Submitted:
23 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
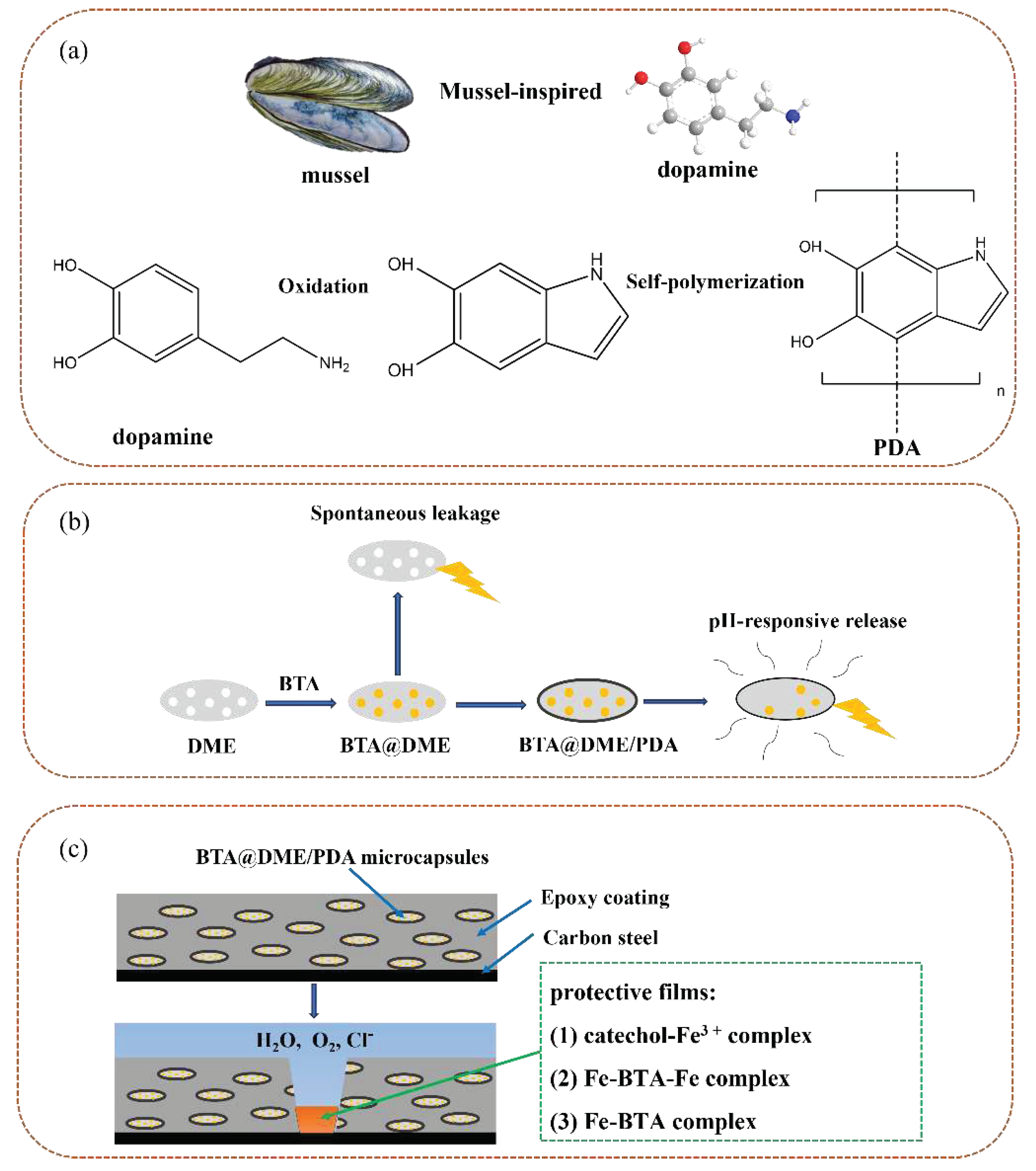
2.2. Preparation of BTA@DME/PDA microcapsules
2.3. Preparation of self-healing coatings
2.4. Characterization
3. Results and discussion
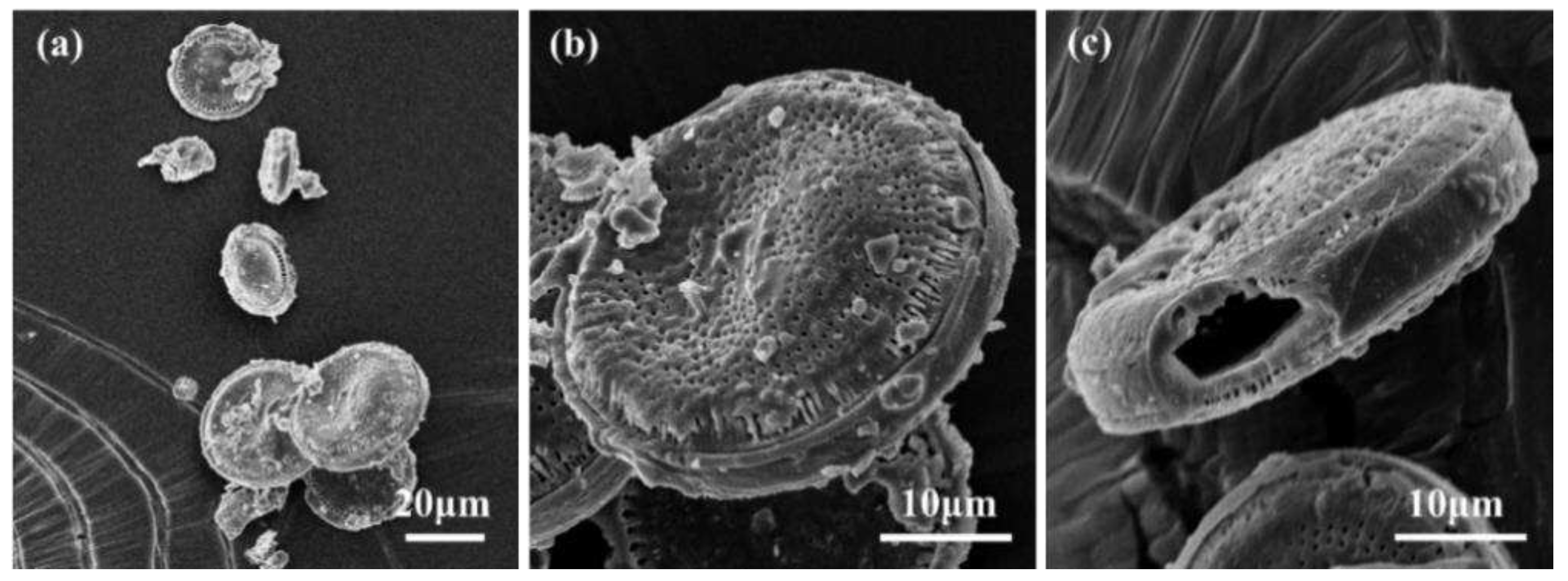
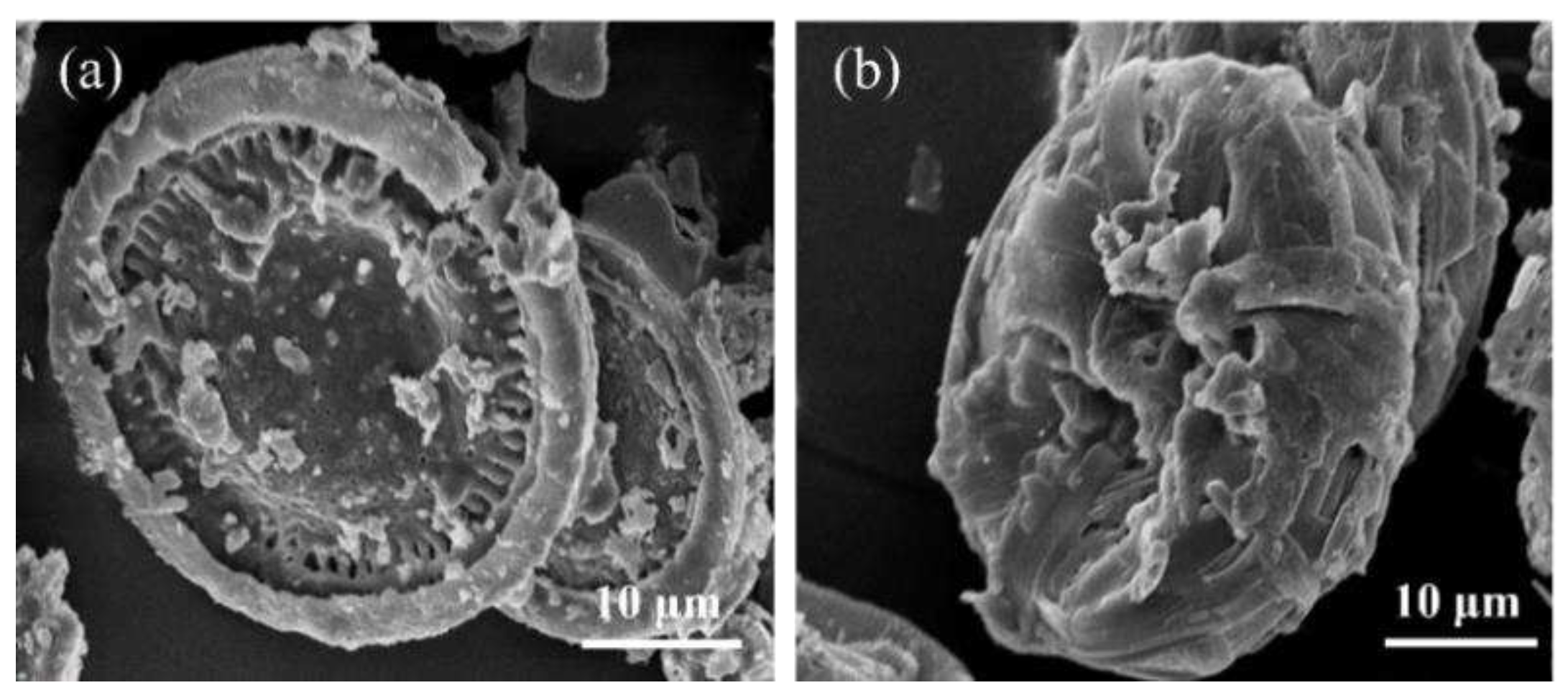
3.1. SEM morphology of microcapsules
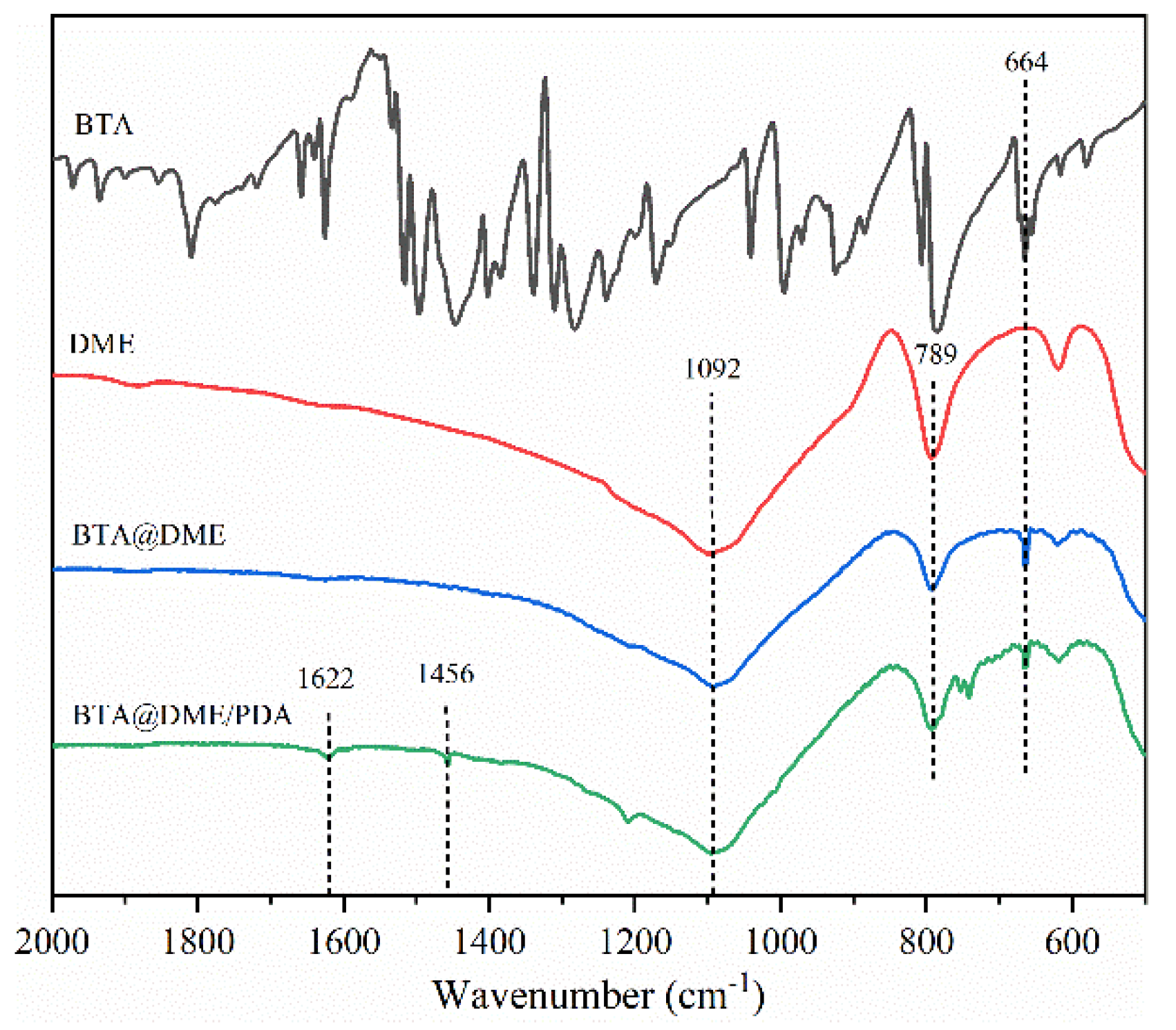
3.2. Chemical structure of BTA@DME/PDA microcapsules
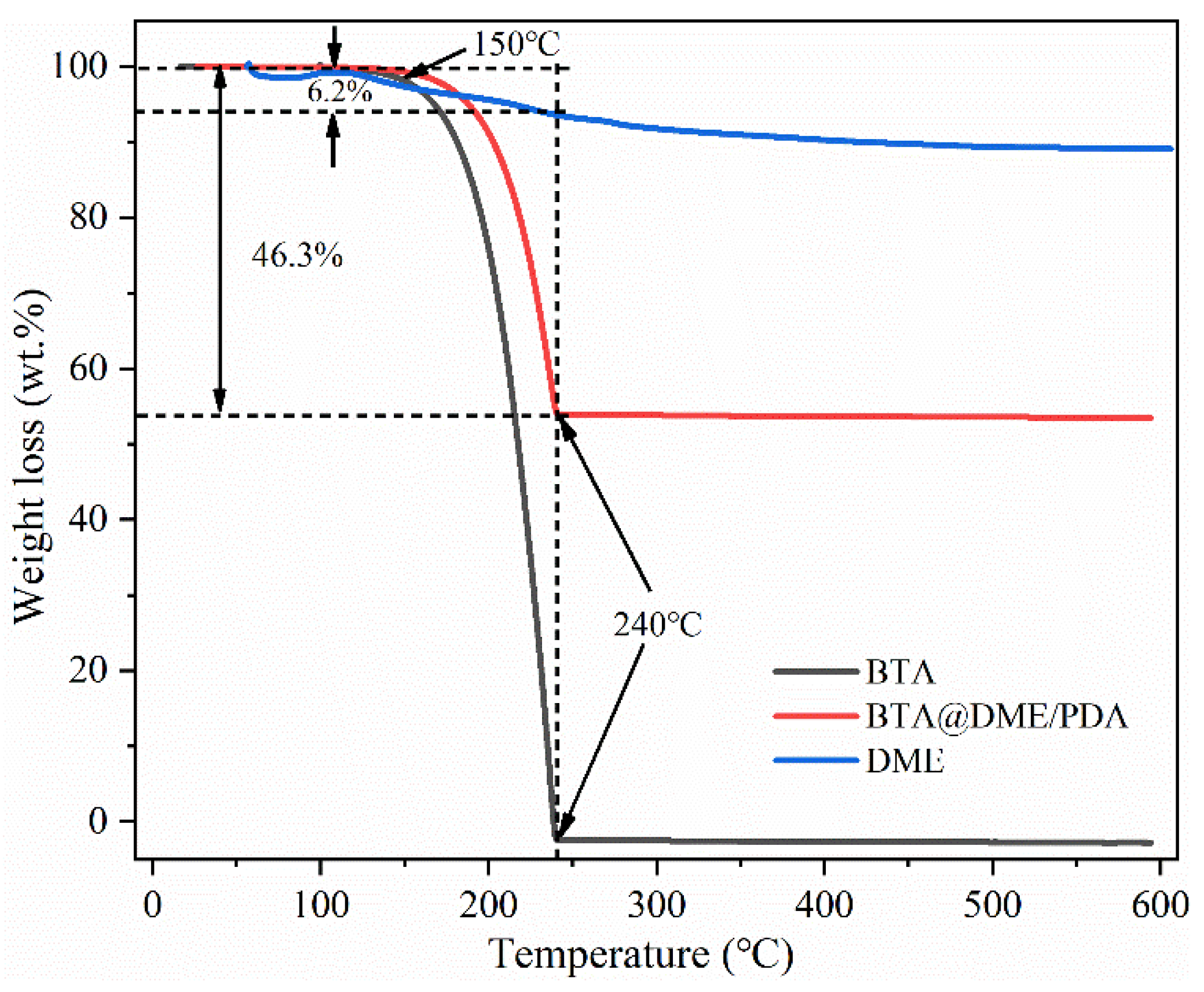
3.3. Loading capacity of BTA@DME/PDA microcapsules
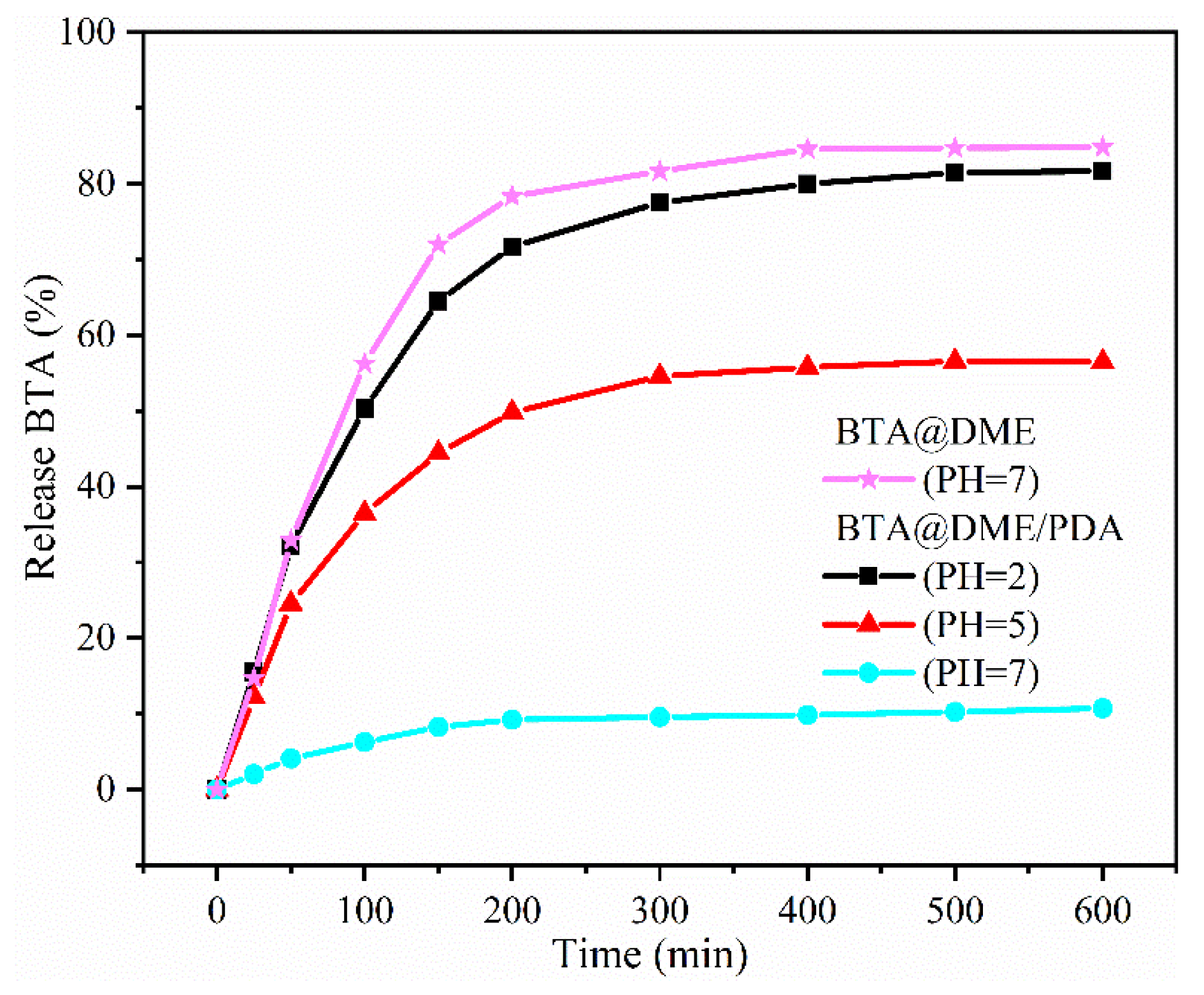
3.4. The pH responsiveness of BTA@DME/PDA microcapsules
3.5. Anticorrosion performance of epoxy coatings containing microcapsules
4. Anticorrosion mechanism of self-healing coating
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Cozzarini L, Marsich L, Schmid C. Ant-nest corrosion failure of heat exchangers copper pipes[J]. Engineering Failure Analysis, 2020, 109: 104387. [CrossRef]
- Xue S, Shen R, Chen W, et al. Corrigendum to "Corrosion fatigue failure analysis and service life prediction of high strength steel wire" [J]. Engineering Failure Analysis, 2021, 129: 105665. [CrossRef]
- Samiee R, Ramezanzadeh B, Mahdavian M, et al. Designing a non-hazardous nano-carrier based on graphene oxide@ Polyaniline-Praseodymium (III) for fabrication of the Active/Passive anti-corrosion coating[J]. Journal of Hazardous Materials, 2020, 398: 123136. [CrossRef]
- Li P, He X, Huang T C, et al. Highly effective anti-corrosion epoxy spray coatings containing self-assembled clay in smectic order[J]. Journal of Materials Chemistry A, 2015, 3(6): 2669-2676. [CrossRef]
- Dehghani A, Bahlakeh G, Ramezanzadeh B. Designing a novel targeted-release nano-container based on the silanized graphene oxide decorated with cerium acetylacetonate loaded beta-cyclodextrin (β-CD-CeA-MGO) for epoxy anti-corrosion coating[J]. Chemical Engineering Journal, 2020, 400: 125860. [CrossRef]
- Cui J, Li X, Pei Z, et al. A long-term stable and environmental friendly self-healing coating with polyaniline/sodium alginate microcapsule structure for corrosion protection of water-delivery pipelines[J]. Chemical Engineering Journal, 2019, 358: 379-388. [CrossRef]
- Blaiszik B J, Kramer S L B, Olugebefola S C, et al. Self-healing polymers and composites[J]. Annual review of materials research, 2010, 40(1): 179-211. [CrossRef]
- Shchukin D G, Möhwald H. Self-repairing coatings containing active nanoreservoirs[J]. small, 2007, 3(6): 926-943. [CrossRef]
- Grigoriev D, Akcakayiran D, Schenderlein M, et al. Protective organic coatings with anticorrosive and other feedback-active features: micro-and nanocontainers-based approach[J]. Corrosion, 2014, 70(5): 446-463. [CrossRef]
- Shchukin D G, Zheludkevich M, Yasakau K, et al. Layer-by-layer assembled nanocontainers for self-healing corrosion protection[J]. Advanced Materials, 2006, 18(13): 1672-1678. [CrossRef]
- Shchukin D G, Grigoriev D O, Möhwald H. Application of smart organic nanocontainers in feedback active coatings[J]. Soft Matter, 2010, 6(4): 720-725. [CrossRef]
- Ullah H, M Azizli K A, Man Z B, et al. The potential of microencapsulated self-healing materials for microcracks recovery in self-healing composite systems: A review[J]. Polymer reviews, 2016, 56(3): 429-485 . [CrossRef]
- Suryanarayana C, Rao K C, Kumar D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings[J]. Progress in organic coatings, 2008, 63(1): 72-78. [CrossRef]
- Brown E N, White S R, Sottos N R. Microcapsule induced toughening in a self-healing polymer composite[J]. Journal of Materials Science, 2004, 39(5): 1703-1710. [CrossRef]
- Tavandashti N P, Ghorbani M, Shojaei A, et al. Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects[J]. Corrosion Science, 2016, 112: 138-149. [CrossRef]
- Qian B, Zheng Z, Michailids M, et al. Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection[J]. ACS applied materials & interfaces, 2019, 11(10): 10283-10291. [CrossRef]
- Joo J B, Zhang Q, Lee I, et al. Mesoporous anatase titania hollow nanostructures though silica-protected calcination[J]. Advanced Functional Materials, 2012, 22(1): 166-174. [CrossRef]
- Zheng Z, Schenderlein M, Huang X, et al. Influence of functionalization of nanocontainers on self-healing anticorrosive coatings[J]. ACS Applied Materials & Interfaces, 2015, 7(41): 22756-22766. [CrossRef]
- Konuklu Y, Ersoy O, Gokce O. Easy and industrially applicable impregnation process for preparation of diatomite-based phase change material nanocomposites for thermal energy storage[J]. Applied Thermal Engineering, 2015, 91: 759-766. [CrossRef]
- Zhang L, Zhibo H U , Sun Z , et al. Research on Diatom Mineral Content of Each Grain Size from Niger Diatomite[J]. China Powder Science and Technology, 2015.
- Y. Noshi,A. Kobayashi,T. Uda. Model for Predicting Bathymetric and Grain Size Changes Considering Equilibrium Slopes Corresponding to Composition of Grain Size and Each Grain Size[J]. Journal of Coastal Research,2009. [CrossRef]
- Liu J, Zhao D F. The present situation and development of diatomite[J]. Environmental Science and Management, 2009, 34(5): 104-106.
- Wen R, Zhang X, Huang Z, et al. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2018, 178: 273-279. [CrossRef]
- Rao Z, Zhang G, Xu T, et al. Experimental study on a novel form-stable phase change materials based on diatomite for solar energy storage[J]. Solar energy materials and solar cells, 2018, 182: 52-60. [CrossRef]
- Rao Z, Zhang G, Xu T, et al. Experimental study on a novel form-stable phase change materials based on diatomite for solar energy storage[J]. Solar energy materials and solar cells, 2018, 182: 52-60. [CrossRef]
- Han J, Liu S. Myristic acid-hybridized diatomite composite as a shape-stabilized phase change material for thermal energy storage[J]. RSC advances, 2017, 7(36): 22170-22177. [CrossRef]
- Hollamby M J, Fix D, Dönch I, et al. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces[J]. Advanced materials, 2011, 23(11): 1361-1365. [CrossRef]
- Shchukin D G. Container-based multifunctional self-healing polymer coatings[J]. Polymer Chemistry, 2013, 4(18): 4871-4877. [CrossRef]
- Li S, Zou Q, Li Y, et al. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly[J]. Journal of the American Chemical Society, 2018, 140(34): 10794-10802. [CrossRef]
- Ryu J H, Messersmith P B, Lee H. Polydopamine surface chemistry: a decade of discovery[J]. ACS applied materials & interfaces, 2018, 10(9): 7523-7540. [CrossRef]
- Cheng L, Liu C, Wu H, et al. A mussel-inspired delivery system for enhancing self-healing property of epoxy coatings[J]. Journal of Materials Science & Technology, 2021, 80: 36-49. [CrossRef]
- Lee H, Dellatore S M, Miller W M, et al. Mussel-inspired surface chemistry for multifunctional coatings[J]. science, 2007, 318(5849): 426-430. [CrossRef]
- Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields[J]. Chemical reviews, 2014, 114(9): 5057-5115. [CrossRef]
- Lee H, Rho J, Messersmith P B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings[J]. Advanced materials, 2009, 21(4): 431-434. [CrossRef]
- Cui J, Yan Y, Such G K, et al. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules[J]. Biomacromolecules, 2012, 13(8): 2225-2228. [CrossRef]
- Yu B, Wang D A, Ye Q, et al. Robust polydopamine nano/microcapsules and their loading and release behavior[J]. Chemical communications, 2009 (44): 6789-6791. [CrossRef]
- Liu X, Cao J, Li H, et al. Mussel-inspired polydopamine: a biocompatible and ultrastable coating for nanoparticles in vivo[J]. ACS nano, 2013, 7(10): 9384-9395. [CrossRef]
- Cheng L, Liu C, Wu H, et al. A mussel-inspired delivery system for enhancing self-healing property of epoxy coatings[J]. Journal of Materials Science & Technology, 2021, 80: 36-49. [CrossRef]
- Xia N N, Xiong X M, Wang J, et al. A seawater triggered dynamic coordinate bond and its application for underwater self-healing and reclaiming of lipophilic polymer[J]. Chemical science, 2016, 7(4): 2736-2742. [CrossRef]
- Gattinoni C, Michaelides A. Understanding corrosion inhibition with van der Waals DFT methods: the case of benzotriazole[J]. Faraday discussions, 2015, 180: 439-458. [CrossRef]
- Chen Z, Huang L, Zhang G, et al. Benzotriazole as a volatile corrosion inhibitor during the early stage of copper corrosion under adsorbed thin electrolyte layers[J]. Corrosion Science, 2012, 65: 214-222. [CrossRef]
- Cho B J, Shima S, Hamada S, et al. Investigation of cu-BTA complex formation during Cu chemical mechanical planarization process[J]. Applied Surface Science, 2016, 384: 505-510. [CrossRef]
- Eklund G S. Initiation of pitting at sulfide inclusions in stainless steel[J]. Journal of the Electrochemical Society, 1974, 121(4): 467. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




