Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental site
2.2. Soil type
2.3. Fertilizers application
2.4. The Experiments
2.4.1. The first experiment
2.4.2. The second experiment
2.5. Irrigation water requirements
2.6. The studied characters
2.7. Statical and data analysis
3. Results and Discussion
3.1. Irrigation treatment and ascorbic acid (AsA) experiment
3.1.1. Response of grain yield and its components to water stress, AsA, and their interaction
3.1.2. Response on water productivity (WP) and irrigation water productivity (IWP) to water stress, AsA, and their interaction across the two seasons
3.2. Irrigation treatment and vitamin B2 (Riboflavin) experiment
3.2.1. Response on yield and yield components to water stress, VB2, and their interaction across the two seasons
3.2.2. Response on water productivity (WP) and irrigation water productivity (IWP) to water stress, vitamin B2, and their interaction
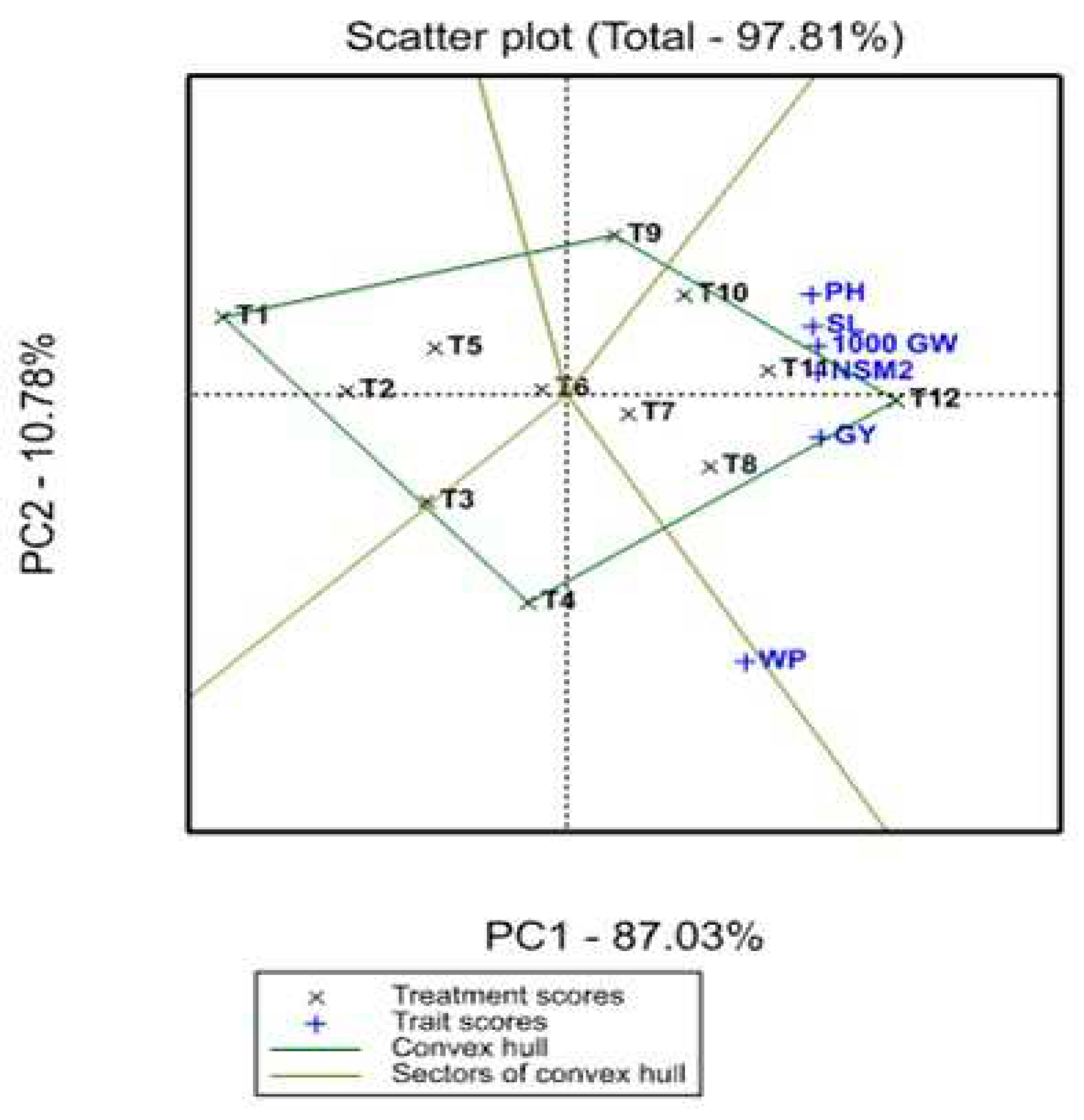
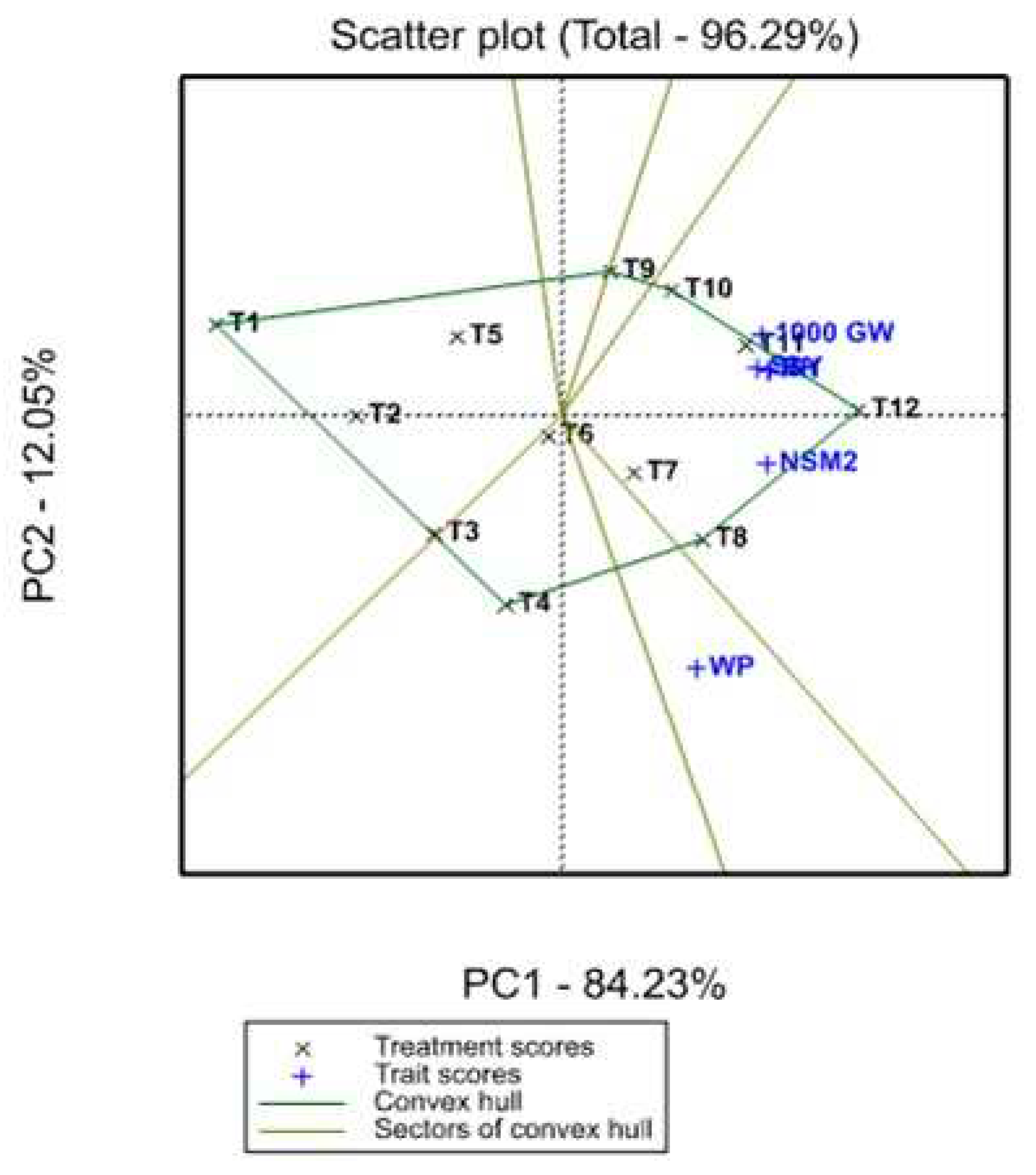
3.3. Treatment x Traits (TT) biplot
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO, F. The future of food and agriculture–Trends and challenges. Ann Report 2017, 296, 1–180. [Google Scholar]
- Abdelkhalik, S.A.M.; Hagras, A.A.E.-A. Behavior of some Egyptian bread wheat genotypes under different natural photo-thermal environments. Egypt J Agri Res 2021, 99, 142–157. [Google Scholar] [CrossRef]
- El-Soukkary, F. Evaluation of pumpkin seed products for bread fortification. Plant Food Hum Nutr 2001, 56, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Kheir, A.M.; Kassie, B.T.; Hoogenboom, G.; Abdelaal, A.I.; Haman, D.Z.; Ruane, A.C. Can Egypt become self-sufficient in wheat? Environ Res Lett 2018, 13, 094012. [Google Scholar] [CrossRef]
- Gomaa, M.; Kandil, E.E.; El-Dein, A.A.; Abou-Donia, M.E.; Ali, H.M.; Abdelsalam, N.R. Increase maize productivity and water use efficiency through application of potassium silicate under water stress. Sci Rep 2021, 11, 1–8. [Google Scholar] [CrossRef]
- ICARDA, A. Water Agri Egypt; 2011.
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Comm 2021, 100228. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Q.; Sun, P.; Song, C. Impact of droughts on winter wheat yield in different growth stages during 2001–2016 in Eastern China. Intern J Disaster Risk Sci 2018, 9, 376–391. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Applied Sciences 2020, 10, 5692. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Roitsch, T.; Liu, F. Activities of leaf and spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC plant biology 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M.; Arvin, M.J. Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination. Crop Pasture Science 2019, 70, 36–43. [Google Scholar] [CrossRef]
- Bekele, S.; Tilahun, K. Regulated deficit irrigation scheduling of onion in a semiarid region of Ethiopia. Agricultural water management 2007, 89, 148–152. [Google Scholar] [CrossRef]
- Eissa, M.A.; Rekaby, S.A.; Hegab, S.A.; Ragheb, H.M. Effect of deficit irrigation on drip-irrigated wheat grown in semi-arid conditions of Upper Egypt. Journal of Plant Nutrition 2018, 41, 1576–1586. [Google Scholar] [CrossRef]
- Noreldin, T.; Ouda, S.; Mounzer, O.; Abdelhamid, M.T. CropSyst model for wheat under deficit irrigation using sprinkler and drip irrigation in sandy soil. Journal of Water and Land Development 2015. [Google Scholar] [CrossRef]
- Ouda, S.; Noreldin, T.; Alarcón, J.J.; Ragab, R.; Caruso, G.; Sekara, A.; Abdelhamid, M.T. Response of spring wheat (Triticum aestivum) to deficit irrigation management under the semi-arid environment of Egypt: field and modeling study. Agriculture 2021, 11, 90. [Google Scholar] [CrossRef]
- Ashour, M.; El Attar, S.; Rafaat, Y.; Mohamed, M. Water resources management in Egypt. Journal of Engineering Sciences 2009, 37, 269–279. [Google Scholar] [CrossRef]
- Moghazy, N.H.; Kaluarachchi, J.J. Sustainable agriculture development in the Western Desert of Egypt: a case study on crop production, profit, and uncertainty in the Siwa region. Sustainability 2020, 12, 6568. [Google Scholar] [CrossRef]
- Fahad, M.; Wajid, S.A.; Ahmad, A.; Cheema, M.J.M. Response of wheat cultivars to deficit irrigation under semiarid conditions of Faisalabad. International Journal of Agriculture and Biology 2019, 21, 1004–1012. [Google Scholar]
- Sarkar, P.; Talukder, M.; Biswas, S.; Khatun, A. Growth and yield of wheat (Triticum aestivum) under deficit irrigation. Bangladesh Journal of Agricultural Research 2013, 38, 719–732. [Google Scholar] [CrossRef]
- Malik, S.; Ashraf, M. Exogenous application of ascorbic acid stimulates growth and photosynthesis of wheat (Triticum aestivum L.) under drought. Soil & Environment 2012, 31. [Google Scholar]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in plant science 2017, 8, 613. [Google Scholar] [CrossRef]
- Akram, N.; Khan, I.; Javed, Z.; Khan, Z.; Mahmood, S. Modulation of some key biochemical parameters in drought-stressed pea (Pisum sativum L.) treated with different plant growth regulators. Modulation of some key biochemical parameters in drought-stressed pea (Pisum sativum L.) treated with different plant growth regulators, 2018; 337–351. [Google Scholar]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiologiae Plantarum 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Dolatabadian, A.; SANAVY, S.A.M.M.; Asilan, K.S. Effect of ascorbic acid foliar application on yield, yield component and several morphological traits of grain corn under water deficit stress conditions. Notulae Scientia Biologicae 2010, 2, 45–50. [Google Scholar] [CrossRef]
- Mori, T.; Sakurai, M. Effects of riboflavin and increased sucrose on anthocyanin production in suspended strawberry cell cultures. Plant Science 1995, 110, 147–153. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Q.; Huang, B. Ascorbic acid mitigation of water stress-inhibition of root growth in association with oxidative defense in tall fescue (Festuca arundinacea Schreb.). Frontiers in Plant Science 2015, 6, 807. [Google Scholar] [CrossRef]
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Guhr, A.; Horn, M.A.; Weig, A.R. Vitamin B2 (riboflavin) increases drought tolerance of Agaricus bisporus. Mycologia 2017, 109, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. Journal of plant physiology 2010, 167, 201–208. [Google Scholar] [CrossRef]
- Deng, B.; Jin, X.; Yang, Y.; Lin, Z.; Zhang, Y. The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant growth regulation 2014, 72, 269–277. [Google Scholar] [CrossRef]
- Yan, W. Crop variety trials: Data management and analysis; John Wiley & Sons: 2014.
- Akcura, M.; Kokten, K. Variations in grain mineral concentrations of Turkish wheat landraces germplasm. Quality Assurance and Safety of Crops & Foods 2017, 9, 153–159. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements; FAO Irrigation and drainage paper 56. Fao, Rome, 300(9), p.D05109: 1998.
- Israelsen, O.; Hansen, V. Irrigation Practices and Principles; John Wiley and Sons, Inc., New York: 1962.
- Ali, M.; Hoque, M.; Hassan, A.; Khair, A. Effects of deficit irrigation on yield, water productivity, and economic returns of wheat. Agricultural water management 2007, 92, 151–161. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Guidelines for predicting crop water requirements; 1977; Volume 24.
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; Mc Graw Hill Book Co.: New York, 1997. [Google Scholar]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Palo Alto, CA: Stanford University Press.: 1960; pp. 278–292.
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop science 2002, 42, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.; Abdelraouf, R.; Ahmed, M.; El Karamany, M. Effect of drought stress and ascorbic acid foliar application on productivity and irrigation water use efficiency of wheat under newly reclaimed sandy soil. Journal of Applied Sciences Research 2012, 8, 4552–4558. [Google Scholar]
- Helsper, J.P.; Kagan, L.; Hilby, C.L.; Maynard, T.M.; Loewus, F.A. L-Ascorbic acid biosynthesis in Ochromonas danica. Plant Physiology 1982, 69, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Kotb, M.; Elhamahmy, M. Foliar application of ascorbic acid improved drought tolerance and productivity of wheat (Triticum aestivum L.). Journal of Plant Production Sciences; Suez Canal University 2013, 1, 1–16. [Google Scholar]
- Hussein, Z.K.; Khursheed, M.Q. Effect of Foliar Application of Ascorbic Acid on Growth, Yield Components and Some Chemical Constituents of Wheat under Water Stress Conditions. Jordan Journal of Agricultural Sciences 2014, 173, 1–16. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Kamel, H.; Dawood, M.G. Response of non-nodulating, nodulating, and super-nodulating soybean genotypes to potassium fertilizer under water stress. Journal of plant nutrition 2011, 34, 1675–1689. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Saudy, H.S.; Abdelhamid, M.T. Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital J Agrometeorol 2021, 2, 81–90. [Google Scholar]
- Abdelhamid, M.T.; Palta, J.A.; Veneklaas, E.J.; Atkins, C.; Turner, N.C.; Siddique, K.H. Drying the surface soil reduces the nitrogen content of faba bean (Vicia faba L.) through a reduction in nitrogen fixation. Plant and Soil 2011, 339, 351–362. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; Abdelraouf, R.E.; Ahmed, M.A.; Mounzer, O.; Alarcón, J.J.; Abdelhamid, M.T. Response Of Wheat (L.) Crop And Broad-Leaved Weeds To Different Water Requirements And Weed Management In Sandy Soils. Agriculture (Pol'nohospodárstvo) 2015, 61, 22–32. [Google Scholar] [CrossRef]
- Ouda, S.A.; Noreldin, T.; Mounzer, O.H.; Abdelhamid, M.T. CropSyst model for wheat irrigation water management with fresh and poor quality water. Journal of Water and Land Development 2015. [Google Scholar] [CrossRef]
- Yaghoubian, H.; Moghadam, H.R.T.; Kasraie, P.; Zahedi, H. Effect of Foliar Application of Salicylic Acid on Physiological and Biochemical Characteristics of Corn (Zea mays L.) Under Withholding Irrigation at Different Growth Stages. Journal of Applied Science and Agriculture 2014, 9, 27–34. [Google Scholar]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.; Gharib, H. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Intern J Plant Prod 2016, 10, 579–596. [Google Scholar]
- Abo-Marzoka, E.; El-Mantawy, R.; Soltan, I. Effect of irrigation intervals and foliar spray with salicylic and ascorbic acids on maize. J. Agric. Res. Kafr El-Sheikh Univ 2016, 42, 506–518. [Google Scholar]
- Taha, A.; Ibrahim, M.; Mosa, A.; EL-Komy, M. Water productivity of wheat crop as affected by different sowing dates and deficit irrigation treatments. J Soil Sci Agri Engin 2017, 8, 521–529. [Google Scholar] [CrossRef]
- Alsayim, H.E.; El-Mahdi, A.R.A.; Nayel, M.H. Water-use efficiency of two wheat cultivars (Triticum aestivum L.) under tropical high terrace soil conditions. Asian Journal of Agriculture and Food Sciences 2013, 1. [Google Scholar]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. Journal of plant research 2019, 132, 881–901. [Google Scholar] [CrossRef]
- M'hamed, H.C.; Rezig, M.; Naceur, M.B. Water use efficiency of durum wheat (Triticum durum Desf) under deficit irrigation. Journal of Agricultural Science 2015, 7, 238. [Google Scholar]
- Azooz, M. Foliar application with riboflavin (Vitamin B2) enhancing the resistance of Hibiscus sabdariffa L.(Deep red sepals variety) to salinity stress. J. Biol. Sci 2009, 9, 109–118. [Google Scholar] [CrossRef]
- Darwish, M.A.; Nassar, R.M.; Abdel-Aziz, N.G.; Abdel-Aal, A.S. Riboflavin minimizes the deleterious effects of salinity stress on growth, chloroplast pigments, free proline, activity of antioxidant enzyme catalase and leaf anatomy of Tecomacapensis (Thumb.) Lindl. Middle East Journal of Agriculture 2017, 6, 757–765. [Google Scholar]
- Karrou, M.; Oweis, T.; Abou El Enein, R.; Sherif, M. Yield and water productivity of maize and wheat under deficit and raised bed irrigation practices in Egypt. African Journal of Agricultural Research 2012, 7, 1755–1760. [Google Scholar]
- Abdrabbo, M.; Ouda, S.; Noreldin, T. Modeling the effect of irrigation scheduling on wheat under climate change conditions. Nature and Science Journal 2013, 11, 10–18. [Google Scholar]
- Beutler, E. Effect of flavin compounds on glutathione reductase activity: in vivo and in vitro studies. The Journal of Clinical Investigation 1969, 48, 1957–1966. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Science 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE biplot analysis: A graphical tool for breeders, geneticists, and agronomists; CRC press: 2003.
- Rasul, G.A.M.; Ahmed, S.; Ahmed, M.Q. Influence of different organic fertilizers on growth and yield of wheat. American-Eurasian J Agric Environ Sci 2015, 15, 1123–1126. [Google Scholar]
- Sabaghnia, N.; Janmohammadi, M. Interrelationships Among some Morphological Traits of Wheat (Triticum aestivum L.) Cultivars using Biplot. Botanica Lithuanica (1392-1665) 2014, 20. [Google Scholar] [CrossRef]
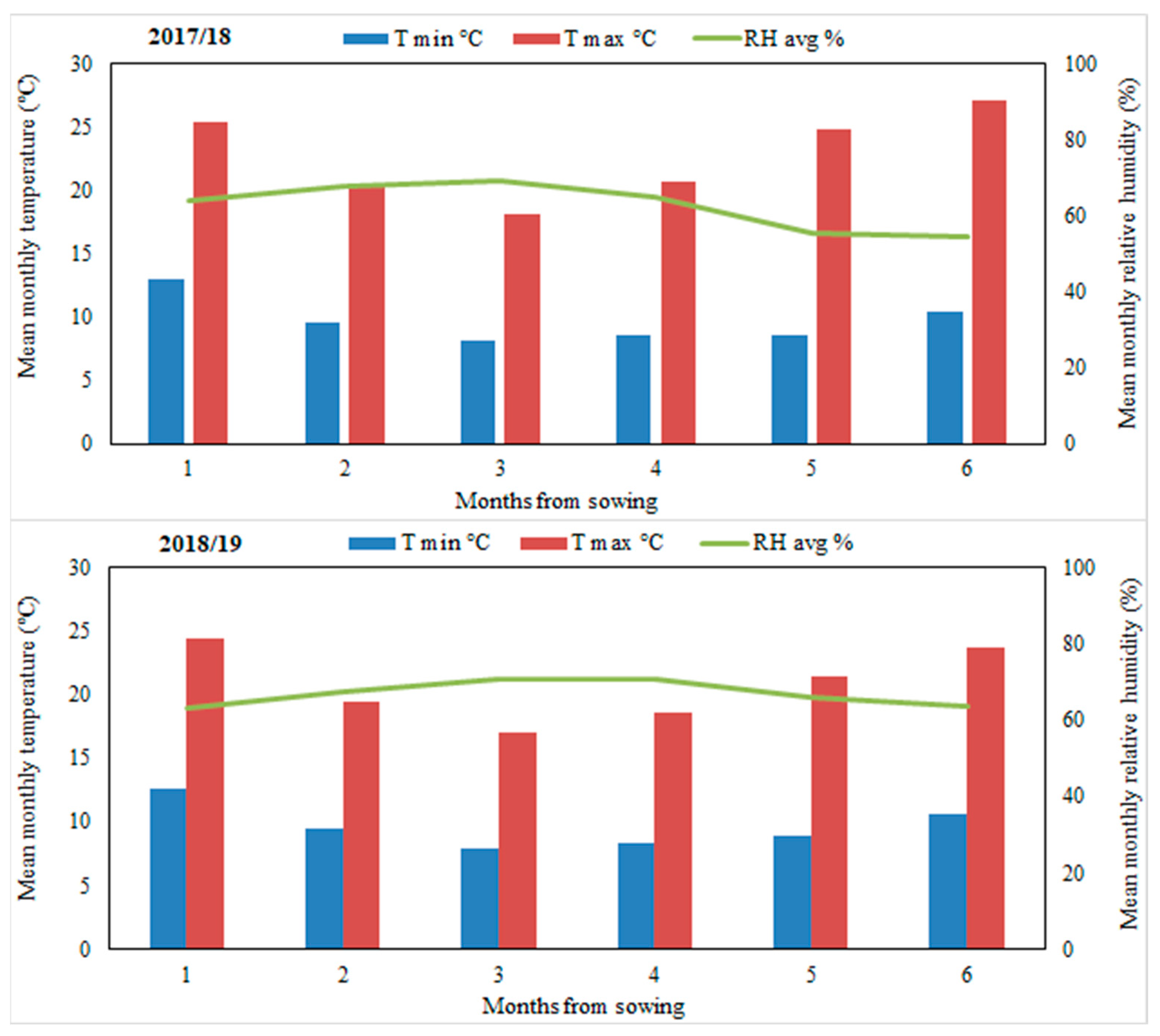
| Texture | Soil depth (cm) | Bulk density (Mg m-3) |
Hydraulic conductivity (cm h-1) |
Particle size distribution | ||
| Sand % | Silt % | Clay % | ||||
|
Sandy loam |
0 – 15 15 - 30 30 - 45 45 – 60 |
1.28 1.26 1.27 1.27 |
3.74 3.12 3.61 3.05 |
58.9 60.3 56.7 57.0 |
24.2 24.5 26.1 25.5 |
16.9 15.2 17.2 17.5 |
| Soil depth (cm) |
pH | E.C. (dS/m) |
CaCO3 (%) |
Soluble cations ( meq/l) |
Soluble anions (meq/l) |
||||||
| K+ | Na+ | Mg2+ | Ca2+ | Cl- | SO42- | HCO3- | |||||
| 0 – 30 | 8.30 | 1.52 | 25.9 | 1.20 | 6.50 | 2.50 | 5.00 | 8.90 | 2.11 | 4.19 | |
| 30 – 60 | 8.50 | 2.10 | 24.9 | 1.56 | 8.04 | 3.24 | 8.20 | 9.40 | 2.84 | 8.80 | |
| Irrigation | ETo (mm/ha) |
C.U. (mm/ha) |
A.I.W. (mm ha-1) |
Ecu (%) |
|||||||
| 2017/18 | 2018/19 | 2017/18 | 2018/19 | 2017/18 | 2018/19 | 2017/18 | 2018/19 | ||||
| IR60 | 316.8 | 297.9 | 191.1 | 181.5 | 420.2 | 393.9 | 45.5 | 46.1 | |||
| IR80 | 422.4 | 397.2 | 209.0 | 210.1 | 576.7 | 541.0 | 36.2 | 38.8 | |||
| IR100 | 528.0 | 496.5 | 230.2 | 230.0 | 733.1 | 688.1 | 31.4 | 33.4 | |||
| Treatment | Plant height | No. of spikes | Spike length | 1000 grain weight | |
|---|---|---|---|---|---|
| (cm) | /m2 | (cm) | (g) | ||
| Irrigation (I.R.) | |||||
| IR60 | 103.9 | 498 | 5.3 | 46.3 | |
| IR80 | 111.2 | 564 | 5.6 | 48.6 | |
| IR100 | 117.5 | 598 | 6.0 | 51.2 | |
| LSD 0.05 | 4.0 | 50 | 0.3 | 3.7 | |
| Ascorbic acid | |||||
| Control | 108.3 | 521 | 5.4 | 47.0 | |
| 2.0 mM | 109.9 | 541 | 5.6 | 48.6 | |
| 4.0 mM | 110.7 | 567 | 5.7 | 49.4 | |
| 6.0 mM | 114.6 | 584 | 5.8 | 50.0 | |
| LSD 0.05 | 2.9 | 31 | 0.4 | 2.9 | |
| Irrigation x Ascorbic acid interaction | |||||
| IR60 | Control | 102.4 | 454 | 5.1 | 44.6 |
| 2.0 mM | 103.2 | 482 | 5.4 | 46.0 | |
| 4.0 mM | 103.6 | 516 | 5.3 | 47.1 | |
| 6.0 mM | 106.3 | 538 | 5.4 | 47.6 | |
| IR80 | Control | 107.4 | 531 | 5.4 | 46.7 |
| 2.0 mM | 109.8 | 554 | 5.5 | 48.8 | |
| 4.0 mM | 112.4 | 578 | 5.7 | 49.3 | |
| 6.0 mM | 115.3 | 594 | 5.8 | 49.8 | |
|
IR100 |
Control | 115.0 | 579 | 5.8 | 49.7 |
| 2.0 mM | 116.6 | 587 | 5.8 | 51.0 | |
| 4.0 mM | 116.0 | 606 | 6.0 | 51.7 | |
| 6.0 mM | 122.2 | 619 | 6.3 | 52.4 | |
| LSD 0.05 | 5.0 | 53 | 0.7 | 5.0 | |
| Treatment | Grain yield | Straw yield | Biological yield | |
|---|---|---|---|---|
| (Kg ha-1) | (Kg ha-1) | (Kg ha-1) | ||
| Irrigation (IR) | ||||
| IR60 | 4399 | 14870 | 19269 | |
| IR80 | 5123 | 16765 | 21889 | |
| IR100 | 5650 | 18567 | 24217 | |
| LSD 0.05 | 464 | 1405 | 1304 | |
| Ascorbic acid | ||||
| Control | 4461 | 15274 | 19735 | |
| 2.0 mM | 4857 | 16525 | 21381 | |
| 4.0 mM | 5240 | 17039 | 22279 | |
| 6.0 mM | 5672 | 18099 | 23771 | |
| LSD 0.05 | 461 | 1277 | 1529 | |
| Irrigation x Ascorbic acid interaction | ||||
| IR60 | Control | 3720 | 13350 | 17070 |
| 2.0 mM | 4167 | 14781 | 18948 | |
| 4.0 mM | 4612 | 15272 | 19883 | |
| 6.0 mM | 5097 | 16079 | 21176 | |
| IR80 | Control | 4629 | 15408 | 20038 |
| 2.0 mM | 4988 | 16574 | 21561 | |
| 4.0 mM | 5256 | 17116 | 22372 | |
| 6.0 mM | 5620 | 17963 | 23583 | |
|
IR100 |
Control | 5035 | 17064 | 22098 |
| 2.0 mM | 5416 | 18219 | 23635 | |
| 4.0 mM | 5852 | 18730 | 24581 | |
| 6.0 mM | 6299 | 20256 | 26555 | |
| LSD 0.05 | 798 | 2211 | 2648 | |
| Treatment | WP | IWP | |
|---|---|---|---|
| (kg mm–1 ha–1) | (kg mm–1 ha–1) | ||
| Irrigation (IR) | |||
| IR60 | 23.68 | 10.85 | |
| IR80 | 24.45 | 9.19 | |
| IR100 | 24.56 | 7.97 | |
| LSD 0.05 | NS | 1.00 | |
| Ascorbic acid | |||
| Control | 21.33 | 8.18 | |
| 2.0 mM | 23.25 | 8.95 | |
| 4.0 mM | 25.11 | 9.69 | |
| 6.0 mM | 27.22 | 10.52 | |
| LSD 0.05 | 2.20 | 0.80 | |
| Irrigation x Ascorbic acid interaction | |||
| IR60 | Control | 20.01 | 9.16 |
| 2.0 mM | 22.42 | 10.27 | |
| 4.0 mM | 24.83 | 11.37 | |
| 6.0 mM | 27.46 | 12.58 | |
| IR80 | Control | 22.09 | 8.29 |
| 2.0 mM | 23.80 | 8.94 | |
| 4.0 mM | 25.08 | 9.43 | |
| 6.0 mM | 26.82 | 10.09 | |
|
IR100 |
Control | 21.88 | 7.09 |
| 2.0 mM | 23.54 | 7.64 | |
| 4.0 mM | 25.43 | 8.26 | |
| 6.0 mM | 27.38 | 8.89 | |
| LSD 0.05 | 3.80 | 3.80 | |
| Treatment | Plant height | No. of spikes /m2 | Spike length | 1000 grain weight | |
|---|---|---|---|---|---|
| (cm) | (cm) | (g) | |||
| Irrigation (IR) | |||||
| IR60 | 101.5 | 455 | 5.0 | 44.8 | |
| IR80 | 108.0 | 508 | 5.2 | 47.7 | |
| IR100 | 112.1 | 524 | 5.7 | 50.8 | |
| LSD 0.05 | 4.3 | 38 | 0.3 | 3.3 | |
| Vitamin B2 (Riboflavin) | |||||
| Control | 104.8 | 463 | 5.1 | 46.0 | |
| 0.5 mM | 105.9 | 486 | 5.2 | 48.0 | |
| 1.0 mM | 107.9 | 509 | 5.3 | 48.3 | |
| 2.0 mM | 110.2 | 526 | 5.6 | 48.9 | |
| LSD 0.05 | 3.3 | 21 | 0.3 | 2.1 | |
| Irrigation x vitamin B2 (Riboflavin) interaction | |||||
| IR60 | Control | 97.7 | 411 | 4.7 | 43.4 |
| 0.5 mM | 100.6 | 443 | 5.1 | 44.6 | |
| 1.0 mM | 102.8 | 471 | 5.0 | 45.2 | |
| 2.0 mM | 104.9 | 496 | 5.0 | 46.0D | |
| IR80 | Control | 106.3 | 473 | 5.1 | 45.7 |
| 0.5 mM | 107.2 | 501 | 5.0 | 48.1 | |
| 1.0 mM | 108.1 | 522 | 5.4 | 48.4 | |
| 2.0 mM | 110.5 | 538 | 5.5 | 48.6 | |
|
IR100 |
Control | 110.3 | 504 | 5.4 | 48.9 |
| 0.5 mM | 110.0 | 515 | 5.5 | 51.2 | |
| 1.0 mM | 112.9 | 534 | 5.6 | 51.4 | |
| 2.0 mM | 115.1 | 544 | 6.1 | 51.9 | |
| LSD 0.05 | 5.8 | 37 | 0.5 | 3.7 | |
| Treatment | Grain yield | Straw yield | Biological yield | |
|---|---|---|---|---|
| (kg ha-1) | (kg ha-1) | (kg ha-1) | ||
| Irrigation (IR) | ||||
| IR60 | 4217 | 13693 | 17910 | |
| IR80 | 4863 | 15879 | 20742 | |
| IR100 | 5267 | 17594 | 22861 | |
| LSD 0.05 | 314 | 1109 | 1242 | |
| Vitamin B2 (Riboflavin) | ||||
| Control | 4501 | 14638 | 19138 | |
| 0.5 mM | 4696 | 15387 | 20083 | |
| 1.0 mM | 4878 | 16121 | 21000 | |
| 2.0 mM | 5054 | 16743 | 21796 | |
| LSD 0.05 | 418 | 1126 | 1100 | |
| Irrigation x vitamin B2 (Riboflavin) interaction | ||||
| IR60 | Control | 3892 | 12087 | 15980 |
| 0.5 mM | 4129 | 13369 | 17498 | |
| 1.0 mM | 4356 | 14310 | 18666 | |
| 2.0 mM | 4491 | 15004 | 19496 | |
| IR80 | Control | 4574 | 14968 | 19542 |
| 0.5 mM | 4812 | 15535 | 20347 | |
| 1.0 mM | 4956 | 16282 | 21238 | |
| 2.0 mM | 5110 | 16732 | 21842 | |
|
IR100 |
Control | 5036 | 16858 | 21894 |
| 0.5 mM | 5148 | 17256 | 22404 | |
| 1.0 mM | 5323 | 17772 | 23095 | |
| 2.0 mM | 5560 | 18491 | 24052 | |
| LSD 0.05 | 725 | 1950 | 1905 | |
| Treatment | WP (kg mm–1 ha–1) | IWP (kg mm–1 ha–1) | |
|---|---|---|---|
| Irrigation (IR) | |||
| IR60 | 22.68 | 10.39 | |
| IR80 | 23.20 | 8.72 | |
| IR100 | 22.89 | 7.43 | |
| LSD 0.05 | NS | 0.66 | |
| Vitamin B2 (Riboflavin) | |||
| Control | 21.55 | 8.30 | |
| 0.5 mM | 22.51 | 8.69 | |
| 1.0 mM | 23.40 | 9.04 | |
| 2.0 mM | 24.23 | 9.36 | |
| LSD 0.05 | 2.00 | 0.80 | |
| Irrigation x vitamin B2 (Riboflavin) interaction | |||
| IR60 | Control | 20.94 | 9.59 |
| 0.5 mM | 22.21 | 10.17 | |
| 1.0 mM | 23.42 | 10.73 | |
| 2.0 mM | 24.15 | 11.06 | |
| IR80 | Control | 21.82 | 8.21 |
| 0.5 mM | 22.96 | 8.63 | |
| 1.0 mM | 23.65 | 8.89 | |
| 2.0 mM | 24.38 | 9.17 | |
|
IR100 |
Control | 21.89 | 7.10 |
| 0.5 mM | 22.37 | 7.26 | |
| 1.0 mM | 23.14 | 7.51 | |
| 2.0 mM | 24.16 | 7.84 | |
| LSD 0.05 | NS | 1.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





