Submitted:
23 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. AMPs from Plants
2.1. Groups of Plant AMPs.
| AMP | Plant Species | Application and activity | Reference |
|---|---|---|---|
| Thi2.1 (Thionin) | Tomato (Lycopersicon esculentum) | Crop protection | [17,25] |
| Mj-AMP2 (Knottin) | Rice (Oryza sativa) | Resistance to fungal pathogens | [17,26] |
| Lipid Transfer Proteins (LTPs) | Tobacco (Nicotina tabacum) | Resistance to pathogens | [17,27] |
| Petunia Floral defensins | Banana (Musa spp.) | Effective resistance against pathogenic fungal Fusarium oxysporum | [17,28] |
| PmAMP1 (cysteine-rich protein) | Canola (Brassica napus) | Resistance against fungal pathogens (Leptosphaeria maculans) | [17,29] |
| SN-1 (Snakin) | Wheat (Triticum aestivum) | Antifungal activity in vitro and enhanced resistance to fungus (Gaeumannomyces graminis) | [17,30] |
| Pro-SmAMP2 (Heiven-like peptide) | Potato (Solanum tuberosum) | Crop protection from Alternaria sp. and Fusarium sp. | [17,31] |
2.2. AMPs Present in Vegetables
2.2.1. AMPs in Tomato
2.2.2. AMPs in Onion
2.2.3. AMPs in Garlic
2.2.4. AMPs in Chili Pepper
| Vegetable AMPs | Mode of action | Active against |
|---|---|---|
| Tomato (snakin SN2) | Pore formation, agglomeration of the cells | S. cerevisiae |
| Onion (Ba-49) | Disruption of the cell membrane, triggering the production of ROS, preventing the formation of biofilm and degrading the formation of mature biofilm | S. aureus |
| Garlic (F3-3-a, F3-3-b, F3-3-c) | Disruption of the cell membrane | E. coli, S. aureus, Salmonella enteritidis, B. subtilis |
| Chili pepper (F3 fraction) | Membrane permeabilization, production of ROS | S. cerevisiae, C. guilliermondii, C. parapsilosis, K. marxiannus, P. membranifaciens, C. tropicalis, C. albicans |
| Vegetable/plant vegetative organ | Inhibition zone d on B. subtilis NIBMCC 8752 | Inhibition zone d on E. coli NIBMCC 8751 |
|---|---|---|
| Parsley (leaves) | 2 | 0 |
| Tomato (seeds) | 5 | 0 |
| Cayenne pepper (tissue discs) | 24 | 25 |
| Cayenne pepper (seeds) | 7 | 11 |
| Onion orange skin (mature bulbs) | 27 | 3 |
| Onion red skin (mature bulbs) | 25 | 3 |
| Onion young (fresh bulbs) | 0 | 0 |
| Garlic (mature bulbs) | 7 | 30 |
| Garlic young (fresh bulbs) | 2 | 0 |
3. AMPs from Animals
| Animals | AMPs | Reference |
|---|---|---|
| Mammalians | Cathelicidins
Defensins Platelet antimicrobial proteins Dermicidins Hepcidins |
[6,18,46] |
| Reptiles | Defensins Cathelicidins |
[6,18] |
| Fish | β-defensins Cathelicidins Hepicidins (HAMP1 and HAMP2) Histone-derived peptides Piscidins (1-7) |
[6,18] |
| Amphibians | Magainins Cancrins |
[1,6,18] |
| Crustaceans | Crustins | [6,18] |
4. AMPs from Humans
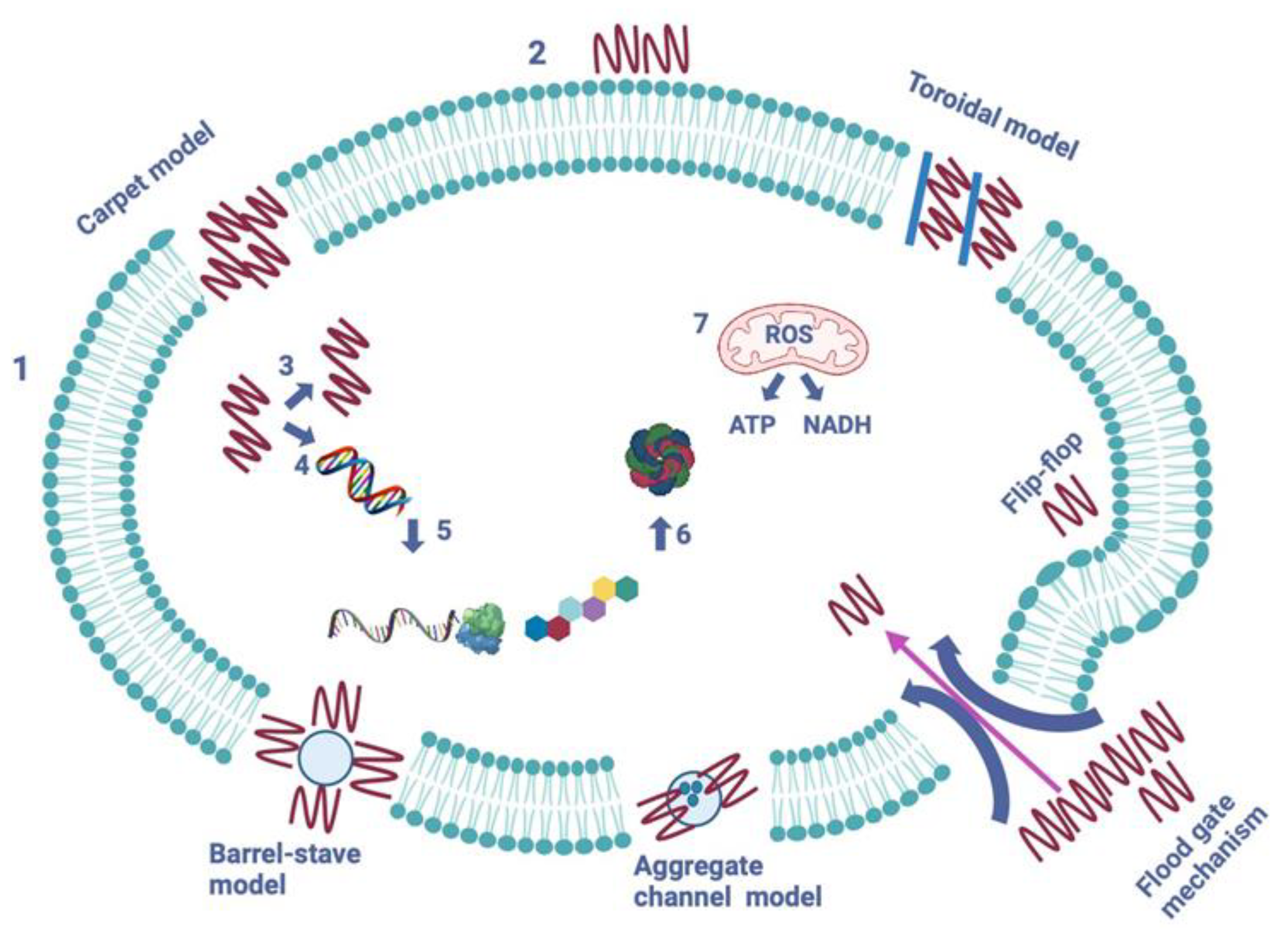
5. Modes of Action and Mechanisms
5.1. Antiviral AMPs
5.2. Antibacterial AMPs
5.3. Antifungal AMPs
5.4. Membrane-Targeting AMPs
5.5. Non-Membrane Targeting
| AMP | Source | Sequence | Reference |
|---|---|---|---|
| LL-37 | Human (Homo sapiens) | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | [62] |
| Indolicidin | Cattle (Bos taurus) | ILPWKWPWWPWRR-amide | |
| Crotamine | South American rattlesnake (Crotalus durissus) | YKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGSG, Cys4-Cys36, Cys11-Cys30, Cys18-Cys37 | [62] |
| Cancrin | Crab-eating frog (Rana cancrivora) | GSAQPYKQLHKVVNWDPYG | [63] |
| Melittin | Honey bee (Apis mellifera) | GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 | [64] |
| Buforin II | Asian toad (Duttaphrynus melanostictus) | TRSSRAGLQFPVGRVHRLLRK | [65] |
| HNP-1 | Human (Homo sapiens) | ACYCRIPACIAGERRYGTCIpYQGRLWAFCC | [62] |
| HBD-2 | Human (Homo sapiens) | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | [62] |
| Protegrin | Pig (Sus scrofa) | RGGRLCYCRRRFCVCVGR-amide | [62] |
| Magainin 2 | African clawed frog (Xenopus laevis) | GIGKFLHSAKKFGKAFVGEIMNS | [62] |
| Cecropin A | Cecropia moth (Hyalophora cecropia) | KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK-amide | [62] |
| NpRS | Garlic (Allium sativum) | RSLNLLMFR | [39] |
| SN1 | Potato (Solanum tuberosum) | MKLFLLTLLLVTLVITPSLIQTTMAGSNFCDSKCKLRCSKAGLADRCLKYCGICCEECKCVPSGTYGNKHECPCYRDKKNSKGKSKCP | [61] |
| SN2 | Tomato (Solanum lycopersicum) | MAISKALFASLLLSLLLLEQVQSIQTDQVSSNAISEGADSYKKIDCGGACAARCRLSSRPRLCHRACGTCCARCNCVPPGTSGNTETCPCYASLTTHGNKRKCP | [61] |
| CC-AMP1 | Ghost Pepper (Capsicum chinense × frutescens) | ZETLDPICMAKCVLKCGKKAWCLTKCIAGCVL | [40] |
| γ-Purothionin | Wheat (Triticum turgidum) | KICRRRSAGF KGPCMSNKNCAQVCQQEGWG GGNCDGPFRRCKCIRQC | [61,66,67] |
| α-1-Purothionin (precursor) | Bread wheat (Triticum aestivum) | MGSKGLKGVMVCLLILGLVL EQVQVEGKSCCRTTLGRNCYNLCRSRGAQK LCSTVCRCKLTSGLSCPKGFPKLALESNSDEPDTIEYCNLGCRSSVCDYMVNAAADDEEM KLYVENCGDACVNFCNGDAGLTSLDA | [61] |
| Thi2.1 | Thale cress (Arabidopsis thaliana) | MKGRILILSLLIMSLVMAQVQVEAKICCPSNQARNGYSVCRIRFSKGRCMQVSGCQNSDTCPRGWVNAILENSADATNEHCKLGCETSVCGAMNTLQNSDASEIVNGASEQCAKGCSIFCTKSYVVPPGPPKLL | [61] |
| Mj-AMP2 | Garden four-o’clock (Mirabilis jalapa) | MAKVPIAFLKFVIVLILFIAMSGMIEACIGNG GRCNENVGPPYCCSGFCLRQPNQGYGVCRNR | [61] |
| Lipid transfer protein | Common tobacco (Nicotiana tabacum) | MEMVGKIA CFVVLCMVVVAPHAEALSCGQVQSGLAPCLPYLQGRGPLGSCCGGVKGLLGAAKSLSDRKTACTCLKSAANAIKGIDMGKAAGLPGACGVNIPYKISPSTDCSKVQ | [61] |
| Flower -derived plant defensin 2 | Petunia x hybrida | MARSICFFAVATLALMLFAAYEAEAATCKAECPTWDGICINKGPCVKCCKAQPEKFTDGHCSKVLRRCLCTKPCATEEATATLANEVKTMAEALVEEDMME | [61] |
| PmAMP1 | Western white pine (Pinus monticola) | METKHLAYVMFVLVSLFLAMAQPSQASYFSAWVGPGCNNHNARYNKCGCSNISHNVHGGYEFVYQGQAPTAYNTNNCKGVAQTRFSSNVNQACSNFAWKSVFIQC | [61] |
| SmAMP2 | Chickweed (Stellaria media) | MLNMKSFALLMLFATLVGVTIAYDPNGKCGRQYGKCRAGQCCSQYGYCGSGSKYCAHNTPLSEIEPTAAGQCYRGRCSGGLCCSKYGYCGSGPAYCGLGMCQGSCLPDMPNHPAQIQARTEAAQAEAQAEAYNQANEAAQVEAYYQAQTQAQPQVEPAVTKAP | [61] |
| Crustin | Red swamp crayfish (Procambarus clarkii) | MLRVLVLSMLVVAALGHLPRPKPPQPGCNYYCTKPEGPNKGAKYCCGPQFLPLIREEKHNGFCPPPLKDCTRILPPQVCPHDGHCPINQKCCFDTCLDLHTCKPAHFYIN | [61] |
| Hepicidin HAMP2.3 | Gilthead seabream (Sparus aurata) | MKTFSVAVAVAIVLTFICLQESSAVSFTEVQELEEPMSNDGPIAAYKEMPEDSWKMGYGSRRWKCRFCCRCCPRMRGCGLCCRF | [61] |
| Histone -derived, partial | Catla (Labeo catla) | MSGRGKTGGKARAKAKTRSSRAGLQFPVGRVHRLLRKGNYAERVGAGAPVYLAAVLEYLTAEILELAGNAARDNKKTRIIP | [61] |
| Piscidin 2 | Schlegel’s black rockfish (Sebastes schlegelii) | MRFIMLFLVLSMVVLMAEPGEAFIHHIFGAIKRIFGDKQRDMADQQELDQRAFDRERAFN | [61] |
| Proline-rich AMP | Green mud crab (Scylla paramamosain) | MRLLWLLVALAAVVPAAMPASAGYFPGRPPFPRPFPRPPSRPFPRPPFPGPFPRPYPWR |
[61] |
| Attacin | House fly (Musca domestica) | MFTKSIAIIVFLATLAVVNAQFGGSITSNS RGGADVFARLGHQFGDNKRNFGGGVFAAGNTLGGPVTRGAFLSGNADRFGGSLSHSRTDNFGSTFSQKLNANLFQNDKHKLDANAFHSRTNLDNGFKFNTVGGGLDYNHANGHGASVTASRIPQLNMNTVDVTGKANLWK SADRATSLDLTGGVSKNFGG PLDGQTNKHI GVGLSHDF |
[61] |
| Oh-Cath | King cobra (Ophiophagus hannah) | MEGFFWKTLLVVGALAIGGTSSLPHKPLTY EEAVDLAVSIYNSKSGEDSLYRLLEAVPPPEWDPLSESNQELNFTIKETVCLVAEERSLEECDFQEDGAI MGCTGYYFFGESPPVLVLTCKPVGEEEEQK QEEGNEEEKEVEKEEKEEDEKDQPRRVKRF KKFFKKLKNSVKKRAKKFFKKPRVIGVSIPF |
[61] |
| TBD-1 | European pond turtle (Emys orbicularis) | YDLSKNCRLRGGICYIGKCPRRFFRSGSCS RGNVCCLRFG | [61] |
| Pelovaterin | Chinese soft-shelled turtle (Pelodiscus sinensis) | DDTPSSRCGSGGWGPCLPIVDLLCIVHVTV GCSGGFGCCRIG | [61] |
| avian β-defensin 1 | Japanese quail (Coturnix japonica) | MKIVYLLFPFILLLAHGAAGSSRDLGKREQ CYRQKGFCAFLKCPSLTIISGKCSRFHVCCKNIWG | [61] |
| α-defensin 5, Paneth cell-specific | Human (Homo sapiens) | MRTIAILAAI LLVALQAQAESLQERADEAT TQKQSGEDNQDLAISFAGNGLSALRTSGSQARATCYCRTG RCATRESLSGVCEISGRLYRLCCR | [61] |
| θ-defensin-1 | Rhesus monkey (Macaca mulatta) | RCICTRGFCRCLCRRGVC | [61] |
6. Discussion on the benefits and limitations of AMPs
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Chrom, C.L.; Renn, L.M.; Caputo, G.A. Characterization and Antimicrobial Activity of Amphiphilic Peptide AP3 and Derivative Sequences. Antibiotics 2019, 8, 20. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, G.; Yang, Y.; Ding, W. Current Advancements in Sactipeptide Natural Products. Frontiers in Chemistry 2021, 9. [Google Scholar] [CrossRef]
- Iacovelli, R.; Bovenberg, R.A.L.; Driessen, A.J.M. Nonribosomal Peptide Synthetases and Their Biotechnological Potential in Penicillium Rubens. Journal of Industrial Microbiology and Biotechnology 2021, 48. (7-8). [Google Scholar] [CrossRef]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021, 62, 5. [CrossRef]
- Pizzo, E.; Cafaro, V.; Donato, A.D.; Notomista, E.Cryptic Antimicrobial Peptides: Identification Methods and Current Knowledge of Their Immunomodulatory Properties. Current Pharmaceutical Design 24 (10), 1054–1066. [CrossRef]
- Fesenko, I.; Azarkina, R.; Kirov, I.; Kniazev, A.; Filippova, A.; Grafskaia, E.; Lazarev, V.; Zgoda, V.; Butenko, I.; Bukato, O.; Lyapina, I.; Nazarenko, D.; Elansky, S.; Mamaeva, A.; Ivanov, V.; Govorun, V. Phytohormone Treatment Induces Generation of Cryptic Peptides with Antimicrobial Activity in the Moss Physcomitrella Patens. BMC Plant Biology 2019, 19((1)). [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Costa, B.; Franco, O.L. Cryptic Host Defense Peptides: Multifaceted Activity and Prospects for Medicinal Chemistry. Current Topics in Medicinal Chemistry 2020, 20((14)), 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Bosso, A.; Maro, A.D.; Cafaro, V.; Donato, A.D.; Notomista, E.; Pizzo, E. Enzymes as a Reservoir of Host Defence Peptides. Current Topics in Medicinal Chemistry 2020, 20((14)), 1310–1323. [Google Scholar] [CrossRef]
- Bosso, A.; Gaglione, R.; Di Girolamo, R.; Veldhuizen, E.J.A.; García-Vello, P.; Fusco, S.; Cafaro, V.; Monticelli, M.; Culurciello, R.; Notomista, E.; Arciello, A.; Pizzo, E. Human Cryptic Host Defence Peptide GVF27 Exhibits Anti-Infective Properties against Biofilm Forming Members of the Burkholderia Cepacia Complex. Pharmaceuticals 2022, 15((2)), 260. [Google Scholar] [CrossRef]
- Ciociola, T.; Zanello, P.P.; D’Adda, T.; Galati, S.; Conti, S.; Magliani, W.; Giovati, L. A Peptide Found in Human Serum, Derived from the C-Terminus of Albumin, Shows Antifungal Activity in Vitro and in Vivo. Microorganisms 2020, 8((10)), 1627. [Google Scholar] [CrossRef]
- Pizzo, E.; Pane, K.; Bosso, A.; Landi, N.; Ragucci, S.; Russo, R.; Gaglione, R.; Torres, M.D.T.; de la Fuente-Nunez, C.; Arciello, A.; Di Donato, A.; Notomista, E.; Di Maro, A. Novel Bioactive Peptides from PD-L1/2, a Type 1 Ribosome Inactivating Protein from Phytolacca Dioica L. Evaluation of Their Antimicrobial Properties and Anti-Biofilm Activities. Biochimica et Biophysica Acta (BBA) - Biomembranes 2018, 1860((7)), 1425–1435. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Bulaon, C.J.I.; Malla, A.; Phoolcharoen, W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules 2021, 26, 4032. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; De Biasi, M.-G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef] [PubMed]
- Höng, K.; Austerlitz, T.; Bohlmann, T.; Bohlmann, H. The Thionin Family of Antimicrobial Peptides. PLOS ONE 2021, 16((7)), e0254549. [Google Scholar] [CrossRef]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant Defensins from a Structural Perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef]
- PDB ID 6NOM Pinheiro-Aguiar, R.; do Amaral, V.S.G.; Pereira, I.B.; Kurtenbach, E.; Almeida, F.C.L. Nuclear Magnetic Resonance Solution Structure of Pisum Sativum Defensin 2 Provides Evidence for the Presence of Hydrophobic Surface-Clusters. Proteins 2020, 88((1)), 242–246. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Rogozhin, E.A. Defense Peptides from the α-Hairpinin Family Are Components of Plant Innate Immunity. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Han, M.; Cao, D.; Xu, M. Molecular and Biological Properties of Snakins: The Foremost Cysteine-Rich Plant Host Defense Peptides. J. Fungi 2020, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-L.; Prasad, V.; Sanjaya; Kuei Hung Chen; Po Chang Liu; Chan, M.-T.; Cheng, C.-P. Transgenic Tomato Plants Expressing an Arabidopsis Thionin (Thi2.1) Driven by Fruit-Inactive Promoter Battle against Phytopathogenic Attack. 2005, 221 (3), 386–393. [CrossRef]
- Prasad, B.D.; Jha, S.; Chattoo, B.B. Transgenic Indica Rice Expressing Mirabilis Jalapa Antimicrobial Protein (Mj-AMP2) Shows Enhanced Resistance to the Rice Blast Fungus Magnaporthe Oryzae. Plant Science 2008, 175((3)), 364–371. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, K.D.; Hwang, B.K.; Ok, S.H.; Shin, J.S. Overexpression of Lipid Transfer Protein (LTP) Genes Enhances Resistance to Plant Pathogens and LTP Functions in Long-Distance Systemic Signaling in Tobacco. Plant Cell Reports 2008, 28((3)), 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Petunia Floral Defensins with Unique Prodomains as Novel Candidates for Development of Fusarium Wilt Resistance in Transgenic Banana Plants. PLoS ONE 2012, 7((6)), e39557. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Yajima, W.; Rahman, M.; Shah, S.; Liu, J.-J.; A.K.M. Ekramoddoullah; Nat. A Cysteine-Rich Antimicrobial Peptide from Pinus Monticola (PmAMP1) Confers Resistance to Multiple Fungal Pathogens in Canola (Brassica Napus). Plant Molecular Biology 2012, 79 (1-2), 61–74. [CrossRef]
- Rong, W.; Qi, L.; Wang, J.; Du, L.; Xu, H.; Wang, A.; Zhang, Z. Expression of a Potato Antimicrobial Peptide SN1 Increases Resistance to Take-All Pathogen Gaeumannomyces Graminis Var. Tritici in Transgenic Wheat. Functional & Integrative Genomics 2013, 13((3)), 403–409. [Google Scholar] [CrossRef]
- Vetchinkina, E.M.; Komakhina, V.V.; Vysotskii, D.A.; Zaitsev, D.V.; Smirnov, A.N.; Babakov, A.V.; Komakhin, R.A. [Expression of Plant Antimicrobial Peptide Pro-SmAMP2 Gene Increases Resistance of Transgenic Potato Plants to Alternaria and Fusarium Pathogens. Genetika 2016, 52((9)), 1055–1068. [Google Scholar] [CrossRef]
- Diz, M.S.S.; Carvalho, A.O.; Rodrigues, R.; Neves-Ferreira, A.G.C.; Da Cunha, M.; Alves, E.W.; Okorokova-Façanha, A.L.; Oliveira, M.A.; Perales, J.; Machado, O.L.T.; Gomes, V.M. Antimicrobial Peptides from Chili Pepper Seeds Causes Yeast Plasma Membrane Permeabilization and Inhibits the Acidification of the Medium by Yeast Cells. Biochimica Et Biophysica Acta 2006, 1760((9)), 1323–1332. [Google Scholar] [CrossRef]
- Herbel, V.; Sieber-Frank, J.; Wink, M. The Antimicrobial Peptide Snakin-2 Is Upregulated in the Defense Response of Tomatoes (Solanum Lycopersicum) as Part of the Jasmonate-Dependent Signaling Pathway. Journal of Plant Physiology 2017, 208, 1–6. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Abashina, T.N.; Odintsova, T.I. Synthetic Oligopeptides Mimicking γ-Core Regions of Cysteine-Rich Peptides of Solanum lycopersicum Possess Antimicrobial Activity against Human and Plant Pathogens. Curr. Issues Mol. Biol. 2021, 43, 1226–1242. [Google Scholar] [CrossRef]
- Taggar, R.; Singh, S.; Bhalla, V.; Bhattacharyya, M.S.; Sahoo, D.K. Deciphering the Antibacterial Role of Peptide from Bacillus Subtilis Subsp. Spizizenii Ba49 against Staphylococcus Aureus. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef]
- Ramita Taggar; Manoj Jangra; Dwivedi, A.; Bansal, K.; Patil, P.B.; Mani Shankar Bhattacharyya; Hemraj Nandanwar; Sahoo, D.K. Bacteriocin Isolated from the Natural Inhabitant of Allium Cepa against Staphylococcus Aureus. World Journal of Microbiology & Biotechnology 2021, 37 (2). [CrossRef]
- Chidike Ezeorba, T.P.; Ezugwu, A.L.; Chukwuma, I.F.; Anaduaka, E.G.; Udenigwe, C.C. Health-Promoting Properties of Bioactive Proteins and Peptides of Garlic (Allium Sativum). Food Chemistry 2024, 435, 137632. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, Z.; Xue, Z.; Jia, Y.; Guo, Q.; Ma, Q.; Zhang, M.; Chen, H. Identification and Antimicrobial Activity Evaluation of Three Peptides from Laba Garlic and the Related Mechanism. Food & Function 2019, 10((8)), 4486–4496. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Zhou, J.; Wang, J.; Zhang, M.; Chen, H. Structural Characterization, Cytotoxicity, and the Antifungal Mechanism of a Novel Peptide Extracted from Garlic (Allium sativa L.). Molecules 2023, 28, 3098. [Google Scholar] [CrossRef] [PubMed]
- Culver, K.D.; Allen, J.L.; Shaw, L.N.; Hicks, L.M. Too Hot to Handle: Antibacterial Peptides Identified in Ghost Pepper. Journal of natural products 2021, 84((8)), 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.F.F.; Carvalho, A.O.; Da Cunha, M.; Rodrigues, R.; Cruz, L.P.; Melo, V.M.M.; Vasconcelos, I.M.; Melo, E.J.T.; Gomes, V.M. Isolation and Characterization of Novel Peptides from Chilli Pepper Seeds: Antimicrobial Activities against Pathogenic Yeasts. Toxicon 2007, 50((5)), 600–611. [Google Scholar] [CrossRef]
- de Azevedo dos Santos, L.; Taveira, G.; da Silva, M.; da Silva Gebara, R.; da Silva Pereira, L.; Perales, J.; Teixeira-Ferreira, A.; de Oliveira Mello, É.; de Oliveira Carvalho, A.; Rodrigues, R.; Gomes, V. Antimicrobial Peptides from Capsicum Chinense Fruits: Agronomic Alternatives against Phytopathogenic Fungi. Bioscience Reports 2020, 40((8)). [Google Scholar] [CrossRef] [PubMed]
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Bioactive Peptides and Dietary Polyphenols: Two Sides of the Same Coin. Molecules 2020, 25, 3443. [Google Scholar] [CrossRef]
- Satchanska, G. Antibacterial Activity of Plant Polyphenols. Secondary Metabolites - Trends and Reviews 2022. IntechOpen. [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Molecular Biology Reports 2012, 39((12)), 10957–10970. [Google Scholar] [CrossRef]
- Chang, T.L.; Vargas, J.; DelPortillo, A.; Klotman, M.E. Dual Role of α-Defensin-1 in Anti–HIV-1 Innate Immunity. Journal of Clinical Investigation 2005, 115((3)), 765–773. [Google Scholar] [CrossRef] [PubMed]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent Evolution of Defensin Sequence, Structure and Function. Cellular and Molecular Life Sciences 2016, 74((4)), 663–682. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Boidin-Wichlacz, C.; Melnyk, O.; Zeppilli, D.; Landon, C.; Thomas, F.; Cambon, M.-A.; Lafond, M.; Mabrouk, K.; Massol, F.; Hourdez, S.; Maresca, M.; Jollivet, D.; Tasiemski, A. The Diversification of the Antimicrobial Peptides from Marine Worms Is Driven by Environmental Conditions. Science of The Total Environment 2023, 879, 162875. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrobial Agents and Chemotherapy 2017, 61((4)). [Google Scholar] [CrossRef] [PubMed]
- PDB ID 2MAG Gesell, J.; Zasloff, M.; Opella, S.J. Journal of Biomolecular NMR 1997, 9 (2), 127–135. [CrossRef]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, Evolution and Medical Applications of Insect Antimicrobial Peptides. Philosophical Transactions of the Royal Society B: Biological Sciences 2016, 371((1695)), 20150290. [Google Scholar] [CrossRef] [PubMed]
- PDB ID 1G89 Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the Bovine Antimicrobial Peptide Indolicidin Bound to Dodecylphosphocholine and Sodium Dodecyl Sulfate Micelles. Biochemistry 2000, 39((51)), 15765–15774, PDB. [Google Scholar] [CrossRef]
- Kalle Pärn; Elo Eriste; Langel, Ü. The Antimicrobial and Antiviral Applications of Cell-Penetrating Peptides. Springer eBooks 2015, 223–245. [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Military Medical Research 2021, 8, 48. [Google Scholar] [CrossRef]
- Kapil, S.; Sharma, V. D-Amino Acids in Antimicrobial Peptides: A Potential Approach to Treat and Combat Antimicrobial Resistance. Canadian Journal of Microbiology 2020. [Google Scholar] [CrossRef]
- Hamza Olleik; Perrier, J.; Hijazi, A.; Baydoun, E.; Maresca, M. Antimicrobial Peptides and Peptidomimetics as Treatment Option for Helicobacter Pylori Infection. WORLD SCIENTIFIC eBooks 2023, 25–56. [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11((6)). [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Frontiers in Cellular and Infection Microbiology 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- NCBI. National Center for Biotechnology Information. Nih.gov. https://www.ncbi.nlm.nih.gov/.
- Almeida, P.F.; Pokorny, A. 5.10 Interactions of Antimicrobial Peptides with Lipid Bilayers. ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/B9780123749208005154 (accessed 2023-11-06).
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Frontiers in Microbiology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and Evolution of Melittin, the Quintessential Membrane Active Peptide. Biochemical Pharmacology 2021, 193, 114769. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-Activity Analysis of Buforin II, a Histone H2A-Derived Antimicrobial Peptide: The Proline Hinge Is Responsible for the Cell-Penetrating Ability of Buforin II. Proceedings of the National Academy of Sciences of the United States of America 2000, 97((15)), 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F. The Antimicrobial Properties of the Puroindolines, a Review. World Journal of Microbiology & Biotechnology 2019, 35((6)). [Google Scholar] [CrossRef]
- Colilla, F.J.; Rocher, A.; Mendez, E. γ-Purothionins: Amino Acid Sequence of Two Polypeptides of a New Family of Thionins from Wheat Endosperm. FEBS Letters 1990, 270((1-2)), 191–194. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Frontiers in Cellular and Infection Microbiology 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; Falabella, P. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Frontiers in Cellular and Infection Microbiology 2021, 11. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial Peptides as Natural Bio-Preservative to Enhance the Shelf-Life of Food. Journal of Food Science and Technology 2016, 53((9)), 3381–3394. [Google Scholar] [CrossRef]
- Czelej, M.; Czernecki, T.; Garbacz, K.; Wawrzykowski, J.; Jamioł, M.; Michalak, K.; Walczak, N.; Wilk, A.; Waśko, A. Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties. Foods 2023, 12, 3394. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Critical Reviews in Biotechnology 2020, 40((7)), 978–992. [Google Scholar] [CrossRef]
- Lai, Z.; Yuan, X.; Chen, H.; Zhu, Y.; Dong, N.; Shan, A. Strategies Employed in the Design of Antimicrobial Peptides with Enhanced Proteolytic Stability. Biotechnology Advances 2022, 59, 107962. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, A.; Leone, L.; Lombardi, A.; Pizzo, E.; Bosso, A.; Winter, R.; Petraccone, L.; Del Vecchio, P.; Oliva, R. The Impact of N-Glycosylation on the Properties of the Antimicrobial Peptide LL-III. Scientific Reports 2023, 13((1)), 3733. [Google Scholar] [CrossRef]
- He, S.; Yang, Z.; Li, X.; Wu, H.; Zhang, L.; Shan, A.; Wang, J. Boosting Stability and Therapeutic Potential of Proteolysis-Resistant Antimicrobial Peptides by End-Tagging β-Naphthylalanine. Acta Biomaterialia 2023, 164, 175–194. [Google Scholar] [CrossRef]
- Meinberger, D.; Drexelius, M.G.; Grabeck, J.; Hermes, G.; Roth, A.; Elezagic, D.; Neundorf, I.; Streichert, T.; Klatt, A.R. Modified CLEC3A-Derived Antimicrobial Peptides Lead to Enhanced Antimicrobial Activity against Drug-Resistant Bacteria. Antibiotics 2023, 12, 1532. [Google Scholar] [CrossRef]
- Cafaro, V.; Bosso, A.; Di Nardo, I.; D’Amato, A.; Izzo, I.; De Riccardis, F.; Siepi, M.; Culurciello, R.; D’Urzo, N.; Chiarot, E.; et al. The Antimicrobial, Antibiofilm and Anti-Inflammatory Activities of P13#1, a Cathelicidin-like Achiral Peptoid. Pharmaceuticals 2023, 16, 1386. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. American Journal of Translational Research 2019, 11((7)), 3919–3931. [Google Scholar]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Shamseddine, L.; Roblin, C.; Veyrier, I.; Basset, C.; De Macedo, L.; Boyeldieu, A.; Maresca, M.; Nicoletti, C.; Brasseur, G.; Kieffer-Jaquinod, S.; Courvoisier-Dezord E.; Amouric, A.; Carpentier, P.; Campo, N.; Berge, M.; Polard, P.; Perrier, J.; Duarte, V.; Lafond, M. Mechanistic and functional aspectsof the Ruminococcin C sactipeptide isoforms. iScience 2023, 26, 107563, 1-20. [CrossRef]
- Toke, O. Antimicrobial Peptides: New Candidates in the Fight against Bacterial Infections. Biopolymers 2005, 80((6)), 717–735. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




