Submitted:
21 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
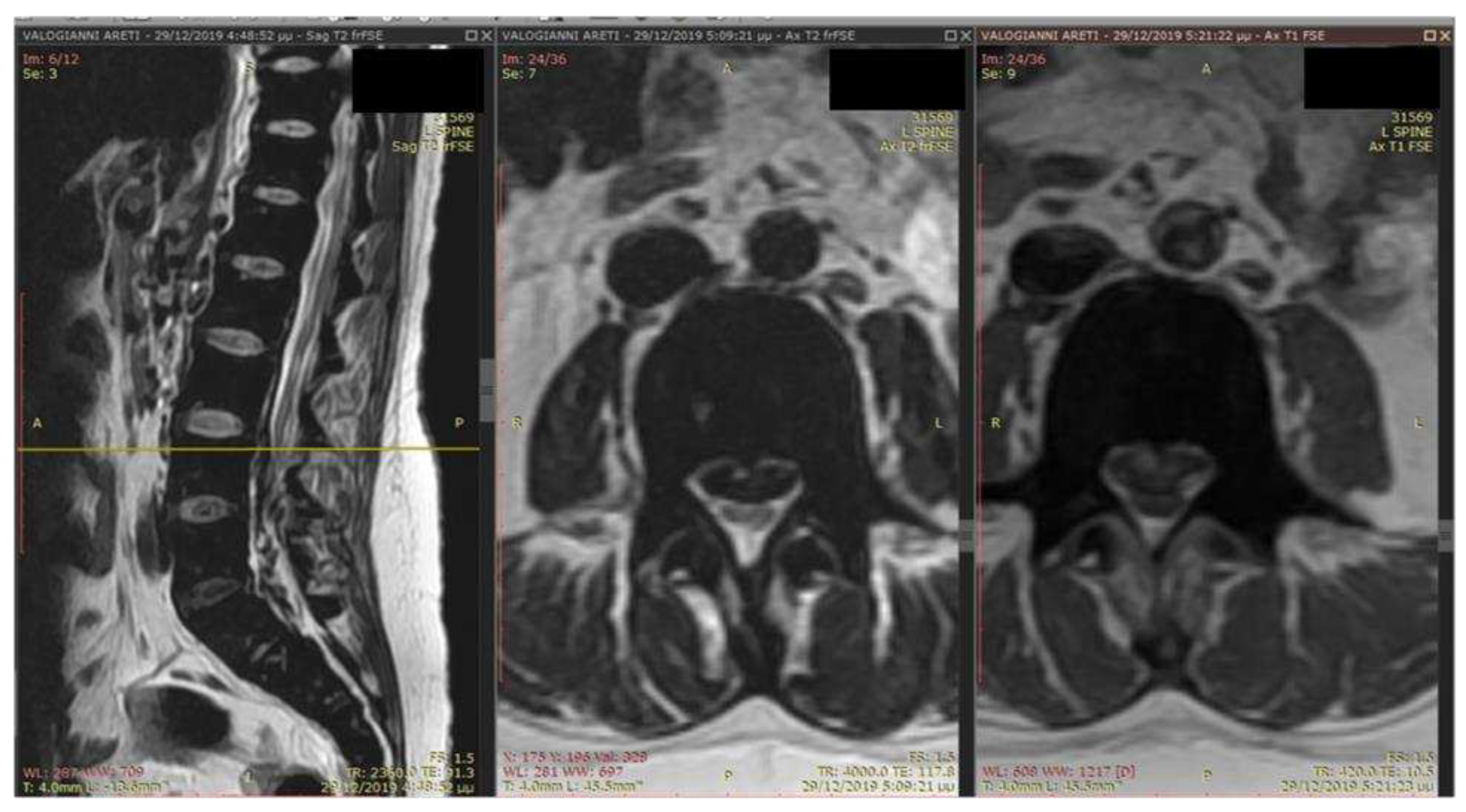
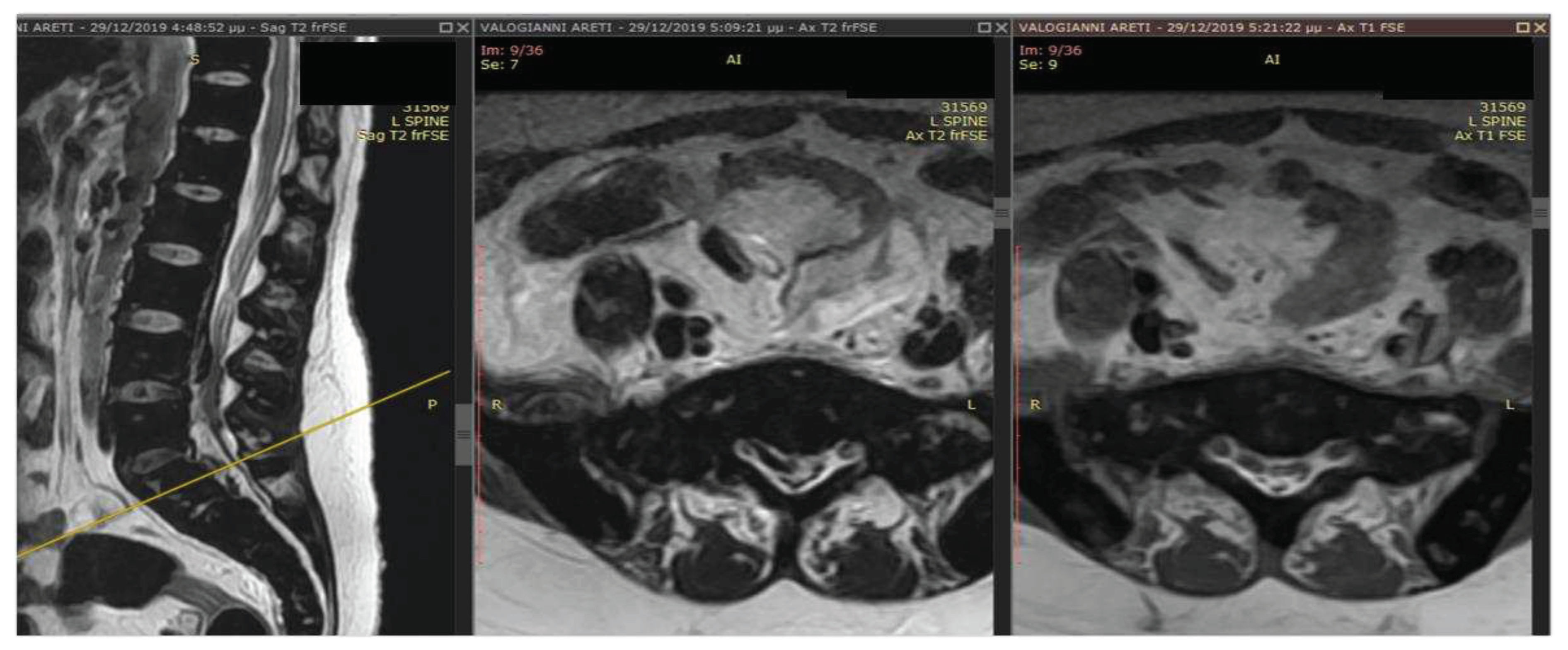
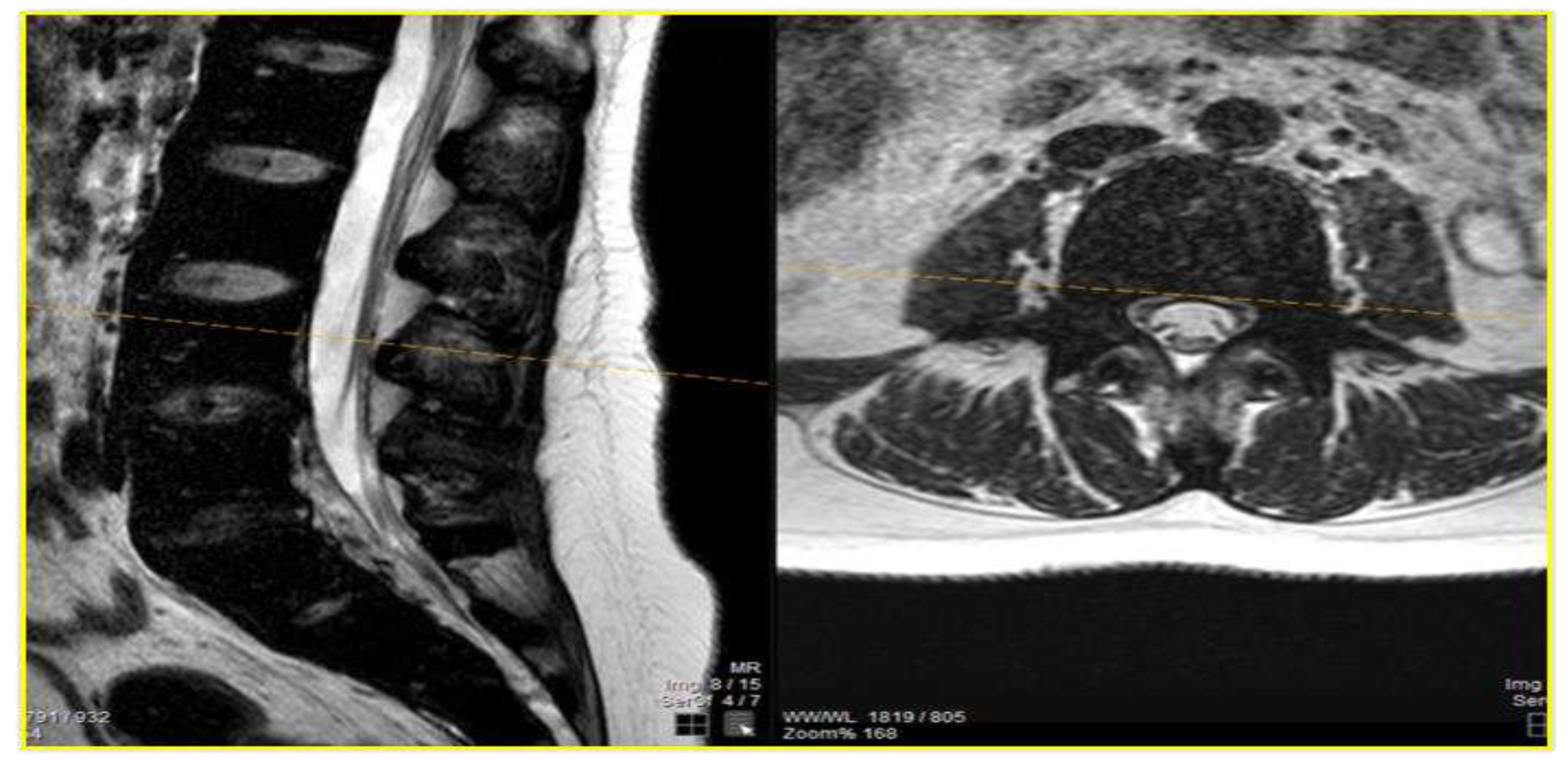
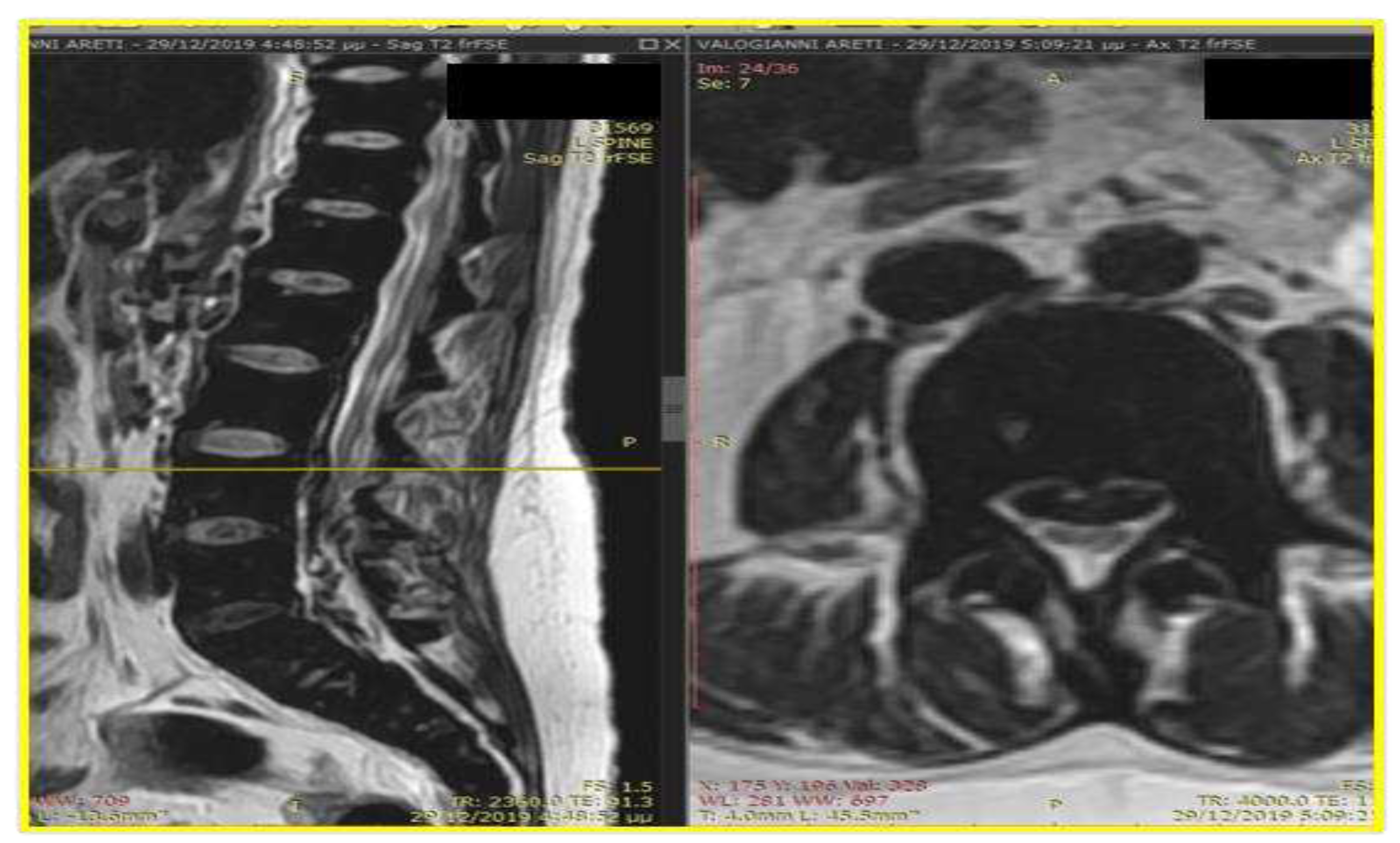
Case Presentation
Discussion
Conclusion
Author Contributions
Funding
Availability of Data and Materials
Conflict of Interest Statement
References
- Haidar, R.; Mhaidli, H.; Taher, A.T. Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. European Spine Journal 2010, 19, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Vichinsky, E.; Musallam, K.; Cappellini, M.D.; Viprakasit, V. Guidelines for the management of non-transfusion dependent thalassemia (NTDT). 2014. [Google Scholar]
- Bolaman, Z.; et al. Intrathoracic extramedullary hematopoiesis resembling posterior mediastinal tumor. The American journal of medicine 2002, 112(9), 739–741. [Google Scholar] [CrossRef] [PubMed]
- Zherebitskiy, V.; Morales, C.; Del Bigio, M.R. Extramedullary hematopoiesis involving the central nervous system and surrounding structures. Human pathology 2011, 42(10), 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; et al. The mechanisms of pathological extramedullary hematopoiesis in diseases. Cellular and Molecular Life Sciences 2020, 77, 2723–2738. [Google Scholar] [CrossRef] [PubMed]
- Malla, S.; et al. Marrow outside marrow: imaging of extramedullary hematopoiesis. Clinical Radiology 2020, 75(8), 565–578. [Google Scholar] [CrossRef] [PubMed]
- Kalchiem-Dekel, O.; Greenbaum, U. Extramedullary hematopoiesis in β-thalassemia. Mayo Clinic Proceedings 2015, 90(11). [Google Scholar] [CrossRef] [PubMed]
- Moulopoulos, L.A.; Koutoulidis, V. Bone marrow MRI; Springer Verlag, 2016. [Google Scholar]
- Subahi, E.A.; et al. Comprehensive Review: Extramedullary Hematopoiesis in Patients with Beta Thalassemia Major (transfusion dependent thalassemia). Blood 2021, 138, 4163. [Google Scholar] [CrossRef]
- Ata, F.; et al. Protocol for extramedullary hematopoiesis in patients with transfusion-dependent β-thalassemia (TDT): A systematic review. Health Science Reports 2021, 4(4), e429. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.; et al. Systematic literature review of the burden of disease and treatment for transfusion-dependent β-thalassemia. Clinical Therapeutics 2020, 42(2), 322–337. [Google Scholar] [CrossRef] [PubMed]
- Doctor, P.N.; Merchant, R.; Pandey, A.K. Paraspinal extramedullary hematopoiesis in beta thalassemia Intermedia treated with low dose radiotherapy. 2020; 452–454. [Google Scholar]
- Cianciulli, P.; et al. Hydroxyurea therapy in paraparesis and cauda equina syndrome due to extramedullary hematopoiesis in thalassemia: improvement of clinical and hematological parameters. European journal of hematology 2000, 64(6), 426–429. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; et al. Combined radiotherapeutic and surgical management of a spinal cord compression by extramedullary hematopoiesis in a patient with hemoglobin E beta-thalassemia. Acta haematologica 1994, 91(3), 154–157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




