1. Introduction
Osteoarthritis (OA) is a prominent contributor to disability and a significant driver of societal expenditure among the older population. The prevalence of this condition is on the rise due to a combination of factors, including an aging population and a growing prevalence of obesity, trauma, abnormal joint morphology, developmental dysplasia of the joint, muscle weakness of the joint, occupation (high-impact physical activity or sport), sedentarity, etc. [
1,
2].
The hip and knee joints, which bear the weight of the body, are the most prominently affected [
3]. Consequently, they stand as the primary drivers of disability among the elderly population. The prevalence of OA shows a consistent upward trajectory with advancing age, affecting approximately 20% of individuals aged 60 or older [
4].
The rehabilitation process holds significant importance for patients who have been diagnosed with hip and knee OA following total hip or knee replacement [
5,
6]. Historically, the examination and management of these individuals have predominantly relied on supervised clinical assessments conducted within controlled clinical environments [
7]. Nevertheless, it is important to acknowledge that these assessments possess inherent limits in their ability to fully capture the intricate nature of real-world experiences encountered during the process of rehabilitation [
8]. Furthermore, traditional methods evaluate data at a specific moment, assuming the stability of parameters over time. However, the intricate dynamics of real-life scenarios encompass physical, and temporal variations throughout the day, week, and so forth, particularly in the individual-level factors such as pain, stress, emotion, and motivation.
The use of technology-assisted rehabilitation is gaining popularity due to its ability to provide objective and automated assessments of patients’ motor function and therapy adherence, incorporating factors like kinematics, activity level, muscle activity, and more [
9]. Mobile health technologies, including wearable and portable sensors, are now being used to assess mobility in unsupervised, real-world situations, offering a more patient-relevant and ecologically valid approach compared to routine clinical tests [
10]. This approach helps overcome the limitations of traditional clinical assessments where the outcomes used in the research study, mainly subjective evaluated by the clinicians, may not be sensitive enough to detect subtle modifications of the patients because of low resolution and ceiling effect [
11]. Quantitative outcomes used to validate the intervention are, most of the time, based on clinical or laboratory assessment which are both quite artificial situations not reflecting the activities of daily living and the real-world evidence [
10].
The implementation of automated unsupervised evaluations signifies a substantial shift in the approach to evaluating and providing support for patients during their rehabilitation process. The potential advantages are diverse, encompassing enhanced precision in assessments, the possibility of tailored, patient-centred therapies, and heightened patient involvement in their own rehabilitation journey [
12,
13]. The objective of this study was to examine the potential of automated technology in augmenting the capacities of patients and rehabilitation specialists.
Remote health monitoring, facilitated by non-invasive wearable sensors and modern communication technologies, ensures patient safety at home while enabling continuous data collection between sessions [
14]. This data can detect even minor changes in a patient’s status, providing more precise and sensitive outcomes, known as digital biomarkers [
15]. Digital biomarkers, collected through various sensors such as accelerometers, smartwatches, connected insoles, and smartphones, offer continuous and objective measurements of biological and physiological data [
16]. They can reveal disease characteristics not easily observable in clinical settings. Digital biomarkers can be categorized as active (supervised) or passive (unsupervised) [
17].
The widespread adoption of smartphones equipped with built-in accelerometers and gait-detection algorithms has introduced a higher level of detail in monitoring mobility data. Recent research efforts have applied accelerometry techniques to individuals undergoing joint arthroplasty [
15,
18]. Given the positive impact of physical activity on enhancing functionality, promoting bone healing, and ensuring the stability of implants, the focus of these studies has been on tracking step count recovery. This assessment aims to determine if post-operative activity levels surpass those recorded before the surgery and the duration required to achieve this [
19].
This method capitalizes on smartphones’ ability to capture activity data in real-world conditions, spanning a diverse range of activities [
20]. However, it’s crucial to recognize that these assessments are susceptible to variations influenced by how subjects carry their phones [
21]. Encouragingly, the self-administered six-minute walk test (6MWT) has exhibited satisfactory accuracy, reproducibility, and acceptability in both healthy individuals and those with varying degrees of congestive heart failure severity [
22]. Nevertheless, the exploration of unintentional walk testing, a method analyzing free-living physical activity data, remains an unexplored area in current research, despite its potential to more accurately portray daily functional status.
Therefore, in the present study we investigated the use of activity tracker on quantifying patient activities of daily living and follow-up. The primary objective of this study was to examine the incorporation of unsupervised evaluations within the rehabilitation process in order to obtain a more multidimensional evaluation of the patient’s progression throughout their recovery trajectory, taking into account the dynamic obstacles they encounter throughout their reintegration into their regular routines.
This study proposes the incorporation of automated unsupervised assessments as an essential element in the rehabilitation process for persons after hip and knee replacement. This change exhibits the potential to enhance the accuracy of assessments, improve the management of these patients and therefore the outcome of the rehabilitation.
2. Materials and Methods
2.1. Data Source
We conducted a retrospective observational study using anonymized depersonalized data. A cohort of 1144 patients who underwent elective total knee and hip arthroplasty was selected from a population of 1339 patients. Patients were included in the study if they used the digital application for at least 6 weeks after surgery and completed their patient reported outcome measures preoperatively. 82,189 days of activities were recorded and analyzed. Each patient provided written informed consent for the scientific use of their anonymized data.
2.2. Recording Device and Outcomes
All data collection was facilitated via the moveUP® application (moveUP®, Brussels, Belgium), which is registered as a medical device. The database comprises data from patients who underwent hip and knee arthroplasty across Belgium, France, and the Netherlands. This application operates on a smart virtual platform designed for digital monitoring, utilizing both objective and subjective patient data. The platform consists of two main components: a patient-facing mobile application and a web-based dashboard utilized by the care provider. Objective data were collected using a commercial activity tracker (Garmin Vivofit 4) worn 24/7 by the patients. Objective data consisted of the number of steps per day and the number of steps per minute throughout the day. Those data were collected during the rehabilitation period for which frequent interactions are occurring between the patient and care providers (Time in system -
Table 1). Clinical data and patients reported outcomes were also measured outside of the rehabilitation period. Patient reported outcomes such as the Oxford Knee Score, Forgotten Joint Score (FJS), Hip Osteoarthritis Outcome score (KOOS), Knee Osteoarthritis Outcome score (KOOS), UCLA Activity Scale (UCLA) and the EuroQol 5-Dimension (EQ5D).
2.3. Procedure
The preoperative parameters were first explored and the impact of demographics of surgery type. Then we explored the variability of the activity parameters throughout the recovery process by assessing the intraweek variability.
To explore the impact of quick or slow recovery trajectories on activity data, we used the JFS Minimal Clinically Important Difference (MCID) as a threshold at 3 months to divide the hip and knee patients into two groups: MCID achieved (MCID+) and MCID non-achieved (MCID-). The thresholds for MCIDs are 17.5 for THA [
23], 16.6 for TKA [
24] and 12,5 for unicondylar [
23]. The flowchart on patient’s selection is presented in
Figure 1. The complete characteristics of the patients are presented in
Table 2.
Finally, we investigated the impact of the type of knee surgery on activity metrics.
2.4. Data Processing
The Garmin activity tracker allows the user to collect the total number of steps each day, but the focal point of data collection was the step-per-minute data [
25]. This continuous stream of data was automatically stored in the moveUP mobile application via an SDK connection, providing real-time access to participants’ dynamic activity profiles. To derive meaningful insights from the step-per-minute data, two key parameters were extracted: Peak 1-Minute cadence (P1M), and Peak 6-Minute consecutive cadence (P6MC). Those metrics were computed for each day of recording.
2.4.1. Peak 1-Minute Cadence (P1M)
The Garmin activity tracker allowed for the identification of the highest step count within any given minute throughout the day [
26,
27]. This metric, termed the P1M served as an indicator of participants’ maximal exertion or bursts of activity during their daily routines. Peak 1-min may represent one’s ‘best natural effort’, or rather the free-living walking cadence of which an individual is capable. Peak-1 min cadence is highly dependent on age, physical activity level (i.e., steps/day), physical function, and body mass index (BMI) [
25,
28,
29].
2.4.2. Peak 6-Minute Consecutive Cadence (P6MC)
The highest continuous activity during 6 minutes is detected in step data, using a sliding 6 min window with 1 min overlap [
30]. The one with the largest number of steps is chosen as the most representative, in order to get the highest intensity reached during 6 consecutives minutes.
With the first minute of day ≤ j ≤ last minute of day -6
2.4.3. Intensity
Walking cadence is a valid proxy of physical activity intensity. Moderate intensity is defined as activity above 3 metabolic equivalents (METs) which correspond to a threshold of 100 steps/min [
31]. Light intensity physical activity is defined as activity between 1,6 and 2,9 METs [
32]. Activities under 20 steps/min are considered as incidental movements and were considered as sedentary behavior [
29]. Results were presented as the minutes spent per week to be consistent with the World Health Organization recommendation about the minimal level of physical activity [
33].
2.4.4. Outliers Removal
To ensure the integrity and accuracy of the step-per-minute data, a rigorous outlier removal process was implemented. Instances where step-per-minute data exceeded 150 were considered outliers, as such values were deemed unrealistic for the targeted population undergoing knee and hip arthroplasty rehabilitation. These outliers were systematically identified and removed from the dataset to prevent skewing of results and to maintain the reliability of the recorded activity metrics.
2.5. Statistical Analysis
The normality of each parameter was checked using graphical methods (boxplots, histograms and Q–Q plots). Data were presented as mean (standard deviation) or median [p25; p75] according to the type of distribution.
To determine the most stable indicators of the gross motor function of the patients, we assessed the intraweek variability of the parameters using the coefficient of variation across the seven days of the week.
We analyzed the different outcomes of both knee and hip OA patients using mixed models [
34]. For each outcome, a mixed model with random intercept was used. The values from each day were treated as repeated measures. The model equation is:
With
where α and
,
and
were employed as fixed effects, while
was used to represent random errors. The parameter
was utilized to quantify the random effect.
Our analysis incorporated fixed effects related to recovery, days after surgery (spanning from 1 to 60 for hip and 1 to 90 for knee surgeries), as well as the interaction between the two. To ensure comparability, we imposed constraints on the estimated baseline measures. This was achieved by normalizing all groups, subtracting the mean values of each group’s first session from all subsequent sessions. Essentially, this constraint allowed for the adjustment of baseline measurements and accommodated variations in the relationship between baseline and follow-up scores across different sessions.
It is important to note that centering the explanatory variables using mean values facilitated the direct interpretation of this effect as an intergroup effect [
35].
We then computed the time, and the associated 95% confidence intervals, needed to differentiate between the recovery status.
Statistical analyses were performed at an overall significance level of 0.05. Statistical analyses were conducted in RStudio (version 2023.09.0) with R version 4.4.2., using the LME4 package to run the mixed effect models [
36].
3. Results
The details of the patient population are displayed in
Table 2.
Significant differences were observed between the hip and knee population for the age, body mass index (BMI), patients reported outcomes, and the time of using the recording system. Age difference was 1.4 years [95%CI -2.43; -0.26], patients undergoing hip surgery being younger than for total knee replacement.
3.1. Preoperative Scores
First, we analyzed the data from the preoperative evaluation. Interestingly, when analyzing the overall number of steps per day we did not find any statistically significant difference between hip and knee OA patients. However, when using the P6MC and the P1M we did find statistically significant differences with higher cadences in patients with hip OA. The complete results were presented in
Table 3.
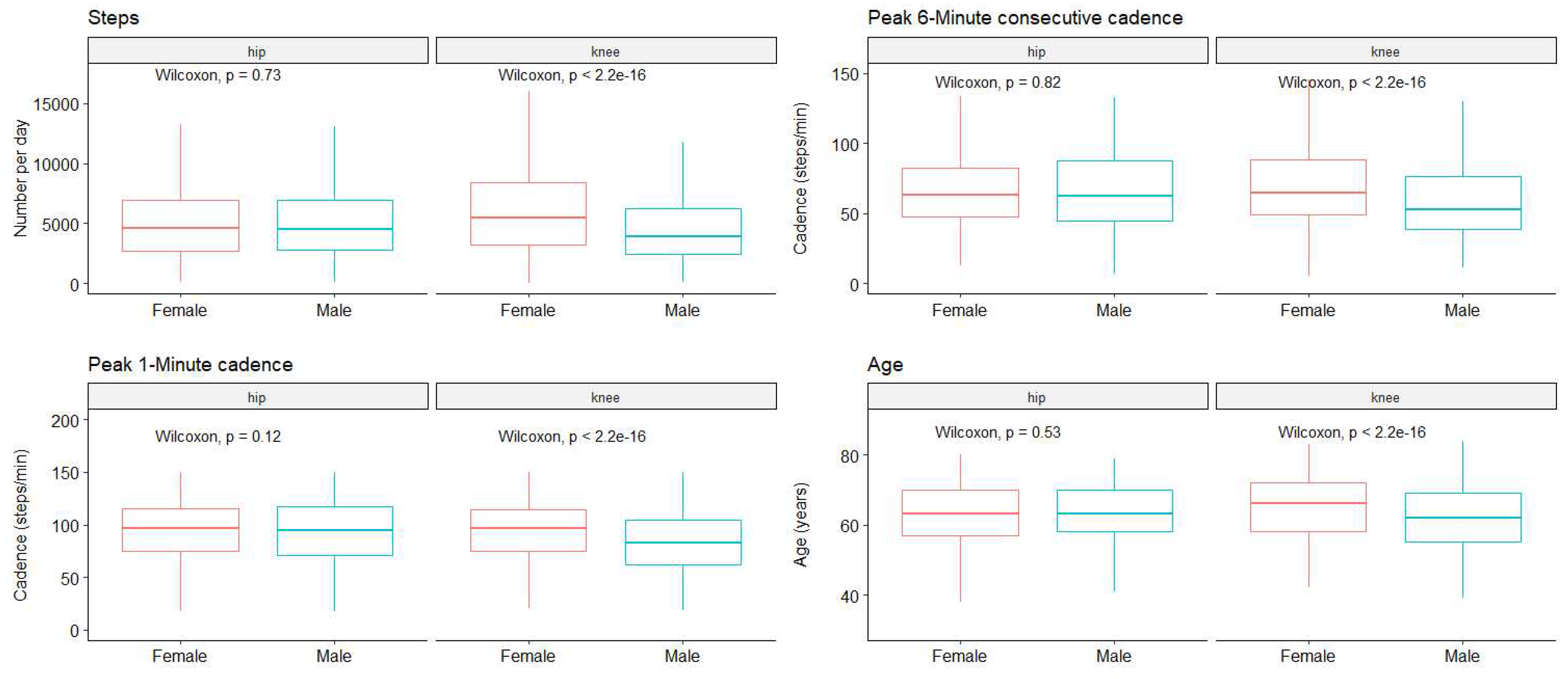
Next, to determine whether a separate analysis is necessary for female and male patients, we compared the pre-operative data based on gender (
Figure 2).
On the one hand, for hip OA we did not find statistically significant differences for the P1M (97 [75 - 115] and 95 [71 - 117] for female and male respectively, p = 0.12), the P6MC (63 [48 - 83] and 62 [45 - 88], p = 0.81) or for the total number of steps per day (4628 [2673 - 6920] and 4508 [2814 - 6939], p = 0.73), nor for age (62.7 (8.9) and 62.6 (9.4), p = 0.83).
On the other hand, for knee OA statistically significant differences were found for the total number of steps (5453 [3190 - 8444] and 3917 [2432 - 6217] for female and male respectively, p < 0.001), the P6MC (65 [49 - 88] and 53 [39 - 76], p < 0.001), the P1M (97 [75 - 115] and 83 [62 - 105], p < 0.001). Interestingly we found a statistically significant difference in the age at surgery, women being older than men (65.1 (8.5) and 61.7 (8.3), p < 0.001).
To further evaluate the potential influence of the age difference observed in knee patients we performed linear regression to determine if age has an influence on the different studied parameters. Scatter plots are presented in
Figure 3.
For female participants, concerning the total number of steps per day the interaction between age and joint is not significant (p = 0.07), but there is a significant effect of age (β = -86 (8), p < 0.001) and joint (β = 1000 (141), p < 0.001). For the P6MC there is no interaction between age and OA location (p = 0.23), but there is a significant effect of both age (β = -0.47 (0.05), p < 0.001) and joint (β = 3.2 (0.9), p = 0.001). For the P1M there is a significant interaction between age and joint (β = 0.26 (0.12), p = 0.031) as well as a significant effect of age (β = -0.54 (0.07), p < 0.001) and joint (-16 (8), p = 0.042).
For male participants, concerning the total number of steps per day the interaction between age and joint is not significant (p = 0.44), and there is no statistically significant effect of age (β = 0.33 (0.61), p = 0.96) but a significant effect of joint (β = -720 (120), p < 0.001). For the P6MC there is no interaction between age and joint (p = 0.79), nor a significant effect of age (β = -0.01 (0.05), p = 0.85), but a significant effect of joint (β = -8.3 (0.9), p < 0.001). For the P1M there is no interaction between age and joint (p = 0.09) but significant effects of age (β = -0.02 (0.06), p < 0.001) and joint (-9.8 (1.1), p < 0.001).
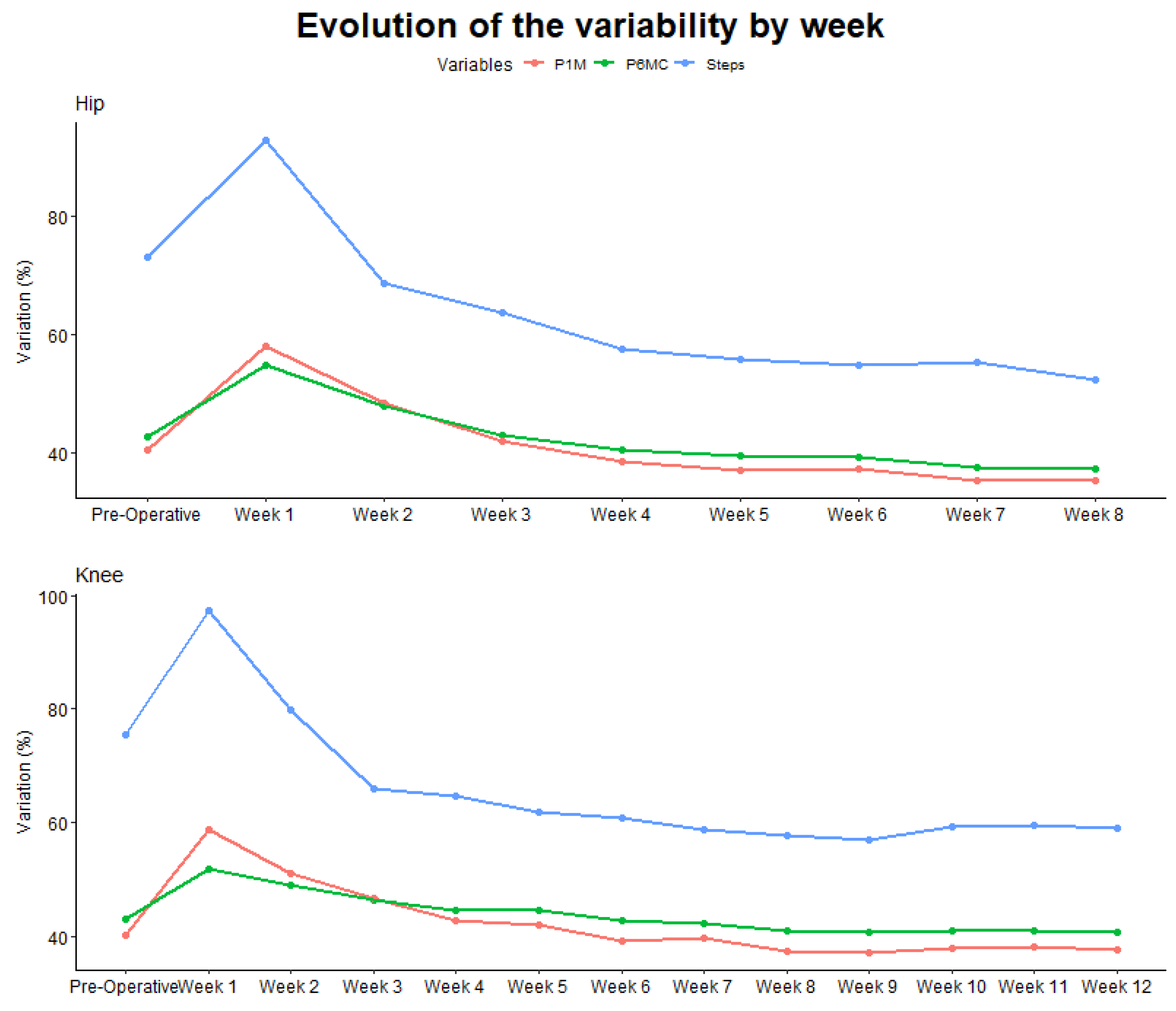
3.2. Variability of the Outcomes
As presented in
Figure 4 we can clearly see a difference in terms of variability between on the one hand the total number of steps per day presenting a lot of variability and on the other hand the cadence during the P6MC and the P1M being much more consistent through the day.
3.3. Evolution of the Parameters during the Rehabilitation Process
3.3.1. According to Recovery
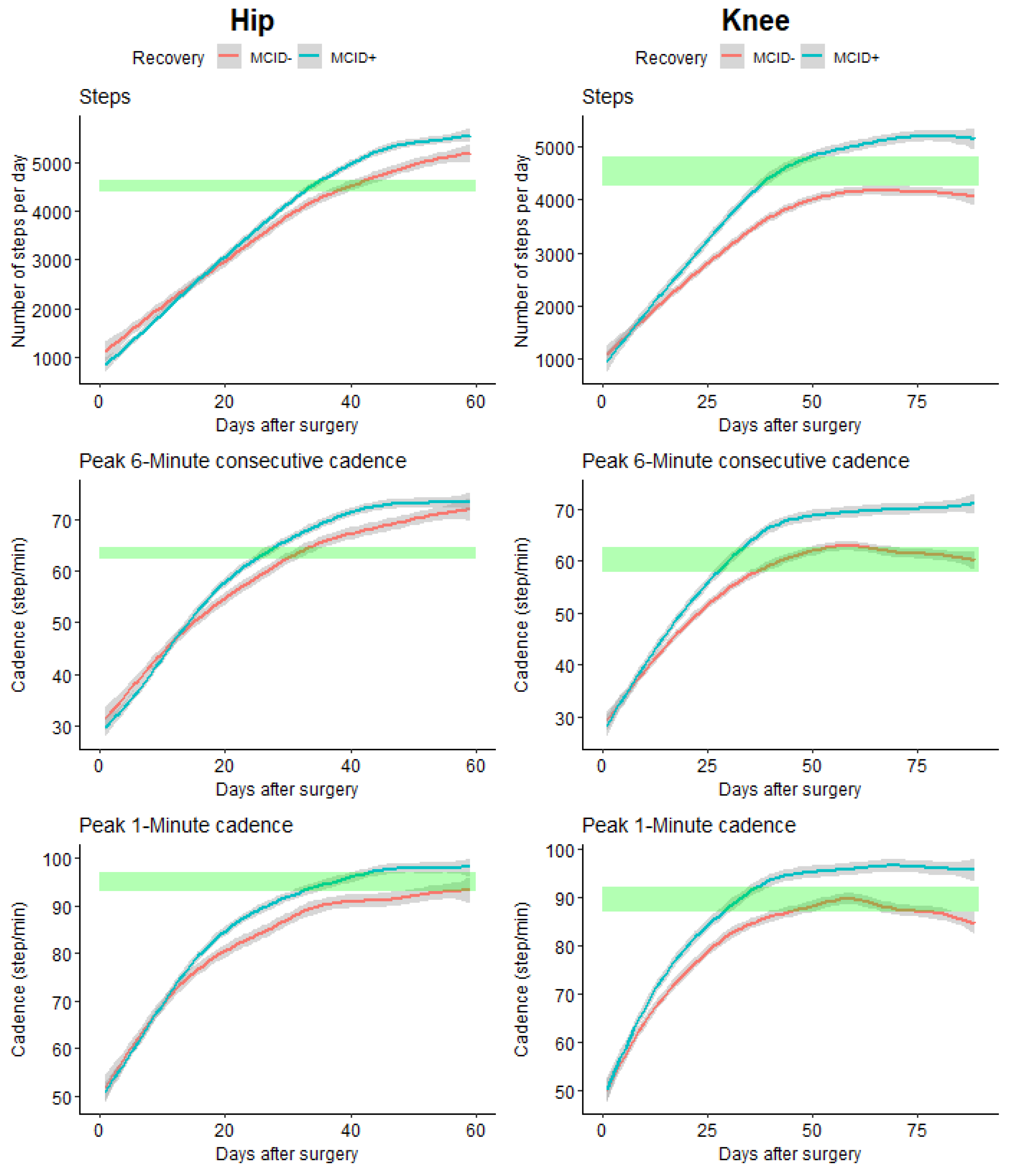
When comparing the time needed to get back to initial (pre-operative) values, important differences were observed for the computed outcomes (see
Figure 5).
THA patients who did not reach their MCID at 3 months (MCID-) took statistically 7 days more to recover their pre-operative activity level (number of steps) than patients who reached their MCID at 3 months (MCID+) (33 days for MCID+ and 40 days for MCID-), for the P6MC a difference of 6 days (26 days for MCID+ and 32 days for MCID-), for P1M the MCID+ regains the initial value after 35 days while the MCID- did not reach the initial value after the 60 days of follow-up.
For TKA, for the number of steps only the MCID+ group reaches the initial value after 40 days, for the P6MC a difference of 10 days (29 days for MCID+ and 39 days for MCID-), for P1M a difference of 12 days (38 days for MCID+ and 50 days for MCID-).
When comparing the trajectory of the evolution in the different groups we observed that for both THA and TKA the newly developed outcomes, P6MC and P1M, allowed for early identification of differences in comparison with the total number of steps per day (
Table 4).
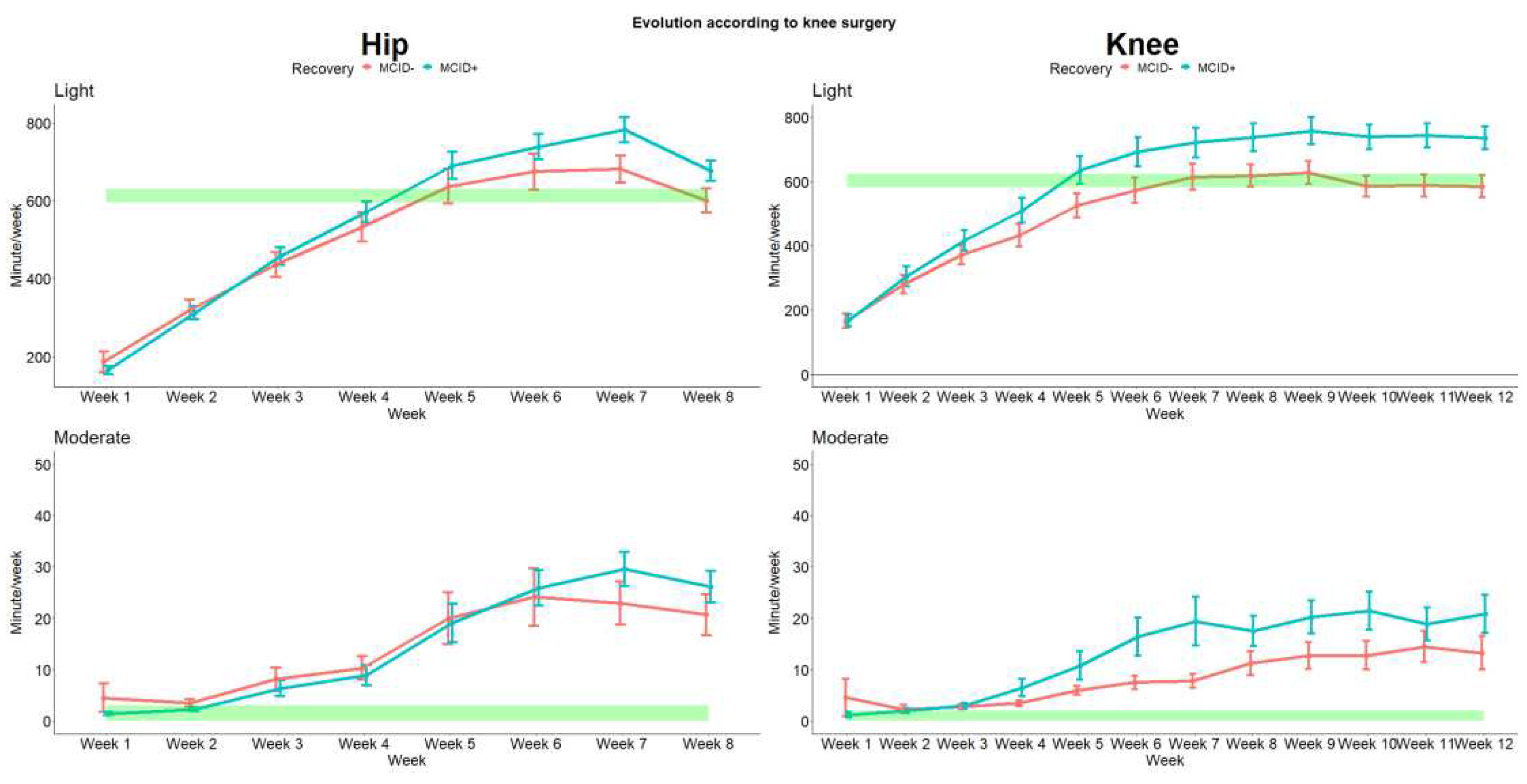
Then we analyzed the intensity of the activities through the rehabilitation process. The evolution for light and moderate activities are presented in
Figure 6 (due to the quasi absence of vigorous activities these results were not presented). As for the number of steps and cadence we observed differences between the recovery status. Interestingly we also observed that both THA and TKA patients are quickly able to recover higher level of moderate activities, which were barely absent pre-surgery.
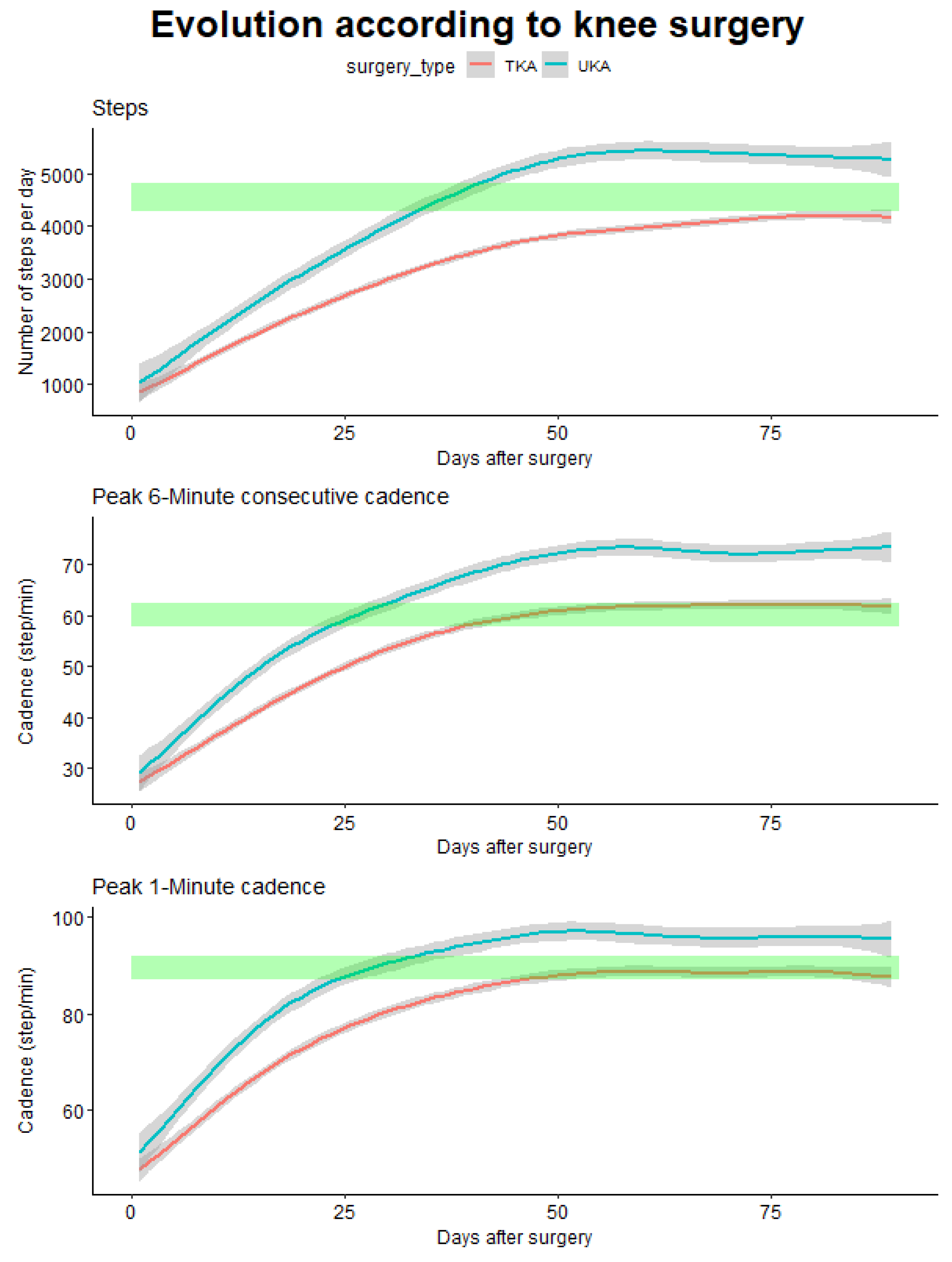
3.3.2. According to the Type of Surgery
For patients with knee prosthesis, we were able to perform comparisons according to the type of surgery, which is an important point given the different clinics and symptomatology’s of these patients. There was no statistically significant difference in age between both interventions (63.7 (6.2) and 62.7 (7.4) for TKR and UKA intervention respectively, p = 0.44) and equal gender distribution (χ²=0.71, p = 0.70).
When comparing the time needed to get back to pre-operative values, important differences were observed for the computed outcomes (
Figure 7).
For the total number of steps only patients with UKA surgery get back to their initial values after 36 days and then continue to progress above pre-operative values while patients with TKR barely get to their initial value after the 90 days. On the other hand, for the P6MC a difference of 16 days was observed (25 days for UKA and 41 days for TKR), as well as for the P1M where a difference of 23 days was observed (28 days for UKA and 51 days for TKR).
Again, when comparing the different outcomes to differentiate the two interventions we observed that early differences are detected using the P6MC and P1M in comparison with the total number of steps.
4. Discussion
4.1. Main results
The results of this study provide insights into the preoperative functional characteristics, variability of outcomes, and the evolution of activity parameters through the rehabilitation process in patients with hip and knee OA undergoing THA and TKA.
The initial noteworthy difference observed in preoperative parameters was the capacity of P6MC and P1M to distinguish between populations with knee and hip OA conditions. In contrast, the number of steps did not exhibit such discriminatory capacity. Gender-based analysis also revealed interesting nuances in preoperative characteristics. While no significant differences were observed in hip OA patients based on gender, significant differences were found in knee OA patients, including the total number of steps, P6MC cadence, P1M cadence, and age at surgery. Female knee OA patients exhibited higher values in these parameters. For both female and male patients, age and OA location showed significant effects on the total number of steps per day, P6MC cadence, and P1M cadence. These findings underscore the importance of considering age as a contributing factor in patients with hip and knee OA.
Intraweek variability analysis revealed notable differences among the studied parameters. While it is established that daily step count is correlated with cadence metrics [
28], our study revealed that the variability in the number of steps is twice as substantial as that observed in cadence metrics. This suggests that cadence measurements as P6MC and P1M may offer more stable indicators of gross motor function compared to the total number of steps per day.
Concerning the time required to return to preoperative values based on the type of arthroplasty (THA or TKA). Significant differences were observed in the recovery trajectories, with the cadence metrics (P6MC and P1M) showing early identification of differences compared to the total number of steps per day. These results highlighted the potential of P6MC and P1M as sensitive measures for assessing the rehabilitation progress in both THA and TKA patients. The median value for P1M was similar to normative value identified in older adults (106±16 and 97±20 for male and female respectively [
37]. Concerning the number of steps, our results aligned with patterns observed in numerous prior studies [
38]. Patients typically resumed their pre-operative activity levels approximately six to ten weeks after undergoing surgery, which is consistent with findings from previous accelerometry investigations [
19,
39]. Notably, patients who underwent TKA experienced a slower recovery compared to those who received partial knee arthroplasty. This outcome mirrors the trends observed in existing literature. Additionally, patients who underwent THA tended to recover gait quality more rapidly and demonstrated greater improvements compared to their pre-operative levels in line with previous studies, although most of these studies primarily focus on total step counts rather than walking session durations or patterns of activity accumulation in these patient populations [
40,
41].
The cadence is often regarded as a reasonable proxy-indicator of ambulatory intensity, with a cadence value of ≥100 steps/min in adults consistently identified as a heuristic for
‘good walking’ [
29,
42]. The newly introduced metric, P6MC, as compared to P1M and P30, exhibits an intriguing difference, wherein P6MC values are 20 to 30 steps lower than P1M, highlighting the distinction between these metrics. Considering established cadence thresholds: 40-59 for purposeful stepping, 60-79 for slow walking, 80-99 for medium walking, 100-119 for brisk walking, and >120 for fast walking [
29]; it becomes apparent that our OA population only demonstrated recovery in slow walking during the 6-minute duration, while brisk walking was restored within one minute. This could still be construed as a diminished functional capacity during the period assessed in this study.
Previous studies showed good correlation between P6MC cadence and the official P6MC distance, however, the direct transformation from cadence to distance is still challenging [
30]. The sensitivity of this outcome in detecting older individuals with functional capacity has been demonstrated [
43]. A gait cadence of 107 steps/min during a 6MWT has been identified as threshold to distinguish older adults with an inability to walk a minimum functional threshold (defined as 370 m) with reasonably high sensitivity (80%) and modest specificity (57%). Interestingly it has also been shown that the unsupervised version of the 6-minute walk test (random walk) give the same results as the official (supervised) 6-minute walk test [
44].
The utilization of wearable sensors for assessing knee arthroplasty procedures is becoming increasingly prevalent [
45]. However, the discernible clinical value of this technology is still a subject of ongoing investigation. One potential indicator under scrutiny is the variability in physical activity following surgery. Notably, patients exhibiting excessive variation in physical activity have been shown to experience more pronounced postoperative pain [
46].
It is noteworthy that only a limited number of patients with knee or hip OA adhere to the physical activity guidelines set by the WHO [
47]. In the context of TKA and THA, previous studies indicated a rise in light activity at 6 months postoperatively, with no concomitant change in moderate or vigorous intensity [
40]. In a comparative study the P1M improved from 70.0±23.7 preoperatively to 91.5±26 at 1 year postoperatively after TKA [
48]. Our investigation suggests that this improvement is realized much earlier than the conventional one-year postoperative timeframe. The exploration of variables related to physical activity holds significance, given that heightened physical activity has been associated with improvements in gait function after TKA. Notably, a suggested cutoff point of 3053 steps per day emerged as a potentially valuable predictive factor for gait function following TKA [
49].
Patients’ trajectories following arthroplasty display considerable variability, and extensive discussions have revolved around categorizing individuals as slow or quick progressors. Notably, these discussions have primarily centered on subjective questionnaires like patient reported outcome measures [
50]. The substantial variability in physical activity among patients has prompted calls for the development of stratification tools [
51]. In our study, we categorized the patient population based on MCID of the FJS at the three-month mark. This time point aligns with routine medical consultations and, in some countries, marks the conclusion of bundled payments. Our analysis demonstrates that activity data holds the potential to partially predict which patients will achieve this milestone early in the recovery process [
52].
4.2. Strengths and limitations
This study demonstrates several notable strengths that enhance its robustness and scientific significance. Foremost among these strengths is the study’s commitment to real-world relevance, evident in its recognition of the critical importance of assessing patients in ecological environment and conditions [
53,
54,
55,
56]. This approach stands in stark contrast to conventional clinical tests, providing a more ecologically valid perspective on the rehabilitation process.
A pivotal strength lies on the utilization of objective measurements facilitated by digital biomarkers collected through wearable sensors. This methodology not only ensures the continuous acquisition of real-time data but also offers a more nuanced and sensitive evaluation compared to subjective assessments by clinicians [
57,
58,
59]. Furthermore, the integration of technology enables continuous monitoring between sessions, affording the ability to detect even subtle changes in a patient’s status [
60,
61,
62]. This feature significantly enhances the precision of the rehabilitation process, ensuring a proactive response to evolving patient needs.
But the results of this study also have to be seen with various limitations. Firstly, we did not monitor wear time or adherence to wearable, this might have an impact on total number of steps, while it should have fewer impacts on cadence metrics. Secondly, concerning the P6MC, previous studies have shown that this could be aggregated as an unsupervised 6 minutes walking test. However, it cannot be sure that the activity was walking [
30,
63]. But the cumulative step count over the 6-minute duration provides valuable information about participants’ endurance and functional capacity. Thirdly, a notable study limitation is the potential variation in smartphone usage among participants. The study assumes a certain level of familiarity and consistent use of smartphones, which could introduce confounding factors if participants differ in their comfort and proficiency with such technology (i.e., digital literacy) [
64]. Additionally, the success of the proposed approach relies on the widespread adoption and accessibility of smartphones and wearable sensors, which may not be universally available to all individuals undergoing rehabilitation [
65]. This raises concerns about the generalizability of the study findings to diverse populations, potentially introducing biases based on socio-economic or demographic factors.
4.3. Future works
As various factors influencing activity recovery after surgery such as the surgical approach [
66], the use of crutches [
39], and BMI [
67] - it would be interesting to integrate these parameters in more complex model to better predict the evolution of the patients through the rehabilitation process.
Future research presents exciting opportunities for advancements and refinement of this methodology. Firstly, research into user engagement strategies with wearable devices could explore innovative approaches to enhance adherence without direct supervision. This could involve the development of user-friendly interfaces, personalized feedback systems, or even incorporating elements of gamification to promote sustained and consistent use.
Moreover, future research could focus on expanding the scope of wearable technology beyond wearables, smartphones, exploring alternative devices or communication methods to cater to individuals who may not have access to wearables. This inclusivity-driven approach would contribute to a more comprehensive understanding of the benefits and challenges associated with wearables in diverse populations.
To bolster the validity and reliability of wearable sensors, future research could delve into advancements in sensor technology, refining algorithms, and conducting thorough validation studies. Comparative assessments against established clinical measures can provide a clearer understanding of the accuracy and potential limitations of these devices.
Additionally, exploring the integration of artificial intelligence and machine learning algorithms for data analysis could open new avenues for deriving meaningful insights from the vast amount of data collected through wearables. These technologies could help identify patterns, predict rehabilitation progress, and tailor interventions based on individual patient needs.
5. Conclusions
This study explores the capacity for technology-assisted rehabilitation to bring about significant changes, particularly through the incorporation of automated, unsupervised evaluations utilizing wearable sensors in the rehabilitation of patients who have undergone hip and knee replacement surgeries and suffer from OA. The study emphasizes the constraints of conventional clinical evaluations and the changing nature of real-life rehabilitation encounters. Using wearable sensors provides a patient-centered and ecologically sound method, addressing the limitations of subjective evaluations conducted in clinics.
The results highlight the importance of cadence measurements, the P6MC and the P1M, in differentiating between individuals with hip and knee conditions and monitoring their recovery progress. These indicators demonstrate sensitivity and surpass the total step count, offering useful insights into the functional capacity and progress of patients throughout rehabilitation.
The use of technology-based, self-directed evaluations represents a fundamental change in rehabilitation methods, offering improved accuracy, tailored treatments, and heightened patient involvement.
Author Contributions
Conceptualization, JL and BB; methodology, JL, AP, BB; software, AP; formal analysis, KD, BB; data curation, AP, LD; writing—original draft preparation, JL, KD, BB; writing—review and editing, JL, KD, JM, HP, BB; visualization, KD, BB; supervision, BB. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Regulatory guidelines were followed with no involvement of institutional review board (IRB) approval as this study used anonymized patient-level data. This study was registered at Clinical Trials.gov (NCT06157190).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
JL, AP and LD are employee of the company who developed the recording device, the other authors declare no conflict of interest.
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.J.; Eyles, J.P.; Hunter, D.J. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv Ther 2016, 33, 1921–1946. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis: Literature Update. Curr Opin Rheumatol 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kontio, T.; Heliövaara, M.; Viikari-Juntura, E.; Solovieva, S. To What Extent Is Severe Osteoarthritis Preventable? Occupational and Non-Occupational Risk Factors for Knee and Hip Osteoarthritis. Rheumatology 2020, 59, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- van Doormaal, M.C.M.; Meerhoff, G.A.; Vliet Vlieland, T.P.M.; Peter, W.F. A Clinical Practice Guideline for Physical Therapy in Patients with Hip or Knee Osteoarthritis. Musculoskeletal Care 2020, 18, 575–595. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Goh, S.-L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Efficacy and Potential Determinants of Exercise Therapy in Knee and Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Ann Phys Rehabil Med 2019, 62, 356–365. [Google Scholar] [CrossRef]
- Wade, D.T. What Is Rehabilitation? An Empirical Investigation Leading to an Evidence-Based Description. Clin Rehabil 2020, 34, 571–583. [Google Scholar] [CrossRef]
- Bonnechère, B.; Sholukha, V.; Omelina, L.; Van Vooren, M.; Jansen, B.; Van Sint Jan, S. Suitability of Functional Evaluation Embedded in Serious Game Rehabilitation Exercises to Assess Motor Development across Lifespan. Gait Posture 2017, 57, 35–39. [Google Scholar] [CrossRef]
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-Term Unsupervised Mobility Assessment in Movement Disorders. Lancet Neurol 2020, 19, 462–470. [Google Scholar] [CrossRef]
- Chen, H.-M.; Chen, C.C.; Hsueh, I.-P.; Huang, S.-L.; Hsieh, C.-L. Test-Retest Reproducibility and Smallest Real Difference of 5 Hand Function Tests in Patients With Stroke. Neurorehabil Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef]
- Adans-Dester, C.; Hankov, N.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.; Zafonte, R.; Dy, J.; Lee, S.I.; Bonato, P. Enabling Precision Rehabilitation Interventions Using Wearable Sensors and Machine Learning to Track Motor Recovery. NPJ Digit Med 2020, 3, 121. [Google Scholar] [CrossRef]
- Lin, D.J.; Stein, J. Stepping Closer to Precision Rehabilitation. JAMA Neurol 2023, 80, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Berkemeyer, K.; Wijndaele, K.; White, T.; Cooper, A.J.M.; Luben, R.; Westgate, K.; Griffin, S.J.; Khaw, K.T.; Wareham, N.J.; Brage, S. The Descriptive Epidemiology of Accelerometer-Measured Physical Activity in Older Adults. Int J Behav Nutr Phys Act 2016, 13, 2. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Papapetropoulos, S.; Xiong, M.; Kieburtz, K. The First Frontier: Digital Biomarkers for Neurodegenerative Disorders. Digital Biomarkers 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Adams, J.L.; Dinesh, K.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Sheth, N.; Aranyosi, A.J.; Zhu, W.; Goldenthal, S.; Biglan, K.M.; et al. Multiple Wearable Sensors in Parkinson and Huntington Disease Individuals: A Pilot Study in Clinic and at Home. Digital Biomarkers 2017, 1, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Crizer, M.P.; Kazarian, G.S.; Fleischman, A.N.; Lonner, J.H.; Maltenfort, M.G.; Chen, A.F. Stepping Toward Objective Outcomes: A Prospective Analysis of Step Count After Total Joint Arthroplasty. The Journal of Arthroplasty 2017, 32, S162–S165. [Google Scholar] [CrossRef]
- Lyman, S.; Hidaka, C.; Fields, K.; Islam, W.; Mayman, D. Monitoring Patient Recovery After THA or TKA Using Mobile Technology. HSS Jrnl 2020, 16, 358–365. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, W.; McDonough, D.J.; Zeng, N.; Lee, J.E. The Dilemma of Analyzing Physical Activity and Sedentary Behavior with Wrist Accelerometer Data: Challenges and Opportunities. JCM 2021, 10, 5951. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Beer, Y.; Mor, A.; Elbaz, A. Smartphone-Based Inertial Sensors Technology - Validation of a New Application to Measure Spatiotemporal Gait Metrics. Gait Posture 2022, 93, 102–106. [Google Scholar] [CrossRef]
- Brooks, G.C.; Vittinghoff, E.; Iyer, S.; Tandon, D.; Kuhar, P.; Madsen, K.A.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Accuracy and Usability of a Self-Administered 6-Minute Walk Test Smartphone Application. Circ Heart Fail 2015, 8, 905–913. [Google Scholar] [CrossRef]
- Longo, U.G.; De Salvatore, S.; Piergentili, I.; Indiveri, A.; Di Naro, C.; Santamaria, G.; Marchetti, A.; Marinis, M.G.D.; Denaro, V. Total Hip Arthroplasty: Minimal Clinically Important Difference and Patient Acceptable Symptom State for the Forgotten Joint Score 12. IJERPH 2021, 18, 2267. [Google Scholar] [CrossRef]
- Clement, N.D.; Scott, C.E.H.; Hamilton, D.F.; MacDonald, D.; Howie, C.R. Meaningful Values in the Forgotten Joint Score after Total Knee Arthroplasty: Minimal Clinical Important Difference, Minimal Important and Detectable Changes, and Patient-Acceptable Symptom State. The Bone & Joint Journal 2021, 103-B, 846–854. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Rowe, D.A. Using Cadence to Study Free-Living Ambulatory Behaviour. Sports Med 2012, 42, 381–398. [Google Scholar] [CrossRef]
- Kang, M.; Kim, Y.; Rowe, D.A. Measurement Considerations of Peak Stepping Cadence Measures Using National Health and Nutrition Examination Survey 2005–2006. Journal of Physical Activity and Health 2016, 13, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Barreira, T.V.; Katzmarzyk, P.T.; Johnson, W.D.; Tudor-Locke, C. Cadence Patterns and Peak Cadence in US Children and Adolescents: NHANES, 2005-2006. Med Sci Sports Exerc 2012, 44, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.M.; Gibson, A.L.; Kang, H.; Zuhl, M.N.; Sharma, H.; Blair, C.K. Self-Selected Walking Cadence after 16-Week Light-Intensity Physical Activity Intervention for Older Cancer Survivors. International Journal of Environmental Research and Public Health 2022, 19, 4768. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Camhi, S.M.; Leonardi, C.; Johnson, W.D.; Katzmarzyk, P.T.; Earnest, C.P.; Church, T.S. Patterns of Adult Stepping Cadence in the 2005-2006 NHANES. Prev Med 2011, 53, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sokas, D.; Paliakaitė, B.; Rapalis, A.; Marozas, V.; Bailón, R.; Petrėnas, A. Detection of Walk Tests in Free-Living Activities Using a Wrist-Worn Device. Front Physiol 2021, 12, 706545. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, E.J.; Mora-Gonzalez, J.; Ducharme, S.W.; Moore, C.C.; Gould, Z.R.; Chase, C.J.; Amalbert-Birriel, M.A.; Chipkin, S.R.; Staudenmayer, J.; Zheng, P.; et al. Cadence-Based Classification of Moderate-Intensity Overground Walking in 41- to 85-Year-Old Adults. Scand J Med Sci Sports 2023, 33, 433–443. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A.; et al. Physical Activity and Public Health: Updated Recommendation for Adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef]
- WHO Global Status Report on Physical Activity 2022 2022.
- Di Brisco, A.M.; Migliorati, S. A New Mixed-effects Mixture Model for Constrained Longitudinal Data. Statistics in Medicine 2020, 39, 129–145. [Google Scholar] [CrossRef]
- Herle, M.; Micali, N.; Abdulkadir, M.; Loos, R.; Bryant-Waugh, R.; Hübel, C.; Bulik, C.M.; De Stavola, B.L. Identifying Typical Trajectories in Longitudinal Data: Modelling Strategies and Interpretations. Eur. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Barreira, T.V.; Brouillette, R.M.; Foil, H.C.; Keller, J.N. Preliminary Comparison of Clinical and Free-Living Measures of Stepping Cadence in Older Adults. J Phys Act Health 2013, 10, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Castro, A.L.; Surmacz, K.; Aguilera-Canon, M.C.; Anderson, M.B.; Van Andel, D.; Redfern, R.E.; Cook, C.E. Early Post-Operative Walking Bouts Are Associated with Improved Gait Speed and Symmetry at 90 Days. Gait & Posture 2023. [Google Scholar] [CrossRef]
- Lebleu, J.; Poilvache, H.; Mahaudens, P.; De Ridder, R.; Detrembleur, C. Predicting Physical Activity Recovery after Hip and Knee Arthroplasty? A Longitudinal Cohort Study. Braz J Phys Ther 2021, 25, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, E.; McVeigh, J.A.; van der Jagt, D.; Mokete, L.; Kaoje, Y.S.; Tikly, M.; Meiring, R.M. Light Intensity Physical Activity Increases and Sedentary Behavior Decreases Following Total Knee Arthroplasty in Patients with Osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2019, 27, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.B.; Chesworth, B.; Davis, A.; Mahomed, N.; Charron, K. Comparing Patient Outcomes After THA and TKA: Is There a Difference? Clinical Orthopaedics & Related Research 2010, 468, 542–546. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Han, H.; Aguiar, E.J.; Barreira, T.V.; Schuna, J.M.; Kang, M.; Rowe, D.A. How Fast Is Fast Enough? Walking Cadence (Steps/Min) as a Practical Estimate of Intensity in Adults: A Narrative Review. Br J Sports Med 2018, 52, 776–788. [Google Scholar] [CrossRef]
- Rubin, D.S.; Ranjeva, S.L.; Urbanek, J.K.; Karas, M.; Madariaga, M.L.L.; Huisingh-Scheetz, M. Smartphone-Based Gait Cadence to Identify Older Adults with Decreased Functional Capacity. Digit Biomark 2022, 6, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Scherrenberg, M.; Bonneux, C.; Yousif Mahmood, D.; Hansen, D.; Dendale, P.; Coninx, K. A Mobile Application to Perform the Six-Minute Walk Test (6MWT) at Home: A Random Walk in the Park Is as Accurate as a Standardized 6MWT. Sensors 2022, 22, 4277. [Google Scholar] [CrossRef]
- Small, S.R.; Bullock, G.S.; Khalid, S.; Barker, K.; Trivella, M.; Price, A.J. Current Clinical Utilisation of Wearable Motion Sensors for the Assessment of Outcome Following Knee Arthroplasty: A Scoping Review. BMJ Open 2019, 9, e033832. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kako, M.; Suzuki, K.; Takagi, Y.; Terai, C.; Yasuda, S.; Kadono, I.; Seki, T.; Hiraiwa, H.; Ushida, T.; et al. Impact of Variation in Physical Activity after Total Joint Replacement. J Pain Res 2018, 11, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.A.; Webster, K.E.; Levinger, P.; Taylor, N.F. What Proportion of People with Hip and Knee Osteoarthritis Meet Physical Activity Guidelines? A Systematic Review and Meta-Analysis. Osteoarthritis Cartilage 2013, 21, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Webber, S.C.; Strachan, S.M.; Pachu, N.S. Sedentary Behavior, Cadence, and Physical Activity Outcomes after Knee Arthroplasty. Med Sci Sports Exerc 2017, 49, 1057–1065. [Google Scholar] [CrossRef]
- Taniguchi, M.; Sawano, S.; Kugo, M.; Maegawa, S.; Kawasaki, T.; Ichihashi, N. Physical Activity Promotes Gait Improvement in Patients With Total Knee Arthroplasty. The Journal of Arthroplasty 2016, 31, 984–988. [Google Scholar] [CrossRef]
- Hesseling, B.; Mathijssen, N.M.C.; van Steenbergen, L.N.; Melles, M.; Vehmeijer, S.B.W.; Porsius, J.T. Fast Starters, Slow Starters, and Late Dippers: Trajectories of Patient-Reported Outcomes After Total Hip Arthroplasty: Results from a Dutch Nationwide Database. J Bone Joint Surg Am 2019, 101, 2175–2186. [Google Scholar] [CrossRef]
- Luna, I.E.; Kehlet, H.; Wede, H.R.; Hoevsgaard, S.J.; Aasvang, E.K. Objectively Measured Early Physical Activity after Total Hip or Knee Arthroplasty. J Clin Monit Comput 2019, 33, 509–522. [Google Scholar] [CrossRef]
- Carmichael, H.; Overbey, D.M.; Hosokawa, P.; Goode, C.M.; Jones, T.S.; Barnett, C.C.; Jones, E.L.; Robinson, T.N. Wearable Technology-A Pilot Study to Define “Normal” Postoperative Recovery Trajectories. J Surg Res 2019, 244, 368–373. [Google Scholar] [CrossRef]
- Bonnechère, B.; Timmermans, A.; Michiels, S. Current Technology Developments Can Improve the Quality of Research and Level of Evidence for Rehabilitation Interventions: A Narrative Review. Sensors (Basel) 2023, 23, 875. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A.; Shah, R.F.; Bendich, I.; Patterson, J.T.; Hwang, K.M.; Zaid, M.B. Machine Learning Algorithms Can Use Wearable Sensor Data to Accurately Predict Six-Week Patient-Reported Outcome Scores Following Joint Replacement in a Prospective Trial. J Arthroplasty 2019, 34, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Emmerzaal, J.; De Brabandere, A.; van der Straaten, R.; Bellemans, J.; De Baets, L.; Davis, J.; Jonkers, I.; Timmermans, A.; Vanwanseele, B. Can the Output of a Learned Classification Model Monitor a Person’s Functional Recovery Status Post-Total Knee Arthroplasty? Sensors 2022, 22, 3698. [Google Scholar] [CrossRef] [PubMed]
- Babaei, N.; Hannani, N.; Dabanloo, N.J.; Bahadori, S. A Systematic Review of the Use of Commercial Wearable Activity Trackers for Monitoring Recovery in Individuals Undergoing Total Hip Replacement Surgery. Cyborg Bionic Syst 2022, 2022, 9794641. [Google Scholar] [CrossRef]
- Park, C.; Mishra, R.; Sharafkhaneh, A.; Bryant, M.S.; Nguyen, C.; Torres, I.; Naik, A.D.; Najafi, B. Digital Biomarker Representing Frailty Phenotypes: The Use of Machine Learning and Sensor-Based Sit-to-Stand Test. Sensors (Basel) 2021, 21, 3258. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Lin, E.; Galea, V.; Mathew, A.J.; Panda, N.; Vetter, I.; Haynes, A.B. Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review. J Pers Med 2020, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Motahari-Nezhad, H.; Al-Abdulkarim, H.; Fgaier, M.; Abid, M.M.; Péntek, M.; Gulácsi, L.; Zrubka, Z. Digital Biomarker-Based Interventions: Systematic Review of Systematic Reviews. J Med Internet Res 2022, 24, e41042. [Google Scholar] [CrossRef]
- Hoogland, J.; Wijnen, A.; Munsterman, T.; Gerritsma, C.L.; Dijkstra, B.; Zijlstra, W.P.; Annegarn, J.; Ibarra, F.; Zijlstra, W.; Stevens, M. Feasibility and Patient Experience of a Home-Based Rehabilitation Program Driven by a Tablet App and Mobility Monitoring for Patients After a Total Hip Arthroplasty. JMIR Mhealth Uhealth 2019, 7, e10342. [Google Scholar] [CrossRef]
- Dias Correia, F.; Nogueira, A.; Magalhães, I.; Guimarães, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Pires, J.; Seabra, R.; et al. Digital Versus Conventional Rehabilitation After Total Hip Arthroplasty: A Single-Center, Parallel-Group Pilot Study. JMIR Rehabil Assist Technol 2019, 6, e14523. [Google Scholar] [CrossRef]
- Bell, K.M.; Onyeukwu, C.; Smith, C.N.; Oh, A.; Devito Dabbs, A.; Piva, S.R.; Popchak, A.J.; Lynch, A.D.; Irrgang, J.J.; McClincy, M.P. A Portable System for Remote Rehabilitation Following a Total Knee Replacement: A Pilot Randomized Controlled Clinical Study. Sensors 2020, 20, 6118. [Google Scholar] [CrossRef] [PubMed]
- Kontaxis, S.; Laporta, E.; Garcia, E.; Martinis, M.; Leocani, L.; Roselli, L.; Buron, M.D.; Guerrero, A.I.; Zabala, A.; Cummins, N.; et al. Automatic Assessment of the 2-Minute Walk Distance for Remote Monitoring of People with Multiple Sclerosis. Sensors 2023, 23, 6017. [Google Scholar] [CrossRef] [PubMed]
- Verweel, L.; Newman, A.; Michaelchuk, W.; Packham, T.; Goldstein, R.; Brooks, D. The Effect of Digital Interventions on Related Health Literacy and Skills for Individuals Living with Chronic Diseases: A Systematic Review and Meta-Analysis. Int J Med Inform 2023, 177, 105114. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B.; Kossi, O.; Mapinduzi, J.; Panda, J.; Rintala, A.; Guidetti, S.; Spooren, A.; Feys, P. Mobile Health Solutions: An Opportunity for Rehabilitation in Low- and Middle Income Countries? Front Public Health 2022, 10, 1072322. [Google Scholar] [CrossRef]
- Toogood, P.A.; Abdel, M.P.; Spear, J.A.; Cook, S.M.; Cook, D.J.; Taunton, M.J. The Monitoring of Activity at Home after Total Hip Arthroplasty. Bone Joint J 2016, 98-B, 1450–1454. [Google Scholar] [CrossRef]
- Twiggs, J.; Salmon, L.; Kolos, E.; Bogue, E.; Miles, B.; Roe, J. Measurement of Physical Activity in the Pre- and Early Post-Operative Period after Total Knee Arthroplasty for Osteoarthritis Using a Fitbit Flex Device. Medical Engineering & Physics 2018, 51, 31–40. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).