INTRODUCTION
Timor-Leste is one of the youngest countries in the world after got its freedom totally under United Nation control in 2002. The country occupying the eastern half of Timor island with total population of 1.341,926 in 2022 (Rangel, G., et al., 2022).
The country has 12 municipalities and 1 special autonomous region, 65 administrative post, 442 villages and 2.225 hamlets. The country compromised of 13 municipalities, 1 autonomous special region, 65 administrative posts, 442 villages and 2.225 hamlets. The extent of the territory is 14.874 square kilometres (Melissa, B. et al., 2023). It has 4 distinct spoken languages such as Portuguese and Tetum are as national languages, English and Bahasa Indonesia are working languages (Rangel, G et al, 2022. P.1).
Timor-Leste is 22 years of age in 2024. The country has a poor road, limited clean water, limited qualified infrastructures for government institutions in municipality level and also administrative posts area. Minimum education infrastructure to guarantee qualified education system in rural area, lack of agriculture facilities to improve and modernize agriculture system to produce rice, corn and other local products, limited local tourism places and hotels to accommodate tourists and as well as health system coverage in the country (Rangel, G, et al, 2023. P.2). However, in this work, the authors only focus to describe malaria microscopy validations process especially quality assurance of malaria microscopy in Timor-Leste.
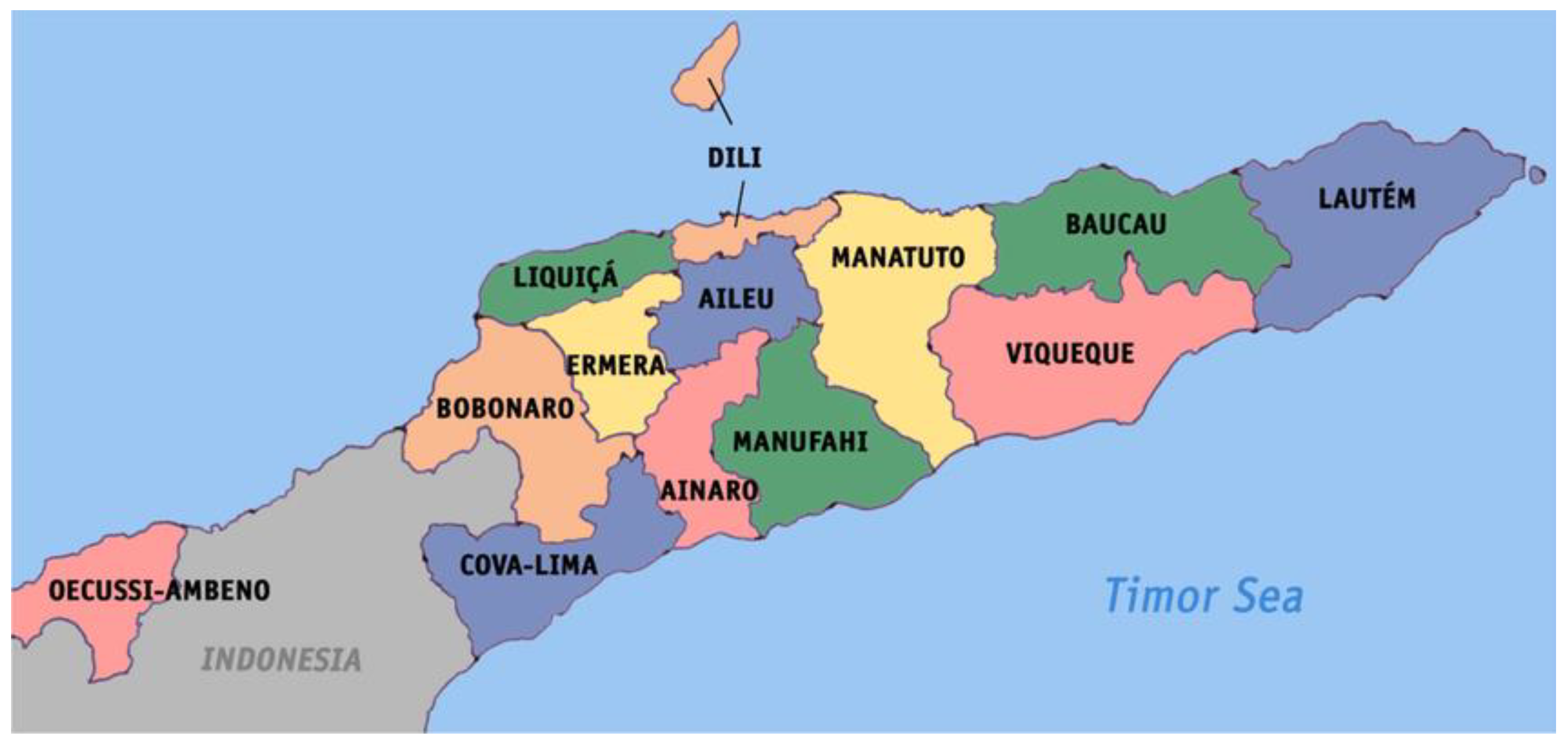
Figure 1.
Map of Timor-Leste (Source Wikipedia).
Figure 1.
Map of Timor-Leste (Source Wikipedia).
The health care system in Timor-Leste still low level if compared with other southeast Asian country. There is one national hospital situated in Capital Dili, one regional hospital located in Baucau Municipality, three referral hospital which placed in border areas with Indonesia such as Suai Referral hospital, Maliana Referral hospital, and Oe-cusse referral hospital. There are 70 community health centres and 442 health posts (Timor-Leste National Health Sector Strategic Plan [NHSSP], 2020. P.135-136).
The health situation in the country still in process of developing and far from flawless because of lack of human resources, infrastructure and medical instruments which affecting inaccurate treatment. This was occurred due to lack of trained laboratory technicians to diagnose various communicable disease in the country. As a result, the mortality rate of pregnant women, children under 5 years and other age of group were high due to limited qualified health treatment in the country. The high number cases of communicable diseases such as tuberculosis, diarrheal diseases and increasing number of non-communicable diseases (WHO, 2022). Malaria was as a main issue in Timor-Leste among other top 10 diseases for 10 years ago. The clinically treated cases of malaria in 2006 was 185,106, confirmed case 37,896, plasmodium falciparum was 24,219 cases, plasmodium vivax was 13,477 cases and mixed infection was 200 cases. From these all malaria cases, 58 patients were died. The last indigenous case was reported in June 2017 and imported cases were reported in 2018 (Mannel, Y, et al 2020. P.2).
The QA of malaria microscopy is a system used to improve the efficiency and cost-effectiveness and accuracy test. In this case, laboratory diagnosis of malaria should be based on clinical symptoms, an accurate sample collection and correct laboratory diagnosis to avoid false positive and also false negative (WHO, 2009. P.1). To respond to the requirement, the quality assurance malaria microscopy at the National Health Laboratory was established by national malaria control program (NMCP), Ministry of Health (MoH) in 2009 due to lack of trained malaria microscopy technicians to work and guarantee the implementation of daily malaria microscopy examination, sending malaria blood smear for validation (Maguire et al, 2006. P.2) at the quality assurance of malaria microscopy and other important work-related malaria microscopy. There are few trained malaria microscopies who are certified through World Health Organization program “external competence assessment” to guarantee the quality of malaria microscopy validators at the national level by helping with an international laboratory technician that hired by NMCP. As a result, most of Timorese laboratory technicians who were classified as qualified validators (Huber, L. 2007. P.1) to validate malaria blood smears, facilitate malaria microscopy diagnosis training, supervision and monitoring, implementation of therapeutic efficacy studies efficacy of drug resistance such as chloroquine for plasmodium vivax, and plasmodium falciparum for the drug of artemether/lumefantrine. The therapeutic study efficacy was implemented in 2012-2013.
THE QUALITY ASURANCE (QA) OF MALARIA MICROSCOPY PROCESS
The Management of Quality Assurance Malaria Microscopy
The QA of malaria microscopy unit, national health laboratory, in Timor-Leste is started from 2009 up to now. To guarantee the implementation of QA of malaria microscopy unit in the country, national malaria control program (NMCP) had been elaborated annual action plan for supplying and procurement for laboratory instrument including microscope, staining racks, box slides. Moreover, allocated also fund for supplying laboratory consumable, durables and room expansion to accommodate national malaria validators. Furthermore, NMCP also allocated more fund for malaria microscopy capacity building, supervision and monitoring program annually (Luckett, R., Mugizi, R., Lopes, S., Etossi, R. C., & Allan, R. 2016. P.2).
To recognize the effort and quality of national validators, NMCP had been hired one international laboratory technician to assist national QA validators to validate all malaria blood smears in the country after crosschecked by national validators. To proof the capacity and progress of the national malaria microscopy validators, NMCP with all effort to hire an international malaria microscopy expert to realize malaria microscopy external competency assessment on malaria microscopy. National validators who are participating in the external competency assessment of malaria microscopy are national malaria microscopy validators and all senior analyst in the country. This was realized as a part of human resources development in malaria microscopy.
On the other hand, NMCP allocates also budget for monitoring and supervision to guarantee regular implementation of malaria microscopy diagnosis in all health facilities start from community health centres (CHC) without bed at administrative posts level, CHCs with bed at municipality level, referral hospitals and national hospital. The QA malaria microscopy validation process is a blinded system, which all blood smears can be validated without knowing where the malaria slides came from, whose slide and from which municipality. All malaria blood smears were sent from all municipality level and referral hospitals after collected by senior analyst and sent it regularly every month to the QA malaria microscopy unit for further QA validation. The feedback of the validation provided back to the relevant health facility for further improvement with highlighted recommendation.
The QA of malaria microscopy validators
The QA malaria microscopy unit validators are qualified laboratory technicians who have attended various capacity building overseas which focus on malaria parasites identification, parasite counting, microscopy maintenance, giemsa stain control, capacity building program certification, monitoring and supervision, reporting and archiving. The malaria microscopy validators are working as team to guarantee quality work of validation, training programs, supervision and monitoring programs.
Senior analyst at the municipality level responsible for the malaria blood smear collection and sent it to the national malaria QA unit for validation process. The QA in malaria consist of pre-analytical, analytical and post analytical process. In pre-analytical process, QA malaria team need to evaluate the request format test of malaria daily, sample collection, materials preparation, labelling, giemsa stain preparation, staining and washing. Analytical process includes malaria blood smears diagnosis or specimen testing. To proof the correct diagnosis, NMCP also had been provided immunochromatography test method to compare with microscopy detection. If the Rapid diagnostic test was indicated positive, laboratory technicians continue to confirm the existence of parasitaemia level in the microscopy. Post-analytical process which include reporting test results, interpretation, follow up storage and retesting or re-examine if needed.
During the supervision, the malaria QA validators supervise and monitor the quality of malaria blood smears, giemsa stain quality, methanol, crosschecking malaria blood smear, maintenance of microscope (World Health Organization. 2016. P. 1), stock of consumables and other relevant necessities at the health facilities level. The QA malaria validators at national level who are responsible for monitoring, supervision, capacity building and other relevant activities that described in the competences, role and responsibilities as national validators at national level.
The quality assurance of malaria microscopy process
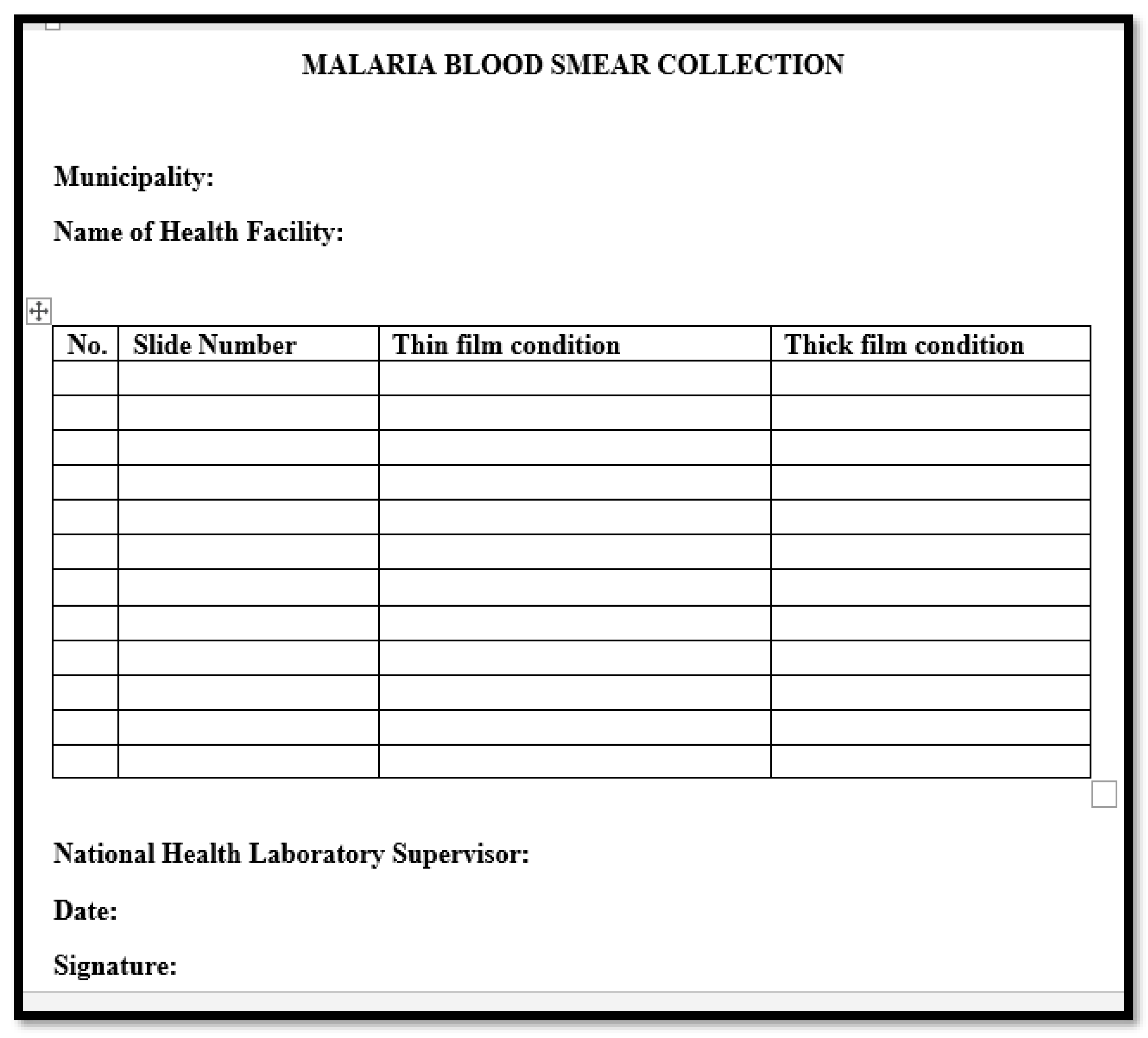
National malaria quality assurance unit had been established monthly sending format in both English and Tetum to collect malaria blood smears and sent it to National Health Laboratory for further validation or crosschecking. The malaria slides sending format as indicated in the
Figure 2. The form used to collect malaria blood smears.
Various malaria cases had been tested and validated such plasmodium falciparum; plasmodium vivax due to only these two malaria plasmodium species exist in Timor-Leste. For the emergency case response, NMCP also had been recruited and collocated malaria assistant in all health facilities from CHC without bed up to national hospital in the country. List of malaria cases had ever been tested and validated as displayed in the
Figure 3.
The malaria cases when reported from all over health facilities in the country, national malaria officer, municipality malaria officer and national malaria quality assurance validators need to go to relevant area for further investigation. The action that needed was investigated the malaria patients during 7-14 days ago if went to some endemic area and other relevant information need to answered by malaria patients. Meanwhile, national malaria validator needs to confirm or validate malaria smears for further treatment.
The importance of quality assurance of malaria microscopy
The QA malaria microscopy unit was established to respond malaria clinical treatment in Timor-Leste. Many malaria clinical cases were treated with malaria antibiotics without laboratory diagnosis results. Therefore, NMCP was established national malaria QA unit at National Health Laboratory to guarantee capacity building for all laboratory technicians in the country, validation malaria blood smears after received from municipality level, monitoring and supervision. As a result, since QA malaria unit established in Timor-Leste, no more treatment clinical malaria cases without laboratory diagnosis. To guarantee the accurate diagnosis, all malaria slides collected and sent to QA malaria microscopy unit for further validation.
The role of QA malaria microscopy
The clinical symptoms of malaria quite similar with other infectious disease such as COVID-19 (Rangel, G., Pamoket, P., Fucharoen, S., & Wanram, S. 2023. P.1), diarrheal and TBC. The clinical symptoms of malaria include headache, cold, vomiting and feel uncomfortable. Therefore, laboratory diagnosis (Bailey, J. W., Williams, J., Bain, B. J., Parker-Williams, J., Chiodini, P. L., & General Haematology Task Force of the British Committee for Standards in Haematology. 2013. P.1) is required to confirm the existence of malaria parasites or the clinical symptoms caused from other infectious diseases. In this case, laboratory technicians play an extremely critical role during serving patients at emergency unit and all health facilities in the country. Available immunochromatography methods (Garcia, M., Kirimoama, S., Marlborough, D., Leafasia, J., & Rieckmann, K. H. 1996) and giemsa stain method (Sathpathi, S., Mohanty, A. K., Satpathi, P., Mishra, S. K., Behera, P. K., Patel, G., & Dondorp, A. M. 2014. P. 2) to diagnose malaria cases in the country.
CHALLENGES AND OPPORTUNITEIS ENCOUNTERED BY QA MALARIA MICROSCOPY
Current Opportunities
The QA malaria microscopy hopes to improve the capacity of detection of malaria cases in the future. The QA malaria unit, National Health laboratory expects to recruit more malaria experts who have great knowledge to work on malaria polymerase chain reaction (PCR) confirmation after malaria blood smear detection, allocated more budget to buy PCR machines and other important necessities therefore QA malaria team can work on it by themselves. The QA malaria team have covering all health facilities around the country since 2009 up to date with the purpose to guarantee laboratory diagnosis on malaria with confident and qualified.
The Referral Hospital directors and municipality directors are supporting the existence of QA malaria microscopy team through deliver information to the head of department and director at the referral hospitals and community health centres (CHCs) managers to cooperate with QA malaria team during monitoring and supervision at laboratory section. The CHCs and all private clinics in the country all well cooperate with QA malaria team in combating malaria cases in Timor-Leste since its establishment.
Challenges encountered
The QA malaria team is facing some internal and external challenges:
Firstly, unavailable post graduate workers in QA malaria unit, most of workers are permanent staffs and basic diploma in medical laboratory holders. Secondly, unavailable allocated budget for annual evaluation mainly laboratory section. Thirdly, inadequate infrastructures for the QA malaria unit at the national to have PCR confirmation test in the future. Fourthly, unavailable budget allocation for QA malaria unit, therefore, the unit still funded by Global fund through national malaria program.
CONCLUSION
In the summary, various points highlighted by authors as follow (1) QA malaria unit has no post graduate workers, unallocated budget and inadequate infrastructure for molecular test confirmation, (2) In order to achieve malaria elimination cases in the country, QA malaria need more budget allocation, fuel, vehicle and other important logistic need to guarantee any new cases of malaria imported into Timor-Leste (3) It concludes that various malaria cases had been successfully tested, treated and under prevention and control since national malaria control program established (4) The study indicated that some challenges that faced by QA malaria unit is still inadequate infrastructures, post graduate health workers and inadequate funds to cover all QA malaria unit necessities, currently.
Biography of principal investigator

Gregorio Rangel is one civil servant of National Health Laboratory, Ministry of Health, Timor-Leste. He was completed his post graduate diploma in applied parasitology and entomology at institute for medical research (IMR), Kuala Lumpur, Malaysia in 2014. He was concluded his master degree in Biomedical Science in Ubon Ratchathani University, Thailand in 2018. In government institutions, He was work with National Malaria Program, Ministry of Health from 2009-2013 and National Health Laboratory from 2013-2021.
In partnership development, He has been worked as national consultant for World Health Organization Timor-Leste from November – December 2020 with project “Infection Prevention and Control”. He has also been worked as national consultant for World Health Organization Timor-Leste from September 2021-July 2022, project entitled “Essential Health Services during COVID-19 period”. He has been also worked as national assessor for National Agency for Academic Assessment and Accreditation (NAAAA) from July-September 2022.
He is a young scientist in medical laboratory. He has contributed various research and publication in biomedical science. Currently, he is working as head of department (equivalent national director) of exacts and natural science, National Institute for science and technology, Timor-Leste. He also a full-time lecturer at department of Biomedical Laboratory Science, Dili Health Science Institute, Timor-Leste. Meanwhile, he is working as principal investigator in a collaborative study between Menzies School of Health Research in Timor-Leste, Ministry of Health and Dili Health Science Institute with project entitled” The Use of Highly Sensitive Diagnostic and Typing/Sequencing Technology in the Pursuit of Malaria Elimination in Timor-Leste”. From March-July 2023, he is working as national consultant for WHO country office to run a project entitle: Situational analysis of health ageing in Timor-Leste in 2023.
References
- Timor-Leste population, 2022: Census Uma Kain 2022. [online] available in https://www.statistics.gov.tl/2015-Timor-Leste-population-and-housing-census-data-sheet/. Accessed in May 21, 2023.
- Gregorio Rangel et al 2022: Assessment of human resources in medical laboratory in Timor-Leste [online] available in Revista Ciencia e Tecnologia. 2022 p.1-7. https://www.researchgate.net/profile/Gregorio-Rangel/publication/366425791_Revista_de_Ciencias_e_Technologia_de_Timor-Leste_ORIGINAL_ARTICLE_ASSESSMENT_OF_HUMAN_RESOURCES_IN_MEDICAL_LABORATORY_SCIENCES_IN_TIMOR-LESTE/links/63a1706940358f78eb04230b/Revista-de-Ciencias-e-Technologia-de-Timor-Leste-ORIGINAL-ARTICLE-ASSESSMENT-OF-HUMAN-RESOURCES-IN-MEDICAL-LABORATORY-SCIENCES-IN-TIMOR-LESTE.pdf. Accessed in May 20, 2023.
- Melissa, B, Rangel, G., Sarakbi, D., et al (2023). Country Learning on Maintaining Quality Essential Health Services (EHS) during COVID-19 in Timor-Leste: A mixed methods qualitative analysis. [online] available in https://www.scienceopen.com/document_file/fbf90151-a13d-4a54-b444-6280888b303e/PubMedCentral/fbf90151-a13d-4a54-b444-6280888b303e.pdf. Accessed in July 18, 2023.
- Timor-Leste National Health Sector, 2015: National Health Sector Plan. [online] available in https://cdn.who.int/media/docs/default-source/searo/timor-leste/national-health-sector-plan.pdf?sfvrsn=70870918_2. Accessed in 20 May 2023.
- WHO. Timor Leste Country Profile. Geneva, World Health Organization. https://www.who.int/countries/tls/en/. Accessed on 20 July 2023.
- WHO, 2009. Malaria Microscopy quality assurance manual version 1 [online] available in…… Accessed on 21 July 2023.
- Maguire et al, 2006: “Production and validation of durable, high quality standardized malaria microscopy slides for teaching, testing and quality assurance during an era of declining diagnostic proficiency”. [online] available in https://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-5-92. Accessed 16 November 2023.
- Huber, L. (2007). Validation and qualification in analytical laboratories. CrC Press. [Online] available in https://www.taylorfrancis.com/books/mono/10.3109/9780849382680/validation-qualification-analytical-laboratories-ludwig-huber. Accessed November 23, 2023.
- Luckett, R., Mugizi, R., Lopes, S., Etossi, R. C., & Allan, R. (2016). The role of laboratory supervision in improving the quality of malaria diagnosis: a pilot study in Huambo, Angola. The American Journal of Tropical Medicine and Hygiene, 94(3), 659. [Online] available in https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4775904/. Accessed on November 23, 2023.
- World Health Organization. (2016). Use, care and maintenance of microscopes (No. WHO/HTM/GMP/MM/SOP/2016.12). World Health Organization. [Online] available in https://iris.who.int/bitstream/handle/10665/340472/WHO-HTM-GMP-MM-SOP-2016.12-eng.pdf. Accessed on November 23, 2023.
- Rangel, G., Pamoket, P., Fucharoen, S., & Wanram, S. (2023). Bioinformatics Analysis of Significant Host Im-mune Response Genes as Potential Biomarkers in COV-ID-19 Infection. J Gastro Hepato, 9(20), 1-5. [Online] available in https://jajgastrohepto.org/wp-content/uploads/2023/05/JJGH-v9-1852.pdf. Accessed on November 23, 2023.
- Bailey, J. W., Williams, J., Bain, B. J., Parker-Williams, J., Chiodini, P. L., & General Haematology Task Force of the British Committee for Standards in Haematology. (2013). Guideline: the laboratory diagnosis of malaria. British journal of haematology, 163(5), 573-580. [Online] available in https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.12572. Accessed on November 23, 2023.
- Garcia, M., Kirimoama, S., Marlborough, D., Leafasia, J., & Rieckmann, K. H. (1996). Immunochromatographic test for malaria diagnosis. The Lancet, 347(9014), 1549. [online] available https://www.researchgate.net/profile/Karl-Rieckmann/publication/14517878_Immunochromatographic_test_for_malaria_diagnosis_Letter/links/5d2718f2458515c11c25df44/Immunochromatographic-test-for-malaria-diagnosis-Letter.pdf. Accessed on November 23, 2023.
- Sathpathi, S., Mohanty, A. K., Satpathi, P., Mishra, S. K., Behera, P. K., Patel, G., & Dondorp, A. M. (2014). Comparing Leishman and Giemsa staining for the assessment of peripheral blood smear preparations in a malaria-endemic region in India. Malaria journal, 13(1), 1-5. [online] available in https://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-13-512. Accessed on November 23, 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 Gregorio Rangel is one civil servant of National Health Laboratory, Ministry of Health, Timor-Leste. He was completed his post graduate diploma in applied parasitology and entomology at institute for medical research (IMR), Kuala Lumpur, Malaysia in 2014. He was concluded his master degree in Biomedical Science in Ubon Ratchathani University, Thailand in 2018. In government institutions, He was work with National Malaria Program, Ministry of Health from 2009-2013 and National Health Laboratory from 2013-2021.
Gregorio Rangel is one civil servant of National Health Laboratory, Ministry of Health, Timor-Leste. He was completed his post graduate diploma in applied parasitology and entomology at institute for medical research (IMR), Kuala Lumpur, Malaysia in 2014. He was concluded his master degree in Biomedical Science in Ubon Ratchathani University, Thailand in 2018. In government institutions, He was work with National Malaria Program, Ministry of Health from 2009-2013 and National Health Laboratory from 2013-2021.