Abbreviations: a*, green to red range intensity; b*, blue to yellow range intensity; CEC, cation exchange capacity; ECse, saturation extract electrical conductivity; Fv/Fm, ratio of variable to maximum chlorophyll fluorescence; GAE, gallic acid equivalent; IAD, index of absorbance difference; INM, integrated nutrient management; L*, lightness; MAPs, medicinal-aromatic plants; RUE, rutin equivalent; SAR, sodium absorption ratio
1. Introduction
Genomic approaches supported by molecular biology and bioinformatics are of critical importance to document and conserve biodiversity [
1]. DNA sequences used as “barcodes” to define genetically biological entities (species) represent a fast, low-cost, reliable, and uncomplicated solution for plant species identification, further allowing insight into their phylogenetic relations [
2,
3]. In various terrestrial medicinal-aromatic plants (MAPs), molecular barcoding has been widely applied, using short regions of nuclear or chloroplast DNA to genetically characterize and identify taxa at species or even at subspecies level [
4]. Previous studies in members of Lamiaceae (major family of MAPs worldwide) have used various molecular markers and DNA tools for successful identification of many members belonging to different genera of Lamiaceae with some genera receiving more attention than others due to complex taxonomical issues, such as members of the genus
Sideritis [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17].
The first molecular DNA study for
Sideritis taxa was initiated in 1999 using RAPDs for fingerprinting 15 plant populations of
S. pusilla (Lange) Pau and their putative parental species
S. hirsuta L. and
S. leucantha Cav. [
5], and the first DNA sequences submitted in GenBank were back in 2002 for the
trnL gene of cpDNA for five different
Sideritis species [
6]. Another significant phylogenetic study has deposited in GenBank datasets focused on 23 Macaronesian
Sideritis taxa (species and subspecies) using the ITS,
trnL and
trnT-
trnL molecular markers [
9], and more recently another 93 specimens of Greek
Sideritis taxa have been deposited for ITS,
psbA-
trnH (PopSet: 972386283),
rbcL (PopSet: 972385939) and
matK (PopSet: 972386103) molecular markers [
18]. The nuclear molecular marker ITS has been broadly used in such studies [
6,
14,
15,
17], whereas multiple regions from the cpDNA have also been used for species identification [
6,
10,
11,
12,
13,
16]. Many fingerprinting studies to date have employed genetic analyses using Amplified Fragment Length Polymorphisms (AFLPs) for several subpopulations of the local Greek endemic
S. euboea Heldr. [
8], Random Amplified Polymorphic DNA (RAPD) markers [
19,
20] and 12 URP to investigate inter-population genetic diversity of
S. raeseri Boiss. & Heldr. [
7].
Species-wise, this investigation was focused on a perennial local endemic plant of Crete (Greece), namely
S. syriaca L. subsp.
syriaca (Lamiaceae), commonly called in Crete ‘malotira’ (or Cretan Mountain tea). This taxon is wild-growing on rocky substrates at high altitudes (1000-2200 m) of the major mountain massifs of Crete, and flowers in June and July [
21]. Malotira represents a contemporary yet traditional herbal medicinal product which is widely used for tea preparation and as food ingredient [
22] with well-documented therapeutic indications [
23] approved by the European Medicines Agency (
https://www.ema.europa.eu/en/medicines/herbal/sideritis-herba, accessed 30 November 2023). Although it has been recently cultivated at small scale in Omalos plateau (Chania, Crete) as a valuable locally native MAP [
24], it is however threatened with extinction [
25] due to over-harvesting of sizeable volumes of plant material directly sourced from wild-growing populations of Lefka Ori in Chania, Crete (
https://flashnews.gr/post/694054/ena-ntokimanter-gia-tin-kritiki-malotira-eftase-mechri-ti-germania-vinteo/, accessed 30 November 2023); such harvested (or cultivated at lesser extent) material is traded in local markets of Crete and is also exported as a local MAP product [
26].
In many countries, traditional and complementary medicine is still a major health resource with herbal materials used for therapeutic purposes [
27,
28]. In China alone, ca. 40% of the administered medicines are of herbal origin [
29]. The latter, is also coupled with an emerging global trend of using MAPs and natural products in Western societies [
30]. Notably, several industrially produced (pharmaceutical) drugs are still based on MAPs [
28] and the market and public demand on a wide range of plant-derived chemicals (e.g., fragrances, cosmetics, color/ flavor additives, etc.) continues to rise [
29,
31,
32,
33]. In this context, the growing request for MAPs, however, inevitably imperil their wild-growing populations [
29,
34] like those of the threatened focal taxon in this study. It is known that unmonitored or poorly-controlled trade, coupled with unsuitable harvesting methods, and over-exploitation pressure on wild-growing populations may imperil several range-restricted species such as the local endemic
S. syriaca subsp.
syriaca [
17,
26] as well as may jeopardize the ecological equilibrium at habitat/ecosystem level [
34]. Nonetheless, introducing focal species of conservation concern and economic interest in agricultural settings such as the focal taxon herein can satisfy the growing demand for specific MAPs without further depleting wild-growing phytogenetic resources. To this end, applied research is needed such as documentation of origin and consolidated taxonomic identity of plant material used, species-specific propagation protocols, cultivation guidelines, and fertilization regimes, thus facilitating conservation actions and enabling sustainable exploitation in different economic sectors [
24,
35].
Cultivation-wise, any plant demand for nutrients is often conventionally met by chemical (inorganic) fertilizers. Accumulative evidence, however, suggests that over-application or inefficient utilization of chemical fertilizers is a major environmental pollution driver with cumulative alarms [
36,
37]. Typical examples include degradation of soil quality, and nutrient loading of water bodies with deleterious effects on aquatic ecosystems [
36,
38]. A more eco-friendly and more sustainable mode of satisfying plant nutrient needs is using organic fertilizers which produce a rather limited environmental impact compared to chemical ones [
39]. Fertilization management can be further improved by applying biostimulants [
40] which may increase root nutrient acquisition, enhancing in this way fertilization efficiency [
40]. In this perspective, the joint addition of chemical fertilizers, organic fertilizers and biostimulants (collectively termed as integrated nutrient management; INM) can potentially ensure plant growth and productivity at a lower environmental cost [
41,
42]. Such applied research lines have also been suggested as indispensable stepping stones for the sustainable exploitation and pilot cultivations of neglected and underutilized local Cretan endemic plants with potential economic interest [
24].
Market-wise, the herbal material quality is initially shaped by the fertilization scheme applied [
39] and includes a wide range of features, the relative importance of which is determined by the intended use [
32,
33]. The level of antioxidant compounds (often referred as antioxidants) is commonly an important index of plant material quality [
38,
43]. Antioxidants are widely recognized as potential health promoters, and they have well-documented preventative and therapeutic effects against several chronic diseases [
44,
45]. These include carotenoids, flavonoids, and phenolics, which are characterized by powerful antioxidant ability [
38], and the herein focal taxon has also rich content in such beneficial compounds [
23]. It has been suggested that organic fertilizers and integrated nutrient management (INM) strategies may stimulate the herbal material antioxidant profile more than chemical fertilizers [
44,
46,
47,
48,
49,
50]. Furthermore, as these strategies help in avoiding societal charges of nitrogen losses, the paybacks of their use might considerably shade their expenses, rendering them extremely suggested for incorporation in fertilization schemes [
51]. Such fertilization regimes have also been associated with enhanced chlorophyll content [
52]. Chlorophyll content determines leaf greenness, which is another measure of herbal material quality, being traditionally employed by processors, distributors, and consumers [
32].
Context-wise, the present investigation aimed to genetically characterize the local endemic
S. syriaca subsp.
syriaca (malotira or Cretan Mountain tea) using seven plastid molecular markers, four of which are employed for the first time in members of genus
Sideritis. The molecular markers selected as suitable plant DNA barcodes are widely used, are suggested by several studies and have been routinely tested in terms of amplification and sequencing success (universality), intra- / inter-specific variation, and resolving power [
3,
53,
54]. These new DNA sequences can be used for reliable taxonomic identification and traceability of commercial products of
Sideritis herba [
23], also supporting the genetic authentication and identity consolidation of related commercial products. Another aim of this study in response to absence of species-specific data was to comparatively investigate the effects of different fertilization treatments and application techniques in a pilot field cultivation of
S. syriaca subsp.
syriaca in Heraklion, Crete; the latter was used to define a species-specific fertilization protocol able to promote plant growth and/or key herbal quality features (i.e., colour intensity, antioxidant metabolite content) for sustainable and commercial exploitation.
2. Results
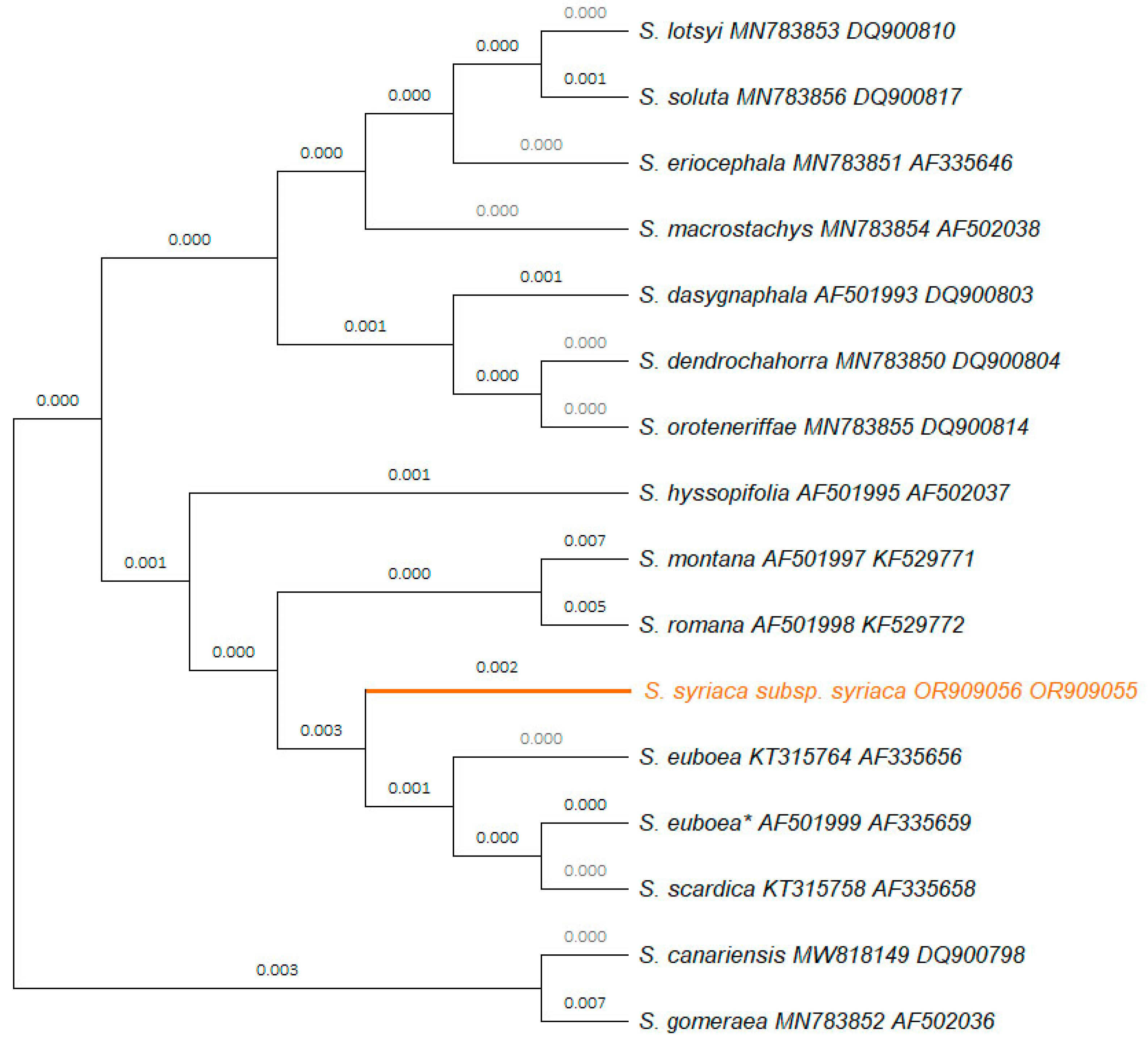
2.1. Molecular characterization of the studied Sideritis syriaca subsp. syriaca
The DNA sequences of S. syriaca subsp. syriaca GR-1-BBGK-15,5939 generated in the present study for the molecular markers petB/petD (999 bp), rbcL (718 bp), trnL/trnF (695 bp), rpoC1 (476 bp), psbA/trnH (534 bp), psbK/psbI (329 bp) and atpF/atpH (498 bp) were deposited in the Genbank obtaining the accession numbers OR909054-OR909060.
No data was available in the GenBank for
Sideritis taxa for the molecular markers
petB/
petD,
rpoC1,
psbK/
psbI and
atpF/
atpH, thus first-time furnished herein for
S. syriaca subsp.
syriaca. However, there were 161 entries available on the Genbank for the
trnL/
trnF molecular marker corresponding to 49
Sideritis taxa, 96 entries belonging to 8
Sideritis taxa for
psbA/
trnH, and 45 entries of 22 Sideritis taxa for
rbcL [
18]. Therefore, a unified phylogenetic tree was constructed for the molecular markers
trnL/
trnF and
rbcL for a total of 15
Sideritis taxa (
Figure 1). The concatenated sequence used for this phylogenetic tree had a length of 1233 bp; within this sequence, a total of 43 single nucleotide polymorphisms (SNPs), a polyT (9 Ts) region and one indel (insertion of TGAA for
S. romana L.) have been identified (Supplementary Material
Table S2). In the phylogenetic tree,
S. syriaca subsp.
syriaca was clearly separated from closely related species such as
S. euboea and
S. scardica (
Figure 1).
The DNA sequence of the present study (OP909056) for
rbcL was identical (100%) to
S. syriaca subsp.
syriaca (KT315757) and identical (100%) to another 19 specimens corresponding to
S. raeseri subsp.
raeseri,
S. euboea and
S. scardica Griseb. originated from Greece; it was also similar (99.86%) to
S. euboea AF501999 from Mt Dirphys which was wrongly labeled as
S. syriaca as well as to
S. hyssopifolia L. (AF501995) (Supplementary Material
Table S3.A). The DNA sequence OP909054 of the present study for
psbA-
trnH was identical (100% similarity) to the respective sequences of
S. syriaca subsp.
syriaca deposited in the GenBank (KT633327, KT633328, KT633350) [
18]. Surprisingly, there were nucleotide differences among 90 more
Sideritis taxa originated from Greece with results shown to be highly problematic (Supplementary Material 3.B).
Basic Local Alignment (BLAST) results showed that the
petB/
petD molecular marker exhibited a 98.60% similarity with
Stenogyne haliakalae Wawra (NC_029817). The sequence generated for the
trnL/
trnF region in this study matched with
S. scardica (AF335658) by 99.81%, whereas the
rpoC1 sequence matched to
Stachys byzantina K.Koch (NC_029825) by 99.37%. The sequence for the
psbK/
psbI intergenic spacer matched with
Anisomeles indica (L.) Kuntze (NC_046781) and
Leucosceptrum canum Sm. (NC_051966) by 94.84%. Finally, the sequence for
atpF/
atpH matched
Stachys byzantina (NC_029825) by 98.20% [
18].
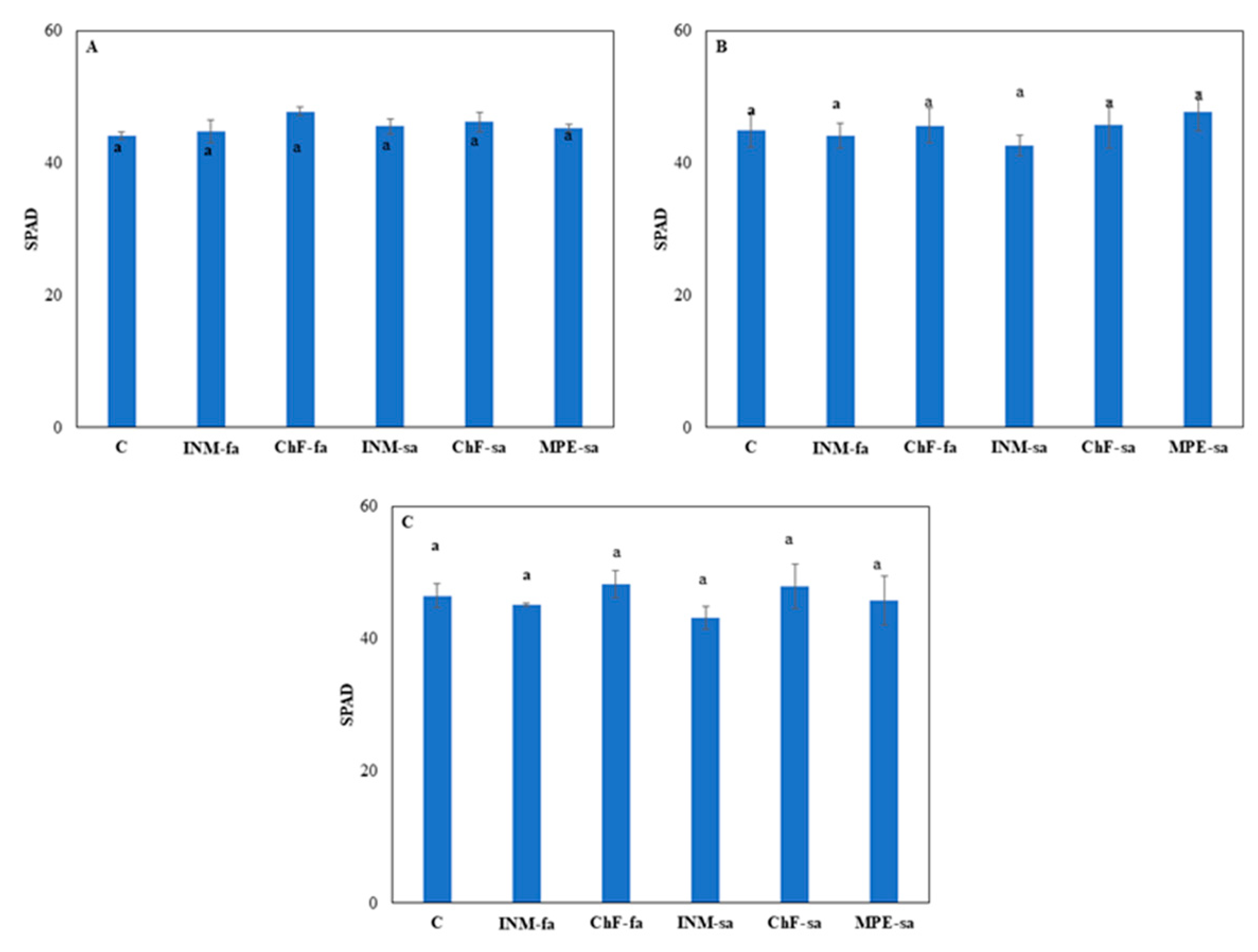
2.2. Fertilization scheme exerted minor effects on leaf colour
At all three growth stages, leaf SPAD value was not affected by the fertilization treatment (
Figure 2). At vegetative stage, plants receiving INM-fa had higher I
AD value (i.e., being less green) from all treatments besides INM-sa (
Suppl. Figure S1A). At early flowering stage, plants receiving ChF-sa showed higher I
AD value from plants treated with ChF-fa and MPE-sa (
Suppl. Figure S1B). At full flowering stage, plants receiving ChF-sa had higher I
AD value as compared to plants treated with ChF-fa, INM-sa or MPE-sa (
Suppl. Figure S1C).
At all three growth stages, leaf L value was not affected by the fertilization regime (
Suppl. Figure S2). At vegetative stage, plants receiving ChF-sa had lower a* value as compared to controls plants or plants receiving ChF-fa or INM-sa (
Suppl. Figure S3A). At early flowering stage, plants receiving INM-sa or ChF-sa showed lower a* value than control plants or plants treated with ChF-fa (
Suppl. Figure S3B). At full flowering stage, plants receiving INM-sa had lower a* value than plants receiving INM-fa or ChF-fa (
Suppl. Figure S3C).
At early flowering stage, plants receiving INM-sa had higher b* value as compared to control plants (
Suppl. Figure S4B). At vegetative and full flowering stages, the fertilization treatment did not affect leaf b* value (
Suppl. Figure S4A, C).
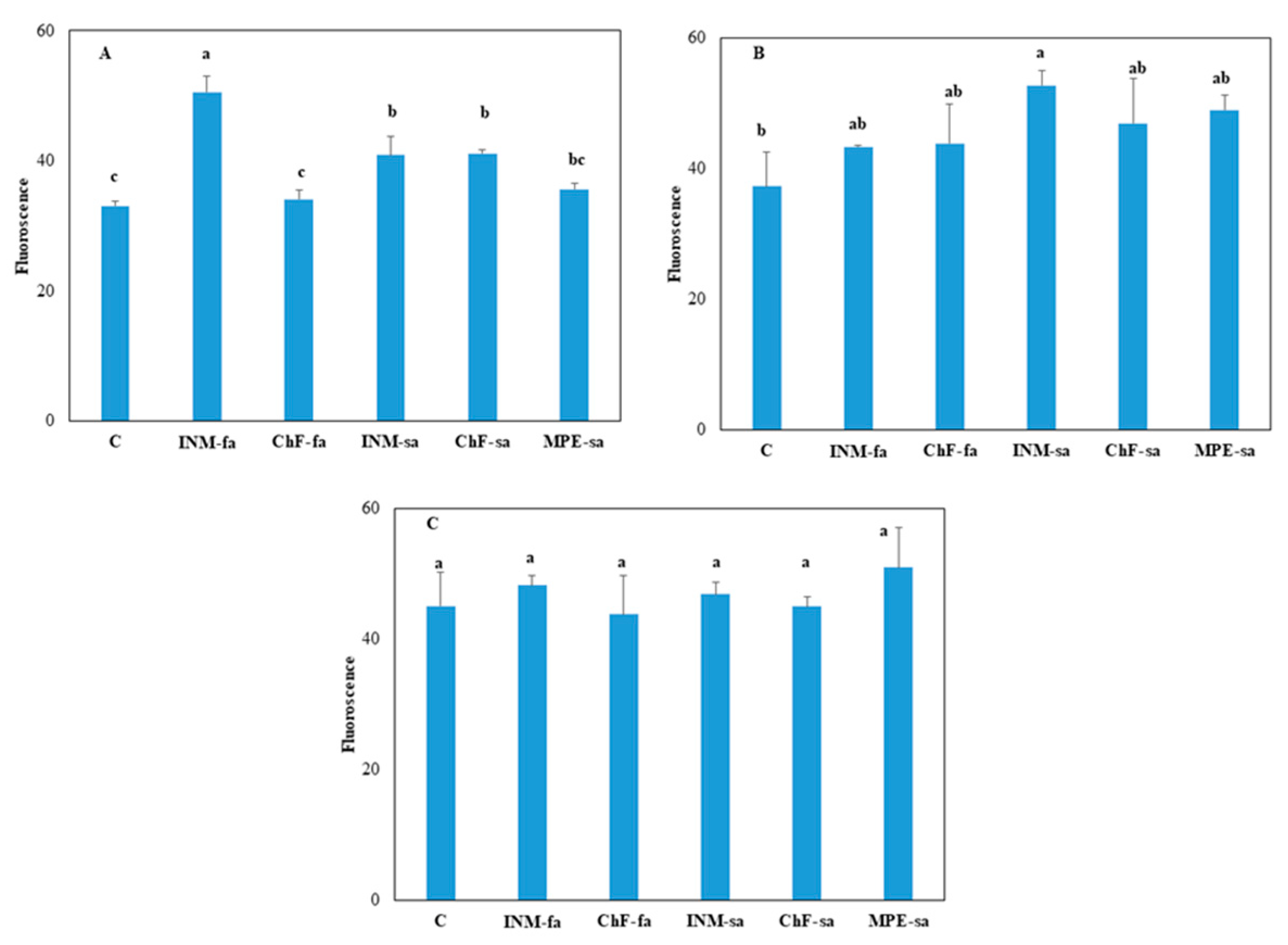
2.3. Fertilization scheme induced limited effects on leaf photosynthetic performance
At three growth stages, the effect of fertilization treatment on overall photosynthetic efficiency was assessed by
in situ F
v/F
m measurements (
Figure 3). At vegetative stage, plants receiving INM-fa had the highest F
v/F
m value (
Figure 3A). At early flowering stage, plants receiving INM-sa had higher F
v/F
m value, as compared to control plants (
Figure 3B). At full flowering stage, leaf F
v/F
m value was not affected by the fertilization regime (
Figure 3C).
2.4. Fertilization induced distinct leaf shape profiles
To determine the effect of fertilization regime on leaf form, four shape indicators (aspect ratio, circularity, roundness, solidity) were employed. Control plants showed a higher aspect ratio as compared to plants receiving ChF-sa or MPE-sa (
Figure 4A). The lowest circularity was noted in control plants (
Figure 4B). Plants receiving MPE-sa had the highest circularity from all treatments, besides ChF-sa (
Figure 4B). Plants under MPE-sa had higher roundness than control plants (
Figure 4C). The lowest solidity was noted in control plants (
Figure 4D). Plants treated with MPE-sa had the highest solidity of all treatments, besides plants receiving INM-sa (
Figure 4D).
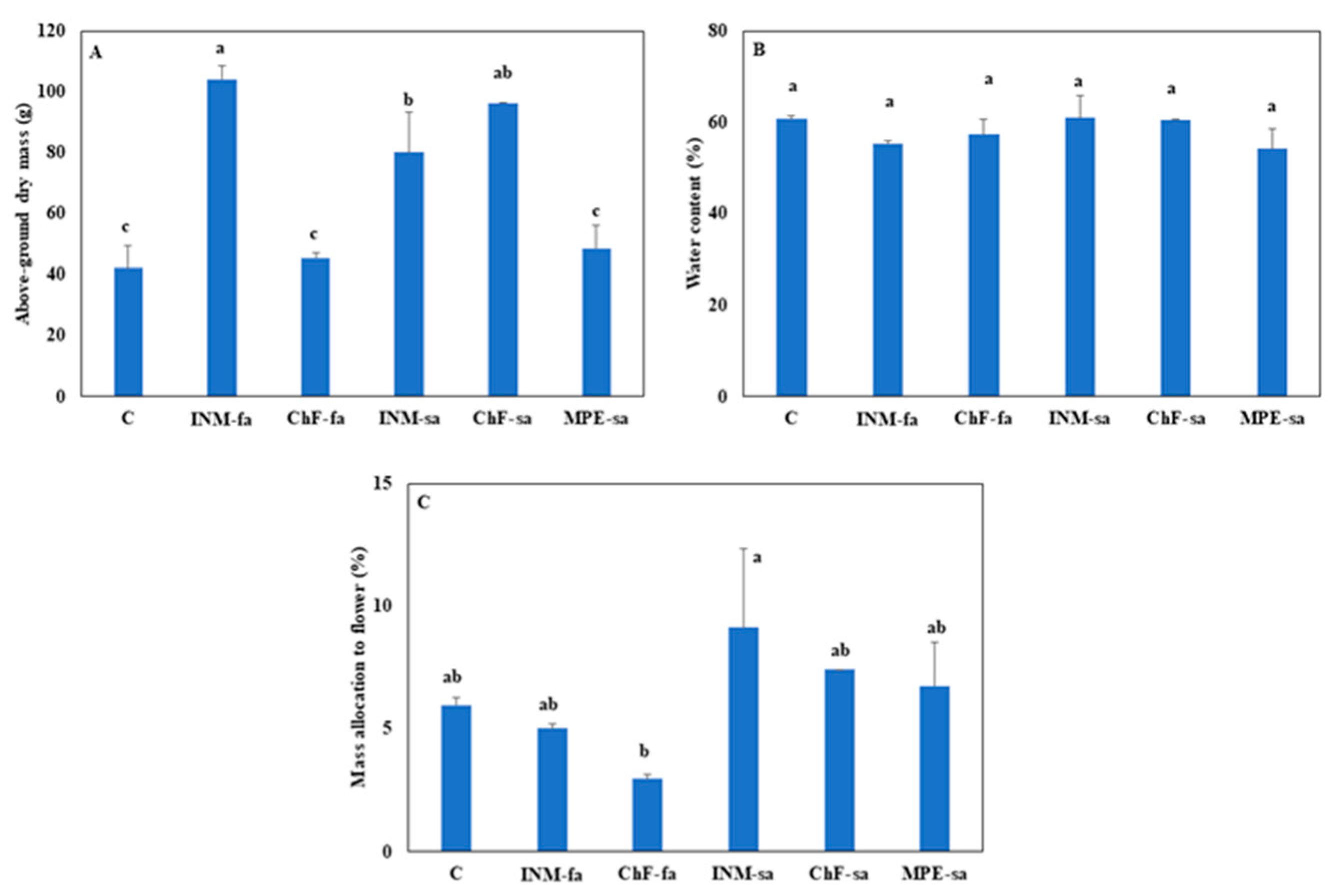
2.5. INM and ChF-sa stimulated plant growth, without affecting biomass allocation to generative organs
INM with either application method (i.e., INM-fa or INM-sa) and ChF-sa improved biomass accumulation as compared to the remaining treatments including the controls (
Figure 5A). These differences in above-ground dry mass were not associated with variation in tissue relative water content (
Figure 5B). Plants receiving INM-sa had higher partitioning to generative organs, as compared to plants receiving ChF-fa (
Figure 5C).
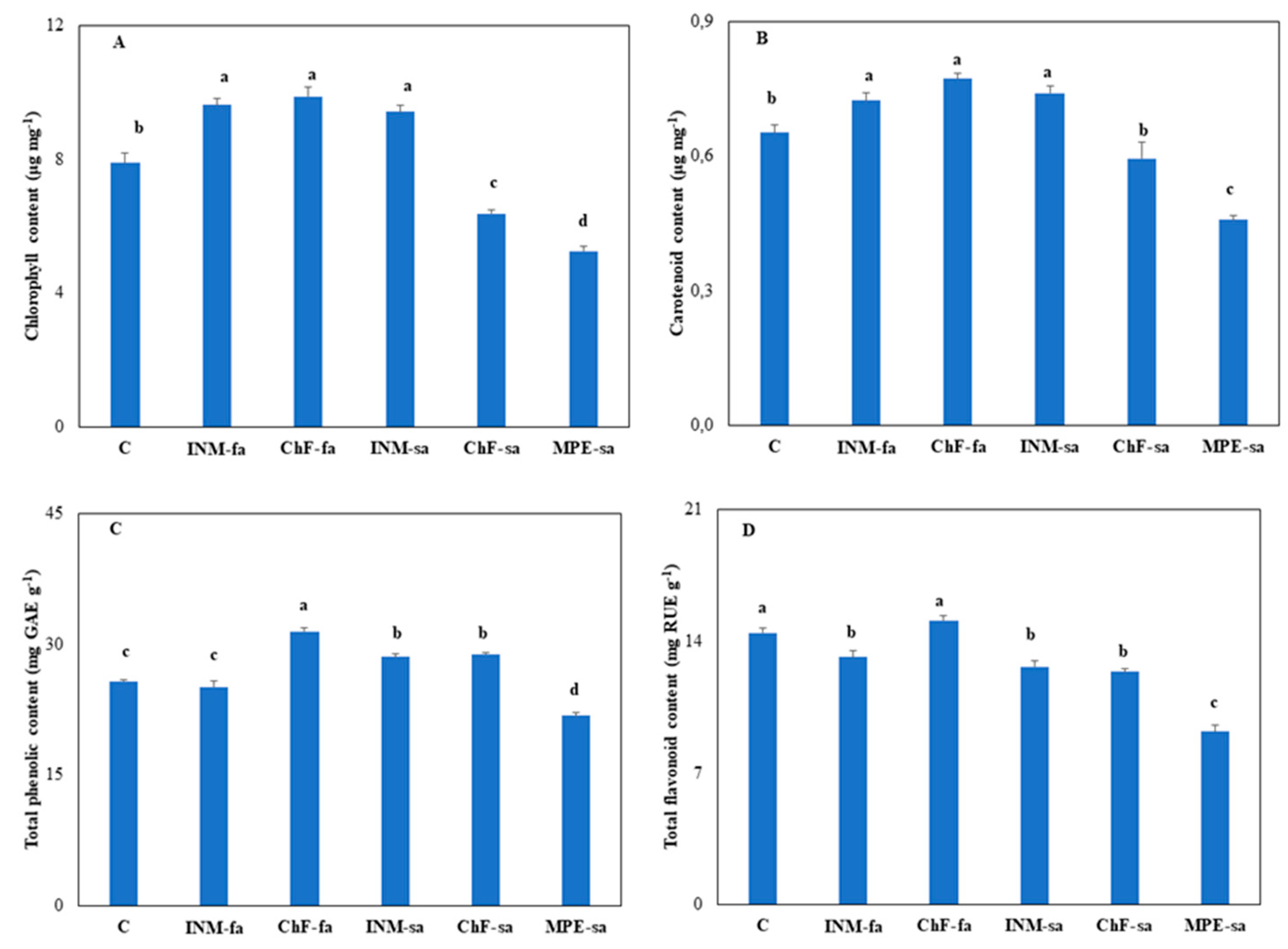
2.6. Fertilization regime affected leaf chlorophyll content
Plant receiving foliar fertilization (i.e., INM-fa, ChF-fa) or INM-sa had higher leaf chlorophyll content as compared to controls (
Figure 6A). Plants receiving ChF-sa or MPE-sa had lower leaf chlorophyll content as compared to controls, while the latter had the lowest content (
Figure 6A).
2.7. Fertilization regime affected leaf antioxidant compound content
Carotenoids, flavonoids, and phenols are critical non enzymatic antioxidants. Plant receiving foliar fertilization (i.e., INM-fa, ChF-fa) or INM-sa had higher leaf carotenoid content as compared to controls (
Figure 6B). Plants receiving MPE-sa had lower leaf carotenoid content as compared to controls (
Figure 6B).
The highest leaf total phenolic content was noted in plants receiving ChF-fa, followed by plants treated with INM-sa or ChF-sa (
Figure 6C). Plants treated with INM-sa or ChF-sa had higher total phenolic content as compared to controls, whereas the ones receiving MPE-sa had the lowest total phenolic content (
Figure 6C).
Plants receiving INM-fa, INM-sa or ChF-sa had lower leaf flavonoid content, as compared to controls (
Figure 6D). The lowest leaf flavonoid content was noted in plants receiving MPE-sa (
Figure 6D).
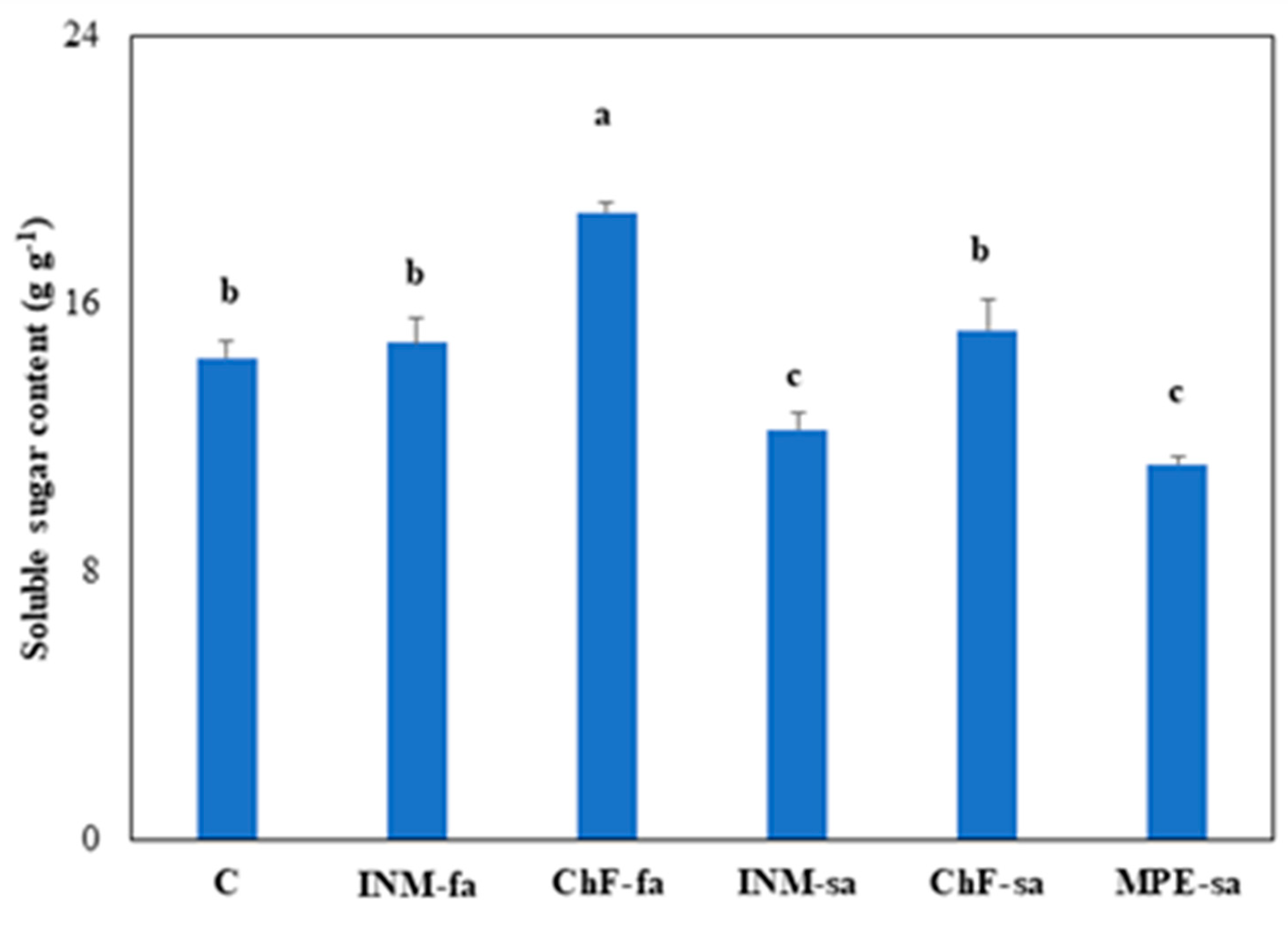
2.8. Fertilization regime affected leaf soluble sugar content
Plants receiving ChF-sa had higher leaf soluble sugar content than controls, whereas the ones receiving INM-sa or MPE-sa had lower leaf soluble sugar content (
Figure 7).
2.9. Leaf and inflorescence mineral analysis
Among all treatments (including control), the highest N concentration in leaves was observed when plants cultivated under integrated nutrient management fertilization applied to soil (INM-sa). Conversely, the highest K concentration was noted by foliar application of conventional fertilizers (ChF-fa). This trend was also observed for P, although it is noteworthy that similar results were also seen with the foliar application of INM fertilization (INM-fa). In contrast, no significant differences were observed in Ca or Mg concentrations with any of the applied treatments (
Table 1).
On the other hand, the results regarding the leaf concentration of micronutrients in
S. syriaca subsp.
syriaca were less conclusive. Specifically, no treatment effects were evident for Zn, and the same held true for Cu, where although ANOVA results indicated significant differences between treatments, none of them significantly differed from the control. On the contrary, the integrated nutrient management (INM) fertilization along with the biostimulant treatment (MPE) applied through soil application exhibited the highest concentrations for Fe, while the conventional fertilization by foliar application (ChF-fa) proved to be more effective than all other treatments in supplying the highest Mn leaf concentration (
Table 2).
ANOVA of floral mineral analysis data showed differences among treatments (
Table 3 and
Table 4). The highest N content was observed in INM-sa, whereas regarding P the highest contents were found in INM-sa and ChF-sa. The latter also had the highest K content (
Table 3).
Regarding inflorescence micronutrient content of
Sideritis syriaca subsp.
syriaca (
Table 4), the highest B content was observed in INM-sa and the lowest value in ChF-sa treatment, which were similar to control (
Table 4).
3. Discussion
3.1. Molecular characterization of the studied Sideritis syriaca subsp. syriaca
The genetic identification of plant species combining taxonomic identification and modern molecular techniques based on DNA sequences has the power to effectively characterize both morphologically and genetically given specimens [
56]. To date, DNA barcoding has been widely used as a powerful tool in combination with bioinformatic analysis in ecological, taxonomic, comparative phylogenetic and biodiversity conservation studies across various taxonomic levels [
3] including distinction of members of the Lamiaceae family [
15,
57], and more specifically of the genus
Sideritis [
11,
13,
14].
DNA barcoding was a valid technique for the discrimination of the herein studied
S. syriaca subsp.
syriaca genotype, further enhancing the classical taxonomic identification based on morphological features and offering insight into the phylogenetic relationships of closely related taxa. In the frame of this study, seven new sequences (i.e.,
petB/
petD,
rbcL,
trnL/
trnF,
rpoC1,
psbA/
trnH,
psbK-
psbI and
atpF/
atpH) were generated and deposited in GenBank for
S. syriaca subsp.
syriaca GR-1-BBGK-15,5939, obtaining unique accession numbers and creating a genetic documentation for the focal taxon while four DNA sequences of the plastid molecular markers
petB/
petD,
rpoC1,
psbK-
psbI and
atpF/
atpH have been reported and deposited to the GenBank for the first time. The phylogenetic analysis with the molecular markers
rbcL and
trnL/
trnF confirmed the identity of the specimen and indicated its phylogenetic position compared to other
Sideritis taxa (
Figure 1).
S. syriaca subsp.
syriaca GR-1-BBGK-15,5939 was classified in a group together with
S. montana L.
, S. romana, S. euboea and
S. scardica, which are also found in Greece ([
55];
Figure 1). The
Sideritis specimen annotated with an asterisk in
Figure 2 is denoted as
S. syriaca in the GenBank database; however, a reevaluation is warranted for this accession. Considering its collection locality (Greece: Mt. Dirfys; [
6]), it is suggested that the appropriate identification should be
S. euboea which is the local endemic mountain tea species of Mt. Dirphys. This recommendation is substantiated by the restricted geographical distribution of
S. syriaca subsp.
syriaca on the island of Crete as a local single-island endemic plant [
55]. Similar issues of misidentification or inaccurate taxonomy of specimens related with the focal taxon is extensively addressed in [
17] for the case of the ITS2 molecular marker.
The Basic Local Alignment (BLAST) results indicated the highest similarity of the new DNA sequences generated for
S. syriaca subsp.
syriaca GR-1-BBGK-15,5939 to the sequences retrieved from the GenBank. Since there was no previous information on sequence data for any
Sideritis taxon for the cpDNA regions
petB/
petD,
rpoC1,
psbK-
psbI and
atpF/
atpH, the BLAST showed only other closely related taxa. The
trnL/
trnF showed the highest similarity to
S. scardica (99.81%). The
rbcL molecular marker of the present study was identical (100%) to
S. syriaca subsp.
syriaca and to another 19 specimens corresponding to the Balkan endemics
S. raeseri subsp.
raeseri and
S. scardica and the local Greek endemic
S. euboea, all originated from Greece; in addition, high similarity was shown to the
S. euboea and
S. hyssopifolia which is wild-growing in France, Italy, Sicilia, Spain and Switzerland [
55]. It is worth mentioning that the molecular marker
rbcL exhibited identical sequences across a notable array of
Sideritis taxa such as
S. perfoliata L. subsp
. perfoliata, S. scardica, S. syriaca subsp
. syriaca, S. clandestina (Bory & Chaub.) Hayek subsp
. clandestina and
S. euboea, provided that all the available sequences for this molecular marker pertain to merely eight taxa. Although
psbA/
trnH was excluded from the phylogenetic analysis herein, its utilization is rather anticipated to yield inconclusive outcomes in terms of species differentiation. This assumption comes in contrast with previous studies [
13] suggesting that the
matK and
psbA/
trnH could serve as potential single-region barcodes for Lamiaceae species. Additional phylogenetic analysis for another 94 specimens of genus
Sideritis from Greece showed identical sequences of three specimens of
S. syriaca subsp
. syriaca, however, the rest of these specimens presented controversial matching and nucleotide differences to the assigned
Sideritis species and subspecies (Supplementary material
Table S3.B). Recently, a new
Sideritis species has been described from Bulgaria namely
S. elica Aneva, Zhelev & Bonchev that was clearly distinct from
S. scardica based on morphological and molecular data [
16]; in that case, the
trnH-psbA molecular marker showed a 6.8% polymorphism [
16]. Previous studies have also investigated the phylogeny of selected
Sideritis taxa using only one nuclear molecular marker [
14,
17,
58], thus compromising the genetic information for comparison with the current investigation.
The computation herein suggests that the combination of two or more molecular markers provides the potential for more informative species’ differentiation. In this study, we furnished for the first time genetic characterization based on seven molecular markers, thus consolidating the identity and authenticating the studied Cretan local endemic germplasm of
S. syriaca subsp.
syriaca which is threatened with extinction [
25].
3.2. Fertilization effects supporting the sustainable exploitation of S. syriaca subsp. syriaca
Plant species become extinct at a rate which is two to three orders higher than the expected natural one [
59]. Overexploitation and habitat destruction or alteration currently threaten over 15,000 plant taxa with extinction [
60], with a large part of their wild-growing populations currently depleted [
61]. For species with increasing demand, adaptation to agricultural environments stands out as a sustainable conservation option. Successful cultivation, however, depends on the development of cultivation protocols, a major part of which is the fertilization scheme. The present field study reports the primary stages of establishing
S. syriaca subsp.
syriaca (endemic to Crete, Greece) into systematic cultivation, and evaluates the fertilization regime which is optimal for improving plant growth as well as in terms of critical features of herbal material quality. In this perspective, emphasis was placed into the use of fertilizers having low environmental footprint, which is of vital importance in the respective MAP market area [
62].
As compared to controls, INM and ChF-sa improved plant growth (
Figure 5A). In INM, this effect was more prominent under foliar application (
Figure 5A). This increase in biomass accumulation was not associated with variation in tissue relative water content (
Figure 5B). In this way, the efficiency of processing (moisture content reduction through drying [
32,
33] was not affected by the fertilization regime. The partitioning to the inflorescences was not significantly different among the three growth-promoting fertilization treatments (INM-fa, INM-sa, ChF-sa;
Figure 5C). Considering yield, the current findings suggest that INM (especially through foliar application) and ChF-sa ought to be employed in
S. syriaca subsp.
syriaca cultivation.
Recent research on the pilot cultivation of Cretan endemic species in northern Greece (including
S. syriaca subsp.
syriaca) has also revealed noteworthy yield improvements with the implementation of comparable fertilization methods [
63]. However, such enhanced yield was accompanied by distinct concentration patterns of macro- and micro-nutrients during the harvest stage. Specifically, a previous own study have highlighted that chemical fertilization through foliar application (ChF-fa) may lead to elevated levels of metallic micronutrients at the harvest stage, specifically Cu and Zn [
63]. Conversely, the same treatment when applied to the soil has been shown to result in a significant increase in Fe and Mn [
63]. Interestingly, N concentration is reported to show minimal variation across different treatments compared to the control, whereas P concentration may rise with foliar application using conventional fertilizers, and K can exhibit positive responses with the integrated nutrient management (INM) fertilization approach through foliar application [
63].
The dissimilarities between the present study’s findings in Crete and the aforementioned pilot case of cultivation in northern Greece could be attributed to the respective differences on properties of the initial soil used as a medium, especially in terms of soil fertility. The soil in the present study (Crete) had elevated concentrations of soil-available macro- and micronutrients, exceeding their sufficiency levels [
64,
65]. In contrast, the soil utilized in the previously published own study [
63] in northern Greece lacked sufficient soil-available potassium, falling below critical sufficiency levels, while the soil phosphorus content was only marginally sufficient [
63]. The above indicates that any fertilization scheme suggestion in the future should be carefully adjusted with the initial amounts of soil available nutrients taking also into consideration the complexity of soil-soil solution interactions that may interfere in the uptake process by plant’s root system.
Regarding the mineral analysis of the inflorescences consisting the major part of
Sideritis herba [
23], it was found that all treatments tended to increase the macronutrient content with the exception of P. The higher N content was observed in INM-sa, whereas INM-fa and ChF-sa showed the highest P values. The latter tended also to had higher K and Ca content, suggesting overall that ChF-sa and INM-fa may favor the accumulation of macronutrients in the inflorescences of
S. syriaca subsp.
syriaca. Regarding inflorescence micronutrient content, it seems that no significant enrichment of inflorescences can be referred in terms of Fe, Zn and B contents. No relative information was found in literature to compare and evaluate the macro- and micro-nutrient contents in inflorescences of
S. syriaca subsp.
syriaca. Thus, further work is needed for a greater period of time and under different experimental conditions to evaluate the partitioning of nutrients to inflorescences and the response of
S. syriaca subsp.
syriaca to fertilization.
Leaf green colour intensity is an important visually perceived quality index throughout the supply chain [
32]. In this study, leaf coloration was evaluated for the first time by three devices (SPAD meter, DA meter, Chroma Meter), indicating a different potential in discriminating the minor fertilization treatment effects (
Figure 2, and Suppl. Figs. S1–S4). At full flowering stage, for instance, no difference among treatments was apparent when examining leaf SPAD value (
Figure 2), L value (a measure of lightness;
Suppl. Figure S2C) or b value (a measure of blue to yellow range intensity;
Suppl. Figure S4C). Although little differences were noted in I
AD value (
Suppl. Figure S1C) and a value (a measure of green to red range intensity;
Suppl. Figure S3C) among treatments, plant receiving fertilization did not differ with control plants. Therefore, it is concluded that the fertilization scheme did not affect leaf colour aspects in
S. syriaca subsp.
syriaca. Besides colour, visual inspection in herbal material grading includes leaf form. As compared to control plants, fertilization increased both circularity and solidity (
Figure 4B, D). Differences in circularity are translated to variation in lobing and serration, while differences in solidity to deep lobes or marked petiole morphology [
66]. In this regard, trained personnel, or automated identification protocols [
33,
67] are expected to be capable of visually discriminating
S. syriaca subsp.
syriaca herbal material that has received fertilization during cultivation based on shape indicators, since colour differences were either absent or minor.
Since the beneficial effects of antioxidant intake are increasingly recognized by consumers, the antioxidant content of natural products (such as
Sideritis herba) is becoming more prominent as an index of herbal material quality [
38,
68]. In this investigation, three critical antioxidants (carotenoids, flavonoids, phenols) were quantified and comparatively evaluated for the first time. MPE-sa was associated with reduced content in all three metabolites (
Figure 6B, C, D), thus consistently downgrading quality. Fertilization mostly promoted carotenoid and total phenolic contents, whereas it decreased flavonoid content (
Figure 6B, C, D). The fertilization regime stimulating antioxidant content was metabolite-specific (
Figure 8B, C, D). Considering all three antioxidants together, ChF-fa was the most stimulatory scheme. Contrary to our results, INM has been previously associated with enhanced antioxidant compound content in other taxa [
49,
50].
Although the present study focused only on one growth cycle in Crete, it offered for the first time reference data for growth/yield aspects and several herbal quality aspects in S. syriaca subsp. syriaca. When the primary target is yield, INM (especially through foliar application) and ChF-sa ought to be employed. When antioxidant compound content is the primary goal, ChF-fa is required while MPE-sa ought to be avoided in S. syriaca subsp. syriaca cultivation.
4. Materials and Methods
4.1. Plant material
Several botanical expeditions were conducted in the mountain ranges of Crete in the frame of the research project “Conservation and sustainable utilization of rare-threatened endemic plants of Crete for the development of new products with innovative precision fertilization” (acronym: PRECISE-M, Τ1ΕΔΚ-05380) with the aim to locate wild-growing populations of
S. syriaca subsp.
syriaca (
Figure 8). Seed collections for ex situ conservation were authorized through a special permit of the Institute of Plant Breeding and Phytogenetic Resources, Hellenic Agricultural Organization Demeter (Permit 82336/879 of 18 May 2019 and 26895/1527, 21 April 2021) obtained yearly by the Greek Ministry of Environment and Energy. The collected seeds and voucher samples were taxonomically identified, and consequently unique IPEN (International Plant Exchange Network) accession numbers were assigned to each of them (GR-1-BBGK-08,4544; GR-1-BBGK-13,5743; GR-1-BBGK-13,5738; GR-1-BBGK-13,5907; GR-1-BBGK-14,5798; GR-1-BBGK-15,5939; GR-1-BBGK-17,6029; GR-1-BBGK-19,1100) according to the protocols of the Balkan Botanic Garden of Krousia, Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization, Demeter. Adequate number of plants were raised ex situ for the field experiment from seedlings and/or cuttings from mother plants of GR-1-BBGK-15,5939 with the aid of a specific propagation protocol [
14]. All plant material employed in the experimental procedure were transplanted in 2 L plastic pots by the company AFI GLAVAKI KE SIA OE Tree & Plant Nurseries, Aridea, PELLAS, GR-58400, Greece.
4.2. DNA barcoding and molecular analysis
Molecular analyses were conducted using fresh juvenile leaves of
S. syriaca subsp.
syriaca GR-1-BBGK-15,5939. The methodology employed for DNA extraction, amplification, and subsequent DNA sequence analysis adhered to the procedures outlined by [
69]. The oligonucleotide primers (5′ to 3′) (InVitrogen Inc., Paisley, Scotland, UK) used for PCR amplification of
S. syriaca subsp.
syriaca were the following: ACTCGCACACACTCCCTTTCC and GCTTTTATGGAAGCTTTAACAAT for
atpF/
atpH [
70], TTGACYCGTTTTTATAGTTTAC and AATTTAGCYCTTAATACAGG for
petB/
petD [
71], CGCGCATGGTGGATTCACAATCC and GTTATGCATGAACGTAATGCTC for
trnH/
psbA [
72], TTAGCCTTTGTTTGGCAAG and AGAGTTTGAGAGTAAGCAT [
73] for
psbK-
psbI, ATGTCACCACAAACAGAGACTAAAGC and CTTCTGCTACAAATAAGAATCGATCTC for
rbcL [
74], GGCAAAGAGGGAAGATTTCG and CCATAAGCATATCTTGAGTTGG for
rpoC1 [
75], ATTTGAACTGGTGACACGAG and CGAAATCGGTAGACGCTACG for
trnL/
trnF [
76]. Annealing temperature for each pair of primers was 54 to 60°C, depending on sequence temperature estimation.
Alignment of each generated sequence was completed by employing the Basic Local Alignment Tool (BLAST), comparing them with existing sequences available in the GenBank. DNA sequences corresponding to the above-mentioned molecular markers for other
Sideritis taxa were retrieved from GenBank and were subsequently aligned for each marker using Mega11 software [
77]. A unified phylogenetic tree was constructed using the Neighbor-Joining statistical method and Maximum Composite Likelihood substitution model. To ensure accessibility and transparency, all newly generated sequences were submitted to the GenBank obtaining the accession numbers OR909054-OR909060. The evaluation of Evolutionary Divergence between Sequences for each molecular marker involved the calculation of pair-wise distances, conducted within the Mega11 software [
77].
4.3. Field experiment and experimental design
The field experiment was established at the beginning of March 2021 within a 20 × 25 m fenced area of the campus of the Hellenic Mediterranean University, Heraklion, Crete (35° 19’ N, 25° 6’ E) at low altitude (60 m above sea level). The planting distance between individuals was 40 cm and 80 cm between separate rows 20 m long arranged at east-west direction and including as “guard plants” other local Cretan endemics, i.e., plant individuals of Origanum dictamnus L., Origanum microphyllum (Benth.) Vogel, Carlina diae (Rech.f.) Meusel & A.Kástner, and Verbascum arcturus L.
The experimental design incorporated completely randomized blocks of 10 plants of
S. syriaca subsp.
syriaca per block, and three blocks per treatment randomly arranged in different rows. To provide the same starting point for all experimental plants, all individuals were trimmed at 5 cm above ground level at the end of April. At the end of May (i.e., 30 d after trimming), six fertilization treatments were introduced weekly till final harvest. An automatic irrigation system was employed with 2 L h
-1 adjustable drippers to supply water to the plants three times per week. Pest and disease control was not deemed necessary during experimentation, but removal of weeds was regularly required and manually performed. Final harvest was carried out at the end of June 2021. The soil properties of the pilot field are described in previous own studies [
65,
78].
4.4. Fertilization treatments
The pilot cultivation of
S. syriaca subsp.
syriaca followed weekly fertilization treatments using water (control), chemical (ChF), biostimulant and INM in liquid or soluble granule fertilizers administered with foliar and soil applications (
Supplementary materials Methods S1). The foliar applications were performed using a 5 L plastic handheld sprayer (low pressure) until apparent wetness, and the soil applications were manually performed (100 mL nutrient solution per plant). The INM and biostimulants were supplied with four special fertilizers from THEOFRASTOS company, Industrial area of Korinthos, GR-20100 Korinthos, Greece (
Supplementary materials Methods S1). These polysaccharide-based semi-organic fertilizers are made from high quality organic edible raw materials and plant extracts, resulting in a full supplement of plants with amino acids, vitamins, sugars, and macro-microelements. The INM polysaccharides are aimed to contribute to the formation of stable soil aggregates formed in connection with active soil microorganisms; INM polysaccharides when foliar-sprayed, they are expected to be rapidly absorbed by the plants. The chemical fertilizers were all in soluble powder or granule form, except the liquid fertilizer for micronutrients (Plex Mix, AGRI.FE.M. LTD Fertilizers, Greece). Two foliar fertilization treatments (INM and chemical), three soil application treatments (INM, chemical and a biostimulator) and a control for the pilot cultivation of
S. syriaca subsp.
syriaca in the field were employed in the experimentation (
Supplementary materials Methods S1).
4.5. Plant measurements
All plant and leaf level measurements were regularly conducted until 25 May 25 2021, ending with completion of flowering. Regarding leaf measurements, fully-expanded leaves grown under direct light were sampled within 15 min. When this was not feasible, samples were placed in vials, were flash-frozen in liquid nitrogen and were transferred to a freezer (-80oC) for storage. All replicate leaves were sampled from separate plant individuals.
4.5.1. Non-invasive evaluation of leaf coloration at three growth stages
Since leaf coloration depends on the fertilization regime affecting both photosynthetic capacity and visually perceived quality [
79], this variable was assessed by employing three methods:
(i) Leaf SPAD value, approximating chlorophyll content determined by using a SPAD-502 (Konica Minolta Corp., Solna, Sweden).
(ii) Index of absorbance difference (I
AD) accurately evaluating fruit ripeness, since it is closely associated with outer mesocarp chlorophyll content [
80]; I
AD was computed as the difference between the absorbance values at 670 and 720 nm, near the chlorophyll absorbance peak [
80]. The potential of I
AD in reflecting respective differences in leaves has not been previously evaluated. In this investigation, I
AD was determined in leaves by using the DA meter (tr DA Meter, T.R. Turoni, Italy).
(iii) Leaf colour quantified by using a Chroma Meter (Model CR-400, Minolta Corp., Japan); CIE L*a*b* coordinates were recorded using D65 illuminants and a 10° Standard Observer as a reference system; L* [a measure of lightness, ranging from 0 (black) to 100 (white)], a* (a measure of intensity in the green to red range, where negative values refer to green and positive to red), and b* (a measure of representing intensity in the blue to yellow range, where negative values refer to blue and positive to yellow) [
33].
These measurements were in situ conducted in attached leaves of intact plant individuals, at three different growth stages (vegetative, early flowering, and full flowering). Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
4.5.2. Non-invasive evaluation of photosynthetic performance in growth stages
As a valid indicator of leaf photosynthetic performance [
81,
82], the ratio of variable to maximum chlorophyll fluorescence (F
v/F
m) was assessed. Measurements were performed by using a chlorophyll fluorometer (OS-30P, Op-tiSciences, Hudson, NH, USA). Prior to evaluation, leaves were dark-adapted (≥ 20 min) by employing leaf clips. F
v/F
m was assessed by applying a saturated photosynthetic photon flux density of 3000 µmol m
−2 s
−1.
These measurements were in situ conducted in attached leaves of intact plant individuals, and at vegetative, early flowering, and full flowering stages. Three points were recorded per replicate leaf and were further averaged. Three replicate leaves were assessed per treatment.
4.5.3. Leaf shape indicators
A morphometric analysis was performed by analyzing leaf form. Leaf shape traits were derived from images acquired by a digital camera (Sony DSC-W830, Sony Corporation, Tokyo, Japan) under non-reflective glass from a distance of 0.5 m, employing a copy stand. Using specialized software (ImageJ; Wayne Rasband/NIH, Bethesda, MD, USA), leaf lamina outlines were processed to estimate the following four (dimensionless) metrics of leaf form: (a) aspect ratio [(major axis) / (minor axis); axes of the best-fitted ellipse], (b) circularity [(4π × area) / (perimeter)
2], (c) roundness [(4 × area) / [4π × (major axis)
2]], and (d) solidity [(area) / (convex area)] [
64,
83]. Each metric was used to quantify a distinct feature of leaf shape. Aspect ratio and roundness are affected by the length to width ratio, while circularity and solidity are depend on serration and lobing [
66]. Aspect ratio ranges from 1 (circle) to value without upper bound (infinitely narrow). Roundness ranges from 0 (infinitely narrow) to 1 (circle). Circularity ranges from 0 (infinitely narrow) to 1 (circle). Solidity ranges from 0 to 1, being inversely related to boundary irregularities. Solidity is sensitive to leaves with deep lobes or a distinct petiole and can be employed to detect leaves lacking such structures [
66]. Solidity, unlike circularity, is not greatly influenced by serrations and minor lobing [
66] . Thirty leaves (5 per plant × 6 plant individuals) were analyzed per treatment.
4.5.4. Plant growth and biomass partitioning to generative organs
Plant growth and biomass partitioning to generative organs were determined. Above-ground plant and inflorescence (fresh and dry) masses were determined (± 0.01 g; MXX-412; Denver Instruments, Bohemia, NY, USA). For measuring dry weight, samples were placed in a forced-air drying oven for 72 h at 80°C. Six replicate plants were evaluated per treatment.
4.5.5. Leaf chlorophyll and carotenoid contents
Chlorophyll content is important for leaf coloration and photosynthetic performance and carotenoids represent key non enzymatic antioxidants [
68,
84,
85]. The effect of growth conditions on chlorophyll and carotenoid contents was therefore assessed. Following fine chopping, leaf portions (0.5 g) were homogenized with the addition of 10 mL of 80% acetone. This primary acetone extract was then filtered, and the filtered extract was diluted by adding 2 mL of 80% acetone per mL of extract. Since chlorophyll is light sensitive, extraction took place in a dark room [
84,
85]. The obtained extract was subjected to reading on a spectrophotometer (Mapada UV-1800; Shanghai. Mapada Instruments Co., Ltd., Shanghai, China). Total chlorophyll and carotenoid contents were calculated [
86]. Three leaves were assessed per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
4.5.6. Leaf total phenolic and total flavonoid contents
Phenols and flavonoids are critical non enzymatic antioxidants [
68,
84,
85] . Leaf samples (0.1 g) were extracted with 1 mL of 80% aqueous methanol in an ultrasonic bath (10 min) and were then centrifuged (15000 g for 10 min). The contents of total phenolics and total flavonoids were determined by using the Folin-Ciocalteu assay and the aluminum chloride colorimetric assay, respectively [
68]. The absorbance against prepared reagent blank was determined using a microplate reader (Infinite 200 PRO, TECAN, Switzerland). For total phenolic content, gallic acid was used as the standard reference and gallic acid equivalent (GAE) was expressed as mg per g fresh mass. For total flavonoid content, rutin was used as the standard reference and rutin equivalent (RUE) was expressed as mg per g fresh mass. Three leaves were assessed per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
4.5.7. Leaf soluble sugar content
The carbohydrate status is related to photosynthetic activity. The effect of fertilization scheme on carbohydrate status was therefore assessed. Leaf samples (0.1 g) were incubated with 1 mL deionized water in a water bath (100
oC for 30 min). The homogenate was centrifuged (15000 g for 15 min) at room temperature (25
oC). Then, 0.1 mL of the solution was mixed with anthranone ethyl acetate and sulphuric acid. Soluble sugar content was assayed in the supernatant according to [
87] by measuring the absorbance at 630 nm using a spectrophotometer (Mapada UV-1800; Mapada Instruments Co., Ltd., Shanghai, China). These measurements were conducted on three replicates per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
4.5.8. Leaf and inflorescence mineral analysis
To assess the role of fertilization regime on mineral uptake by plants, leaf and inflorescence mineral analysis was conducted. The samples were washed with distilled water, dried at 70°C, weighed, ground, and then analyzed for total N by the Kjeldahl method [
88]. In addition, sub-samples were ash-burned at 500°C for at least 4 h (Mills and Benton-Jones, 1996); the ash was dissolved in 2 M HCl, filtered, and P, K, Ca, Mg, Cu, Zn, Fe, Mn, and B were determined in the filtrate, flame photometry, atomic absorption spectromentry and UV-Vis spectrometry, depending on the element. Mineral content was expressed on a dry weight basis. Three replicates were evaluated per treatment. For each replicate, four samples (collected from different plant individuals) were pooled, and the assay was performed twice.
4.6. Statistical analysis
Data analyses of the fertilization experiment were carried out using the SAS statistical software (SAS Institute, Cary, NC, USA). Data were tested for homogeneity of variances (Duncan’s test). Subsequently, estimated least significant differences (LSD) of treatment effects were determined (P = 0.05).
5. Conclusions
In this study, a consolidated genetic fingerprinting based on seven molecular markers (four markers used for the first time in members of genus Sideritis) and DNA barcoding using two molecular markers was generated for the first time for S. syriaca subsp. syriaca, a threatened local endemic plant of Crete (Greece) with depleting wild-growing populations due to overharvesting from the natural environment, thus consolidating its molecular identity, and enabling the traceability of related commercial products. Due to limited cultivation/fertilization data, the optimal fertilization regime for plant growth/yield and herbal material quality was investigated in S. syriaca subsp. syriaca in a field study to facilitate its local cultivation in Crete as a valuable MAP with strong medicinal value approved by the European Medicines Agency. Five fertilization regimes were examined including chemical fertilizers (foliar/ root application), INM (foliar/ root application), and INM with biostimulant (root application). Leaf colour was not altered by the fertilization scheme, but leaf shape was indeed affected. In this way, the visually perceived quality was influenced by fertilization. INM (especially through foliar application) and chemical fertilizer (by soil application) improved yield and did not alter tissue water content or biomass partitioning to generative organs. Chemical fertilizers (foliar application) were the best treatment for enhanced antioxidant compound content, whereas INM with biostimulant was the worst one. In conclusion, different fertilization choices may be employed when targeting either yield or antioxidant compound accumulation in S. syriaca subsp. syriaca. For the former, INM (foliar application) and chemical fertilization (soil application) were found suitable, whereas for the latter the best scheme found was chemical fertilization (foliar application). The data furnished herein are aimed to further facilitate the sustainable exploitation of this valuable medicinal-aromatic plant still suffering from overharvesting directly from wild-growing populations threatened with extinction.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Methods S1. Chemical composition of fertilizers used in the experimental procedure (Theofrastos company, Korinthos, Greece) and fertilization treatments applied in the pilot cultivation of
Sideritis syriaca subsp.
syriaca with other Cretan endemic plants [
64,
65] at the premises of the Hellenic Mediterranean University;
Table S2. Alignment presenting the nucleotide differences among 15
Sideritis species based on the molecular plastid markers
rbcL and
trnL/
trnF. Accession numbers of DNA sequences obtained in this study from the GenBank are indicated next to taxon names. The clade marked in orange represents
S. syriaca subsp.
syriaca GR-1-BBGK-15,5939 studied herein. The taxon marked with asterisk (*) is characterized as
S. syriaca in the database, but based on the origin of the specimen, it should be identified as
S. euboea which is local endemic of Mt Dirphys and not as
S. syriaca subsp.
syriaca which is a local single-island endemic of Crete (Greece);
Table S3.A. Phylogenetic analysis of
Sideritis taxa using the plastid molecular marker
rbcL. Alignment of 20
Sideritis DNA sequences retrieved from GenBank compared to
Sideritis syriaca subsp.
syriaca GR-1-BBGK-15,5939 specimen of the current study (OP909056); the conserved areas (100%) are toggled;
Table S3.B. Phylogenetic analysis of
Sideritis taxa using the plastid molecular marker
psbA-
trnH. Alignment of 94
Sideritis DNA sequences retrieved from GenBank compared to
Sideritis syriaca subsp.
syriaca GR-1-BBGK-15,5939 specimen of the current study (OP909054); the conserved areas (100%) are toggled;
Figure S1. Effect of fertilization regime through different (root/foliar) application methods on leaf index of absorbance difference of
Sideritis syriaca subsp.
syriaca at vegetative (A), early flowering (B), and full flowering (C) stage;
Figure S2. Effect of fertilization regime through different (root/foliar) application methods on leaf L value of
Sideritis syriaca subsp.
syriaca at vegetative (A), early flowering (B), and full flowering (C) stage;
Figure S3. Effect of fertilization regime through different (root/foliar) application methods on leaf a value of
Sideritis syriaca at vegetative (A), early flowering (B), and full flowering (C) stage;
Figure S4. Effect of fertilization regime through different (root/foliar) application methods on leaf b value of
Sideritis syriaca subsp.
syriaca at vegetative (A), early flowering (B), and full flowering (C) stage.
Author Contributions
Conceptualization, G.T. (Georgios Tsoktouridis), and N.K.; Methodology, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., I.T., I.S., G.T. (Georgios Tsoktouridis), K.G., and T.M.; Software, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., I.I., and T.M.; Validation, D.F., V.A.T., F.B., E.S., K.K., F.J.D., I.I., T.M., K.G., and N.K.; Formal Analysis, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., I.I, I.T., I.S., and T.M.; Investigation, K.P., D.F., G.T. (Georgios Tsoktouridis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., I.T., I.S., K.G., G.T. (Georgios Tsaniklidis), T.M.; Resources, K.P., T.M., I.I., V.A.T., and G.T. (Georgios Tsoktouridis); Data Curation, K.P., D.F., G.T. (Georgios Tsaniklidis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., I.T., I.S., G.T. (Georgios Tsoktouridis), and T.M.; Writing—Original Draft Preparation, K.P., D.F., G.T. (Georgios Tsoktouridis), I.S., V.A.T., and N.K.; Writing—Review & Editing, K.P., D.F., G.T. (Georgios Tsoktouridis), V.A.T., F.B., E.S., K.K., F.J.D., I.I., I.S., G.T. (Georgios Tsaniklidis), T.M., and N.K.; Visualization, V.A.T., D.F., and N.K.; Supervision, T.M., D.F., G.T. (Georgios Tsoktouridis); Project Administration, G.T. (Georgios Tsoktouridis); Funding Acquisition, K.P., K.G., N.K., T.M., and G.T. (Georgios Tsoktouridis). All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed during 2018-2021 by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-05380) entitled “Conservation and sustainable utilization of rare threatened endemic plants of Crete for the development of new products with innovative precision fertilization” (Acronym: PRECISE-M).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the results of this study are included in the article and its supplementary materials. Original datasets are also available upon request All newly generated DNA sequences were submitted to the GenBank under the accession numbers OR909054-OR909060.
Acknowledgments
We are grateful to the laboratory staff and the undergraduate students at the Hellenic Mediterranean University for their contributions, continued diligence, and dedication to their craft. We are also thankful to the staff of the Balkan Botanic Garden of Kroussia and the Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization Demeter for the ex situ conservation of mother plants of S. syriaca subsp. syriaca. The valuable comments of the editor and two anonymous reviewers are greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Casiraghi, M.; Labra, M.; Ferri, E.; Galimberti, A.; De Mattia, F. DNA barcoding: a six-question tour to improve users’ awareness about the method. Brief. Bioinform. 2010, 11, 440–453. [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proceedings. Biol. Sci. 2003, 270, 313–321. [CrossRef]
- Kress, W.J.; Erickson, D.L. DNA barcodes: genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2761–2762. [CrossRef]
- Kehie, M.; Kumaria, S.; Devi, K.S.; Tandon, P. Genetic diversity and molecular evolution of Naga King Chili inferred from internal transcribed spacer sequence of nuclear ribosomal DNA. Meta Gene 2016, 7, 56–63. [CrossRef]
- Vazquez, J.L.H.; Gómez-Mercado, F.; Guerrero, J.-L.G.; Rodriguez-García, I.; García-Maroto, F. Genetic relationships and population structure within taxa of the endemic Sideritis pusilla (Lamiaceae) assessed using RAPDs. Bot. J. Linn. Soc. 1999, 129, 345–358. [CrossRef]
- Lindqvist, C.; Albert, V.A. Origin of the Hawaiian endemic mints within North American Stachys (Lamiaceae). Am. J. Bot. 2002, 89, 1709–1724. [CrossRef]
- Patelou, E.; Chatzopoulou, P.; Polidoros, A.N.; Mylona, P. V. Genetic diversity and structure of Sideritis raeseri Boiss. & Heldr. (Lamiaceae) wild populations from Balkan Peninsula. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100241. [CrossRef]
- Sarrou, E.; Doukidou, L.; Avramidou, E. V.; Martens, S.; Angeli, A.; Stagiopoulou, R.; Fyllas, N.M.; Tourvas, N.; Abraham, E.; Maloupa, E.; et al. Chemodiversity is closely linked to genetic and environmental diversity: Insights into the endangered populations of the local endemic plant Sideritis euboea Heldr. of Evia Island (Greece). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100426. [CrossRef]
- Barber, J.C.; Finch, C.C.; Francisco-Ortega, J.; Santos-Guerra, A.; Jansen, R.K. Hybridization in Macaronesian Sideritis (Lamiaceae): Evidence from incongruence of multiple independent nuclear and chloroplast sequence datasets. Taxon 2007, 56, 74–88. [CrossRef]
- Sevindik, E. Comparative and phylogenetic analysis of Rubisco large subunit (rbcL) proteins in some Sideritis L. (Lamiaceae) species: A bioinformatic approach. Genetica 2019, 51, 69–80. [CrossRef]
- Barber, J.C.; Ortega, J.F.; Santos-Guerra, A.; Marrero, A.; Jansen, R.K. Evolution of endemic Sideritis (Lamiaceae) in Macaronesia: Insights from a chloroplast DNA restriction site analysis. Syst. Bot. 2000, 25, 633–647. [CrossRef]
- Bendiksby, M.; Thorbek, L.; Scheen, A.C.; Lindqvist, C.; Ryding, O. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon 2011, 60, 471–484. [CrossRef]
- Theodoridis, S.; Stefanaki, A.; Tezcan, M.; Aki, C.; Kokkini, S.; Vlachonasios, K.E. DNA barcoding in native plants of the Labiatae (Lamiaceae) family from Chios Island (Greece) and the adjacent Çeşme-Karaburun Peninsula (Turkey). Mol. Ecol. Resour. 2012, 12, 620–633. [CrossRef]
- Kalivas, A.; Ganopoulos, I.; Xanthopoulou, A.; Chatzopoulou, P.; Tsaftaris, A.; Madesis, P. DNA barcode ITS2 coupled with high resolution melting (HRM) analysis for taxonomic identification of Sideritis species growing in Greece. Mol. Biol. Rep. 2014, 41, 5147–5155. [CrossRef]
- Salmaki, Y.; Heubl, G.; Weigend, M. Towards a new classification of tribe Stachydeae (Lamiaceae): Naming clades using molecular evidence. Bot. J. Linn. Soc. 2019, 190, 345–358. [CrossRef]
- Aneva, I.; Zhelev, P.; Bonchev, G. Sideritis elica, a new species of Lamiaceae from Bulgaria, revealed by morphology and molecular biology. Plants (Basel) 2022, 11(21), 2900. [CrossRef]
- Kloukina, C.; Tomou, E.-M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Skaltsa, H. Non-polar secondary metabolites and essential oil of ex situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crops Prod. 2020, 158, 112957. [CrossRef]
- NCBI National Center for Biotechnology Information (NCBI)[Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on Nov 15, 2023).
- Grdiša, M.; Radosavljević, I.; Liber, Z.; Stefkov, G.; Ralli, P.; Chatzopoulou, P.S.; Carović-Stanko, K.; Šatović, Z. Divergent selection and genetic structure of Sideritis scardica populations from southern Balkan Peninsula as revealed by AFLP fingerprinting. Sci. Rep. 2019, 9, 12767. [CrossRef]
- Nemli, S.; Subaşi, Ü.; Eroglu, V.; Şenol, S.; Muhammed, B.; Tanyolac, B. High Levels of genetic variation as detected by AFLP in Sideritis tmolea from Western Turkey. Turkish J. Field Crops 2014, 192, 247–254. [CrossRef]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Part 2: Maps.; Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, 2016; ISBN 978 392 180 097 3. [CrossRef]
- Stikoudi, M.; Maloupa, E.; Lazari, D.; Krigas, N. Aromagarden and Cooking for Wellness: Discovering secrets of Mediterranean plants; i-Print: Thessaloniki, 2016.
- EMA/HMPC European Union herbal monograph on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba (Final). Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub_en.pdf (accessed on Nov 30, 2023).
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology (Basel). 2021, 10. [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology (Basel). 2021, 10, 195. [CrossRef]
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Chapter 4 - Sustainable use of mediterranean medicinal-aromatic plants. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press, 2020; pp. 57–74 ISBN 978-0-12-814700-9. [CrossRef]
- Bodeker, G.; Burford, G.; Volkov, A. Integrative, traditional and complementary medicine. In International Encyclopedia of Public Health; 2017; pp. 288–295 ISBN 9780128037089. [CrossRef]
- Jain, D.; Chaudhary, P.; Kotnala, A.; Hossain, R.; Hossain, M. Hepatoprotective activity of medicinal plants: A mini review. J. Med. Plants Stud. 2020, 8, 183–188. [CrossRef]
- Hoareau, L.; Dasilva, E.J. Medicinal plants: a re-emerging health aid. Electron. J. Biotechnol. 1999, 2, 0. http://dx.doi.org/10.4067/S0717-34581999000200002.
- Dutta, T.; Anand, U.; Saha, S.; Mane, A.; Prasanth, D.; Kandimalla, R.; Prockow, J.; Dey, A.; Cooke, S. Advancing urban ethnopharmacology: a modern concept of sustainability, conservation and cross-cultural adaptations of medicinal plant lore in the urban environment. Conserv. Physiol. 2022, 9, 1–20. [CrossRef]
- Hassanvand, F.; Rezaei Nejad, A.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crops Prod. 2019, 134, 19–25. [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11. [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [CrossRef]
- Malik, A.; Suryapani, S.; Ahmad, J. Chemical vs organic cultivation of medicinal and aromatic plants: The choice is clear. Int. J. Med. Aromat. Plants 2011, 1, 5–13.
- Ninou, E.G.; Paschalidis, Konstantinos A. , Mylonas, I.G.; Christos, V.; Mavromatis, A.G. The effect of genetic variation and nitrogen fertilization on productive characters of Greek oregano. Act. Agric. Scand. 2017, 67, 372–379. [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [CrossRef]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10. [CrossRef]
- Rehim, A.; Amjad Bashir, M.; Raza, Q.-U.-A.; Gallagher, K.; Berlyn, G.P. Yield enhancement of biostimulants, vitamin B12, and CoQ10 compared to inorganic fertilizer in Radish. Agronomy 2021, 11. [CrossRef]
- Selim, M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int. J. Agron. 2020, 2020, 1–14. [CrossRef]
- Gezahegn, A.M. Role of integrated nutrient management for sustainable maize production. Int. J. Agron. 2021, 2021, 9982884. [CrossRef]
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, I.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology and Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer Netherlands: Dordrecht, 2009; pp. 207–228 ISBN 978-90-481-2305-6. [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [CrossRef]
- Zhang, Y.; Ntagkas, N.; Fanourakis, D.; Tsaniklidis, G.; Zhao, J.; Cheng, R.; Yang, Q.; Li, T. The role of light intensity in mediating ascorbic acid content during postharvest tomato ripening: A transcriptomic analysis. Postharvest Biol. Technol. 2021, 180, 111622. [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Rusaczonek, A.; Rembiałkowska, E. Antioxidant content in black currants from organic and conventional cultivation. Electron. J. Polish Agric. Univ. Ser. Food Sci. Technol. 2008, 11.
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [CrossRef]
- Tõnutare, T.; Moor, U.; Mölder, K.; Põldma, P. Fruit composition of organically and conventionally cultivated strawberry “Polka.” Agron. Res. 2009, 7, 755–760.
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [CrossRef]
- Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [CrossRef]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G.; et al. GIS-facilitated germination of stored seeds from five wild-growing populations of Campanula pelviformis Lam. and fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9. [CrossRef]
- Amujoyegbe, B.; Opabode, J.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum Sorghum bicolour (L.) Moench). Afr. J. Biotechnol. (ISSN 1684-5315) Vol 6 Num 16 2010, 6. [CrossRef]
- Hollingsworth, P.; For; Rest, L.; Spouge, J.; Hajibabaei, M.; Ratnasingham, S.; Bank, M.; Chase, M.; Cowan, R.; Erickson, D.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. 2009, 106, 12794–12797. [CrossRef]
- Hollingsworth, P.; Graham, S.; Little, D. Choosing and using a plant DNA barcode. PLoS One 2011, 6, e19254. [CrossRef]
- POWO. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on Jul 2, 2023).
- Tsaballa, A.; Kelesidis, G.; Krigas, N.; Sarropoulou, V.; Bagatzounis, P.; Grigoriadou, K. Taxonomic identification and molecular DNA barcoding of collected wild-growing orchids used traditionally for salep production. Plants 2023, 12(17), 3038. [CrossRef]
- Nazar, N.; Howard, C.; Slater, A.; Sgamma, T. Challenges in medicinal and aromatic plants DNA barcoding; Lessons from the Lamiaceae. Plants 2022, 11(1), 137. [CrossRef]
- Tezcan, M.; Vlachonasios, K.; Aki, C. DNA barcoding study on Sideritis trojana Bornm. An endemic medicinal plant of IDA mountain, Turkey. Freesenius Environ. Bull. 2010, 19, 1352–1355.
- Pimm, S.; Russell, G.; Gittleman, J.; Brooks, T. The future of biodiversity. Science 1995, 269, 347–350. [CrossRef]
- Bentley, R. Medicinal Plants; Domville-Fife Press: London, 2010.
- Ross, I. Medicinal plants of the world: Chemical constituents, traditional and modern medicinal uses; Humana Press Inc.: New Jersey, 2005.
- Laird, S..; Pierce, A.. Promoting sustainable and ethical botanicals. Strategies to improve commercial raw material sourcing: Results from the sustainable botanicals pilot project industry surveys, case studies and standards collection; Rainforest Alliance: New York, 2002.
- Bilias, F.; Ipsilantis, I.; Samara, E.; Tsoktouridis, G.; Glavakis, E.; Grigoriadou, K.; Krigas, N.; Matsi, T. From the wild to the field: Effect of foliar or soil application of inorganic or semi-organic fertilizers on various parameters of four local endemic plant species of Crete (Greece). Braz. J. Bot. 2023. [CrossRef]
- Fanourakis, D.; Kazakos, F.; Nektarios, P.A. Allometric individual leaf area estimation in Chrysanthemum. Agronomy 2021, 11(4), 795. [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13(24), 14030. [CrossRef]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweetpotato (Ipomoea batatas): The influence of genetics, environment, and G×E. New Phytol. 2019, 225(5), 2183-2195. [CrossRef]
- Nasiri, A.; Taheri-Garavand, A.; Fanourakis, D.; Zhang, Y.-D.; Nikoloudakis, N. Automated grapevine cultivar identification via leaf imaging and deep convolutional neural networks: A proof-of-concept study employing primary Iranian varieties. Plants 2021, 10(8), 1628. [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [CrossRef]
- Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Kampouropoulou, S.; Papanastasi, K.; Grigoriadou, K.; Menexes, G.; Maloupa, E. Micropropagation and molecular characterization of Thymus sibthorpii Benth. (Lamiaceae), an aromatic-medicinal thyme with ornamental value and conservation concern. In Vitr. Cell. Dev. Biol. - Plant 2019, 55, 647–658. [CrossRef]
- Yamane, K.; Kawahara, T. Intra- and interspecific phylogenetic relationships among diploid Triticum - Aegilops species (Poaceae) based on base-pair substitutions, indels, and microsatellites in chloroplast noncoding sequences. Am. J. Bot. 2005, 92, 1887–1898. [CrossRef]
- Löhne, C.; Borsch, T. Molecular evolution and phylogenetic utility of the petD group II intron: A case study in basal angiosperms. Mol. Biol. Evol. 2005, 22, 317–332. [CrossRef]
- Ledford, H. Botanical identities. Nature 2008, 451, 616. [CrossRef]
- Lee, H.-L.; Jansen, R.K.; Chumley, T.W.; Kim, K.-J. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [CrossRef]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One 2007, 2, 1–10. [CrossRef]
- Chase, M.W.; Cowan, R.S.; Hollingsworth, P.M.; van den Berg, C.; Madriñán, S.; Petersen, G.; Seberg, O.; Jørgsensen, T.; Cameron, K.M.; Carine, M.; et al. A proposal for a standardised protocol to barcode all land plants. Taxon 2007, 56, 295–299. [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12(1), 94. [CrossRef]
- Javadi Asayesh, E.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7(11), 485. [CrossRef]
- Costa, G.; Fiori, G.; Torrigiani, P.; Noferini, M. Use of Vis/NIR spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. In Fresh Produce, Vol. 3; Global Science Books, UK, 2009; pp. 35-41. Available online: https://www.researchgate.net/publication/277217074_Use_of_VisNIR_Spectroscopy_to_Assess_Fruit_Ripening_Stage_and_Improve_Management_in_Post-Harvest_Chain#fullTextFileContent (accessed 12 December 2023).
- Sørensen, H.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Rezaei Nejad, A.; Ottosen, C.-O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. (Amsterdam) 2020, 267, 109354. [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 2021, 11. [CrossRef]
- Koubouris, G.; Bouranis, D.; Vogiatzis, E.; Rezaei Nejad, A.; Giday, H.; Tsaniklidis, G.; Ligoxigakis, E.; Blazakis, K.; Kalaitzis, P.; Fanourakis, D. Leaf area estimation by considering leaf dimensions in olive tree. Sci. Hortic. (Amsterdam) 2018, 240, 440-445. [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2022, 97, 346–360. [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [CrossRef]
- Lichtenthaler, H.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [CrossRef]
- Bremmer, J.M. Part 3: Chemical methods. In Methods of soil analysis; Sparks, D.L., Ed.; SSSA, ASA: Madison, WI, 1996; pp. 1085–1121.
Figure 1.
Phylogenetic tree illustrating the relationships among 15
Sideritis species based on the molecular plastid markers
rbcL and
trnL/
trnF. The tree was constructed using the Neighbor-Joining method and Maximum Composite Likelihood substitution model. Accession numbers of DNA sequences obtained in this study are indicated next to taxon names. The clade marked in orange represents
Sideritis syriaca subsp.
syriaca GR-1-BBGK-15,5939 studied herein. The taxon marked with asterisk (*) is characterized as
S. syriaca in the database, however, based on its original collection area (Mt. Dirphys in Evia, Greece), it should be identified as the unique local endemic mountain tea plant of this mountain namely
S. euboea and not as
S. syriaca subsp.
syriaca which is a single-island endemic of Crete Island, Greece [
55].
Figure 1.
Phylogenetic tree illustrating the relationships among 15
Sideritis species based on the molecular plastid markers
rbcL and
trnL/
trnF. The tree was constructed using the Neighbor-Joining method and Maximum Composite Likelihood substitution model. Accession numbers of DNA sequences obtained in this study are indicated next to taxon names. The clade marked in orange represents
Sideritis syriaca subsp.
syriaca GR-1-BBGK-15,5939 studied herein. The taxon marked with asterisk (*) is characterized as
S. syriaca in the database, however, based on its original collection area (Mt. Dirphys in Evia, Greece), it should be identified as the unique local endemic mountain tea plant of this mountain namely
S. euboea and not as
S. syriaca subsp.
syriaca which is a single-island endemic of Crete Island, Greece [
55].
Figure 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf SPAD value of Sideritis syriaca subsp. syriaca at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences. .
Figure 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf SPAD value of Sideritis syriaca subsp. syriaca at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences. .
Figure 3.
Effect of fertilization regime through different (root/foliar) application methods on chlorophyll fluorescence value of Sideritis syriaca subsp. syriaca at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 3.
Effect of fertilization regime through different (root/foliar) application methods on chlorophyll fluorescence value of Sideritis syriaca subsp. syriaca at vegetative (A), early flowering (B), and full flowering (C) stage. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization regime through different (root/foliar) application methods on four leaf shape factors of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 4.
Effect of fertilization regime through different (root/foliar) application methods on four leaf shape factors of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization regime through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 5.
Effect of fertilization regime through different (root/foliar) application methods on above-ground dry weight (A), water content (B) and dry weight partitioning to inflorescences (C) of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of six replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization regime through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 6.
Effect of fertilization regime through different (root/foliar) application methods on leaf chlorophyll (A), carotenoid (B), total phenol (C) and total flavonoid (D) content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization regime through different (root/foliar) application methods on leaf soluble sugar content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 7.
Effect of fertilization regime through different (root/foliar) application methods on leaf soluble sugar content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the means of three replicates ± SEM. Within each plot, different letters indicate significant differences.
Figure 8.
(A) Sideritis syriaca subsp. syriaca wild-growing individual at full flowering on rocky crevices of Sfakianes Madares in Lefka Ori, Chania, Crete (Greece); (B) Densely hairy leaf indumentum and (C) Pilot field with S. syriaca subsp. syriaca experimental individuals cultivated at the premises of the Hellenic Mediterranean University, Heraklion, Crete (Greece).
Figure 8.
(A) Sideritis syriaca subsp. syriaca wild-growing individual at full flowering on rocky crevices of Sfakianes Madares in Lefka Ori, Chania, Crete (Greece); (B) Densely hairy leaf indumentum and (C) Pilot field with S. syriaca subsp. syriaca experimental individuals cultivated at the premises of the Hellenic Mediterranean University, Heraklion, Crete (Greece).
Table 1.
Effect of fertilization regime through different (root/foliar) application methods on leaf essential macronutrient content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulator by soil application (THEOMASS). Values represent the mean of three replicates ± SE.
Table 1.
Effect of fertilization regime through different (root/foliar) application methods on leaf essential macronutrient content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulator by soil application (THEOMASS). Values represent the mean of three replicates ± SE.
| Treatment |
N |
P |
K |
Ca |
Mg |
| |
(g kg-1) |
| C |
13.4 ± 0.4c* |
2.3 ± 0.1b |
18.9 ± 0.3b |
23.0 ± 1.4a |
3.2 ± 0.1a |
| INM-fa |
21.8 ± 1.7ab |
3.4 ± 0.0a |
18.7 ± 0.4b |
18.7 ± 0.5a |
2.7 ± 0.0a |
| ChF-fa |
18.3 ± 0.3abc |
3.5 ± 0.0a |
22.4 ± 0.5a |
22.9 ± 1.8a |
3.0 ± 0.1a |
| INM-sa |
22.8 ± 0.8a |
2.5 ± 0.1b |
18.7 ± 0.5b |
22.1 ± 1.7a |
2.9 ± 0.1a |
| ChF-sa |
12.8 ± 1.6c |
2.7 ± 0.0b |
20.4 ± 0.4ab |
23.3 ± 1.5a |
4.2 ± 0.5a |
| MPE-sa |
16.0 ± 1.5bc |
2.3 ± 0.2b |
19.1 ± 0.5b |
25.6 ±1 .0a |
3.2 ± 0.1a |
|
p F-test |
0.021 |
< 0.001 |
0.029 |
NS#
|
NS#
|
Table 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf essential micronutrient content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SE.
Table 2.
Effect of fertilization regime through different (root/foliar) application methods on leaf essential micronutrient content of Sideritis syriaca subsp. syriaca. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as biostimulant by soil application (THEOMASS). Values represent the mean of three replicates ± SE.
| Treatment |
Cu |
Zn |
Fe |
Mn |
B |
| |
(mg kg-1) |
| C |
14.9 ± 0.1abc*
|
26.5 ± 1.4a |
1058 ± 89cd |
52.0 ± 1.6c |
27.5 ± 0.8b |
| INM-fa |
13.2 ± 0.4c |
23.8 ± 0.5a |
1057 ± 49cd |
62.8 ± 1.3bc |
19.7 ± 0.3d |
| ChF-fa |
14.5 ± 0.5bc |
27.5 ± 0.4a |
1277 ± 79bc |
83.0 ± 5.0a |
26.9 ± 0.3bc |
| INM-sa |
15.8 ± 0.3ab |
24.7 ± 0.6a |
1703 ± 111a |
68.4 ± 1.1b |
25.8 ± 0.3bc |
| ChF-sa |
15.5 ± 0.6ab |
23.3 ± 0.1a |
871 ± 19d |
37.0 ± 0.4d |
24.1 ± 0.8c |
| MPE-sa |
16.9 ± 0.2a |
26.8 ± 1.0a |
1578 ± 24ab |
54.0 ± 1.5c |
35.0 ± 0.6a |
|
p F-test |
0.033 |
NS#
|
0.003 |
< 0.001 |
< 0.001 |
Table 3.
Effect of fertilization regime through different (root/foliar) application methods on essential macronutrient content of Sideritis syriaca subsp. syriaca inflorescences. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as bioreactor by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
Table 3.
Effect of fertilization regime through different (root/foliar) application methods on essential macronutrient content of Sideritis syriaca subsp. syriaca inflorescences. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as bioreactor by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
| Treatment |
N |
P |
K |
Ca |
Mg |
Na |
| |
(g kg-1) |
| C |
8.65±0.00c |
1.31±0.45b |
12.67±2.22ab |
1.84±0.19bc |
1.04±0.20ab |
0.52±0.11b |
| INM-fa |
10.14±0.36bc |
2.40±0.38a |
13.95±0.44ab |
2.75±0.31ab |
1.28±0.01a |
0.85±0.02a |
| ChF-fa |
10.28±2.07bc |
1.72±0.12ab |
9.19±1.24b |
1.22±0.01c |
0.61±0.12b |
0.51±0.08b |
| INM-sa |
16.63±1.68a |
1.26±0.31b |
12.21±1.16ab |
3.20±0.69ab |
1.27±0.29a |
0.55±0.16b |
| ChF-sa |
10.46±0.00bc |
2.64±0.00a |
16.84±0.00a |
3.49±0.00a |
1.40±0.00a |
0.68±0.00ab |
| MPE-sa |
13.38±1.92ab |
1.61±0.53ab |
12.52±2.52ab |
2.56±1.04ab |
1.36±0.35a |
0.44±0.10b |
|
pF-test
|
0.014 |
0.075 |
0.081 |
0.083 |
NS#
|
NS |
Table 4.
Effect of fertilization regime through different (root/foliar) application methods on essential micronutrient content of Sideritis syriaca subsp. syriaca inflorescences. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as bioreactor by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
Table 4.
Effect of fertilization regime through different (root/foliar) application methods on essential micronutrient content of Sideritis syriaca subsp. syriaca inflorescences. C: Control (water); INM-fa: Integrated nutrient management (INM) by foliar application; ChF-fa: Chemical fertilization by foliar application; INM-sa: INM by soil application; ChF-sa: Chemical fertilization by soil application; MPE-sa: Mixture of plant extracts as bioreactor by soil application (THEOMASS). Values represent the mean of three replicates ± SEM.
| Treatment |
Β |
Zn |
Fe |
| |
|
mg kg-1
|
|
| C |
6.93±0.12a |
14.81±3.20ab |
149.86±43.79ab |
| INM-fa |
5.60±0.20b |
15.32±1.41ab |
201.35±42.26a |
| ChF-fa |
6.60±0.34ab |
10.78±2.93b |
49.94±23.79b |
| INM-sa |
6.82±0.78a |
14.30±1.20ab |
135.70±38.84ab |
| ChF-sa |
5.49±0.00b |
20.20±1.00a |
157.32±10.00ab |
| MPE-sa |
6.29±0.18ab |
17.51±4.47ab |
148.68±56.23ab |
|
pF-test
|
0.068 |
NS |
NS |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).