Submitted:
22 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Experimental design
Sample collection
Biochemical parameters
Meat quality
Fecal microbiota profiling
Determination of fecal metabolites
Statistical analysis
Results
Growth performance and serum biochemical parameters
Meat quality
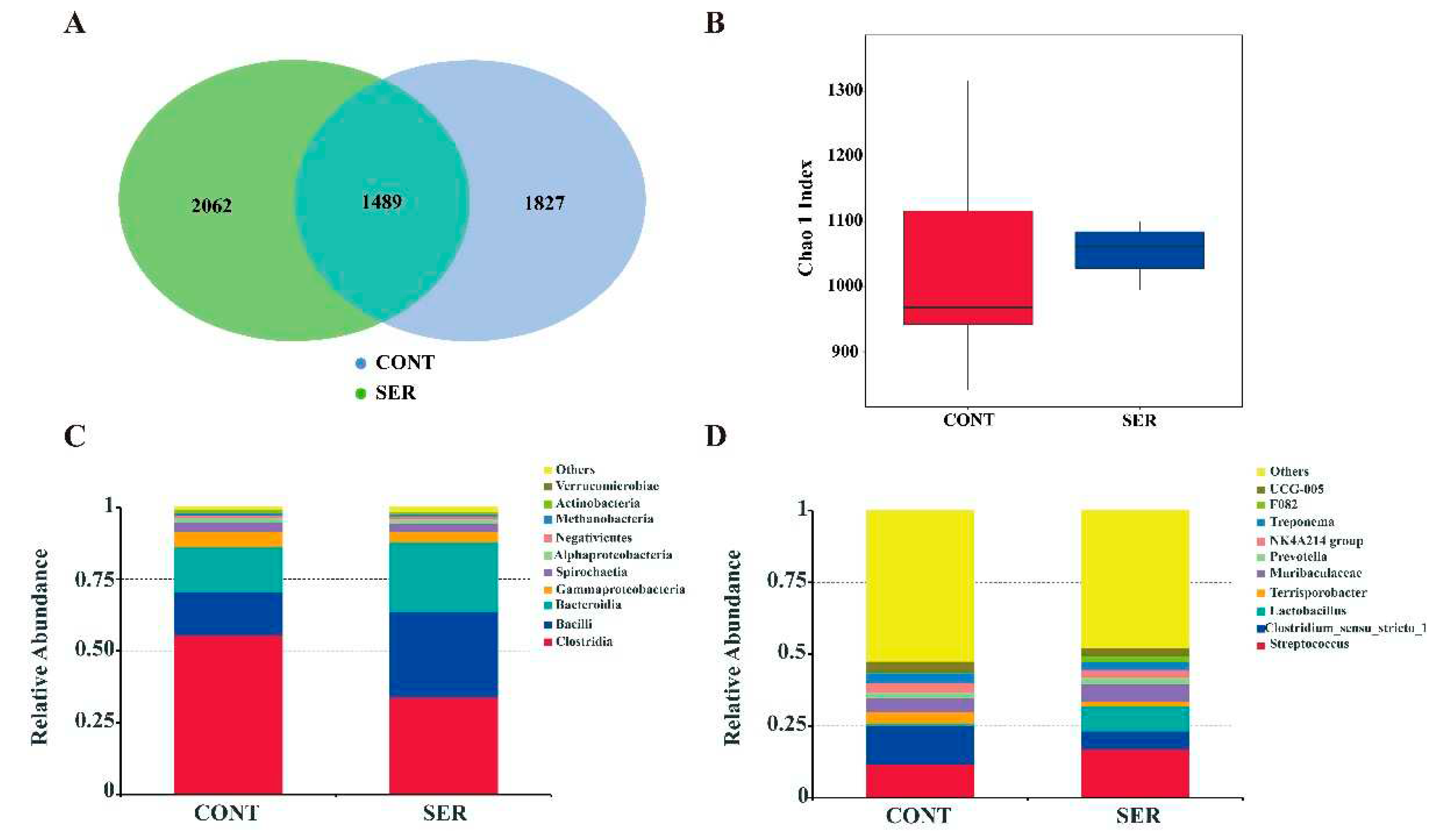
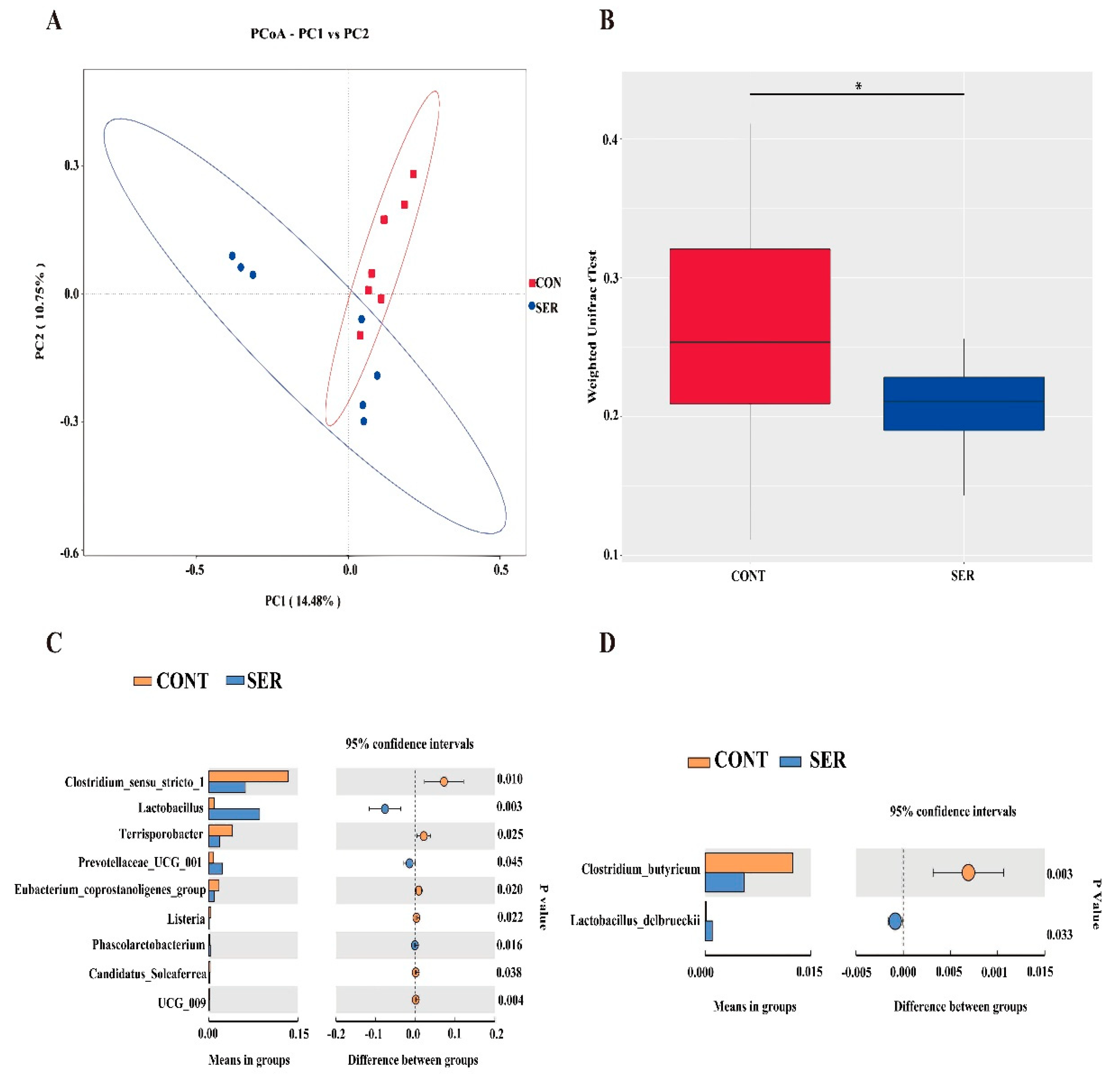
Fecal microbiota composition
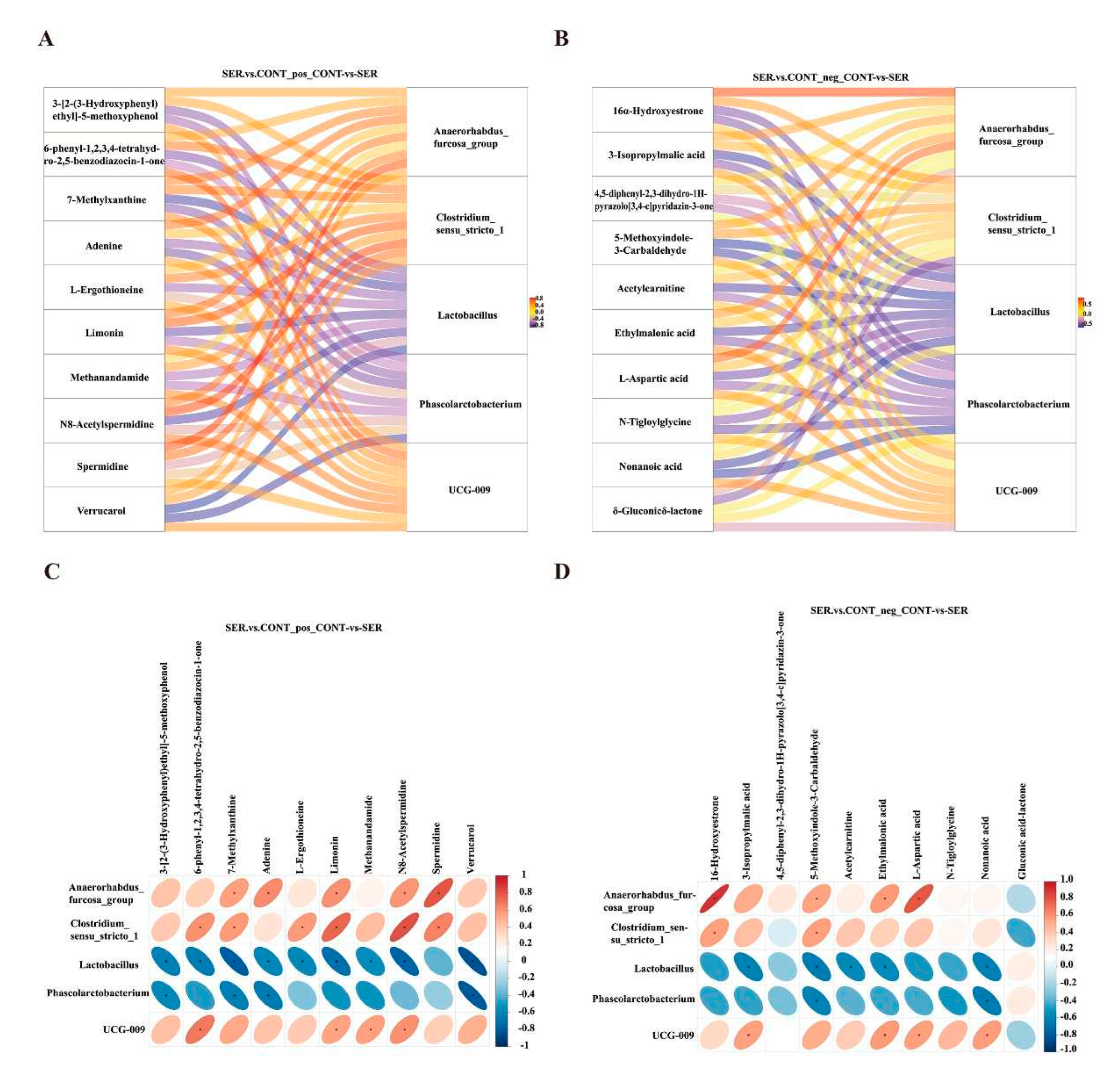
Fecal metabolites
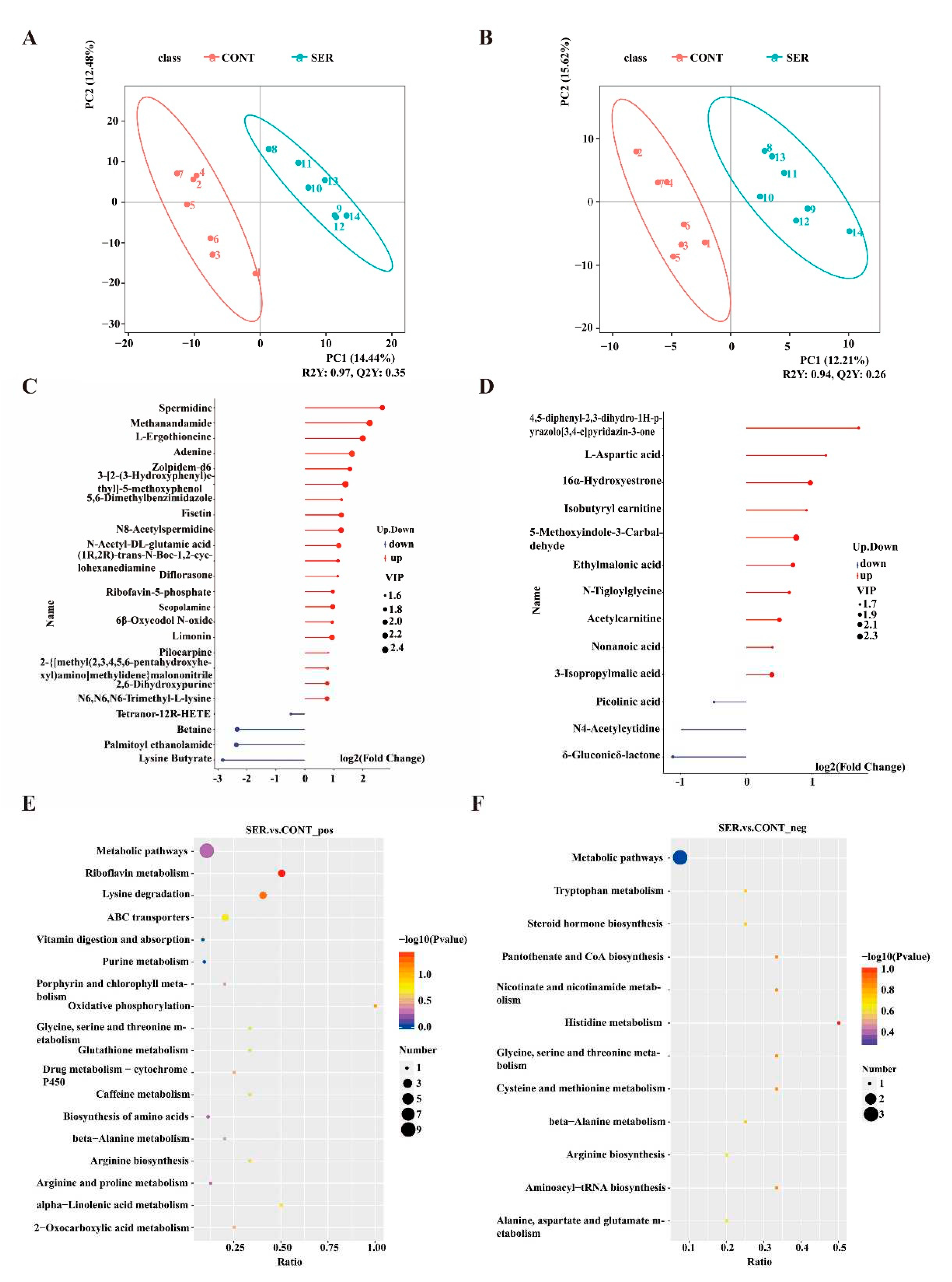
Correlation between microbiota and metabolites
Discussion
- Animal Welfare Statement:The study was conducted according to the principles of the animal welfare committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences and was approved by the animal welfare committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
- Informed Consent Statement: Informed consent was obtained from all individual participants included in the study.
- Data Availability Statement: The 16S rDNA gene sequence data have been deposited in the NCBI Bioproject database (https://www.ncbi.nlm.nih.gov/bioproject/), registration number for PRJNA994863.
- Acknowledgments: This work was supported by the Hunan Provincial Science and Technology Department(2021JJ30320), Science and Technology Innovation Program of Hunan Province (2023RC1074), Youth Innovation Promotion Association CAS and China Agriculture Research System of MOF and MARA (CARS-35).
References
- Liu, B.; Chen, Y.; Li, Q.; Zhong, Z.; Tan, Y.; Zhang, S.; Zhu, L. Effects of Breed and Gender Effect on Pork Quality Traits. Southwest China Journal of Agricultural Sciences 2019, 32, 2222–2225. [Google Scholar]
- Wang, Y.; Thakali, K.; Morse, P.; Shelby, S.; Chen, J.; Apple, J.; Huang, Y. Comparison of Growth Performance and Meat Quality Traits of Commercial Cross-Bred Pigs versus the Large Black Pig Breed. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Chen, B.L.; Li, D.Y.; Leng, D.; Kui, H.; Bai, X.; Wang, T. Gut microbiota and meat quality. Frontiers in Microbiology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Huang, Z.; Su, Y.; Li, F.; Yin, J. Research Progress of Nutritional Regulation for Meat Quality of Pigs. Chinese Journal of Animal Nutrition 2020, 32, 4555–4564. [Google Scholar]
- Luo, J.; Zeng, D.; Cheng, L.; Mao, X.; Yu, J.; Yu, B.; Chen, D. Dietary beta-glucan supplementation improves growth performance, carcass traits and meat quality of finishing pigs. Anim Nutr 2019, 5, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Deng, D.; Cui, Y.; Chen, W.; Yu, M.; Ma, X. Diet supplemented with fermented okara improved growth performance, meat quality, and amino acid profiles in growing pigs. Food Sci Nutr 2020, 8, 5650–5659. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Liu, Z.; Deng, D.; Cui, Y.; Tian, Z.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J Food Sci Technol 2020, 57, 404–412. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, H.; Na, L.; Zhou, X.; Li, X.; Zhao, Y.; Wen, Z.; He, Q. Methionine restriction at the post-weanling period promotes muscle fiber transition in piglets and improves intramuscular fat content in growing-finishing pigs. Amino Acids 2019, 51, 1657–1666. [Google Scholar] [CrossRef]
- Hu, C.J.; Jiang, Q.Y.; Zhang, T.; Yin, Y.L.; Li, F.N.; Deng, J.P.; Wu, G.Y.; Kong, X.F. Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. Journal of animal science 2017, 95. [Google Scholar] [CrossRef]
- He, L.; Long, J.; Zhou, X.; Liu, Y.; Li, T.; Wu, X. Serine is required for the maintenance of redox balance and proliferation in the intestine under oxidative stress [J]. FASEB J 2020, 34, 4702–4717. [Google Scholar] [CrossRef]
- He, L.; Ding, Y.; Zhou, X.; Li, T.; Yin, Y. Serine signaling governs metabolic homeostasis and health. Trends Endocrinol Metab 2023, 34, 361–372. [Google Scholar] [CrossRef]
- Lionaki, E.; Gkikas, I.; Daskalaki, I.; Ioannidi, M.-K.; Klapa, M.I.; Tavernarakis, N. Mitochondrial protein import determines lifespan through metabolic reprogramming and de novo serine biosynthesis. Nature Communications 2022, 13. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhang, L.; Kong, X.; Li, F. Serine-to-glycine ratios in low-protein diets regulate intramuscular fat by affecting lipid metabolism and myofiber type transition in the skeletal muscle of growing-finishing pigs. Anim Nutr 2021, 7, 384–392. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Liu, D.; Feng, Y.; Yin, J.; Zhou, X. Exogenous and Endogenous Serine Deficiency Exacerbates Hepatic Lipid Accumulation. Oxid Med Cell Longev 2021, 2021, 4232704. [Google Scholar] [CrossRef]
- Nguyen Cong, O.; Bernard, T.; Pham Kim, D.; Do Duc, L.; Nassim, M.; Nguyen Thi, H.; Nguyen Hoang, T.; Georges, D.; Jerome, B.; Vu Dinh, T.; et al. Growth performance, carcass quality characteristics and colonic microbiota profiles in finishing pigs fed diets with different inclusion levels of rice distillers' by-product [J]. Animal science journal = Nihon chikusan Gakkaiho 2019, 90, 948–960. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Guo, J.; Wang, Y.; Wang, L.; Xu, D.; Yan, E.; Zhang, X.; Yin, J. Effects of Dietary Yeast beta-Glucan Supplementation on Meat Quality, Antioxidant Capacity and Gut Microbiota of Finishing Pigs [J]. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, R.; Zhang, B.; Zhang, X.; Yang, H.; Zhou, X. Serine Alleviates Dextran Sulfate Sodium-Induced Colitis and Regulates the Gut Microbiota in Mice [J]. Front Microbiol 2018, 9, 3062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Du, W.; Fu, A.K.; Zhang, X.P.; Huang, Y.; Lee, K.H.; Yu, K.; Li, W.F.; Li, Y.L. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef Microbes 2016, 7, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Zhou, X.; Wang, Q.; Xie, C.; Li, F.; Fan, Z.; Zhang, B.; Ruan, Z.; Chen, X.; Wu, X.; et al. Dietary supplementation of Lonicera macranthoides leaf powder improves amino acid profiles in serum and longissimus thoracis muscle of growing-finishing pigs. Anim Nutr 2016, 2, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. The Impact of Grouping on Skin Lesions and Meat Quality of Pig Carcasses. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Henriquez-Rodriguez, E.; Tor, M.; Pena, R.N.; Estany, J. A polymorphism in the stearoyl-CoA desaturase gene promoter increases monounsaturated fatty acid content in dry-cured ham. Meat Sci 2015, 106, 38–43. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Guan, P.; He, L.; Zhou, X. Serine Administration Improves Selenium Status, Oxidative Stress, and Mitochondrial Function in Longissimus Dorsi Muscle of Piglets with Intrauterine Growth Retardation. Biol Trace Elem Res 2023, 201, 1740–1747. [Google Scholar] [CrossRef]
- Ogbuagu, N.E.; Ayo, J.O.; Aluwong, T.; Akor-Dewu, M.B. L-serine improves lipid profile, performance, carcass weight and intestinal parameters in feed restricted broiler chickens during the hot-dry season. Trop. Anim. Health Prod. 2022, 54, 12. [Google Scholar] [CrossRef]
- Lewis, Caroline A.; Parker, Seth J.; Fiske, Brian P.; McCloskey, D.; Gui, Dan Y.; Green, Courtney R.; Vokes, Natalie I.; Feist, Adam M.; Vander Heiden, Matthew G.; Metallo, Christian M. Tracing Compartmentalized NADPH Metabolism in the Cytosol and Mitochondria of Mammalian Cells. Molecular Cell 2014, 55, 253–263. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; He, F.; Fu, J.; Xin, Z.; Deng, B.; He, L.; Zhou, X.; Ren, W. Serine Supports IL-1β Production in Macrophages Through mTOR Signaling. Frontiers in immunology 2020, 11. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W. Role of mTOR in Glucose and Lipid Metabolism. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, F.; Lin, G.; Xiong, Y.L. Modulation of muscle antioxidant enzymes and fresh meat quality through feeding peptide-chelated trace minerals in swine production. Food Bioscience 2021, 42. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Wu, C.; Zhang, Y.; Wu, X.; Yin, Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol Nutr Food Res 2017, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xie, J.; Zhang, Y.; Wang, J.; Sun, X.; Zhang, H. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol 2013, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Tian, Y.; Zhou, F.; Ma, J.; Xia, S.; Yang, T.; Ma, L.; Zeng, Q.; Liu, G.; et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innovation (Camb) 2023, 4, 100486. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xin, Y.; Ma, Y.; Xu, X.; Zhao, S.; Cao, J. Screening of Microbes Associated With Swine Growth and Fat Deposition Traits Across the Intestinal Tract [J]. Frontiers in Microbiology 2020, 11. [Google Scholar] [CrossRef]
- Izzo, L.T.; Trefely, S.; Demetriadou, C.; Drummond, J.M.; Mizukami, T.; Kuprasertkul, N.; Farria, A.T.; Nguyen, P.T.T.; Murali, N.; Reich, L.; et al. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci. Adv. 2023, 9, 20. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Chen, L.; Zhang, Y.; Wang, J.; Li, H.; Yan, X.; Xia, L.; Yao, G. Differences in meat quality between Angus cattle and Xinjiang brown cattle in association with gut microbiota and its lipid metabolism. Frontiers in Microbiology 2022, 13. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.J.; Zuo, S.X.; Peng, S.J.; Wang, Z.J.; Zhang, Y.J.; Luo, H.L. Untargeted and Targeted Metabolomics Profiling of Muscle Reveals Enhanced Meat Quality in Artificial Pasture Grazing Tan Lambs via Rescheduling the Rumen Bacterial Community. J. Agric. Food Chem. 2021, 69, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Goodarzi, P.; Pezeshki, A.; Hagen, D.E. RNA-seq reveals insights into molecular mechanisms of metabolic restoration via tryptophan supplementation in low birth weight piglet model. Journal of animal science 2022, 100. [Google Scholar] [CrossRef] [PubMed]
- Zubiri-Gaitán, A.; Blasco, A.; Hernández, P. Plasma metabolomic profiling in two rabbit lines divergently selected for intramuscular fat content. Communications Biology 2023, 6. [Google Scholar] [CrossRef] [PubMed]
| Item | 20~30 kg | 30~60kg | 60~120 kg |
|---|---|---|---|
| Corn, % | 65.00 | 72.30 | 59.00 |
| Wheat bran, % | 6.00 | 3.00 | / |
| Rice, % | / | / | 20.00 |
| Soybean meal#, % | 25.00 | 19.50 | 14.00 |
| Soybean oil, % | / | 1.20 | 3.00 |
| Limestone, % | 1.00 | 0.80 | 0.80 |
| calcium hydrogen phosphate, % | 0.80 | 0.70 | 0.50 |
| NaCl, % | 0.40 | 0.45 | 0.30 |
| L-lysine hydrochloride, % | 0.30 | 0.60 | 0.25 |
| DL-methionine, % | 0.10 | 0.10 | / |
| L-threonine, % | 0.20 | 0.20 | / |
| L-tryptophan, % | / | 0.03 | / |
| Zeolite powder | 0.08 | / | 1.03 |
| Premix*, % | 1.12 | 1.12 | 1.12 |
| Total, % | 100 | 100 | 100 |
| Calculated nutrient level | |||
| DE(MJ/kg) | 13.86 | 14.28 | 14.03 |
| CP, % | 17.51 | 15.57 | 12.55 |
| Ca, % | 0.64 | 0.53 | 0.46 |
| Total P, % | 0.50 | 0.43 | 0.38 |
| P, % | 0.23 | 0.20 | 0.17 |
| Lysine, % | 1.03 | 1.12 | 0.71 |
| Methionine, % | 0.36 | 0.33 | 0.2 |
| Threonine, % | 0.73 | 0.65 | 0.37 |
| Tryptophan, % | 0.15 | 0.15 | 0.10 |
| CONT | SER | |
|---|---|---|
| Initial BW, kg | 20.95±0.76 | 20.35±0.38 |
| Final BW, kg | 116.8±7.1 | 125.2±4.9 |
| ADG, kg | 0.806±0.059a | 0.871±0.026b |
| ADFI, kg | 2.25±0.16 | 2.29±0.04 |
| F/G | 2.80±0.05 | 2.63±0.10 |
| CONT | SER | |
|---|---|---|
| GSH, ng/mL | 129.4±17.4a | 196.9±19.1b |
| SOD, ng/mL | 197.6±29.3a | 286.4±17.2b |
| MDA, nmol/mL | 4.06±0.40a | 1.91±0.24b |
| CONT | SER | |
|---|---|---|
| Color | ||
| L | 45.23±1.56 | 44.11±1.28 |
| a | 13.86±0.33 | 14.48±0.48 |
| b | 5.40±0.57 | 5.08±0.46 |
| pH45min | 6.60±0.09 | 6.42±0.11 |
| pH24h | 5.60±0.07 | 5.53±0.07 |
| Drip loss | 3.58±0.50a | 2.24±0.33b |
| Intramuscular fat | 2.26±0.18a | 2.94±0.17b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





