Submitted:
22 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
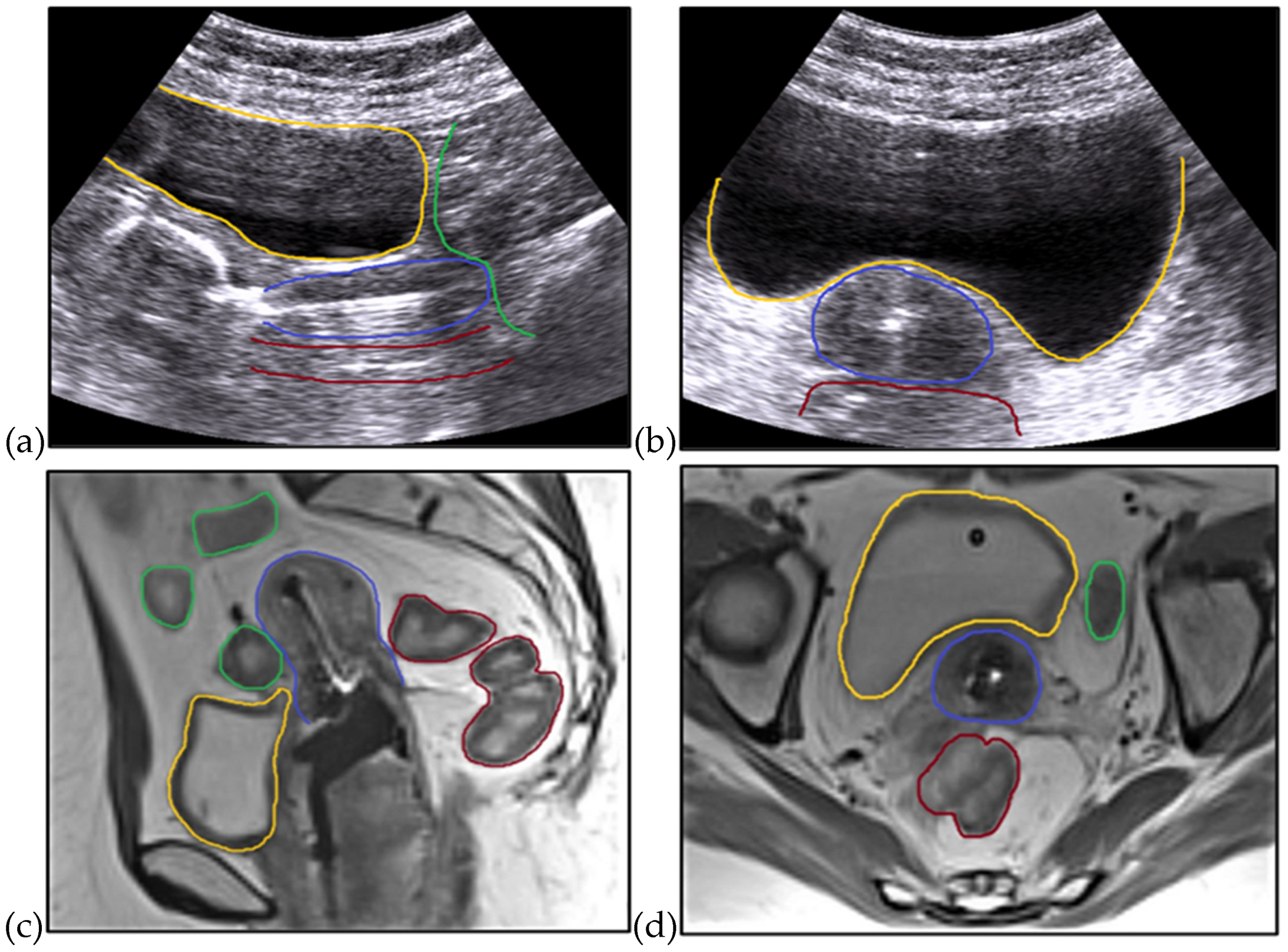
2. Materials and Methods
3. Results
| Imaging modality | X-ray | Ultrasound | Tomography | Magnetic resonance | Positron emission tomography |
|---|---|---|---|---|---|
| Soft-tissue resolution | Poor | Good | Good@ | Excellent | Good |
| Geometric accuracy | Good | Good | Good* | Good# | Excellent |
| Image quality | Protocol dependent | Operator and protocol dependent | Sequence and protocol dependent | Sequence and protocol dependent& | Sequence and protocol dependent& |
| Artifacts | Metal | Multiple types and causes | Multiple types and causes | Multiple types and causes | Multiple types and causes |
| Slice orientation | Single planar | Multi planar | Trans axial | Multi planar | Trans axial |
| Usage in cervical cancer | Yes | Yes | Yes | Yes | Rarely used |
| Accurate visualization and reconstruction of brachytherapy applicators | Yes | Yes | Yes | Yes | Yes |
| Type of applicators | Metal or plastic with metal X-ray guides | Metal, plastic | Metal, Plastic | Metal%, Plastic | Metal, Plastic |
| Possibility of radiation dose calculation | No | NA | Yes | NA | Yes |
| Portability (potential for intraoperative use) | Yes | Yes | Sometimes available | Sometimes available | No |
| Time to obtain image | Seconds | Minutes | Seconds | Hour | Hours |
| Availability | Low | Low | Medium | High | High |
| Cost of equipment | Low | Low | High | High | High |
| Cost of scan | Low | Low | High | High | High |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent, and A. Jemal, “Global cancer statistics, 2012,” CA Cancer J Clin, vol. 65, no. 2, pp. 87–108, Mar. 2015. [CrossRef]
- Z. Li, S. Yang, L. Liu, and S. Han, “A comparison of concurrent chemoradiotherapy and radiotherapy in Chinese patients with locally advanced cervical carcinoma: a multi-center study,” Radiat Oncol, vol. 9, no. 1, Sep. 2014. [CrossRef]
- H. Lukka et al., “Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer - A meta-analysis,” Clin Oncol, vol. 14, no. 3, pp. 203–212, 2002. [CrossRef]
- K. Tanderup, P. J. Eifel, C. M. Yashar, R. Pötter, and P. W. Grigsby, “Curative radiation therapy for locally advanced cervical cancer: Brachytherapy is NOT optional,” International Journal of Radiation Oncology Biology Physics, vol. 88, no. 3. Elsevier Inc., pp. 537–539, Mar. 01, 2014. [CrossRef]
- R. Pötter et al., “Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer,” Radiotherapy and Oncology, vol. 100, no. 1, p. 116, Jul. 2011. [CrossRef]
- C. Haie-Meder et al., “Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV,” Radiotherapy and Oncology, vol. 74, no. 3, pp. 235–245, Mar. 2005. [CrossRef]
- T. P. Hellebust et al., “Recommendations from Gynaecological (GYN) GEC-ESTRO working group: Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy,” Radiotherapy and Oncology, vol. 96, no. 2, pp. 153–160, Aug. 2010. [CrossRef]
- A. N. Viswanathan et al., “American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy,” Brachytherapy, vol. 11, no. 1, pp. 47–52, Jan. 2012. [CrossRef]
- J. C. A. Dimopoulos et al., “Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV) - Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy,” Radiotherapy & Oncology, 2012.
- R. Pötter et al., “Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy - 3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology,” Radiotherapy and Oncology, vol. 78, no. 1, pp. 67–77, Jan. 2006. [CrossRef]
- K. Tanderup et al., “Image guided intensity modulated External beam radiochemotherapy and MRI based adaptive BRAchytherapy in locally advanced CErvical cancer EMBRACE-II,” 2015. Accessed: Oct. 27, 2022. [Online]. Available: https://www.embracestudy.dk/UserUpload/PublicDocuments/EMBRACE II Protocol.pdf.
- D. Van Elburg et al., “Clinical implementation of 3D transvaginal ultrasound for intraoperative guidance of needle implant in template interstitial gynecologic high-dose-rate brachytherapy,” Brachytherapy, Nov. 2023. [CrossRef]
- World Health Organization, “Cancer Today.” Accessed: Jun. 05, 2023. [Online]. Available: https://gco.iarc.fr/today/home.
- M. Gherghe et al., “Quantitative Analysis of SPECT-CT Data in Metastatic Breast Cancer Patients—The Clinical Significance,” Cancers (Basel), vol. 14, no. 2, Jan. 2022. [CrossRef]
- Tr. Pãtrascu, H. Doran, E. Catrina, O. Mihalache, D. Degeratu, and G. Predescu, “Tumori sincrone ale tractului digestiv,” Chirurgia (Bucur), vol. 0, no. 1, Feb. 2010.
- C. Cirimbei, V. Rotaru, E. Chitoran, and S. Cirimbei, “Laparoscopic Approach in Abdominal Oncologic Pathology, Proceedings of the 35th Balkan Medical Week, Athens, Greece, 25-27 September 2018,” Proceedings of the 35th Balkan Medical Week, Athens, Greece. Accessed: Jul. 20, 2023. [Online]. Available: https://www.webofscience.com/wos/woscc/full-record/WOS:000471903700043.
- V. Rotaru, E. Chitoran, C. Cirimbei, S. Cirimbei, and L. Simion, “Preservation of Sensory Nerves During Axillary Lymphadenectomy, Proceedings of the 35th Balkan Medical Week, Athens, Greece, 25-27 September 2018,” Proceedings of the 35th Balkan Medical Week, Athens, Greece. Accessed: Jul. 20, 2023. [Online]. Available: https://www.webofscience.com/wos/woscc/full-record/WOS:000471903700045.
- L. Simion et al., “Simultaneous Approach Of Colo-Rectal And Hepatic Lesions In Colo-Rectal Cancers With Liver Metastasis - A Single Oncological Center Overview,” Chirurgia (Bucur), vol. 118, no. 3, 2023.
- L. Simion et al., “Indocyanine Green (ICG) and Colorectal Surgery: A Literature Review on Qualitative and Quantitative Methods of Usage,” Medicina (B Aires), vol. 59, no. 9, p. 1530, Aug. 2023. [CrossRef]
- L. Simion et al., “Analysis of Efficacy-To-Safety Ratio of Angiogenesis-Inhibitors Based Therapies in Ovarian Cancer: A Systematic Review and Meta-Analysis,” Diagnostics, vol. 13, no. 6. MDPI, Mar. 01, 2023. [CrossRef]
- L. Simion et al., “Breast Reconstruction Following Mastectomy for Breast Cancer or Prophylactic Mastectomy-Therapeutic Options and Results,” 2023. [CrossRef]
- L. Simion et al., “A Decade of Therapeutic Challenges in Synchronous Gynecological Cancers from the Bucharest Oncological Institute,” Diagnostics, vol. 13, no. 12, p. 2069, Jun. 2023. [CrossRef]
- L. Simion et al., “Inequities in Cervical Cancer Screening and HPV Vaccination Programs and Their Impact on Incidence/Mortality Rates and the Severity of Disease in Romania,” Jul. 2023. [CrossRef]
- C. E. Condrat, L. Filip, M. Gherghe, D. Cretoiu, and N. Suciu, “Maternal HPV infection: Effects on pregnancy outcome,” Viruses, vol. 13, no. 12. MDPI, Dec. 01, 2021. [CrossRef]
- D. Berger, S. Van Dyk, L. Beaulieu, T. Major, and T. Kron, “Modern Tools for Modern Brachytherapy,” Clin Oncol, Aug. 2023. [CrossRef]
- L. Simion et al., “Inequities in Screening and HPV Vaccination Programs and Their Impact on Cervical Cancer Statistics in Romania,” 2023. [CrossRef]
- R. Vojtíšek, F. Mouryc, D. Čechová, R. Ciprová, J. Ferda, and J. Fínek, “[MRI based 3D brachytherapy planning of the cervical cancer - our experiences with the use of the uterovaginal Vienna Ring MR CT applicator],” Klin Onkol, vol. 27, no. 1, pp. 45–51, 2014. [CrossRef]
- C. Onal et al., “Comparison of conventional and CT-based planning for intracavitary brachytherapy for cervical cancer: target volume coverage and organs at risk doses,” J Exp Clin Cancer Res, vol. 28, no. 1, p. 95, 2009. [CrossRef]
- W. Y. Song, J. L. Robar, B. Morén, T. Larsson, Å. Carlsson Tedgren, and X. Jia, “Emerging technologies in brachytherapy,” Phys Med Biol, vol. 66, no. 23, p. 23TR01, Nov. 2021. [CrossRef]
- T. P. Hellebust, “Place of modern imaging in brachytherapy planning,” Cancer/Radiothérapie, vol. 22, no. 4, pp. 326–333, Jun. 2018. [CrossRef]
- INTERNATIONAL ATOMIC ENERGY AGENCY, “The Transition from 2-D Brachytherapy to 3-D High Dose Rate Brachytherapy,” The Transition from 2-D Brachytherapy to 3-D High Dose Rate Brachytherapy, pp. 1–33, 2015, Accessed: Aug. 28, 2023. [Online]. Available: https://www.iaea.org/publications/10705/the-transition-from-2-d-brachytherapy-to-3-d-high-dose-rate-brachytherapy.
- H. Zhang et al., “Clinical implementation, logistics and workflow guide for MRI image based interstitial HDR brachytherapy for gynecological cancers,” J Appl Clin Med Phys, vol. 20, no. 11, pp. 37–49, Nov. 2019. [CrossRef]
- A. Torresin et al., “Review of potential improvements using MRI in the radiotherapy workflow,” Z Med Phys, vol. 25, no. 3, pp. 210–220, Sep. 2015. [CrossRef]
- H. Kim et al., “Workflow and efficiency in MRI-based high-dose-rate brachytherapy for cervical cancer in a high-volume brachytherapy center,” Brachytherapy, vol. 17, no. 5, pp. 753–760, Sep. 2018. [CrossRef]
- M. Arenas et al., “Individualized 3D scanning and printing for non-melanoma skin cancer brachytherapy: A financial study for its integration into clinical workflow,” J Contemp Brachytherapy, vol. 9, no. 3, pp. 270–276, Jun. 2017. [CrossRef]
- ICRU, “Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix,” 10.1093/jicru_ndw027, vol. 13, no. 1–2, pp. 1-10, Apr. 2013. [CrossRef]
- M. Abdel-Wahab, E. Zubizarreta, A. Polo, and A. Meghzifene, “Improving Quality and Access to Radiation Therapy—An IAEA Perspective,” Semin Radiat Oncol, vol. 27, no. 2, pp. 109–117, Apr. 2017. [CrossRef]
- W. Small, J. B. Strauss, C. S. Hwang, L. Cohen, and J. Lurain, “Should Uterine Tandem Applicators Ever Be Placed Without Ultrasound Guidance? No: A Brief Report and Review of the Literature,” International Journal of Gynecologic Cancer, vol. 21, no. 5, pp. 941–944, Jul. 2011. [CrossRef]
- M. C. Jacobsen et al., “Contemporary image-guided cervical cancer brachytherapy: Consensus imaging recommendations from the Society of Abdominal Radiology and the American Brachytherapy Society,” Brachytherapy, vol. 21, no. 4, pp. 369–388, Jul. 2022. [CrossRef]
- S. van Dyk et al., “Clinical outcomes from an innovative protocol using serial ultrasound imaging and a single MR image to guide brachytherapy for locally advanced cervix cancer,” Brachytherapy, vol. 15, no. 6, pp. 817–824, Nov. 2016. [CrossRef]
- E. Tharavichitkul et al., “Intermediate-term results of trans-abdominal ultrasound (TAUS)-guided brachytherapy in cervical cancer,” Gynecol Oncol, vol. 148, no. 3, pp. 468–473, Mar. 2018. [CrossRef]
- S. van Dyk, S. Kondalsamy-Chennakesavan, M. Schneider, D. Bernshaw, and K. Narayan, “Assessing changes to the brachytherapy target for cervical cancer using a single MRI and serial ultrasound,” Brachytherapy, vol. 14, no. 6, pp. 889–897, Nov. 2015. [CrossRef]
- Z. Zhang, N. Zhang, and G. Cheng, “Application of three-dimensional multi-imaging combination in brachytherapy of cervical cancer,” Radiologia Medica, vol. 128, no. 5, pp. 588–600, May 2023. [CrossRef]
- R. Banerjee and M. Kamrava, “Brachytherapy in the treatment of cervical cancer: a review,” Int J Womens Health, pp. 6–555, 2014. [CrossRef]
- S. Van Dyk, K. Narayan, R. Fisher, and D. Bernshaw, “Conformal Brachytherapy Planning for Cervical Cancer Using Transabdominal Ultrasound,” Int J Radiat Oncol Biol Phys, vol. 75, no. 1, pp. 64–70, Sep. 2009. [CrossRef]
- K. Narayan, S. van Dyk, D. Bernshaw, P. Khaw, L. Mileshkin, and S. Kondalsamy-Chennakesavan, “Ultrasound guided conformal brachytherapy of cervix cancer: Survival, patterns of failure, and late complications,” J Gynecol Oncol, vol. 25, no. 3, pp. 206–213, 2014. [CrossRef]
- S. Addley, M. Persic, R. Kirke, and S. Abdul, “Combined direct hysteroscopic and real-time ultrasound guidance facilitating safe insertion of intra-uterine brachytherapy applicator for locally advanced cervical cancer with significant endocervical stenosis: A novel collaborative approach,” Gynecol Oncol Rep, vol. 47, Jun. 2023. [CrossRef]
- M. T. M. Davidson, J. Yuen, D. P. D’Souza, J. S. Radwan, J. A. Hammond, and D. L. Batchelar, “Optimization of high-dose-rate cervix brachytherapy applicator placement: The benefits of intraoperative ultrasound guidance,” Brachytherapy, vol. 7, no. 3, pp. 248–253, Jul. 2008. [CrossRef]
- A. N. Viswanathan and B. A. Erickson, “Three-Dimensional Imaging in Gynecologic Brachytherapy: A Survey of the American Brachytherapy Society,” Int J Radiat Oncol Biol Phys, vol. 76, no. 1, pp. 104–109, Jan. 2010. [CrossRef]
- R. Y. Kim, D. S. Levy, D. J. Brascho, and K. D. Hatch, “Uterine Perforation During Intracavitary Application Prognostic Significance in Carcinoma of the Cervix,” Radiology, vol. 147, pp. 249–251, 1983.
- M. D. Rotmensch, M. D. Waggoner, and M. D. Quiet, “Ultrasound Guidance for Placement of Difficult Intracavitary Implants,” Gynecol Oncol, vol. 54, no. 2, pp. 159–162, 1994. [CrossRef]
- I. Sahinler et al., “Tandem application with transvaginal ultrasound guidance,” Int J Radiat Oncol Biol Phys, vol. 59, no. 1, pp. 190–196, May 2004. [CrossRef]
- R. G. Stock, K. Chan, M. Terk, J. K. Dewyngaert, N. N. Stone, and P. Dotting, “A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance,” Int J Radiat Oncol Biol Phys, vol. 37, no. 4, pp. 819–825, Mar. 1997. [CrossRef]
- D. N. Sharma, G. K. Rath, S. Thulkar, S. Kumar, V. Subramani, and P. K. Julka, “Use of transrectal ultrasound for high dose rate interstitial brachytherapy for patients of carcinoma of uterine cervix,” J Gynecol Oncol, vol. 21, no. 1, pp. 12–17, Mar. 2010. [CrossRef]
- U. Mahantshetty et al., “Trans-abdominal ultrasound (US) and magnetic resonance imaging (MRI) correlation for conformal intracavitary brachytherapy in carcinoma of the uterine cervix,” Radiotherapy and Oncology, vol. 102, no. 1, pp. 130–134, Jan. 2012. [CrossRef]
- P. Petric and C. Kirisits, “Potential role of TRAns Cervical Endosonography (TRACE) in brachytherapy of cervical cancer: Proof of concept,” J Contemp Brachytherapy, vol. 8, no. 3, pp. 215–220, 2016. [CrossRef]
- N. Nesvacil, M. P. Schmid, R. Pötter, G. Kronreif, and C. Kirisits, “Combining transrectal ultrasound and CT for image-guided adaptive brachytherapy of cervical cancer: Proof of concept,” Brachytherapy, vol. 15, no. 6, pp. 839–844, Nov. 2016. [CrossRef]
- S. van Dyk, P. Khaw, M. Y. Lin, D. Chang, and D. Bernshaw, “Ultrasound-guided Brachytherapy for Cervix Cancer,” Clin Oncol, vol. 33, no. 9, pp. e403–e411, Sep. 2021. [CrossRef]
- Y. Hu, Y. Jin, D. Wang, and Y. Luo, “Observation of hemostatic effectiveness and safety of ultrasound-CT guided 3D intracavitary and interstitial brachytherapy in the treatment of larger cervical cancer with bleeding: A retrospective study,” Medicine, vol. 102, no. 37, p. E34904, Sep. 2023. [CrossRef]
- C. Pintakham, E. Tharavichitkul, S. Wanwilairat, and W. Nobnop, “Comparative dosimetry of brachytherapy treatment planning between a volume-based plan by CT and a point-based plan by TAUS in CT datasets for brachytherapy,” J Radiother Pract, vol. 22, no. 2, Nov. 2023. [CrossRef]
- S. Sridhara, K. V. Rana, S. Naware, G. Singh, K. Nagare, and G. Gupta, “Ultrasound training in surgical residency: Is it feasible?,” Medical Journal of Dr. D.Y. Patil University, vol. 7, no. 3, pp. 284–288, May 2014. [CrossRef]
- K. Hsieh et al., “Dose and fractionation regimen for brachytherapy boost in cervical cancer in the US,” Gynecol Oncol, vol. 180, pp. 55–62, Jan. 2024. [CrossRef]
- P. St-Amant, W. Foster, M. A. Froment, P. Noel, S. Aubin, and L. Beaulieu, “PD-0180: Use of 3D-ultrasound for cervical cancer brachytherapy: an imaging technique to improve contouring,” Radiotherapy and Oncology, vol. 115, p. S89, Apr. 2015. [CrossRef]
- W. Lee and 李蘊恩, “Implementation of 3D printing in photon, electron radiotherapy and brachytherapy,” 2023, Accessed: Dec. 15, 2023. [Online]. Available: http://hub.hku.hk/handle/10722/335584.
- R. Kumar et al., “Disparities in brachytherapy utilization in cervical cancer in the United States: A comprehensive literature review,” Gynecol Oncol, vol. 179, pp. 79–84, Dec. 2023. [CrossRef]
- “Study Details | Clinical Trial of Molecular Biomarkers in Women With Uterine Cervix Cancer | ClinicalTrials.gov.” Accessed: Dec. 15, 2023. [Online]. Available: https://www.clinicaltrials.gov/study/NCT05462951?cond=Cervical%20Cancer&intr=brachytherapy&rank=1.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





