1. Introduction
Over the last several decades, more sustainable plastics have been developed with the objective of reducing the use of non-renewable resources in their production. This is due to increased ecological knowledge about the need to reduce the environmental impact that conventional plastics have on the environment (
Figure 1). The goal of sustainable development is to replace conventional petroleum-based polymers with more environmentally friendly materials in a variety of industrial sectors. As a result, increased production and use of bio-based polymers in recent years has placed biopolymers as one of the most promising ways to achieve this goal. Valorization of agro-food wastes, biopolymers produced by yeast, algae, or bacterial fermentation, as well as those extracted directly from biomass, such as polysaccharides, proteins, and lipids, have all been discussed and created a lot of interest, especially for medical products, food packaging, agricultural films, membrane process applications, sustainable clothing, and other areas that, despite recent successful developments in bio-based polymers, continue to require improvement[
1].
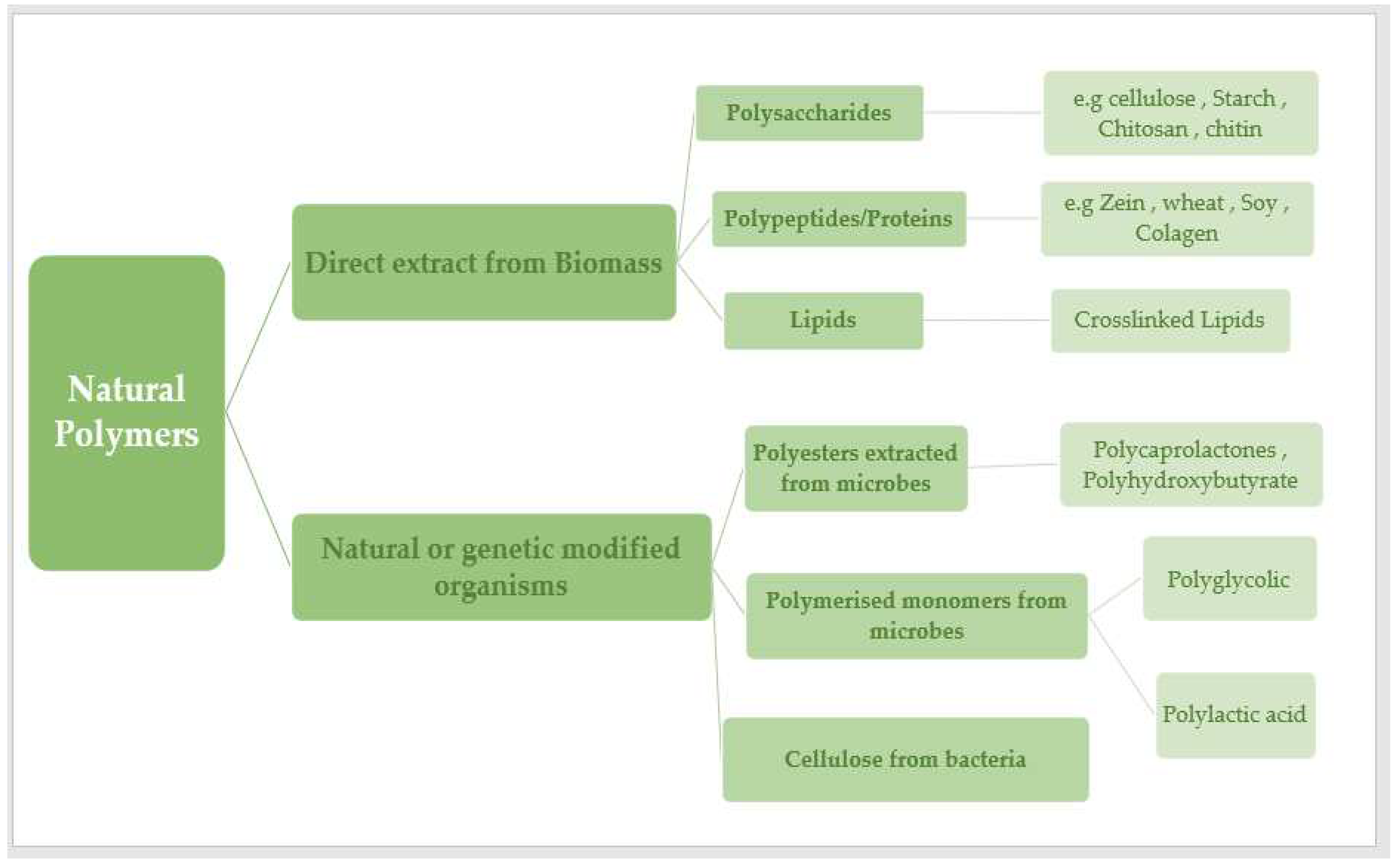
Depending on their natural origin, natural polymers can be divided into six major groups: polysaccharides, proteins, polynucleotides, polyesters, lignin, and polyisopreness [
2].
Polysaccharides and polypeptides are frequently employed as drug carriers in cancer therapy. Excellent biocompatibility, accessibility, stability, non-toxicity, cost-effectiveness, and biodegradability are a part of natural polymers, particularly those based on polysaccharides [
3].Several polysaccharides, including starch, dextran, pullulan, alginate, pectin, chitin, chitosan, hyaluronic acid, albumin, gelatin, and guar gum, are employed to produce nanocarriers for cancer therapy, according to the literature.
Polyelectrolyte complexation, hydrophobically modified polymer self-assembly, covalent or ionic cross-linking, and other processes are used to create polysaccharide-based nanoparticles [
4]. Several natural polymers have been found to exhibit anticancer properties on their own. Chitosan, for examples, has anticancer properties because it may perforate the membranes of tumor cells and induce apoptosis. Several papers have extensively investigated the anticancer potential of chitosan against sarcomas and hepatocarcinomas [
5]. Less soluble treatments have been attached to and transported to tumor locations using natural polymers as a basis.
The high degree of flexibility of these polymers offers the possibility of reducing drugs loss and toxicity to healthy tissue.
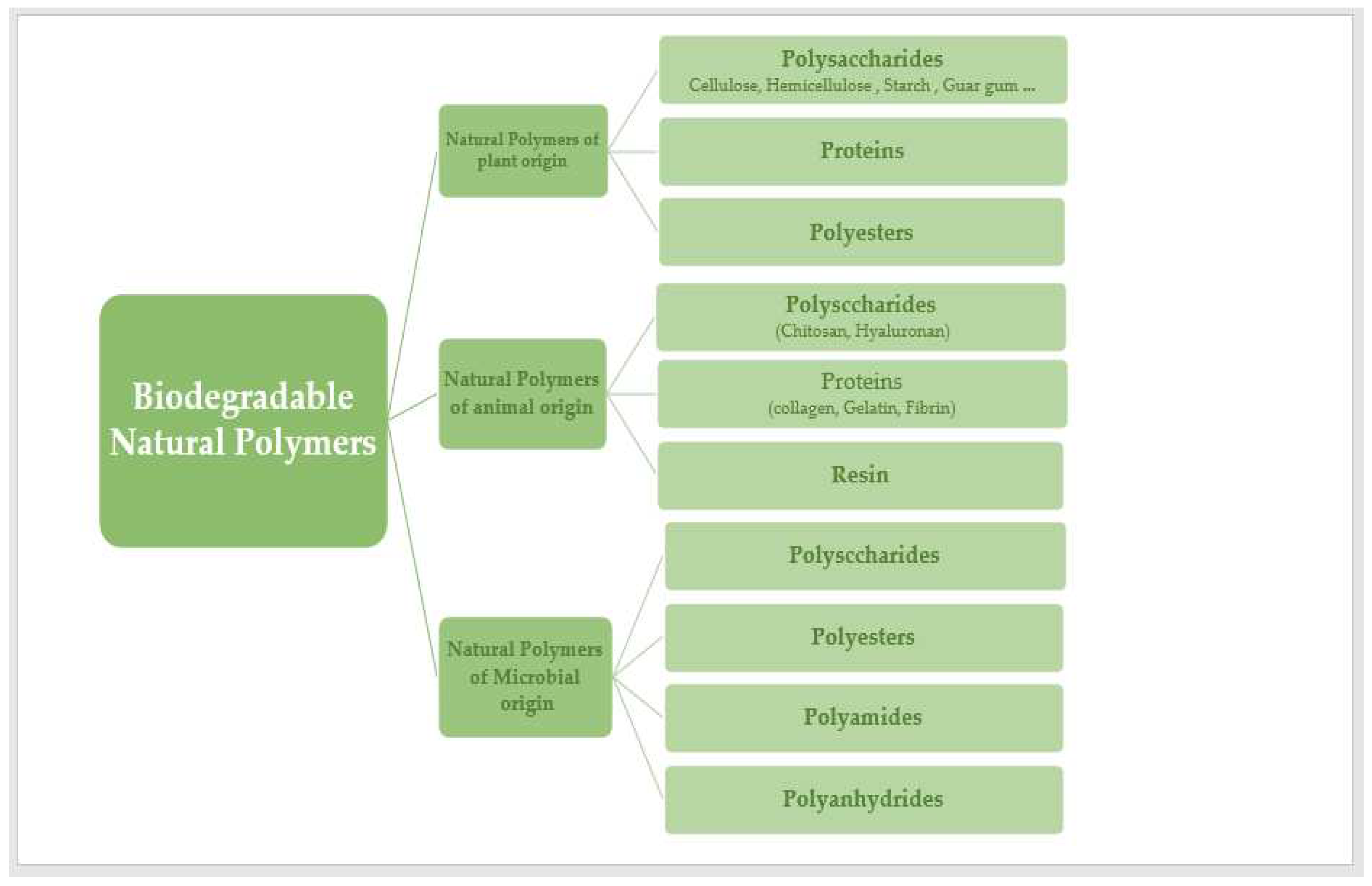
Most naturally occurring polymers are condensation polymers, which form rapidly as a byproduct of the joining of monomer units to produce small molecules (often water). Other polymers are those that are formed without by-products by mixing the monomer units that comprise the polymer. Polymers found in nature may be classified into six broad groups based on their origins: polyesters, lignin, polyisoprenes, polysaccharides, polynucleotides, and proteins [
6]. Polysaccharides and proteins have both been extensively investigated in the DDS due to their biocompatibility and processability. Natural biopolymers can be produced by algae, plants, animals, bacteria, and microorganisms. This article discusses several polymers produced by plants, animals, and microorganisms (
Figure 2).
Excipients produced from natural polymers are used in pharmaceuticals. Anti-foaming agents, such as silicone, vegetable gums, cellulose, starch, and polyvinyl acetate (PVAc) are used in emulsions. Their major purpose is to prevent unfavourable organoleptic properties [
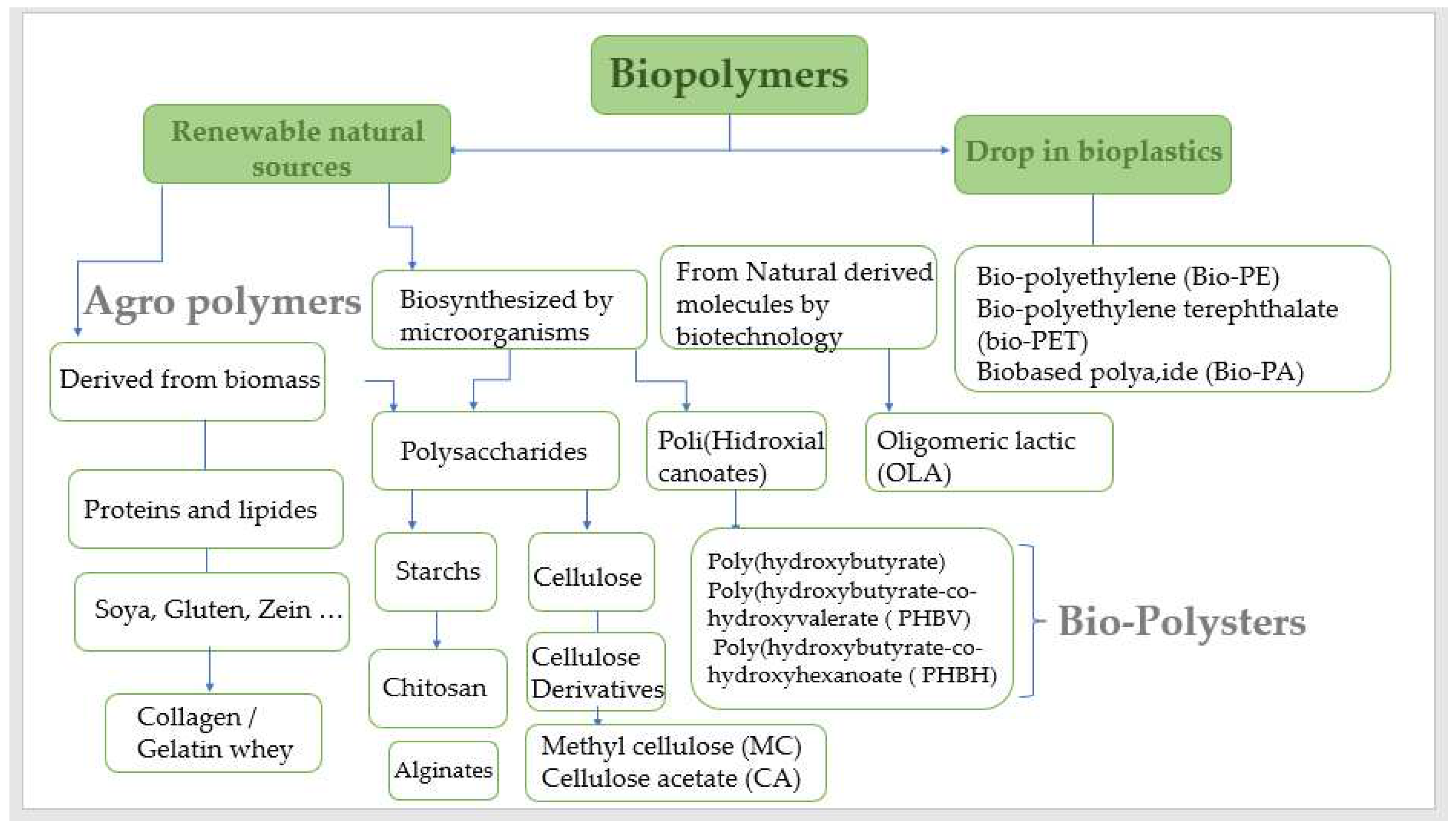
8]. There are three categories of biodegradable biopolymers derived from renewable resources:
1. Plant-based polymers, such as polysaccharides (such as cellulose, starch, chitin, and chitosan) and proteins. 2. Microorganisms derived, such as polyhydroxybutyrate (PHB). 3. Polymers are created indirectly through the polymerization of monomers. These polymers are frequently derived from plant resources such as lactic acid, are formed when sugars ferment or when reactive monomeric compounds derived from vegetable oils combine with sugars. A variety of biopolymers are employed in medical sectors. Polymers used in sutures and medical implants include polylactides (PLA), polyglycolides (PGA), and polylactide-coglycolide co-polymers (PLGA) [
9].
These biopolymers are non-toxic and well tolerated by the body. Other biopolymers suitable for pharmaceutical uses include cellulose, polyamino acids, and polyhydroxyal kanoates (PHA) [
10].
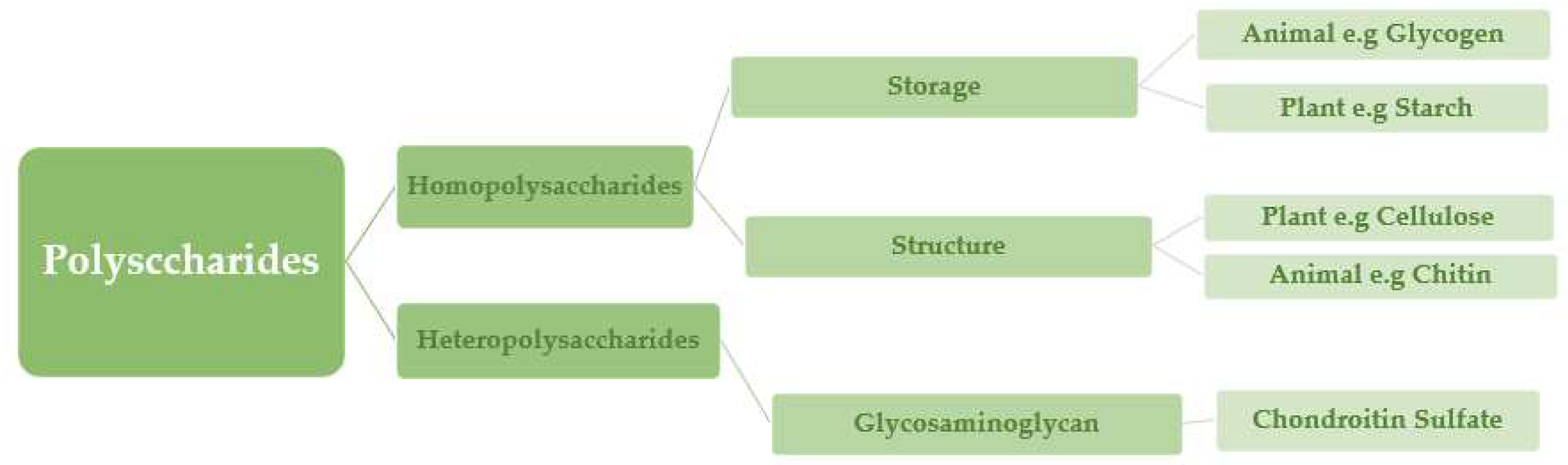
2. Classification of Natural Polysaccharides
Polysaccharides, which belong to the third main class of biopolymers (carbohydrates) (
Figure 3), are important for the immune system, blood coagulation, fertilization, pathogenesis prevention, and therapeutic effectiveness [
11].Structure support, energy storage, lubrication, and cell signal transduction are only a few of the biological functions that polysaccharides have an impact on in cells [
12].
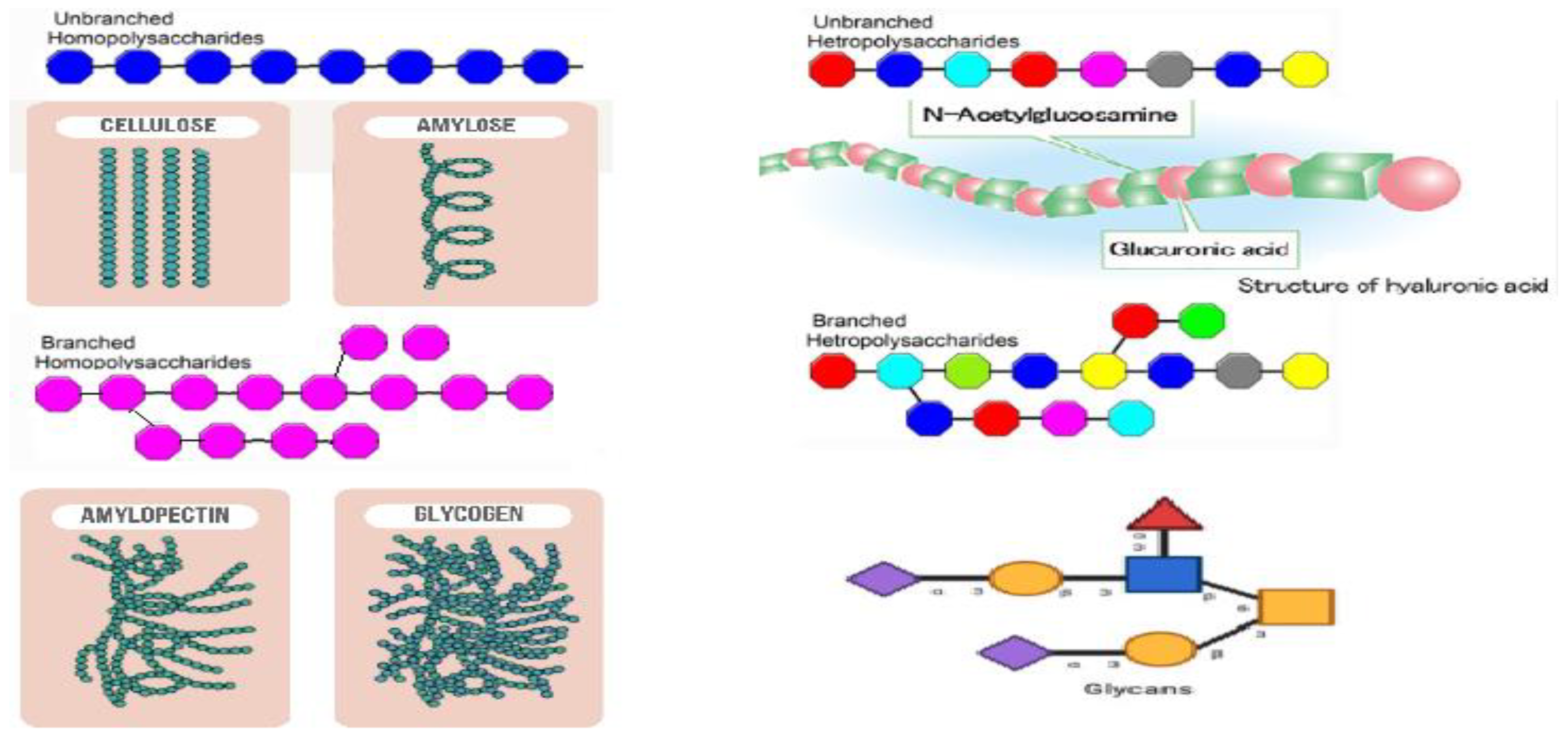
Based on their chemical structure, which consists of monosaccharide units joined by glycosidic linkages, polysaccharides—the most prevalent type of carbohydrates in nature—are categorized [
14].They may also establish covalent bonds with other structures like lipids, peptides, and amino acids. In contrast to heteropolysaccharides, which are heteroglycans made up of several monosaccharides, homopolysaccharides are homoglycans composed of the same monosaccharide (
Figure 4)[
13].
Acetic linkages are used to join homopolymers of glucose or amino sugars. Polysaccharides are the world's most abundant renewable resource, according to current statistics. Each year, photosynthesis produces several orders of magnitude more carbohydrate than is generated artificially [
16].
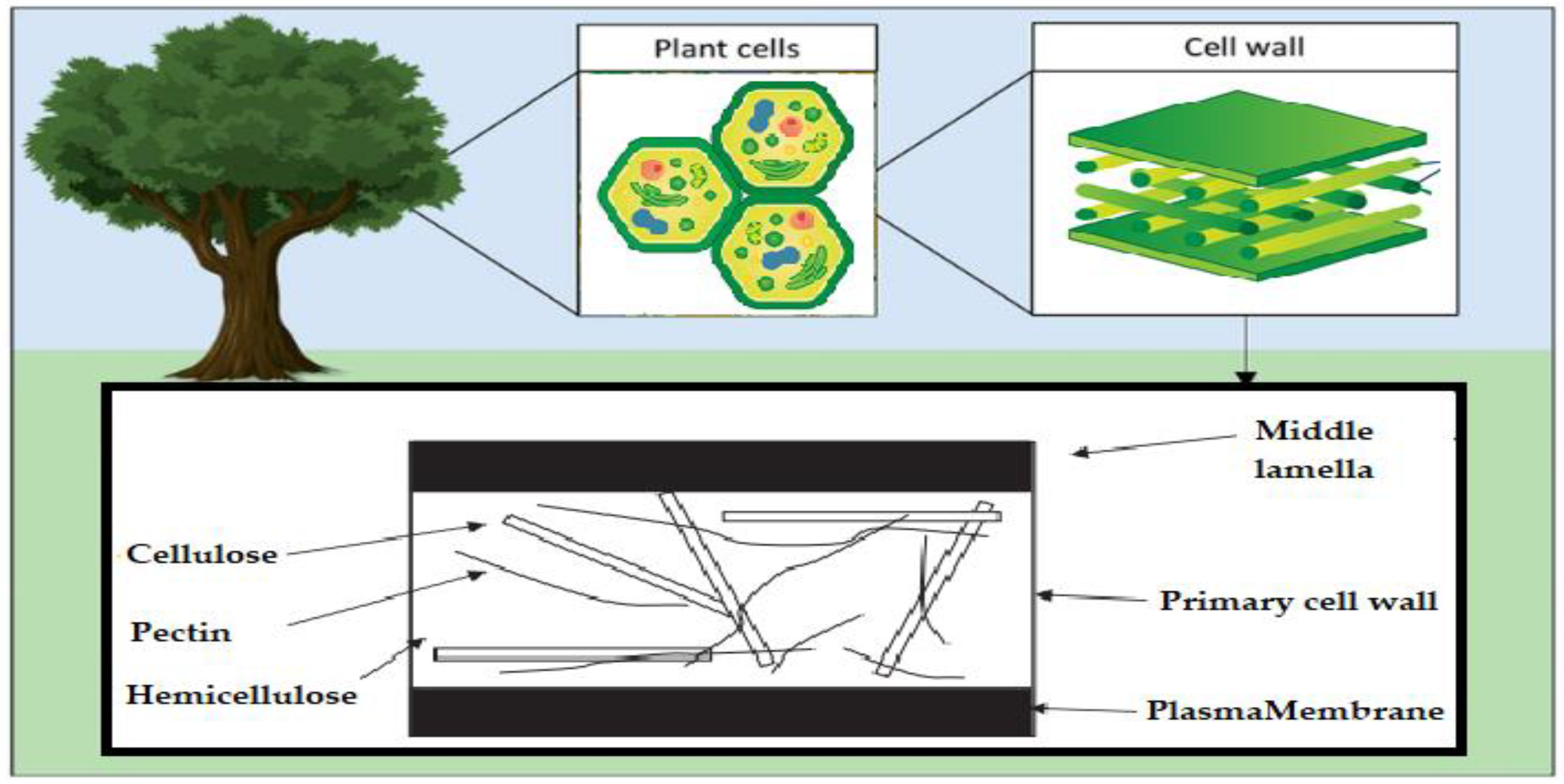
Figure 5.
Schematic of the polysaccharide components of the plant cell wall.
Figure 5.
Schematic of the polysaccharide components of the plant cell wall.
Polysaccharides are classified into various classes based on their structure or function (
Table 1). There are three basic categories: Polysaccharides include structural polysaccharides like cellulose and chitin, storage polysaccharides like starch and glycogen, and gel-forming polysaccharides like alginic acid and mucopolysaccharides. Ionic or nonionic (cationic and anionic) polymers can be found as well as branched or straight-chained polymers [
17].
Natural polysaccharides, such as cellulose (
Figure 6), have stability and physical structure, which adds to their ability to retain food, such as starch [
14]. Various examples of negatively charged polysaccharides are pectin, heparin, hyaluronic acid, and alginate. Chitosan is a polysaccharide that is positively charged, whereas pectin, pectinol, and alginate are polyelectrolyte-based polysaccharides [
13].
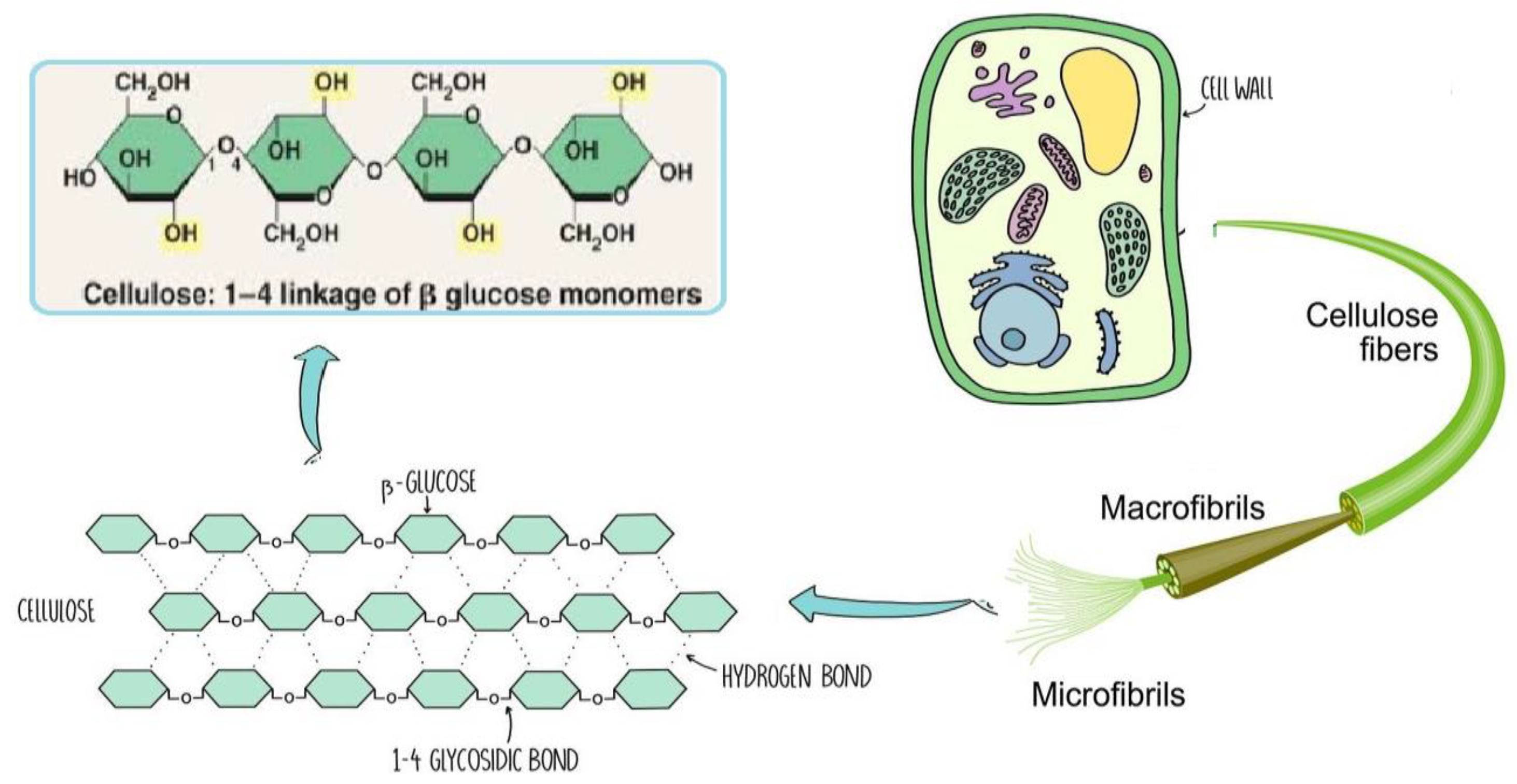
2.1. Cellulose
Since it constitutes the majority of plant cell walls and almost half of the biomass in photosynthetic species, cellulose may be the most common chemical on the planet. Several materials, including cotton, linen, wood, hemp, jute, kenaf, sugar beet cereal straws, and flax, have long been solid sources of cellulose. Other cellulose sources include bacteria (such as
Acetobacter), algae ( like
Valonia and
Microdicyon), and marine animals of the
Ascite family [
18].
The chemical structure of cellulose is illustrated in
Figure 7 as having three hydroxyl groups per AGU, with the exception of the terminal ends. These hydroxyl groups collaborate to generate intra- and inter-hydroxyl hydrogen bonds, resulting in a supramolecular polymer with crystalline (ordered) sections [
19]. When cellulose molecules are randomly organised, they form amorphous patches that connect the ordered crystalline areas together [
20] .The structure of cellulose has a significant impact on its reactivity. The hydroxyl groups on the surface of the cellulose molecule can create both intramolecular and intermolecular hydrogen bonds [
21]. Many of the physical and chemical features of the polymeric chains are dictated by the hydrogen bonds formed between them, which may increase their linear integrity. Nonetheless, cellulose has several intrinsic disadvantages, including low crease resistance, poor solvent solubility, and a lack of thermoplasticity. To improve the properties of cellulose, controlled chemical or physical surface modification is necessary [
22]. The biosynthesized component of natural cellulose, in general, cannot be processed as a synthetic polymer [
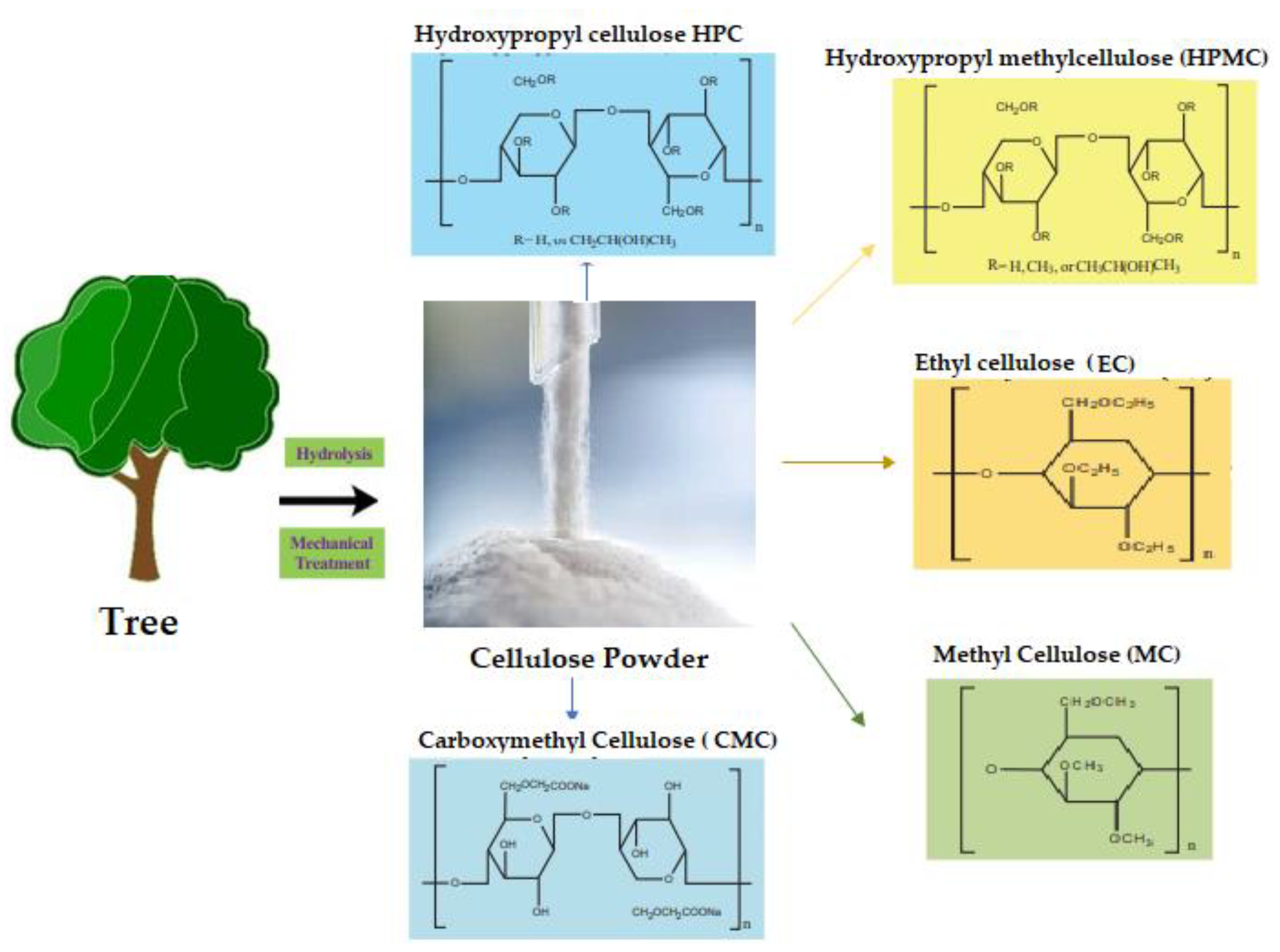
23]. One proposed solution is to surface-functionalize cellulose molecules with foreign groups. This enables the cellulose surface chemistry to be modified without affecting the drug's various critical intrinsic properties, including self-assembly, controlled dispersion inside a variety of matrix polymers, and increasing particle-particle and particle-matrix bond strengths. Numerous drug delivery methods use conventional cellulose and its derivatives, including cellulose ethers, cellulose esters, and oxycellulose. Sodium carboxymethyl cellulose (CMC), methylcellulose (MC), ethylcellulose (EC), hydroxypropyl cellulose (HPC), and hydroxypropyl methyl cellulose (HPMC) are among the several cellulose ethers that can be found in nature (
Figure 8) [
24].
2.2. Hemicelluloses
Another important component of plant cells is hemicelluloses, which form a matrix around cellulose microfibrils, are composed of a variety of molecules, including xyloglucans, xylans, mannans, and (1-3)-(1-4)-glucans [
26,
27,
28].Chemical interactions like hydrogen bonds and van der Waals forces are frequently used to link them to the cellulose microfibrils. Hemicelluloses also function as metabolic reserves and signalling molecules in cells [
29], with a global yearly production of over 60 billion tonnes, is the second-most abundant renewable component of lignocellulosic biomass after cellulose [
30,
31].
2.3. Pectin
Plant cell walls also contain cellulose, hemicelluloses, and lignin in addition to pectin. It belongs to the same group of polysaccharides as agar and mucopolysaccharides which form gels [
32]. One of the most crucial hydrocolloids for industry is pectin, which is employed in many different food products. Citrus and apple pectin are the main industrial sources of high-methoxyl (>50%) pectin, whereas sunflower pectin is a naturally occurring source of low-methoxyl (50%) pectin [
33].
2.4. Starch
Pure starch is an odourless white powder. The polysaccharide is composed of the molecules of amylose and amylopectin. Each has a varied percentage depending on the source and kind of starch, although amylose and amylopectin generally account for 20-25% and 75-80% of total starch, respectively [
34]. Starch, which is present in fruits, seeds, roots in the form of grains in leaves, tubers, stem core, and rhizomes, is the most significant polysaccharide for storing energy in plants [
35,
36,
37]. Similar to potatoes, rice, wheat, maize, and cassava, it constitutes the majority of the human diet's carbohydrate intake [
38].
2.5. Glycogen
Another type of carbohydrate that stores energy is called glycogen. It similar to other storage polysaccharides like amylopectin [
17] but is more compacted and branched. In general, the cytoplasm of animal cells contains glycogen, which is a type of glucose. Although glycogen is essential for physiological metabolism, it has no industrial use and is only included here for completeness' purposes [
39].
2.6. Chitin
Chitin is a linear polysaccharide obtained from animal that is extremely hydrophobic and includes acetyl and amino groups inside its unit. It is insoluble in water and conventional organic solvents, but soluble in specialised solvents such as dimethylacetamide with 5% lithium chloride and chloroalcohols in aqueous mineral acid solutions. Another frequent structural polysaccharide, it may be found in fungi's mycelia and spores, as well as the exoskeletons of insects, crustaceans, and other invertebrates [
40,
41].
2.7. Hyaluronic Acid
Hyaluronic acid is one type of glycoprotein, known as mucopolysaccharides or mucins. In comparison to other polysaccharides like heparin, it is one among the few polysaccharides discovered in vertebrate tissue and is more frequently found in developing embryos. These are polysaccharides that have been covalently bonded to proteins. Proteoglycans are one of the others. Hyaluronic acid, frequently known as hyaluronan, is a straight-chain polysaccharide with a molecular mass of about 7 × 10
6 g/mol that is frequently tightly linked to proteins by a hydrogen bond such those of water [
42]. As a result, extraction and isolation are difficult tasks. It has certain biological applications; such as being used in surgical applications with alginate to improve wound healing [
43,
44].
2.8. Alginate
The seaweed alginate is a long-chain hydrophilic polymer that gives flexibility and strength to the cell walls of the algae. Since 600 B.C., it has been used as food.
However, it wasn't until 1896 that Akrefting was able to effectively extract pure alginate from seaweed. For the first time, alginate was used as a stabilising component in ice cream by the company Kelco in 1929, and it became a commercially accessible product [
40]. Alginate is a safe and natural food ingredient. Alginate has excellent biological properties compared to other seaweed polysaccharides, including ion cross-linking, pH sensitivity, biocompatibility, and biodegradability, which have been extensively used in the food and nutraceutical industries. Alginate is the only polysaccharide with carboxyl groups naturally present in each component residue [
45].
3. Processing and Characterization of Natural Polymers
The two basic steps in polymer processing are the conversion of polymers into final products with the necessary structure, such as scaffolds, microneedles, and food packaging sheets, as well as the conversion of polymers into powders and pellets for additional processing. In contrast to the former, which may include extrusion and mixing, the latter may make use of processes like injection molding or film casting. This chapter will go through these concepts. It is necessary to characterise polymers in order to determine their properties such as mechanical strength, thermal conductivity, microstructure, and density, as well as their utility, quality assurance, and safety [
46].
3.1. Blends and Composites
Polymers can be employed more widely as fillers or as matrices in blends and composites. A composite is a system made up of two or more different elements linked together to form a multiphase system with many components while the individual elements maintain their physical and chemical identities. A composite is made up of a filler, which may be a woven fabric, metal, ceramic, or polymer fiber, and a polymer matrix. As a result, polymers can serve as a matrix or filler in composites. Because simpler, mono-component synthetic or natural materials cannot give the needed physical and/or physicochemical qualities, these systems are required [
15].
Polymers can be employed more widely as fillers or as matrices in blends and composites. A composite is a system made up of two or more different elements linked together to form a multiphase system with many components while the individual elements maintain their physical and chemical identities. A composite is made up of a filler, which may be a woven fabric, metal, ceramic, or polymer fiber, and a polymer matrix. Polymers can therefore be used in composites as a matrix or filler. Because simpler, mono-component synthetic or natural materials cannot give the needed physical and/or physicochemical qualities, these systems are required [
47].
Composites can be made from polymers, both synthetic and natural. Natural and synthetic polymers are blended to develop a new breed of materials with more diverse properties that may be used in a wider range of applications. The production of synthetic polymers, while offering advantages like product consistency and ease of production, has also raised environmental concerns due to their nonbiodegradability and potential toxicity, which has led to an increase in the popularity of natural polymer-based composites in recent years. When two or more polymers are physically combined, either while molten or while dissolving in a suitable solvent, blends are created. Different polymer blends can be used to create a variety of structures, including molecular composites, miscible one-phase and separated phase, compatible and incompatible alloys, interpenetrating and semi-interpenetrating polymer networks, and more (Environmental Impact of Polymer Fiber Manufacture).
Polymer mixtures are classified into two categories: compatible blends and incompatible blends. Incompatible mixes are immiscible mixtures with distinctly differentiated phases. The mechanical qualities of these blends are often poor. Compatible blends are those that combine to create a single phase in which the individual components are morphologically indistinguishable. Compared to the component polymers, these polymer blends are able to provide excellent mechanical properties. Incompatible polymer mixtures, contrary to popular opinion, are more common [
47].
Table 2.
Natural/synthetic composites and compatibilizers.
Table 2.
Natural/synthetic composites and compatibilizers.
| Synthetic polymer |
Natural polymer |
Compatibilizer |
Processing technique |
Reference |
| Polypropylene |
Sawdust |
Maleic anhydride |
Extrusion |
[15] |
| Polypropylene |
Wood fibers |
Ethylene–propylene or ethylene–propylidene copolymer Maleate polypropylene Calcium stearate |
Injection forming |
[47] |
| Low density polyethylene |
Lignocellulosic fibers Sawdust |
Ionomer polyethylene Maleate polypropylene Low molecular weight polypropylnene Maleic anhydride |
Extrusion Injection |
[47] |
| Polyurethane |
Mechanical pulp |
Isocyanates |
Pressing |
[47] |
| Phenol formaldehyde |
Lignocellulose |
Chemical modified fibers |
Pressing |
[47] |
| Polyester + PE + PP |
Wood fibers |
Phenol resins |
Pressing |
[47] |
| Carboxylated Nitrile Rubber |
Natural rubber |
Maleic anhydride grafted polyisoprene epoxy resin |
Roll milling |
[48] |
| Chlorinated Polyethylene |
Natural rubber |
Maleic anhydrided grafted ethylene propylene diene rubber EPDM-g-MA |
Thermal mixing followed by roll milling |
[49] |
| Carboxylated nitrile rubber |
Natural rubber |
Bis(disopropyl) thiophosphoryl polysulphides |
Thermal mixing followed by roll milling |
[50] |
| Poly(lactic acid) |
Natural rubber |
Poly(lactic acid)- natural rubber tri block copolymer |
|
[51] |
3.2. Processing Techniques
The technology used to manufacture polymer composites is determined by a number of factors, including the nature of the fibre and polymer, the intended purpose, which might be for construction or the fabrication of car components, or biological uses such as scaffolding and wound healing.
3.2.1. Extrusion Molding
Extrusion is the most typical method for molding extruded polymers. To obtain homogeneity of the polymer with or without the addition of other polymers and additives, polymers are melted under heat and shear to create blends or composites. To achieve homogeneity and the required shape, polymers must often be processed in their melt form when being used as matrix materials for engineering or medical applications. Injection molding also calls for the polymer to flow into a cavity and cool there, whereas extrusion molding primarily relies on the flow of molten polymer through a screw [
52,
53].
3.2.2. Solvent Casting
This method is frequently used to create polymer films. Numerous uses, including packaging, transdermal drug delivery, and wound healing, depend on the ability of polymers to form films. Coatings can also be produced using films. One of the most important properties of a film for any application is its consistency. Certain techniques are employed to achieve consistency in the production of films from neat polymers or mixtures. Self-absorption of monolayers (SAM), spin coating, thermal spraying, solvent casting, floating technique, and Langmuir-Blodgett film formation are all techniques for making films. The two most used techniques are solvent casting and spin coating. In this paper, we look into nanocellulose as a polymer composite reinforcement, focusing on the manufacturing processes needed to make such a composite. The polymer matrix is comprised of fish skin gelatin, and the reinforcing component is cotton linter cellulose whiskers whiskers [
54].
3.2.3. Cellulose Nanoparticles
The reduction of particle size to the micro- or nanoscale can significantly enhance the polymer's functioning properly. Nanocellulose is presently the subject of intensive research with the goal of developing high-performance composites. Some bacteria, such as
Acetobacter species, create nanocellulose using the bottom-up technique. They can also be produced from plants via a top-down technique that includes the breakdown of plant components [
55,
56]. Algae and tunicate are other sources of nanocellulose [
57,
58,
59].
3.3. Characterization
Structural characterisation, which determines the conformation of polymers like polysaccharides, is based on an understanding of the usual energy release for particular types of linkages, as determined by the angle of rotation about the linkage. This works especially effectively for compounds with well-known conformations, such as poly- and oligosaccharides. A polysaccharide or oligosaccharide chain's exact geometry can be determined, for instance, by understanding the dihedral angles of rotation of the monosaccharide links [
60] .
A polysaccharide having different rotations at each monosaccharide link, for example, is anticipated to have a random structure. The possibility of a random conformation is constrained by interactions between and within chains because they providing less potential for independent rotation. Conformation is essential for the polymer to work effectively. Consider cellulose and amylose as two polysaccharides with identical monomer units and are (14)-d-linked poly-d-glucans. However, the helix of amylose is swaying, but the zigzag chain conformation of cellulose is stretched. Because cellulose and amylose have distinct conformations, this is inedible and water insoluble. Polymer characterization also aids in identifying a polymer's crystalline or amorphous nature. Compounds that form crystallize often have much stronger structures. Unlike proteins, which can maintain their crystal structures even in solutions, polysaccharides rarely form crystal structures [
60].
4. Cellulose and starch extraction, purification, and modification.
The therm "polymer" means "many pieces" (it derived from the Greek words poly, which means "many," and meros, which means "parts"). Large molecules known as polymers have molar weights ranging from dozens to millions. Synthetic polymers like plastics, material filaments, and specialty rubbers make up around 80% of the organic chemicals market. By combining numerous small molecules into one large molecule, polymers are created. Small molecules known as monomers are utilized to create polymers. Expanding polymers are made from monomer units that are specifically joined together, whereas building polymers are made from monomer units that join together such a way that a small molecule, generally water, is provided during each reaction Polymers are abundantly dispersed in nature. There are several natural polymers, including proteins, cellulose, gelatine, starch, chitin, and chitosan, are found in the bodies of plants, animals, and humans. These polymers can be isolated, refined, and modified to improve their performance in a wide range of applications [
61].
4.1. Extraction, Purification and Modification of Cellulose
4.1.1. Cellulose Extraction
The cellulose -glucopyranosyl residues are linked together by 1 → 4 links. Cellulose crystallizes into monoclinic, rod-shaped crystals. The unit cell's long b-axis is constructed by chains that are oriented parallel to the fibre direction. It is assumed that the chains are pleated to facilitate the creation of intrachain hydrogen bridges between O-4 and O-6 and between O-3 and O-5. While hydrophobic interactions are present along the c-axis, intermolecular hydrogen bridges along the a-axis stabilize the parallel chains. 60% of the original cellulose can be found in the crystalline sections. Amorphous gel patches that can crystallize when moisture is removed divide these sections. These areas appear to include the linkages that are acid- or alkali-labile as well. These bonds are hydrolyzed, resulting in the formation of microcrystalline cellulose. The molecular weight of this partially depolymerized cellulose product is 30–50 kD, although it lacks a fibrose structure. It is nonetheless water insoluble [
62].
4.1.2. Cellulose Modification
Due to their high strength and stiffness, biodegradability, and renewability, as well as their production and use in composites, cellulose macro- and nanofibers are becoming increasingly prevalent. A relatively recent field of research is the use of cellulose nanofibers in the production of composites. Cellulose macro- and nanofibers are suitable for usage as reinforcement in composite materials due to their superior mechanical, thermal, and biodegradation properties. Because cellulose fibres are naturally hydrophilic, increasing their surface roughness is critical for producing composites with enhanced properties [
63].
4.2. Starch Extraction, Purification, and Modification
4.2.1. Extraction Methods of Starch
Several plant organs use starch as a common form of stored carbohydrate. Being part of numerous meals makes it the most significant source of carbohydrates in the human diet. Starch and its by-products are also important for industry, particularly in the paper and textile industries. The sources are typically concentrated in areas where starch is isolated. Wheat, potatoes, maize and potatoes produced 99% of the world's starch, in both natural and modified forms. Certain alternative starches are also accessible commercially. Starches produced from legumes (peas, lentils) have lately gained interest due to properties that appear to make them a viable alternative for chemically modified starches in a number of commodities [
62].
Various foods and commercial goods include rice starch as an additive. Desserts and bakery goods are among of the preferred uses of processed meals owing to the inherent benefits of rice starch, which include its small and uniform size distribution, white colour, and clean odour. However, rice protein binds strongly to the starch granules' surface in the endosperm, which raises the cost of starch separation compared to other starches. Rice protein can be removed from rice flour using alkaline solvents, surfactants, or protein hydrolyzing enzymes [
64]. Protein extraction for starch separation frequently makes use of alkaline solvents like NaOH and surfactants like sodium lauryl sulfate (SLS) and dodecylbenzene sulfonate (DoBS). These solvents induce the oligomeric protein structures to separate and change into soluble forms. For isolating rice starch, an aqueous 1.2% DoBS solution with 0.12% sodium sulfite outperformed 0.2% NaOH or 1.2% SLS including 0.12% sodium sulfite. The effectiveness of protein removal may be increased even more by repeating rapid extraction processes (1-2 h) with new solution. The protein extractability ascended lightly but the starch loss increased significantly, hence raising the extraction temperature was not advised. The paste's ability to adhere to surfaces had a substantial impact on the remaining protein content of rice starch; less protein made the paste viscous and reduced the pasting temperature [
65].
4.2.2. Modification of Starch
Through physical and chemical processes, the characteristics of starch, amylose, and amylopectin can be improved or "tailored" to suit or adapt to a particular application or food product. The amorphous component rises when starch granules are broken down by grinding or applying pressure at various water contents, boosting dispersibility and swellability in cold water, lowering the temperature at which gelatinization occurs by 5 to 10 °C, and increasing enzymatic vulnerability. For instance, amylase breakdown and water absorption are both more and higher in bread dough formed from flour with damaged starch. Extruded starches have a lower viscosity, are more soluble, and dispersible. Chemical changes occur at temperatures ranging from 185 to 200 °C, as evidenced by the partial breakdown of amylase when heated correctly. Other sugars found in addition to maltose were isomaltose, gentiobiose, sophorose, and 1,6-anhydroglucopyranose [
62].
5. Biological Potential of Natural Polymers
5.1. Anti-inflammatory Activity
Natural polysaccharides are frequently utilized in nanotechnology for the treatment of inflammatory diseases [
66], and their anti-inflammation properties have been investigated [
67]. For example, TCM polysaccharides mainly block chemotactic and adhesion factor expression as well as the activity of key enzymes involved in the inflammatory process [
68] . While other polysaccharides inhibit inflammatory-related mediators like cytokines (IL-1b, IL-6, TNF-), NO (nitric oxide), and NO (nitric oxide), and reduce inflammatory cell infiltration, sulphated polysaccharides derived from algae have an anti-inflammatory effect by preventing leukocyte migration to sites of inflammation [
69]. Due to their potential therapeutic benefits, Nutritional supplements derived from plants and animals have been intensively explored in the development of new drugs [
70]. Natural products, their derivatives, and biomacromolecules constitute around 50 percent of the current drugs on the market [
71].These supplements are widely recommended, because of their variety of chemical components and lack of side effects compared to synthetic varieties.
Certain plants include natural substances that may have the ability to act as NSAID-like anti-inflammatory agents and be used as osteoarthritis drugs. Curcumin, capsaicin, berberine, sinomenine, rapamycin, white willow bark, and other natural substances have all proven pharmacological effects [
72] .
5.2. Hypoglycemic and Hypocholesterolemic Activities
It is possible to treat hyperglycemia, hyperlipidemia, hyperinsulinemia, and insulin resistance with ganoderma atrum polysaccharide, and it can also assist in avoiding kidney damage in type 2 diabetics. Since the 1980s, polysaccharides have been extensively researched for their hypoglycemic and hypocholesterolemic effects in clinical trials. To increase the therapeutic efficacy of loaded proteins and increase their stability, natural polysaccharides can be employed as nanocarriers. Orally administered insulin-loaded dextran-chitosan nanoparticulate polyelectrolyte complex had a better bioavailability and a more prolonged hypoglycemic impact [
12]. Additional examples of polysaccharides having hypoglycemic and hypocholesterolemic properties include chitosan, kefiran, and sulfated polysaccharides from Bullacta exarate [
73], [
74], and [
75].
According to a number of studies,
α-glucosidase and
α-amylase are crucial for the insulin regulation. As a result, it is believed that inhibiting these enzymes is a good choice for treating diabetes. Inhibiting these enzymes can, in fact, limit carbohydrate digestion of and delay the absorption of glucose [
76]. In this context, acarbose and plant extracts (which may include numerous biologically active substances) have been described as hypoglycemic drugs that exhibit α-glucosidase inhibitory action and can reduce blood glucose level (BGL) [
77].
Concerning actions that decrease cholesterol It is generally recognized that dietary intake and cholesterol production, absorption, and secretion frequently have an impact on plasma cholesterol levels, especially LDL-c. In fact, several studies have reported evidence that different dietary proteins and their hydrolysates can improve blood lipid profiles, for instance, diets with soy [
78], milk proteins [
79], and fish proteins [
80].
5.3. Anticoagulant Activity
Sulfated polysaccharides called unfractionated and low molecular weight heparins are employed as anticoagulants, however they have side effects include bleeding and thrombocytopenia [
81]. Among the several polysaccharide properties, anticoagulant action has received much attention. Polysaccharides, particularly sulfated polysaccharides, have been shown to exhibit biological effects such as anti-tumor, antioxidant, and anticoagulant characteristics [
73].The anticoagulant action of these sulfated polysaccharides is significantly influenced by the high sulfate concentration [
82]. Natural polysaccharides from a variety of marine sources, including corals, marine fungi, microalgae, and shellfish (shrimp, crab, squilla, lobster, and crayfish) [
83], may be regarded as anticoagulant agents [
84] .
5.4. Antiviral Activity
The ability of sulfated polysaccharides from seaweeds to block the replication of enveloped viruses such the herpes simplex virus (HSV), human immunodeficiency virus (HIV), human cytomegalovirus, dengue virus, and respiratory syncytial virus has been known since the 1950s [
69]. Sulfated exopolysaccharides, which have a crucial biological role as antiviral agents, can be produced by a variety of microalgae species [
82]. Chinese traditional medicine-derived polysaccharides have been utilized as antiviral medications for a long time because they can boost and strengthen the immune system by stimulating macrophagocytes to enhance their phagocytic activity and trigger the release of IFN- and antibodies [
68]. Biochemical characteristics of microalgal polysaccharides include antioxidant, antibacterial, and antiviral action [
85].
6. Application of Natural Polymers in Food
Every food product contains macromolecules, and great majority of which are polymers. Simple polymer molecules are covalently bonded linear chains of monomers. The number of times these monomers are repeated along the polymer chain determines the degree of polymerization, the molar mass of most polymers varies. If many monomers are present, the structure may become more complicated. Most polymers varied in molar mass as a result of the degree of polymerization. The structure may grow more complex if there are several monomers involved. The fact that polymers can be branched and that individual monomers might have distinct characteristics, like charge (in the case of polyelectrolytes) and hydrophobicity (in the case of amphipathic polymers), adds to the complexity. Polymers are widely used in food because of their higher order structures «. Polysaccharides (i.e., starches, celluloses), polyamides (proteins), and minor amounts of polynucleotides (DNA, RNA) are the three principal types of naturally occurring polymers that make up the majority of meals [
86].
Proteins are linear complex heteropolyelectrolytes that have a distinctive sequence of up to 20 different amino acid monomers with a variety of charge, hydrophobicity, and aromatic structural configurations. In live cells and animals, protein, which has an average degree of polymerization of about 103, performs a variety of biological functions. Meals typically contain some form of protein, which also serves as a source of amino acids for our own metabolism. They have the ability to produce and stabilize emulsions, foams, and gels, as well as enhance viscosity and water holding capacity. Despite their use, proteins are more expensive than other natural biopolymers, particularly starches and celluloses, which restricts their use in technology. Some of the key components include gelatine, milk proteins, egg proteins, and plant proteins [
87].
Heteropolymers of sugars and their derivatives are known as polysaccharides. Many are polyelectrolytes that can be linear or branched. Polysaccharides generally have a degree of polymerization between 103 to 104."Nutritional" functions, according to biological processes, serve as energy storage for metabolism (in particular starch in plants and glycogen in animals) and "building material" (such as cellulose in plants and chitin in fungus cell walls and the exoskeletons of arthropods like crustaceans).The latter category, known as structural polysaccharides, is composed mostly of mixed and very complex structures and occurs in several forms [
88].
Starch and protein are both essential dietary components that are rich in macronutrients. This section will concentrate on natural polymers as food additives and components in food formulations having a specialized purpose beyond basic nutritional value.
These specific applications of natural polymers in food may be divided into two basic categories:
Stabilizing food microstructures through gelling, thickening, emulsion, and foaming as well as using processing aids including cryoprotectants to increase freeze-thaw stability, drying aids, and encapsulant material.
Additional physiological and biological functionality, such as those provided by functional foods with specific health claims including lowering blood cholesterol levels, raising satiety, enhancing bioavailability, and inhibiting microbial growth [
89].
Table 3.
Sources of commercially important hydrocolloids used in the food industry [
88].
Table 3.
Sources of commercially important hydrocolloids used in the food industry [
88].
| Botanical |
Plants
Trees
Tree gum exudates
Seeds
Tubers |
Starch, pectin, cellulose
Cellulose
Gum arabic (acasia), gum tragacanth, karaya
Guar gum, tara gum, locust bean gum,
Konjac mannan (glucomannan), potato starch |
| Algal |
Red seaweed
Brown seaweed |
Agar, carrageenan
Alginate |
| Microbial |
|
Xanthan gum, dextran, gellan gum, cellulose |
| Animal |
|
Gelatin, caseinate, whey protein, chitosan |
6.1. Regulatory Aspects
Natural polymers that are classified as either additives or ingredients, with the bulk falling under the additive category. Gelatine, on the other hand, is categorized as an ingredient. Ingredients are listed by name in the product's list of ingredients, whereas additives must be identified by a distinctive number (the E-number in the European Union and the INS number abroad) and are optionally identified by name. In the US, additives are governed by the JECFA (Joint/ WHO Expert Committee on Food Additives) and the European Commission. The legislation is used to protect consumer health and to ensure ethical behaviour in the food sector [
90].
The goal of additives regulation is to establish acceptable ingestion limits and purity criteria for diverse compounds. An acceptable daily intake (ADI) and an international number (INS, International Number System) or an E-number are assigned to the additive when it has been approved. The INS/E-number validates its acceptability. The European Food and Safety Agency (EFSA) is in charge of monitoring health claims made for natural polymers used as additives in the EU. The EFSA assesses whether or not the scientific evidence currently available for the health claim is sufficient. In the EU, only EFSA-approved health claims may be advertised on food packaging. Insufficient research (RCTs are preferred), inadequate investigations, or insufficient test subjects used in the studies result in the majority of applications being refused. Many natural polymers, however, passed EFSA evaluation and had their health claims approved [
91].
Table 4.
EFSA-approved health claims for products made with natural polymers.
Table 4.
EFSA-approved health claims for products made with natural polymers.
| Claim |
Hydrocolloid |
| Maintenance of normal blood cholesterol concentrations |
Beta-glucan, konjacmannan glucomannan, pectins, guar gum |
| Maintenance or achievement of a normal body weight |
Konjacmannan glucomannan |
| Reduction of postprandial glycaemic responses |
Beta-glucan, pectins |
7. Pharmaceutical Applications of Natural Polymers.
Natural polymers are used for a wide range of applications in both the polymer and pharmaceutical industries. Because the pharmaceutical industry is so extensive, there is a continual need to consider different applications. As a result, knowing how natural polymers are utilised in the pharmaceutical sector would logically benefit the polymer business in recognising the broader uses of these polymers and adding the essential requirements to fulfil the end applications (e.g. give varied functions). The pharmaceutical industry's polymer applications are largely focused on medication delivery systems. The following section of this chapter discusses drug entrance sites into the body and gives an outline of the potential applications of natural polymers.
7.1. Transdermal Drug Delivery Devices
Polymers are commonly used in transdermal medicine administration procedures. They function as crucial packaging components, coatings, penetration enhancers, ease of handling drug device handling, structural support for the device in the form of a backing layer, and control drug release rate control. They are frequently used in transdermal patches because to their distinctive properties. The development of transdermal drug delivery device components from more readily accessible natural polymers becomes essential as petrochemical-based resources for the manufacturing of synthetic polymers grow more expensive and scarcer. Following a description of the various components of the transdermal drug delivery system, the usage of natural polymers in each element, the matrix, adhesive layer, rate-controlling membrane, backing layer, release liner, and penetration improver will be discussed. Polymers are used more than any other material in transdermal drug delivery (TDD) because they contain features that are significant in the drug delivery process [
92]. They can help with pharmaceutical release control from carrier formulations [
93]. Silicones, polyvinyl alcohol, chitosan, polyacrylates, polyesters including PLGA, PELA, and PLA, and cellulose derivatives are among the polymers often used in TDD.
Natural polymers are the ideal choice in TDD because they are easily available, inexpensive, potentially biodegradable, and biocompatible. They may also be treated to various chemical and surface modifications to match the requirements of the TDD system. A TDD system combines one or more polymers with an embedded medication for regulated and sustained drug delivery into or through the skin[
94]. High analytical product yields demand for the chemical inertness and purity of polymers utilized in TDD systems. Additionally, it must have physical properties that are sufficient for the intended use. The material must be suitable for processing and must not degrade. In addition, because a TDD patch system would be in touch with a person's skin for a prolonged period of time, biodegradability and safety are paramount properties to include in the design [
95].
7.2. Natural Polymers in Transdermal Drug Delivery
Polymers have been used in transdermal medicine delivery since the 1980s. The drug is to be absorbed into the skin from a matrix of cross-linked linear polymer chains seen in the majority of transdermal patches[
94]. Transdermal drug delivery polymers include silicones, chitosan, polyvinyl alcohol, polyvinylparrolidone, polyacrylates, and cellulose derivatives. Both natural and synthetic polymers have been used as matrices, gelling agents, emulsifiers, penetration enhancers, and adhesives in transdermal delivery systems [
96], for example, demonstrated successful transdermal testosterone delivery to lab rats using a silicone elastomer synthetic polymer matrix. Another group studied the use of pectin hydrogels for insulin transdermal delivery. Diabetes-prone rats with type 2 diabetes mellitus were administered insulin-loaded pectin hydrogels [
97]. Diabetes-prone rats with type 2 diabetes mellitus were administered insulin-loaded pectin hydrogels The outcomes demonstrated that the transdermal patch effectively and pharmacologically administered insulin across the skin in a dose-dependent manner [
98,
99] studied the use of natural polymers such as rubber latex as the adhesive for the backing layer in nicotine patches. As a result, more research into the potential of natural polymers for transdermal medication administration is required.
Natural polymers derived from plants and animals are emerging as a preferable option to synthetic polymers in the development of TDD systems, because they are biocompatible, biodegradable, and degrading into non-toxic monomers, as well as being more easily accessible Synthetic polymers are increasingly being employed in the development of TDD systems [
2,
26]. Synthetic polymers derived from petroleum and synthetically altered polypeptides are known to have limited therapeutic uses due to their toxicity and slow biodegradation rates [
92,
100,
101]. Natural-derived polymeric polysaccharides outperformed traditional carriers like polylactic acid (PLA), poly (lactic-co-glycolic acid) (PLGA), and polyvinyl pyrrolidone (PVP) in terms of drug delivery due to their high-water retention, lack of toxicity, good biocompatibility, biodegradability, and several of other important biological properties. For example, sodium alginate could be used for drug encapsulation by cross-linking with metal ions to prepare nanoparticles and thereby improve skin penetration of drugs [
102]; chitosan (CS) has significant slow-release properties for drug delivery [
103]; hyaluronic acid (HA) can increase skin hydration and involve cell signalling to promote tissue regeneration and wound healing [
104], etc.
7.3. Natural Polymers in Topical Delivery Systems
Mucoadhesive microspheres, liposomes, solutions, gels, and mucoadhesive hydrogels are commonly used to deliver drugs through the nose. Starch, chitosan, alginate, dextran, hyaluronic acid, and gelatine are examples of natural polymers utilised for nasal medication administration [
105]. The interaction of the drug formulation with the mucin surface is known as mucoadhesion. By increasing the contact duration between the drug formulation and nasal mucosa layers in the cavity, the idea behind mucoadhesion is to enable sustained drug administration in nasal membranes [
106]. Mucoadhesion reduces the chance of complete mucociliary clearance while promoting drug absorption [
107]. Starch is a biodegradable polysaccharide that may easily be converted into microspheres [
108]. Examples of starch-loaded intranasal medications in the development stage include insulin [
106], morphine [
109], inactivated influenza [
110], and salbutamol [
111]. Chitosan, a naturally occurring polysaccharide with mucoadhesive characteristics, exhibits excellent adhesion to nasal epithelial cells and the overlying mucus layer [
112].
Compared to chitosan, PLA, and carboxymethyl cellulose, alginate is a divalent cation-induced rapid gelation, natural polysaccharide with higher mucoadhesion strength [
107]. Usually, mucoadhesive polymers are combined with alginate gel to increase the vehicle's strength and drug loading efficiency [
113].
Polysaccharides have recently gained a great deal of interest in the area of tissue engineering [
114], cosmetics, and wound healing [
115] They are also extensively applied as drug carriers, building blocks for drug delivery, bioactive materials, and excipients to increase drug delivery [
116]. Natural polysaccharides are a potential candidate for numerous uses, including the delivery of medications and vaccinations, due to their versatility in structuring and modification to achieve specific goals. Microorganism-derived polysaccharides including scleroglucan, gellan gum, and xanthan gum have all been intensively researched to be utilized for drug delivery [
117]. Drugs can be incorporated into bioadhesive polysaccharide nanoparticle carriers to improve their absorption [
19]. Pectin, guar gum, amylose, inulin, dextran, chitosan, and chondroitin sulphate are other naturally occurring polysaccharides that have been studied for their potential to be employed as pharmaceutical excipients and for colon-specific drug release [
118]. In addition, chitin and chitosan are low-immunogenic and tissue compatible polysaccharides that have demonstrated effectiveness in the delivery of drugs and vaccines [
119], as well as the transport of pharmaceuticals to the colon as prodrugs or coating tablets [
120]. Chitosan is one of the most biopolymers that has been widely used as a drug and vaccine delivery system in many preparations (
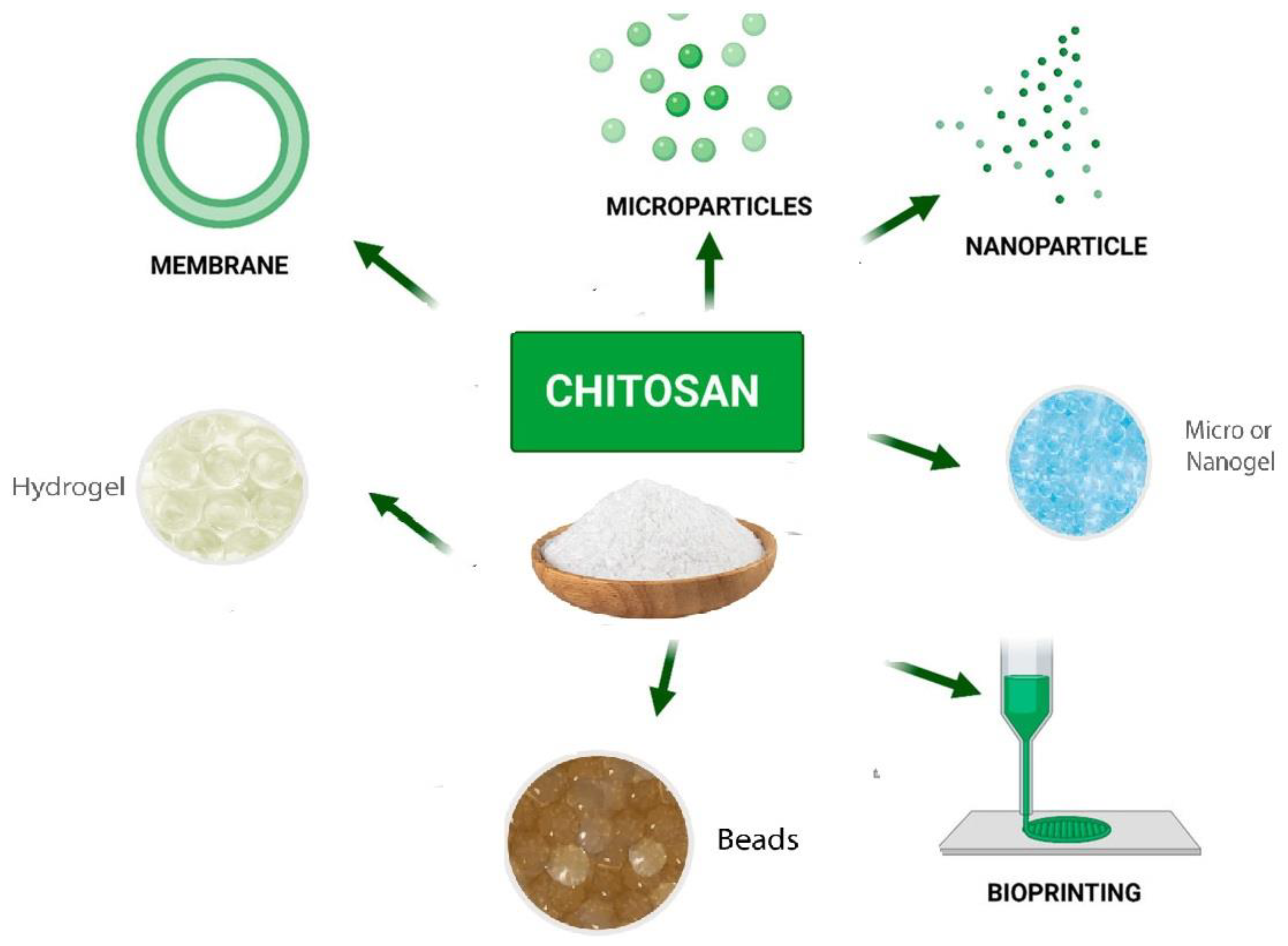
Figure 9) [
121]. This is due to the wide variety of antigen that could be encapsulated under mild conditions and without using organic solvents, which avoids the degradation and denaturation of the antigen during processing or after loading [
122].
7.4. Natural Polymer Implants
Natural polymer uses in implants are particularly appealing for the same reasons that every other biomedical application has been [
124]. Because these materials will be embedded in the body for extended periods of time, they must be biocompatible and functional for the entire time they will be there until they are removed or degrade into the body and release non-toxic, biocompatible degradation products that can be safely eliminated from the body.
Table 5.
Examples of natural polymers used in implants.
Table 5.
Examples of natural polymers used in implants.
| Polymer |
API/implant |
References |
| Chitosan |
Flurbiprofen
Timolol maleaic
Thymol
Quercetin
Dexamethason
Vancomycin |
[125]
[125]
[126]
[126]
[125]
[127] |
| Hyaluronic acid/chitosan multilayer coating |
Chitosan Imidazole/siRNA nanoplex |
[128] |
| Chitosan/carbonnanotube |
Titanium implant |
[129] |
| Collagen/cellulose |
Juca extract |
[130] |
| Agarose |
Thymolol
Quercetin |
[126] |
| Poly (Lactic acid) |
Ibuprofen
Acetylsalicylic acid |
[130] |
8. Environmental Impact of Natural Polymers.
"Natural" and "green" are not equivalent terms. As their name signifies, natural polymers, often referred to as biopolymers, are substances that are produced by biological organisms such as cellulose, silk, chitin, protein, and DNA. Examples of natural polymers include silk, cellulose, chitin, and protein. It is possible to artificially create natural polymers from natural raw materials in a larger scale. The output of natural polymers is rising gradually although it continues to produce approximately less than 1% of the 300 million tonnes of plastics made annually. From
$3.3 billion in 2012 to
$4.6 billion in 2016 [
131,
132] .the demand for natural polymers in the US is anticipated to increase 6.9% annually. The term "green chemistry," which first originated in the 1990s, refers to the production of green polymers, also known as sustainable or green chemistry. The International Union of Pure and Applied Chemistry (IUPAC) defines "green chemistry" as "the design of chemical products and processes that decrease or eliminate the use or creation of compounds harmful to humans, animals, plants, and the environment [
133].The concept of bio-based feedstocks is not new in the chemical industry. At least since the start of the twenty-first century, they have proven widely applicable in industries. Bio-based products, however, were not prioritized for a very long time because the price of oil was so low and the growth of oil-based products offered so many chances. The limited availability and variability of fossil fuel materials, environmental concerns, and technological advancements are among many of the causes that have pushed the creation of bio-based polymers and products. Building a chemical industry based on fossil fuels took more than a century. The bio-based polymer sector is quickly catching up with the fossil fuel-based chemical industry, despite its remarkable growth since the late 1990s [
133].
Because of advancements in white biotechnology, the production of bio-based polymers and other chemicals from renewable resources is now a possibility. First-generation techniques mainly focused on food resources like corn, starch, rice, etc. to produce bio-based polymers. As the discussion over food vs. fuel intensified, technology began to concentrate on cellulose-based feedstocks, including waste from the food, wood, and paper sectors, as well as stems and leaves and solid municipal waste streams. More and more of these technologies are being developed to supplement those previous waste streams; however, it may take a few decades to fully develop the variety of chemicals based on these technologies. Examination of the motivations behind the green and renewable polymer sectors in further detail. Rolf Mülhaupt of the University of Freiburg in Germany states that the growth of the green polymer sector is unstoppable because, at the turn of the century, renewable polymers are enjoying a renaissance and there is a significant push towards the development of macromolecular materials derived from biological sources [
134].
The proposed transition from petrochemistry to bioeconomy is motivated by a number of factors.
To achieve the desired transition from petrochemistry to the bioeconomy. Economically, the small quantity of oil is expected to bring up the price of oil even further, especially given the expected growth in global energy consumption. For no other reason than that Earth's population is rapidly expanding, there is an increase in energy consumption on a global level. The affordability and competitiveness of plastics might be significantly impacted. By switching the production of chemical raw materials to renewable resources like coal, the synthesis of plastics could be safeguarded from the projected future oil problem. The need for sustainable and "green" products is therefore made even more important by the growing public concern about global warming. The creation of ecologically friendly products with a reduced carbon footprint is also being accelerated by a wave of environmental laws and regulations[
135].
Green manufacturing practices for polymers include the following:
High percentage of raw materials in the product;
Clean (waste-free) and efficient production methods;
The reduction of greenhouse gas emissions;
Avoiding using additional chemicals, such as organic solvents;
High manufacturing energy efficiency;
A product with a high raw material content;
8.1. Renewable Polymers
Polymerization of either naturally occurring biopolymers or bio-based monomers can be used to create sustainable polymers. Examples of biomaterials that experienced chemical modification to meet polymer manufacturing and uses include proteins, terpenes, polysaccharides, and polyesters (
Figure 10) [
136].
8.1.1. Polylactic Acid.
Despite being discovered in 1845, polylactic acid (PLA) was not commercialised until the beginning of 1990. PLA is a member of the aliphatic polyester family and its primary ingredient is lactic acid. Hydroxyl carboxylic acid, the monomer of lactic acid, is generated by bacteria from corn starch or sugars supplied from renewable resources. Corn has the advantage of offering a high-quality feedstock for fermentation that yields a high-purity lactic acid, which is required for an efficient synthesis process, even though alternative renewable resources may be used. Depending on the microorganism utilized, the fermentation process results in either L-lactic acid or D-lactic acid [
137].
PLA is a commercially desirable polymer because it has some similarities to hydrocarbon polymers like polyethylene terephthalate in several ways (PET). High rigidity, exceptional transparency, a glossy appearance, and the capacity to withstand a variety of processing conditions are just some of its distinctive qualities. The ease of melt processing has made it possible to synthesize PLA fibers, which are becoming increasingly used in a variety of textiles, including furniture, curtains, soft nonwoven baby wipes, and tough landscape fabrics. These textiles can perform better than conventional fabrics comprised of synthetic substitutes. Implants used for cell growth employ bioresorbable scaffolds made of PLA and different PLA compositions. PLA has been approved by the FDA for a few particular human clinical. Additionally, bone support splints have been made using PLA-based polymers [
138].
8.1.2. Bio-polyethylene
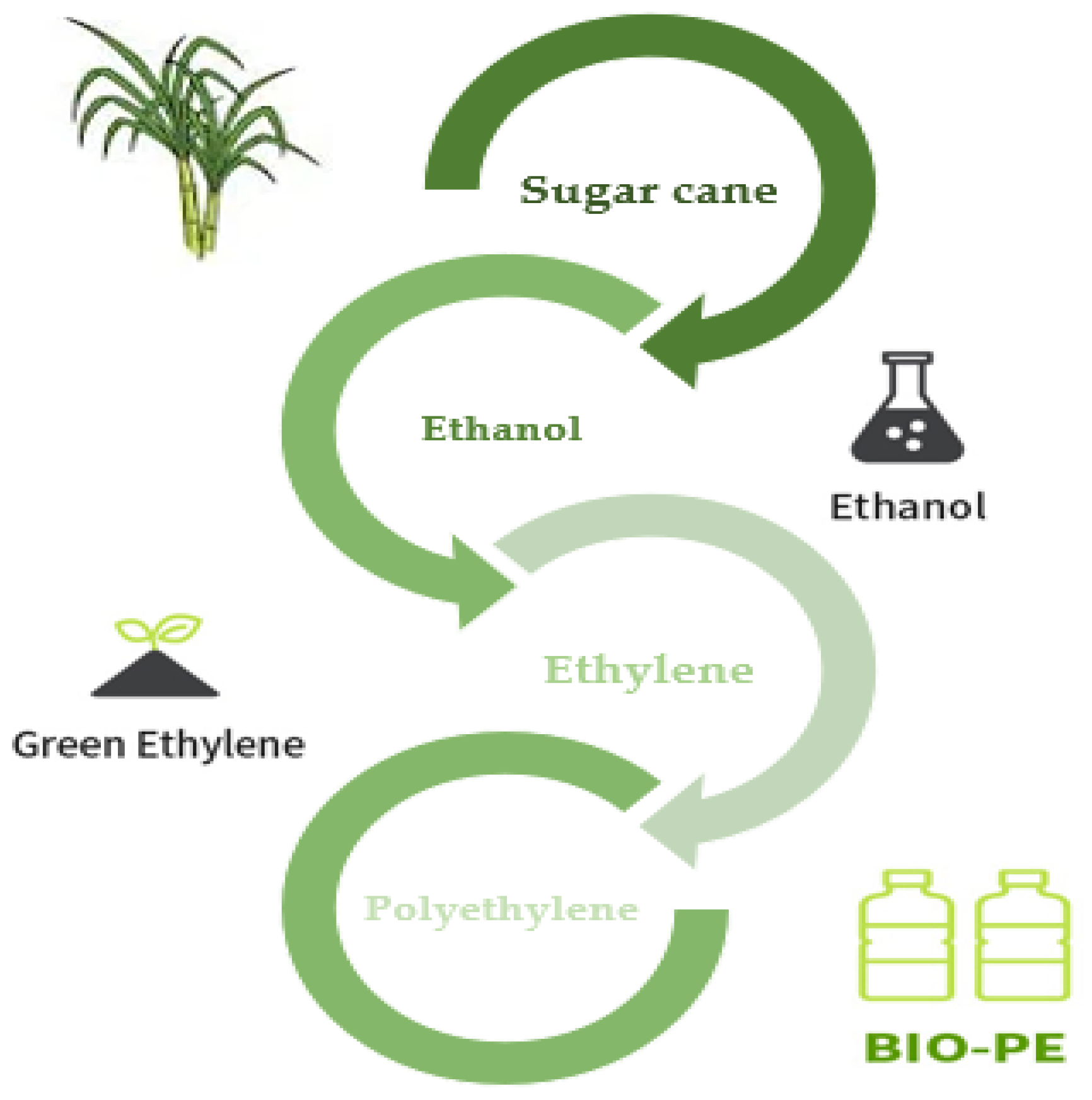
Polyethylene (PE) is an essential technological polymer that was previously derived from fossil resources. Ethylene is polymerized under pressure, heat, and the presence of a catalyst to create PE. Either steam cracking of naphtha or other heavy oils or ethanol dehydration traditionally yield ethylene. Currently, microbial PE, also known as green PE, is made from ethanol produced by microbial fermentation. The idea of creating PE from bioethanol is not especially novel. Braskem produced bio-PE and bio-PVC from bioethanol throughout the 1980s. However, the technique was unappealing at the time due to low oil prices and the limits of the biotechnology procedures [
139].
Sugarcane is now used to make bio-PE on an industrial scale from bioethanol. Through a microbial strain and biological fermentation process, bioethanol may also be produced from biorenewable feedstocks such as sugar beet, starch crops like maize, wood, wheat, and other plant wastes. Traditionally, extracted sugarcane juice with a high sucrose concentration is anaerobically fermented to create ethanol. An azeotropic mixture of ethanol + water with a high concentration of the former is produced when ethanol is distilled to remove water. To create ethylene and eventually polyethylene, ethanol is thoroughly dehydrated at high temperatures over a solid catalyst (
Figure 11) [
140].
8.1.3. Cellulose
Cellulose constitutes the majority of the cell walls in all plants. It has a crystalline structure and is a complex carbohydrate. In contrast to starch, which contains primarily -1,4 linkages, cellulose possesses
ß-1,4-glycosidic bonds that all of the glucose units. Cotton and wood fibers are the two main sources of raw materials for the manufacture of cellulosic plastics. Alkali and carbon disulfide are used to dissolve plant fibers, resulting in viscose that is then transformed into cellulose in the form of cellophane in a sulfuric acid and salt sulphate solution. Currently, two procedures are used to extract cellulose from the other parts of wood (
Figure 12). Pre-hydrolysis and sulfite Chemicals and high pressure are used in the kraft pulping process to separate cellulose from lignin and hemicellulose, yielding cellulose with a purity of more than 97%. The three principal cellulose derivatives utilised in industry are cellulose acetate, cellulose esters (used in extrusion, moulding, and films), and regenerated cellulose for fibres [
142].
8.1.4. Alginates
Alginates are used in several industrial applications as viscosifiers, stabilisers, and agents that form gels, films, or water-binding polymers. Printing on fabrics, ceramics, welding rods, and water treatment are among the applications [
143,
144,
145]. The polymer dissolves in cold water and forms thermostable gels. Custard creams and restructured food are two examples of food products that use these properties. Alginates are also used as stabilizers and thickeners in a variety of drinks, ice creams, emulsions, and sauces [
146].
9. Economic Impacts of Natural Polymers
The body's natural extracellular matrix (ECM) is mostly composed of natural polymers such collagen, elastin, and fibrinogen. In the production of a range of medications, natural materials like as protein, enzymes, muscle fibers, polysaccharides, and gummy exudates are used efficiently. Well-known natural polymers utilized in the pharmaceutical industry and other fields include chitosan, carrageenan, ispaghula, acacia, agar, gelatine, shellac, guar gum, and gum karaya. These natural polymers are popular in the pharmaceutical business as emulsifying agents, adjuvants, and packaging adhesives; they are also suitable for the production of pharmaceutical and cosmetic products [
147].
Synthetic polymer contamination in the environment has reached catastrophic proportions in developing countries. Petroleum-based plastics are not readily destroyed by microorganisms, and as a result, they build up in the environment. Additionally, there has been a significant rise in oil prices recently. These features have piqued people's interest in biodegradable polymers. In the 1980s, the first biodegradable plastics and polymers were introduced. The requirement for polymers made from renewable resources has increased significantly over the past 20 years, largely due to two important factors: first, environmental concerns, and second, the realization that our petroleum supplies are limiting. Synthetic and natural polymers are just two of the elements that can be used to create biodegradable plastics. Natural polymers can be obtained in significant numbers from renewable sources, as compared to synthetic polymers, which are made from non-renewable petroleum resources. Polymer erosion occurs when hydrolytically or enzymatically sensitive linkages in polymeric biomaterials are cleaved. A variety of biodegradable polymers have lately been developed, as have numerous microbes and enzymes capable of breaking them down [
148] .
According to studies, the United States leads the global market for natural polymers. Africa suffers behind despite global trends toward the production of natural polymers and expanding markets in Latin America, Europe, and Asia. Over the course of several decades, substitutes for cotton and natural rubber have decreased trade profits for developing nations and their comparative advantages in global industry. The development of natural polymers has had an impact on the scientific, technical, and technological fields of agriculture, medicine, pharmaceuticals, and the packaging industry, as well as the economies of countries, primarily in the West, through a variety of cutting-edge materials used in food and beverage, healthcare, and personal care products [
149].
9.1. Certain important natural polymers for economy.
Biodegradable polymers are divided into groups, among other criteria, based on their chemical structure, place of origin and synthesis, processing techniques, economic relevance, and usage. Depending on where they are derived from, biodegradable polymers can be divided into two groups: natural polymers (obtained from natural resources), and synthetic polymers (made from oil). The
Figure 13 depicts the global production capacity of various biopolymers in 2016 [
150] .
9.2. Industry Use of Natural Biodegradable Polymers
Biopolymers, also known as natural polymers, are polymers that come naturally during all stages of organism development. Activated monomers, which are often created inside of cells through intricate metabolic processes, are most frequently used in enzyme-catalyzed chain growth polymerization reactions in their creation (
Table 6) [
149].
10. Future Perspectives
10.1. Natural Polymer Bases in Gums for Food Applications
The incorporation of polyisoprenes in food, especially chewing gum. Initially, chewing gums were made from naturally occurring polymer-based materials like gutta and other gums like chicle that may be found in nature. More chewing gum companies started focused on using synthetic polymers to make chewing gums, such as polyethylene, polyvinyl acetate, styrene-butadiene copolymers, and isobutylene-isoprene copolymers, as chewing gum demand became more sophisticated and interest in synthetic polymers increased. Previously, natural guayule rubber was coupled with polyvinyl acetate and hydrogenated vegetable oil as plasticizers, emulsifiers, solvents, and inorganic fillers to make a chewing gum based on natural polymers [
6]. However, synthetic polymers are currently used in the great majority of chewing gum products on the market. Chewing gums produced of synthetic polymers are hydrophobic, which causes them to stick to surfaces despite their chosen consistency and capacity to attain desirable qualities.
Natural polymers offer more hydrophilic possibilities, enabling the production of non-stick chewing gum from natural substrates like natural rubber. The health risks associated with chewing gum swallowing, whether done on purpose or accidentally, are also expected to be lower with natural polymer-based chewing gums because they are more likely to be ingested. One of the disadvantages of using natural-based polymers for food applications, such as polyisoprene, is the presence of trans and cis isomeric forms and protein residues that may induce allergic reactions. In order to better eliminate these allergens from natural polymers, processing techniques have been improved. As a result, more food products will be utilised [
153].
10.2. Natural Polymer Trends and Prospects in the Cosmetics Industry
Biopolymers, also known as natural polymers, are polymers that occur spontaneously during all stages of organism development. They are generally created by enzyme-catalyzed chain growth polymerization reactions of activated monomers, which are typically created within cells through complicated metabolic processes [
149].
Numerous examples, but because to economic competitiveness, scientific study for physicochemical or sensory features of such polymer connections is restricted .looked into the possible connections between the rheological characteristics of xanthan gum XG and hydroxypropyl guar (HPG) and their filament-stretching abilities in both pure and mixed aqueous solutions as well as in cosmetic emulsions. Different rheological characteristics between XG and HPG were seen in pure solution. For concentrations below 1% w/w, XG solutions had higher stretchability values than HPG, while at higher concentrations, the filament stretching capabilities of both polymers were comparable. A significant synergistic impact for XG/HPG solution at a 25/75 ratio was seen in mixed solutions regardless of total concentration tested (below 0.5% w/w) [
154].
The effect of the synergy on the stretching characteristics is noteworthy because the interaction between XG and HPG increases the maximum filament length. Polysaccharides also have the benefit of resembling biological macromolecules, such as the extracellular matrix (ECM), which increases their potential for use in cell therapeutic approaches [
155].
In recent years, there has been a surge in interest in employing polysaccharide materials in tissue engineering [
156].Future tumor treatments may benefit from the use of polysaccharides derived from mushrooms, such as chizophyllan, lentinan, grifolan, polysaccharide-peptide complex (PSP), and polysaccharide-protein complex (PSK), which can stimulate the immune system and have an anticancer effect [
157]. Although they have been used for a very long time, mushrooms have recently become more popular in the treatment of cancer. Polysaccharides' use will most certainly increase in the future due to their safety. Several additional organic synthesizes and chemical modifications caused significant pollution, which might be decreased by switching to natural polysaccharides [
158]. Polysaccharides have been employed more and more in a variety of ways to regulate the distribution of various drugs, particularly due to their biocompatibility and biodegradability properties [
159].The various functional groups present on polysaccharide structures, including the hydroxyl, amino, and carboxylic acid groups, could be further modified to function as a particular biological tool in a variety of fields, such as vaccine adjuvants, drug carriers, tissue engineering scaffolds, and many other pharmacological activities [166]. Natural polysaccharides like glycogen, cellulose, and starch were engineered onto biologically superior molecules to support their biopharmaceutical candidacy using a variety of techniques, including chemical modification, co-polymer grafting, and atom transfer radical polymerization (ATRP) [
160].
Drugs with hydrophobic groups can be conjugated with polysaccharides that have hydrophilic groups, creating amphiphilic prodrugs that can self-assemble into nanostructures with improved water solubility. There are significant limitations and challenges for polysaccharides in the pharmaceutical and biological industries, nevertheless, because they are typically taken from natural sources. Examples include changes in viscosity from batch to batch, microbial contamination, viscosity reduction during storage, thickening, and an uncontrolled rate of hydration. Fortunately, these limitations may be avoided through modifications such grafting, cross-linking, and mixing with other natural, synthetic, and semi-synthetic polymers [
161].
In addition to polysaccharides naturally forming in combination with other molecules such as proteins and lipids, polysaccharide diversity in nature adds an additional significant obstacle to the extraction and purification of these polysaccharides. Their separation needs the employment of effective and accurate procedures to avoid co-extraction and contamination with other substances. Furthermore, discovering and fully appreciating the relationship between polysaccharide structure and activity may open up new avenues for utilization in biological and pharmaceutical applications [
162].
11. Conclusion
The majority of natural biopolymers are polysaccharides, which have a variety of chemical and physical properties that make them a strong candidate for use in numerous biological applications. In comparison to other synthetic polymers, polysaccharides have a number of benefits, such as being secure, affordable, stable, hydrophilic, biocompatible, and biodegradable. Additionally, they can be chemically modified and tailored for particular uses in a variety of applications, such as the creation of pharmaceutical materials, drug release agents, and plasma substitutes. Numerous biological functions of polysaccharides exist, such as immunoregulatory, anti-tumor, anti-virus, anti-inflammatory, antioxidative, and hypoglycemic effects. Polysaccharides have received a lot of attention in recent years, emerging as one of the most successful alternatives to traditional medicine. Carbohydrate-based pharmaceuticals have been proved to be successful in a number of disciplines, but they are not attracting the same level of attention as those based on proteins or nucleic acids. We completed our work by urging more research because there are many unanswered issues and undiscovered biological features of various polysaccharides. Further investigation and characterization of the structural activity relation of polysaccharides is necessary to fully explore their potential applications and get a better understanding of the precise mechanisms behind polysaccharides biological activities.
Acknowledgments
This work was also funded by the Programa Operacional Regional do Centro (CEN-TRO-04-3559-FSE-000162) within the European Social Fund (ESF), CICS-UBI (UIDP/00709/2020) financed by National Funds from Fundação para a Ciência e a Tecnologia (FCT), Community Funds (UIDB/00709/2020), by Fundação La Caixa and Fundação para a Ciência e Tecnologia (FCT) under the Programa Promove Project PD21-00023, and project PRR-C05-i03-I-000143.
References
- Balart, R.; Garcia-Garcia, D.; Fombuena, V.; Quiles-Carrillo, L.; Arrieta, M.P. Biopolymers from Natural Resources. Polymers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Singh, V.; Arora, A. Natural Biodegradable Polymers as Matrices in Transdermal Drug Delivery. International Journal of Drug Development and Research 2011, 3. [Google Scholar]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide Hydrogels for Modified Release Formulations. Journal of Controlled Release 2007, 119. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate Nanoparticles as Antituberculosis Drug Carriers: Formulation Development, Pharmacokinetics and Therapeutic Potential. Indian J Chest Dis Allied Sci 2006, 48. [Google Scholar]
- Qi, L.; Xu, Z. In Vivo Antitumor Activity of Chitosan Nanoparticles. Bioorg Med Chem Lett 2006, 16. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.; Olubowale, M.; Okereke, C. Microneedle-Assisted Transdermal Delivery of Acetylsalicylic Acid (Aspirin) from Biopolymer Films Extracted from Fish Scales. Polymer Bulletin 2018, 75. [Google Scholar] [CrossRef]
- Biodegradable Natural Polymers. Available online: Https://Link.Springer.Com/Chapter/10.1007/978-3-319-12478-0_2.
- Zhou, C.Y.; Wang, Y.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. The Effect of ATP Marination on the Depolymerization of Actin Filament in Goose Muscles during Postmortem Conditioning. Poult Sci 2018, 97, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Paquot, M.; Dubois, P. Les Polymères Issus Du Végétal: Matériaux à Proprieties Spécifiques Pour Des Applications Ciblées En Industrie Plastique. Biotechnology, Agronomy and Society and Environment 2006, 10.
- Pillai, O.; Dhanikula, A.B.; Panchagnula, R. Drug Delivery: An Odyssey of 100 Years. Curr Opin Chem Biol 2001, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, S.; Wang, Z. Pre-Treatment Optimization and Properties of Gelatin from Freshwater Fish Scales. Food and Bioproducts Processing 2011, 89. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv Drug Deliv Rev 2008, 60. [Google Scholar] [CrossRef] [PubMed]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef]
- Mohammed, A.M.A.; Yunus, N.Z.M.; Hezmi, M.A.; Rashid, A.S.A. Sequestration of Carbon Dioxide Using Ground Granulated Blast Furnaces Slag and Kaolin Mixtures. Global Nest Journal 2021, 23, 105–111. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility, Second Edition. CRC Press 2005.
- Yui, T.; Ogawa, K. X-Ray Diffraction Study of Polysaccharides. In Polysaccharides; 2004.
- Pérez, S.; Mazeau, K. Conformations, Structures, and Morphologies of Celluloses. In Polysaccharides; 2004.
- Habitat Suitability Index Models: Pronghorn.
- Kulasinski, K.; Keten, S.; Churakov, S. V.; Derome, D.; Carmeliet, J. A Comparative Molecular Dynamics Study of Crystalline, Paracrystalline and Amorphous States of Cellulose. Cellulose 2014, 21. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. Journal of Physical Chemistry 1995, 99. [Google Scholar] [CrossRef]
- Hebeish, A.; Guthrie, J.T. The Chemistry and Technology of Cellulosic Copolymers; 1981.
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose Modification by Polymer Grafting: A Review. Chem Soc Rev 2009, 38. [Google Scholar] [CrossRef] [PubMed]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem Rev 2015, 115. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Xu, B.; Chrusciel, M.; Gao, J.; Bazert, M.; Stelmaszewska, J.; Xu, Y.; Zhang, H.; Pawelczyk, L. Erratum: Functional Characterization of MicroRNA-27a-3p Expression in Human Polycystic Ovary Syndrome" (Endocrinology (2018) 159:1 (297-309) DOI: 10.1210/En.2017-00219). Endocrinology 2019, 159. [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur Polym J 2010, 46. [Google Scholar] [CrossRef]
- Zielke, C.; Stradner, A.; Nilsson, L. Characterization of Cereal β-Glucan Extracts: Conformation and Structural Aspects. Food Hydrocoll 2018, 79. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, J.; Zhao, Q.; Zheng, J. Structure and Characteristic of β-Glucan in Cereal: A Review. J Food Process Preserv 2015, 39. [Google Scholar] [CrossRef]
- Kuki, H.; Yokoyama, R.; Kuroha, T.; Nishitani, K. Xyloglucan Is Not Essential for the Formation and Integrity of the Cellulose Network in the Primary Cell Wall Regenerated from Arabidopsis Protoplasts. Plants 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, X. Strategies to Modify Physicochemical Properties of Hemicelluloses from Biorefinery and Paper Industry for Packaging Material. Rev Environ Sci Biotechnol 2018, 17. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog Polym Sci 2023, 140, 101675. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Annapure, U.S. Trends in “Green” and Novel Methods of Pectin Modification - A Review. Carbohydr Polym 2022, 278, 118967. [Google Scholar] [CrossRef] [PubMed]
- KAJIWARA, K.; MIYAMOTO, T. ChemInform Abstract: Progress in Structural Characterization of Functional Polysaccharides. ChemInform 2010, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, A. Microstructure, Thermodynamics and Rheological Properties of Different Types of Red Adzuki Bean Starch. Quality Assurance and Safety of Crops and Foods 2020, 12. [Google Scholar] [CrossRef]
- Farooq, U.; Di Mattia, C.; Faieta, M.; Flamminii, F.; Pittia, P. Colloidal Properties and Stability of Olive Oil-in Water Emulsions Stabilized by Starch Particles. Italian Journal of Food Science 2021, 33. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health Benefits of Resistant Starch: A Review of the Literature. J Funct Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Tan, L.; Kong, L. Starch-Guest Inclusion Complexes: Formation, Structure, and Enzymatic Digestion. Crit Rev Food Sci Nutr 2020, 60. [Google Scholar] [CrossRef]
- Zhu, Y.; Delbianco, M.; Seeberger, P.H. Automated Assembly of Starch and Glycogen Polysaccharides. J Am Chem Soc 2021, 143. [Google Scholar] [CrossRef]
- Jolly, G.; Kuscu, M.C.; Kokate, P.; Younis, M. A Low-Energy Key Management Protocol for Wireless Sensor Networks. In Proceedings of the Proceedings - IEEE Symposium on Computers and Communications; 2003.
- Kulkarni, V.; Butte, K.; Rathod, S. Natural Polymers – A Comprehensive Review. International Journal of Research in Pharmaceutical and Biomedical Sciences 2012, 3. [Google Scholar]
- Guizzardi, S.; Uggeri, J.; Belletti, S.; Cattarini, G. Hyaluronate Increases Polynucleotides Effect on Human Cultured Fibroblasts. Journal of Cosmetics, Dermatological Sciences and Applications 2013, 03. [Google Scholar] [CrossRef]
- Taravel, F.; Mazeau, K.; Tvaros¡ka, I. Computer Modeling of Polysaccharide–Polysaccharide Interactions. In Polysaccharides; 2004.
- Oerther, S.; Le Gall, H.; Payan, E.; Lapicque, F.; Presle, N.; Hubert, P.; Dexheimer, J.; Netter, P.; Lapicque, F. Hyaluronate-Alginate Gel as a Novel Biomaterial: Mechanical Properties and Formation Mechanism. Biotechnol Bioeng 1999, 63, 206–215. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar Drugs 2022, 20. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic Starches: Properties, Challenges, and Prospects. Starch/Staerke 2013, 65.
- I., C.; G., M.-S.; H., G.; A., B.-L.; A., D.; H., T.; A., F.; C., M.; F., L.; F., H. Causality and Preventability of Adverse Drug Reactions of Analgesics. Drug Saf 2013, 36.
- Onyeagoro, G.N. Reactive Compatibilization of Natural Rubber ( NR )/ Carboxylated Nitrile Rubber ( XNBR ) Blends By Maleic Anhydride-Grafted-Polyisoprene ( MAPI ) And Epoxy Resin Dual Compatibilizers. International Refereed Journal of Engineering and Science (IRJES) ISSN (Online) 2013, 2.
- Sirisinha, C.; Saeoui, P.; Guaysomboon, J. Oil and Thermal Aging Resistance in Compatibilized and Thermally Stabilized Chlorinated Polyethylene/Natural Rubber Blends. Polymer (Guildf) 2004, 45. [Google Scholar] [CrossRef]
- Naskar, N.; Debnath, S.C.; Basu, D.K. Novel Method for Preparation of Carboxylated Nitrile Rubber-Natural Rubber Blends Using Bis(Diisopropyl)Thiophosphoryl Polysulfides. J Appl Polym Sci 2001, 80. [Google Scholar] [CrossRef]
- Chumeka, W.; Pasetto, P.; Pilard, J.F.; Tanrattanakul, V. Bio-Based Triblock Copolymers from Natural Rubber and Poly(Lactic Acid): Synthesis and Application in Polymer Blending. Polymer (Guildf) 2014, 55. [Google Scholar] [CrossRef]
- Hummel, D.O. Polymeren-Lehrbuch: Polymer Chemistry - an Introduction. Von R. B. Seymour Und C. E. Carraher, Jr. Marcel Dekker Inc. New York-Basel 1981. XVI, 576 S., Zeichn., Geb., SFr. 80,-. Nachrichten aus Chemie, Technik und Laboratorium 1981, 29. [CrossRef]
- Sowunmi, S.; Ebewele, R.O.; Peters, O.; Conner, A.H. Differential Scanning Calorimetry of Hydrolysed Mangrove Tannin. Polym Int 2000, 49. [Google Scholar] [CrossRef]
- Santos, T.M.; Souza Filho, M. de S.M.; Caceres, C.A.; Rosa, M.F.; Morais, J.P.S.; Pinto, A.M.B.; Azeredo, H.M.C. Fish Gelatin Films as Affected by Cellulose Whiskers and Sonication. Food Hydrocoll 2014, 41. [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated Cellulose, a New Cellulose Product: Properties, Uses, and Commercial Potential. J. Appl. Polym. Sci., Appl. Polym. Symp. 1983, 37.
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated Cellulose: Morphology and Accessibility. J. Appl. Polym. Sci., Appl. Polym. Symp. 1983, 37.
- Preston, R.D.; Nicolai, E.; Reed, R.; Millard, A. An Electron Microscope Study of Cellulose in the Wall of Valonia Ventricosa. Nature 1948, 162. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Tanner, S.F.; Cartier, N.; Chanzy, H. High-Resolution Solid-State 13C Nuclear Magnetic Resonance Spectroscopy of Tunicin, an Animal Cellulose. Macromolecules 1989, 22. [Google Scholar] [CrossRef]
- Lee, K.Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the Use of Nanocellulose as Reinforcement in Polymer Matrix Composites. Compos Sci Technol 2014, 105. [Google Scholar] [CrossRef]
- Núñez, O.; Ikegami, T.; Kajiwara, W.; Miyamoto, K.; Horie, K.; Tanaka, N. Preparation of High Efficiency and Highly Retentive Monolithic Silica Capillary Columns for Reversed-Phase Chromatography by Chemical Modification by Polymerization of Octadecyl Methacrylate. J Chromatogr A 2007, 1156. [Google Scholar] [CrossRef]
- David, A.; Lewandrowski, K.U.; Josten, C.; Ekkernkamp, A.; Clasbrummel, B.; Muhr, G. Surgical Correction of Talipes Equinovarus Following Foot and Leg Compartment Syndrome; 1996.
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; 2009.
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int J Polym Sci 2011, 2011. [Google Scholar] [CrossRef]
- Maniñgat, C.C.; Juliano, B.O. Properties of Lintnerized Starch Granules from Rices Differing in Amylose Content and Gelatinization Temperature. Starch - Stärke 1979, 31. [Google Scholar] [CrossRef]
- Lim, S.T.; Lee, J.H.; Shin, D.H.; Lim, H.S. Comparison of Protein Extraction Solutions for Rice Starch Isolation and Effects of Residual Protein Content on Starch Pasting Properties. Starch/Staerke 1999, 51. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Lazim, N.A.M.; Selvakumaran, S. Natural Polysaccharide-Based Composites for Drug Delivery and Biomedical Applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; 2019.
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized Extraction of Pectin-like Polysaccharide from Suaeda Fruticosa Leaves: Characterization, Antioxidant, Anti-Inflammatory and Analgesic Activities. Carbohydr Polym 2018, 185. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.X. Strategies to Diversify Natural Products for Drug Discovery. Med Res Rev 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 2016, 79. [Google Scholar] [CrossRef] [PubMed]
- Bost, J.; Maroon, A.; Maroon, J. Natural Anti-Inflammatory Agents for Pain Relief. Surg Neurol Int 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr Polym 2018, 183. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Ahmed, S.A.; Tabish, M.; Hasnain, M.S. Natural Polysaccharides in Tissue Engineering Applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; 2019.
- Moradi, Z.; Kalanpour, N. Kefiran, a Branched Polysaccharide: Preparation, Properties and Applications: A Review. Carbohydr Polym 2019, 223. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-Amylase Inhibitory Activities of Nepalese Medicinal Herb Pakhanbhed (Bergenia Ciliata, Haw.). Food Chem 2008, 106. [Google Scholar] [CrossRef]
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Hypoglycemic and Antioxidant Potential of Coconut Water in Experimental Diabetes. In Proceedings of the Food and Function; 2012; Vol. 3.
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Arnoldi, A.; Kurowska, E.; Carroll, K.K.; Sirtori, C.R. Soy Protein Peptides Regulate Cholesterol Homeostasis in Hep G2 Cells. Journal of Nutrition 2000, 130. [Google Scholar] [CrossRef]
- O’connor, L.E.; Berry, J.W.; Weiss, J.; Gilbert, P. Guilt, Fear, Submission, and Empathy in Depression; 2002; Vol. 71;
- Berge, R.K.; Tronstad, K.J.; Berge, K.; Rost, T.H.; Wergedahl, H.; Gudbrandsen, O.A.; Skorve, J. The Metabolic Syndrome and the Hepatic Fatty Acid Drainage Hypothesis. In Proceedings of the Biochimie; 2005; Vol. 87.
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological Activities of Sulfated Polysaccharides from Tropical Seaweeds. Biomedicine and Pharmacotherapy 2010, 64. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides; Springer International Publishing, 2014; pp. 1–38.
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Rajan, D.K. Potential Uses of Fungal Polysaccharides as Immunostimulants in Fish and Shrimp Aquaculture: A Review. Aquaculture 2019, 500, 250–263. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Koryakina, L.P.; Vladimirov, L.N. Bitter Gentian Teas: Nutritional and Phytochemical Profiles, Polysaccharide Characterisation and Bioactivity. Molecules 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Usman, B.; Kaushik, N.; Hoffman, J.; Wang, D.; Saenko, K. VisDA: The Visual Domain Adaptation Challenge. 2017.
- Walstra, Pieter. Physical Chemistry of Foods; Marcel Dekker, 2003; ISBN 0824793552.
- Telis, V.R.N. Biopolymer Engineering in Food Processing; 2012.
- Phillips, G.O. Handbook of Hydrocolloids; Woodhead Pub, 2009; ISBN 9781845694142.
- Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Navarro-Rodríguez de Vera, C.; Fernández-López, M.; Viuda-Martos, M.; Fernández-López, J. Sustainability and Gender Perspective in Food Innovation: Foods and Food Processing Coproducts as Source of Macro- and Micro-Nutrients for Woman-Fortified Foods. Foods 2022, 11. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Viebke, C.; Al-Assaf, S.; Phillips, G.O. Food Hydrocolloids and Health Claims. Bioactive Carbohydrates and Dietary Fibre 2014, 4. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.J.; Chung, J.Y.; Lee, J.H.; Young, S.B.; Kim, Y.H. Natural and Synthetic Biomaterials for Controlled Drug Delivery. Arch Pharm Res 2014, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cleary, G.W. Transdermal Delivery Systems: A Medical Rationale. In Topical Drug Bioavailability, Bioequivalence, and Penetration; 1993.
- Fujimoto, T.; Enomoto, K.; Matuo, K.; Tojo, K. In Vivo Evaluation of Skin Permeability of Drugs after Applying Adhesive Transdermal Patches. In Proceedings of the AIChE Annual Meeting, Conference Proceedings; 2006.
- Sirbubalo, M.; Tucak, A.; Muhamedagic, K.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Cekic, A.; Begic-Hajdarevic, D.; Husic, M.C.; Dervišević, A.; et al. 3d Printing—a “Touch-Button” Approach to Manufacture Microneedles for Transdermal Drug Delivery. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tojo, K.; Chien, Y.W. Kinetics and Thermodynamics of Drug Permeation through Silicone Elastomers (II) Effect of Penetrant Lipophilicity. Drug Dev Ind Pharm 1986, 12. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Munjeri, O.; Bwititi, P.; Osim, E.E. Orally Administered, Insulin-Loaded Amidated Pectin Hydrogel Beads Sustain Plasma Concentrations of Insulin in Streptozotocin-Diabetic Rats. Journal of Endocrinology 2000, 164. [Google Scholar] [CrossRef]
- Suksaeree, J.; Boonme, P.; Taweepreda, W.; Ritthidej, G.C.; Pichayakorn, W. Characterization, in Vitro Release and Permeation Studies of Nicotine Transdermal Patches Prepared from Deproteinized Natural Rubber Latex Blends. Chemical Engineering Research and Design 2012, 90. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Tufts, M.A.; Mapanga, R.F. Synergistic Antihyperglycemic Effects between Plant-Derived Oleanolic Acid and Insulin in Streptozotocin-Induced Diabetic Rats. Ren Fail 2010, 32, 832–839. [Google Scholar] [CrossRef]
- Shi, W.; Dumont, M.J.; Ly, E.B. Synthesis and Properties of Canola Protein-Based Superabsorbent Hydrogels. Eur Polym J 2014, 54. [Google Scholar] [CrossRef]
- Deming, T.J. Synthetic Polypeptides for Biomedical Applications. Progress in Polymer Science (Oxford) 2007, 32. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J Pharm Sci 2022, 111. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int J Biol Macromol 2020, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and Delivery Mechanisms of Hyaluronic Acid Used for Topical/Transdermal Delivery – A Review. Int J Pharm 2020, 578. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kumar, M.; Pathak, K. A Review on Mucoadhesive Polymer Used in Nasal Drug Delivery System. J Adv Pharm Technol Res 2011, 2, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Mao, S. New Strategies to Improve the Intranasal Absorption of Insulin. Drug Discov Today 2010, 15. [Google Scholar] [CrossRef]
- Patil, S.B.; Sawant, K.K. Development, Optimization and in Vitro Evaluation of Alginate Mucoadhesive Microspheres of Carvedilol for Nasal Delivery. J Microencapsul 2009, 26. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, O.; Gedanken, A. The Development and Characterization of Starch Microspheres Prepared by a Sonochemical Method for the Potential Drug Delivery of Insulin. Macromol Chem Phys 2010, 211. [Google Scholar] [CrossRef]
- Illum, L.; Watts, P.; Fisher, A.N.; Hinchcliffe, M.; Norbury, H.; Jabbal-Gill, I.; Nankervis, R.; Davis, S.S. Intranasal Delivery of Morphine. Journal of Pharmacology and Experimental Therapeutics 2002, 301. [Google Scholar] [CrossRef]
- Coucke, D.; Schotsaert, M.; Libert, C.; Pringels, E.; Vervaet, C.; Foreman, P.; Saelens, X.; Remon, J.P. Spray-Dried Powders of Starch and Crosslinked Poly(Acrylic Acid) as Carriers for Nasal Delivery of Inactivated Influenza Vaccine. Vaccine 2009, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.Y.; Guo, J.; Xu, Y.; Li, H.Y.; Seville, P.C. Influence of Excipients on Spray-Dried Powders for Inhalation. Powder Technol 2014, 256. [Google Scholar] [CrossRef]
- Illum, L. Nasal Drug Delivery - Possibilities, Problems and Solutions. In Proceedings of the Journal of Controlled Release; 2003; Vol. 87.
- Pal, D.; Nayak, A.K. Novel Tamarind Seed Polysaccharide-Alginate Mucoadhesive Microspheres for Oral Gliclazide Delivery: In Vitro-in Vivo Evaluation. Drug Deliv 2012, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Williams, C.G.; Sun, D.D.N.; Wang, J.; Leong, K.; Elisseeff, J.H. Photocrosslinkable Polysaccharides Based on Chondroitin Sulfate. J Biomed Mater Res A 2004, 68. [Google Scholar] [CrossRef] [PubMed]
- Bragd, P.L.; Van Bekkum, H.; Besemer, A.C. TEMPO-Mediated Oxidation of Polysaccharides: Survey of Methods and Applications. Top Catal 2004, 27. [Google Scholar] [CrossRef]
- Ngwuluka, N.C. Responsive Polysaccharides and Polysaccharides-Based Nanoparticles for Drug Delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers; 2018.
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv Drug Deliv Rev 2013, 65. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for Colon Targeted Drug Delivery. Drug Delivery: Journal of Delivery and Targeting of Therapeutic Agents 2004, 11.
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem Rev 2013, 113. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Sinha, M.; Burgert, S. Reinfusion of Drained Blood as an Alternative to Homologous Blood Transfusion after Total Knee Replacement. Int Orthop 2001, 25, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent Development of Chitosan-Based Polyelectrolyte Complexes with Natural Polysaccharides for Drug Delivery. Int J Biol Macromol 2014, 64. [Google Scholar] [CrossRef]
- Arca, H.Ç.; Günbeyaz, M.; Şenel, S. Chitosan-Based Systems for the Delivery of Vaccine Antigens. Expert Rev Vaccines 2009, 8. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J Polym Environ 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Natural Polymer Biomaterials: Advanced Applications. In New Functional Biomaterials for Medicine and Healthcare; 2014.
- Braga, M.E.M.; Pato, M.T.V.; Silva, H.S.R.C.; Ferreira, E.I.; Gil, M.H.; Duarte, C.M.M.; de Sousa, H.C. Supercritical Solvent Impregnation of Ophthalmic Drugs on Chitosan Derivatives. Journal of Supercritical Fluids 2008, 44. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; De Sousa, H.C. Development of Natural-Based Wound Dressings Impregnated with Bioactive Compounds and Using Supercritical Carbon Dioxide. Int J Pharm 2011, 408. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, C.C.; Liao, J.W.; Yen, S.K. Vancomycin-Chitosan Composite Deposited on Post Porous Hydroxyapatite Coated Ti6Al4V Implant for Drug Controlled Release. Materials Science and Engineering C 2013, 33. [Google Scholar] [CrossRef]
- Hartmann, H.; Hossfeld, S.; Schlosshauer, B.; Mittnacht, U.; Pêgo, A.P.; Dauner, M.; Doser, M.; Stoll, D.; Krastev, R. Hyaluronic Acid / Chitosan Multilayer Coatings on Neuronal Implants for Localized Delivery of SiRNA Nanoplexes. Journal of Controlled Release 2013, 168. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.A.; Fekry, A.M.; Farghali, R.A. A Study of Calcium Carbonate/Multiwalled-Carbon Nanotubes/Chitosan Composite Coatings on Ti-6Al-4V Alloy for Orthopedic Implants. Appl Surf Sci 2013, 285. [Google Scholar] [CrossRef]
- Ma, S.L.; Lu, Z.W.; Wu, Y.T.; Zhang, Z.B. Partitioning of Drug Model Compounds between Poly(Lactic Acid)s and Supercritical CO2 Using Quartz Crystal Microbalance as an in Situ Detector. Journal of Supercritical Fluids 2010, 54. [Google Scholar] [CrossRef]
- Market for Bioplastics.
- Angeles de Frutos Carolina Manzano, M.; Rovira Virgili, U.; Angeles de Frutos, M.; Manzano Universitat Rovira Virgili, C. U Market Transparency, Market Quality and Sunshine Trading Col·lecció “DOCUMENTS DE TREBALL DEL DEPARTAMENT D’ECONOMIA-CREIP” DEPARTAMENT D’ECONOMIA-CREIP OVIRA I IRGILI DEPARTAMENT D’ECONOMIA DEPARTAMENT D’ECONOMIA-CREIP Facultat d’Economia i Empresa Market Transparency, Market Quality and Sunshine Trading; 2013.
- Mülhaupt, R. Green Polymer Chemistry and Bio-Based Plastics: Dreams and Reality. Macromol Chem Phys 2013, 214. [Google Scholar] [CrossRef]
- Carus, M.; Carrez, D.; Kaeb, H.; Venus, J. Level Playing Field for Bio-Based Chemistry and Materials. Nova Institute 2011. [Google Scholar]
- Gawel, E.; Pannicke, N.; Hagemann, N. A Path Transition towards a Bioeconomy-The Crucial Role of Sustainability. Sustainability (Switzerland) 2019, 11. [Google Scholar] [CrossRef]
- Brostow, W.; Datashvili, T.; Jiang, P.; Miller, H. Recycled HDPE Reinforced with Sol-Gel Silica Modified Wood Sawdust. Eur Polym J 2016, 76, 28–39. [Google Scholar] [CrossRef]
- Erwin, P.; Pohlen, M.; Beckman, J.E. The Outer Disks of Early-Type Galaxies. I. Surface-Brightness Profiles of Barred Galaxies. Astronomical Journal 2008, 135, 20–54. [Google Scholar] [CrossRef]
- Dorozhkin, S. V. Calcium Orthophosphate-Based Biocomposites and Hybrid Biomaterials. In Proceedings of the Journal of Materials Science; 2009; Vol. 44.
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; De Guzman, L.I. Breeding for Resistance to Varroa Destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Jiao, F.; Yuan, Q. Catalytic Dehydration of Bioethanol to Ethylene over TiO2/γ-Al2O3 Catalysts in Microchannel Reactors. Catal Today 2007, 125. [Google Scholar] [CrossRef]
- Available online: Http://Www.Sojitz.Com/En/News/2012/07 /20120705.Php.
- Brostow, W.; Datashvili, T.; Miller, H. WOOD AND WOOD DERIVED MATERIALS; 2010; Vol. 32;
- Teli, M.D.; Chiplunkar, V. Role of Thickeners in Final Performance of Reactive Prints. 1986.
- Qin, Y.; Cai, L.; Feng, D.; Shi, B.; Liu, J.; Zhang, W.; Shen, Y. Combined Use of Chitosan and Alginate in the Treatment of Wastewater. J Appl Polym Sci 2007, 104. [Google Scholar] [CrossRef]
- Xie, Z.P.; Huang, Y.; Chen, Y.L.; Jia, Y. A New Gel Casting of Ceramics by Reaction of Sodium Alginate and Calcium Iodate at Increased Temperatures. J Mater Sci Lett 2001, 20. [Google Scholar] [CrossRef]
- F-35 Joint Strike Fighter Structural Prognosis and Health Management an Overview.
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers (Basel) 2010, 2. [Google Scholar] [CrossRef]
- Holland, N.D.; Clague, D.A.; Gordon, D.P.; Gebruk, A.; Pawson, D.L.; Vecchione, M. “Lophenteropneust” Hypothesis Refuted by Collection and Photos of New Deep-Sea Hemichordates. Nature 2005, 434, 374–376. [Google Scholar] [CrossRef]
- Anyika, M.; Gholami, H.; Ashtekar, K.D.; Acho, R.; Borhan, B. Point-to-Axial Chirality Transfer - A New Probe for “Sensing” the Absolute Configurations of Monoamines. J Am Chem Soc 2014, 136. [Google Scholar] [CrossRef]
- MA Sayed Patwary; Kazi M Maraz; Shahirin Shahida; Arwah Ahmed; Ruhul A Khan A Review on the Properties and Applications of Biodegradable Polymers. GSC Advanced Research and Reviews 2021, 9. [CrossRef]
- Natural Polymers || Economic Impacts of Natural Polymers. [CrossRef]
- Percival, S.L.; Thomas, J.; Linton, S.; Okel, T.; Corum, L.; Slone, W. The Antimicrobial Efficacy of Silver on Antibiotic-Resistant Bacteria Isolated from Burn Wounds. Int Wound J 2012, 9, 488–493. [Google Scholar] [CrossRef]
- Farber, T.M.; Clewell, A.E.; Endres, J.R.; Hauswirth, J.; Van Gemert, M.; Schauss, A.G.; Sheane, C.A. Safety Assessment of a Novel Ingredient for Removable Chewing Gum. Food and Chemical Toxicology 2010, 48. [Google Scholar] [CrossRef]
- Jamshidian, M.; Jalal, S.; Jansen, C. MissMech: An R Package for Testing Homoscedasticity, Multivariate Normality, and Missing Completely at Random (MCAR); 2014; Vol. 56;
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and Polypeptide Based Injectable Thermo-Sensitive Hydrogels for Local Biomedical Applications. Int J Biol Macromol 2019, 133. [Google Scholar] [CrossRef]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for Tissue Engineering: Current Landscape and Future Prospects. Carbohydr Polym 2019, 205. [Google Scholar] [CrossRef]
- Pandya, U.; Dhuldhaj, U.; Sahay, N.S. Bioactive Mushroom Polysaccharides as Antitumor: An Overview. Nat Prod Res 2019, 33. [Google Scholar] [CrossRef]
- Maciel, J. V.; Durigon, A.M.M.; Souza, M.M.; Quadrado, R.F.N.; Fajardo, A.R.; Dias, D. Polysaccharides Derived from Natural Sources Applied to the Development of Chemically Modified Electrodes for Environmental Applications: A Review. Trends in Environmental Analytical Chemistry 2019, 22. [Google Scholar] [CrossRef]
- Layek, B.; Mandal, S. Natural Polysaccharides for Controlled Delivery of Oral Therapeutics: A Recent Update. Carbohydr Polym 2020, 230. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomedicine and Pharmacotherapy 2018, 107. [Google Scholar] [CrossRef] [PubMed]
- Saidin, N.M.; Anuar, N.K.; Meor Mohd Affandi, M.M.R. Roles of Polysaccharides in Transdermal Drug Delivery System and Future Prospects. J Appl Pharm Sci 2018, 8. [Google Scholar]
- Van Dam, J.E.G.; Van Den Broek, L.A.M.; Boeriu, C.G. Polysaccharides in Human Health Care. Nat Prod Commun 2017, 12. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).