1. Introduction
In recent years, infections caused by the multidrug-resistant extended-spectrum β-lactamase (ESBL)-producing Enterobacterales (ESBL-E) have become a serious issue in companion animals, as well as in humans [1,2]. Carbapenems are frequently used for the treatment of ESBL-E infections in human medicine but there is a risk of developing carbapenem-resistant Enterobacterales (CRE) [3,4]. Although the true prevalence of CRE in companion animals is unknown, there have been several reports on CRE isolation in dogs and cats worldwide [5,6]. Such prevalence of CRE in companion animals represents not only a serious concern in veterinary medicine but also potential public health threats by transmitting to surrounding people through close contact [7]. Therefore, the search for alternative drugs for the treatment of ESBL-E infections is a high-priority issue in veterinary medicine.
Flomoxef (FMX) is an oxacephem antibiotic that is resistant to degradation by ESBLs because of its characteristic structure, with a methoxy group at the 7S position [8]. In human medicine, flomoxef is an effective alternative to carbapenems for the treatment of ESBL-E infections [9–11]. Furthermore, we previously reported the high in vitro efficacy of FMX against ESBL-E derived from companion animals [12,13]. These findings suggest that FMX may be a potential alternative to carbapenems in companion animal medicine. However, there are insufficient reports on the pharmacokinetics (PK) of FMX in dogs, and a regimen of FMX for ESBL-E infections in dogs has yet to be established.
Recently, PK–pharmacodynamics (PD) analysis using Monte Carlo Simulation (MCS) has been used to study appropriate dosing regimens of antimicrobial drugs [14,15]. MCS can establish large virtual populations via the randomization of PK and PD indices and thereby estimate the probability of achieving antimicrobial efficacy (probability of target attainment, PTA) by dosage regimen [14,15]. The nonclinical PK–PD cutoff value is based on MCS analysis [16,17], which is a mathematical method that randomizes PK and PD indices by repeated random sampling. This allows estimating the PTA of antimicrobial efficacy and evaluating antimicrobial efficacy of the dosing regimen [16]. In this study, we first determined the PK parameters of FMX by administration experiments in healthy dogs. Next, based on the PK–PD relationship analyzed by MCS, we determined the nonclinical PK–PD cutoff values for dogs and proposed dosing regimens of FMX which can be clinically effective for ESBL-E infection in dogs.
2. Results
2.1. PK parameters of FMX in dogs
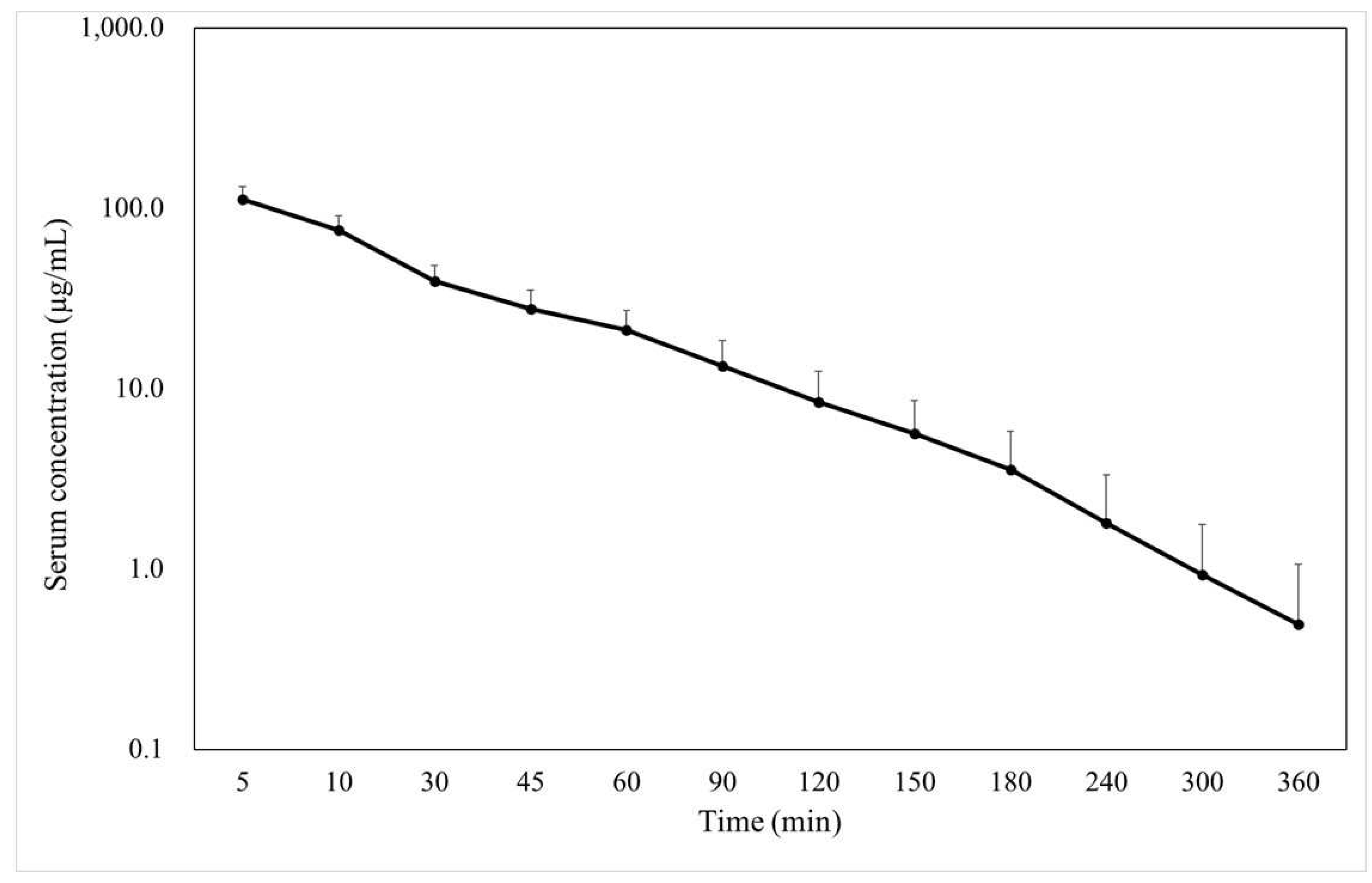
The blood concentration-time curve and PK parameters of FMX when bolusly intravenous administrated at 40 mg/kg are shown in
Figure 1 and
Table 1, respectively. Serum FMX concentration at 5 min was 111.82 ± 19.60 μg/mL, which decreased gradually.
We previously determined MICs of FMX in a total of 308 ESBL-producing isolates of Escherichia coli (n = 90), Klebsiella pneumoniae (n = 120), Proteus mirabilis (n = 29), and Enterobacter cloacae (n = 69), using the agar dilution method according to the Clinical and Laboratory Standards Institute guideline [12,13]. These investigations showed that the MIC50 and MIC90 (minimum concentrations that can inhibit 50% and 90% of isolates, respectively) of FMX were 0.125 and 4 µg/mL for Escherichia coli, 0.125 and 1 µg/mL for K. pneumoniae, 0.25 and 1 µg/mL for P. mirabilis, and 8 and >256 µg/mL for Enterobacter cloacae. The mean blood concentration was below the MIC90 for ESBL-K. pneumoniae and P. mirabilis after 150 min and that for ESBL-Escherichia coli after 180 min. Contrarily, for ESBL-Enterobacter cloacae, even the mean blood concentrations at 5 min did not exceed the MIC90.
2.2. Nonclinical PK-PD cutoff value of FMX in dogs
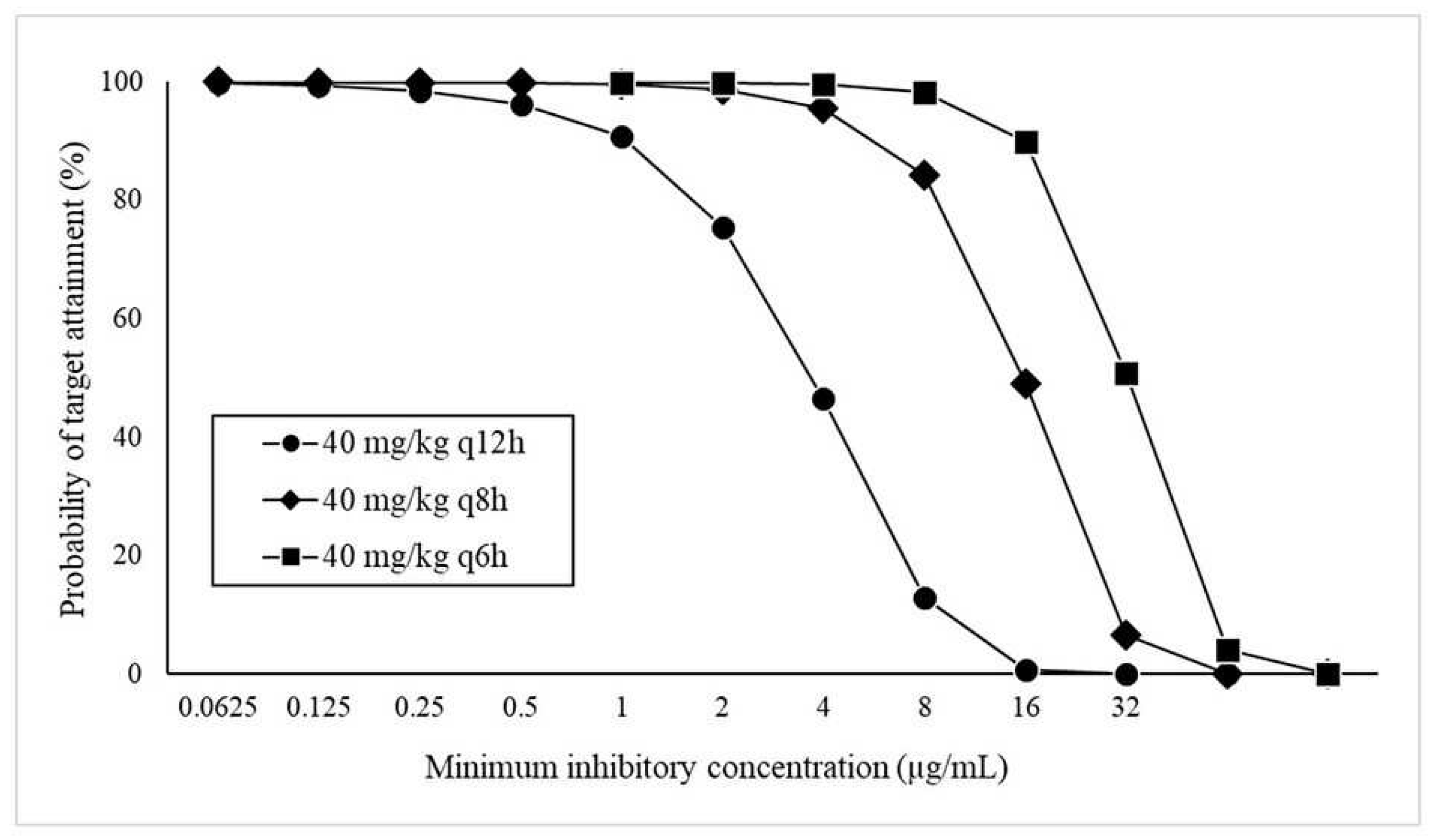
The PTA results of FMX at each MIC when administered at 40 mg/kg q12h, q8h, and q6h are shown in
Figure 2. All regimens achieved a PTA of more than 90% at MIC of ≤0.5 μg/mL but not at an MIC of ≥16 µg/mL. Based on the calculated PTA, the nonclinical PK–PD cutoff values at 40 mg/kg at every 12 hour (q12h), 8 hour (q8h), and 6 hour (q6h) were ≤0.5, ≤2, and ≤8 µg /mL, respectively.
2.2. CFR of FMX for ESBL-E infections in dogs
Table 2 shows the results of CFR calculated based on the wild-type MIC distribution of ESBL-E. Considering the estimated CFR, the regimens of 40 mg/kg q8h and q6h were optimal, and that of 40 mg/kg q12h was moderately successful for dogs infected with ESBL-
Escherichia coli, -
K. pneumoniae, and -
P. mirabilis. In contrast, none of regimens achieved CFR <80% for ESBL-
Enterobacter cloacae-infected dogs.
3. Discussion
While various alternatives to carbapenems have been investigated in humans, few have been studied in companion animals such as canines. This study is the first to report the usefulness of FMX, an oxacephem antibiotic used in humans, against ESBL-E infections in dogs, based on a PK–PD approach.
Although antimicrobial susceptibility breakpoints are essential as indicators for appropriate antimicrobial therapy, the breakpoint for FMX has not yet been established in both humans and animals. In this study, we attempted to establish canine-specific nonclinical PK–PD cutoff values by using MCS analysis. Our data showed that nonclinical PK–PD cutoff values for FMX increase with shorter dosing intervals, as previously reported in humans [23]. In addition, these nonclinical PK–PD cutoff values are higher than the MIC90 of ESBL-K. pneumoniae and -P. mirabilis (1 µg/mL each) and that of ESBL-Escherichia coli (4 µg/mL) [12,13] when administered at q8h and q6h, respectively. In addition, the CFR simulated in this study suggests that the q6h and q8h regimens of FMX are appropriate for treatment of ESBL-Escherichia coli, -K. pneumoniae and -P. mirabilis infections in dogs. The similar dosing intervals were proposed to achieve bactericidal concentrations against ESBL-E infections as found in human patients based on PK–PD simulation [23]. These findings in our study indicate that FMX administration at shorter dose intervals can be an alternative treatment for ESBL-Escherichia coli, -K. pneumoniae and -P. mirabilis infections in dogs.
In contrast, for, all of nonclinical PK–PD cutoff values calculated in this study were lower than FMX MIC90 for ESBL-Enterobacter cloacae (>256 µg/mL) [13]. This finding supports that the CFR for ESBL-Enterobacter cloacae was not even moderately successful. It is known that Enterobacter cloacae has an inducible chromosomal AmpC β-lactamase, which can be induced by cephamycins, including oxacephems [24,25]. Therefore, FMX is unlikely to be a candidate drug for ESBL-Enterobacter cloacae infections in dogs. However, infection with ESBL-Enterobacter cloacae is less prevalent in companion animals [26].
The optimal dose of FMX in dogs has not yet been established. In this study, we used 40 mg/kg per dose, referencing the human dosage (i.e., a maximum of 37.5 mg/kg four times per day) and investigated the blood PK of FMX in dogs when bolusly administered at this dose. The results revealed similar values for elimination half-life and clearance per body weight, compared with those in healthy human subjects, 44.2–46.2 min and 15.14 L/h, respectively [27,28]. This implies that the elimination rate of FMX in dogs is comparable to that in humans, although the protein binding rate is much lower in dogs (8%) than in humans (36.2%) [28]. Mitsuzono et al. [29] estimated that the no-observed-effect level of FMX in dogs is 200 mg/kg/day based on a 6-month intravenous toxicity study. Therefore, we believe that the dosing regimens in this study (40 mg/kg q12h, q8h, and q6h) are fully acceptable from the viewpoint of safety.
This study has several limitations. First, we used only a small number of dogs to calculate the PK parameters because of animal welfare concerns. However, we increased the reliability of these parameters by using bootstrap replicates. Second, PK parameters were calculated using healthy beagle dogs and may differ from those in dogs with renal failure, as reported in a human study [30]. Third, PK parameters of FMX are also different among body tissues [29], underlining the need to investigate the parameters in sites other than blood in dogs. Nevertheless, we believe that FMX has clinical efficacy for dogs with urinary tract infections as well as bacteremia, and possibly soft-tissue infection because almost all of the drug metabolites are excreted into the urine [31] as a result of the extremely low protein binding rate [19].
4. Materials and Methods
4.1. Animals
The animal experiments in this study were conducted under an ethics committee-approved protocol in accordance with the Tottori University Animal Use Committee (Approval No. 19-T-17). Five beagle dogs were used in this study (four males and one female, aged 6.2±1.8 years and weighing 13.6±1.7 kg, SHIMIZU Laboratory Supplies Co., Ltd., Kyoto, Japan). The dogs were individually housed in each cage and confirmed to be clinically healthy based on physical tests, blood tests, and image examination prior to the study. They did not receive any medications in the 6 months prior to the examination. They were fed the same commercial food (Aiken Genki, Unicharm Corporation, Tokyo, Japan) and were individually housed in separate cages in the same room at the experiment animal facility.
4.2. Drug administration and serum sampling
A central venous catheter (Covidien Japan, Inc., Tokyo) was placed in the jugular vein under general anesthesia on the day before the drug administration. Anesthesia was induced by intravenously administering propofol (4 mg/kg body weight, Propoflo, DS Pharma Animal Health Co., Ltd., Osaka, Japan), and subsequently intubated with a cuffed endotracheal tube. The vaporizer was adjusted to deliver 2% isoflurane (ISOFLURANE Inhalation Solution, Mylan EPD G.K., Tokyo, Japan) at an oxygen flow rate of 2 L/min. Flomoxef (FMX) (Shionogi Co. Ltd, Osaka, Japan) was dissolved in water for injection (Nissin Pharmaceutical Co., Ltd., Yamagata, Japan) and was bolusly administered at 40 mg/kg through the radial skin vein. Three-mL blood samples were collected from a central venous catheter before administration and 2 mL at 5, 10, 30, 45, 60, 90, 120, 150, 180, 240, 300, and 360 min after administration. Serum samples were obtained after centrifugation at 1,300× g for 10 min after coagulation and stored at −80°C until analysis.
4.3. Determination of serum concentrations of FMX in dogs
Calculation of FMX concentration in serum samples was outsourced to NDTS, Inc. (Hokkaido, Japan). Briefly, as an internal standard (IS), 200 µL of latamoxef (LMX) sodium (Shionogi, Osaka, Japan) solution (1 µg/mL) was added to the same volume of serum. After 100 μL of 20% sulfosalicylic acid was added, the IS was mixed vigorously for 30 sec and centrifuged at 12,000× g for 5 min. Then 250 μL of supernatant was collected and mixed with 250 μL of 100 mM acetic acid solution. The mixture was subjected to solid-phase extraction using Oasis HLB (1 cc, 30 mg; Waters, USA). After loading, each sample was washed with 1 ml of 20 mM aqueous acetic acid solution, followed by elution with 1 mL of methanol. The eluted solution was dried at 35°C under a stream of nitrogen and then dissolved into 100 μL of methanol. High-performance liquid chromatograph-tandem mass spectrometry (LC–MS/MS) was performed on a high-performance liquid chromatography-mass spectrometer (Prominence and LCMS-8045 tandem mass spectrometer, Shimadzu Corporation, Kyoto, Japan). Separation by high-performance liquid chromatography was performed using two solutions: mobile phase A, 10 mM ammonium formate solution, and mobile phase B, 10 mM ammonium formate plus methanol, with the following gradient conditions: 5% (0 min)–40% (6 min)–100% (8 min)–100% (10 min)–5% (10.5 min). After 5 µL of sample was injected, target molecules were separated on a C18 reversed-phase column (Cadenza CD-C18, 3.0 mm i.d. × 150 mm, intact, Kyoto, Japan), which was controlled at a temperature under 40°C. Mass spectrometry was performed in electrospray ionization (positive) and multiple monitoring reaction mode at a capillary voltage of 4.5 Kv, source (DL) temperature of 250°C, nebulization gas 180 L/hr, and drench gas 10 L/min. LMX was detected at monitor ion m/z = 521 > 137, collision energy 27 V, and FMX at m/z = 497 > 137, collision energy 26 V. The area under the peak was determined by the analytical software LCMS solution (Shimadzu Corporation, Kyoto, Japan). The FMX concentration in each sample was calculated using a calibration curve with the serum obtained before drug administration, to which a known concentration of FMX sodium (Shionogi Co., Ltd.) had been added.
4.4. Calculation of PK parameters
MCS was performed using commercial software (Oracle Crystal Ball version 11.1.2.4.850, Kozo Keikaku Engineering Inc., Tokyo, Japan) to calculate PTA based on the PK and PD parameters of FMX at a 40 mg/kg bolus dose at q12h, q8h, and q6h. The PK parameters from the non-compartment model were calculated using the package PK (ver. 4.0.3) of R software [18] based on serum FMX concentrations in five dogs.
4.5. Monte Carlo simulation
Based on log-normally distributed PK parameters, 10,000 virtual patients were generated for each dosing regimen to construct drug serum concentration-time profiles. The percentage of time that the unbound drug concentration was above the MIC (fTAM), based on the serum protein binding rate of 8% [19], was employed as the PK–PD index to determine the optimal dosing regimen. The PK–PD target value was set as ≥40% according to a previous study [20]. The nonclinical PK–PD cutoff was calculated as the highest MIC that achieved a PTA of ≥90% [17,21]. The cumulative fraction of response (CFR) was calculated based on the wild-type MIC distribution, of which FMX in ESBL-E (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae) isolates from companion animals were determined in the previous studies [12,13]. A regimen with a CFR of ≥90% is defined as optimal, and a regimen with a CFR of 80–90% is defined as moderately successful [22].
5. Conclusions
In conclusion, we calculated nonclinical PK–PD cutoff values at 40 mg/kg FMX q12h, q8h, and q6h by MCS and estimated CFR based on the MIC distribution of wild-type ESBL-E. Our results indicated that q8h and q6h dosage regimens of 40 mg/kg FMX are effective non-carbapenem treatment options for infections with ESBL-Escherichia coli, -P. mirabilis, and -K. pneumoniae. However, ESBL-Enterobacter cloacae infection in dogs cannot be treated with FMX. We believe that these results provide a basis for the use of FMX in dogs with ESBL-E infections. However, further clinical studies are required to confirm the efficacy of FMX.
Author Contributions
Conceptualization, K.H. and T.M.; methodology, M.K., M.J, and K.H.; software, T.M. and K.H..; validation, M.K. and K.H..; formal analysis, K.H.; investigation, M.K., M.J. and K.H..; resources, M.K. and K.H.; data curation, M.K. and M.J.; writing—original draft preparation, M.K.; writing—review and editing, T.M. and K.H.; supervision, K.H..; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant number 21K05917 (Japan Society for the Promotion of Science).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Tottori University Animal Use Committee of Tottori University (Approval number: 19-T-17).
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors would like to thank Shionogi Co., Ltd. for providing FMX sodium.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing Escherichia coli in dogs and cats - A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, Q.; Zheng, B.; Ji, J.; Ying, C.; Yu, X.; Wang, H.; Xiao, Y. Simulating moxalactam dosage for extended-spectrum β-lactamase-producing Enterobacteriaceae using blood antimicrobial surveillance network data. Infect. Drug Resist. 2019, 12, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Giamarellou, H. Carbapenem-sparing strategies for ESBL producers: When and How. Antibiotics (Basel) 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- KuKanich, K.; Burklund, A.; McGaughey, R.; Muturi, N.; Thomason, S.; Chengappa, M.M.; Garrison, I.; Stacey, B.; Zhang, S.; Gull, T. One Health approach for reporting veterinary carbapenem-resistant Enterobacterales and other bacteria of public health concern. Emerg. Infect. Dis. 2023, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roscetto, E.; Varriale, C.; Galdiero, U.; Esposito, C.; Catania, M.R. Extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacterales in companion animals and animal-assisted intervention dogs. Int. J. Environ. Res. Public Health 2021, 18, 12952. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A.; Carreras, I. Activities of β-lactam antibiotics against Escherichia coli strains producing extended spectrum β-lactamases. Antimicrob. Agents Chemother. 1990, 34, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Su, L.H.; Tang, Y.F.; Liu, J.W. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J. Antimicrob. Chemother. 2006, 58, 1074–1077. [Google Scholar] [CrossRef]
- Horie, A.; Nariai, A.; Katou, F.; Abe, Y.; Saito, Y.; Koike, D.; Hirade, T.; Ito, T.; Wakuri, M.; Fukuma, A. Increased community-acquired upper urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli in children and the effficacy of flomoxef and cefmetazole. Clin. Exp. Nephrol. 2019, 23, 1306–1314. [Google Scholar] [CrossRef]
- Darlow, C.A.; Hope, W. Flomoxef for neonates: extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J. Antimicrob. Chemother. 2022, 77, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Harada, K.; Tsuyuki, Y.; Kimura, Y.; Miyamoto, T.; Hatoya, S.; Hikasa, Y. In vitro efficacy of 16 antimicrobial drugs against a large collection of β-lactamase-producing isolates of extraintestinal pathogenic Escherichia coli from dogs and cats. J. Med. Microbiol. 2017, 66, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Kanao, Y.; Narita, H.; Jitsuiki, M.; Iyori, K.; Tsunoi, M.; Tsuyuki, Y.; Torii, K.; Harada, K. In vitro efficacy of cephamycins against multiple extended-spectrum β-lactamase-producing Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae isolates from dogs and cats. J. Vet. Med. Sci. 2023, 85, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Matsuo Tatsumi, Y.; Wajima, T.; Nakamura, R.; Tsuji, M. Evaluation of antibacterial activities of flomoxef against ESBL producing Enterobacteriaceae analyzed by Monte Carlo simulation. Jpn. J. Antibiot. 2013, 66, 71–86. [Google Scholar] [PubMed]
- Yamada, T.; Minami, K.; Oda, K.; Suzuki, K.; Nishihara, M.; Uchiyama, K.; Ukimura, A. Probability of target attainment of oral antimicrobials for Escherichia coli and Klebsiella pneumoniae based on Monte Carlo simulations. Diagn. Microbiol. Infect. Dis. 2022, 103, 115662. [Google Scholar] [CrossRef] [PubMed]
- Trang, M.; Dudley, M.N.; Bhavnani, S.M. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr. Opin. Pharmacol. 2017, 36, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Clinical and laboratory Standards Institute. Development of In Vitro Susceptibility Test Methods, Breakpoints, and Quality Control Parameters. 6th ed. CLSI guideline M23. 2023.
- Jaki, T.; Wolfsegger, M.J. Estimation of pharmacokinetic parameters with the R package PK. Pharmaceut. Statist. 2010, 10, 284–288. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakashimizu, H.; Nakano, M.; Otsubo, R.; Matsubara, H.; Yoshida, T. Pharmacokinetic characterization of 6315-S (flomoxef) in experimental animals. Chemotherapy 1987, 35 (Suppl. 1), 226–250. [Google Scholar]
- Tashiro, S.; Hayashi, M.; Takemura, W.; Igarashi, Y.; Liu, X.; Mizukami, Y.; Kojima, N.; Enoki, Y.; Taguchi, K.; Yokoyama, Y.; Nakamura, T.; Matsumoto, K. Pharmacokinetics/pharmacodynamics evaluation of flomoxef against extended-spectrum beta-lactamase-producing Escherichia coli in vitro and in vivo in a murine thigh infection model. Pharm. Res. 2021, 38, 27–35. [Google Scholar] [CrossRef]
- Papich, M.G. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimens for the prudent use of antimicrobial drugs. Vet. Microbiol. 2014, 171, 480–486. [Google Scholar] [CrossRef]
- Wang, G.; Yu, W.; Cui, Y.; Shi, Q.; Huang, C.; Xiao, Y. Optimal empiric treatment for KPC-2-producing Klebsiella pneumoniae infections in critically ill patients with normal or decreased renal function using Monte Carlo simulation. BMC Infect. Dis. 2021, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Ohge, H.; Ikawa, K.; Uegami, S.; Watadani, Y.; Shigemoto, N.; Yoshimura, K.; Kitagawa, H.; Kaiki, Y.; Morikawa, N.; Takahashi, S. Pharmacokinetics of flomoxef in plasma, peritoneal fluid, peritoneum, and subcutaneous adipose tissue of patients undergoing lower gastrointestinal surgery: dosing considerations based on site-specific pharmacodynamic target attainment. J. Infect. Chemother. 2023, 29, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C.; Chin, N.X. In vitro activity and β-lactamase stability of a new difluoro oxacephem, 6315-S. Antimicrob. Agents Chemother. 1986, 30, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC β-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High prevalence of extended-spectrum β-lactamase producing Enterobacteriaceae among clinical isolates from cats and dogs admitted to a veterinary hospital in Switzerland. Front. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, K.; Okamoto, Y.; Maehara, K.; Mase, K.; Iida, Y.; Yoshioka, S.; Yamada, H.; Yoshida, T.; Oguma, T.; Kimura, Y.; Hirauchi, M. Clinical study on 6315-S (flomoxef). Chemotherapy (Tokyo) 1987, 35 (Suppl. 1), 494–517. [Google Scholar]
- Hamada, Y.; Kasai, H.; Suzuki-Ito, M.; Matsumura, Y.; Doi, Y.; Hayakawa, K. Pharmacokinetic/pharmacodynamic analysis and dose optimization of cefmetazole and flomoxef against extended-spectrum β-lactamase-producing Enterobacterales in patients with invasive urinary tract infection considering renal function. Antibiotics (Basel) 2022, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzono, T.; Isowa, K.; Ogawa, H.; Kashima, H.; Aoyama, N.; Ohbara, D.; Ishimura, K.; Hatano, M.; Harada, Y. Intravenous chronic toxicity study of 6315-S (flomoxef) in beagles. Chemotherapy (Tokyo) 1987, 35 (Suppl. 1), 292–314. [Google Scholar]
- Andrassy, K.; Koderisch, J.; Gorges, K.; Sonntag, H.; Hirauchi, K. Pharmacokinetics and hemostasis following administration of a new, injectable oxacephem (6315-S, flomoxef) in volunteers and in patients with renal insufficiency. Infection 1991, 19 (Suppl 5), S296–302. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakashimizu, H.; Nakano, M.; Otsubo, R.; Matsubara, H.; Yoshida, T. Pharmacokinetic characterization of 6315-S (flomoxef) in experimental animals. Chemotherapy 1987, 35 (Suppl. 1), 161–175. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).