1. Introduction
Control over the composition of III-V ternary materials has been a subject of extensive research for decades [
1,
2]. More recently, III-V ternary nanowires (NWs) and NW-based heterostructures attracted great interest due their fundamental properties and potential applications in Si-integrated optoelectronics and other fields [
2,
3,
4,
5,
6]. Most III-V NWs are grown by different epitaxy techniques by the VLS method with a catalyst droplet, often Au [
7], which can be replaced with a group III metal (Ga) in the self-catalyzed VLS approach [
8]. From the fundamental viewpoint, the VLS growth of a ternary A
xB
1-xC NW is a complex process where the vapor phase containing A, B and C species condenses in a quaternary liquid phase consisting of A, B, C and Au atoms (in the case of Au catalyst) and then crystallizes into a ternary A
xB
1-xC NW [
9,
10,
11,
12,
13,
14,
15,
16,
17]. The composition of a quaternary liquid in a catalyst droplet is described by three independent variables, for example, (i) the fraction of A atoms in liquid,
where
and
are the atomic concentrations of A and B atoms in liquid, (ii) the total concentration of A and B atoms in liquid,
, and (iii) the concentration of atoms C in liquid
, with
. In the self-catalyzed VLS growth, the droplet is a ternary alloy, and the number of independent variables is reduced to two in view of
. The vapor phase producing three atomic fluxes of A, B and C atoms
,
and
can be described by the fraction of A atoms in vapor,
the total flux of A and B atoms
, and the flux ratio
. The liquid-solid distribution
links the solid and liquid composition, whereas the vapor-solid distribution
links the solid and vapor composition [
10,
12].
Most models for the composition of VLS III-V ternary NWs developed so far treat the liquid-solid growth and hence the liquid-solid distributions, considering liquid as an isolated mother phase without any material exchange with vapor [
11,
12,
13,
14,
15,
16,
17]. Here, we study VLS ternary NWs based on group V intermix, with A and B atoms belonging to group V and C atoms belonging to group III. In this case, the liquid-solid growth occurs under group III rich conditions, because the total concentration of highly volatile group V atoms in the droplet
is always much smaller than
. According to Ref. [
10], this yields the kinetic liquid-solid distribution of III-V ternary NWs based on group V intermix, given by
Here,
is the pseudo-binary interaction parameter of AC and BC pairs in solid in thermal units,
are the diffusion coefficients of
A, B atoms in liquid,
are the interaction terms in the chemical potentials of A and B atoms in liquid,
are the chemical potentials of pure
A, B and C liquids, and
are the chemical potentials of pure solid binaries AC and BC. The expressions for the parameters
and
in Eqs. (3) are given in the two equivalent forms, with
and
as the chemical potential differences for pure binaries.
The functional form of the kinetic liquid-solid distribution given by Eqs. (3) is the same as the kinetic vapor-solid distribution for III-V ternary materials based on group III intermix, which are grown under group V rich conditions without any droplet [
18]. However, the coefficients in Eqs. (3) are modified and contain the parameters of liquid rather than vapor. While the interaction terms
depend only on
with neglect of small corrections containing
and
(see Ref. [
10] for a detailed discussion), the
term is inversely proportional to
. Unfortunately, the very low concentrations of group V elements in the droplet (~0.01 or even less [
9]) are below the detection limit of any characterization technique and cannot be measured during or after growth. Furthermore, there is almost no chance that the value of
is kept constant under varying vapor fluxes
and
during the VLS growth of a ternary NW, which is why even the use of
as a fitting constant cannot be justified. This uncertainty was not circumvented in Ref. [
10], where the obtained vapor-solid distribution contained
. This uncertainty makes the liquid-solid distribution given by Eqs. (3) almost useless for the compositional control over VLS ternary NWs based on group V intermix.
In Ref. [
19], a rather general approach was developed which resulted in the analytic vapor-solid distribution of III-V ternary materials:
This vapor-solid distribution is the sum of the purely kinetic (
) and equilibrium (
distributions, whose weighs are regulated by the effective atomic V/III ratio
related to
. Thermodynamic function
contains the pseudo-binary interaction constant and the affinity parameter
which will be given below. When
is close to unity, the growth of a ternary is kinetically controlled, whereas at
the growth occurs under C-poor conditions and the vapor-solid distribution becomes close-to-equilibrium (or nucleation-limited [
14,
15]). This expression fits very well the compositional data on InSb
xAs
1-x [
2] and AlSb
xAs
1-x [
20] epi-layers as well as Au-catalyzed VLS InSb
xAs
1-x NWs [
21], although no droplet on the NW top was considered in the model of Ref. [
19]. In Ref. [
21], Borg and coauthors fitted the VLS data using the numerical model of Biefeld [
2], which is based on similar considerations as the model of Ref. [
19]. Due to the additional diffusion flux of group III (C) atoms from the NW sidewalls to the droplet, the fitting values of the V/III ratios obtained in Refs. [
21] and [
19] are much smaller than the V/III ratios in vapor. This fundamental observation will be used in this work.
The compositions of VLS III-V ternary NWs based on group V intermix were experimentally studied in many material systems including InSb
xAs
1-x [
21,
22,
23,
24], GaSb
xAs
1-x [
25], InP
xAs
1-x [
26,
27], and GaP
xAs
1-x [
28,
29,
30,
31,
32], using different epitaxy techniques such as Au-catalyzed metal-organic vapor phase epitaxy (MOVPE) [
21,
22,
25,
27], Au-catalyzed chemical beam epitaxy (CBE) [
26], Ag-catalyzed [
26] and self-catalyzed [
23,
28,
29,
30,
31] molecular beam epitaxy (MBE) on different substrates, or even the substrate-free Au-catalyzed aerotaxy by MOVPE [
32] (see Refs. [
11,
12] for comprehensive reviews). A limited number of the measured vapor-solid distributions, for example, Au-catalyzed InP
xAs
1-x [
26] and self-catalyzed GaP
xAs
1-x [
29] followed the simplest kinetic Langmuir-McLean shape (see below), with only one parameter describing the different incorporation rates of A and B atoms into a droplet. The comprehensive experimental study of Borg and coauthors [
21] revealed the transition from linear
dependence of Au-catalyzed InSb
xAs
1-x NWs at low V/III ratios to a non-linear close-to-equilibrium shape at high V/III ratios. Such a transition was observed much earlier by Biefeld in InSb
xAs
1-x epi-layers [
2] and predicted to be a general phenomenon in Ref. [
19] (see Eq. (5)). However, the models of Refs. [
2] and [
19] considered the vapor-solid growth without any droplet, and their use for modeling the compositions of VLS NWs requires a justification.
Overall, the achieved level of the growth and compositional modeling of VLS III-V ternary NWs based on group V intermix is insufficient for quantitative comparison with the data and even for qualitative understanding of some compositional trends. The principally unknown parameters of the liquid phase should be either fully eliminated or expressed through the known parameters of vapor in the final expressions. Consequently, here we develop a fully self-consistent growth model of such NWs which, under rather general assumptions, leads to the vapor-solid distributions with the circumvented uncertainties in the infinitely low group V concentrations in the droplet. It will be shown that, using some reasonable simplifications, the vapor-solid distribution can be reduced to an approximation which is very close to Eq. (5), where the parameter
accounts for surface diffusion of group III atoms. The model fits quite well the available compositional data for different VLS NWs based on group V intermix. It justifies the use of the vapor-solid distribution similar to Eq. (5) for VLS NWs [
21] and provides a basis for modeling and the compositional tuning of such NWs in general.
2. Model
We consider the steady-state VLS growth of an A
xB
1-xC NW based on group V intermix under the following assumptions. First, we neglect desorption of group III atoms C from the droplet. This is usual in modeling the VLS growth by MBE [
9,
33] and MOVPE [
34], and supported by the data of Ref. [
35] showing that group III atoms can re-emit from a masked surface but not from the NW sidewalls or droplet. As a result, a NW ensemble of sufficient volume is able to collect all group III atoms sent from vapor. The absence of group III desorption from the droplet is also supported by the measured vapor-solid distributions of III-V ternary NWs based on group III intermix, whose shape is close to the Langmuir-McLean shape in most cases [
10]. Second, we assume that the droplet volume does not change with time, at least after a certain incubation stage where the measured NW composition can be different from its steady-state value. This assumption is usual in modeling of Au-catalyzed VLS growth [
9,
33,
34]. Self-catalyzed VLS growth is different, because the droplet serves as a non-stationary reservoir of group III atoms which can either swell or shrink depending on the effective V/III ratio [
36,
37]. However, the droplet volume should self-equilibrate to a steady-state value corresponding to equal group III and group V flows and stay constant after that [
36,
37,
38]. Third, we assume that group V atoms are not diffusive and enter NWs only through their droplets [
8,
36,
37,
38,
39]. Fourth, we consider that the arriving group V species are A
2 and B
2 dimers, as usual in MBE [
39]. This assumption is not critical. The model can be re-arranged, for example, for A
4 and B
4 tetramers or group V precursors containing only one group V atom, such as AsH
3 or PH
3. However, these precursors most probably decompose in vapor before reaching the droplet surface, resulting in the fluxes of V
2 dimers or V
4 tetramers depending on the growth temperature.
Under these assumptions, the steady-state VLS growth of a ternary NW based on group V intermix is described by the two equations:
Here,
and
are the vapor-liquid incorporation rates or, more precisely, the effective adsorption coefficients giving the ratio of the number A or B atoms entering the droplet over the total number of these atoms impinging onto the droplet surface. They account for a possible difference in A and B beam angles in the directional deposition techniques such as MBE. These
and
in our notation do not include desorption. Similarly,
is the effective collection efficiency of group III atoms on the droplet surface, NW sidewalls and possibly the substrate surface. For III-V NWs,
may be much larger than
and
, because most group III atoms are collected by the droplet from solid surfaces surrounding the droplet [
9,
21,
26,
33,
34,
35,
36,
37]. The
and
denote the vapor fluxes of A
2 and B
2 dimers, brining 2 group V atoms each, whereas
and
denote the desorption fluxes of A and B atoms. The vapor composition for the fluxes of group V dimers is given by
which is the same as Eq. (2) due to
and
.
Equations (6) are similar to the ones considered in Ref. [
10], However, there is one important difference. In Ref. [
10], we used the unknown NW growth rate
instead of
in the left-hand-side, which was then eliminated by dividing one equation to the other. This did not allow us to circumvent the uncertainty in the unknown total concentration of group V atoms in the droplet, which remained in the vapor-solid distribution. Now, Eqs. (6) contain the known group III flux
, which determines the NW growth rate in the absence of desorption. It equals the total influx of A and B atoms minus their total desorption fluxes. This follows from summing up the two Eqs. (6). Our aim is to express the unknown group V concentrations in the droplet
and
(or, equivalently,
and
) through the vapor fluxes. To do that, we need to find the desorption fluxes as functions of
and
. We define the desorption fluxes as the vapor fluxes which are at equilibrium with liquid at a given composition, like in Ref. [
39] for a binary III-V NW. The vapor-liquid equilibrium corresponds to
where
and
are the chemical potentials of A
2 and B
2 dimers in vapor.
Considering that vapor is a mixture of perfect gases, the chemical potentials of A
2 and B
2 dimers are logarithmic functions of the fluxes
Here, we prefer to use the reference states of A
2 and B
2 vapors corresponding to the fluxes
and
that are at equilibrium with pure A and B liquids (having the chemical potentials
and
). We choose the reference fluxes with the same incorporation rates
and
as for a quaternary droplet. It will be shown later that using the reference fluxes
and
(corresponding to
) does not affect the final result.
Using Eqs. (9) and Eqs. (4) for the chemical potentials
and
in Eqs. (8), we obtain the desorption fluxes in the form
According to these expressions, the desorption fluxes are proportional to the squared concentrations of A and B atoms in liquid, because group V atoms always desorb in the form of dimers [
39,
40]. Substitution of these desorption fluxes into Eqs. (6), along with the definitions for
given by Eq. (1) and
by Eq. (7), leads to
This gives two equations for the two unknowns
and
which contain, however, the vapor composition
and the solid composition
. Summing up Eqs. (11) and (12), we find
with
as the ratio of the vapor-liquid condensation rates of A and B atoms. Importantly,
is independent of the vapor composition
. However, it depends on the liquid composition
and the solid composition
, becoming
-independent only when
.
Inferring
from Eqs. (11) and (12), we get
Using Eq. (13), after some simple manipulations we obtain the main result of this work in the form
with the parameters
Clearly, the parameter
determines the effective ratio of the total flux of group V atoms over the flux of group III atoms entering the droplet. In the simplest model for surface diffusion of group III adatoms [
9], the
ratio is given by
, where
is the diffusion length of group III adatoms on the NW sidewalls,
is the NW radius and
is a constant related to the droplet contact angle and the epitaxy technique. Therefore,
in III-V NWs is largely reduced with respect to the atomic V/III flux ratio in vapor , particularly for thin NWs with .
3. Results and discussion
In our model, the effective V/III ratio is allowed to vary in the range
to preserve the steady-state VLS growth conditions with a constant droplet volume. At
, the incoming group V and III fluxes equal each other and all the arriving atoms are incorporated into the NW, meaning that the group V desorption fluxes are negligible. In this kinetic VLS regime, the vapor-solid distribution given by Eq. (16) is reduced to the one-parametric Langmuir-McLean formula
For larger
, a fraction of A
2 and B
2 dimers must desorb from the droplet surface. In this case, the vapor-solid distribution is described by Eq. (16), in which the liquid composition
should be calculated using Eqs. (3). The previously unknown
in the parameter
is now given by Eq. (13). Therefore,
becomes a function of
and
. Inferring the explicit dependence
from Eq. (13) requires the solution of a quadratic equation for
. Substitution of the obtained
into Eq. (16) yields the analytic vapor-solid distribution
. This
is a function of vapor fluxes and the parameters of the liquid phase which depend only on
. Therefore, the general vapor-solid distribution at intermediate
contains a parametric dependence on
, which can be measured during [
41] or after [
9] growth.
This complicated procedure is not required for practical purposes. We now show that the parameters of liquid can be fully circumvented in the following approximation. The limiting behavior at
corresponds to no-growth conditions where the arriving fluxes of A
2 and B
2 atoms are equalized by the desorption fluxes. In this case, AC and BC pairs in liquid should also be at equilibrium with solid. The liquid-solid equilibrium in a ternary system corresponds to [
13]
where
and
are the composition-dependent chemical potentials of AC and BC pairs in solid. Using Eqs. (4) and the same expression for C atoms,
, along with the regular solution model for the chemical potentials in solid,
and
(Refs. [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]), Eqs. (21) are equivalent to
Upon substitution of these expressions into Eqs. (3), the simple calculation shows that the kinetic liquid-solid distribution is reduced to the equilibrium one [
10,
13,
14,
15]:
where
is the same as in Eq. (3). For the equilibrium liquid-solid distribution, we have
where the equilibrium function
is the same as in Eq. (5), and the affinity parameter is given by
Using the approximation
in Eq. (16), the analytic vapor-solid distribution is obtained in the form:
where
is given by Eq. (14) and
is given by Eq. (25). At
, it is reduced to the result of Ref. [
19] given by Eq. (4). If we re-write Eqs. (9) as
with
and
as the equilibrium fluxes at
, all the results remain, with
modified to
Thus, the analytic vapor-solid distribution of VLS III-V ternary NWs based on group V intermix is given by Eq. (26) and is very close to the vapor-solid distribution for III-V
x-V
1-x materials grown in the vapor-solid mode without any droplet [
19]. The main difference is in the
parameter, which equals the atomic V/III flux ratio in vapor for the vapor-solid growth, while for VLS NWs it accounts for the fact that a catalyst droplet is able to collect much more group III atoms from the surrounding surfaces (as given, for example, by Eq. (19)). The other difference is in the parameter
that describes the effect of different condensation rates of A
2 and B
2 dimers into the droplet. These rates are usually assumed equal for the vapor-solid growth, corresponding to
. The obtained result is similar to Ref. [
42], where it was shown that the vapor-solid distribution of VLS III-V ternary NWs based on group III intermix is kinetic, despite of the fact that the corresponding liquid-solid distribution is close-to-equilibrium [
10]. Equation (26) is approximate, because it uses the equilibrium shape of the liquid-solid distribution at intermediate
which, strictly speaking, is valid only under no-growth conditions at
. Similar approximation was used in Ref. [
19] for obtaining Eq. (3).
The shape of the vapor-solid distribution given by Eq. (26) is determined by the two thermodynamic parameters
and
, and the two kinetic parameters
and
. The effective V/III ratio can easily be changed in the VLS growth experiments. The other parameters are independent of
in the first approximation and determined primarily by the material system, growth catalyst and temperature.
Figure 1 shows the vapor-solid distributions obtained from Eq. (26) for a model system with a fixed
1.6,
0.3,
2 and different
. Although the miscibility gap is absent (
), the equilibrium distribution and the distribution at a large
are non-linear. They are shifted to the right due to a small
, meaning that obtaining a noticeable fraction of AC pairs in a NW requires a much larger fraction of A atoms in vapor. As the effective V/III ratio decreases, the curves get closer to the kinetic Langmuir-McLean shape, which favors the vapor-liquid incorporation of A atoms with respect to B atoms at
2. In principle, any vapor-solid distribution between the equilibrium and kinetic curves is possible, and can be achieved by tuning the total V/III ratio at a fixed temperature (for example, by changing the total group V flux at a fixed group III flux).
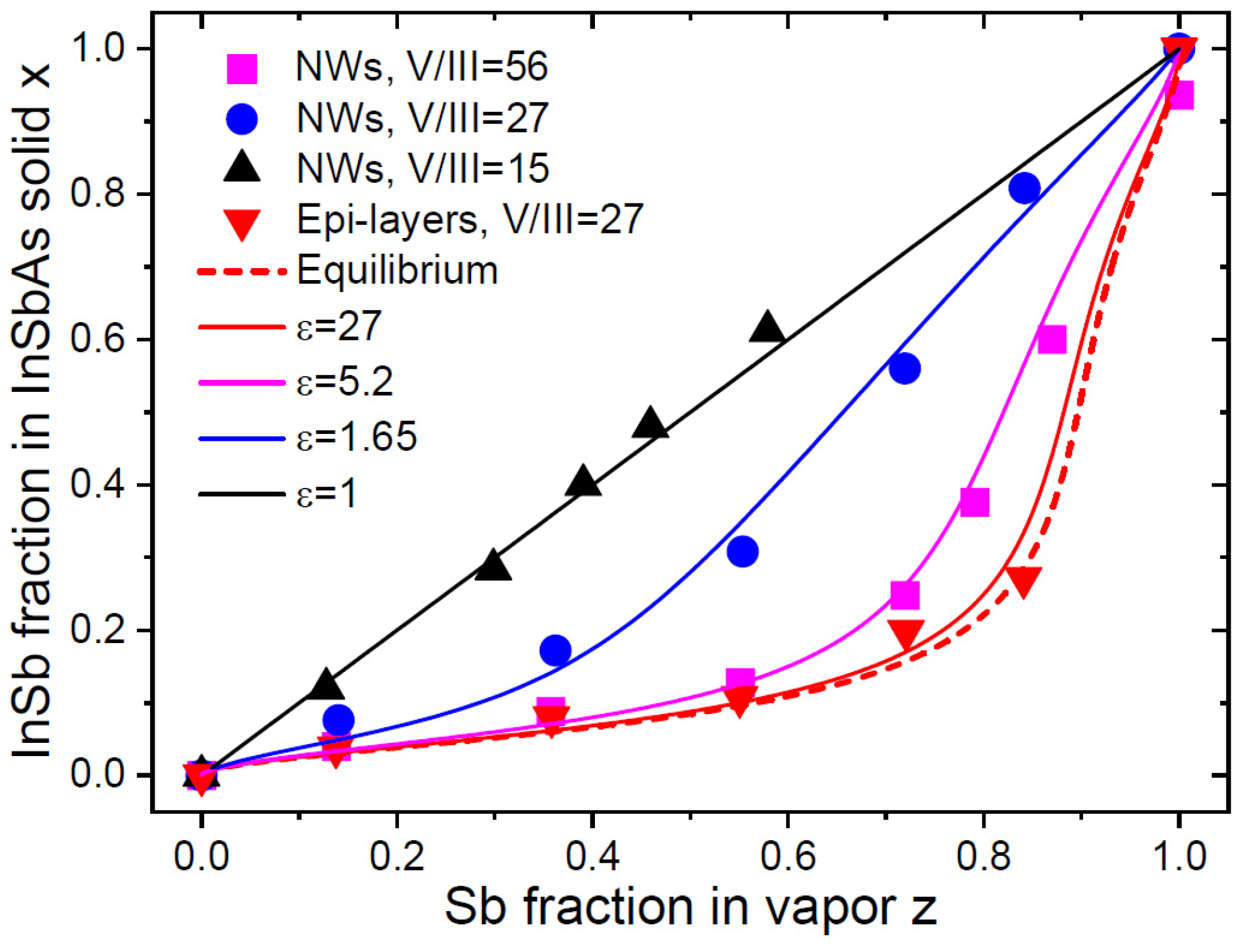
Such a behavior was observed in InSb
xAs
1-x epi-layers [
2], AlSb
xAs
1-x epi-layers [
20], and more recently in Au-catalyzed VLS InSb
xAs
1-x NWs [
21]. These NWs were grown by MOVPE on InAs(111)B substrates at 450
oC using TMIn, TMSb and AsH
3 precursors, with 50 nm diameter colloidal Au nanoparticles as the VLS growth seeds. The total V/III flux ratio in vapor
was set to 15, 27 and 56 by varying group V fluxes at a constant TMIn flux. These vapor-solid distributions were analyzed in our recent work [
19]. Here, we extend the analysis by considering the vapor-solid distributions of InSb
xAs
1-x NWs together with epi-layers that were grown concomitantly with the NWs [
21].
Figure 2 shows the measured vapor-solid distributions of InSb
xAs
1-x NWs and epi-layers. The
value at 450
oC is well-known and equals 1.566 [
19,
43]. The vapor-solid growth of epi-layers at a high
of 27 must yield a close-to-equilibrium shape of the corresponding distribution. This allows us to choose a
value of 0.34, which is close to the equilibrium constant of 0.429 given in Ref. [
1] and used for modeling in Ref. [
21]. The kinetic curve, obtained for NWs at
, is linear. This should correspond to
, that is, equal incorporation rates of Sb and As into the droplet. Assuming that
is the same for epi-layers and NWs (which is not guaranteed in the general case), the different behaviors of the vapor-solid distributions in
Figure 2 are entirely due to different
values in Eq. (26). For epi-layers, the fitting value of
is the same as
is vapor. For NWs, the fitting values of
are 11-16 times smaller than
is vapor, which is explained by the additional fluxes of diffusive In adatoms from the surrounding surfaces as compared to the surrounding vapor. This observation was made in the original work [
21].
Before discussing the data on VLS InP
xAs
1-x and GaP
xAs
1-x NWs, we note that the parameter
given by Eq. (25) or Eq. (28) contains the exponential of the well-known difference of chemical potentials for pure binaries
[
44,
45,
46], while the pre-exponential factor (for example,
in Eq. (28)) is less obvious. It is different from what is usually considered in the equilibrium constants for surface reactions [
1,
2,
20]. These constants describe the equilibrium of binary or more complex vapors with binary solids, while our
and
are the equilibrium fluxes for pure group V liquids. Our
also includes the unknown parameter
. An accurate analysis of these factors is beyond the scope of this work. In what follows, we will use
as a fitting value, but taking into account the thermodynamic trend that follows from the exponential factor
in the affinity parameter.
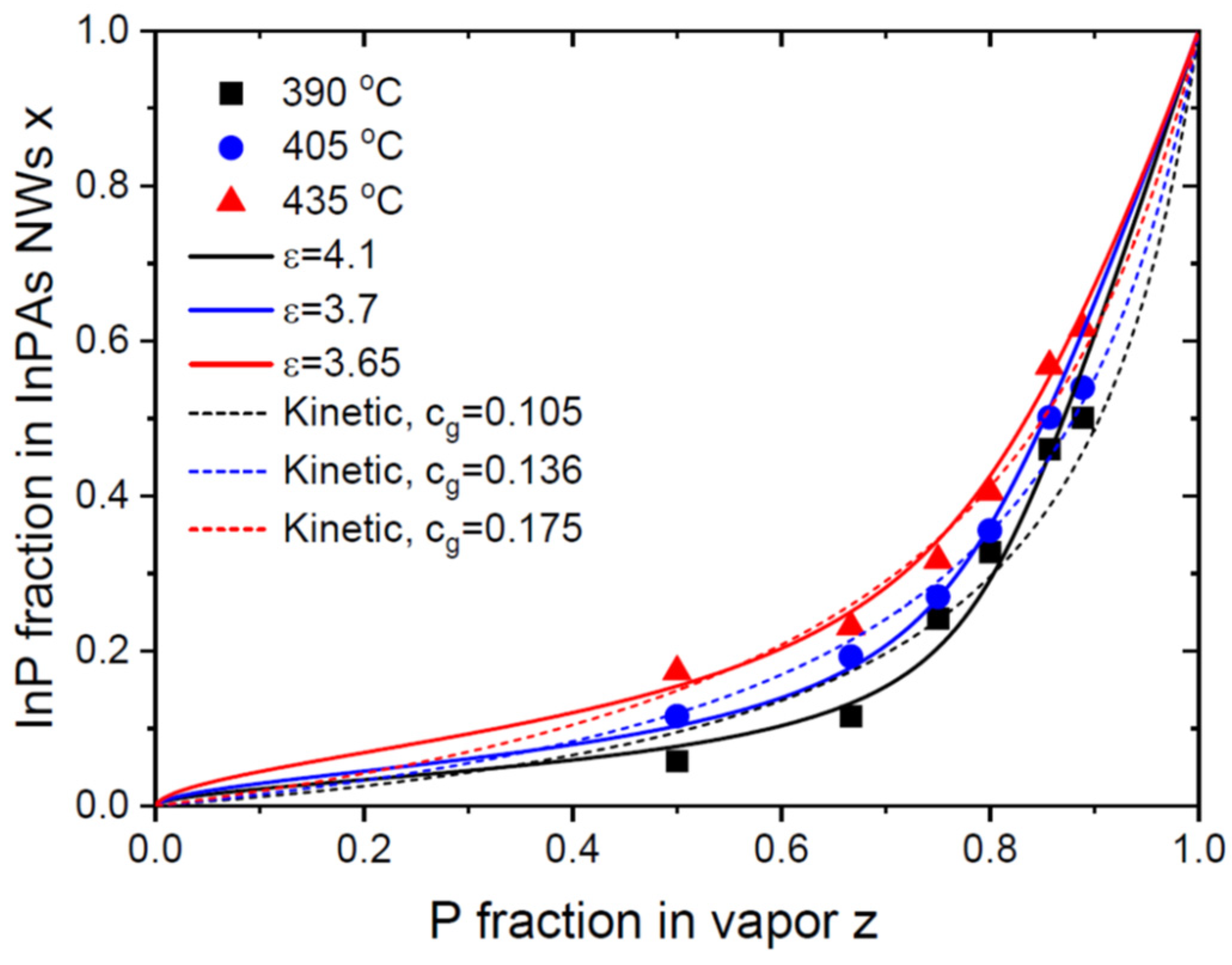
Figure 3 shows the vapor-solid distributions of Au-catalyzed InP
xAs
1-x NWs obtained by Persson and coauthors [
26]. These NWs were grown by CBE on InAs(111)B substrates using 50 nm diameter colloidal Au droplets, which resulted in ~ 60 nm diameter NWs. The growth started with InAs NW stems and continued with InP
xAs
1-x sections grown at three different temperatures of 390
oC, 405
oC and 435
oC. The total V/III flux ratio in vapor during growth of InPAs sections was in the range from 30 to 45. It is seen that the values of
are systematically larger than
, meaning that the incorporation of P atoms is lower than for As atoms. The authors fitted the data using the kinetic Langmuir-McLean Eq. (20) with the low
values that increased from 0.105 at 390
oC to 0.175 at 435
oC (dashed lines in
Figure 3). The values of
equal 0.233 at 390
oC, 0.2375 at 405
oC, and 0.244 at 435
oC [
44,
45,
46]. This shows a thermodynamic trend for having a smaller fraction of P atoms in vapor than in solid in the whole temperature domain studied in Ref. [
26]. Very high V/III flux ratios employed in this work should lead to desorption of the excessive P and As atoms from the droplet surface, as in the previous case of InSb
xAs
1-x NWs. Therefore, we fit the data using the general equation Eq. (26), using the
values that are noticeably larger than unity. They appear close to InSbAs NWs under similar V/III flux ratios in vapor. The best fits are obtained with
at 390
oC, 0.13 at 405
oC, and 0.2 at 435
oC, and
in all cases (solid lines in
Figure 3). These curves provide slightly better fits than the Langmuir-McLean formula.
It is interesting to note that these fitting values are very close to the effective ratios of the P over As incorporation rates obtained in Ref. [
26]. This is most probably explained by relatively weak interactions of InP and InAs pairs in solid, corresponding to the low
values given in
Table 1. In this case, the equilibrium distribution in Eq. (26) is close to the Langmuir-McLean shape. This property follows directly from Eq. (26) for
at
. Therefore, fitting the vapor-solid distributions of III-V ternary NWs with low pseudo-binary interaction parameters
by the one-parametric Langmuir-McLean formula is entirely possible [
13,
14,
15,
47]. The effective ratio of the incorporation rates of different group V atoms must, however, include the differences in the desorption rates and the dependence on the total V/III flux ratio, as in our model.
GaPAs is another example of a ternary material with low
, which are in the range from 0.64 to 0.7 in the typical growth temperature window of 550-630
oC (see
Table 1). In contrast to InPAs, the difference of chemical potentials
is positive, yielding the values of
ranging from 1.849 at 550
oC to 1.782 at 630
oC [
44,
45]. This should favor faster incorporation of P atoms relative to As atoms and, consequently, a larger P fraction in vapor relative to solid in close-to-equilibrium growth regimes under high V/III flux ratios.
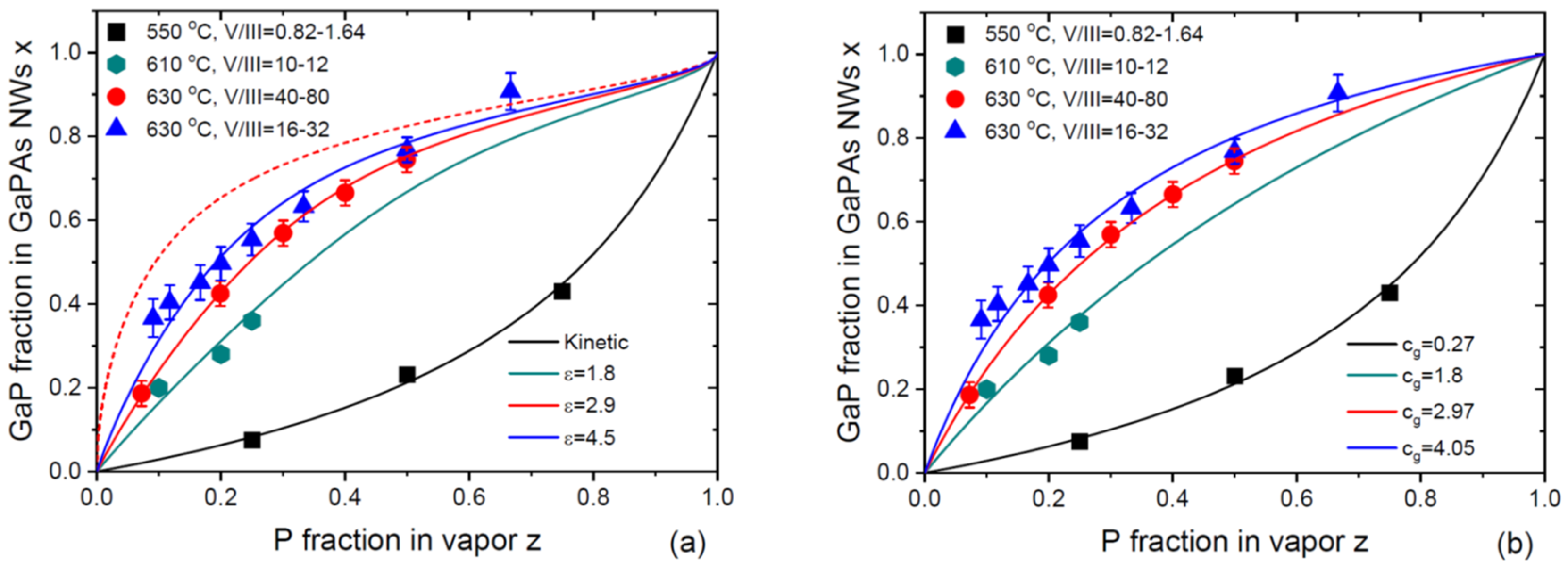
Figure 4 show the compilation of the vapor-solid distributions of VLS GaP
xAs
1-x NWs from the four works. Metaferia and coauthors grew the NWs by Au-catalyzed MOVPE using the substrate-free aerotaxy at 550
oC, under a low total V/III flux ratios in vapor from 0.82 to 1.64 [
32]. Other GaP
xAs
1-x NWs [
28,
30] or GaP
xAs
1-x sections in GaP NWs [
31] were grown by the self-catalyzed MBE (with Ga droplets) on Si(111) substrates. Himwas and coauthors [
28] grew the NWs at 610
oC under the total V/III flux ratios ranging from 10 to 12. Zhang and coauthors [
30] and Bolshakov and coauthors [
31] grew the NWs at 630
oC under higher total V/III ratios, ranging from 40 to 80 in Ref. [
30] and from 16 to 32 in Ref. [
31]. Different procedures for preparation of Ga droplets were used and resulted in different NW surface densities, diameters and lengths. The vapor-solid distribution of the NWs grown by aerotaxy at low V/III ratios corresponds to a lower incorporation rate of P atoms, while the other NWs grown at much higher V/III ratios exhibit the opposite trend. The vapor-solid distributions obtained by Zhang and coauthors [
30] and Bolshakov and coauthors [
31] at 630
oC are very close to each other.
Figure 4 (a) shows the fits to the whole set of data obtained from Eq. (26) with different
. The data of Ref. [
32] at low
are fitted with a minimum
corresponding to the Langmuir-McLean shape at
, as in the original work. The MBE data of Refs. [
28], [
30] and [
31] are fitted with large
of 1.8, 2.97 and 4.5, using the same parameter
in the equilibrium distribution and the same
. The value of
is not critical for these fits. The MBE data can be well-fitted using, for example,
using slightly different
values. This figure shows the same trend as in Figs. 1 and 2, that is, transitioning of the kinetic distribution to the equilibrium shape with increasing the total V/III ratio.
Figure 4 (b) shows that the equally good fits can be obtained using the Langmuir-McLean formula with different
for all the data. The fitting value of the effective ratio of the incorporation rates of P over As atoms increases from 0.27 to 4.05 (the fitting value of 2.97 was obtained by Zhang and coauthors in Ref. [
30] for their data). It would be difficult to explain this trend without considering desorption of the excessive group V atoms in MBE growths under very high V/III ratios. As in the previous case, the Langmuir-McLean shapes provide the excellent fits due to a low
values in this material system.