You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Causal Relationship Investigation between Iron Status and Metabolic Disorders by Mendelian Randomization Studies Revealed Increased Risks of Hypertension and Hypertriglyceridemia but a Protective Role against Hyperglycemia among Taiwanese Han Chinese
Altmetrics
Downloads
146
Views
49
Comments
0
This version is not peer-reviewed
Abstract
A number of epidemiological studies have implicated elevated iron levels with higher risks of cardiometabolic diseases, including the metabolic syndrome (MetS). MetS is a pathologic clustering of metabolic derangements characterized by central obesity, insulin resistance, hypertension, and dyslipidemia, which can all lead to increased cardiovascular disease morbidity and mortality. However, it remains unclear whether a causal association exists between higher iron status and MetS and its associated risk components. Using the data from the Taiwan Biobank (TWB) (19,012 males; 42,340 females) and of the European Whites from the UK Biobank (UKB) (128,549 males; 142,817 females), we first constructed ethnic- and sex-specific hemoglobin-polygenic risk scores (Hb-PRS) as instrumental variable (IV) for iron status with the Hb-associated SNPs we previously identified from genome-wide association studies (GWAS). We then conducted Mendelian randomization (MR) analyses to elucidate the causal role of elevated iron (either Hb-PRS quintiles or continuous Hb-PRS) on various metabolic components. MR-Egger regressions determined the potential pleiotropic effects of the constructed Hb-PRSs, while the causal relationship of Hb-PRS with anemia served as a control in the analyses. The mean Hb concentration range corresponding to the Hb-PRS quintiles is relatively narrow in both UKB (14.91-15.21 g/dL for males and 13.39-13.71 g/dL for females) and TWB (14.79-15.28 g/dL for males and 12.78-13.21 g/dL for females). In these windows of Hb-PRSs, we found differential associations of Hb-PRS with diabetes (T2D) or hyperglycemia (HGY) as compared to other MetS components. The highest quintiles of Hb-PRSs were causally associated with greater risks of hypertension (HTN) and hypertriglyceridemia (DLP) (i.e., [Taiwanese HC males: Q5 vs. Q1 ORHTN = 1.10 (1.00–1.21), p = 0.0473, Q4 vs. Q1 ORDLP = 1.10 (1.00–1.22), p = 0.0485; European White males: ORHTN = 1.05 (1.01–1.09), p = 0.0194, ORDLP = 1.04 (1.00–1.08), p = 0.0468]). Interestingly, we obtained a consistent negative causal association between increasing Hb-PRS quintiles and T2D or HGY risk across all sex subgroups (i.e., [Taiwanese HC females: ORHGY = 0.89 (0.84–0.95), 0.88 (0.83–0.94), 0.87 (0.82–0.93), 0.86 (0.81–0.92) from Q2 to Q5; European White females: ORHGY = 0.92 (0.87–0.98), 0.91 (0.86–0.97), 0.93 (0.87–0.98), 0.87 (0.82–0.93) from Q2 to Q5; all p < 0.05]. The positive causal relationships between Hb-PRSs and diastolic blood pressure (DBP) and triglycerides (TG) but inverse causal association with glucose (GLU) and glycated hemoglobin (HbA1c) further corroborated such findings. There is, however, a lack of causal association with central obesity (CO), while a U-shaped type of causality was observed for gout (GT) or hyperuricemia (HUA). Sensitivity analyses, which excluded subjects based on self-reports of health conditions or medications and hospital episode statistics data, revealed similar results. Our MR studies confirm a modest causal relationship between propensity of elevated iron status and increased risk of HTN and DLP. We have further observed that modestly lower iron levels causally contribute to the development of T2D. Further validation and elucidation of the underlying biological mechanisms are highly warranted.
Keywords:
Subject: Biology and Life Sciences - Life Sciences
1. Introduction
Iron is an essential micronutrient that has several vital functions in the human body [1]. Primarily, iron facilitates oxygen transport as an integral component of the hemoglobin (Hb) protein. Around 65% of body iron is integrated in the Hb molecules in circulating red blood cells, which partially reflects the largest amount of functional iron in the body [2]. Additionally, iron functions as a transport medium for electrons within cells and is a crucial requirement for DNA synthesis and cell division. Hence, dysregulated iron homeostasis often results in the most common human disorders that range from anemia at the negative end of the iron status spectrum (i.e., nutritional iron deficiency), to hemochromatosis at the other extreme (i.e., iron overload) [3].
Systemic iron levels and iron nutrition status, however, are modifiable human traits. As an important and essential micronutrient, iron must be provided by diet and, in some cases, through supplementation or food fortification. Heme iron in red meat is a superb exogenous source of iron as compared to that of the plant sources [4]. It is estimated to contribute more than 40% of the total absorbed iron in meat-eating population groups [5]. Nevertheless, dietary iron absorption will always depend on systemic iron needs, such that more iron is absorbed during states of deficiency while less is absorbed when iron body stores are replete. Hepcidin, the key systemic iron-regulatory hepatic hormone, drives the regulation of intestinal iron absorption [6].
Iron overnutrition, which is commonly associated with the increased intakes of iron from high consumption of red meat (namely beef, lamb, organ meats, and processed meats), had drawn attention because of its potential as a risk factor for cardiovascular disease and chronic metabolic conditions such as diabetes [7]. It has been postulated from the “iron hypothesis” that iron depletion may protect against heart disease; whereas high levels of body iron stores promote cardiovascular and metabolic diseases through oxidative damage that takes place in the different tissues of the body [8]. In the development of atherosclerosis, for instance, high amounts of iron will catalyze the Fenton reaction that generates free radicals oxidizing the low-density lipoproteins, eventually leading to the deposition of atherosclerotic plaque [9].
The cardiometabolic syndrome, also commonly referred to as the metabolic syndrome (MetS), has been defined by the World Health Organization (WHO) as an aggregation of metabolic dysfunctions that mainly include insulin resistance or impaired glucose tolerance, dyslipidemia (namely elevated triglycerides and low high-density lipoprotein cholesterol levels), high blood pressure, and central obesity [10]. Each of these associated risk factors has an independent effect; but altogether, they lead to a substantial increase in cardiovascular disease morbidity and mortality [11]. A continuous increase in MetS global prevalence has also been observed among men and women across all age groups for some time [12], which is thought to be driven by genetic susceptibility and lifestyle changes. Nonetheless, the exact mechanisms as to how iron in excess contributes to MetS and its individual risk components is yet to be fully elucidated.
For several years, there has been accumulating reports on the possible links of either altered iron status, red meat consumption, or dietary intakes of iron (heme, in particular) on the pathophysiology of the MetS risk components and cardiovascular diseases [7,13–22]. Inferring causal effects, however, cannot be achieved from these associations due to unmeasured or residual confounding and reverse causation. For instance, it has been difficult to disentangle the coherent mechanistic actions of both heme iron and saturated fatty acids from high intakes of red meat in relation to cardiometabolic derangements [23]. These two may have been interplaying in the complex etiology of atherosclerotic diseases as the storage of saturated fatty acids in the body has been deemed as the culprit of hypercholesterolemia and coronary artery disease [24]. Epidemiological studies cannot tease apart the attributing effects due to the high collinearity between these two factors.
Mendelian randomization is a genetic epidemiology method which has recently been used to examine the causality between iron in excess and cardiometabolic risks. It has become a valuable tool in human nutrition, especially when the conduct of randomized controlled trials is not feasible to infer causality between dietary factors and biomarkers of health outcomes [25]. MR provides causal estimates between an exposure to a modifiable environmental risk factor and a health outcome, wherein genetic variants in the form of single nucleotide polymorphisms (SNPs) are used as instrumental variables for the said risk factor [26]. A polygenic risk score (PRS) is constructed and used to predict the risk of health outcomes. The MR technique is said to overcome biases that are inherent in conventional epidemiological studies, such as confounding and reverse causation, since alleles of genetic loci are, in principle, randomly assigned during conception [27]. As with all epidemiological approaches, however, the plausibility of MR findings will depend on specific assumptions [28]. First is the ‘relevance’ assumption, which requires that the genetic variants (i.e., IV) associate with the risk factor-of-interest. Second is the ‘independence’ assumption, for which there should be an independence between the genetic variants and confounders of the risk factor-outcome association. Lastly, is the ‘exclusion restriction’ assumption, which indicates that the genetic variants do not affect the outcome except through the risk factor (i.e., vertical pleiotropy).
Few MR studies have provided evidence to support that iron status, as a modifiable risk factor, can causally influence the etiology of cardiovascular diseases [29–31] and metabolic conditions [32,33]. Initial MR data were also observed to be contradicting among some studies. In a study conducted by Gill and colleagues [30], a protective effect of higher iron propensity (measured by a linear combination of SNPs predicting serum iron, transferrin saturation, ferritin, and transferrin) was shown to reduce coronary artery disease risk. Conversely, in another study [31], increased iron propensity was rather shown to have a detrimental effect on stroke risk, particularly on cardioembolic stroke. Such opposing observations, although for different CVD outcomes, were derived using the same set of IVs utilized in both studies, namely HFE rs1800562 and rs1799945 and TMPRSS6 rs855791 (i.e., three of the most strongly associated genetic variants of iron status [34]). Nevertheless, the unique genetic differences across ethnicities may have contributed to such findings and should be taken into consideration [35]. On the contrary, most of the results from observational association studies depicted elevated iron levels as a risk factor for cardiometabolic conditions. We have also recently shown in our sex-specific observational association studies that higher iron, as depicted by deciles of Hb levels, increases the risks of MetS and its metabolic components in the Taiwanese Han Chinese and European Whites [36]. Thus, investigating the predictability of genetic propensity score for iron status, and comparing it between ethnicities or across population groups, becomes immensely important as it may provide further insights on the role of iron nutrition (i.e., both in deficiency or excess) in the etiology of cardiometabolic diseases.
To date, no MR study has evaluated the causality between iron status and the key metabolic components of MetS altogether in one study. In this regard, we conducted ethnic- and sex-specific MR studies among the Taiwanese HC and European Whites in order to elucidate the causal relationships between iron status and the risks of MetS and its components (i.e., CO, HTN, DLP, and T2D or HGY). We have included GT or HUA as a risk component due to the increasing evidence that supports its association with the pathogenesis of MetS [37,38]. We further investigated the causal role of iron levels on several quantitative metabolic traits, namely waist circumference (WC), systolic (SBP) and diastolic blood pressure (DBP), fasting blood glucose (FBG) or random glucose (GLU), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and uric acid (UA) levels, along with the related phenotypes glycated hemoglobin (HbA1c), cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels. Our findings shall provide additional insights on the etiology of MetS risk components and, at the same time, help better identify at-risk individuals based on the genetic propensity for iron status. Precision iron nutrition, in the future, could prevent further complications associated with MetS and ultimately lead to the improvement of public health.
2. Materials and Methods
2.1. Study Design, Study Populations, and Ethical Considerations
A one-sample Mendelian randomization design was applied in this study. As the standard design for an MR analysis, SNPs (i.e., IVs), exposure, and outcome are measured in the same individuals and extracted from a single dataset of participants [28]. Ethnic-specific MR studies between the Taiwanese HC of TWB and European Whites of UKB cross-validated the causal inferences in these two large ethnicities that are known to differ in iron status profile. Sex stratification further accounted for the known differences in iron status between males and females.
Information on the TWB and UKB Projects can be obtained at https://www.twbiobank.org.tw/ (accessed on 2 January 2023) and https://www.ukbiobank.ac.uk/ (accessed on 2 January 2023), respectively [39,40]. The inclusion-exclusion criteria for the study populations of our MR studies followed that of our previously conducted two-stage genome-wide association studies (2S-GWAS) [35].
The TWB initially consisted of 20,764 male and 46,751 female Taiwanese HC (N=67,515). Participants were aged from 30 to 70 years old and were generally healthy at the time of assessment. We removed subjects who had self-reported kidney failure or cancer and had missing Hb data.
The UKB, on the other hand, consisted of 229,134 males and 273,402 females (N=502,536) who resided in the United Kingdom from 2006 to 2010. Subjects were aged from 40 to 70 years old during assessment. We initially selected the participants who identified themselves as White (i.e., British, Irish, or any other White background) and were born in the UK. We then excluded subjects who: (1) had inconsistent reported and genetic sex, (2) were pregnant, (3) had self-reported kidney failure, cancer, hereditary or genetic hematological disorder, clotting disorder or excessive bleeding, acquired immunodeficiency syndrome, tuberculosis, or any tropical and travel-related infections [41]. We further removed individuals with a recorded fasting time of 0 (i.e., non-fasted) or beyond 24 h (i.e., over-fasted) during the blood sample collection.
We finally included the TWB and UKB participants whom we were able to construct Hb-PRSs on, as derived from the sets of ethnic-specific and sex-specific Hb-associated SNPs we identified in the 2S-GWAS of Hb [35]. A total of 61,352 Taiwanese HC (19,012 males; 42,340 females) were included in the TWB MR studies, whereas a final sample size of 271,366 European Whites (128,549 males; 142,817 females) were used in the UKB MR studies.
All the work performed in this study has been carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol has been given ethical clearance by the Institutional Review Board of Academia Sinica, with reference number AS-IRB 02. The ethics approval of the UKB Project was provided by the UK National Health Service’s National Research Ethics Committee, with reference number 11/NW/0382. Duly signed informed consents were collected from the participants. All available data and information were treated with the utmost confidentiality.
2.2. Data Collection in the Taiwan Biobank and UK Biobank
Details on the data collection in TWB and UKB and the ascertainment of metabolic risk outcomes were fully described in previous studies [35,36].
Briefly, venous blood samples were collected from the participants at baseline assessment for the genetic data, hematological indices, and blood biochemistry parameters. Blood collection in the UKB was done at random and participants were non-fasted [42]. Hemoglobin and hematological indices were immediately analyzed following blood draw using routinely calibrated automated clinical hematology analyzers. Biochemical markers that include FBG or GLU, TG, TC, HDL-C and LDL-C, and UA were measured via enzymatic assays using clinical chemistry analyzers. HbA1c level was specifically determined through high-performance liquid chromatography.
Anthropometric parameters (i.e., weight, body mass index (BMI), WC) and blood pressure (BP) readings were measured twice following standard procedures. A third measurement of BP level was taken if the first two readings differed by more than 10 mmHg.
Information about the socio-demographic profile, lifestyle behaviors, personal and family medical history, environmental risk factors, and other health-related information were obtained through a structured face-to-face interview in the TWB and through a comprehensive touchscreen questionnaire in the UKB [39,40]. A verbal interview with a trained nurse was done afterward for some variables that needed verification (i.e., type and frequency of prescription medications) in the UKB.
2.3. Ascertainment of Metabolic Risk Outcomes
We followed the definition of NCEP-ATP III as the diagnostic criteria to ascertain the MetS, namely: (1) WC > 102 cm in males or >88 cm in females; (2) TG > 150 mg/dL (>1.70 mmol/L) or taking triglyceride-lowering drugs; (3) HDL-C < 40 mg/dL (<1.00 mmol/L) in males or <50 mg/dL (<1.30 mmol/L) in females or taking statins or other medicines for high cholesterol; (4) SBP/DBP >130/>85 mmHg or current use of anti-hypertensive drugs; and (5) FBG > 100 mg/dL (>5.6 mmol/L) or current use of anti-hyperglycemic drugs [43]. The WC cut-off point for Asians set by the International Diabetes Federation (i.e., WC > 90 cm in males and >80 cm in females) was specifically used to define CO in the Taiwanese HC group [44].
With respect to the individual MetS component, we first ascertained the disease outcomes in both TWB and UKB using self-reported physician-diagnosed health conditions and ethnic-specific cut-off values for WC, SBP/DBP, FBG or GLU, HbA1c, TG, HDL-C, and UA. Additionally, in the UKB, we utilized the data on prescription medications and hospital episode statistics (i.e., International Classification of Diseases 10th (ICD10) and 9th (ICD9) revisions) in the ascertainment of HTN, DLP, T2D or HGY, and GT or HUA.
CO was defined as a WC > 90 cm in males or >80 cm in females for East Asians and a WC > 102 cm in males or >88 cm in females for Europeans [44,45]. HTN was diagnosed based on an SBP/DBP reading of >140/>90 mmHg [46,47]. T2D was ascertained from an FBG level > 126 mg/dL (>7.0 mmol/mol) [48] or an HbA1c > 6.5% (>48 mmol/mol) [49]. However, because of the non-fasting state of participants in the UKB, we followed the algorithms of Eastwood and co-authors, which used a slightly higher GLU level of > 11.1 mmol/L (>200 mg/dL) in order to exclude false positives when capturing hyperglycemia in the European White group [50]. We defined DLP in this study as having either hypertriglyceridemia (HTG) (i.e., TG > 150 mg/dL or >1.70 mmol/L), low levels of HDL-C (i.e., HDL-C < 40 mg/dL or <1.00 mmol/L in males; <50 mg/dL or <1.30 mmol/L in females), or a combination of both [51,52]. Hypercholesterolemia (HCL) and high LDL-C levels were then determined as TC > 240 mg/dL or >6.20 mmol/L and LDL-C > 160 mg/dL or >4.10 mmol/L, respectively [51,52]. GT or HUA was ascertained from a UA level > 7.0 mg/dL or >416.0 μmol/L in males and >6.0 mg/dL or >357.0 μmol/L in females [53].
2.4. Genetic Data and Two-Stage Genome-Wide Association Studies of Hemoglobin
2S-GWAS have been conducted to identify the SNPs associated with Hb among 60,518 Taiwanese HC (18,824 males; 41,694 females) and 269,451 European Whites (127,726 males; 141,725 females). The quality control of genetic data and the 2S-GWAS protocols were described in detail in a previous paper [35].
Genomic DNAs were extracted from the collected blood samples of TWB and UKB participants. A total of about 650,000 SNPs were genotyped in the TWB using a ThermoFisher Scientific Axiom Genome-Wide Array Plate (TWB 2.0) platform [54]. UKB genetic data, on the other hand, included more than 800,000 markers that were genotyped using the Applied Biosystems UK BiLEVE Axiom Array and the closely related Applied Biosystems UK Biobank Axiom Array [55,56].
2.5. Construction of Hemoglobin-Polygenic Risk Scores
Ethnic- and sex-specific Hb-PRSs were constructed as a combination of the Hb-associated SNPs using the allelic scoring function in PLINK 1.9 (https://www.cog-genomics.org/plink/1.9/ (accessed on 6 February 2023)) [57,58]. The strategy of aggregating SNPs into a single score relies on the assumption that the effect of all independent SNPs could be combined by their individual effect sizes and the resulting PRS may predict exposures more accurately than smaller subsets of these SNPs [59]. We followed the strategy of Choi and team in constructing the PRS (https://choishingwan.github.io/PRS-Tutorial/ (accessed on 6 February 2023)) [60].
In order to capture the most approximate causal independent genetic variants, linkage disequilibrium (LD) between Hb-associated SNPs was first investigated and accounted for. LD-based clumping was carried out in PLINK, which retained the SNPs that were weakly correlated and largely independent of each other (i.e., r2 < 0.5 across 250 kb) while preferentially selecting the SNPs that were mostly associated with Hb concentration. SNPs that flank pseudogenes were removed. In accordance with the “iron hypothesis”, some alleles were flipped to ensure that all effect alleles were risk-increasing (i.e., positive beta) [29]. Finally, 36 out of 61 Hb-associated SNPs were used in the construction of Hb-PRS for the male Taiwanese HC, 58 out of 82 SNPs for the female Taiwanese HC, 698 out of 1,982 SNPs for the male European Whites, and 805 out of 2,248 SNPs for the female European Whites.
Hb-PRS was calculated as the summation of the number of effect alleles per SNP (i.e., 0, 1, or 2) for each individual, multiplied by the effect size for a particular SNP (i.e., beta-coefficient relating Hb concentration to number of alleles) as obtained from the linear regression models of the 2S-GWAS joint analyses [60]: Hb-PRS = β1 × Allelic Number (SNP1) + β2 × Allelic Number (SNP2) + … + βn × Allelic Number (SNPn).
2.6. One-Sample Mendelian Randomization Analyses
Independent one-sample MR analyses that involved the Taiwanese HC and European White cohorts were performed, wherein Hb-PRSs served as the IVs (i.e., genetically instrumented Hb concentration) for iron status being the modifiable risk factor-of-interest in this study.
For describing the characteristics of subjects, continuous variables were expressed as mean ± s.d., whereas categorical characteristics were in counts and percentage values. The normal distribution of quantitative parameters was assessed, and extreme outliers were removed. We categorized subjects according to quintiles of ethnic- and sex-specific Hb-PRS. Trends in quantitative characteristics and prevalence across Hb-PRS quintiles were respectively tested by linear regression and the Cochran–Armitage trend test.
The main MR analyses utilized logistic and linear regressions wherein males and females were analyzed separately. Logistic regression models determined and compared between the two ethnic groups the associations across quintiles of Hb-PRS and the risks for each binary outcome, namely: MetS, CO, HTN, DLP, T2D (or T2D and HGY), and GT (or GT and HUA). The lowest Hb-PRS quintile served as the reference. Age, age-squared, and the first ten genetic PCs were adjusted in the first model. Smoking and alcohol drinking status (as well as menopausal status in females) were further adjusted in the second model, since years of life and certain lifestyle factors may modify the inherent effects of Hb-associated genetic loci. The categorization of smoking status (i.e., never smoked, stopped smoking, occasionally smoking, or currently smoking), alcohol drinking status (i.e., never drank, stopped drinking, occasionally drinking, or currently drinking), and menopausal status in females (i.e., non-menstruating, unsure of menopausal status, or had age-at-menopause <44, 45–49, 50–54, or >55 years) were all previously described in detail [36].
Additionally, the MetS metabolic traits WC, SBP, DBP, FBG or GLU, TG, HDL-C, and UA, along with the related phenotypes HbA1c, TC, and LDL-C, were analyzed as quantitative outcomes. We examined the potential causality of Hb-PRS on such traits through linear regressions, which similarly adjusted for potential confounders in the models. Variables with skewed distribution were log-transformed prior to analyses.
All MR analyses were carried out in SAS v9.4. The criteria for statistical significance were a p-value of <0.05.
2.7. MR-Egger Regressions and Sensitivity Analyses
We conducted MR-Egger regressions to investigate whether the causal estimates obtained from the MR analyses were influenced by pleiotropy. MR-Egger was implemented using the package “Mendelianrandomization” in R v4.1.1 (https://www.r-project.org/ (accessed on 5 June 2023)) [61].
Briefly, the associations of IVs with various metabolic risk outcomes and metabolic traits were first analyzed in PLINK. Beta-coefficients (i.e., log odds ratio) and standard errors were derived from univariable regression analyses. MR-Egger was then initiated through the mr_input() function of MRInput object, where the betas and standard errors of the SNPs-Hb association (i.e., bx, bxse) and the SNPs-metabolic risk outcomes/traits association (i.e., by, byse) were entered. Genetic associations were aligned to the same effect alleles. MR-Egger estimates and all SNPs, including those possibly violating the IV assumption, were displayed though the mr_plot() function. The MR-Egger intercept parameter can be interpreted as an estimate of the overall directional pleiotropy, whereas the slope can provide pleiotropy-corrected causal estimates [62].
To further test the robustness of the main MR findings in a sensitivity analyses, we excluded subjects who were ascertained from either self-reported physician-diagnosed health conditions or medications (i.e., regular prescriptions) or hospital in-patient records. This validated the causal relationships upon excluding data sources that may potentially introduce biases and errors (e.g., misclassification of categories and subcategories of health conditions when using ICD codes) [32,50].
Finally, since the inverse relationship between Hb levels and anemia risk has been well established at the lower end of Hb distribution [63], the causal association between Hb-PRS and anemia (i.e., Hb < 13.0 g/dL in males and <12.0 g/dL in females) served as a control in the analyses.
3. Results
3.1. Discovery and Selection of Hemoglobin-Associated SNPs for the Construction of Hemoglobin-Polygenic Risk Scores
We previously conducted 2S-GWAS that involved 60,518 Taiwanese Han Chinese (18,824 males; 41,694 females) of the TWB and 269,451 European Whites (127,726 males; 141,725 females) of the UKB and identified common and ethnic-specific SNPs that are associated with blood Hb concentration [35]. In the current MR studies, the IVs for iron status were constructed from the clumped sets of ethnic- and sex-specific Hb-associated SNPs we discovered previously: 36 for male Taiwanese HC, 58 for female Taiwanese HC, 698 for male European Whites, and 805 for female European Whites (Tables S1–S4, respectively).
Table S5 provides the means and standard deviation of the Hb-PRSs as well as the regression coefficients relating Hb-PRS to Hb in g/dL, whereas the sex-specific distributions of Hb-PRSs and the scatterplots of Hb concentrations against Hb-PRSs are presented in Figure S1. In both ethnic groups, the mean Hb-PRSs of males were slightly higher than those of the females. Between the Taiwanese HC and European Whites, however, we observed generally higher mean Hb-PRSs in the former despite the very much larger numbers of SNPs used to construct the IVs in UKB than in TWB. The F-statistics were 404.0 and 659.5, respectively, for the male- and female-specific beta estimates of Hb-PRSs in the Taiwanese HC group, and 1,478.9 and 2,284.7, respectively, for the male- and female-specific estimates in the European White group. Around 1.5–2.1% of the variance in Hb levels could be explained by the constructed Hb-PRSs in TWB, whereas the resulting r2 values in UKB were at 1.1–1.6%.
Majority of the Hb-associated SNPs in TWB and UKB that were used to construct the IVs had very small effect sizes (i.e., beta <0.1) [35]. Nonetheless, the abovementioned regression parameters indicated a favorable relationship between the IVs and Hb [64].
3.2. Characteristics of Subjects in the Mendelian Randomization Studies
The characteristics of subjects in the TWB and UKB MR studies as stratified by quintiles of Hb-PRS and sex are presented in Tables S6 and S7, respectively. The mean Hb levels of male subgroups (15.0 g/dL) were found to be higher than the female Taiwanese HC (13.01 g/dL) and female European (13.52 g/dL) subgroups. As expected, we observed significant continuous increases in the mean Hb values, but decreasing anemia prevalence rates, with increasing quintiles of Hb-PRSs for all sex subgroups in both ethnic groups (p <0.0001).
The mean ages were at 50–51 years old for male and female Taiwanese HC and around 56–57 years for the European White counterparts. There were no significant differences on the mean age by Hb-PRS quintiles, except among male Europeans, wherein very small increments in age were noted. We also did not find any significant differences on the percentages of females who had entered menopause, as well as with their mean age at menopause, and the frequency or levels of physical activity of subjects. However, majority of the female subjects had already entered menopause during assessment, while some of the subjects were engaged in regular exercises or had moderate- to heavy- intensity exercises. Smoking status by Hb-PRS quintiles was found to be significantly different among male Taiwanese HC, particularly as the percentages of those who never smoked became larger toward higher quintiles of Hb-PRS. Alcohol consumption by Hb-PRS was also significant, but only among male Europeans, wherein the proportion of current drinkers generally decreased across Hb-PRS quintiles.
In both ethnic groups, there were highly significant continuous increases in mean DBP levels with Hb-PRSs. A similar trend was observed with the TG levels of European Whites, whereas a U- or J-shaped trend could be noted among the Taiwanese HC. The mean WC and SBP levels of male Taiwanese HC and the mean UA levels of female Taiwanese HC were also significantly increasing with Hb-PRS quintiles. Specific to the European subgroups, the mean GLU and HbA1c values were instead found to be remarkably decreasing with Hb-PRS quintiles. These data initially showed that causal associations between Hb-PRSs and some metabolic traits may exist. On the contrary, there were no significant differences on mean FBG and HDL-C by Hb-PRS quintiles in the Taiwanese HC subgroups, whereas mean WC, SBP, HDL-C and UA levels were not significant in the Europeans.
3.3. Trends on Prevalence Rates of Metabolic Disorders and the Causal Role of Iron Status on Metabolic Risk Components among Taiwanese Han Chinese and European Whites
Tables S8 and S9 show the overall distributions of metabolic risk outcomes in the TWB and UKB, respectively (Tables S10–S11 provide the frequency distributions of metabolic conditions based on measured health indicators by Hb-PRS and sex in the TWB and UKB; Tables S12–S13 present the percentages of cases based on self-reported data, and; Table S14 shows the percentages from hospital in-patient records that were only currently available in the UKB). The prevalence of MetS following the NCEP-ATP III criteria was 23.8% among Taiwanese HC and 40.3% among Europeans. Of all the MetS risk components, CO was found to be highly prevalent in both ethnicities (i.e., 44.7% among Taiwanese HC and 56.1% among Europeans). This was followed by DLP (34.3%; 62.3%), HTN (23.9%; 55.6%), GT (20.2%; 14.1%), and T2D (10.2%; 7.0%). DLP seemed to be relatively common among Europeans, but the non-fasting state of UKB participants may have contributed to this data. Between sexes, higher prevalence rates of MetS and all metabolic components, except CO, were observed among male than among female subjects in both ethnic groups.
There were apparent differences in the trends of prevalence rates of MetS and metabolic components by Hb-PRS in both ethnic groups. The MetS prevalence with increasing Hb-PRS quintiles was generally increasing among Taiwanese HC (p = 0.0066 in males; p = 0.0134 in females) and female Europeans (p = 0.0097). We observed highly significant continuous increases in the proportions of male and female Europeans with HTG (p <0.0001 and p = 0.0001, respectively), while high TG levels among male Taiwanese HC was also generally increasing (p = 0.0125). A steady increase in HTN prevalence based on SBP/DBP readings was initially observed in the female Taiwanese HC group only (p = 0.0302). However, based on self-reports of physician-diagnosed HTN and hospital diagnoses, significant positive trends have been similarly observed among Europeans. The prevalence rates of T2D based on either high FBG (GLU) or HbA1c levels, diagnosis by a doctor, or hospital records were all found to be consistent but rather remarkably decreasing toward higher quintiles of Hb-PRS.
MR analyses showed evidence to support the causal associations between elevated iron status and higher risks of HTN and DLP (Figure 1; Tables S16–S17) among Taiwanese HC and European Whites. Causal associations could not be concluded, however, between Hb-PRS quintiles and CO (Figure 2; Table S18). Hb-PRS was associated with GT and HUA but implicated a U-shaped type of causality in the two ethnicities (Figure 3; Table S19). On the other hand, our findings on T2D and HGY (Figure 4; Table S20–S21) were different from the other risk components as increasing Hb-PRS quintiles were observed to be causally associated with decreasing risks. Relatively low iron levels were causally associated with T2D development.
3.3.1. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Hypertension and Dyslipidemia
We found consistent positive causal associations between higher levels of genetically instrumented Hb and stronger HTN risks, as compared to the first quintiles (i.e., reference), in all sex groups of both ethnicities (Figure 1). The odds ratios (OR) obtained for HTN among Taiwanese HC upon fully adjusting for age, age-squared, smoking status, and alcohol drinking status (and menopausal status in females) are as follows: OR for Q5 = 1.10 (1.00–1.21, p = 0.0473) in males; OR for Q4 = 1.09 (1.00–1.19, p = 0.0033) in females. Among Europeans, the ORs for Q3 and Q5 in the male subgroup were respectively 1.06 (1.02–1.10, p = 0.0052) and 1.05 (1.01–1.09, p = 0.0194), whereas the ORs for Q4 and Q5 in the female subgroup were respectively 1.04 (1.00–1.08, p = 0.0495) and 1.07 (1.03–1.11, p = 0.0004) (Table S16).
There were also positive causal relationships observed between Hb-PRS quintiles and risk of DLP (Figure 1). In the male Taiwanese HC group, Q4 was found significant [OR = 1.10 (1.00–1.22, p = 0.0485)]. Conversely, among male Europeans, we attained significant but modest causal associations with DLP risk from Q2 to Q4 as follows: 1.04 (1.00–1.08, p = 0.0305), 1.05 (1.01–1.09, p = 0.0166), 1.04 (1.00–1.08, p = 0.0468). An OR for Q5 = 1.04 (1.01–1.08, p = 0.0220) was obtained among female Europeans (Table S17).
There was not much difference in the odds ratios between the crude and adjusted models. ORs among Europeans were relatively lower than among the Taiwanese HC despite a larger sample size and higher prevalence rates of HTN and DLP in the former. Between sex groups, the trend and risk elevations for the higher Hb-PRS quintiles were found to be relatively consistent in females.
3.3.2. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Central Obesity and Gout or Hyperuricemia Risk
As compared to HTN and DLP, we did not obtain any significant causal association between Hb-PRS quintiles and the risk of CO among Taiwanese HC and European Whites (Figure 2; Table S18). On the other hand, the causal associations with GT or HUA followed a distinct U-shaped or curvilinear type of association (Figure 2; Table S19). This was more evident among Europeans, wherein the middle quintiles resulted in ORs < 1.0. Particularly among male Europeans, ORs were at 0.92 (0.88−0.96, p = 0.0002), 0.90 (0.87−0.94, p <0.0001), and 0.93 (0.89−0.97, p = 0.0012) for Q2, Q3, and Q4 of Hb-PRS, respectively. An OR for Q3 = 0.93 (0.88−0.98, p = 0.0122) was obtained for the female European group. On the contrary, in the female Taiwanese HC group, the highest Hb-PRS quintile rather resulted in an OR of 1.14 (1.01−1.28, p = 0.0321).
3.3.3. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Type 2 Diabetes or Hyperglycemia
The causal role of increasing genetically instrumented Hb concentration levels on T2D or HGY risk was interesting. Here we obtained causal, protective effects of increasing Hb-PRS quintiles against T2D or HGY, which were consistent for all sex groups of both the Taiwanese HC and European Whites (Figure 3; Table S20). Very highly significant ORs (p <0.0001) for the highest Hb-PRS quintiles were obtained as follows: 0.83 (0.75−0.91) among male Taiwanese HC; 0.86 (0.81−0.92) among female Taiwanese HC; 0.88 (0.83−0.93) among male Europeans, and; 0.87 (0.82−0.93) among female Europeans. For both female subgroups, the highly significant negative causal associations with T2D or HGY were identified starting from Q2 up to the last. Even with diabetes alone as an outcome, the decreased risks at higher Hb-PRS quintiles remained evident (Table S21).
3.4. Causal Associations between Hb-PRS and Quantitative Metabolic Traits
Table 1 and Table 2 provide the sex-specific causal estimates of associations between Hb-PRS and quantitative metabolic traits among the Taiwanese HC and European Whites, respectively, as we further examined the association of Hb-PRS as a continuous IV on the different quantitative MetS metabolic traits WC, SBP, DBP, FBG or GLU, TG, HDL-C, and UA, along with the related phenotypes HbA1c, TC, and LDL-C.
Consistent with the results obtained from determining the causality between Hb-PRS and the metabolic risk components, we found that the Hb-PRSs were significantly and positively associated with DBP and TG levels among the Taiwanese HC (Table 1) and European Whites (Table 2). Genetically instrumented Hb corresponded to betas of 53.6 (p = 0.0008) and 69.1 (p = 0.0003) for DBP among male and female Taiwanese HC, respectively; while higher estimates at 131.3 and 221.4 (p <0.0001) were obtained for male and female Europeans. A positive causal association with SBP was only identified in the male Taiwanese HC group (β = 52.5, p = 0.0324), as the association in the female group was marginally significant. Beta values for TG were 543.5 (p = 0.0027) among male Taiwanese HC and 9.6 and 12.9 among Europeans (p <0.0001). Associations with HDL-C were inconsistent and not significant.
Although we did not previously identify a causal relationship between Hb-PRS and CO risk, a positive association with WC has been obtained in the male Taiwanese HC group (β = 33.4, p = 0.0199). Hb-PRS was also found causally and positively associated with UA but only among the female Taiwanese HC (β = 4.8, p = 0.0224).
The direction of causality between Hb-PRS and GLU among Europeans was consistently negative and very highly significant (β = −11.7 in males and −12.1 in females, p <0.0001). HbA1c, a highly correlated phenotype with FBG or GLU, depicted the same negative and highly significant association with Hb-PRS. Interestingly, Hb-PRS also demonstrated negative causal associations with LDL-C and TC in both ethnicities.
3.5. MR-Egger Regressions
Sex-specific MR-Egger regressions examined the presence of pleiotropy for the sets of genetic variants utilized as IVs for iron status. Tables S22 and S23 present the results from the MR-Egger regressions for the Hb-PRS−metabolic outcomes associations and Hb-PRS−metabolic traits associations, respectively. Figures S1-S4 are the scatterplots of sex-specific genetic associations and causal estimates of representative SNPs, which indicated the possible pleiotropic Hb-associated SNPs that may have violated the IV assumptions.
MR-Egger estimates provided further evidence that iron status was a causal risk factor for HTN and DLP among Europeans, but a causally protective risk factor against T2D or HGY for both ethnicities (Table S22). Additionally, the slope was significant and positive for DBP among Europeans and female Taiwanese HC, as well as with TG in male Europeans (Table S23). The slope of the MR-Egger regression can provide pleiotropy-corrected causal estimates. However, it is considered underpowered unless the SNPs combine to explain a large proportion of the variance in the exposure with varying effect sizes [65].
There was no apparent directional pleiotropy based on the MR-Egger intercepts except only for T2D or HGY in male Europeans alone (p = 0.042). All of the other intercept estimates did not significantly differ from zero. Moreover, the MR-Egger scatter plots (Figures S1-S4) suggested that the sets of SNPs used to construct the ethnic- and sex-specific Hb-PRSs do not indirectly affect MetS, the individual risk components, and various metabolic traits through other pathways or mechanisms.
3.6. Sensitivity Analyses
Sensitivity analyses were carried out to further validate the main MR findings, which similarly depicted the causal roles of genetically instrumented Hb concentration levels on the metabolic risk outcomes upon exclusion of subjects based on self-reported data and hospital in-patient records. A comparison of OR plots between the main MR and sensitivity analyses are presented in Figures S5 and S6 for the Taiwanese HC and European Whites, respectively. Corresponding OR tables are in Tables S24–S28. Sensitivity analyses for the causality between iron status and metabolic traits are then reported in Tables S29–S30.
Results of the sensitivity analyses revealed similar trends and findings. The evidence to support a positive causality of iron status on increased risks of HTN and DLP and a U-shaped causality for GT or HUA, particularly among Europeans, remained. The consistent causal associations between increasing Hb-PRS but decreased risks against T2D or HGY also still held true even with the sensitivity analyses. On the other hand, Hb-PRS remained positively associated (i.e., highly significant) with DBP and TG in both ethnicities and with UA particularly among female Taiwanese HC, but negatively related with GLU, HbA1c, TC, and LDL-C levels. No causal association with HDL-C was identified.
Finally, we utilized the causality between Hb-PRS and anemia risk as another sensitivity analyses and control for the main MR analyses. Causal, negative associations between Hb-PRS quintiles and anemia risk followed the well-established inverse relationship between blood Hb levels and anemia (Figure 4; Table S31). Monotonic decreases in anemia risk with increasing Hb-PRS quintiles were very highly significant (p <0.0001) across all sex groups, with the highest quintiles resulting in low ORs for anemia as follows: 0.44 (0.36−0.55) among male Taiwanese HC; 0.52 (0.48−0.57) among female Taiwanese HC; 0.54 (0.48−0.61) among male Europeans, and; 0.62 (0.57−0.67) among female Europeans. In this regard, the relationship of increasing genetically determined Hb against T2D or HGY (Figure 3; Table S20–S21) supports the causal role of relatively lower iron levels on T2D development. Further validation is, however, needed.
4. Discussion
Our ethnic- and sex-specific Mendelian randomization studies among the Taiwanese Han Chinese of Taiwan Biobank and the European Whites of UK Biobank elucidated the causal but differential relationship between iron status and several metabolic components. Here, ethnic- and sex-specific polygenic risk scores of blood hemoglobin concentration were constructed and served as instruments to represent iron status. We found that Hb-PRSs correspond to a relatively narrow range of Hb concentration (i.e., 14.91-15.21 g/dL for males and 13.39-13.71 g/dL for females in the UKB; 14.79-15.28 g/dL for males and 12.78-13.21 g/dL for females in the TWB). Within these relatively narrow mid-range of the Hb distribution, our findings support the causality of higher iron levels on stronger risks of hypertension and dyslipidemia for both ethnicities, which were further corroborated by the positive causal relationships with diastolic blood pressure and blood triglyceride levels. A distinct U-shape causality was obtained for gout or hyperuricemia, whereas no causality existed for central obesity. In contrast, we discovered an inverse causal association of increasing increments of iron level with decreasing risk of diabetes or hyperglycemia in both ethnicities, which, interestingly, implied a protective role for higher iron status against this specific metabolic condition alone and, at the same time, supports the causal role of relatively lower iron levels on the development of type 2 diabetes. We do not know whether there would be a risk rising at the higher end of Hb-PRS similar to our previous U-shaped finding on Hb concentration and diabetes in the observational study [36], since there were not enough participants with very high Hb-PRS. In addition, we did not present the association between Hb-PRS and the MetS due to the inconsistency that exists among the five components.
4.1. Causal Role of Elevated Iron on Increased Risks of Hypertension and Dyslipidemia
A number of cross-sectional and prospective studies have demonstrated positive associations between elevated iron status or dietary iron intakes (particularly heme iron from red meat) and stronger risk or higher incidence of HTN [17,22,36], DLP [18,21,36], or the MetS in general [19−21,36]. However, investigation of whether a true causality exists or not remained scarce.
The first MR-PheWAS of systemic iron status among Europeans in the UKB did not detect any causality between genetically predicted iron status and cardiometabolic phenotypes across the phenome, except for an evidence of a causal, protective effect of higher iron on lower hypercholesterolemia risk [32]. Our current MR studies and the first MR-PheWAS both utilized the hospital episode statistics data in UKB. However, the MR-PheWAS used only three iron status genetic instruments (i.e., HFE rs1800562, rs1799945; TMPRSS6 rs855791 [34]), whilst we constructed IVs using population-specific SNPs from a GWAS in our MR studies [35]. It may largely explain why we were able to derive evidence of causality for iron-HTN and iron-DLP associations. The consistent findings we obtained between the Taiwanese HC and European Whites strengthened the evidence for a causal role of genetically determined higher iron status on HTN and DBP, as well as on DLP and blood TG (but not HDL-C). On the other hand, the lack of causal effect of iron status on HDL-C levels in our MR studies has been consistent with the result of another MR-PheWAS of blood iron on lipid-related metabolic conditions among Europeans in the UKB [66]. In addition to this, the negative causal role of iron status on LDL-C or TC levels has been similar to the abovementioned finding of the first MR-PheWAS of systemic iron status among Europeans [32]. These require further validation.
There are two widely accepted plausible mechanisms underlying the association of elevated iron status with metabolic derangements. First, excess iron has been long believed to be a contributor to overall oxidative stress, as free iron facilitates the excessive formation of toxic reactive oxygen species (ROS) and catalyzes cellular reactions that can damage the cell [9−10]. The pro-oxidant property of heme iron could affect several tissues, particularly the pancreatic beta cells, muscles, and adipocytes; thus, inducing insulin resistance, beta cell dysfunction, altered endothelial dysfunction, and the release of iron from the ferritin stores. Iron is also suggested to inhibit the body’s oxidative defenses such as that of the superoxide dismutase, one of the main antioxidant defense enzymes [67]. The second mechanistic action of excess iron points to the enhancement of inflammation in the body [10,68−69]. As such, the relationship of iron overload with infectious and inflammatory chronic diseases has been well documented. It is thought that the secretion of proinflammatory adipokines is mediated by the expression of hepcidin, which is influenced by intracellular and extracellular iron concentrations [6]. Chronic low-grade inflammation is recognized as a pathogenic event of any MetS risk component in genetically or metabolically predisposed individuals, resulting in cytokine hypersecretion and the production of other inflammatory mediators [68].
In hypertension, the direct link between iron and blood pressure, for the most part, remains unknown [17]. Oxidative stress and chronic inflammation from excessive iron is presumed to lead to atherosclerotic events by initially causing endothelial damage and vascular dysfunction that subsequently induce high blood pressure [70]. Another proposed mechanism relates to the action of high Hb levels, which is also considered as the most important determinant of whole blood viscosity [71]. Hyperviscosity or the decreased blood flow throughout the body, following a prolonged state of oxidative stress, could raise the blood pressure systemically [72].
In dyslipidemia, the excessive biosynthesis of ROS may lead to lipid peroxidation and alterations in lipid metabolism. Lipid peroxidation causes the depletion of the cellular content of reduced glutathione, an important player in preventing cell damage [73]. It was also hypothesized that the increased risk of iron overload due to high intakes of iron may reduce the insulin-mediated suppression of lipase (i.e., enzyme responsible for mobilization of triglyceride), which, then, can increase intracellular lipolysis, the plasma levels of free fatty acids, and its transport to the liver [18,21,69]. Increment in the levels of liver free fatty acids stimulates triglyceride-rich lipoprotein production.
Since both HTN and DLP, as components of MetS or as separate metabolic risk factors, have been linked to the onset of cardiovascular diseases, further work is warranted to unravel the mechanistic details of higher iron status, either from genetic or dietary causes, and its effect on hypertension and lipid metabolism.
4.2. Non-Causality of Iron for Central Obesity and a U-Shape Causality for Gout or Hyperuricemia
There were no causal associations found between genetically determined iron levels and CO risk. These MR findings were inconsistent with those of the observational studies [19,36]. Central obesity is considered a predominant risk factor (i.e., core component) of MetS and a marker of onset of a dysmetabolic state [10,44]. Although oxidative stress from excess iron is deemed to play a causative role in CO and obesity per se, we were unable to derive evidence of causality for CO. Therefore, we surmise that the mechanisms of action of too much iron in relation to MetS is probably independent of abdominal fat accumulation or via a separate pathway independent of obesity. For example, red meat intake is a contributing factor to both high hemoglobin concentration and obesity [74]. It is likely that it is the high fat content of red meat which causes obesity, and that bioavailable iron from heme induces other metabolic disorders such as hypertension and hypertriglyceridemia. Nevertheless, it is also plausible that obesity can be both a result of and a cause of oxidative stress [75]. It may be visceral adiposity instead that could lead to changes in iron homeostasis, making higher iron status a marker rather than a causal factor [76]. This complexity requires further investigation.
On the other hand, the MR findings on iron-GT/HUA causality corroborate the results of our observational studies on the previously unreported curvilinear effects of iron status on GT or HUA [36]. This highlights that the genetic predisposition to either too much or too less iron can cause a detrimental role on risk of GT or HUA, and that maintaining iron status within the normal range is most beneficial. In gout, the stimulation of iron-induced oxidative stress begins when iron cations form complexes with monosodium urate crystals. This contributes to granulocyte and complement activation, which eventually leads to gouty inflammation [77]. Urate, on the other hand, is a well-known antioxidant in humans that reduces oxidative stress by acting as a chelator of iron. Accordingly, elevation in uric acid levels was hypothesized to be a response to counter an increased oxidative stress in the body [78]. Although the mechanism of increased iron on uric acid metabolism and gout remains to be fully understood, looking further into the causal role of iron on the pathology of HUA or GT will be highly beneficial, especially that hyperuricemia has been recently proposed as an additional cause and component of the MetS [37].
4.3. Causal Role of Lower Iron on Risks of Diabetes or Hyperglycemia
Negative causal associations of genetically predicted higher iron status on T2D or HGY risk, and on FBS/GLU or HbA1c levels, were obtained in the current MR studies, implicating a causal protective effect of elevated iron within the normal range against glucose metabolism derangements and, concomitantly, a causal role for relatively lower iron levels on T2D development. Results from epidemiological studies, however, have mostly presented a positive relationship between iron and diabetes [7,15−16]. In contrast, our recently published observational study yielded a distinct U-shaped association between increasing Hb and T2D risk [36]. Conflicting findings between observational association studies and MR analyses suggest that the results from the former were likely influenced by a number of environmental or lifestyle factors, while those from the MR studies potentially indicate the true causal effects of both iron deficiency and excess in the complex etiology of T2D.
The positive link between increased iron status or high iron intakes and hyperglycemia (or diabetes) in cross-sectional and prospective studies has been robustly attributed to iron being a strong pro-oxidant. Pancreatic beta cells, in particular, have a low expression of antioxidants, lending these cells to be highly sensitive to ROS [70]. This eventually leads to changes in glucose metabolism, a decrease in the synthesis and secretion of pancreatic insulin, impaired insulin signaling, and iron-dependent apoptosis (i.e., ferroptosis) [75]. Insulin resistance is regarded as another core component of MetS [10,44], and several metabolic manifestations are explained from this dysfunction [67]. For instance, hyperinsulinemia during ROS-induced insulin resistance states exacerbates the endothelial damage and vascular dysfunction that lead to a systemic increase in blood pressure (i.e., hypertension), as well as the suppression of lipase that stimulates the production of triglyceride-rich lipoproteins (i.e., dyslipidemia).
Our MR finding of relatively higher iron status causally reducing the risk of T2D or HGY may be biologically plausible and is of considerable clinical relevance, as diabetes has been well known to increase the risk of morbidity and mortality related to atherosclerotic and cardiovascular diseases. One MR study has similarly provided evidence on the negative correlation between iron status and T2D risk [33]. Few MR studies have further shown that higher genetically determined iron status in the normal range is protective against atherosclerotic disease and hypercholesterolemia risk [30,32]. These findings may be explained by a hyperoxic state due to elevated Hb and iron status since reduced iron stores leading to hypoxia could increase red blood cell turnover, which, then, has been linked to the increased glycation of Hb [79].
On the other hand, iron deficiency or lower-than-normal iron levels also affect diabetes risk through several putative molecular mechanisms [80]. Both iron deficiency and iron excess have been shown to increase ROS production that causes mitochondrial DNA damage [81]. Lower iron status contributes to many long-term micro- and macrovascular complications through the chronic impairment of insulin action and low-grade inflammation [82]. Additionally, lower iron-T2D risk association, which we had earlier demonstrated in our observational association studies [36], may represent an end-stage anemia phenomenon that results in poor blood circulation, decreased oxidative capacity, and pancreatic islet malfunction [79]. Interestingly, in the current MR studies, we were able to further provide evidence that supports the causal role of lower iron status in the etiology of diabetes since the other MetS components did not depict a clear negative association. Nevertheless, since the constructed IVs (i.e., Hb-PRS) only defined small variations in iron status within the normal range [32], careful consideration must be taken when interpreting our data.
From an alternative perspective, another mechanism that may underlie this iron-T2D relationship could be attributed to the effects of the genetic variants along the MHC region. This may be especially true among Europeans, wherein majority of the SNPs utilized as IVs for this group were found along this region. Removal of the MHC region in IV construction could be considered as a sensitivity analyses in future MR studies. Whether the iron-T2D causal associations would be robust or not to such analyses will implicate the genes within the MHC region as the ones predominantly driving the causality [83]. Further validation is highly warranted.
4.4. Strengths and Limitations of the Study
Our MR studies has several strengths. To our knowledge, we are the first to establish a causality inference between iron status and various metabolic disorders (as well as MetS metabolic traits) altogether. Whilst the genetic propensity to elevated iron status may be considered a modest causal factor for either HTN, DLP, or GT or HUA, we have discovered a strong inverse or negative causality for T2D or HGY. This supplemented and even leveraged the results we obtained from the Hb-MetS observational association studies [36]. Our findings may pave the way to further elucidating the link between iron and metabolic dysfunctions, and, in the future, to coming up with novel preventive or therapeutic strategies such as genetic-based dietary interventions (i.e., precision nutrition) [84]. Second, our ethnic-specific MR analyses involved two of the largest and major ethnic groups in the world, the Han Chinese and Europeans. We have cross-validated our findings between two population groups that uniquely differ in iron status profile (i.e., anemia, thalassemia, and other hemoglobinopathies are more common among Asians; hemochromatosis or iron overload in the body is prevalent among Caucasians). At the same time, we have addressed the underrepresentation of Asians and non-European descents in genetic/epidemiological studies [85] with the findings obtained from the Taiwanese HC group. Third, our MR studies employed relatively large sample sizes of TWB and UKB cohorts, which enabled us to further carry out sex-specific analyses without sacrificing the robustness of the results and the statistical power of our studies. A very large sample size is also a major requirement for MR studies in evaluating the likelihood and magnitude of any causal association. Estimated reliable genetic associations are known to be generally modest because individual genetic variants are likely to explain only a very small proportion of the variation in phenotypic traits [25]; hence, the need for adequate statistical power. Fourth, we were able to satisfy the key assumption of vertical pleiotropy for an MR study based on the MR-Egger regression results, which, when violated, will bias any inference of the causal effect [28]. The sensitivity analyses also yielded results consistent with the main MR analyses. Both analyses further strengthened the causal inferences obtained in this study. Lastly, in constructing the IVs, we opted to utilize population-specific SNPs identified from a previously conducted GWAS of Hb concentration [35]. This approach allowed the inclusion of genetic variants that contain additional information (i.e., multiple genetic backgrounds), which may result in predicting exposures more accurately while reducing weak instrument bias [59].
Our MR studies has some limitations as well. First, as we have employed a one-sample MR framework, the assumption of independence between the gene-exposure and gene-outcome association estimates may have been violated as these were measured in the same individuals [87]. Most MR studies at present follow a two-sample design (i.e., two independent samples for separate measurements of exposure and outcome), which could be more advantageous than one-sample MR due to increased statistical power [28]. However, the assumption of homogenous samples and population stratification can seriously affect estimates when gene-exposure and gene-outcome associations were derived from different populations [25,86]. Second, we did not further perform a reverse MR analysis to particularly determine whether HGY or T2D would cause a lowering in iron levels (i.e., “outcome” to “exposure”), despite a prior knowledge that the relationship between these two is bi-directional or reciprocating [87]. This approach may help tease apart the correlation between iron status and diabetes, but the interpretation of results will be more plausibly difficult due to the existence of feedback loops between exposure and outcome variables [88]. It would also be interesting to validate another hypothesis that the onset of metabolic disorders leads to changes in iron homeostasis, probably through increased inflammatory status, which corresponds to a continuous cycle between these two [70]. Third, the generalizability of our findings to other ethnicities may not hold. It is highly recommended to further validate causality in other population groups, especially that both iron status and genetic susceptibility to diet-related chronic metabolic conditions differ across ethnicities [89−90]. Fourth, similar to the Hb-MetS observational association studies, we were unable to screen and adjust for subjects who were vegetarians, taking iron supplements, or had consumed more red meats, as these data were incomplete or unavailable in the TWB and UKB at the time of analyses. Other variables that may have confounded the results such as total dietary energy, saturated fats, iron absorption enhancers or inhibitors, concurrent acute or chronic infections, and autoimmune diseases were also unadjusted for. Nonetheless, biases from confounding should not influence the MR estimates [28]. Lastly, we have utilized Hb concentration as the surrogate of iron status. Hb may only act as a good and acceptable iron biomarker from the lower end of the iron status spectrum to within normal levels [2]. Transferrin saturation becomes the ideal biomarker at the higher end, while serum ferritin levels could be exploited for the entire spectrum of iron status. It would finally be worthwhile to utilize the genetic variants associated with serum hepcidin as instruments for iron status. Hepcidin, being the principal iron-regulatory hormone, is a promising candidate to link genetic variation, dietary iron, and metabolic derangements.
5. Conclusions
Our ethnic- and sex-specific MR studies among the Taiwanese Han Chinese and European Whites have provided evidence to support that genetic predisposition to higher iron levels has a causal role on increasing the risks of HTN and DLP while relatively lower iron levels can causally contribute to the development of T2D. Taken together, our MR findings will help to further our understanding on the role of iron dysregulation in human health and better identify at-risk individuals. Since iron status is modifiable and could be optimized through dietary and other interventions, complications associated with chronic metabolic disorders could be prevented for genetically and metabolically susceptible individuals in the near future – one of the ultimate goals of precision nutrition.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: List of 36 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Male Taiwanese Han Chinese of Taiwan Biobank, Table S2: List of 58 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Female Taiwanese HC of TWB, Table S3: List of 698 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Male European Whites of UK Biobank, Table S4: List of 805 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Female European Whites of UKB, Table S5: Comparison of constructed Hb-PRSs and regression parameters between Taiwanese HC of TWB and European Whites of UKB, Table S6: Characteristics of subjects in TWB (N=61,352) across quintiles of Hb-PRS, Table S7: Characteristics of subjects in UKB (N=271,366) across quintiles of Hb-PRS, Table S8: Overall distributions of metabolic outcomes in TWB, Table S9: Overall distributions of metabolic outcomes in UKB, Table S10: Distributions of different metabolic conditions among subjects in TWB across quintiles of Hb-PRS, Table S11: Distributions of different metabolic conditions among subjects in UKB across quintiles of Hb-PRS, Table S12: Distributions of self-reported metabolic conditions among subjects in TWB across quintiles of Hb-PRS, Table S13: Distributions of self-reported metabolic conditions among subjects in UKB across quintiles of Hb-PRS, Table S14: Cases of metabolic conditions from hospital in-patient records of UKB subjects across quintiles of Hb-PRS, Table S15: Odds ratios of metabolic syndrome across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S16: Odds ratios of hypertension across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S17: Odds ratios of dyslipidemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S18: Odds ratios of central obesity across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S19: Odds ratios of gout or hyperuricemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S20: Odds ratios of diabetes or hyperglycemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S21: Odds ratios of type 2 diabetes across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S22: Sex-specific MR-Egger regressions of associations between iron status and risks of metabolic outcomes among Taiwanese HC and European Whites, Table S23: Sex-specific MR-Egger regressions of associations between iron status and metabolic traits among Taiwanese HC and European Whites, Table S24: Odds ratios from sensitivity analyses of causal associations between MetS and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S25: Odds ratios from sensitivity analyses of causal associations between HTN and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S26: Odds ratios from sensitivity analyses of causal associations between DLP and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S27: Odds ratios from sensitivity analyses of causal associations between GT or HUA and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S28: Odds ratios from sensitivity analyses of causal associations between T2D or HGY and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S29: Causal estimates from sensitivity analyses of associations between genetically instrumented Hb concentration and metabolic traits among Taiwanese HC, Table S30: Causal estimates from sensitivity analyses of associations between genetically instrumented Hb concentration and metabolic traits among European Whites, Table S31: Odds ratios of anemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Figure S1: Overlay histograms of sex-specific Hb-PRSs and scatterplots of sex-specific Hb-PRSs against Hb concentration among Taiwanese HC of TWB and European Whites of UKB, Figure S2: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for binary metabolic outcomes among Taiwanese HC, Figure S3: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for binary metabolic outcomes among European Whites, Figure S4: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for quantitative metabolic traits among Taiwanese HC, Figure S5: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for quantitative metabolic traits among European Whites, Figure S6: Comparison of OR plots between main MR and sensitivity analyses among Taiwanese HC, depicting causal roles of iron status on MetS and other metabolic outcomes, Figure S7: Comparison of OR plots between main MR and sensitivity analyses among European Whites, depicting causal roles of iron status on MetS and other metabolic outcomes.
Author Contributions
Conceptualization, W.−H.P. and V.J.T.−G.; methodology, V.J.T.−G., K.−M.C., Y.−T.H. and W.−H.P.; software, V.J.T.−G. and K.−M.C.; validation, K.−M.C.; formal analysis, V.J.T.−G.; investigation, V.J.T.−G.; resources, H.−C.Y. and W.−H.P.; data curation, V.J.T.−G.; writing–original draft preparation, V.J.T.−G.; writing–review and editing, W.−H.P.; visualization, V.J.T.−G.; supervision, Y.−T.H., H.−C.Y. and W.−H.P.; project administration, K.−M.C. and W.−H.P.; funding acquisition, W.−H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Cloud Project of Academia Sinica with grant number AS-PH-109-02.
Institutional Review Board Statement
The UK National Health Service’s National Research Ethics Committee gave ethical clearance to the UKB Project with reference number 11/NW/0382. The Institutional Review Board of Academia Sinica approved the TWB study and protocols on June 2019 with reference number AS-IRB02-108226. This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We thank all the participants and investigators of the Taiwan Biobank and UK Biobank. We thank the Institute of Statistical Science of Academia Sinica and the Multidisciplinary Health Cloud Research Program Project – the Statistical Science unit and the Humanities and Social Sciences unit for the facility and financial support. This study has been undertaken using the UK Biobank resource under Application Number 48272.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Geissler, C.; Singh, M. Iron, meat and health. Nutrients 2011, 3, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Bangkok, Thailand, 1998; Available online: https://apps.who.int/iris/handle/10665/42716 (accessed on 02 May 2023).
- Monsen, E.R.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.D.; Mertz, W.; Finch, C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Basuli, D.; Stevens, R.G.; Torti, F.M.; Torti, S.V. Epidemiological associations between iron and cardiovascular disease and diabetes. Front. Pharmacol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Clark, L. Iron, Oxidative Stress, and Disease Risk. Nutr. Rev. 2004, 62, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. (2010). The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef] [PubMed]
- van der A, D.L.; Peeters, P.H.; Grobbee, D.E.; Marx, J.J.; van der Schouw, Y.T. Dietary haem iron and coronary heart disease in women. Eur. Heart J. 2005, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, S.; Liu, G.; Yan, F.; Ma, X.; Huang, Z.; Tian, H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS ONE 2012, 7, e41641. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Brown, I.J.; Chan, Q.; Van Horn, L.; Ueshima, H.; Zhao, L.; Stamler, J.; Elliott, P.; International Collaborative Research Group on Macro-/Micronutrients and Blood Pressure. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ 2008, 337, a258. [Google Scholar] [CrossRef]
- Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Habibi-Moeini, A.S.; Azizi, F. Red meat and dietary iron intakes are associated with some components of metabolic syndrome: Tehran Lipid and Glucose Study. J. Transl. Med. 2019, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Sorlí, M.; Bulló, M.; Basora, J.; Ibarrola-Jurado, N.; Fernández-Ballart, J.; Martínez-González, M.A.; Serra-Majem, L.; González-Pérez, R.; Salas-Salvadó, J.; Nureta-PREDIMED Investigators. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk: cross-sectional and 1-year follow-up assessment. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 200–207. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Dos Santos Vieira, D.A.; Hermes Sales, C.; Galvão Cesar, C.L.; Marchioni, D.M.; Fisberg, R.M. Influence of Haem, Non-Haem, and Total Iron Intake on Metabolic Syndrome and Its Components: A Population-Based Study. Nutrients 2018, 10, 314. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Bo, Y.; Liu, Y. Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 830–836. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: an overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Qi, L. Mendelian randomization in nutritional epidemiology. Nutr. Rev. 2009, 67, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Janss, L.L.; Burgess, S.; Kiemeney, L.A.; den Heijer, M.; de Graaf, J.; Holewijn, S.; Benyamin, B.; Whitfield, J.B.; Swinkels, D.W.; Vermeulen, S.H. Iron and hepcidin as risk factors in atherosclerosis: what do the genes say? BMC Genet. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Del Greco, M.F.; Walker, A.P.; Srai, S.K.S.; Laffan, M.A.; Minelli, C. The Effect of Iron Status on Risk of Coronary Artery Disease: A Mendelian Randomization Study-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1788–1792. [Google Scholar] [CrossRef]
- Gill, D.; Monori, G.; Tzoulaki, I.; Dehghan, A. Iron Status and Risk of Stroke. Stroke 2018, 49, 2815–2821. [Google Scholar] [CrossRef]
- Gill, D.; Benyamin, B.; Moore, L.S.P.; Monori, G.; Zhou, A.; Koskeridis, F.; Evangelou, E.; Laffan, M.; Walker, A.P.; Tsilidis, K.K.; et al. Associations of genetically determined iron status across the phenome: A mendelian randomization study. PLoS Med. 2019, 16, e1002833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2010, 5, 4926. [Google Scholar] [CrossRef] [PubMed]
- Timoteo, V.J.; Chiang, K.M.; Yang, H.C.; Pan, W.H. Common and ethnic-specific genetic determinants of hemoglobin concentration between Taiwanese Han Chinese and European Whites: findings from comparative two-stage genome-wide association studies. J. Nutr. Biochem. 2023, 111, 109126. [Google Scholar] [CrossRef] [PubMed]
- Timoteo, V.J.; Chiang, K.M.; Pan, W.H. Positive or U-Shaped Association of Elevated Hemoglobin Concentration Levels with Metabolic Syndrome and Metabolic Components: Findings from Taiwan Biobank and UK Biobank. Nutrients 2022, 14, 4007. [Google Scholar] [CrossRef] [PubMed]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and Metabolic Syndrome: A Tangled Web. Current Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.M.; Tsay, Y.C.; Vincent Ng, T.C.; Yang, H.C.; Huang, Y.T.; Chen, C.H.; Pan, W.H. Is Hyperuricemia, an Early-Onset Metabolic Disorder, Causally Associated with Cardiovascular Disease Events in Han Chinese? J. Clin. Med. 2019, 8, 1202. [Google Scholar] [CrossRef]
- Feng, Y.A.; Chen, C.Y.; Chen, T.T.; Kuo, P.H.; Hsu, Y.H.; Yang, H.I.; Chen, W.J.; Su, M.W.; Chu, H.W.; Shen, C.Y.; et al. Taiwan Biobank: A rich biomedical research database of the Taiwanese population. Cell Genom. 2022, 2, 100197. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/259425 (accessed on 02 May 2023).
- Elliott, P.; Peakman, T.C.; UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006; Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensusworldwide-definitionof-the-metabolic-syndrome.html (accessed on 02 May 2023).
- Zimmet, P.Z.; Alberti, K.G. Introduction: Globalization and the non-communicable disease epidemic. Obesity 2006, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.E.; Wang, T.D.; Ueng, K.C.; Lin, T.H.; Yeh, H.I.; Chen, C.Y.; Wu, Y.J.; Tsai, W.C.; Chao, T.H.; Chen, C.H.; et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J. Chin. Med. Assoc. 2015, 78, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; ESC Scientific Document Group; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization & International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/handle/10665/43588 (accessed on 02 May 2023).
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; de Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the Capture and Adjudication of Prevalent and Incident Diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Li, Y.H.; Ueng, K.C.; Jeng, J.S.; Charng, M.J.; Lin, T.H.; Chien, K.L.; Wang, C.Y.; Chao, T.H.; Liu, P.Y.; Writing Group of 2017 Taiwan Lipid Guidelines for High Risk Patients; et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017, 116, 217–248. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Wei, C.Y.; Yang, J.H.; Yeh, E.C.; Tsai, M.F.; Kao, H.J.; Lo, C.Z.; Chang, L.P.; Lin, W.J.; Hsieh, F.J.; Belsare, S.; et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. 2021, 6, 10. [Google Scholar] [CrossRef]
- Wain, L.V.; Shrine, N.; Miller, S.; Jackson, V.E.; Ntalla, I.; Soler Artigas, M.; Billington, C.K.; Kheirallah, A.K.; Allen, R.; Cook, J.P.; et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir. Med. 2015, 3, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; Sham, P.C. PLINK: a tool set for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, D.I.; Kuchenbaecker, K.B.; Shah, S.; Sofat, R.; Holmes, M.V.; White, J.; Mindell, J.S.; Kivimaki, M.; Brunner, E.J.; Whittaker, J.C.; Casas, J.P.; Hingorani, A.D. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int. J. Epidemiol. 2016, 45, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mak, T.S.H.; O’Reilly, P.F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity, Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 02 May 2023).
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Francis, M.; Sun, Y.; Ryu, M.S.; Grider, A.; Ye, K. The Causal Effects of Blood Iron and Copper on Lipid Metabolism Diseases: Evidence from Phenome-Wide Mendelian Randomization Study. Nutrients 2020, 12, 3174. [Google Scholar] [CrossRef]
- Avelar, T.M.T.; Storch, A.S.; Castro, L.A.; Azevedo, G.V.M.M.; Ferraz, L.; Lopes, P.F. Oxidative stress in the pathophysiology of metabolic syndrome: which mechanisms are involved? J. Bras. Patol. Med. Lab. 2015, 51, 231–239. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 2009, 139, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kilani, N.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P. Iron metabolism and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1025–1032. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Chien, S.; Alderman, M.H.; Atlas, S.A.; Laragh, J.H. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation 1990, 81, 107–117. [Google Scholar] [CrossRef]
- Atsma, F.; Veldhuizen, I.; de Kort, W.; van Kraaij, M.; Pasker-de Jong, P.; Deinum, J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 2012, 60, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E. Inflammation, lipids, and free radicals: lessons learned from the atherogenic process. Semin. Reprod. Endocrinol. 1998, 16, 249–261. [Google Scholar] [CrossRef]
- Chang, H.C.; Yang, H.C.; Chang, H.Y.; Yeh, C.J.; Chen, H.H.; Huang, K.C.; Pan, W.H. Morbid obesity in Taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS One 2017, 12, e0169577. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Rao, G.; Cooke, C.L.; Miller, M.J.; Hoidal, J.R. Complexation of iron cation by sodium urate crystals and gouty inflammation. Arch. Biochem. Biophys. 1994, 313, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Soliman, N. Iron deficiency anemia and glucose metabolism. Acta Biomed. 2017, 88, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Astle, W.J.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.L.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell 2016, 167, 1415–1429.e19. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Lawson, H.A. Ironing out the Details: Untangling Dietary Iron and Genetic Background in Diabetes. Nutrients 2018, 10, 1437. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef]
- Minelli, C.; Del Greco, M.F.; van der Plaat, D.A.; Bowden, J.; Sheehan, N.A.; Thompson, J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol. 2021, 50, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Baird, D.; Borges, M.C.; Bowden, J.; Hemani, G.; Haycock, P.; Evans, D.M.; Smith, G.D. Recent Developments in Mendelian Randomization Studies. Curr. Epidemiol. Rep. 2017, 4, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Barad, A.; Clark, A.G.; Wang, Y.; Lin, X.; Gu, Z.; O’Brien, K.O. Ethnic Differences in Iron Status. Adv. Nutr. 2021, 12, 1838–1853. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Shen, J. Gene-environment interactions and susceptibility to metabolic syndrome and other chronic diseases. J. Periodontol. 2008, 79, 1508–1513. [Google Scholar] [CrossRef]
Figure 1.
Odds ratios of HTN and DLP across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 1.
Odds ratios of HTN and DLP across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.

Figure 2.
Odds ratios of CO and GT or HUA across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 2.
Odds ratios of CO and GT or HUA across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
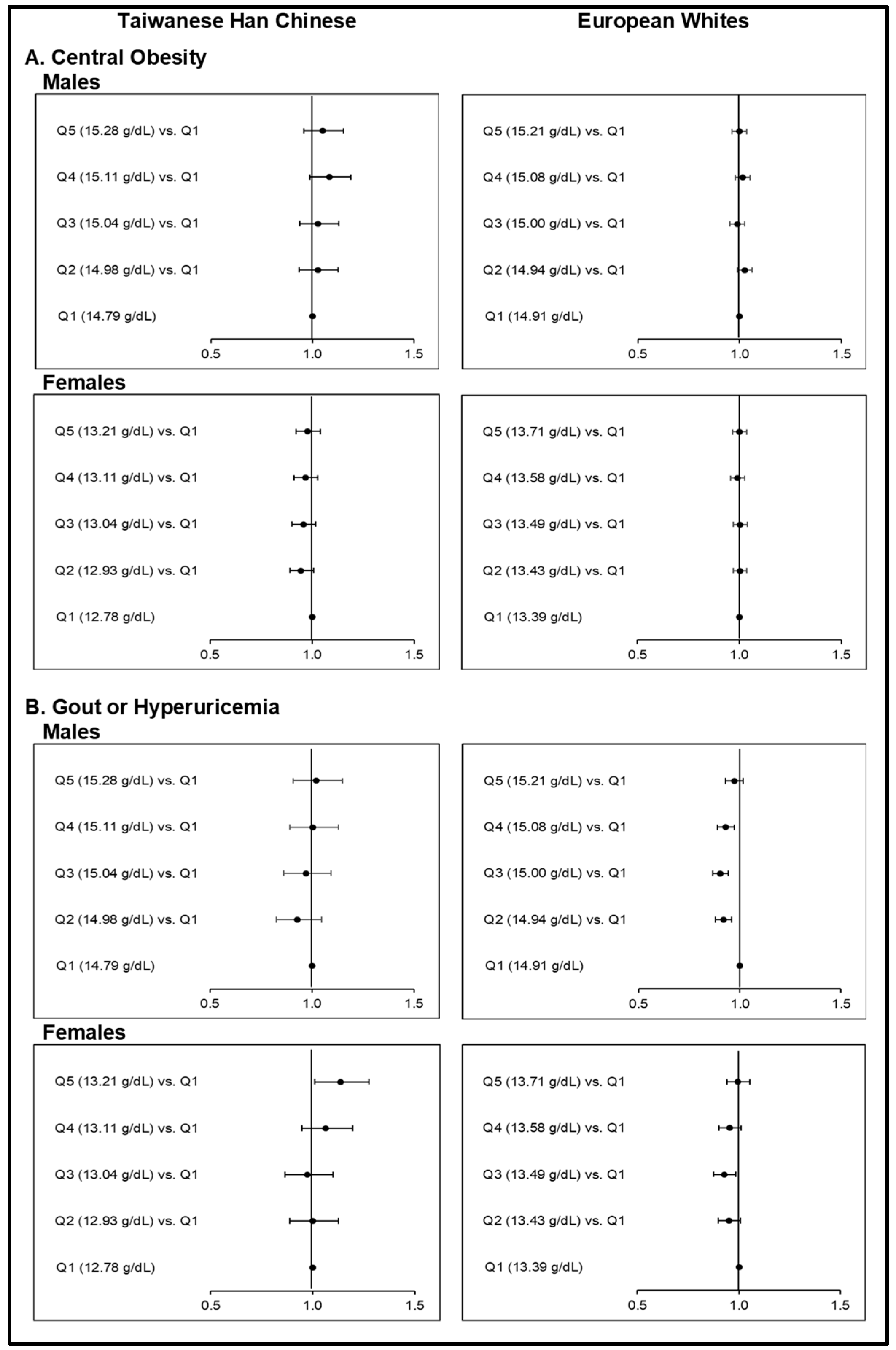
Figure 3.
Odds ratios of T2D or HGY across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 3.
Odds ratios of T2D or HGY across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
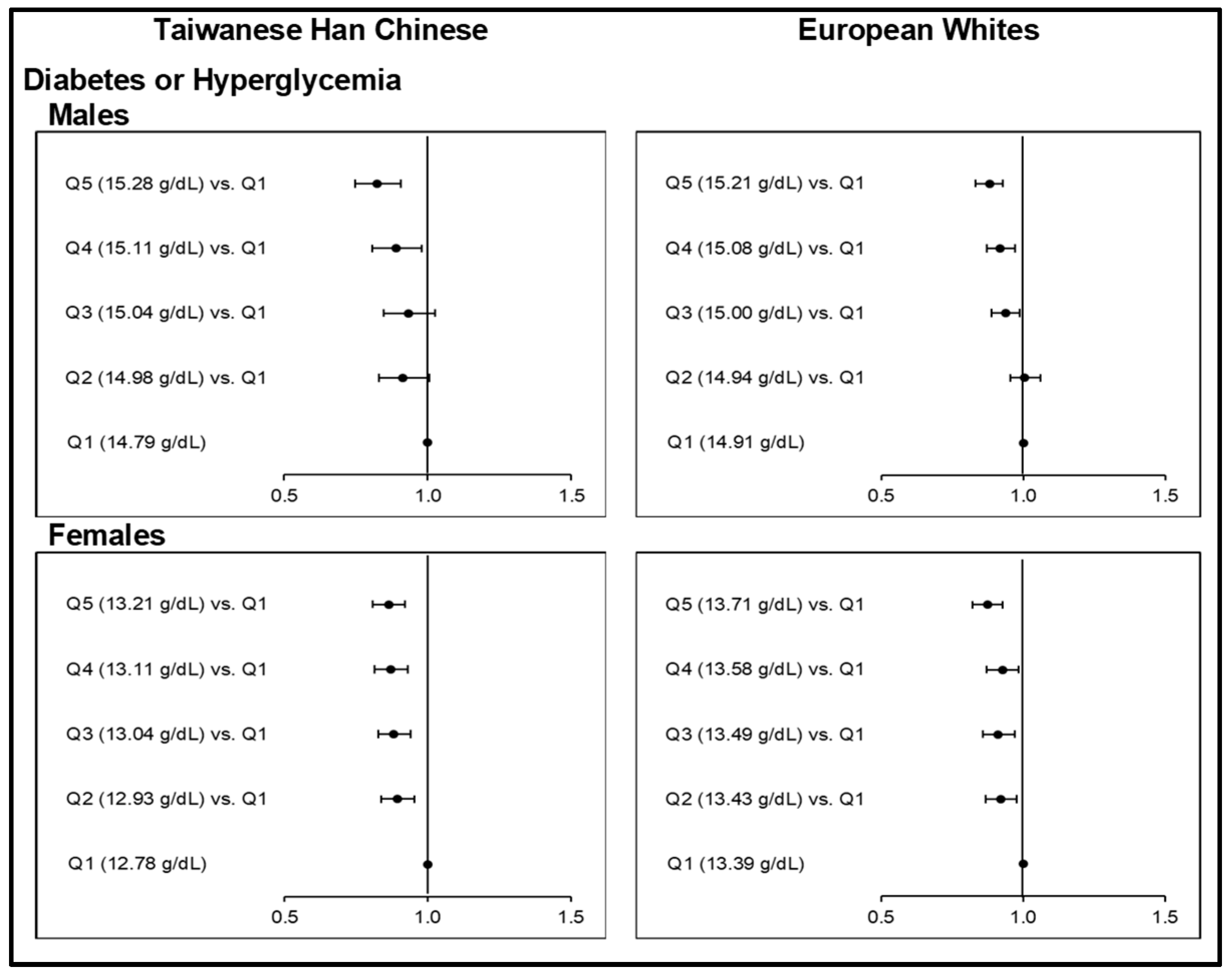
Figure 4.
Odds ratios of anemia across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 4.
Odds ratios of anemia across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
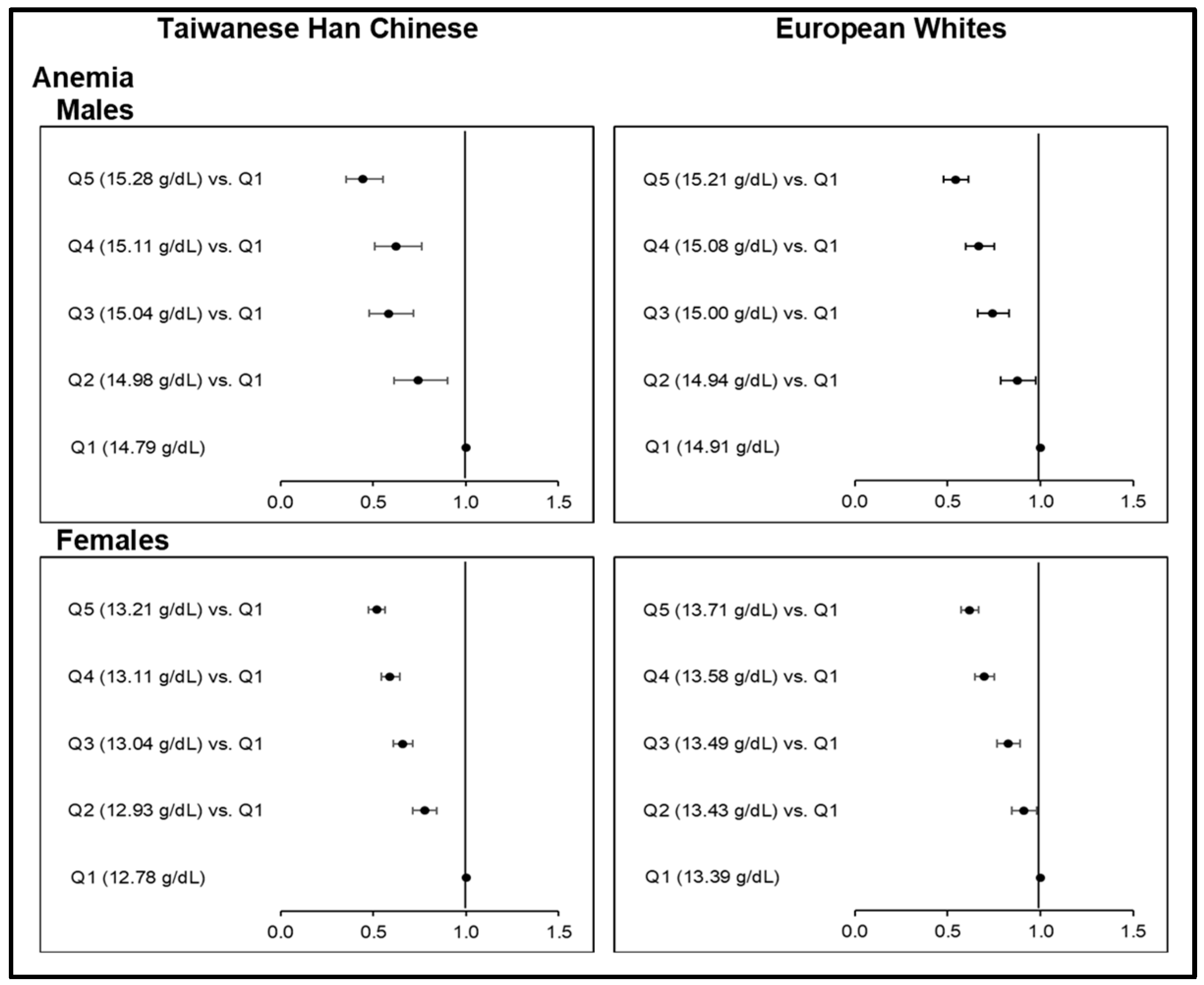
Table 1.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among Taiwanese Han Chinese.
Table 1.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among Taiwanese Han Chinese.
| Metabolic Trait | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Beta | SE | P-value | Beta | SE | P-value | |
| Males (n=19,005) | ||||||
| WC, cm | 31.9 | 14.4 | 0.0268 | 33.4 | 14.3 | 0.0199 |
| SBP, mm Hg | 51.9 | 24.6 | 0.0351 | 52.5 | 24.5 | 0.0324 |
| DBP, mm Hg | 53.5 | 16.1 | 0.0009 | 53.6 | 16.0 | 0.0008 |
| FBG*, mg/dL | 87.6 | 35.8 | 0.0094 | 88.2 | 35.7 | 0.0087 |
| TG*, mg/dL | 533.5 | 183.1 | 0.0005 | 543.5 | 180.9 | 0.0003 |
| HDL-C, mg/dL | -27.1 | 17.1 | 0.1131 | -27.3 | 16.9 | 0.1059 |
| UA, mg/dL | 2.1 | 2.1 | 0.3255 | 2.3 | 2.1 | 0.2690 |
| HbA1c*, % | 0.40 | 1.4 | 0.7771 | 0.36 | 1.4 | 0.7962 |
| TC, mg/dL | -103.3 | 53.5 | 0.0535 | -100.7 | 53.5 | 0.0599 |
| LDL-C, mg/dL | -130.7 | 48.0 | 0.0065 | -129.3 | 48.0 | 0.0071 |
| Females (n=42,330) | ||||||
| WC, cm | 24.6 | 18.3 | 0.1777 | 24.6 | 18.2 | 0.1773 |
| SBP, mm Hg | 55.3 | 31.1 | 0.0751 | 57.0 | 31.0 | 0.0664 |
| DBP, mm Hg | 68.7 | 19.1 | 0.0003 | 69.1 | 19.1 | 0.0003 |
| FBG*, mg/dL | -45.1 | 35.2 | 0.0795 | -46.2 | 35.2 | 0.0736 |
| TG*, mg/dL | 109.2 | 135.4 | 0.2129 | 103.1 | 135.1 | 0.2302 |
| HDL-C, mg/dL | 2.9 | 25.4 | 0.9077 | 2.1 | 25.3 | 0.9352 |
| UA, mg/dL | 5.0 | 2.1 | 0.0188 | 4.8 | 2.1 | 0.0224 |
| HbA1c*, % | -6.1 | 1.4 | <0.0001 | -6.1 | 1.4 | <0.0001 |
| TC, mg/dL | -121.0 | 4.2 | 0.0673 | -126.3 | 65.9 | 0.0551 |
| LDL-C, mg/dL | -44.8 | 59.6 | 0.4515 | -48.1 | 59.4 | 0.4184 |
* Variables with skewed distribution were log-transformed prior to analyses. Model 1 similarly adjusted for age, age-squared, and the first ten genetic PCs; whereas model 2 additionally adjusted for smoking status and alcohol consumption (as well as menopausal status in females). DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Table 2.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among European Whites.
Table 2.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among European Whites.
| Metabolic Trait | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Beta | SE | P-value | Beta | SE | P-value | |
| Males (n=128,549) | ||||||
| WC, cm | -21.9 | 18.6 | 0.2396 | -27.0 | 18.4 | 0.1434 |
| SBP, mm Hg | -30.8 | 28.8 | 0.2842 | -27.7 | 28.8 | 0.3348 |
| DBP, mm Hg | 129.1 | 17.1 | <0.0001 | 131.3 | 17.1 | <0.0001 |
| GLU*, mmol/L | -11.5 | 2.4 | <0.0001 | -11.7 | 2.4 | <0.0001 |
| TG*, mmol/L | 9.9 | 1.9 | <0.0001 | 9.6 | 1.9 | <0.0001 |
| HDL-C, mmol/L | 0.13 | 0.55 | 0.8170 | 0.26 | 0.54 | 0.6262 |
| UA, μmol/L | -139.4 | 120.0 | 0.2450 | -143.8 | 119.1 | 0.2273 |
| HbA1c*, mmol/mol | -177.8 | 11.9 | <0.0001 | -181.1 | 11.8 | <0.0001 |
| TC, mmol/L | -1.2 | 1.9 | 0.5232 | -0.87 | 1.9 | 0.6408 |
| LDL-C, mmol/L | -2.9 | 1.4 | 0.0427 | -2.7 | 1.4 | 0.0618 |
| Females (n=142,817) | ||||||
| WC, cm | -17.7 | 27.4 | 0.5189 | -15.1 | 27.0 | 0.5768 |
| SBP, mm Hg | 41.9 | 40.5 | 0.3013 | 40.6 | 40.5 | 0.3160 |
| DBP, mm Hg | 220.6 | 22.6 | <0.0001 | 221.4 | 22.6 | <0.0001 |
| GLU*, mmol/L | -12.3 | 2.4 | <0.0001 | -12.1 | 2.4 | <0.0001 |
| TG*, mmol/L | 12.6 | 1.9 | <0.0001 | 12.9 | 1.8 | <0.0001 |
| HDL-C, mmol/L | -1.0 | 0.89 | 0.2615 | -1.1 | 0.87 | 0.2026 |
| UA, μmol/L | -19.3 | 144.5 | 0.8936 | -3.4 | 144.1 | 0.9811 |
| HbA1c*, mmol/mol | -220.7 | 12.2 | <0.0001 | -217.8 | 12.1 | <0.0001 |
| TC, mmol/L | -7.9 | 2.4 | 0.0011 | -8.0 | 2.4 | 0.0009 |
| LDL-C, mmol/L | -8.9 | 1.9 | <0.0001 | -8.9 | 1.9 | <0.0001 |
* Variables with skewed distribution were log-transformed prior to analyses. Model 1 similarly adjusted for age, age-squared, and the first ten genetic PCs; whereas model 2 additionally adjusted for smoking status and alcohol consumption (as well as menopausal status in females). DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.docx (4.40MB )
Submitted:
21 December 2023
Posted:
25 December 2023
You are already at the latest version
Alerts
supplementary.docx (4.40MB )
This version is not peer-reviewed
Submitted:
21 December 2023
Posted:
25 December 2023
You are already at the latest version
Alerts
Abstract
A number of epidemiological studies have implicated elevated iron levels with higher risks of cardiometabolic diseases, including the metabolic syndrome (MetS). MetS is a pathologic clustering of metabolic derangements characterized by central obesity, insulin resistance, hypertension, and dyslipidemia, which can all lead to increased cardiovascular disease morbidity and mortality. However, it remains unclear whether a causal association exists between higher iron status and MetS and its associated risk components. Using the data from the Taiwan Biobank (TWB) (19,012 males; 42,340 females) and of the European Whites from the UK Biobank (UKB) (128,549 males; 142,817 females), we first constructed ethnic- and sex-specific hemoglobin-polygenic risk scores (Hb-PRS) as instrumental variable (IV) for iron status with the Hb-associated SNPs we previously identified from genome-wide association studies (GWAS). We then conducted Mendelian randomization (MR) analyses to elucidate the causal role of elevated iron (either Hb-PRS quintiles or continuous Hb-PRS) on various metabolic components. MR-Egger regressions determined the potential pleiotropic effects of the constructed Hb-PRSs, while the causal relationship of Hb-PRS with anemia served as a control in the analyses. The mean Hb concentration range corresponding to the Hb-PRS quintiles is relatively narrow in both UKB (14.91-15.21 g/dL for males and 13.39-13.71 g/dL for females) and TWB (14.79-15.28 g/dL for males and 12.78-13.21 g/dL for females). In these windows of Hb-PRSs, we found differential associations of Hb-PRS with diabetes (T2D) or hyperglycemia (HGY) as compared to other MetS components. The highest quintiles of Hb-PRSs were causally associated with greater risks of hypertension (HTN) and hypertriglyceridemia (DLP) (i.e., [Taiwanese HC males: Q5 vs. Q1 ORHTN = 1.10 (1.00–1.21), p = 0.0473, Q4 vs. Q1 ORDLP = 1.10 (1.00–1.22), p = 0.0485; European White males: ORHTN = 1.05 (1.01–1.09), p = 0.0194, ORDLP = 1.04 (1.00–1.08), p = 0.0468]). Interestingly, we obtained a consistent negative causal association between increasing Hb-PRS quintiles and T2D or HGY risk across all sex subgroups (i.e., [Taiwanese HC females: ORHGY = 0.89 (0.84–0.95), 0.88 (0.83–0.94), 0.87 (0.82–0.93), 0.86 (0.81–0.92) from Q2 to Q5; European White females: ORHGY = 0.92 (0.87–0.98), 0.91 (0.86–0.97), 0.93 (0.87–0.98), 0.87 (0.82–0.93) from Q2 to Q5; all p < 0.05]. The positive causal relationships between Hb-PRSs and diastolic blood pressure (DBP) and triglycerides (TG) but inverse causal association with glucose (GLU) and glycated hemoglobin (HbA1c) further corroborated such findings. There is, however, a lack of causal association with central obesity (CO), while a U-shaped type of causality was observed for gout (GT) or hyperuricemia (HUA). Sensitivity analyses, which excluded subjects based on self-reports of health conditions or medications and hospital episode statistics data, revealed similar results. Our MR studies confirm a modest causal relationship between propensity of elevated iron status and increased risk of HTN and DLP. We have further observed that modestly lower iron levels causally contribute to the development of T2D. Further validation and elucidation of the underlying biological mechanisms are highly warranted.
Keywords:
Subject: Biology and Life Sciences - Life Sciences
1. Introduction
Iron is an essential micronutrient that has several vital functions in the human body [1]. Primarily, iron facilitates oxygen transport as an integral component of the hemoglobin (Hb) protein. Around 65% of body iron is integrated in the Hb molecules in circulating red blood cells, which partially reflects the largest amount of functional iron in the body [2]. Additionally, iron functions as a transport medium for electrons within cells and is a crucial requirement for DNA synthesis and cell division. Hence, dysregulated iron homeostasis often results in the most common human disorders that range from anemia at the negative end of the iron status spectrum (i.e., nutritional iron deficiency), to hemochromatosis at the other extreme (i.e., iron overload) [3].
Systemic iron levels and iron nutrition status, however, are modifiable human traits. As an important and essential micronutrient, iron must be provided by diet and, in some cases, through supplementation or food fortification. Heme iron in red meat is a superb exogenous source of iron as compared to that of the plant sources [4]. It is estimated to contribute more than 40% of the total absorbed iron in meat-eating population groups [5]. Nevertheless, dietary iron absorption will always depend on systemic iron needs, such that more iron is absorbed during states of deficiency while less is absorbed when iron body stores are replete. Hepcidin, the key systemic iron-regulatory hepatic hormone, drives the regulation of intestinal iron absorption [6].
Iron overnutrition, which is commonly associated with the increased intakes of iron from high consumption of red meat (namely beef, lamb, organ meats, and processed meats), had drawn attention because of its potential as a risk factor for cardiovascular disease and chronic metabolic conditions such as diabetes [7]. It has been postulated from the “iron hypothesis” that iron depletion may protect against heart disease; whereas high levels of body iron stores promote cardiovascular and metabolic diseases through oxidative damage that takes place in the different tissues of the body [8]. In the development of atherosclerosis, for instance, high amounts of iron will catalyze the Fenton reaction that generates free radicals oxidizing the low-density lipoproteins, eventually leading to the deposition of atherosclerotic plaque [9].
The cardiometabolic syndrome, also commonly referred to as the metabolic syndrome (MetS), has been defined by the World Health Organization (WHO) as an aggregation of metabolic dysfunctions that mainly include insulin resistance or impaired glucose tolerance, dyslipidemia (namely elevated triglycerides and low high-density lipoprotein cholesterol levels), high blood pressure, and central obesity [10]. Each of these associated risk factors has an independent effect; but altogether, they lead to a substantial increase in cardiovascular disease morbidity and mortality [11]. A continuous increase in MetS global prevalence has also been observed among men and women across all age groups for some time [12], which is thought to be driven by genetic susceptibility and lifestyle changes. Nonetheless, the exact mechanisms as to how iron in excess contributes to MetS and its individual risk components is yet to be fully elucidated.
For several years, there has been accumulating reports on the possible links of either altered iron status, red meat consumption, or dietary intakes of iron (heme, in particular) on the pathophysiology of the MetS risk components and cardiovascular diseases [7,13–22]. Inferring causal effects, however, cannot be achieved from these associations due to unmeasured or residual confounding and reverse causation. For instance, it has been difficult to disentangle the coherent mechanistic actions of both heme iron and saturated fatty acids from high intakes of red meat in relation to cardiometabolic derangements [23]. These two may have been interplaying in the complex etiology of atherosclerotic diseases as the storage of saturated fatty acids in the body has been deemed as the culprit of hypercholesterolemia and coronary artery disease [24]. Epidemiological studies cannot tease apart the attributing effects due to the high collinearity between these two factors.
Mendelian randomization is a genetic epidemiology method which has recently been used to examine the causality between iron in excess and cardiometabolic risks. It has become a valuable tool in human nutrition, especially when the conduct of randomized controlled trials is not feasible to infer causality between dietary factors and biomarkers of health outcomes [25]. MR provides causal estimates between an exposure to a modifiable environmental risk factor and a health outcome, wherein genetic variants in the form of single nucleotide polymorphisms (SNPs) are used as instrumental variables for the said risk factor [26]. A polygenic risk score (PRS) is constructed and used to predict the risk of health outcomes. The MR technique is said to overcome biases that are inherent in conventional epidemiological studies, such as confounding and reverse causation, since alleles of genetic loci are, in principle, randomly assigned during conception [27]. As with all epidemiological approaches, however, the plausibility of MR findings will depend on specific assumptions [28]. First is the ‘relevance’ assumption, which requires that the genetic variants (i.e., IV) associate with the risk factor-of-interest. Second is the ‘independence’ assumption, for which there should be an independence between the genetic variants and confounders of the risk factor-outcome association. Lastly, is the ‘exclusion restriction’ assumption, which indicates that the genetic variants do not affect the outcome except through the risk factor (i.e., vertical pleiotropy).
Few MR studies have provided evidence to support that iron status, as a modifiable risk factor, can causally influence the etiology of cardiovascular diseases [29–31] and metabolic conditions [32,33]. Initial MR data were also observed to be contradicting among some studies. In a study conducted by Gill and colleagues [30], a protective effect of higher iron propensity (measured by a linear combination of SNPs predicting serum iron, transferrin saturation, ferritin, and transferrin) was shown to reduce coronary artery disease risk. Conversely, in another study [31], increased iron propensity was rather shown to have a detrimental effect on stroke risk, particularly on cardioembolic stroke. Such opposing observations, although for different CVD outcomes, were derived using the same set of IVs utilized in both studies, namely HFE rs1800562 and rs1799945 and TMPRSS6 rs855791 (i.e., three of the most strongly associated genetic variants of iron status [34]). Nevertheless, the unique genetic differences across ethnicities may have contributed to such findings and should be taken into consideration [35]. On the contrary, most of the results from observational association studies depicted elevated iron levels as a risk factor for cardiometabolic conditions. We have also recently shown in our sex-specific observational association studies that higher iron, as depicted by deciles of Hb levels, increases the risks of MetS and its metabolic components in the Taiwanese Han Chinese and European Whites [36]. Thus, investigating the predictability of genetic propensity score for iron status, and comparing it between ethnicities or across population groups, becomes immensely important as it may provide further insights on the role of iron nutrition (i.e., both in deficiency or excess) in the etiology of cardiometabolic diseases.
To date, no MR study has evaluated the causality between iron status and the key metabolic components of MetS altogether in one study. In this regard, we conducted ethnic- and sex-specific MR studies among the Taiwanese HC and European Whites in order to elucidate the causal relationships between iron status and the risks of MetS and its components (i.e., CO, HTN, DLP, and T2D or HGY). We have included GT or HUA as a risk component due to the increasing evidence that supports its association with the pathogenesis of MetS [37,38]. We further investigated the causal role of iron levels on several quantitative metabolic traits, namely waist circumference (WC), systolic (SBP) and diastolic blood pressure (DBP), fasting blood glucose (FBG) or random glucose (GLU), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and uric acid (UA) levels, along with the related phenotypes glycated hemoglobin (HbA1c), cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels. Our findings shall provide additional insights on the etiology of MetS risk components and, at the same time, help better identify at-risk individuals based on the genetic propensity for iron status. Precision iron nutrition, in the future, could prevent further complications associated with MetS and ultimately lead to the improvement of public health.
2. Materials and Methods
2.1. Study Design, Study Populations, and Ethical Considerations
A one-sample Mendelian randomization design was applied in this study. As the standard design for an MR analysis, SNPs (i.e., IVs), exposure, and outcome are measured in the same individuals and extracted from a single dataset of participants [28]. Ethnic-specific MR studies between the Taiwanese HC of TWB and European Whites of UKB cross-validated the causal inferences in these two large ethnicities that are known to differ in iron status profile. Sex stratification further accounted for the known differences in iron status between males and females.
Information on the TWB and UKB Projects can be obtained at https://www.twbiobank.org.tw/ (accessed on 2 January 2023) and https://www.ukbiobank.ac.uk/ (accessed on 2 January 2023), respectively [39,40]. The inclusion-exclusion criteria for the study populations of our MR studies followed that of our previously conducted two-stage genome-wide association studies (2S-GWAS) [35].
The TWB initially consisted of 20,764 male and 46,751 female Taiwanese HC (N=67,515). Participants were aged from 30 to 70 years old and were generally healthy at the time of assessment. We removed subjects who had self-reported kidney failure or cancer and had missing Hb data.
The UKB, on the other hand, consisted of 229,134 males and 273,402 females (N=502,536) who resided in the United Kingdom from 2006 to 2010. Subjects were aged from 40 to 70 years old during assessment. We initially selected the participants who identified themselves as White (i.e., British, Irish, or any other White background) and were born in the UK. We then excluded subjects who: (1) had inconsistent reported and genetic sex, (2) were pregnant, (3) had self-reported kidney failure, cancer, hereditary or genetic hematological disorder, clotting disorder or excessive bleeding, acquired immunodeficiency syndrome, tuberculosis, or any tropical and travel-related infections [41]. We further removed individuals with a recorded fasting time of 0 (i.e., non-fasted) or beyond 24 h (i.e., over-fasted) during the blood sample collection.
We finally included the TWB and UKB participants whom we were able to construct Hb-PRSs on, as derived from the sets of ethnic-specific and sex-specific Hb-associated SNPs we identified in the 2S-GWAS of Hb [35]. A total of 61,352 Taiwanese HC (19,012 males; 42,340 females) were included in the TWB MR studies, whereas a final sample size of 271,366 European Whites (128,549 males; 142,817 females) were used in the UKB MR studies.
All the work performed in this study has been carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol has been given ethical clearance by the Institutional Review Board of Academia Sinica, with reference number AS-IRB 02. The ethics approval of the UKB Project was provided by the UK National Health Service’s National Research Ethics Committee, with reference number 11/NW/0382. Duly signed informed consents were collected from the participants. All available data and information were treated with the utmost confidentiality.
2.2. Data Collection in the Taiwan Biobank and UK Biobank
Details on the data collection in TWB and UKB and the ascertainment of metabolic risk outcomes were fully described in previous studies [35,36].
Briefly, venous blood samples were collected from the participants at baseline assessment for the genetic data, hematological indices, and blood biochemistry parameters. Blood collection in the UKB was done at random and participants were non-fasted [42]. Hemoglobin and hematological indices were immediately analyzed following blood draw using routinely calibrated automated clinical hematology analyzers. Biochemical markers that include FBG or GLU, TG, TC, HDL-C and LDL-C, and UA were measured via enzymatic assays using clinical chemistry analyzers. HbA1c level was specifically determined through high-performance liquid chromatography.
Anthropometric parameters (i.e., weight, body mass index (BMI), WC) and blood pressure (BP) readings were measured twice following standard procedures. A third measurement of BP level was taken if the first two readings differed by more than 10 mmHg.
Information about the socio-demographic profile, lifestyle behaviors, personal and family medical history, environmental risk factors, and other health-related information were obtained through a structured face-to-face interview in the TWB and through a comprehensive touchscreen questionnaire in the UKB [39,40]. A verbal interview with a trained nurse was done afterward for some variables that needed verification (i.e., type and frequency of prescription medications) in the UKB.
2.3. Ascertainment of Metabolic Risk Outcomes
We followed the definition of NCEP-ATP III as the diagnostic criteria to ascertain the MetS, namely: (1) WC > 102 cm in males or >88 cm in females; (2) TG > 150 mg/dL (>1.70 mmol/L) or taking triglyceride-lowering drugs; (3) HDL-C < 40 mg/dL (<1.00 mmol/L) in males or <50 mg/dL (<1.30 mmol/L) in females or taking statins or other medicines for high cholesterol; (4) SBP/DBP >130/>85 mmHg or current use of anti-hypertensive drugs; and (5) FBG > 100 mg/dL (>5.6 mmol/L) or current use of anti-hyperglycemic drugs [43]. The WC cut-off point for Asians set by the International Diabetes Federation (i.e., WC > 90 cm in males and >80 cm in females) was specifically used to define CO in the Taiwanese HC group [44].
With respect to the individual MetS component, we first ascertained the disease outcomes in both TWB and UKB using self-reported physician-diagnosed health conditions and ethnic-specific cut-off values for WC, SBP/DBP, FBG or GLU, HbA1c, TG, HDL-C, and UA. Additionally, in the UKB, we utilized the data on prescription medications and hospital episode statistics (i.e., International Classification of Diseases 10th (ICD10) and 9th (ICD9) revisions) in the ascertainment of HTN, DLP, T2D or HGY, and GT or HUA.
CO was defined as a WC > 90 cm in males or >80 cm in females for East Asians and a WC > 102 cm in males or >88 cm in females for Europeans [44,45]. HTN was diagnosed based on an SBP/DBP reading of >140/>90 mmHg [46,47]. T2D was ascertained from an FBG level > 126 mg/dL (>7.0 mmol/mol) [48] or an HbA1c > 6.5% (>48 mmol/mol) [49]. However, because of the non-fasting state of participants in the UKB, we followed the algorithms of Eastwood and co-authors, which used a slightly higher GLU level of > 11.1 mmol/L (>200 mg/dL) in order to exclude false positives when capturing hyperglycemia in the European White group [50]. We defined DLP in this study as having either hypertriglyceridemia (HTG) (i.e., TG > 150 mg/dL or >1.70 mmol/L), low levels of HDL-C (i.e., HDL-C < 40 mg/dL or <1.00 mmol/L in males; <50 mg/dL or <1.30 mmol/L in females), or a combination of both [51,52]. Hypercholesterolemia (HCL) and high LDL-C levels were then determined as TC > 240 mg/dL or >6.20 mmol/L and LDL-C > 160 mg/dL or >4.10 mmol/L, respectively [51,52]. GT or HUA was ascertained from a UA level > 7.0 mg/dL or >416.0 μmol/L in males and >6.0 mg/dL or >357.0 μmol/L in females [53].
2.4. Genetic Data and Two-Stage Genome-Wide Association Studies of Hemoglobin
2S-GWAS have been conducted to identify the SNPs associated with Hb among 60,518 Taiwanese HC (18,824 males; 41,694 females) and 269,451 European Whites (127,726 males; 141,725 females). The quality control of genetic data and the 2S-GWAS protocols were described in detail in a previous paper [35].
Genomic DNAs were extracted from the collected blood samples of TWB and UKB participants. A total of about 650,000 SNPs were genotyped in the TWB using a ThermoFisher Scientific Axiom Genome-Wide Array Plate (TWB 2.0) platform [54]. UKB genetic data, on the other hand, included more than 800,000 markers that were genotyped using the Applied Biosystems UK BiLEVE Axiom Array and the closely related Applied Biosystems UK Biobank Axiom Array [55,56].
2.5. Construction of Hemoglobin-Polygenic Risk Scores
Ethnic- and sex-specific Hb-PRSs were constructed as a combination of the Hb-associated SNPs using the allelic scoring function in PLINK 1.9 (https://www.cog-genomics.org/plink/1.9/ (accessed on 6 February 2023)) [57,58]. The strategy of aggregating SNPs into a single score relies on the assumption that the effect of all independent SNPs could be combined by their individual effect sizes and the resulting PRS may predict exposures more accurately than smaller subsets of these SNPs [59]. We followed the strategy of Choi and team in constructing the PRS (https://choishingwan.github.io/PRS-Tutorial/ (accessed on 6 February 2023)) [60].
In order to capture the most approximate causal independent genetic variants, linkage disequilibrium (LD) between Hb-associated SNPs was first investigated and accounted for. LD-based clumping was carried out in PLINK, which retained the SNPs that were weakly correlated and largely independent of each other (i.e., r2 < 0.5 across 250 kb) while preferentially selecting the SNPs that were mostly associated with Hb concentration. SNPs that flank pseudogenes were removed. In accordance with the “iron hypothesis”, some alleles were flipped to ensure that all effect alleles were risk-increasing (i.e., positive beta) [29]. Finally, 36 out of 61 Hb-associated SNPs were used in the construction of Hb-PRS for the male Taiwanese HC, 58 out of 82 SNPs for the female Taiwanese HC, 698 out of 1,982 SNPs for the male European Whites, and 805 out of 2,248 SNPs for the female European Whites.
Hb-PRS was calculated as the summation of the number of effect alleles per SNP (i.e., 0, 1, or 2) for each individual, multiplied by the effect size for a particular SNP (i.e., beta-coefficient relating Hb concentration to number of alleles) as obtained from the linear regression models of the 2S-GWAS joint analyses [60]: Hb-PRS = β1 × Allelic Number (SNP1) + β2 × Allelic Number (SNP2) + … + βn × Allelic Number (SNPn).
2.6. One-Sample Mendelian Randomization Analyses
Independent one-sample MR analyses that involved the Taiwanese HC and European White cohorts were performed, wherein Hb-PRSs served as the IVs (i.e., genetically instrumented Hb concentration) for iron status being the modifiable risk factor-of-interest in this study.
For describing the characteristics of subjects, continuous variables were expressed as mean ± s.d., whereas categorical characteristics were in counts and percentage values. The normal distribution of quantitative parameters was assessed, and extreme outliers were removed. We categorized subjects according to quintiles of ethnic- and sex-specific Hb-PRS. Trends in quantitative characteristics and prevalence across Hb-PRS quintiles were respectively tested by linear regression and the Cochran–Armitage trend test.
The main MR analyses utilized logistic and linear regressions wherein males and females were analyzed separately. Logistic regression models determined and compared between the two ethnic groups the associations across quintiles of Hb-PRS and the risks for each binary outcome, namely: MetS, CO, HTN, DLP, T2D (or T2D and HGY), and GT (or GT and HUA). The lowest Hb-PRS quintile served as the reference. Age, age-squared, and the first ten genetic PCs were adjusted in the first model. Smoking and alcohol drinking status (as well as menopausal status in females) were further adjusted in the second model, since years of life and certain lifestyle factors may modify the inherent effects of Hb-associated genetic loci. The categorization of smoking status (i.e., never smoked, stopped smoking, occasionally smoking, or currently smoking), alcohol drinking status (i.e., never drank, stopped drinking, occasionally drinking, or currently drinking), and menopausal status in females (i.e., non-menstruating, unsure of menopausal status, or had age-at-menopause <44, 45–49, 50–54, or >55 years) were all previously described in detail [36].
Additionally, the MetS metabolic traits WC, SBP, DBP, FBG or GLU, TG, HDL-C, and UA, along with the related phenotypes HbA1c, TC, and LDL-C, were analyzed as quantitative outcomes. We examined the potential causality of Hb-PRS on such traits through linear regressions, which similarly adjusted for potential confounders in the models. Variables with skewed distribution were log-transformed prior to analyses.
All MR analyses were carried out in SAS v9.4. The criteria for statistical significance were a p-value of <0.05.
2.7. MR-Egger Regressions and Sensitivity Analyses
We conducted MR-Egger regressions to investigate whether the causal estimates obtained from the MR analyses were influenced by pleiotropy. MR-Egger was implemented using the package “Mendelianrandomization” in R v4.1.1 (https://www.r-project.org/ (accessed on 5 June 2023)) [61].
Briefly, the associations of IVs with various metabolic risk outcomes and metabolic traits were first analyzed in PLINK. Beta-coefficients (i.e., log odds ratio) and standard errors were derived from univariable regression analyses. MR-Egger was then initiated through the mr_input() function of MRInput object, where the betas and standard errors of the SNPs-Hb association (i.e., bx, bxse) and the SNPs-metabolic risk outcomes/traits association (i.e., by, byse) were entered. Genetic associations were aligned to the same effect alleles. MR-Egger estimates and all SNPs, including those possibly violating the IV assumption, were displayed though the mr_plot() function. The MR-Egger intercept parameter can be interpreted as an estimate of the overall directional pleiotropy, whereas the slope can provide pleiotropy-corrected causal estimates [62].
To further test the robustness of the main MR findings in a sensitivity analyses, we excluded subjects who were ascertained from either self-reported physician-diagnosed health conditions or medications (i.e., regular prescriptions) or hospital in-patient records. This validated the causal relationships upon excluding data sources that may potentially introduce biases and errors (e.g., misclassification of categories and subcategories of health conditions when using ICD codes) [32,50].
Finally, since the inverse relationship between Hb levels and anemia risk has been well established at the lower end of Hb distribution [63], the causal association between Hb-PRS and anemia (i.e., Hb < 13.0 g/dL in males and <12.0 g/dL in females) served as a control in the analyses.
3. Results
3.1. Discovery and Selection of Hemoglobin-Associated SNPs for the Construction of Hemoglobin-Polygenic Risk Scores
We previously conducted 2S-GWAS that involved 60,518 Taiwanese Han Chinese (18,824 males; 41,694 females) of the TWB and 269,451 European Whites (127,726 males; 141,725 females) of the UKB and identified common and ethnic-specific SNPs that are associated with blood Hb concentration [35]. In the current MR studies, the IVs for iron status were constructed from the clumped sets of ethnic- and sex-specific Hb-associated SNPs we discovered previously: 36 for male Taiwanese HC, 58 for female Taiwanese HC, 698 for male European Whites, and 805 for female European Whites (Tables S1–S4, respectively).
Table S5 provides the means and standard deviation of the Hb-PRSs as well as the regression coefficients relating Hb-PRS to Hb in g/dL, whereas the sex-specific distributions of Hb-PRSs and the scatterplots of Hb concentrations against Hb-PRSs are presented in Figure S1. In both ethnic groups, the mean Hb-PRSs of males were slightly higher than those of the females. Between the Taiwanese HC and European Whites, however, we observed generally higher mean Hb-PRSs in the former despite the very much larger numbers of SNPs used to construct the IVs in UKB than in TWB. The F-statistics were 404.0 and 659.5, respectively, for the male- and female-specific beta estimates of Hb-PRSs in the Taiwanese HC group, and 1,478.9 and 2,284.7, respectively, for the male- and female-specific estimates in the European White group. Around 1.5–2.1% of the variance in Hb levels could be explained by the constructed Hb-PRSs in TWB, whereas the resulting r2 values in UKB were at 1.1–1.6%.
Majority of the Hb-associated SNPs in TWB and UKB that were used to construct the IVs had very small effect sizes (i.e., beta <0.1) [35]. Nonetheless, the abovementioned regression parameters indicated a favorable relationship between the IVs and Hb [64].
3.2. Characteristics of Subjects in the Mendelian Randomization Studies
The characteristics of subjects in the TWB and UKB MR studies as stratified by quintiles of Hb-PRS and sex are presented in Tables S6 and S7, respectively. The mean Hb levels of male subgroups (15.0 g/dL) were found to be higher than the female Taiwanese HC (13.01 g/dL) and female European (13.52 g/dL) subgroups. As expected, we observed significant continuous increases in the mean Hb values, but decreasing anemia prevalence rates, with increasing quintiles of Hb-PRSs for all sex subgroups in both ethnic groups (p <0.0001).
The mean ages were at 50–51 years old for male and female Taiwanese HC and around 56–57 years for the European White counterparts. There were no significant differences on the mean age by Hb-PRS quintiles, except among male Europeans, wherein very small increments in age were noted. We also did not find any significant differences on the percentages of females who had entered menopause, as well as with their mean age at menopause, and the frequency or levels of physical activity of subjects. However, majority of the female subjects had already entered menopause during assessment, while some of the subjects were engaged in regular exercises or had moderate- to heavy- intensity exercises. Smoking status by Hb-PRS quintiles was found to be significantly different among male Taiwanese HC, particularly as the percentages of those who never smoked became larger toward higher quintiles of Hb-PRS. Alcohol consumption by Hb-PRS was also significant, but only among male Europeans, wherein the proportion of current drinkers generally decreased across Hb-PRS quintiles.
In both ethnic groups, there were highly significant continuous increases in mean DBP levels with Hb-PRSs. A similar trend was observed with the TG levels of European Whites, whereas a U- or J-shaped trend could be noted among the Taiwanese HC. The mean WC and SBP levels of male Taiwanese HC and the mean UA levels of female Taiwanese HC were also significantly increasing with Hb-PRS quintiles. Specific to the European subgroups, the mean GLU and HbA1c values were instead found to be remarkably decreasing with Hb-PRS quintiles. These data initially showed that causal associations between Hb-PRSs and some metabolic traits may exist. On the contrary, there were no significant differences on mean FBG and HDL-C by Hb-PRS quintiles in the Taiwanese HC subgroups, whereas mean WC, SBP, HDL-C and UA levels were not significant in the Europeans.
3.3. Trends on Prevalence Rates of Metabolic Disorders and the Causal Role of Iron Status on Metabolic Risk Components among Taiwanese Han Chinese and European Whites
Tables S8 and S9 show the overall distributions of metabolic risk outcomes in the TWB and UKB, respectively (Tables S10–S11 provide the frequency distributions of metabolic conditions based on measured health indicators by Hb-PRS and sex in the TWB and UKB; Tables S12–S13 present the percentages of cases based on self-reported data, and; Table S14 shows the percentages from hospital in-patient records that were only currently available in the UKB). The prevalence of MetS following the NCEP-ATP III criteria was 23.8% among Taiwanese HC and 40.3% among Europeans. Of all the MetS risk components, CO was found to be highly prevalent in both ethnicities (i.e., 44.7% among Taiwanese HC and 56.1% among Europeans). This was followed by DLP (34.3%; 62.3%), HTN (23.9%; 55.6%), GT (20.2%; 14.1%), and T2D (10.2%; 7.0%). DLP seemed to be relatively common among Europeans, but the non-fasting state of UKB participants may have contributed to this data. Between sexes, higher prevalence rates of MetS and all metabolic components, except CO, were observed among male than among female subjects in both ethnic groups.
There were apparent differences in the trends of prevalence rates of MetS and metabolic components by Hb-PRS in both ethnic groups. The MetS prevalence with increasing Hb-PRS quintiles was generally increasing among Taiwanese HC (p = 0.0066 in males; p = 0.0134 in females) and female Europeans (p = 0.0097). We observed highly significant continuous increases in the proportions of male and female Europeans with HTG (p <0.0001 and p = 0.0001, respectively), while high TG levels among male Taiwanese HC was also generally increasing (p = 0.0125). A steady increase in HTN prevalence based on SBP/DBP readings was initially observed in the female Taiwanese HC group only (p = 0.0302). However, based on self-reports of physician-diagnosed HTN and hospital diagnoses, significant positive trends have been similarly observed among Europeans. The prevalence rates of T2D based on either high FBG (GLU) or HbA1c levels, diagnosis by a doctor, or hospital records were all found to be consistent but rather remarkably decreasing toward higher quintiles of Hb-PRS.
MR analyses showed evidence to support the causal associations between elevated iron status and higher risks of HTN and DLP (Figure 1; Tables S16–S17) among Taiwanese HC and European Whites. Causal associations could not be concluded, however, between Hb-PRS quintiles and CO (Figure 2; Table S18). Hb-PRS was associated with GT and HUA but implicated a U-shaped type of causality in the two ethnicities (Figure 3; Table S19). On the other hand, our findings on T2D and HGY (Figure 4; Table S20–S21) were different from the other risk components as increasing Hb-PRS quintiles were observed to be causally associated with decreasing risks. Relatively low iron levels were causally associated with T2D development.
3.3.1. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Hypertension and Dyslipidemia
We found consistent positive causal associations between higher levels of genetically instrumented Hb and stronger HTN risks, as compared to the first quintiles (i.e., reference), in all sex groups of both ethnicities (Figure 1). The odds ratios (OR) obtained for HTN among Taiwanese HC upon fully adjusting for age, age-squared, smoking status, and alcohol drinking status (and menopausal status in females) are as follows: OR for Q5 = 1.10 (1.00–1.21, p = 0.0473) in males; OR for Q4 = 1.09 (1.00–1.19, p = 0.0033) in females. Among Europeans, the ORs for Q3 and Q5 in the male subgroup were respectively 1.06 (1.02–1.10, p = 0.0052) and 1.05 (1.01–1.09, p = 0.0194), whereas the ORs for Q4 and Q5 in the female subgroup were respectively 1.04 (1.00–1.08, p = 0.0495) and 1.07 (1.03–1.11, p = 0.0004) (Table S16).
There were also positive causal relationships observed between Hb-PRS quintiles and risk of DLP (Figure 1). In the male Taiwanese HC group, Q4 was found significant [OR = 1.10 (1.00–1.22, p = 0.0485)]. Conversely, among male Europeans, we attained significant but modest causal associations with DLP risk from Q2 to Q4 as follows: 1.04 (1.00–1.08, p = 0.0305), 1.05 (1.01–1.09, p = 0.0166), 1.04 (1.00–1.08, p = 0.0468). An OR for Q5 = 1.04 (1.01–1.08, p = 0.0220) was obtained among female Europeans (Table S17).
There was not much difference in the odds ratios between the crude and adjusted models. ORs among Europeans were relatively lower than among the Taiwanese HC despite a larger sample size and higher prevalence rates of HTN and DLP in the former. Between sex groups, the trend and risk elevations for the higher Hb-PRS quintiles were found to be relatively consistent in females.
3.3.2. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Central Obesity and Gout or Hyperuricemia Risk
As compared to HTN and DLP, we did not obtain any significant causal association between Hb-PRS quintiles and the risk of CO among Taiwanese HC and European Whites (Figure 2; Table S18). On the other hand, the causal associations with GT or HUA followed a distinct U-shaped or curvilinear type of association (Figure 2; Table S19). This was more evident among Europeans, wherein the middle quintiles resulted in ORs < 1.0. Particularly among male Europeans, ORs were at 0.92 (0.88−0.96, p = 0.0002), 0.90 (0.87−0.94, p <0.0001), and 0.93 (0.89−0.97, p = 0.0012) for Q2, Q3, and Q4 of Hb-PRS, respectively. An OR for Q3 = 0.93 (0.88−0.98, p = 0.0122) was obtained for the female European group. On the contrary, in the female Taiwanese HC group, the highest Hb-PRS quintile rather resulted in an OR of 1.14 (1.01−1.28, p = 0.0321).
3.3.3. Odds Ratios between Upper Quintiles of Hb-PRS and the First Quintile with respect to Type 2 Diabetes or Hyperglycemia
The causal role of increasing genetically instrumented Hb concentration levels on T2D or HGY risk was interesting. Here we obtained causal, protective effects of increasing Hb-PRS quintiles against T2D or HGY, which were consistent for all sex groups of both the Taiwanese HC and European Whites (Figure 3; Table S20). Very highly significant ORs (p <0.0001) for the highest Hb-PRS quintiles were obtained as follows: 0.83 (0.75−0.91) among male Taiwanese HC; 0.86 (0.81−0.92) among female Taiwanese HC; 0.88 (0.83−0.93) among male Europeans, and; 0.87 (0.82−0.93) among female Europeans. For both female subgroups, the highly significant negative causal associations with T2D or HGY were identified starting from Q2 up to the last. Even with diabetes alone as an outcome, the decreased risks at higher Hb-PRS quintiles remained evident (Table S21).
3.4. Causal Associations between Hb-PRS and Quantitative Metabolic Traits
Table 1 and Table 2 provide the sex-specific causal estimates of associations between Hb-PRS and quantitative metabolic traits among the Taiwanese HC and European Whites, respectively, as we further examined the association of Hb-PRS as a continuous IV on the different quantitative MetS metabolic traits WC, SBP, DBP, FBG or GLU, TG, HDL-C, and UA, along with the related phenotypes HbA1c, TC, and LDL-C.
Consistent with the results obtained from determining the causality between Hb-PRS and the metabolic risk components, we found that the Hb-PRSs were significantly and positively associated with DBP and TG levels among the Taiwanese HC (Table 1) and European Whites (Table 2). Genetically instrumented Hb corresponded to betas of 53.6 (p = 0.0008) and 69.1 (p = 0.0003) for DBP among male and female Taiwanese HC, respectively; while higher estimates at 131.3 and 221.4 (p <0.0001) were obtained for male and female Europeans. A positive causal association with SBP was only identified in the male Taiwanese HC group (β = 52.5, p = 0.0324), as the association in the female group was marginally significant. Beta values for TG were 543.5 (p = 0.0027) among male Taiwanese HC and 9.6 and 12.9 among Europeans (p <0.0001). Associations with HDL-C were inconsistent and not significant.
Although we did not previously identify a causal relationship between Hb-PRS and CO risk, a positive association with WC has been obtained in the male Taiwanese HC group (β = 33.4, p = 0.0199). Hb-PRS was also found causally and positively associated with UA but only among the female Taiwanese HC (β = 4.8, p = 0.0224).
The direction of causality between Hb-PRS and GLU among Europeans was consistently negative and very highly significant (β = −11.7 in males and −12.1 in females, p <0.0001). HbA1c, a highly correlated phenotype with FBG or GLU, depicted the same negative and highly significant association with Hb-PRS. Interestingly, Hb-PRS also demonstrated negative causal associations with LDL-C and TC in both ethnicities.
3.5. MR-Egger Regressions
Sex-specific MR-Egger regressions examined the presence of pleiotropy for the sets of genetic variants utilized as IVs for iron status. Tables S22 and S23 present the results from the MR-Egger regressions for the Hb-PRS−metabolic outcomes associations and Hb-PRS−metabolic traits associations, respectively. Figures S1-S4 are the scatterplots of sex-specific genetic associations and causal estimates of representative SNPs, which indicated the possible pleiotropic Hb-associated SNPs that may have violated the IV assumptions.
MR-Egger estimates provided further evidence that iron status was a causal risk factor for HTN and DLP among Europeans, but a causally protective risk factor against T2D or HGY for both ethnicities (Table S22). Additionally, the slope was significant and positive for DBP among Europeans and female Taiwanese HC, as well as with TG in male Europeans (Table S23). The slope of the MR-Egger regression can provide pleiotropy-corrected causal estimates. However, it is considered underpowered unless the SNPs combine to explain a large proportion of the variance in the exposure with varying effect sizes [65].
There was no apparent directional pleiotropy based on the MR-Egger intercepts except only for T2D or HGY in male Europeans alone (p = 0.042). All of the other intercept estimates did not significantly differ from zero. Moreover, the MR-Egger scatter plots (Figures S1-S4) suggested that the sets of SNPs used to construct the ethnic- and sex-specific Hb-PRSs do not indirectly affect MetS, the individual risk components, and various metabolic traits through other pathways or mechanisms.
3.6. Sensitivity Analyses
Sensitivity analyses were carried out to further validate the main MR findings, which similarly depicted the causal roles of genetically instrumented Hb concentration levels on the metabolic risk outcomes upon exclusion of subjects based on self-reported data and hospital in-patient records. A comparison of OR plots between the main MR and sensitivity analyses are presented in Figures S5 and S6 for the Taiwanese HC and European Whites, respectively. Corresponding OR tables are in Tables S24–S28. Sensitivity analyses for the causality between iron status and metabolic traits are then reported in Tables S29–S30.
Results of the sensitivity analyses revealed similar trends and findings. The evidence to support a positive causality of iron status on increased risks of HTN and DLP and a U-shaped causality for GT or HUA, particularly among Europeans, remained. The consistent causal associations between increasing Hb-PRS but decreased risks against T2D or HGY also still held true even with the sensitivity analyses. On the other hand, Hb-PRS remained positively associated (i.e., highly significant) with DBP and TG in both ethnicities and with UA particularly among female Taiwanese HC, but negatively related with GLU, HbA1c, TC, and LDL-C levels. No causal association with HDL-C was identified.
Finally, we utilized the causality between Hb-PRS and anemia risk as another sensitivity analyses and control for the main MR analyses. Causal, negative associations between Hb-PRS quintiles and anemia risk followed the well-established inverse relationship between blood Hb levels and anemia (Figure 4; Table S31). Monotonic decreases in anemia risk with increasing Hb-PRS quintiles were very highly significant (p <0.0001) across all sex groups, with the highest quintiles resulting in low ORs for anemia as follows: 0.44 (0.36−0.55) among male Taiwanese HC; 0.52 (0.48−0.57) among female Taiwanese HC; 0.54 (0.48−0.61) among male Europeans, and; 0.62 (0.57−0.67) among female Europeans. In this regard, the relationship of increasing genetically determined Hb against T2D or HGY (Figure 3; Table S20–S21) supports the causal role of relatively lower iron levels on T2D development. Further validation is, however, needed.
4. Discussion
Our ethnic- and sex-specific Mendelian randomization studies among the Taiwanese Han Chinese of Taiwan Biobank and the European Whites of UK Biobank elucidated the causal but differential relationship between iron status and several metabolic components. Here, ethnic- and sex-specific polygenic risk scores of blood hemoglobin concentration were constructed and served as instruments to represent iron status. We found that Hb-PRSs correspond to a relatively narrow range of Hb concentration (i.e., 14.91-15.21 g/dL for males and 13.39-13.71 g/dL for females in the UKB; 14.79-15.28 g/dL for males and 12.78-13.21 g/dL for females in the TWB). Within these relatively narrow mid-range of the Hb distribution, our findings support the causality of higher iron levels on stronger risks of hypertension and dyslipidemia for both ethnicities, which were further corroborated by the positive causal relationships with diastolic blood pressure and blood triglyceride levels. A distinct U-shape causality was obtained for gout or hyperuricemia, whereas no causality existed for central obesity. In contrast, we discovered an inverse causal association of increasing increments of iron level with decreasing risk of diabetes or hyperglycemia in both ethnicities, which, interestingly, implied a protective role for higher iron status against this specific metabolic condition alone and, at the same time, supports the causal role of relatively lower iron levels on the development of type 2 diabetes. We do not know whether there would be a risk rising at the higher end of Hb-PRS similar to our previous U-shaped finding on Hb concentration and diabetes in the observational study [36], since there were not enough participants with very high Hb-PRS. In addition, we did not present the association between Hb-PRS and the MetS due to the inconsistency that exists among the five components.
4.1. Causal Role of Elevated Iron on Increased Risks of Hypertension and Dyslipidemia
A number of cross-sectional and prospective studies have demonstrated positive associations between elevated iron status or dietary iron intakes (particularly heme iron from red meat) and stronger risk or higher incidence of HTN [17,22,36], DLP [18,21,36], or the MetS in general [19−21,36]. However, investigation of whether a true causality exists or not remained scarce.
The first MR-PheWAS of systemic iron status among Europeans in the UKB did not detect any causality between genetically predicted iron status and cardiometabolic phenotypes across the phenome, except for an evidence of a causal, protective effect of higher iron on lower hypercholesterolemia risk [32]. Our current MR studies and the first MR-PheWAS both utilized the hospital episode statistics data in UKB. However, the MR-PheWAS used only three iron status genetic instruments (i.e., HFE rs1800562, rs1799945; TMPRSS6 rs855791 [34]), whilst we constructed IVs using population-specific SNPs from a GWAS in our MR studies [35]. It may largely explain why we were able to derive evidence of causality for iron-HTN and iron-DLP associations. The consistent findings we obtained between the Taiwanese HC and European Whites strengthened the evidence for a causal role of genetically determined higher iron status on HTN and DBP, as well as on DLP and blood TG (but not HDL-C). On the other hand, the lack of causal effect of iron status on HDL-C levels in our MR studies has been consistent with the result of another MR-PheWAS of blood iron on lipid-related metabolic conditions among Europeans in the UKB [66]. In addition to this, the negative causal role of iron status on LDL-C or TC levels has been similar to the abovementioned finding of the first MR-PheWAS of systemic iron status among Europeans [32]. These require further validation.
There are two widely accepted plausible mechanisms underlying the association of elevated iron status with metabolic derangements. First, excess iron has been long believed to be a contributor to overall oxidative stress, as free iron facilitates the excessive formation of toxic reactive oxygen species (ROS) and catalyzes cellular reactions that can damage the cell [9−10]. The pro-oxidant property of heme iron could affect several tissues, particularly the pancreatic beta cells, muscles, and adipocytes; thus, inducing insulin resistance, beta cell dysfunction, altered endothelial dysfunction, and the release of iron from the ferritin stores. Iron is also suggested to inhibit the body’s oxidative defenses such as that of the superoxide dismutase, one of the main antioxidant defense enzymes [67]. The second mechanistic action of excess iron points to the enhancement of inflammation in the body [10,68−69]. As such, the relationship of iron overload with infectious and inflammatory chronic diseases has been well documented. It is thought that the secretion of proinflammatory adipokines is mediated by the expression of hepcidin, which is influenced by intracellular and extracellular iron concentrations [6]. Chronic low-grade inflammation is recognized as a pathogenic event of any MetS risk component in genetically or metabolically predisposed individuals, resulting in cytokine hypersecretion and the production of other inflammatory mediators [68].
In hypertension, the direct link between iron and blood pressure, for the most part, remains unknown [17]. Oxidative stress and chronic inflammation from excessive iron is presumed to lead to atherosclerotic events by initially causing endothelial damage and vascular dysfunction that subsequently induce high blood pressure [70]. Another proposed mechanism relates to the action of high Hb levels, which is also considered as the most important determinant of whole blood viscosity [71]. Hyperviscosity or the decreased blood flow throughout the body, following a prolonged state of oxidative stress, could raise the blood pressure systemically [72].
In dyslipidemia, the excessive biosynthesis of ROS may lead to lipid peroxidation and alterations in lipid metabolism. Lipid peroxidation causes the depletion of the cellular content of reduced glutathione, an important player in preventing cell damage [73]. It was also hypothesized that the increased risk of iron overload due to high intakes of iron may reduce the insulin-mediated suppression of lipase (i.e., enzyme responsible for mobilization of triglyceride), which, then, can increase intracellular lipolysis, the plasma levels of free fatty acids, and its transport to the liver [18,21,69]. Increment in the levels of liver free fatty acids stimulates triglyceride-rich lipoprotein production.
Since both HTN and DLP, as components of MetS or as separate metabolic risk factors, have been linked to the onset of cardiovascular diseases, further work is warranted to unravel the mechanistic details of higher iron status, either from genetic or dietary causes, and its effect on hypertension and lipid metabolism.
4.2. Non-Causality of Iron for Central Obesity and a U-Shape Causality for Gout or Hyperuricemia
There were no causal associations found between genetically determined iron levels and CO risk. These MR findings were inconsistent with those of the observational studies [19,36]. Central obesity is considered a predominant risk factor (i.e., core component) of MetS and a marker of onset of a dysmetabolic state [10,44]. Although oxidative stress from excess iron is deemed to play a causative role in CO and obesity per se, we were unable to derive evidence of causality for CO. Therefore, we surmise that the mechanisms of action of too much iron in relation to MetS is probably independent of abdominal fat accumulation or via a separate pathway independent of obesity. For example, red meat intake is a contributing factor to both high hemoglobin concentration and obesity [74]. It is likely that it is the high fat content of red meat which causes obesity, and that bioavailable iron from heme induces other metabolic disorders such as hypertension and hypertriglyceridemia. Nevertheless, it is also plausible that obesity can be both a result of and a cause of oxidative stress [75]. It may be visceral adiposity instead that could lead to changes in iron homeostasis, making higher iron status a marker rather than a causal factor [76]. This complexity requires further investigation.
On the other hand, the MR findings on iron-GT/HUA causality corroborate the results of our observational studies on the previously unreported curvilinear effects of iron status on GT or HUA [36]. This highlights that the genetic predisposition to either too much or too less iron can cause a detrimental role on risk of GT or HUA, and that maintaining iron status within the normal range is most beneficial. In gout, the stimulation of iron-induced oxidative stress begins when iron cations form complexes with monosodium urate crystals. This contributes to granulocyte and complement activation, which eventually leads to gouty inflammation [77]. Urate, on the other hand, is a well-known antioxidant in humans that reduces oxidative stress by acting as a chelator of iron. Accordingly, elevation in uric acid levels was hypothesized to be a response to counter an increased oxidative stress in the body [78]. Although the mechanism of increased iron on uric acid metabolism and gout remains to be fully understood, looking further into the causal role of iron on the pathology of HUA or GT will be highly beneficial, especially that hyperuricemia has been recently proposed as an additional cause and component of the MetS [37].
4.3. Causal Role of Lower Iron on Risks of Diabetes or Hyperglycemia
Negative causal associations of genetically predicted higher iron status on T2D or HGY risk, and on FBS/GLU or HbA1c levels, were obtained in the current MR studies, implicating a causal protective effect of elevated iron within the normal range against glucose metabolism derangements and, concomitantly, a causal role for relatively lower iron levels on T2D development. Results from epidemiological studies, however, have mostly presented a positive relationship between iron and diabetes [7,15−16]. In contrast, our recently published observational study yielded a distinct U-shaped association between increasing Hb and T2D risk [36]. Conflicting findings between observational association studies and MR analyses suggest that the results from the former were likely influenced by a number of environmental or lifestyle factors, while those from the MR studies potentially indicate the true causal effects of both iron deficiency and excess in the complex etiology of T2D.
The positive link between increased iron status or high iron intakes and hyperglycemia (or diabetes) in cross-sectional and prospective studies has been robustly attributed to iron being a strong pro-oxidant. Pancreatic beta cells, in particular, have a low expression of antioxidants, lending these cells to be highly sensitive to ROS [70]. This eventually leads to changes in glucose metabolism, a decrease in the synthesis and secretion of pancreatic insulin, impaired insulin signaling, and iron-dependent apoptosis (i.e., ferroptosis) [75]. Insulin resistance is regarded as another core component of MetS [10,44], and several metabolic manifestations are explained from this dysfunction [67]. For instance, hyperinsulinemia during ROS-induced insulin resistance states exacerbates the endothelial damage and vascular dysfunction that lead to a systemic increase in blood pressure (i.e., hypertension), as well as the suppression of lipase that stimulates the production of triglyceride-rich lipoproteins (i.e., dyslipidemia).
Our MR finding of relatively higher iron status causally reducing the risk of T2D or HGY may be biologically plausible and is of considerable clinical relevance, as diabetes has been well known to increase the risk of morbidity and mortality related to atherosclerotic and cardiovascular diseases. One MR study has similarly provided evidence on the negative correlation between iron status and T2D risk [33]. Few MR studies have further shown that higher genetically determined iron status in the normal range is protective against atherosclerotic disease and hypercholesterolemia risk [30,32]. These findings may be explained by a hyperoxic state due to elevated Hb and iron status since reduced iron stores leading to hypoxia could increase red blood cell turnover, which, then, has been linked to the increased glycation of Hb [79].
On the other hand, iron deficiency or lower-than-normal iron levels also affect diabetes risk through several putative molecular mechanisms [80]. Both iron deficiency and iron excess have been shown to increase ROS production that causes mitochondrial DNA damage [81]. Lower iron status contributes to many long-term micro- and macrovascular complications through the chronic impairment of insulin action and low-grade inflammation [82]. Additionally, lower iron-T2D risk association, which we had earlier demonstrated in our observational association studies [36], may represent an end-stage anemia phenomenon that results in poor blood circulation, decreased oxidative capacity, and pancreatic islet malfunction [79]. Interestingly, in the current MR studies, we were able to further provide evidence that supports the causal role of lower iron status in the etiology of diabetes since the other MetS components did not depict a clear negative association. Nevertheless, since the constructed IVs (i.e., Hb-PRS) only defined small variations in iron status within the normal range [32], careful consideration must be taken when interpreting our data.
From an alternative perspective, another mechanism that may underlie this iron-T2D relationship could be attributed to the effects of the genetic variants along the MHC region. This may be especially true among Europeans, wherein majority of the SNPs utilized as IVs for this group were found along this region. Removal of the MHC region in IV construction could be considered as a sensitivity analyses in future MR studies. Whether the iron-T2D causal associations would be robust or not to such analyses will implicate the genes within the MHC region as the ones predominantly driving the causality [83]. Further validation is highly warranted.
4.4. Strengths and Limitations of the Study
Our MR studies has several strengths. To our knowledge, we are the first to establish a causality inference between iron status and various metabolic disorders (as well as MetS metabolic traits) altogether. Whilst the genetic propensity to elevated iron status may be considered a modest causal factor for either HTN, DLP, or GT or HUA, we have discovered a strong inverse or negative causality for T2D or HGY. This supplemented and even leveraged the results we obtained from the Hb-MetS observational association studies [36]. Our findings may pave the way to further elucidating the link between iron and metabolic dysfunctions, and, in the future, to coming up with novel preventive or therapeutic strategies such as genetic-based dietary interventions (i.e., precision nutrition) [84]. Second, our ethnic-specific MR analyses involved two of the largest and major ethnic groups in the world, the Han Chinese and Europeans. We have cross-validated our findings between two population groups that uniquely differ in iron status profile (i.e., anemia, thalassemia, and other hemoglobinopathies are more common among Asians; hemochromatosis or iron overload in the body is prevalent among Caucasians). At the same time, we have addressed the underrepresentation of Asians and non-European descents in genetic/epidemiological studies [85] with the findings obtained from the Taiwanese HC group. Third, our MR studies employed relatively large sample sizes of TWB and UKB cohorts, which enabled us to further carry out sex-specific analyses without sacrificing the robustness of the results and the statistical power of our studies. A very large sample size is also a major requirement for MR studies in evaluating the likelihood and magnitude of any causal association. Estimated reliable genetic associations are known to be generally modest because individual genetic variants are likely to explain only a very small proportion of the variation in phenotypic traits [25]; hence, the need for adequate statistical power. Fourth, we were able to satisfy the key assumption of vertical pleiotropy for an MR study based on the MR-Egger regression results, which, when violated, will bias any inference of the causal effect [28]. The sensitivity analyses also yielded results consistent with the main MR analyses. Both analyses further strengthened the causal inferences obtained in this study. Lastly, in constructing the IVs, we opted to utilize population-specific SNPs identified from a previously conducted GWAS of Hb concentration [35]. This approach allowed the inclusion of genetic variants that contain additional information (i.e., multiple genetic backgrounds), which may result in predicting exposures more accurately while reducing weak instrument bias [59].
Our MR studies has some limitations as well. First, as we have employed a one-sample MR framework, the assumption of independence between the gene-exposure and gene-outcome association estimates may have been violated as these were measured in the same individuals [87]. Most MR studies at present follow a two-sample design (i.e., two independent samples for separate measurements of exposure and outcome), which could be more advantageous than one-sample MR due to increased statistical power [28]. However, the assumption of homogenous samples and population stratification can seriously affect estimates when gene-exposure and gene-outcome associations were derived from different populations [25,86]. Second, we did not further perform a reverse MR analysis to particularly determine whether HGY or T2D would cause a lowering in iron levels (i.e., “outcome” to “exposure”), despite a prior knowledge that the relationship between these two is bi-directional or reciprocating [87]. This approach may help tease apart the correlation between iron status and diabetes, but the interpretation of results will be more plausibly difficult due to the existence of feedback loops between exposure and outcome variables [88]. It would also be interesting to validate another hypothesis that the onset of metabolic disorders leads to changes in iron homeostasis, probably through increased inflammatory status, which corresponds to a continuous cycle between these two [70]. Third, the generalizability of our findings to other ethnicities may not hold. It is highly recommended to further validate causality in other population groups, especially that both iron status and genetic susceptibility to diet-related chronic metabolic conditions differ across ethnicities [89−90]. Fourth, similar to the Hb-MetS observational association studies, we were unable to screen and adjust for subjects who were vegetarians, taking iron supplements, or had consumed more red meats, as these data were incomplete or unavailable in the TWB and UKB at the time of analyses. Other variables that may have confounded the results such as total dietary energy, saturated fats, iron absorption enhancers or inhibitors, concurrent acute or chronic infections, and autoimmune diseases were also unadjusted for. Nonetheless, biases from confounding should not influence the MR estimates [28]. Lastly, we have utilized Hb concentration as the surrogate of iron status. Hb may only act as a good and acceptable iron biomarker from the lower end of the iron status spectrum to within normal levels [2]. Transferrin saturation becomes the ideal biomarker at the higher end, while serum ferritin levels could be exploited for the entire spectrum of iron status. It would finally be worthwhile to utilize the genetic variants associated with serum hepcidin as instruments for iron status. Hepcidin, being the principal iron-regulatory hormone, is a promising candidate to link genetic variation, dietary iron, and metabolic derangements.
5. Conclusions
Our ethnic- and sex-specific MR studies among the Taiwanese Han Chinese and European Whites have provided evidence to support that genetic predisposition to higher iron levels has a causal role on increasing the risks of HTN and DLP while relatively lower iron levels can causally contribute to the development of T2D. Taken together, our MR findings will help to further our understanding on the role of iron dysregulation in human health and better identify at-risk individuals. Since iron status is modifiable and could be optimized through dietary and other interventions, complications associated with chronic metabolic disorders could be prevented for genetically and metabolically susceptible individuals in the near future – one of the ultimate goals of precision nutrition.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: List of 36 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Male Taiwanese Han Chinese of Taiwan Biobank, Table S2: List of 58 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Female Taiwanese HC of TWB, Table S3: List of 698 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Male European Whites of UK Biobank, Table S4: List of 805 clumped Hb-associated SNPs utilized in construction of Hb-PRS among Female European Whites of UKB, Table S5: Comparison of constructed Hb-PRSs and regression parameters between Taiwanese HC of TWB and European Whites of UKB, Table S6: Characteristics of subjects in TWB (N=61,352) across quintiles of Hb-PRS, Table S7: Characteristics of subjects in UKB (N=271,366) across quintiles of Hb-PRS, Table S8: Overall distributions of metabolic outcomes in TWB, Table S9: Overall distributions of metabolic outcomes in UKB, Table S10: Distributions of different metabolic conditions among subjects in TWB across quintiles of Hb-PRS, Table S11: Distributions of different metabolic conditions among subjects in UKB across quintiles of Hb-PRS, Table S12: Distributions of self-reported metabolic conditions among subjects in TWB across quintiles of Hb-PRS, Table S13: Distributions of self-reported metabolic conditions among subjects in UKB across quintiles of Hb-PRS, Table S14: Cases of metabolic conditions from hospital in-patient records of UKB subjects across quintiles of Hb-PRS, Table S15: Odds ratios of metabolic syndrome across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S16: Odds ratios of hypertension across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S17: Odds ratios of dyslipidemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S18: Odds ratios of central obesity across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S19: Odds ratios of gout or hyperuricemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S20: Odds ratios of diabetes or hyperglycemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S21: Odds ratios of type 2 diabetes across quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S22: Sex-specific MR-Egger regressions of associations between iron status and risks of metabolic outcomes among Taiwanese HC and European Whites, Table S23: Sex-specific MR-Egger regressions of associations between iron status and metabolic traits among Taiwanese HC and European Whites, Table S24: Odds ratios from sensitivity analyses of causal associations between MetS and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S25: Odds ratios from sensitivity analyses of causal associations between HTN and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S26: Odds ratios from sensitivity analyses of causal associations between DLP and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S27: Odds ratios from sensitivity analyses of causal associations between GT or HUA and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S28: Odds ratios from sensitivity analyses of causal associations between T2D or HGY and quintiles of Hb-PRS among Taiwanese HC and European Whites, Table S29: Causal estimates from sensitivity analyses of associations between genetically instrumented Hb concentration and metabolic traits among Taiwanese HC, Table S30: Causal estimates from sensitivity analyses of associations between genetically instrumented Hb concentration and metabolic traits among European Whites, Table S31: Odds ratios of anemia across quintiles of Hb-PRS among Taiwanese HC and European Whites, Figure S1: Overlay histograms of sex-specific Hb-PRSs and scatterplots of sex-specific Hb-PRSs against Hb concentration among Taiwanese HC of TWB and European Whites of UKB, Figure S2: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for binary metabolic outcomes among Taiwanese HC, Figure S3: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for binary metabolic outcomes among European Whites, Figure S4: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for quantitative metabolic traits among Taiwanese HC, Figure S5: Sex-specific MR-Egger plots of SNP-outcome estimates against SNP-exposure estimates for quantitative metabolic traits among European Whites, Figure S6: Comparison of OR plots between main MR and sensitivity analyses among Taiwanese HC, depicting causal roles of iron status on MetS and other metabolic outcomes, Figure S7: Comparison of OR plots between main MR and sensitivity analyses among European Whites, depicting causal roles of iron status on MetS and other metabolic outcomes.
Author Contributions
Conceptualization, W.−H.P. and V.J.T.−G.; methodology, V.J.T.−G., K.−M.C., Y.−T.H. and W.−H.P.; software, V.J.T.−G. and K.−M.C.; validation, K.−M.C.; formal analysis, V.J.T.−G.; investigation, V.J.T.−G.; resources, H.−C.Y. and W.−H.P.; data curation, V.J.T.−G.; writing–original draft preparation, V.J.T.−G.; writing–review and editing, W.−H.P.; visualization, V.J.T.−G.; supervision, Y.−T.H., H.−C.Y. and W.−H.P.; project administration, K.−M.C. and W.−H.P.; funding acquisition, W.−H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Cloud Project of Academia Sinica with grant number AS-PH-109-02.
Institutional Review Board Statement
The UK National Health Service’s National Research Ethics Committee gave ethical clearance to the UKB Project with reference number 11/NW/0382. The Institutional Review Board of Academia Sinica approved the TWB study and protocols on June 2019 with reference number AS-IRB02-108226. This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We thank all the participants and investigators of the Taiwan Biobank and UK Biobank. We thank the Institute of Statistical Science of Academia Sinica and the Multidisciplinary Health Cloud Research Program Project – the Statistical Science unit and the Humanities and Social Sciences unit for the facility and financial support. This study has been undertaken using the UK Biobank resource under Application Number 48272.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Geissler, C.; Singh, M. Iron, meat and health. Nutrients 2011, 3, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Bangkok, Thailand, 1998; Available online: https://apps.who.int/iris/handle/10665/42716 (accessed on 02 May 2023).
- Monsen, E.R.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.D.; Mertz, W.; Finch, C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Basuli, D.; Stevens, R.G.; Torti, F.M.; Torti, S.V. Epidemiological associations between iron and cardiovascular disease and diabetes. Front. Pharmacol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Clark, L. Iron, Oxidative Stress, and Disease Risk. Nutr. Rev. 2004, 62, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. (2010). The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef] [PubMed]
- van der A, D.L.; Peeters, P.H.; Grobbee, D.E.; Marx, J.J.; van der Schouw, Y.T. Dietary haem iron and coronary heart disease in women. Eur. Heart J. 2005, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, S.; Liu, G.; Yan, F.; Ma, X.; Huang, Z.; Tian, H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS ONE 2012, 7, e41641. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Brown, I.J.; Chan, Q.; Van Horn, L.; Ueshima, H.; Zhao, L.; Stamler, J.; Elliott, P.; International Collaborative Research Group on Macro-/Micronutrients and Blood Pressure. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ 2008, 337, a258. [Google Scholar] [CrossRef]
- Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Habibi-Moeini, A.S.; Azizi, F. Red meat and dietary iron intakes are associated with some components of metabolic syndrome: Tehran Lipid and Glucose Study. J. Transl. Med. 2019, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Sorlí, M.; Bulló, M.; Basora, J.; Ibarrola-Jurado, N.; Fernández-Ballart, J.; Martínez-González, M.A.; Serra-Majem, L.; González-Pérez, R.; Salas-Salvadó, J.; Nureta-PREDIMED Investigators. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk: cross-sectional and 1-year follow-up assessment. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 200–207. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Dos Santos Vieira, D.A.; Hermes Sales, C.; Galvão Cesar, C.L.; Marchioni, D.M.; Fisberg, R.M. Influence of Haem, Non-Haem, and Total Iron Intake on Metabolic Syndrome and Its Components: A Population-Based Study. Nutrients 2018, 10, 314. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Bo, Y.; Liu, Y. Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 830–836. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: an overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Qi, L. Mendelian randomization in nutritional epidemiology. Nutr. Rev. 2009, 67, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Janss, L.L.; Burgess, S.; Kiemeney, L.A.; den Heijer, M.; de Graaf, J.; Holewijn, S.; Benyamin, B.; Whitfield, J.B.; Swinkels, D.W.; Vermeulen, S.H. Iron and hepcidin as risk factors in atherosclerosis: what do the genes say? BMC Genet. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Del Greco, M.F.; Walker, A.P.; Srai, S.K.S.; Laffan, M.A.; Minelli, C. The Effect of Iron Status on Risk of Coronary Artery Disease: A Mendelian Randomization Study-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1788–1792. [Google Scholar] [CrossRef]
- Gill, D.; Monori, G.; Tzoulaki, I.; Dehghan, A. Iron Status and Risk of Stroke. Stroke 2018, 49, 2815–2821. [Google Scholar] [CrossRef]
- Gill, D.; Benyamin, B.; Moore, L.S.P.; Monori, G.; Zhou, A.; Koskeridis, F.; Evangelou, E.; Laffan, M.; Walker, A.P.; Tsilidis, K.K.; et al. Associations of genetically determined iron status across the phenome: A mendelian randomization study. PLoS Med. 2019, 16, e1002833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2010, 5, 4926. [Google Scholar] [CrossRef] [PubMed]
- Timoteo, V.J.; Chiang, K.M.; Yang, H.C.; Pan, W.H. Common and ethnic-specific genetic determinants of hemoglobin concentration between Taiwanese Han Chinese and European Whites: findings from comparative two-stage genome-wide association studies. J. Nutr. Biochem. 2023, 111, 109126. [Google Scholar] [CrossRef] [PubMed]
- Timoteo, V.J.; Chiang, K.M.; Pan, W.H. Positive or U-Shaped Association of Elevated Hemoglobin Concentration Levels with Metabolic Syndrome and Metabolic Components: Findings from Taiwan Biobank and UK Biobank. Nutrients 2022, 14, 4007. [Google Scholar] [CrossRef] [PubMed]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and Metabolic Syndrome: A Tangled Web. Current Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.M.; Tsay, Y.C.; Vincent Ng, T.C.; Yang, H.C.; Huang, Y.T.; Chen, C.H.; Pan, W.H. Is Hyperuricemia, an Early-Onset Metabolic Disorder, Causally Associated with Cardiovascular Disease Events in Han Chinese? J. Clin. Med. 2019, 8, 1202. [Google Scholar] [CrossRef]
- Feng, Y.A.; Chen, C.Y.; Chen, T.T.; Kuo, P.H.; Hsu, Y.H.; Yang, H.I.; Chen, W.J.; Su, M.W.; Chu, H.W.; Shen, C.Y.; et al. Taiwan Biobank: A rich biomedical research database of the Taiwanese population. Cell Genom. 2022, 2, 100197. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/259425 (accessed on 02 May 2023).
- Elliott, P.; Peakman, T.C.; UK Biobank. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006; Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensusworldwide-definitionof-the-metabolic-syndrome.html (accessed on 02 May 2023).
- Zimmet, P.Z.; Alberti, K.G. Introduction: Globalization and the non-communicable disease epidemic. Obesity 2006, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.E.; Wang, T.D.; Ueng, K.C.; Lin, T.H.; Yeh, H.I.; Chen, C.Y.; Wu, Y.J.; Tsai, W.C.; Chao, T.H.; Chen, C.H.; et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J. Chin. Med. Assoc. 2015, 78, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; ESC Scientific Document Group; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization & International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/handle/10665/43588 (accessed on 02 May 2023).
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; de Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the Capture and Adjudication of Prevalent and Incident Diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Li, Y.H.; Ueng, K.C.; Jeng, J.S.; Charng, M.J.; Lin, T.H.; Chien, K.L.; Wang, C.Y.; Chao, T.H.; Liu, P.Y.; Writing Group of 2017 Taiwan Lipid Guidelines for High Risk Patients; et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017, 116, 217–248. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Wei, C.Y.; Yang, J.H.; Yeh, E.C.; Tsai, M.F.; Kao, H.J.; Lo, C.Z.; Chang, L.P.; Lin, W.J.; Hsieh, F.J.; Belsare, S.; et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. 2021, 6, 10. [Google Scholar] [CrossRef]
- Wain, L.V.; Shrine, N.; Miller, S.; Jackson, V.E.; Ntalla, I.; Soler Artigas, M.; Billington, C.K.; Kheirallah, A.K.; Allen, R.; Cook, J.P.; et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir. Med. 2015, 3, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; Sham, P.C. PLINK: a tool set for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, D.I.; Kuchenbaecker, K.B.; Shah, S.; Sofat, R.; Holmes, M.V.; White, J.; Mindell, J.S.; Kivimaki, M.; Brunner, E.J.; Whittaker, J.C.; Casas, J.P.; Hingorani, A.D. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int. J. Epidemiol. 2016, 45, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mak, T.S.H.; O’Reilly, P.F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity, Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 02 May 2023).
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Francis, M.; Sun, Y.; Ryu, M.S.; Grider, A.; Ye, K. The Causal Effects of Blood Iron and Copper on Lipid Metabolism Diseases: Evidence from Phenome-Wide Mendelian Randomization Study. Nutrients 2020, 12, 3174. [Google Scholar] [CrossRef]
- Avelar, T.M.T.; Storch, A.S.; Castro, L.A.; Azevedo, G.V.M.M.; Ferraz, L.; Lopes, P.F. Oxidative stress in the pathophysiology of metabolic syndrome: which mechanisms are involved? J. Bras. Patol. Med. Lab. 2015, 51, 231–239. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 2009, 139, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kilani, N.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P. Iron metabolism and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1025–1032. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Chien, S.; Alderman, M.H.; Atlas, S.A.; Laragh, J.H. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation 1990, 81, 107–117. [Google Scholar] [CrossRef]
- Atsma, F.; Veldhuizen, I.; de Kort, W.; van Kraaij, M.; Pasker-de Jong, P.; Deinum, J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 2012, 60, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E. Inflammation, lipids, and free radicals: lessons learned from the atherogenic process. Semin. Reprod. Endocrinol. 1998, 16, 249–261. [Google Scholar] [CrossRef]
- Chang, H.C.; Yang, H.C.; Chang, H.Y.; Yeh, C.J.; Chen, H.H.; Huang, K.C.; Pan, W.H. Morbid obesity in Taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS One 2017, 12, e0169577. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Rao, G.; Cooke, C.L.; Miller, M.J.; Hoidal, J.R. Complexation of iron cation by sodium urate crystals and gouty inflammation. Arch. Biochem. Biophys. 1994, 313, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Soliman, N. Iron deficiency anemia and glucose metabolism. Acta Biomed. 2017, 88, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Astle, W.J.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.L.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell 2016, 167, 1415–1429.e19. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Lawson, H.A. Ironing out the Details: Untangling Dietary Iron and Genetic Background in Diabetes. Nutrients 2018, 10, 1437. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef]
- Minelli, C.; Del Greco, M.F.; van der Plaat, D.A.; Bowden, J.; Sheehan, N.A.; Thompson, J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol. 2021, 50, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Baird, D.; Borges, M.C.; Bowden, J.; Hemani, G.; Haycock, P.; Evans, D.M.; Smith, G.D. Recent Developments in Mendelian Randomization Studies. Curr. Epidemiol. Rep. 2017, 4, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Barad, A.; Clark, A.G.; Wang, Y.; Lin, X.; Gu, Z.; O’Brien, K.O. Ethnic Differences in Iron Status. Adv. Nutr. 2021, 12, 1838–1853. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Shen, J. Gene-environment interactions and susceptibility to metabolic syndrome and other chronic diseases. J. Periodontol. 2008, 79, 1508–1513. [Google Scholar] [CrossRef]
Figure 1.
Odds ratios of HTN and DLP across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 1.
Odds ratios of HTN and DLP across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.

Figure 2.
Odds ratios of CO and GT or HUA across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 2.
Odds ratios of CO and GT or HUA across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.

Figure 3.
Odds ratios of T2D or HGY across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 3.
Odds ratios of T2D or HGY across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.

Figure 4.
Odds ratios of anemia across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.
Figure 4.
Odds ratios of anemia across increasing quintiles of genetically instrumented Hb concentration among Taiwanese Han Chinese and European Whites. Sex-specific analyses fully adjusted for age, age-squared, first ten genetic PCs, smoking status, and alcohol consumption, as well as menopausal status in females.

Table 1.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among Taiwanese Han Chinese.
Table 1.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among Taiwanese Han Chinese.
| Metabolic Trait | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Beta | SE | P-value | Beta | SE | P-value | |
| Males (n=19,005) | ||||||
| WC, cm | 31.9 | 14.4 | 0.0268 | 33.4 | 14.3 | 0.0199 |
| SBP, mm Hg | 51.9 | 24.6 | 0.0351 | 52.5 | 24.5 | 0.0324 |
| DBP, mm Hg | 53.5 | 16.1 | 0.0009 | 53.6 | 16.0 | 0.0008 |
| FBG*, mg/dL | 87.6 | 35.8 | 0.0094 | 88.2 | 35.7 | 0.0087 |
| TG*, mg/dL | 533.5 | 183.1 | 0.0005 | 543.5 | 180.9 | 0.0003 |
| HDL-C, mg/dL | -27.1 | 17.1 | 0.1131 | -27.3 | 16.9 | 0.1059 |
| UA, mg/dL | 2.1 | 2.1 | 0.3255 | 2.3 | 2.1 | 0.2690 |
| HbA1c*, % | 0.40 | 1.4 | 0.7771 | 0.36 | 1.4 | 0.7962 |
| TC, mg/dL | -103.3 | 53.5 | 0.0535 | -100.7 | 53.5 | 0.0599 |
| LDL-C, mg/dL | -130.7 | 48.0 | 0.0065 | -129.3 | 48.0 | 0.0071 |
| Females (n=42,330) | ||||||
| WC, cm | 24.6 | 18.3 | 0.1777 | 24.6 | 18.2 | 0.1773 |
| SBP, mm Hg | 55.3 | 31.1 | 0.0751 | 57.0 | 31.0 | 0.0664 |
| DBP, mm Hg | 68.7 | 19.1 | 0.0003 | 69.1 | 19.1 | 0.0003 |
| FBG*, mg/dL | -45.1 | 35.2 | 0.0795 | -46.2 | 35.2 | 0.0736 |
| TG*, mg/dL | 109.2 | 135.4 | 0.2129 | 103.1 | 135.1 | 0.2302 |
| HDL-C, mg/dL | 2.9 | 25.4 | 0.9077 | 2.1 | 25.3 | 0.9352 |
| UA, mg/dL | 5.0 | 2.1 | 0.0188 | 4.8 | 2.1 | 0.0224 |
| HbA1c*, % | -6.1 | 1.4 | <0.0001 | -6.1 | 1.4 | <0.0001 |
| TC, mg/dL | -121.0 | 4.2 | 0.0673 | -126.3 | 65.9 | 0.0551 |
| LDL-C, mg/dL | -44.8 | 59.6 | 0.4515 | -48.1 | 59.4 | 0.4184 |
* Variables with skewed distribution were log-transformed prior to analyses. Model 1 similarly adjusted for age, age-squared, and the first ten genetic PCs; whereas model 2 additionally adjusted for smoking status and alcohol consumption (as well as menopausal status in females). DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Table 2.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among European Whites.
Table 2.
Causal estimates of associations between Hb-PRS and quantitative metabolic traits among European Whites.
| Metabolic Trait | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Beta | SE | P-value | Beta | SE | P-value | |
| Males (n=128,549) | ||||||
| WC, cm | -21.9 | 18.6 | 0.2396 | -27.0 | 18.4 | 0.1434 |
| SBP, mm Hg | -30.8 | 28.8 | 0.2842 | -27.7 | 28.8 | 0.3348 |
| DBP, mm Hg | 129.1 | 17.1 | <0.0001 | 131.3 | 17.1 | <0.0001 |
| GLU*, mmol/L | -11.5 | 2.4 | <0.0001 | -11.7 | 2.4 | <0.0001 |
| TG*, mmol/L | 9.9 | 1.9 | <0.0001 | 9.6 | 1.9 | <0.0001 |
| HDL-C, mmol/L | 0.13 | 0.55 | 0.8170 | 0.26 | 0.54 | 0.6262 |
| UA, μmol/L | -139.4 | 120.0 | 0.2450 | -143.8 | 119.1 | 0.2273 |
| HbA1c*, mmol/mol | -177.8 | 11.9 | <0.0001 | -181.1 | 11.8 | <0.0001 |
| TC, mmol/L | -1.2 | 1.9 | 0.5232 | -0.87 | 1.9 | 0.6408 |
| LDL-C, mmol/L | -2.9 | 1.4 | 0.0427 | -2.7 | 1.4 | 0.0618 |
| Females (n=142,817) | ||||||
| WC, cm | -17.7 | 27.4 | 0.5189 | -15.1 | 27.0 | 0.5768 |
| SBP, mm Hg | 41.9 | 40.5 | 0.3013 | 40.6 | 40.5 | 0.3160 |
| DBP, mm Hg | 220.6 | 22.6 | <0.0001 | 221.4 | 22.6 | <0.0001 |
| GLU*, mmol/L | -12.3 | 2.4 | <0.0001 | -12.1 | 2.4 | <0.0001 |
| TG*, mmol/L | 12.6 | 1.9 | <0.0001 | 12.9 | 1.8 | <0.0001 |
| HDL-C, mmol/L | -1.0 | 0.89 | 0.2615 | -1.1 | 0.87 | 0.2026 |
| UA, μmol/L | -19.3 | 144.5 | 0.8936 | -3.4 | 144.1 | 0.9811 |
| HbA1c*, mmol/mol | -220.7 | 12.2 | <0.0001 | -217.8 | 12.1 | <0.0001 |
| TC, mmol/L | -7.9 | 2.4 | 0.0011 | -8.0 | 2.4 | 0.0009 |
| LDL-C, mmol/L | -8.9 | 1.9 | <0.0001 | -8.9 | 1.9 | <0.0001 |
* Variables with skewed distribution were log-transformed prior to analyses. Model 1 similarly adjusted for age, age-squared, and the first ten genetic PCs; whereas model 2 additionally adjusted for smoking status and alcohol consumption (as well as menopausal status in females). DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Positive or U-Shaped Association of Elevated Hemoglobin Concentration Levels with Metabolic Syndrome and Metabolic Components: Findings from Taiwan Biobank and UK Biobank
Vanessa Timoteo
et al.
Nutrients,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






