Introduction
Urinary tract infections (UTIs) are among the most common bacterial infections in the community and the hospital setting1.In a study conducted in Sri Lanka, the incidence of UTIs in the diabetic population was 27%2. In the South Asian Region, UTIs account for a significant portion of hospital-acquired infections. In noninstitutionalised elderly populations, UTIs are the second most common infection, accounting for nearly 25% of all infections3.
A urinary tract infection (UTI) is an infection in any part of the urinary system, which includes kidneys, ureters, bladder, and urethra. Hence, the infections of the urinary system range from pyelonephritis to urethritis. Acute renal infections include entities such as acute pyelonephritis (APN), Acute focal bacterial nephritis (AFBN), renal and perinephric abscesses, emphysematous pyelonephritis (EPN), and pyonephrosis. In contrast, chronic renal infections include chronic pyelonephritis, xanthogranulomatous pyelonephritis (XGP), malakoplakia, and eosinophilic cystitis4.
Urinary tract infection diagnoses are mostly made clinically and confirmed by abnormal laboratory values5. Hence, imaging is not usually performed and is reserved for complicated situations to assess the type and extent of the renal infection and its complications. Thus, this article mainly focuses on the main radiological modalities used and their radiological features in renal infections to guide treating microbiologists and physicians.
Acute Pyelonephritis
Acute pyelonephritis (APN) is characterised by infection of the renal collecting system and parenchyma, and it is considered a serious entity as it has the potential for renal damage and urosepsis. Renal infection characteristically results from the ascending retrograde spread of organisms. Eighty per cent of encountered organisms causing pyelonephritis are Escherichia coli, followed by other Gram-negatives such as Klebsiella spp, Pseudomonas spp, Enterococcus spp, and some of the Gram-positives include Streptococcus spp and Staphylococcus spp6.
In the majority, treatment for APN usually starts based on clinical and laboratory findings. However, radiological imaging is generally reserved for complicated cases, atypical symptoms, old age, diabetes and other immunocompromised situations, and congenital anomalies7.
Imaging modalities utilised in the diagnostic workup of acute pyelonephritis are diverse. Among them, ultrasound is a valuable initial imaging modality. It can be used in paediatric patients, emergency settings, at the bedside for critically ill patients, as a guide during interventions in complicated cases and for obtaining sequential images to monitor the progression of the disease process
8. Advancements in ultrasound technology, including improved image resolution, better transducers, and enhanced imaging algorithms, have increased the sensitivity rates of detecting APN
5,9,10. The ultrasound features of APN and pathological reasons attributed to the ultrasound features are listed in
Table 1.
Ultrasound is insensitive to detecting acute pyelonephritis since there is a potential to miss subtle changes in mild pyelonephritis, thereby underestimating the severity of the attack11. However, newer applications like tissue harmonic imaging have increased the sensitivity and specificity to 97% and 80%, respectively5.
Contrast-enhanced computed tomography (CECT) is the imaging technique for assessing individuals with acute pyelonephritis
5. According to H. Stunell et al., helical and multislice CT protocol for APN includes three phases. The first phase, non-contrast, will detect parenchymal, calyceal and ureteric calculi, gas formation, haemorrhage and renal enlargement. However, on most occasions, non-contrast images will appear normal. The second phase would be post-IV contrast imaging acquisition of about 50-90 seconds. The third phase, or excretory phase (> 2 minutes post-IV contrast), is optional and only run if a ureteric obstruction is suspected
14. The CECT features of APN and pathological explanations that are attributed to the CECT features are listed in
Table 2.
Some less common findings can be observed in contrast-enhanced computed tomography (CECT), including perirenal fat infiltration, ureteral wall oedema, and the formation of renal abscesses16. These findings are associated with increased tissue destruction, as indicated by clinical and laboratory parameters16.
Furthermore, certain features in CECT scans can provide insights into the pathology of renal infections. For instance, the presence of round peripheral hypo-enhancing parenchymal renal lesions may suggest a hematogenous route of infection. Conversely, the presence of wedge-shaped areas implies an ascending route of infection. However, distinguishing between these routes can become challenging when the entire kidney is involved
7. Contrast-enhanced ultrasound has been shown to have similar sensitivity and specificity as non-contrast CT(NCCT) in diagnosing and following up patients with pyelonephritis
10.
Figure 1
In MRI, features observed are similar to those of CT, such as renal enlargement, signal intensity alterations due to haemorrhage or oedema, striated nephrogram, and perinephric fluid reaction. MRI with contrast helps to show areas of renal parenchymal involvement7.
Radionuclide cortical scintigraphy with 99 mTc-dimercaptosuccinic acid (Tc-99m DMSA) offers visualisation of the renal cortex and may show peripheral areas of decreased uptake related to acute pyelonephritis or scar formation. Currently, its use is limited to paediatrics for detecting renal scarring, and scar formation following acute pyelonephritis is rare in adults17.
If left untreated, acute pyelonephritis can give rise to complications such as renal abscess formation, chronic pyelonephritis, and emphysematous pyelonephritis, the details of which will be discussed under separate subtopics further in this article.
Acute Focal Bacterial Nephritis
Acute focal bacterial nephritis (AFBN), or acute lobar nephronia (ALN), is an intermediate condition between acute pyelonephritis and renal abscess, mainly seen in children, with rare occurrences in adults. Escherichia coli is the predominant organism in both age groups18.AFBN clinically resembles renal abscess, presenting with fever, flank or abdominal pain, dysuria, and pyuria. Early imaging is vital to prevent progression into renal abscesses18. Ultrasonographically, AFBN appears hypoechogenic or hyperechoic with a poorly defined mass. Duplex ultrasonography, with or without contrast enhancement, may reveal localised hypoperfusion19. Ultrasound provides 90% sensitivity and 86.4% specificity in diagnosis, making it a primary imaging choice18.
CT appearance includes a poorly defined wedge-shaped area with reduced contrast enhancement, representing single lobe inflammation, sometimes affecting multiple lobes
19. CT distinguishes simple and complicated AFBN, with simple AFBN displaying striated or wedge-shaped, poorly defined, homogeneous hypodense areas, while complicated AFBN exhibits heterogeneously focal hypodense areas in nephrographic phases
20.
Figure 2
Pathological explanations of ultrasonic and CT findings are listed in
Table 3. In MRI of AFBN, the affected areas are hypointense in T2W images with poor enhancement in post-contrast T1W images.
Renal and Perinephric Abscesses
Renal abscesses are encapsulated pus collections confined to the renal parenchyma, and perinephric abscesses are collections of suppurative material located between Gerota’s fascia and the renal capsule21. The main organisms involved are Gram-negative bacteria, including Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa22.
Diabetes mellitus is the most common contributory factor for renal and perinephric abscesses, followed by other conditions such as liver cirrhosis, renal stones, chronic renal insufficiency, an immunosuppressed state, ureteral obstruction, chronic urinary retention, and iatrogenic causes21.
The imaging modalities used to diagnose renal and perirenal abscesses are ultrasound scan, CT scan, and MRI
23.
Figure 3
Renal abscesses appear enlarged with distorted architecture on ultrasound, presenting as well-defined fluid collections with internal echogenic debris and distinct walls. This clear wall helps to differentiate renal abscess from AFBN. There may be internal septations causing multiple loculations within the abscess7. Perinephric abscess is usually low or mixed echogenic, depending on the content and located outside the renal parenchyma7. Ultrasound guidance is useful in the percutaneous drainage of abscess material for microbiological assessment and placement of pigtail catheters for therapeutic drainage. Percutaneous drainage offers benefits due to its simplicity and rapidity as a procedure, avoidance of general anaesthesia, reduced invasiveness, especially when dealing with a large volume collection, the presence of comorbid factors, and when previous medical treatments have been unsuccessful23.
CECT is considered more sensitive than ultrasonography for diagnosing renal abscesses, with a detection rate of approximately 90-100%23. However, one study showed no superiority of CECT over ultrasound for diagnosing renal abscesses, probably due to recent advancements in ultrasound technology24. Still, the CT technique is more accurate in assessing the abscess size, rest of the renal parenchymal condition and post-therapeutic assessment23.On a CECT, early evolving abscesses are seen as small rounded or wedge-shaped cortical low attenuating non-enhancing lesions. In contrast, mature abscesses are seen as marginated, complex cystic. This appearance may simulate renal malignancy25. However, features such as perilesional oedema within the kidney, variable degree of fat stranding adjacent to the abscess, and thickening of Gerota’s fascia, low attenuating lesion with enhancing wall in CECT with positive history and urine analysis indicate a renal abscess25. CECT is also valuable in the precise evaluation of extrarenal extension into perinephric space, involvement of psoas and posterior abdominal wall muscles and adjacent organs26.
In the case of a difficult ultrasound-aided approach, CT-guided aspiration of abscess material or insertion of a drain tube is the preferred method.
MRI is recognised as more sensitive and specific than CT for surveying renal lesions. MRI is highly sensitive and specific, primarily used in pregnancy and children to avoid CT radiation. Mature abscesses display low T1W and high T2W signal intensities, with enhancing walls. Heterogeneous restricted diffusion in DWI images is observed in acute stages, while resolution shows no diffusion restriction on follow-up images26.
Emphysematous Pyelonephritis.
Emphysematous pyelonephritis (EPN) is a rare, severe necrotising infection in the renal parenchyma and perirenal tissues caused by gas-forming bacteria, leading to gas formation in the renal parenchyma, collecting system, or perinephric tissue
27. Uncontrolled diabetes mellitus is a major predisposing factor in 85-100% of cases, and non-diabetics typically have urinary tract obstruction
7. The most common organisms isolated in EPN are
Escherichia coli, Klebsiella and
Proteus mirabilis27. Timely radiological diagnosis is crucial, as clinical and lab findings only suggest urological sepsis. Imaging findings of EPN are typical; thus, a diagnosis is made easily with plain radiographs, ultrasound, and non-contrast CT(NCCT)
7.
Figure 4
In an X-ray abdomen, mottled or crescent-shaped gas collections are observed within the renal parenchyma and perinephric space28. However, differentiating renal gas from bowel gas on X-ray is challenging, with a low detection rate of only 33%29.
Ultrasonically, gas locules in EPN appear as multiple non-dependent echogenic foci in the renal parenchyma or perinephric spaces. Small gas locules may resemble calculi or bowel gas, while large collections can create high-amplitude echoes with posterior dirty acoustic shadowing (reverberation artefacts). These artefacts may underestimate the depth of renal parenchymal and perinephric involvement, which warrants CT12.
NCCT is considered the gold standard for EPN, providing superior diagnosis, lesion characterisation, severity evaluation, and guidance for drainage and follow-up4. In EPN, the value of contrast-enhanced CT scans is limited because these patients often have impaired renal functions. However, contrast-enhanced CT (CECT) is considered the best modality for differentiating EPN from emphysematous pyelitis, a more benign entity in which the gas formation is limited to the renal collecting system29. CT findings in EPN include enlarged and destructed renal parenchyma, small gas bubbles or linear streaks, fluid collections with gas-fluid levels, and necrotic areas with or without abscesses31.
The EPN is classified into five classes, with class 4 having the worst outcomes based on the extent of air seen on CT findings
31.
Table 4
MRI could be useful in conditions where CT scans are contraindicated or should be used cautiously, such as during pregnancy or for children. Free gas within the renal or perinephric tissue in EPN appears as signal voids on both T1W and T2W images. MRI is used to assess abscesses and the structure of the kidney. Abscess contents are usually hypointense in T1W and heterogeneously hyperintense in T2W images, while diffusion restriction is observed in DWI images32.
Pyonephrosis
Pyonephrosis is a life-threatening urological emergency characterised by accumulating pus or infected material in an obstructed renal collecting system. This obstruction can result from calculi, strictures, tumours, sloughed papillae, or congenital anomalies, necessitating urgent diagnosis and intervention to prevent renal function loss and septic shock5,26.
The most common isolated organisms in pyonephrosis are Escherichia coli, Proteus spp, and Pseudomonas33. Various imaging modalities are used to diagnose and manage pyonephrosis.
Abdominal plain films reveal an enlarged renal outline, absence of the psoas shadow, and urinary tract calculi34.
Ultrasound findings typically include a dilated pelvicalyceal system with internal echogenic debris, fluid-fluid levels, and, occasionally, incomplete (dirty) shadows with gas accumulation. Floating echogenic debris is a reliable diagnostic sign (sensitivity 90%, specificity 97%)5,12.
CECT helps to diagnose pyonephrosis, detect the cause of the obstruction, and assess renal functions. CT findings include renal enlargement, dilatation, obstruction of the pelvicalyceal system, hyperenhancement, thickening of the renal pelvic wall (>2mm), and perinephric fat stranding. Gas-fluid or fluid-fluid levels and layering of contrast material overlying purulent fluid confirm an infected system
7,5. Furthermore, high HU levels in the dilated collecting system on non-contrast CT can differentiate infected from uninfected hydronephrosis. This distinction is especially valuable when CT contrast cannot be used
8.
Figure 5
MRI reveals findings similar to those observed on CECT. However, MRI is the preferred imaging modality over CECT when distinguishing between simple hydronephrosis and pyonephrosis without contrast agents. The DWI sequences may show diffusion restriction of pyogenic material within the collecting system5.
Upon pyonephrosis diagnosis, immediate decompression of the infected dilated system is essential through image-guided percutaneous nephrostomy (PCN) placement. This intervention prevents permanent renal function loss and life-threatening bacteremia.
Xanthogranulomatous Pyelonephritis
Xanthogranulomatous pyelonephritis (XGP) is a rare chronic granulomatous inflammatory condition resulting from chronic urinary tract obstruction, recurrent bacterial infections, and an atypical immune response, causing renal destruction35. The inflammatory process starts in the renal pelvis, extending into the renal medulla, cortex, perinephric space, and retroperitoneum7. The most common causative organisms include Proteus mirabilis and Escherichia coli35.
While histological examination following nephrectomy provides a definitive XGP diagnosis, imaging plays a crucial pre-operative role35. However, imaging features can be misleading, resembling malignancy, tuberculosis, or malakoplakia35. Renal stones, particularly staghorn calculi, are observed in an X-ray KUB. However, not all patients with XGP have renal calculi, nor do all patients with staghorn calculi have XGP35.
The value of USS for the diagnosis of XGP is limited, but it is an option for the initial evaluation. Ultrasonically, XGP exhibits generalised renal enlargement with preservation of renal contour, thinning of renal parenchyma, multiple hypoechoic areas due to dilated calyces with internal echogenic debris or solid granulomatous material, renal pelvic staghorn or parenchymal calculi, suggestive of diffuse XGP12,35. However, focal XGP can be confused with other renal pathologies, such as renal malignancy12.
CECT is the gold standard in diagnosing XGP, showing specific findings and extrarenal extension, which is important in surgical planning
7,36. Classic CECT signs include non-functioning enlarged kidney, staghorn calculus, contracted renal pelvis, dilated multi-loculated calyces, and thinning renal cortex, resembling the "Bear Paw” sign found in 67% of patients
36.
Figure 6
Dilated calyces may have negative Hounsfield unit (HU) numbers due to xanthomatous material7. According to Malek et al., XGP has originally been classified into three stages based on the degree of involvement of nephric and perinephric tissues within a pediatric population. Nevertheless, this classification can also be applied to adults. Stage I (confined to the kidney), Stage II (Gerota fascia infiltration), and Stage III (extension into perinephric space and retroperitoneal structures)37.
MRI findings for XGP are non-specific, with varying signal intensity in different xanthomatous reactions, often overlapping with tuberculosis and renal malignancy35. MRI is not a routine investigation, but post-IV gadolinium (Gd-DTPA) imaging can reveal perinephric extension35.
Furthermore, Tc99m MAG3 or DMPA scintigraphy imaging assesses whether the affected kidney retains residual function. The complete loss of unilateral function indicates diffuse XGP, whereas a kidney with residual function suggests focal XGP38.
Conclusion
Diagnosis of urinary tract infections is primarily established clinically and confirmed through laboratory results. Radiological investigations are typically reserved for complicated renal infections to identify contributing factors, such as renal calculi, diagnose the type of infection, and assess the extent and complications of the renal infection. Furthermore, imaging has become crucial for radiologists during interventional procedures in treating pyonephrosis and renal abscesses. Traditionally, abdominal X-rays identify renal calculi and gas shadows in emphysematous pyelonephritis. Ultrasonography offers an excellent imaging modality for evaluation in emergency settings, pregnancy, and pediatric cases. CECT or NCCT is the imaging method of choice in the most complex situations, allowing for precise assessment of disease burden. Current MRI sequences yield promising results in specific scenarios, such as pregnancy and pediatric subjects, where minimising radiation exposure is a concern.
Author Contributions
SRS formulated the concept, designed the review, conducted the literature review, and contributed to the manuscript writing. CP conducted the literature review and contributed to the manuscript writing. All authors reviewed the manuscript.
Data Availability Statement
Acquired DICOM medical images during the current review are available from the corresponding author upon reasonable request. The subject's identifying information was removed in radiology images.
Acknowledgments
The authors acknowledge Professor Lakmini Wijesooriya, MD, MPhil, for her guidance on critical concepts, design, and manuscript correction for this review. Furthermore, the authors acknowledge the management and radiographers of Leesons Hospital, Ragama, Sri Lanka.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval and Consent
Ethical approval was not considered, as our work is a narrative review. However, we have secured informed consent to publish radiological images from each patient (Figure numbers:
Figure 1A,B,D,
Figure 2,
Figure 3A–D,
Figure 4A,
Figure 5B). For
Figure 1C,
Figure 4B and
Figure 5A, credit has been provided, and the reference is mentioned in the relevant image legend. Additionally, we have intentionally removed identifying information from the radiology images. Consent was obtained in the local and English language, and fully completed consent forms are available upon request.
References
- Foxman, B. The epidemiology of urinary tract infection. Nature Reviews Urology. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sachinthana JG, A.; Kariyawasam, G.M.; Edirisinghe EM, D.T.; Peiris HS, N.; Dayarathna DA, R.K. Incidence of Urinary Tract Infections and Allergic Conditions among Type 2 Diabetes Mellitus Patients in Rural Hospital Clinics in Sri Lanka. International Journal of Diabetes and Clinical Research. 2018, 5, 097. [Google Scholar] [CrossRef]
- Goh, L.P.W.; Marbawi, H.; Goh, S.M.; Asis, A.K.B.A.; Gansau, J.A. The prevalence of hospital-acquired infections in Southeast Asia (1990-2022). The Journal of Infection in Developing Countries. 2023, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Das, C.J.; Ahmad, Z.; Sharma, S.; Gupta, A.K. Multimodality imaging of renal inflammatory lesions. World Journal of Radiology. 2014, 6, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.; Wagner, B.; Travis, M. Pyelonephritis: Radiologic-Pathologic Review. Radio Graphics. 2008, 28, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.M.; Cho, S.Y.M.; Kwon, S.Y.M.; Choi, H.M.; Lee, J.W.M. Clinical and microbiological characteristics of men with nonobstructive acute pyelonephritis. Medicine. 2021, 100, e27386. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.F.J.; Zwirewich, C.; Torreggiani, W.C. Imaging of urinary tract infection in the adult. European Radiology Supplements. 2004, 14, 1–1. [Google Scholar] [CrossRef]
- Tamburrini, S.; Lugarà, M.; Iannuzzi, M.; Cesaro, E.; De Simone, F.; Del Biondo, D.; Toto, R.; Iulia, D.; Marrone, V.; Faella, P.; et al. Pyonephrosis Ultrasound and Computed Tomography Features: A Pictorial Review. Diagnostics. 2021, 11, 331 . [Google Scholar] [CrossRef]
- Chen, K.-C.; Hung, S.-W.; Seow, V.-K.; Chong, C.-F.; Wang, T.-L.; Li, Y.-C.; Chang, H. The role of emergency ultrasound for evaluating acute pyelonephritis in the ED. The American Journal of Emergency Medicine. 2011, 29, 721–724. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, M.H.; Pai, K.S.; Kim, H.G. Diagnostic performance of contrast-enhanced ultrasound for acute pyelonephritis in children. Scientific Reports 2020, 10, 10715. Scientific Reports 2020, 10, 10715. [Google Scholar] [CrossRef]
- Majd, M.; Blask, A.R.N.; Markle, B.M.; Shalaby-Rana, E.; Pohl, H.G.; Park, J.-S.; Chandra, R.; Rais-Bahrami, K.; Pandya, N.; Patel, K.M.; et al. Acute Pyelonephritis: Comparison of Diagnosis with with99mTc-DMSA SPECT, Spiral CT, MR Imaging, and Power Doppler US in an Experimental Pig Model. Radiology. 2001, 218, 101–108 . [Google Scholar] [CrossRef]
- Vourganti, S.; Agarwal, P.K.; Bodner, D.R.; Dogra, V.S. Ultrasonographic evaluation of renal infections. Radiologic clinics of North America. 2006, 44, 763–775. [Google Scholar] [CrossRef]
- Enikeev, D.V.; Glybochko, P.; Alyaev, Y.; Enikeev, M.; Rapoport, L. Imaging Technologies in the Diagnosis and Treatment of Acute Pyelonephritis. Urologia Journal. 2017, 84, 179–184. [Google Scholar] [CrossRef]
- Stunell, H.; Buckley, O.; Feeney, J.; Geoghegan, T.; Browne, R.F.J.; Torreggiani, W.C. Imaging of acute pyelonephritis in the adult. European Radiology. 2007, 17, 1820–1828. [Google Scholar] [CrossRef]
- Saunders, H.S.; Dyer, R.B.; Shifrin, R.Y.; Scharling, E.S.; E Bechtold, R.; Zagoria, R.J. The CT nephrogram: implications for evaluation of urinary tract disease. RadioGraphics. 1995, 15, 1069–1085. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.; Lee, K.W.; Kim, J.M.; Kim, Y.H.; Kim, M.E. Relationship Between Uncommon Computed Tomography Findings and Clinical Aspects in Patients with Acute Pyelonephritis. Korean Journal of Urology. 2014, 55, 482–2 . [Google Scholar] [CrossRef]
- Kaplan, D.M.; Rosenfield, A.T.; Smith, R.C. Advances in the imaging of renal infection. Helical CT and modern coordinated imaging. Infectious Disease Clinics of North America. 1997, 11, 681–705. [Google Scholar] [CrossRef]
- Conley, S.P.; Frumkin, K. Acute Lobar Nephronia: A Case Report and Literature Review. The Journal of Emergency Medicine. 2014, 46, 624–626 . [Google Scholar] [CrossRef]
- Sieger, N.; Kyriazis, I.; Schaudinn, A.; Kallidonis, P.; Neuhaus, J.; Liatsikos, E.N.; Ganzer, R.; Stolzenburg, J.-U. Acute focal bacterial nephritis is associated with invasive diagnostic procedures - a cohort of 138 cases extracted through a systematic review. BMC Infectious Diseases. 2017, 17, 240. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Tsau, Y.-K.; Chen, S.-Y.; Lin, T.-Y. Clinical courses of children with acute lobar nephronia correlated with computed tomographic patterns. Pediatric Infectious Disease Journal. 2009, 28, 300–303. [Google Scholar] [CrossRef]
- Gardiner, R.A.; Gwynne, R.A.; Roberts, S.A. Perinephric abscess. BJU International, 2011; 107, 20–23. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Wang, C.-C.; Liu, Y.-B.; Liu, K. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World Journal of Nephrology 2016, 5, 108–114. [Google Scholar] [CrossRef]
- Rubilotta, E.; Balzarro, M.; Lacola, V.; Sarti, A.; Porcaro, A.B.; Artibani, W. Current Clinical Management of Renal and Perinephric Abscesses: A Literature Review. Urologia Journal. 2014, 81, 144–147. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Huang, G.; Fu, H. Analysis of 17 children with renal abscess. International Journal of Clinical and Experimental Pathology 2019, 12, 3179–3184 PMID: 31934162. [Google Scholar] [PubMed]
- Bhatt, S.; MacLennan, G.; Dogra, V. Renal Pseudotumors. American Journal of Roentgenology. 2007, 188, 1380–1387 . [Google Scholar] [CrossRef]
- El-Ghar, M.A.; Farg, H.; Sharaf, D.E.; El-Diasty, T. CT and MRI in Urinary Tract Infections: A Spectrum of Different Imaging Findings. Medicina. 2021, 57, 32 . [Google Scholar] [CrossRef]
- Ubee, S.; McGlynn, L.; Fordham, M. Emphysematous pyelonephritis. BJU International. 2011, 107, 1474–1478. [Google Scholar] [CrossRef]
- Narlawar, R.; Raut, A.; Nagar, A.; Hira, P.; Hanchate, V.; Asrani, A. Imaging features and guided drainage in emphysematous pyelonephritis: a study of 11 cases. Clinical Radiology. 2004, 59, 192–197 . [Google Scholar] [CrossRef]
- Grayson, D.E.; Abbott, R.M.; Levy, A.D.; Sherman, P.M. Emphysematous Infections of the Abdomen and Pelvis: A Pictorial Review. Radio Graphics. 2002, 22, 543–561 . [Google Scholar] [CrossRef]
- Gonçalves, E.; Maia, B.T.; Versiani, C.M.; Mota, C.T.; Filho, A.G.S. Pielite enfisematosa unilateral: relato de caso. Radiologia Brasileira. 2013, 46, 56–58 . [Google Scholar] [CrossRef]
- Huang, J.; Tseng, C. Emphysematous Pyelonephritis. Archives of Internal Medicine. 2000, 160, 797. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Wu, S.-Y.; Yang, S.-D.; Chang, S.-J. Emphysematous pyelonephritis: classification, management, and prognosis. Tzu Chi Medical Journal. 2022, 34, 297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.P.; Khan, I.; Kishore, A.; Gopal, M.; Behera, V. Pyonephrosis among Patients with Pyelonephritis Admitted in Department of Nephrology and Urology of a Tertiary Care Centre: A Descriptive Cross-sectional Study. Journal of Nepal Medical Association. 2023, 61, 111–114 . [Google Scholar] [CrossRef] [PubMed]
- Watt, I.; Roylance, J. Pyonephrosis. Clinical Radiology. 1976, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Bolger, M.P.; Hennebry, J.; Byrne, C.; Greene, L.; Stroiescu, A.; Heneghan, J.; Ryan, A.G. Xanthogranulomatous Pyelonephritis: A Narrative Review with Current Perspectives on Diagnostic Imaging and Management, Including Interventional Radiology Techniques. International Journal of Nephrology and Renovascular Disease 2021, 14, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, S.; Comune, R.; Lassandro, G.; Pezzullo, F.; Liguori, C.; Fiorini, V.; Picchi, S.G.; Lugarà, M.; Del Biondo, D.; Masala, S. MDCT Diagnosis and Staging of Xanthogranulomatous Pyelonephritis. Diagnostics. 2023, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.; Elder, J. Xanthogranulomatous pyelonephritis: a critical analysis of 26 cases and of the literature. The Journal of Urology. 1978, 119, 589–593. [Google Scholar] [CrossRef]
- Eckoldt, F.; Riebel, T.; Wolke, S. Xanthogranulomatous pyelonephritis in children: diagnostic and therapeutic aspects. Journal of Medical Ultrasonics. 2009, 36, 33–37 . [Google Scholar] [CrossRef]
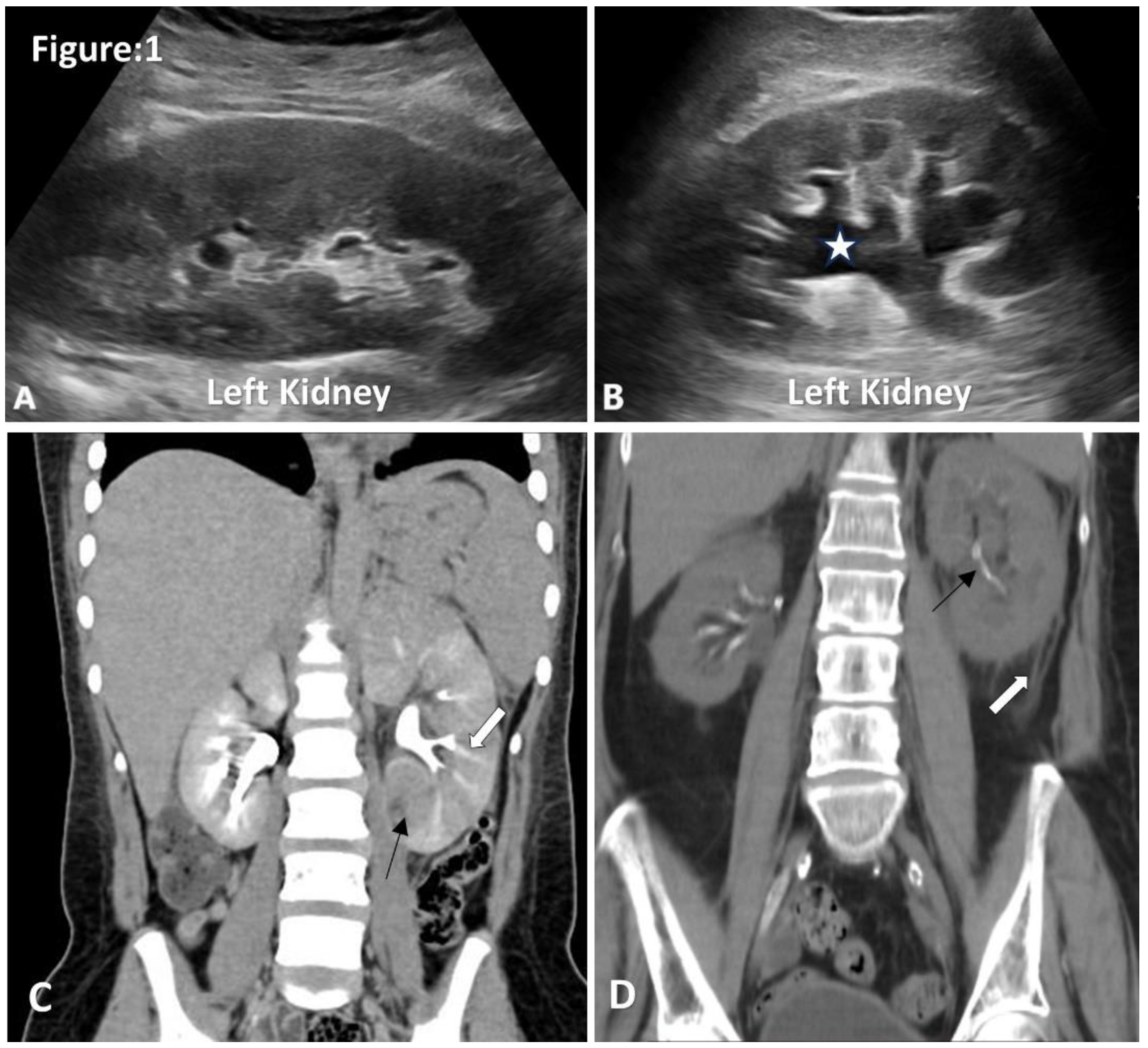
Figure 1.
Ultrasound and CT images of acute pyelonephritis. A. Ultrasonography shows an enlarged left kidney with hypoechoic renal parenchyma, loss of renal fat and loss of corticomedullary differentiation. B. Mild hydronephrosis and proximal hydroureter without obvious obstructive cause. C. Coronal images of contrast CT show an enlarged left kidney with a striated nephrogram(white arrow).Area of poor enhancement of left lower pole renal parenchyma (black arrow) (Image courtesy of Hani Makky Al Salam, Radiopaedia.org, rID: 18306) D. Non-contrast CT coronal images show thickening of Gerotas fascia, perinephric fat stranding and obliteration of perinephric fat planes (white arrow). Note incidentally detected intrarenal arterial calcification (black arrow).
Figure 1.
Ultrasound and CT images of acute pyelonephritis. A. Ultrasonography shows an enlarged left kidney with hypoechoic renal parenchyma, loss of renal fat and loss of corticomedullary differentiation. B. Mild hydronephrosis and proximal hydroureter without obvious obstructive cause. C. Coronal images of contrast CT show an enlarged left kidney with a striated nephrogram(white arrow).Area of poor enhancement of left lower pole renal parenchyma (black arrow) (Image courtesy of Hani Makky Al Salam, Radiopaedia.org, rID: 18306) D. Non-contrast CT coronal images show thickening of Gerotas fascia, perinephric fat stranding and obliteration of perinephric fat planes (white arrow). Note incidentally detected intrarenal arterial calcification (black arrow).
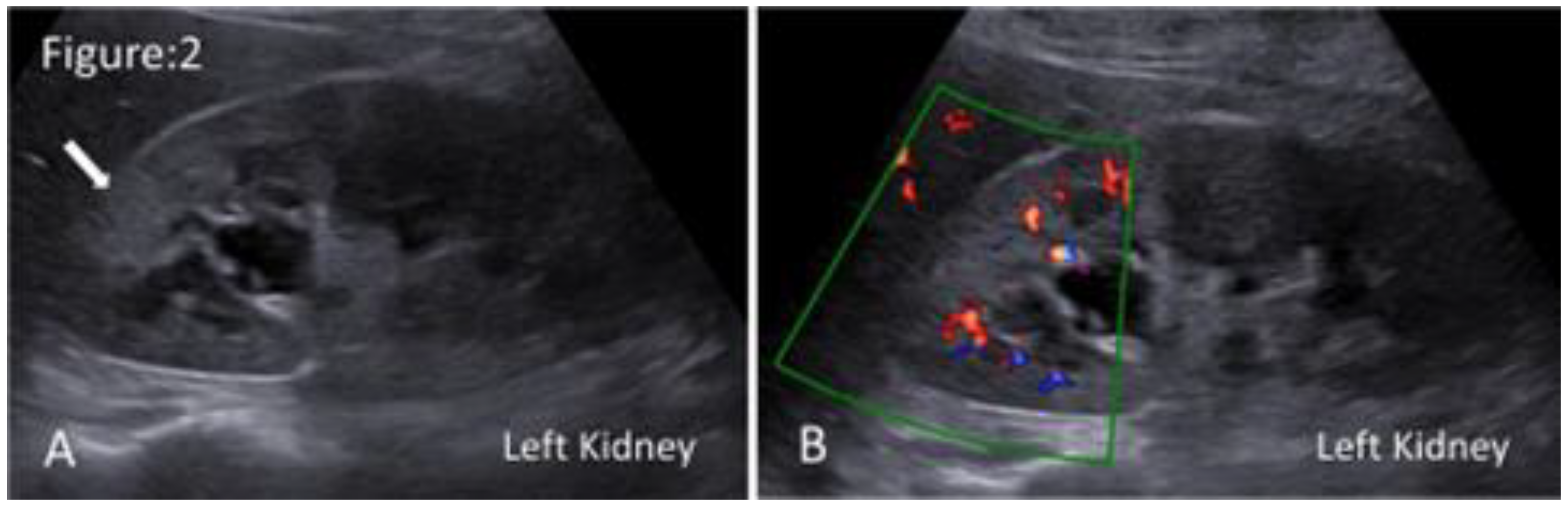
Figure 2.
Ultrasound images of acute focal bacterial nephritis. A. Grey scale ultrasonography shows a localised, poorly defined hyperechoic region in the upper pole of the left kidney(arrow). B. Duplex ultrasonography shows a localised area of hypoperfusion in the affected region.
Figure 2.
Ultrasound images of acute focal bacterial nephritis. A. Grey scale ultrasonography shows a localised, poorly defined hyperechoic region in the upper pole of the left kidney(arrow). B. Duplex ultrasonography shows a localised area of hypoperfusion in the affected region.
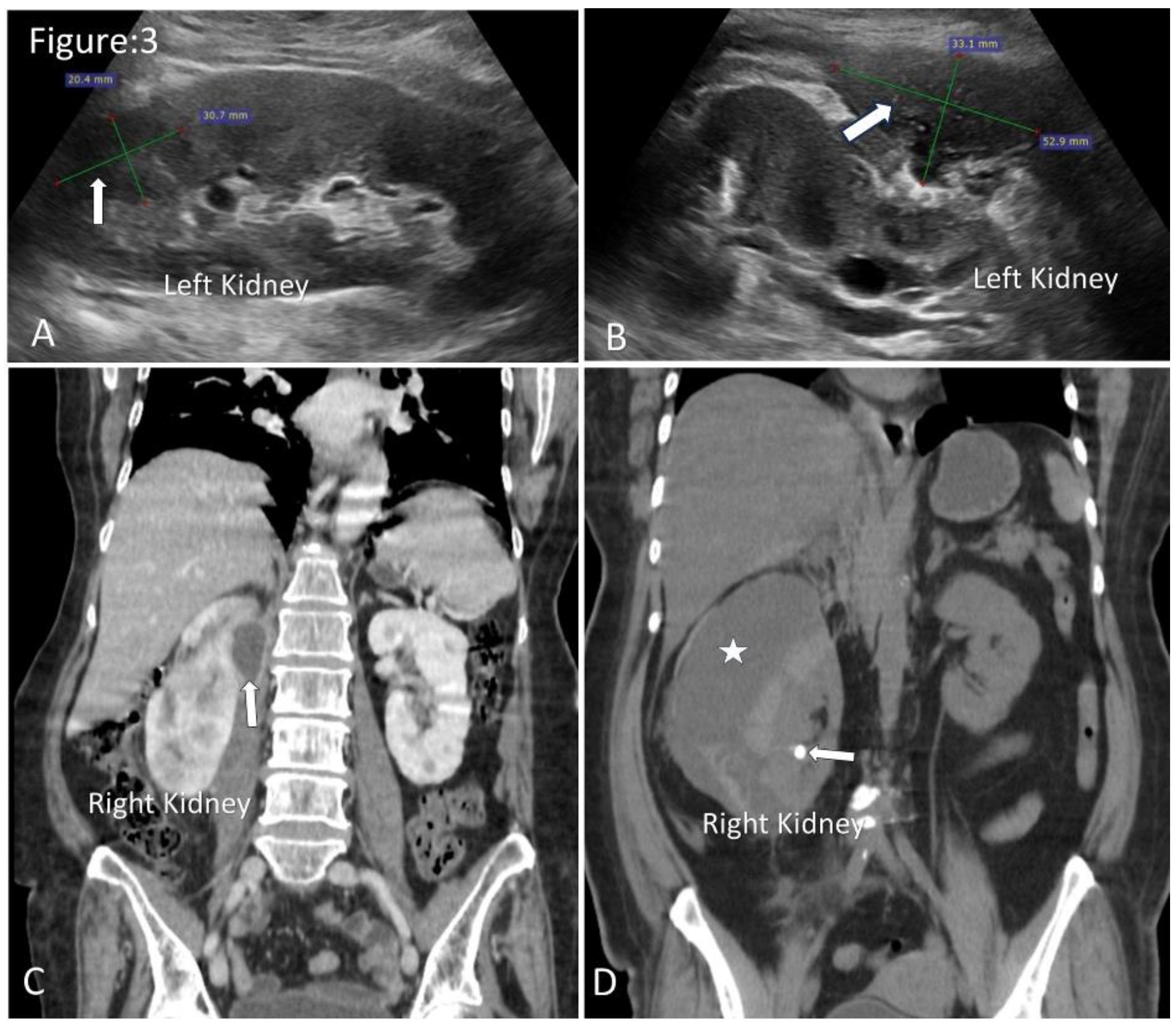
Figure 3.
Ultrasound and CT images of renal and perinephric abscesses. A. Ultrasonography shows well-defined fluid collection with internal echogenic debris in the upper pole of the left kidney (arrow). B. Moderate-size perinephric abscess in relation to the left kidney (arrow). C. Coronal images of contrast CT show a small perinephric abscess in relation to the upper half of the right kidney(arrow). D. Coronal images of non-contrast CT show a large perinephric abscess(asterisk). Note incidentally detected renal calculus (arrow).
Figure 3.
Ultrasound and CT images of renal and perinephric abscesses. A. Ultrasonography shows well-defined fluid collection with internal echogenic debris in the upper pole of the left kidney (arrow). B. Moderate-size perinephric abscess in relation to the left kidney (arrow). C. Coronal images of contrast CT show a small perinephric abscess in relation to the upper half of the right kidney(arrow). D. Coronal images of non-contrast CT show a large perinephric abscess(asterisk). Note incidentally detected renal calculus (arrow).
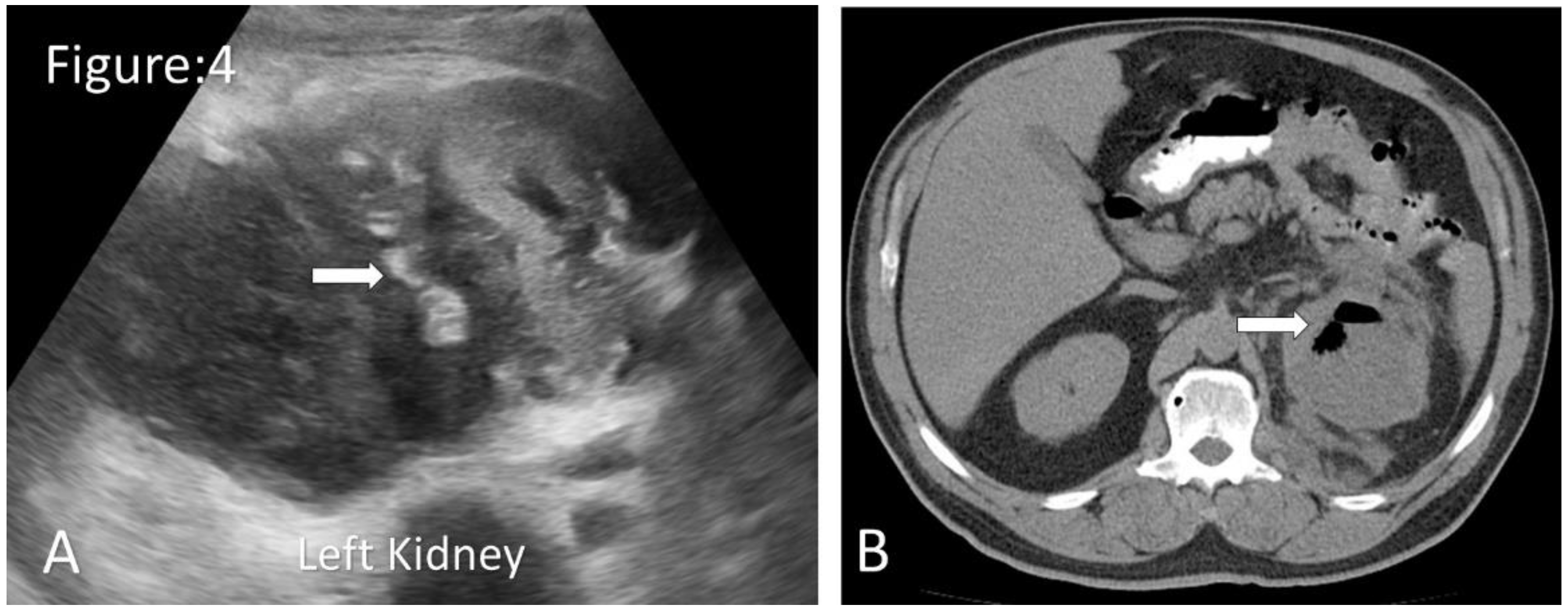
Figure 4.
Ultrasound and CT images of emphysematous pyelonephritis. A. Ultrasonography shows an enlarged left kidney with few gas locules in the kidney( arrow). B. Non-contrast axial CT shows an enlarged left kidney with internal localised gas locules. ( arrow) (Image courtesy of Rafael Lourenço do Carmo, Radiopaedia.org, rID: 50118).
Figure 4.
Ultrasound and CT images of emphysematous pyelonephritis. A. Ultrasonography shows an enlarged left kidney with few gas locules in the kidney( arrow). B. Non-contrast axial CT shows an enlarged left kidney with internal localised gas locules. ( arrow) (Image courtesy of Rafael Lourenço do Carmo, Radiopaedia.org, rID: 50118).
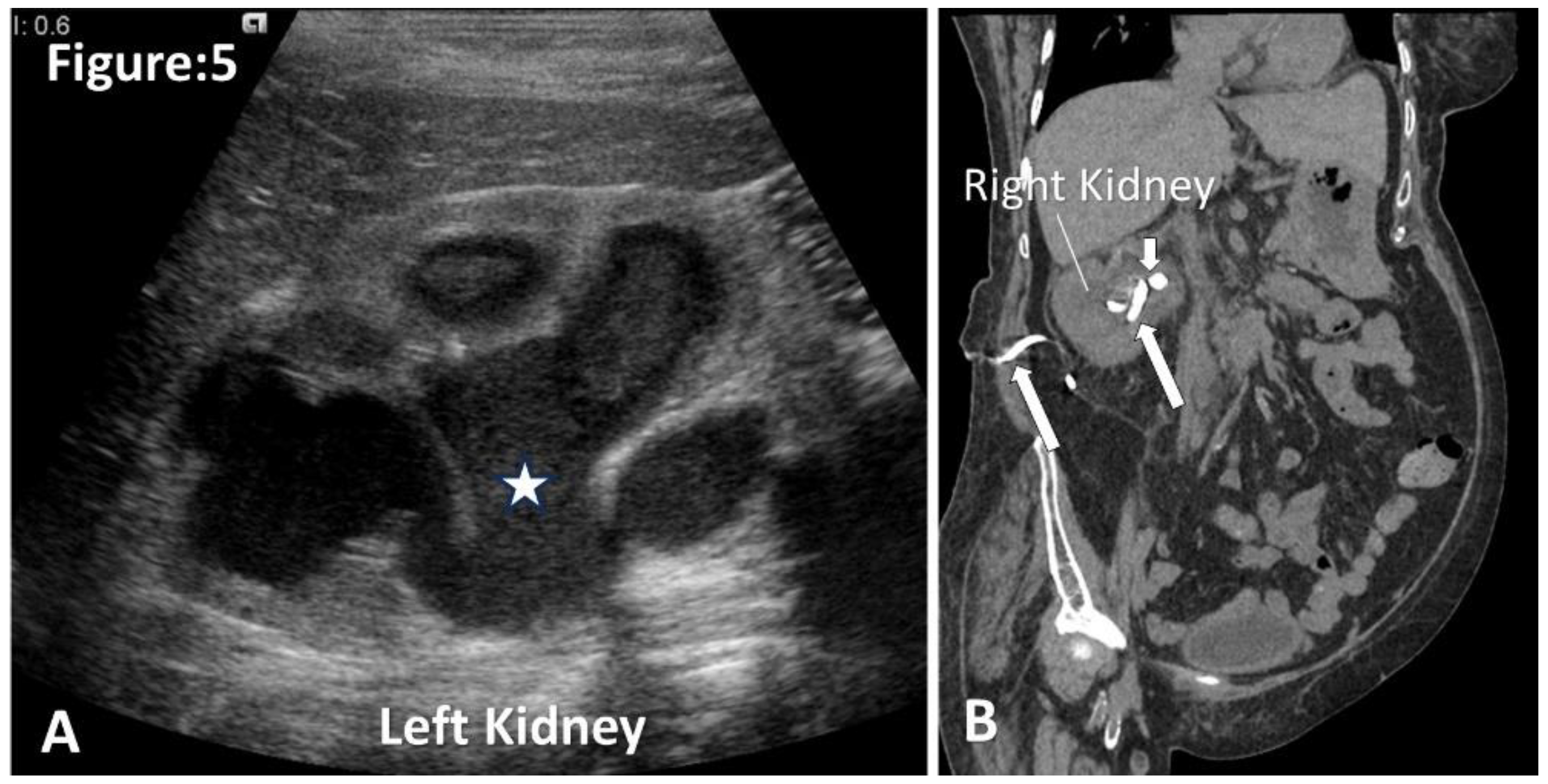
Figure 5.
Ultrasound and CT images of pyonephrosis. A. Ultrasound of left kidney shows dilated pelvicalyceal system with floating echogenic debris (asterisk). (Image courtesy of Andrew Dixon, Radiopaedia.org, rID: 10432). B. Non-contrast coronal CT shows a nephrostomy tube in situ for a patient with complicated pyonephrosis. Note obstructed calculus at the right ureteropelvic junction (short arrow).
Figure 5.
Ultrasound and CT images of pyonephrosis. A. Ultrasound of left kidney shows dilated pelvicalyceal system with floating echogenic debris (asterisk). (Image courtesy of Andrew Dixon, Radiopaedia.org, rID: 10432). B. Non-contrast coronal CT shows a nephrostomy tube in situ for a patient with complicated pyonephrosis. Note obstructed calculus at the right ureteropelvic junction (short arrow).
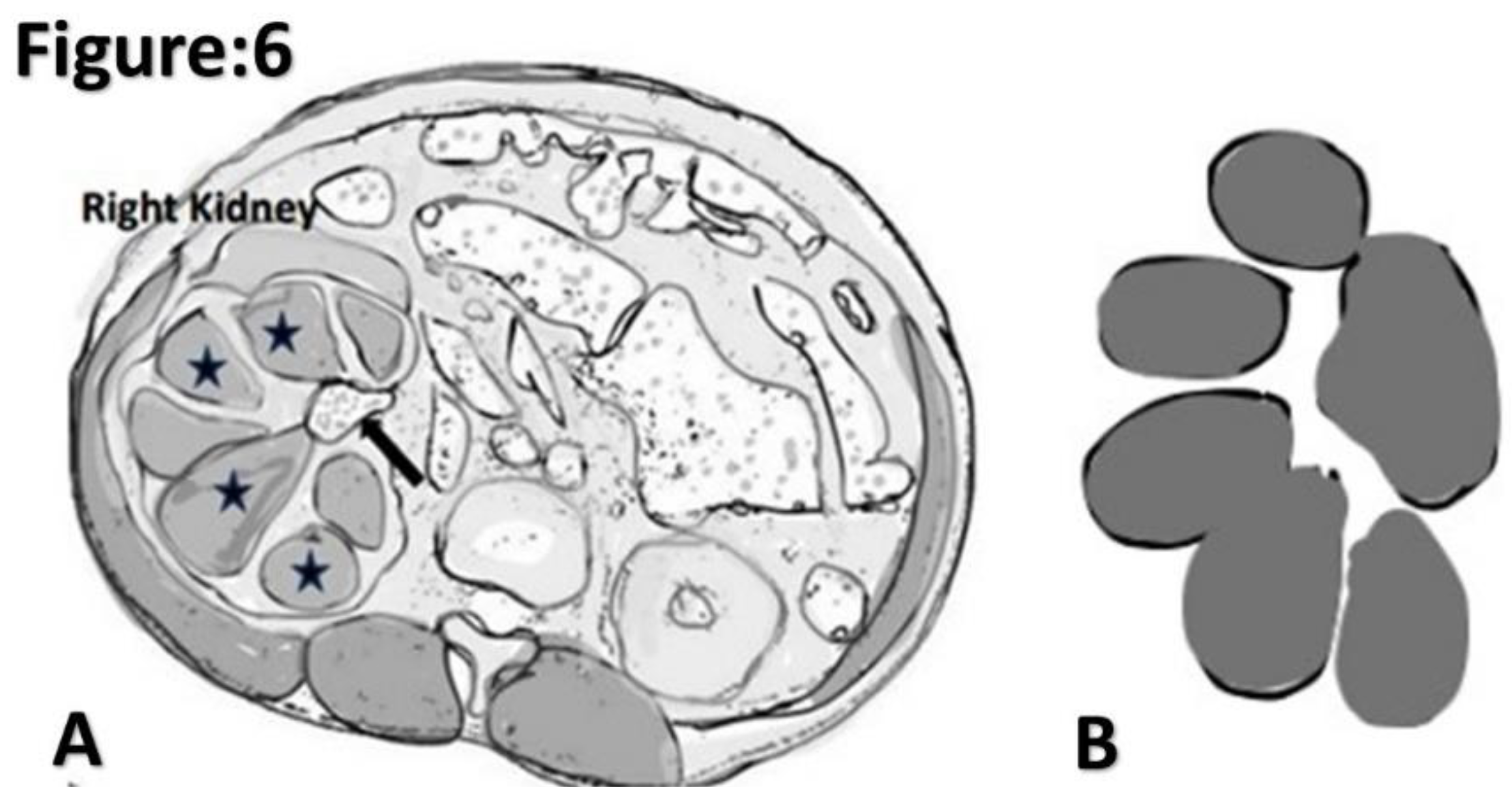
Figure 6.
Illustrated diagram of xanthogranulomatous pyelonephritis based on axial CT. A. dilated multi-loculated calyces (asterisk) with thinning of renal cortex and a staghorn calculus (arrow) giving rise to a classic “Bare Paw” appearance. B. Illustrated diagram of a “ Bare Paw”.
Figure 6.
Illustrated diagram of xanthogranulomatous pyelonephritis based on axial CT. A. dilated multi-loculated calyces (asterisk) with thinning of renal cortex and a staghorn calculus (arrow) giving rise to a classic “Bare Paw” appearance. B. Illustrated diagram of a “ Bare Paw”.
Table 1.
The ultrasound features of APN and pathological reasons attributed to the ultrasound features5,12,13.
Table 1.
The ultrasound features of APN and pathological reasons attributed to the ultrasound features5,12,13.
| Ultrasound Feature |
Pathological Explanation |
| Generalised enlargement of the kidney (the affected kidney is ≥1.5 cm longer than the unaffected kidney). |
Renal parenchymal inflammation results in parenchymal oedema and congestion. |
| Hypoechoic renal parenchyma. |
| Loss of renal sinus fat. |
| Loss of corticomedullary differentiation. |
| Hyperechoic renal parenchyma. |
Focal area of parenchymal haemorrhage. |
| Mild hydronephrosis and proximal hydroureter without obvious obstructive cause. |
Inhibition of ureteric peristaltic motion, caused by bacterial endotoxins, leads to the dilatation of the pelvicalyceal system. |
| Hyperechoic debris within the dilated pelvicalyceal system. |
Renal parenchymal inflammation results in the accumulation of dead neutrophils, bacteria, cellular debris, and proteinaceous material within the dilated pelvicalyceal system. |
| Power Duplex evidence of increased blood flow within the renal vascular system (Flaring kidney). |
Renal parenchymal inflammation leads to dilation of the renal vasculature and increased blood flow. |
| Limited renal movements during respiration compared with unaffected kidney. |
Inflammatory process and associated oedema result in increased stiffness and decreased compliance of the kidney. |
Table 2.
The CECT features of APN and pathological explanations attributed to the CT features15.
Table 2.
The CECT features of APN and pathological explanations attributed to the CT features15.
| CECT Features |
Pathological Explanation |
| In diffuse pyelonephritis, generalised renal enlargement, poor enhancement of renal parenchyma and inadequate excretion of contrast in delayed images. |
Renal parenchymal inflammation results in the accumulation of additional interstitial fluid, causing renal oedema. |
| A radial pattern of alternating high and low attenuation linear bands extends through the corticomedullary layers along the direction of the excretory tubules of the kidney. (Striated nephrogram) |
Striations are areas of hyper-concentrated contrast material within obstructed renal tubules outlined against the background of the edematous renal parenchyma. |
| Thickening of Gerotas fascia, perinephric fat stranding and obliteration of perinephric fat planes. |
Due to perinephric propagation, inflammation, oedema, and thickening of Gerotas fascia occur. Perinephric fat stranding and obliteration of perinephric fat planes due to swelling of perirenal space fat. This results in linear areas of soft tissue |
Table 3.
The Ultrasound and CECT features of acute focal bacterial nephritis and pathological explanations attributed to the imaging features19,20.
Table 3.
The Ultrasound and CECT features of acute focal bacterial nephritis and pathological explanations attributed to the imaging features19,20.
| CECT Features |
Pathological Explanation |
| In focal pyelonephritis, an Ill-defined wedge-shaped area of low attenuation radiates from the renal medulla to the cortical surface. |
Renal parenchymal inflammation results in vascular spasm, tubular obstruction due to inflammatory debris, and interstitial oedema, leading to decreased flow of contrast through renal tubules. |
| Ultrasound features |
Focal area of hypoechogenicity.
Power Duplex evidence of focal absence of blood flow within a localised hypoechoic region. |
Table 4.
Classification of emphysematous pyelonephritis based on CT findings31.
Table 4.
Classification of emphysematous pyelonephritis based on CT findings31.
| Classification |
Radiological Features Based on CT Findings |
| Class 1 |
Gas in the collecting system only |
| Class 2 |
Gas in the renal parenchyma without extension to the extrarenal space |
| Class 3A |
Extension of gas or abscess to the perinephric space |
| Class 3B |
Extension of gas or abscess to the pararenal space |
| Class 4 |
Bilateral EPN or a solitary kidney with EPN |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).