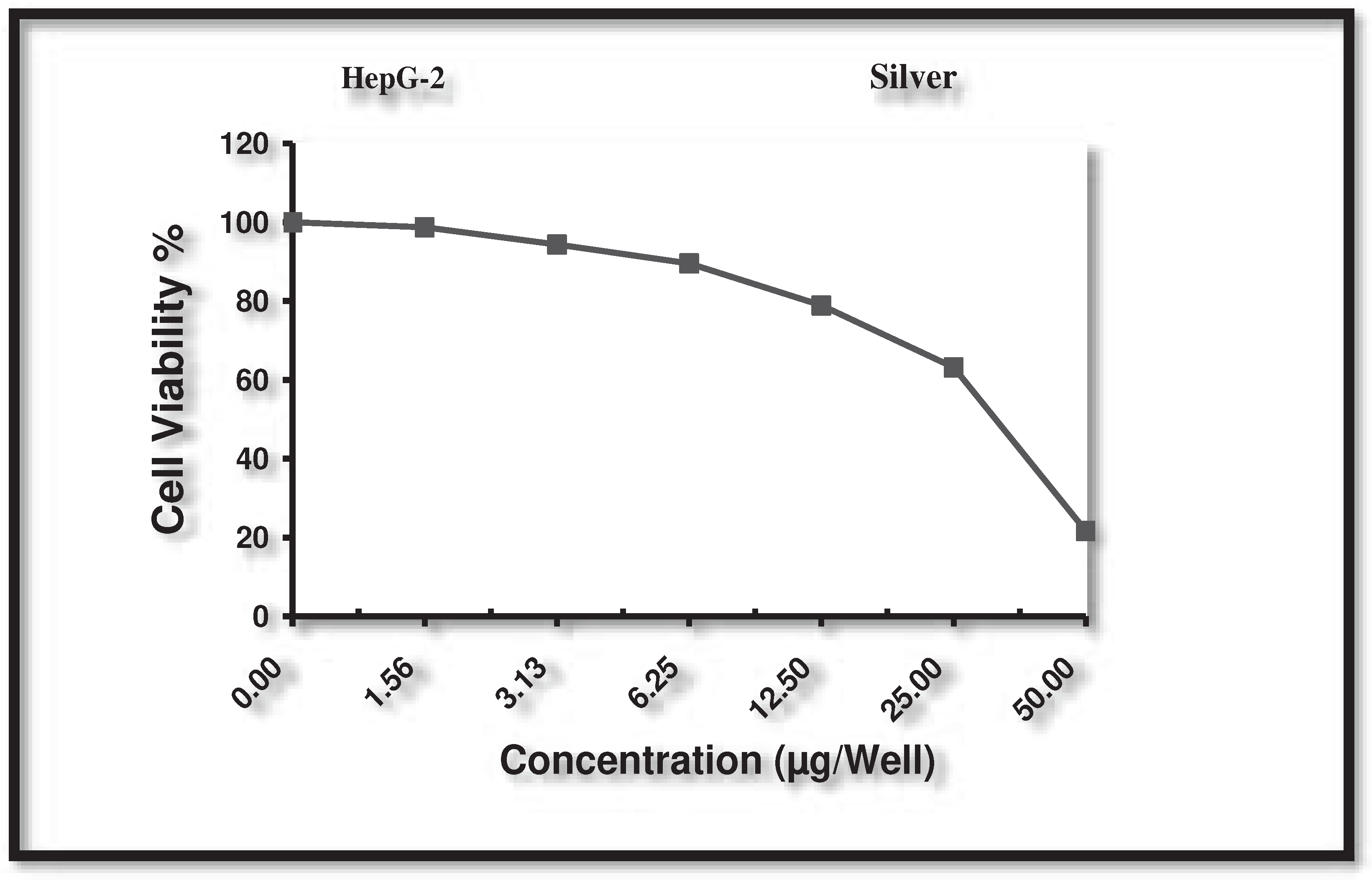
3.10. Cytotoxic activity against human liver cancer cell (HepG-2)
3.11. Wound Healing Assay
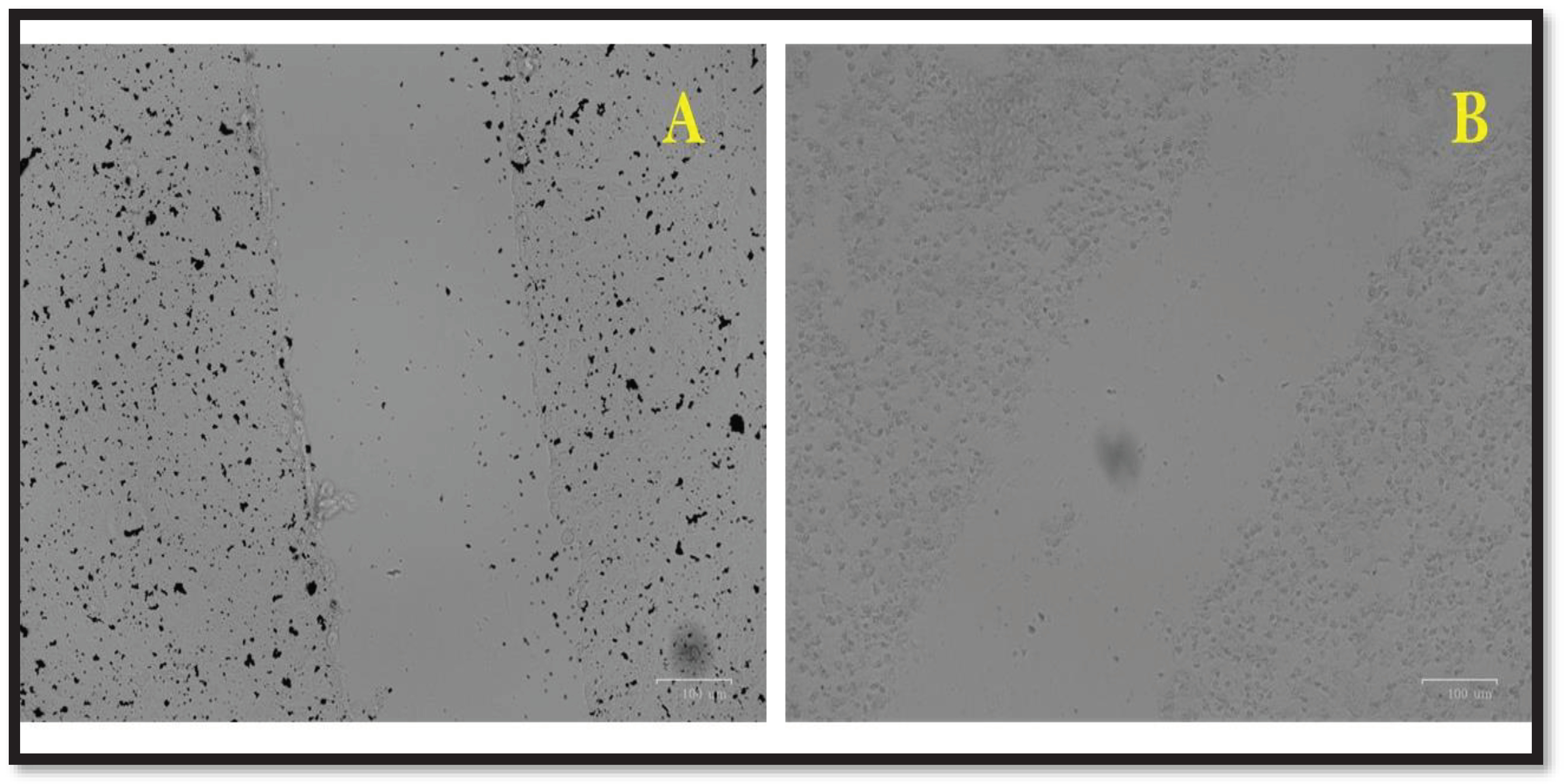
4. Discussion
5. Conclusions
Acknowledgments
References
- Grossart H-P, Van den Wyngaert S, Kagami M, Wurzbacher C, Cunliffe M, Rojas-Jimenez K: Fungi in aquatic ecosystems. Nature Reviews Microbiology 2019, 17(6):339-354. [CrossRef]
- Hyde KD, Jeewon R, Chen Y-J, Bhunjun CS, Calabon MS, Jiang H-B, Lin C-G, Norphanphoun C, Sysouphanthong P, Pem D: The numbers of fungi: is the descriptive curve flattening? Fungal Diversity 2020, 103:219-271. [CrossRef]
- Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai Y-C, Abd-Elsalam KA, Ertz D, Hidayat I: The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal diversity 2015, 74:3-18. [CrossRef]
- Deshmukh SK, Prakash V, Ranjan N: Marine fungi: A source of potential anticancer compounds. Frontiers in microbiology 2018, 8:2536. [CrossRef]
- Lal HM, Thomas S, Li T, Maria HJ: Polymer Nanocomposites Based on Silver Nanoparticles: Springer; 2021. [CrossRef]
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS: Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11(8):902. [CrossRef]
- De Silva C, Nawawi NM, Abd Karim MM, Abd Gani S, Masarudin MJ, Gunasekaran B, Ahmad SA: The mechanistic action of biosynthesised silver nanoparticles and its application in aquaculture and livestock industries. Animals 2021, 11(7):2097. [CrossRef]
- Xu L, Yi-Yi W, Huang J, Chun-Yuan C, Zhen-Xing W, Xie H: Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10(20):8996. [CrossRef]
- Bamal D, Singh A, Chaudhary G, Kumar M, Singh M, Rani N, Mundlia P, Sehrawat AR: Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review. Nanomaterials 2021, 11(8):2086. [CrossRef]
- Mistry H, Thakor R, Patil C, Trivedi J, Bariya H: Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnology Letters 2021, 43:307-316. [CrossRef]
- Nasrollahzadeh M, Sajadi SM, Issaabadi Z, Sajjadi M: Biological sources used in green nanotechnology. In: Interface science and technology. vol. 28: Elsevier; 2019: 81-111. [CrossRef]
- Abdel-Hameed SM, Abd Allah NA, Hamed MM, Soltan OI: Papaya fruit by-products as novel food ingredients in cupcakes. Annals of Agricultural Sciences 2023, 68(1):60-74. [CrossRef]
- Gaddeyya G, Niharika PS, Bharathi P, Kumar PR: Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Advances in Applied Science Research 2012, 3(4):2020-2026.
- Alsohaili SA, Bani-Hasan BM: Morphological and molecular identification of fungi isolated from different environmental sources in the Northern Eastern desert of Jordan. Jordan Journal of Biological Sciences 2018, 11(3).
- Pallavi S, Rudayni HA, Bepari A, Niazi SK, Nayaka S: Green synthesis of Silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saudi Journal of Biological Sciences 2022, 29(1):228-238. [CrossRef]
- Verma VC, Kharwar RN, Gange AC: Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 2010, 5(1):33-40. [CrossRef]
- Mistry H, Thakor R, Patil C, Trivedi J, Bariya H: Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnology Letters 2021, 43(1):307-316. [CrossRef]
- Bao Z, Ikunaga Y, Matsushita Y, Morimoto S, Takada-Hoshino Y, Okada H, Oba H, Takemoto S, Niwa S, Ohigashi K: Combined analyses of bacterial, fungal and nematode communities in andosolic agricultural soils in Japan. Microbes and environments 2012, 27(1):72-79. [CrossRef]
- Hulikere MM, Joshi CG: Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process biochemistry 2019, 82:199-204. [CrossRef]
- Hashem AH, Khalil AMA, Reyad AM, Salem SS: Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biological trace element research 2021:1-11. [CrossRef]
- Hashem AH, Saied E, Amin BH, Alotibi FO, Al-Askar AA, Arishi AA, Elkady FM, Elbahnasawy MA: Antifungal activity of biosynthesized silver nanoparticles (AgNPs) against aspergilli causing aspergillosis: Ultrastructure Study. Journal of Functional Biomaterials 2022, 13(4):242. [CrossRef]
- Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B: Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Materials Science and Engineering: C 2016, 58:44-52. [CrossRef]
- Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, Mohanta TK: Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Frontiers in molecular biosciences 2017, 4:14. [CrossRef]
- Rahman S, Rahman L, Khalil AT, Ali N, Zia D, Ali M, Shinwari ZK: Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Applied microbiology and biotechnology 2019, 103:2551-2569. [CrossRef]
- Gezaf SA, Hamedo HA, Ibrahim AA, Mossa MI: Mycosynthesis of silver nanoparticles by endophytic Fungi: Mechanism, characterization techniques and their applications. Microbial Biosystems 2022, 7(2):48-65. [CrossRef]
- Sandhu SS, Shukla H, Shukla S: Biosynthesis of silver nanoparticles by endophytic fungi: Its mechanism, characterization techniques and antimicrobial potential. African Journal of Biotechnology 2017, 16(14):683-698.
- Guilger-Casagrande M, Lima Rd: Synthesis of silver nanoparticles mediated by fungi: a review. Frontiers in bioengineering and biotechnology 2019, 7:287. [CrossRef]
- Khan N, Khan M, Jameel J, Jameel N, Rheman S: An overview: biological organisms that serves as nanofactories for metallic nanoparticles synthesis and fungi being the most appropriate. Bioceram Dev Appl 2017, 7(101):1-4. [CrossRef]
- Li G, He D, Qian Y, Guan B, Gao S, Cui Y, Yokoyama K, Wang L: Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. International journal of molecular sciences 2011, 13(1):466-476. [CrossRef]
- Alghuthaymi MA, Almoammar H, Rai M, Said-Galiev E, Abd-Elsalam KA: Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnology & Biotechnological Equipment 2015, 29(2):221-236. [CrossRef]
- Rabab M, Raida E, Awatif A: Biosynthesis and characterization of silver nanoparticles using Trichoderm longibrachiatum and their effect on phytopathogenic fungi. Egyptian Journal of Biological Pest Control 2018, 28:28. [CrossRef]
- Elamawi RM, Al-Harbi RE, Hendi AA: Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egyptian journal of biological pest control 2018, 28(1):1-11. [CrossRef]
- Gudikandula K, Vadapally P, Charya MS: Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2:64-78. [CrossRef]
- Nithya R, Ragunathan R: Synthesis of silver nanoparticles using a probiotic microbe and its antibacterial effect against multidrug resistant bacteria. African Journal of Biotechnology 2012, 11(49):11013-11021. [CrossRef]
- Nithya R, Ragunathan R: In vitro synthesis, characterization and medical application of silver nanoparticle by using a lower fungi. Middle-East J Sci Res 2014, 21(6):922-928. [CrossRef]
- San Chan Y, Don MM: Biosynthesis and structural characterization of Ag nanoparticles from white rot fungi. Materials Science and Engineering: C 2013, 33(1):282-288. [CrossRef]
- Balakumaran M, Ramachandran R, Balashanmugam P, Mukeshkumar D, Kalaichelvan P: Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiological research 2016, 182:8-20. [CrossRef]
- Ammar H, El-Desouky T: Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. Journal of Applied Microbiology 2016, 121(1):89-100. [CrossRef]
- Lotfy WA, Alkersh BM, Sabry SA, Ghozlan HA: Biosynthesis of silver nanoparticles by Aspergillus terreus: characterization, optimization, and biological activities. Frontiers in bioengineering and biotechnology 2021, 9:633468. [CrossRef]
- Rai R, Vishwanathan A, Vijayakumar B: Antibacterial Potential of Silver Nanoparticles Synthesized Using Aspergillus hortai. BioNanoScience 2023, 13(1):203-211. [CrossRef]
- Wang D, Xue B, Wang L, Zhang Y, Liu L, Zhou Y: Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Scientific reports 2021, 11(1):10356. [CrossRef]
- Gopa DR, Pullapukuri K: Green synthesis of silver nanoparticles from Aspergillus flavus and their antibacterial performance. Chemical Product and Process Modeling 2023. [CrossRef]
- Al-Zubaidi S, Al-Ayafi A, Abdelkader H: Biosynthesis, characterization and antifungal activity of silver nanoparticles by Aspergillus niger isolate. Journal of Nanotechnology Research 2019, 1(1):23-36. [CrossRef]
- Al-Soub A, Khleifat K, Al-Tarawneh A, Al-Limoun M, Alfarrayeh I, Al Sarayreh A, Al Qaisi Y, Qaralleh H, Alqaraleh M, Albashaireh A: Silver nanoparticles biosynthesis using an airborne fungal isolate, Aspergillus flavus: optimization, characterization and antibacterial activity. Iranian Journal of Microbiology 2022, 14(4):518-528. [CrossRef]
- Bruna T, Maldonado-Bravo F, Jara P, Caro N: Silver nanoparticles and their antibacterial applications. International Journal of Molecular Sciences 2021, 22(13):7202. [CrossRef]
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ: The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16(10):2346. [CrossRef]
- Logeswari P, Silambarasan S, Abraham J: Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. Journal of Saudi Chemical Society 2015, 19(3):311-317. [CrossRef]
- Silambarasan S, Abraham J: Biosynthesis of silver nanoparticles. African Journal of Biotechnology 2013, 12(21).
- Saravanan M, Arokiyaraj S, Lakshmi T, Pugazhendhi A: Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microbial pathogenesis 2018, 117:68-72. [CrossRef]
- Narasimha G, Praveen B, Mallikarjuna K, DEVA PRB: Mushrooms (Agaricus bisporus) mediated biosynthesis of sliver nanoparticles, characterization and their antimicrobial activity. 2011.
- Fouda A, Awad MA, Al-Faifi ZE, Gad ME, Al-Khalaf AA, Yahya R, Hamza MF: Aspergillus flavus-mediated green synthesis of silver nanoparticles and evaluation of their antibacterial, anti-candida, acaricides, and photocatalytic activities. Catalysts 2022, 12(5):462. [CrossRef]
- Akpinar I, Unal M, Sar T: Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Applied Sciences 2021, 3(4):506. [CrossRef]
- Narware J, Yadav R, Keswani C, Singh S, Singh H: Silver nanoparticle-based biopesticides for phytopathogens: Scope and potential in agriculture. In: Nano-biopesticides today and future perspectives. Elsevier; 2019: 303-314.
- Elbahnasawy MA, Shehabeldine AM, Khattab AM, Amin BH, Hashem AH: Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. Journal of Drug Delivery Science and Technology 2021, 62:102401. [CrossRef]
- Ajaz S, Ahmed T, Shahid M, Noman M, Shah AA, Mehmood MA, Abbas A, Cheema AI, Iqbal MZ, Li B: Bioinspired green synthesis of silver nanoparticles by using a native Bacillus sp. strain AW1-2: Characterization and antifungal activity against Colletotrichum falcatum Went. Enzyme and microbial technology 2021, 144:109745. [CrossRef]
- Khalil NM, Abd El-Ghany MN, Rodríguez-Couto S: Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218:477-486. [CrossRef]
- Asghar MA, Zahir E, Asghar MA, Iqbal J, Rehman AA: Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: as an effective antimicrobial and aflatoxin B1 adsorption agents. PloS one 2020, 15(7):e0234964. [CrossRef]
- Keshari AK, Srivastava R, Singh P, Yadav VB, Nath G: Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. Journal of Ayurveda and integrative medicine 2020, 11(1):37-44. [CrossRef]
- Sulaiman GM, Hussien HT, Saleem MM: Biosynthesis of silver nanoparticles synthesized by Aspergillus flavus and their antioxidant, antimicrobial and cytotoxicity properties. Bulletin of Materials Science 2015, 38:639-644. [CrossRef]
- Garg H: An approach for solving constrained reliability-redundancy allocation problems using cuckoo search algorithm. Beni-Suef University Journal of Basic and Applied Sciences 2015, 4(1):14-25. [CrossRef]
- Chi NTL, Veeraragavan GR, Brindhadevi K, Chinnathambi A, Salmen SH, Alharbi SA, Krishnan R, Pugazhendhi A: Fungi fabrication, characterization, and anticancer activity of silver nanoparticles using metals resistant Aspergillus niger. Environmental Research 2022, 208:112721. [CrossRef]
- Liu X, Chen J-L, Yang W-Y, Qian Y-C, Pan J-Y, Zhu C-N, Liu L, Ou W-B, Zhao H-X, Zhang D-P: Biosynthesis of silver nanoparticles with antimicrobial and anticancer properties using two novel yeasts. Scientific Reports 2021, 11(1):1-12. [CrossRef]
- Kumari RM, Kumar V, Kumar M, Pareek N, Nimesh S: Assessment of antibacterial and anticancer capability of silver nanoparticles extracellularly biosynthesized using Aspergillus terreus. Nano Express 2020, 1(3):030011. [CrossRef]
- Waseem M, Nisar MA: Fungal-derived nanoparticles as novel antimicrobial and anticancer agents. In: Functionalized Nanomaterials. IntechOpen; 2016.
- Al-saggaf MS: Formulation of insect chitosan stabilized silver nanoparticles with propolis extract as potent antimicrobial and wound healing composites. International Journal of Polymer Science 2021, 2021:1-9. [CrossRef]
- Paladini F, Pollini M: Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials 2019, 12(16):2540. [CrossRef]
- Chinnasamy G, Chandrasekharan S, Koh TW, Bhatnagar S: Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Frontiers in microbiology 2021, 12:611560. [CrossRef]
- Bold B-E, Urnukhsaikhan E, Mishig-Ochir T: Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Frontiers in Chemistry 2022, 10. [CrossRef]
| Pathogenic organism | CF | AgNo3 | Ag-NPs | Ampicillin (1 mg/ml) |
|---|---|---|---|---|
| Bacillus subtillus ATCC 6633 | 0.0 | 10 ± 0.01a | 28 ± 0.25b | 44 ± 0.01a |
| Staphylococcus aureus ATCC 25923 | 0.0 | 6 ± 0.21b | 30 ± 0.2a | 40 ± 0.01a |
| Bacillus cereus | 0.0 | 6 ± 0.01a | 26 ± 0.01b | 40 ± 0.01a |
| Pseudomonas aeruginosa ATCC 9027 | 0.0 | 8 ± 0.5a | 22 ± 0.2b | 40 ± 0.01a |
| Salmonella typhimurium ATCC 14028 | 0.0 | 6 ± 0.02a | 14 ± 0.1a | 42 ± 0.01a |
| Pseudomonas fluorescens DSM 50090 | 0.0 | 12 ± 0.1a | 30 ± 0.05a | 40 ± 0.01a |
| Aeromonas hydrophila NRRL 914 | 0.0 | 4 ± 0.1a | 14 ± 0.2a | 40 ± 0.01a |
| Pathogenic fungi | AgNo3 | Ag-NPs | Nystatin |
|---|---|---|---|
| A. fumigatus (NIOF-F3) | 8 ± 0.01a | 16 ± 0.12b | 0.0 |
| A. niger (NIOF-F8) | 4 ± 0.21b | 22 ± 0.1a | 0.0 |
| A. flavus (NIOF-F12) | 0.0 | 20 ± 0.02b | 0.0 |
| A. terreus (NIOF-F13) | 0.0 | 18 ± 0.01b | 0.0 |
| A. parasiticus (NIOF-F15) | 8 ± 0.02a | 16 ± 0.02b | 0.0 |
| P. oxalicum (NIOF-F22) | 10 ± 0.1a | 14 ± 0.04a | 0.0 |
| F. solani (NIOF-F48) | 4 ± 0.1b | 16 ± 0.1b | 0.0 |
| F. oxysporum (NIOF-F63) | 6 ± 0.1a | 20 ± 0.1a | 0.0 |
| C. albicans (NIOF-F71) | 10 ± 0.1a | 18 ± 0.2a | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





