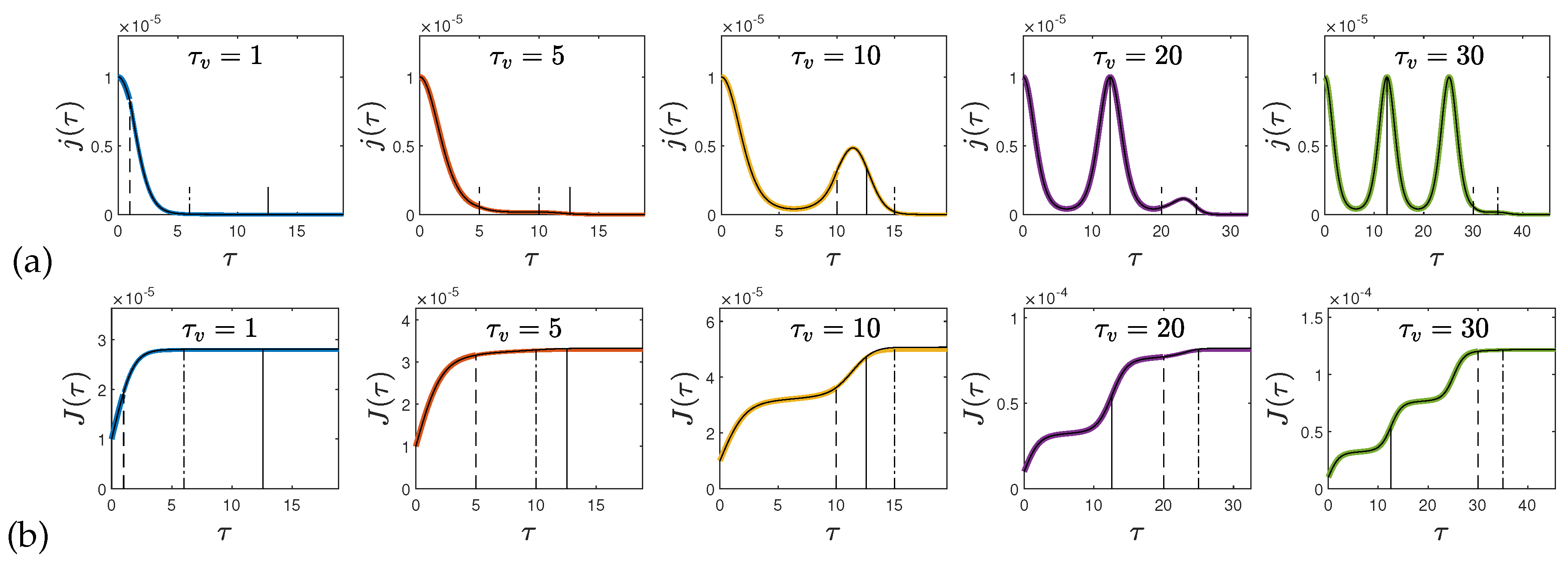1. Introduction
The susceptible-infected-recovered/removed-vaccinated (SIRV) epidemic model [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22] is an important generalization of the simpler susceptible-infected -recovered/removed (SIR) epidemic model [
23,
24,
25,
26,
27] as it accounts for the effects of vaccination campaigns on a considered population, while the original SIR model does not take into account vaccination campaigns. In the SIRV model the time-dependent infection (
), recovery (
) and vaccination (
) rates regulate the transitions between the compartments
,
and
, respectively. Two important key parameters of the SIRV pandemic model are the ratios
of the recovery to infection rate and
of the vaccination to infection rate. Existing analytical solutions to the SIRV equations [
2,
28] have adopted originally stationary values of the ratios
and
, allowing for arbitrary time-dependent infection rates
so that the recovery and vaccination rates have the same time dependence as the infection rate. Here we apply the recently developed analytical approach for the solution of the SIR-epidemics model [
29] to the SIRV-epidemics model. For all times after the start of the epidemic, for which the cumulative fraction of infected persons
is much less then unity, an accurate analytical approximative solution of the SIRV equations is possible for general and arbitrary time dependences of the infection (
), recovery (
) and vaccination (
) rates. As vaccination campaigns automatically reduce the rate of new infections by transferring susceptible persons directly to vaccinated persons, who then no longer can get infected, the limit
is even better fulfilled than in the SIR-epidemics model.
Of high interest, especially from the medical and public health care points of view, are the rate of new infections
and its corresponding cumulative number
, defined by
respectively, after the start of the pandemic outburst at time
, as the hospitalization and death rates are directly proportional to
. Forecasts of the hospitalization and death rates are essential in order to prepare a community for an upcoming pandemic outburst by introducing non-pharmaceutical interventions and/or vaccination campaigns at an optimized time.
The organization of the manuscript is as follows. In Sect. 2 we introduce the starting SIRV-model equations both in terms of the real time t and the reduced time . It is beneficial for the analysis to express the SIRV-equations in a form directly involving the observable quantities such as rate of new infections , the cumulative fraction of infections and the cumulative fraction of vaccinated persons . As shown in Sect. 3 the SIRV-equations in this form allow an approximate analytical solution in the limit of small cumulative fractions . The approximate solution can be written both as function of the real and the reduced time. In Sect. 4 the approximate solutions are compared with the earlier obtained analytical results for the special case of stationary ratios between the recovery to infection rate and the vaccination to infection rate, respectively. In Sects. 5 and 6 we investigate two applications which were inaccessible to analytical treatment before. The considered applications include the cases of stationary ratios with a delayed start of vaccinations (Sect. V), and an oscillating ratio of recovery to infection rate with a delayed vaccination at constant rate (Sect. VI). Here the analytical approximations are compared with the exact numerical solution of the SIRV-equations for these two applications in order to test the accuracy of the analytical approach. A summary and conclusion (Sect. VII) completes the manuscript.
2. SIRV model
The original SIRV-equations read [
1]
obeying the sum constraint
at all times
after the start of the wave at time
with the initial conditions
where
is positive and usually very small,
.
Recently, it has been demonstrated [
30] that the SIRV equations (
2) -() can be expressed as
and
in terms of the reduced time
and the ratios
The great advantage of the SIRV equations written in the form (
8)–(
10) is the direct involvement of observable and monitored quantities, such as the rate of new infections
, the cumulative fraction of new infections
and the cumulative fraction of vaccinated persons
. This has enabled the determination [
30] of the time variation of the ratios
and
from past Covid-19 mutant waves. For completeness we note the SIRV equations (
2)–(5) in terms of the reduced time (
11)
In the following we will derive approximate analytical solutions of the four nonlinear differential equations (
13)–(16) in the limit of small
and prove its accuracy by comparing with the exact numerical solutions of these equations for a number of illustrative examples of the reduced time dependence of the ratios
and
. As will be demonstrated the proposed analytical approximation is self-regulating as the final analytical expression for the cumulative fraction
after infinite time allows us to check the validity of the original assumption
,
3. Approximative analytical solutions
3.1. Solution in the limit of small
Initially at reduced time
the cumulative number of new infections is extremely small. In the limit
, where
, also at later times we use the approximations
to obtain for Eq. (
8)
With the initial condition
for arbitrary but given dependencies
, Eq. (
18) immediately integrates to
which approaches
after infinite time.
Likewise, in the same limit
Eq. (
10) becomes
where we inserted Eq. (
19). With the initial condition
Eq. (
20) integrates to
Because of the adopted smallness
we simplify the approximative solution (
21) in the following as
but we keep the
in the solution (
19) in order not to violate the restriction
.
In terms of the real time in this early time limit the approximative solution (
19) and (
22) read
and
respectively.
3.2. Comparison with the SIR model limit
The SIR model corresponds to the limit of no vaccinations
, corresponding to
. In this limit the solutions (
22) and (
24) reduce to
and
respectively, in perfect agreement with the earlier derived Eqs. (15) and (17) of ref. [
29].
3.3. Properties of the approximative solution (22)
The approximative solution (
22) is predominantly determined by the reduced time variation of the ratios
and
. For the first and second time derivatives of the solution (
22) we obtain
Consequently, extrema of the rate of new infections occur at reduced times
determined by
As the right-hand side of this Eq. is smaller or equal than unity no extrema of infections occur for a sum of variations
greater than unity at all times. As both rates are semi-positive the condition (
30) for no extrema in the rate iof new infections is fulfilled if either the vaccination rate
is greater than the infection rate and/or the recovery rate
is greater than the infection rate.
In the case of reduced time intervals where
we obtain
so that the extrema are maxima if
is positive. Alternatively, the extrema are minima if
is negative. Note that there can be multiple minima and maxima depending on the reduced time variation of the ratios
and
. The extreme values of the rate of new infections in the case
are given by
3.4. Cumulative fraction
Integrating the rate of new infections (
22) provides us with the corresponding cumulative fraction
For general reduced time variations
and
the integral in Eq. (
36) can be reasonably well approximated evaluated using the method of steepest descent [
31,
32] by expanding the argument in the exponential function in Eq. (
36) to second order in
z around its (possible multiple) minimum values
where
With this expansion we obtain for the cumulative fraction (
36)
where the sum of
m accounts for possible multiple minima and
For a minimum the second derivative
has to be positive. The minima occur at times given by
and, as discussed before, see Eqs. (
29)–(
31) only for reduced time intervals where the sum
is less than unity.
4. Special case: stationary ratios
We first consider the approximative solutions (
19) and (
22) in the special case of stationary ratios
considered before [
1]. We readily obtain
and
Provided
the rate of new infections (
44) attains its maximum value at the reduced time
The maximum rate of new infections then is
Equations (
45) and (
46) agree exactly with Eqs. (98) and (100) derived before [
1].
4.1. Cumulative fraction
Integrating Eq. (
44) yields for the cumulative fraction
with the integral
where we substituted
. The integral (
48) can be expressed as the difference of two lower incomplete gamma functions
yielding
so that the cumulative fraction (
47) is given by
For infinitely large times the fraction (
51) approaches the final value
Equations (
51) and (
52) agree exactly with the earlier derived Eqs. (A10) and (102) of ref. [
1], using a different approach.
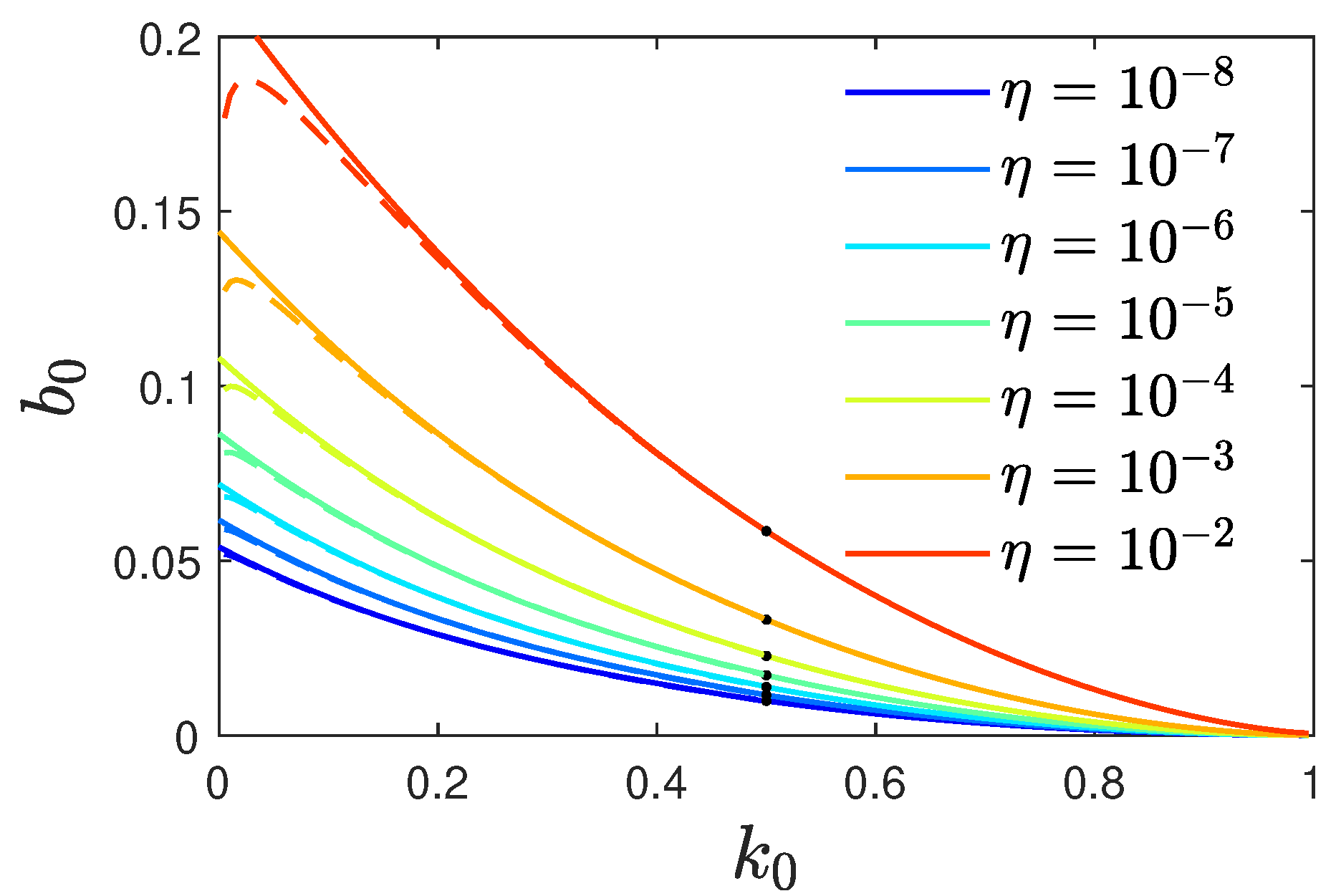
Because the analytical approximations were derived in the limit
, for consistency we have to require
for the values of
and
for which our approximation holds. In
Figure 1 we calculate the required values of
and
fulfilling
using Eq. (
52). The required values depend on the initial condition encoded by
, and are located above the line shown in this figure. For sufficiently large
,
for any ratio
, while at low recovery to infection ratios
, the vaccination to infection rate must be significant to ensure
. The regime of
close to zero is numerically difficult to evaluate using Eq. (
52).
4.2. Limit
In the limit of small
we use relation (
49) and the asymptotic expansion (Eq. 6.5.32 in [
33]) of the upper incomplete gamma function for large arguments
to obtain for
The fraction (
52) then becomes
Using Stirling’s (Eq. 6.1.37 in [
33]) formula for the gamma function
for large
x, Eq. (
55) becomes,
For values of
the fraction (
56) to leading orders is given by
Because one has to require
, or equivalently,
, Eq. (
57) turns into an inequality for
, that can be written in terms of the principal branch
of Lambert’s
W-function, because
is solved for any
and
y by
, leading to
This inequality (
58) ensures
. Along with the information contained in Eq. (
52), it is visualized in
Figure 1.
5. Stationary ratios with delayed start of vaccinations
As first new application of our results we discuss the case of stationary ratio
for all reduced times and the influence of a stationary ratio
starting at the delayed reduced time
, i.e.,
where
and
denotes the step function. We then obtain for Eq. (
19), i.e., in the limit
,
for
and
Likewise the rate (
22) becomes the SIR-rate [
29]
at times without vaccination and
at later times. While the SIR-rate (
61) is exponentially increasing in reduced time, the rate (
62) has a maximum value
provided
, the rate of new infections attains its maximum at the reduced time
We first note that for
the rates (
62) and (
63) correctly reproduce the earlier results (
44) and (
46). We emphasize that the delayed start of the vaccinations increases both the maximum time of the rate of infections and the maximum rate of new infections. Compared to the case of no delay in the start of vaccinations (
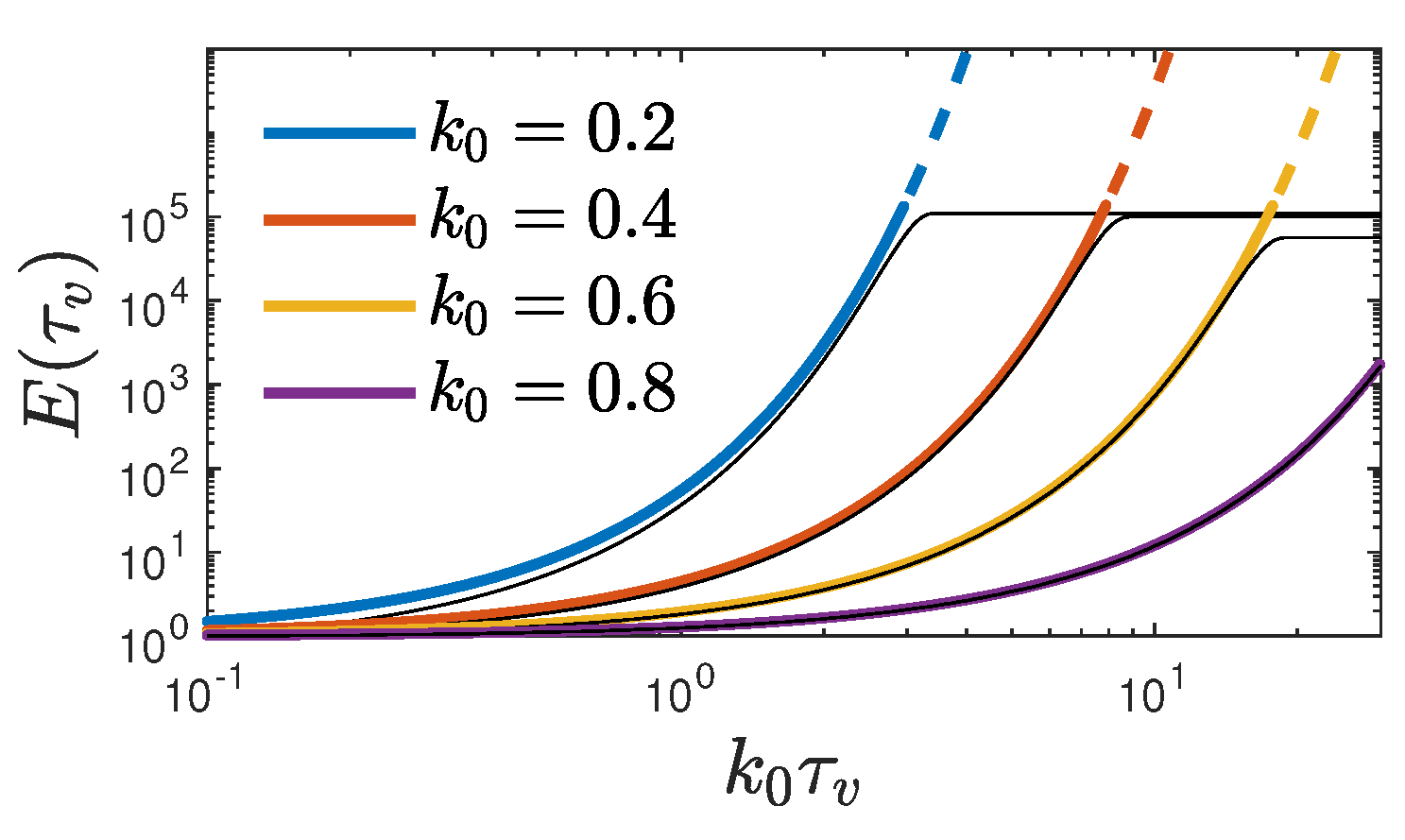
) we introduce the enhancement factor for the maximum rate
shown in
Figure 2, which is independent of the vaccination rate and determined by the values of
and
. Apparently, this exponential enhancement solely results from the new infections before the vaccinations start. While the enhancement factor increases exponentially over a wide range of
, in accord with Eq. (
65), it numerically reaches a plateau as
approaches infinity, whose height increases with decreasing
. This is a clear indications that for large values of the enhancement factor a regime is reached where no longer
is much smaller than unity so that the analytical approximation no longer holds. This explanation is supported by the cumulative fraction at large times (
68) (see below) being directly proportional to the enhancement factor (
65).
Integrating the rates of new injections (
61) and (
63) yields for the cumulative fraction
and
For infinite large times the fraction (
67) approaches the final value
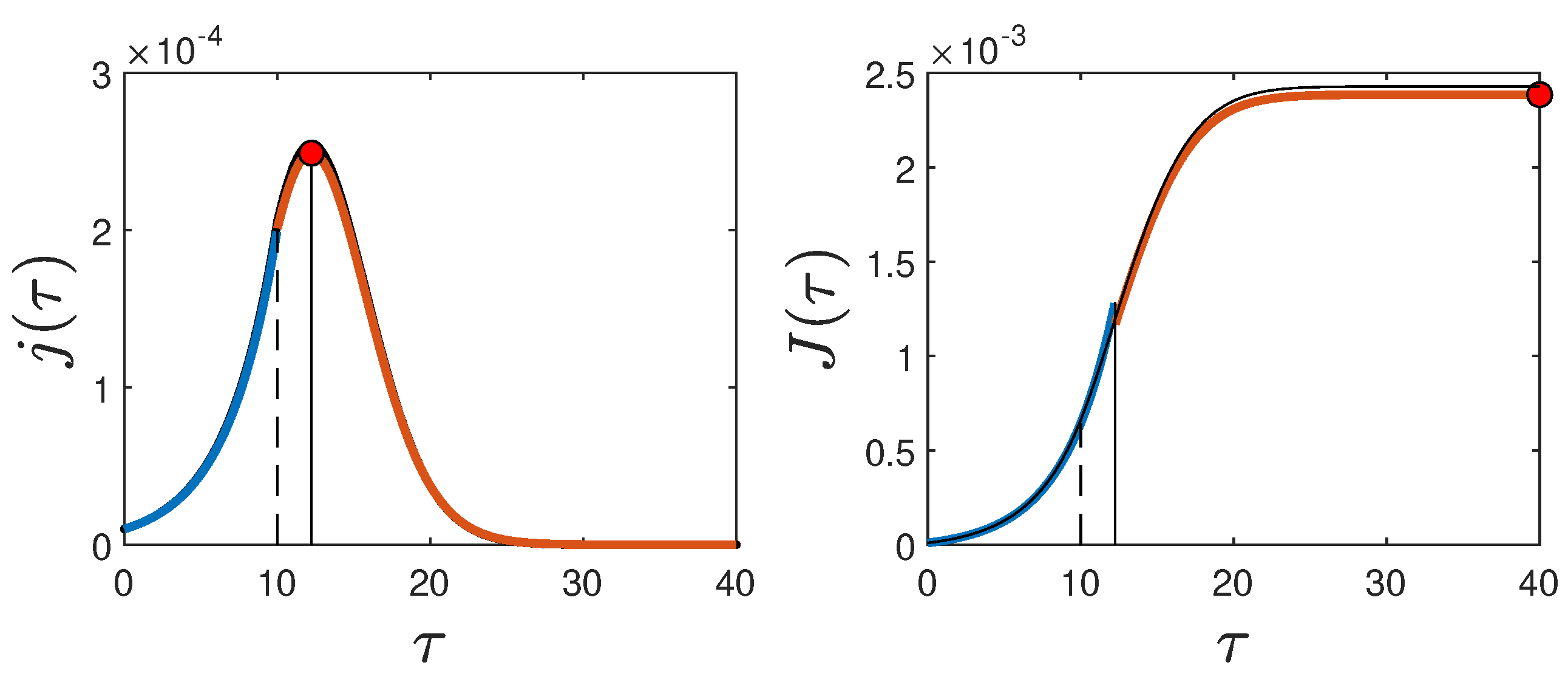
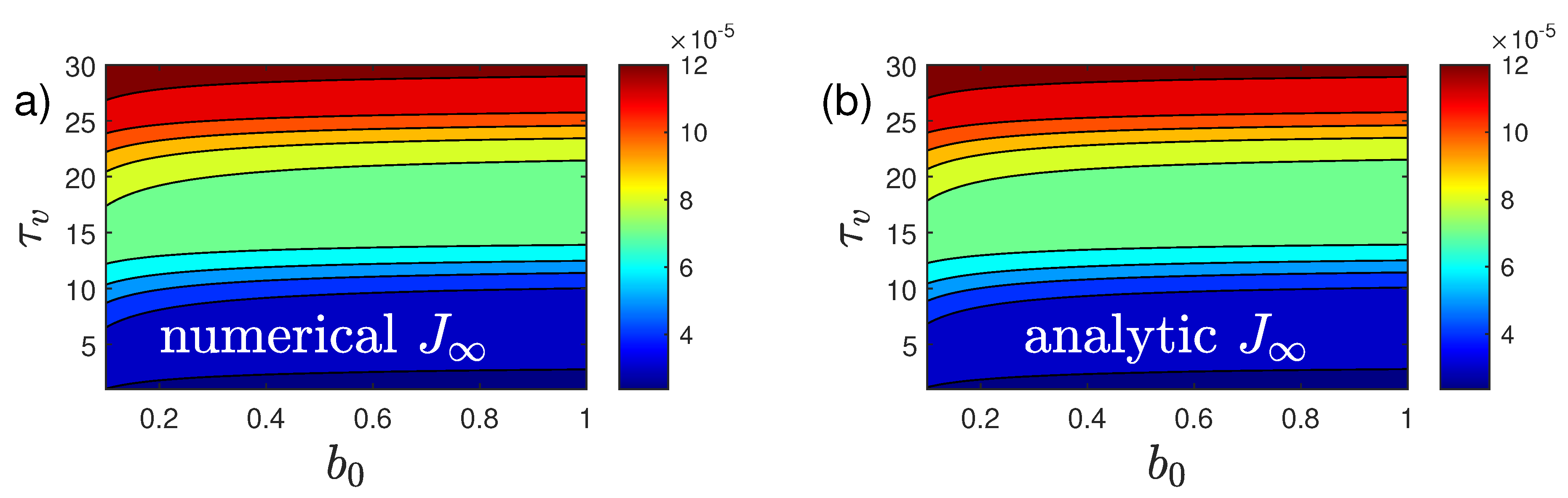
An example showing all quantities calculated analytically in this section, along with the numerical solution for a case with
(
Figure 1), is given in
Figure 3.
6. Oscillating ratio k with delayed vaccinations at constant rate
As second application we investigate the influence of delayed vaccinations with constant rate on the earlier discussed SIR-application [
29] with an oscillating
k ratio and delayed vaccination ratio
b,
with constant values
and
. As noted before [
29] the oscillating ratio (
69) represents a series of repeating pandemic outbursts with equal amplitudes in the rate of new infections. We then obtain for Eq. (
19)
for
and
Likewise the rate (
22) becomes the SIR-rate [
29]
at times without vaccination, and
at later times. In
Figure 4-a we show the rate of new infections (
72)–(
73) in the case
and
for several values of the starting time of vaccinations
and the vaccination rate
. We also compare in each case the analytical approximations with the exact rates of new infections from solving the SIRV equations numerically.
For the corresponding cumulative fractions one finds [
29]
in terms of an infinite series of the modified Bessel function of the first kind
, and
with the integral
where we substituted
and introduced the function
This function (
77) has the following asymptotic behaviors for small and large values of
, i.e.
In the following we therefore approximate the function (
77) as
with
With this approximation we calculate the integral (
76). For values of
we obtain
with
in terms of error functions with complex arguments. The real part in Eq. (
81) is calculated in detail in Appendix A providing
Likewise, in the alternative case
we find
The remaining integrals can be evaluated with the help of
and
Consequently, Eq. (
83) becomes
For the cumulative fraction (
75) we obtain
and
by inserting Eq. (
83) and (
86), respectively. Hence the cumulative fraction after infinite time is given by
which is compared in
Figure 5 with the numerical values. It is sufficient to evaluate the sums up to
; with this setting the calculation of a
value lasts only a fraction of a second.
7. Summary and conclusions
The dynamical equations of the susceptible-infected-recovered/removed-vaccinated (SIRV) epidemics model play an important role to predict and/or analyze the temporal evolution of epidemics outbreaks accounting quantitatively for the influence of vaccination campaigns. Additional to the time-dependent infection () and recovery () rates, regulating the transitions between the compartments and , respectively, the time-dependent vaccination ( accounts for the transition between the compartments of susceptible to vaccinated fractions. Here apparently for the first time a new accurate analytical approximation is derived for arbitrary and different but given temporal dependences of the infection, recovery and vaccination rates, which is valid for all times after the start of the epidemics for which the cumulative fraction of new infections is much less than unity. As vaccination campaigns automatically reduce the rate of new infections by transferring susceptible persons to vaccinated persons, who then no longer can get infected, the limit is even better fulfilled than in the SIR-epidemics model which does not account for vaccinations. The proposed analytical approximation is self-regulating as the final analytical expression for the cumulative fraction after infinite time allows to check the validity of the original assumption , thus indicating the allowed range of parameter values describing the temporal dependence of the ratios and .
The comparison of the analytical approximation for the temporal dependence of the rate of new infections
, the corresponding cumulative fraction of new infections
,and the fraction of vaccinated persons
, respectively, with the exact numerical solution of the SIRV-equations for two different and interesting applications proves the accuracy of the analytical approach. These two applications were not accessible to analytical treatment before. The considered applications include the cases of stationary ratios with a delayed start of vaccinations, and an oscillating ratio of recovery to infection rate with a delayed vaccination at constant rate. The excellent agreement of the analytical approximations with the exact numerical solution of the SIRV-equations for these two applications proves the accuracy of the analytical approach. In the first case the effect of a delayed start of vaccinations on the maximum rate of new infections and on the final cumulative fraction of infected persons is quantitatively calculated demonstrating the importance of an early start of vaccinations during a new epidemic outburst. Moreover, the new analytical approximation agrees favorably well with the earlier obtained analytical approximation [
28] for the case of stationary ratios between the recovery to infection rate and the vaccination to infection rate, respectively, implying that the time dependence of the three rates
,
, and
is the same.
Data Availability Statement
The data that support the findings of this study are available within the article.
Appendix A Reduction of the function W n (τ)
In order to reduce the function
introduced in Eq. (
81) we use for the error function with complex argument their infinite series representation (Eq. 7.1.29 in [
33])
with
and the properties
and
. After straightforward but tedious algebra one obtains for general real values of
A,
B and
C for
Applying Eq. (
A3) to the two error functions in Eq. (
81) then yields Eq. (
82). For
A,
B,
C equally distributed in the range
, the first term
in Eq. (
A3) contributes on average about 97% to the full expression. This feature can be used to write down a simplified expression for
.
References
- Schlickeiser, R.; Kröger, M. Analytical modeling of the temporal evolution of epidemics outbreaks accounting for vaccinations. Physics 2021, 3, 386–426. [CrossRef]
- Babaei, N.A.; Özer, T. On exact integrability of a COVID-19 model: SIRV. Math. Meth. Appl. Sci. 2023, 1, 1–18.
- Rifhat, R.; Teng, Z.; Wang, C. Extinction and persistence of a stochastic SIRV epidemic model with nonlinear incidence rate. Adv. Diff. Eqs. 2021, 2021, 200.
- Ameen, I.; Baleanu, D.; Ali, H.M. An efficient algorithm for solving the fractional optimal control of SIRV epidemic model with a combination of vaccination and treatment. Chaos Solit. Fract. 2020, 137, 109892. [CrossRef]
- Oke, M.; Ogunmiloro, O.M.; Akinwumi, C.T.; Raji, R.A. Mathematical Modeling and Stability Analysis of a SIRV Epidemic Model with Non-linear Force of Infection and Treatment. Commun. Math. Appl. 2019, 10, 717–731.
- Liu, X.D.; Wang, W.; Yang, Y.; Hou, B.H.; Olasehinde, T.S.; Feng, N.; Dong, X.P. Nesting the SIRV model with NAR, LSTM and statistical methods to fit and predict COVID-19 epidemic trend in Africa. BMC Public Health 2023, 23. [CrossRef]
- Mahayana, D. Lyapunov Stability Analysis of Covid-19 SIRV Model. 2022 IEEE 18th Int. Colloquium on Signal Processing & Applications (CSPA 2022). IEEE; Univ Teknologi Mara; IEEE Control Syst Soc; IEEE Control Syst Soc Malaysia Chapter, IEEE, 2022, pp. 287–292. [CrossRef]
- Petrakova, V.S.; Shaydurov, V.V. SIRV-D Optimal Control Model for COVID-19 Propagation Scenarios. J. Siber. Fed. Univ. Math. Phys. 2023, 16, 87–97.
- Zhao, Z.; Li, X.; Liu, F.; Jin, R.; Ma, C.; Huang, B.; Wu, A.; Nie, X. Stringent Nonpharmaceutical Interventions Are Crucial for Curbing COVID-19 Transmission in the Course of Vaccination: A Case Study of South and Southeast Asian Countries. Healthcare 2021, 9. [CrossRef]
- Smith, D.K.; Lauro, K.; Kelly, D.; Fish, J.; Lintelman, E.; McEwen, D.; Smith, C.; Stecz, M.; Ambagaspitiya, T.D.; Chen, J. Teaching Undergraduate Physical Chemistry Lab with Kinetic Analysis of COVID-19 in the United States. J. Chem. Educ. 2022, 99, 3471–3477. [CrossRef]
- Huntingford, C.; Rawson, T.; Bonsall, M.B. Optimal COVID-19 Vaccine Sharing Between Two Nations That Also Have Extensive Travel Exchanges. Front. Public Health 2021, 9. [CrossRef]
- Marinov, T.T.; Marinova, R.S. Adaptive SIR model with vaccination: simultaneous identification of rates and functions illustrated with COVID-19. Sci. Rep. 2022, 12. [CrossRef]
- Beenstock, M.; Felsenstein, D.; Gdaliahu, M. The joint determination of morbidity and vaccination in the spatiotemporal epidemiology of COVID-19. Spatial Spatial-Tempor. Epidem. 2023, 47. [CrossRef]
- Haas, F.; Kröger, M.; Schlickeiser, R. Multi-Hamiltonian structure of the epidemics model accounting for vaccinations and a suitable test for the accuracy of its numerical solvers. J. Phys. A 2022, 55. [CrossRef]
- Li, X.; Li, X.; Zhang, Q. Time to extinction and stationary distribution of a stochastic susceptible-infected-recovered-susceptible model with vaccination under Markov switching. Math. Popul. Stud. 2020, 27, 259–274. [CrossRef]
- Cai, C.R.; Wu, Z.X.; Guan, J.Y. Behavior of susceptible-vaccinated-infected-recovered epidemics with diversity in the infection rate of individuals. Phys. Rev. E 2013, 88. [CrossRef]
- Widyaningsih, P.; Nugroho, A.A.; Saputro, D.R.S. Susceptible Infected Recovered Model with Vaccination, Immunity Loss, and Relapse to Study Tuberculosis Transmission in Indonesia. Int. Conf. Sci. Appl. Sci. (ICSAS 2018); Suparmi, A.; Nugraha, D., Eds. Sebelas Maret Univ, Amer. Inst. Physics, 2018, Vol. 2014, AIP Conf. Proc. [CrossRef]
- Chapman, J.D.; Evans, N.D. The structural identifiability of susceptible-infective-recovered type epidemic models with incomplete immunity and birth targeted vaccination. Biomed. Signal Process. Control 2009, 4, 278–284. [CrossRef]
- Wang, J.; Zhang, R.; Kuniya, T. A reaction-diffusion Susceptible-Vaccinated-Infected-Recovered model in a spatially heterogeneous environment with Dirichlet boundary condition. Math. Comp. Simul. 2021, 190, 848–865. [CrossRef]
- Khader, M.M.; Adel, M. Numerical Treatment of the Fractional Modeling on Susceptible-Infected-Recovered Equations with a Constant Vaccination Rate by Using GEM. Int. J. Nonlin. Sci. Numer. Simul. 2019, 20, 69–75. [CrossRef]
- Dai, Y.; Zhou, B.; Jiang, D.; Hayat, T. Stationary distribution and density function analysis of stochastic susceptible-vaccinated-infected-recovered (SVIR) epidemic model with vaccination of newborns. Math. Meth. Appl. Sci. 2022, 45, 3401–3416. [CrossRef]
- Kiouach, D.; El-idrissi, S.E.A.; Sabbar, Y. The impact of Levy noise on the threshold dynamics of a stochastic susceptible-vaccinated-infected-recovered epidemic model with general incidence functions. Math. Meth. Appl. Sci. 2023. [CrossRef]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. A 1927, 115, 700. [CrossRef]
- Kendall, D.G. Deterministic and stochastic epidemics in closed populations. Proc. Third Berkeley Symp. on Math. Statist. and Prob. 1956, 4, 149. [CrossRef]
- Wu, W.; Teng, Z. Periodic wave propagation in a diffusive SIR epidemic model with nonlinear incidence and periodic environment. J. Math. Phys. 2022, 63, 122701.
- Zhang, S.P.; Yang, Y.R.; Zhou, Y.H. Traveling waves in a delayed SIR model with nonlocal dispersal and nonlinear incidence. J. Math. Phys. 2018, 59, 011513.
- Wu, W.; Zhang, L.; Teng, Z. Wave propagation in a nonlocal dispersal SIR epidemic model with nonlinear incidence and nonlocal distributed delays. J. Math. Phys. 2020, 61, 061512.
- Schlickeiser, R.; Kröger, M. Analytical solution of the SIR-model for the temporal evolution of epidemics: Part B. Semi-time case. J. Phys. A 2021, 54, 175601. [CrossRef]
- Schlickeiser, R.; Kröger, M. Analytical solution of the SIR-model for the not too late temporal evolution of epidemics for general time-dependent recovery and infection rates. Covid 2023, 3, 1781–1796.
- Schlickeiser, R.; Kröger, M. Key epidemic parameters of the SIRV model determined from past COVID-19 mutant waves. Covid 2023, 3, 592–600.
- Morse, P.M.; Feshbach, H. Methods of Theoretical Physics, Part I; McGraw-Hill, New York, 1953.
- Mathews, J.; Walker, R.L. Mathematical Methods in Physics, 2nd ed.; Benjamin, Menlo Park, California, 1970.
- Abramowitz, M.; Stegun, I.A. Handbook of Mathematical Functions; Dover Publ., New York, 1970.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).