2. Methods
2.1. Data collection
The Almazov National Medical Research Center initiated a prospective PAH program for adult patients in January 2006 in the Northwest region of Russia. The inclusion criteria into the registry for patients with precapillary pulmonary hypertension were the following: a mean pulmonary arterial pressure (mean PAP) ≥25 mmHg, pulmonary capillary wedge pressure (PCWP) < 15 mmHg and pulmonary vascular resistance (PVR) ≥3 Wood units.
Exclusion criteria for the study were the following: PAH other etiologies than IPAH, moderate to severe restrictive or chronic obstructive lung disease (COPD) with total lung capacity (TLC<60%), and forced expiratory volume in one second (FEV1<60%) or oxygen dependence due to the lung disease; severe left heart disease defined in presence of heart valve defects, signs of cardiomyopathy, ischemic heart disease (IHD) requiring revascularization, arterial hypertension with blood pressure >160 and 90 mmHg; established malignancies; uncontrolled diabetes mellitus; thyroid disease; mental disorders; end-stage kidney dysfunction. Left heart disease (LHD) phenotype was defined as having more than 3 comorbidities: hypertension, ischemic heart disease, diastolic dysfunction by echocardiography, atrial fibrillation, or obesity (IMT>30 kg/m2). Patients with LHD phenotype and with echocardiography signs of diastolic dysfunction type 2 or 3 type, PCWP≥ 15 mmHg, PVR≤5 WU or with an uncertain PCWP value greater than diastolic PAP were not enrolled into the study.
Demographics, symptoms, comorbidity, 6-minute walk test (6MWT) distance and cardiopulmonary exercise test (CPET), echocardiographic and RHC parameters, lung function testing including diffusing capacity for carbon monoxide (DLCO), laboratory variables (hemoglobin, creatinine with estimated glomerular filtration rate [eGFR], total bilirubin, uric acid, N-terminal pro-brain-type natriuretic peptide [NT-proBNP]), as well as the number of PAH medications were collected. Estimated GFR was calculated according to the CKD-EPI equation.
Baseline risk stratification was performed using 2015 ESC/ERS risk stratification, the REVEAL (Registry to Evaluate Early and Long-Term Disease Management), and the REVEAL 2.0 score [
https://www.pahinitiative.com/hcp/risk–assessment/calculators]. The probability of heart failure with preserved ejection fraction (HFpEF) was assessed using the H2FpEF-score [
11].
According to the Global Burden of Disease Study, the average life expectancy and aging depend a lot on the countries and reflect many aspects, including the socio-demographic index [
12], which is considerably lower in the Russian Federation than in the USA, Western Europe, and Japan [
13]. The official Rosinfostat data provides 71.2 years as the average life expectancy in the Russian Federation from 2010 through 2019 years [
https://rosinfostat.ru/prodolzhitelnost-zhizni/]. We have chosen the age of 60 years as the cut-off for aging because the number of comorbidities considerably increases.
The number of PAH medications was assessed at the time of diagnosis and the end of follow-up. It should be noted that PAH therapy in Russia is limited with endothelin receptor antagonists (ERA) (bosentan, ambrisentan, macitentan), phosphodiesterase type 5 inhibitor (PDEi-5- sildenafil), prostanoids (inhaled iloprost), IP-receptors agonist (selexipag) and soluble guanylate cyclase stimulator (sGC - riociquat). Parenteral prostanoids for PAH treatment have not been registered yet.
The start date of follow-up was the date of hospitalization for the first right heart catheterization (RHC) and the last date of follow-up was the date of death, last patient visit, or telephone contact within three months. The date and cause of death were obtained from medical records, local physicians, or death certificates provided by relatives. The follow-up analysis was limited to February 2021.
By the legislation of the Russian Federation and the Local Acts of the Almazov National Medical Research Center, statistical analysis of registries does not require a specific permission of the Ethics Committee. The study reflects daily clinical practice within the Guidelines for the management of patients with PAH [
3]. Identifiable patient information was not presented in the study.
2.2. Study population
The study population comprised 119 (≥18 years old) Caucasian IPAH patients. All patients were divided according to age of IPAH diagnosis into two groups: group I <60 years old (n=89); group II ≥60 years old (n=30). The entire cohort of IPAH patients was divided into sub-groups based on H2FpEF score: H2FpEF score≤1, H2FpEF score ≥2. Lung function tests were performed in 102 patients, NT-proBNP in 112 patients, 6MWT in 118 patients, and CPET in 76 patients.
2.3. Statistical analysis
Demography, clinical data including comorbidity, PAH functional class (FC) (WHO), PAH drugs number, hemodynamic data, laboratory and lung function test parameters, echocardiography variables and exercise performance parameters were compared in group I and group II as well as in H2FpEF score groups. Numerical parameters with normal distribution were presented as mean ± standard deviation (M±SD), and numerical parameters with abnormal distribution were presented as median and interquartile range (IQR; M±25%, 75%). Categorical variables were presented as absolute numbers and percentages and compared using Fisher exact or Pearson M-L Chi-square tests, as appropriate. Mean values in groups were compared using the Mann-Whitney U-test, and Wilcoxon signed-rank test, as appropriate. Survival analyses were performed using Kaplan-Meier curves and log-rank test to compare survival distribution between group I and II as well as for the patients with- or without left heart disease (LHD) comorbidities. The discrimination power of the different risk stratification scoring systems was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). A statistically significant difference was determined as a two-tailed P<0.05. Statistical analysis of the data was carried out using Statistica for Windows, version 10.0 (StatSoft, USA).
3. Results
3.1. Study population, comorbidity and exercise tolerance
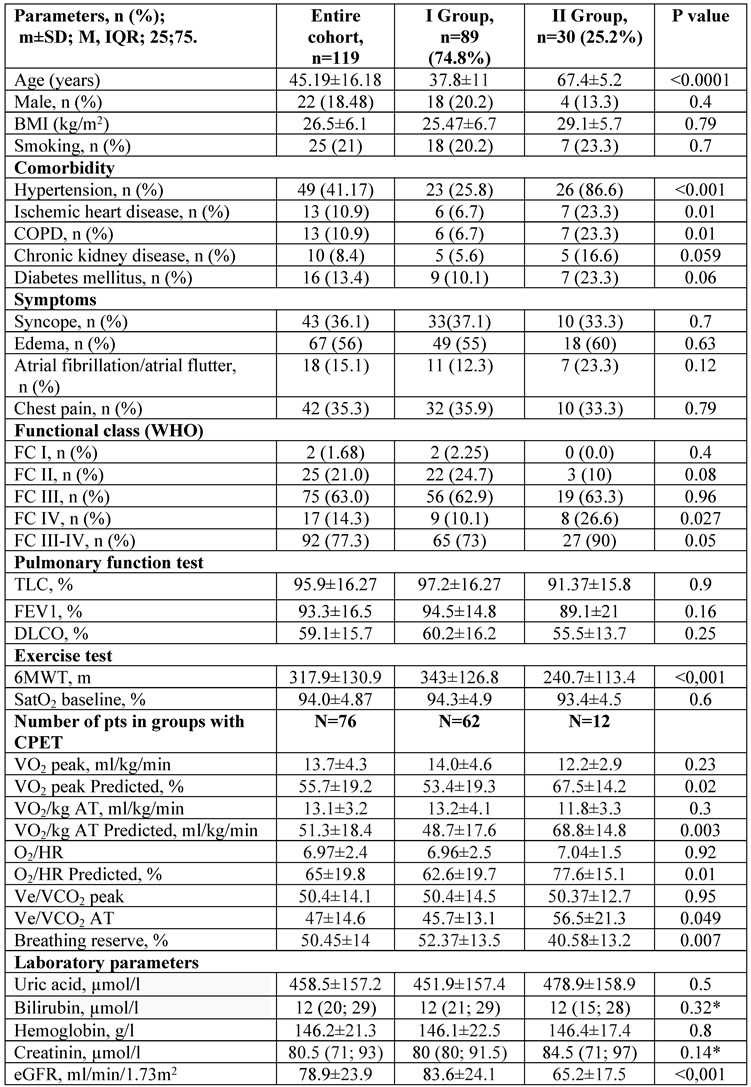
The mean age was 45.19±16.18 years [min-max: 18; 76] with a male-to-female ratio of 1:5.4 in the entire cohort (n=119). The mean time from symptoms to the first RHC was 1.9 (0.9; 3.9) years and the mean follow-up was 2.98 (1.6; 6.1) years in the entire study population.
Left heart disease was more prevalent in group II (≥ 60 years old) when compared with group I patients (χ
2=34.3, p<0.001). Patients with hypertension and COPD had shorter 6MWT distances (280 ±125 vs 343±129 m, p=0.002; and 227±138 vs 329±126 m, p=0.007) when compared with other patients in the entire IPAH population. Diabetes mellitus and chronic kidney disease had a trend toward a more frequent presence in group II patients (≥ 60 years old) (
Table 1).
Patients with III-IV FC (WHO) were more prevalent in group II in comparison with group I. The 6MWT distance was significantly reduced in elderly patients unless the peak O
2 consumption (VO
2), as well as oxygen pulse (O
2/HR) at the aerobic threshold, were comparable between the two groups. To exclude age-dependent differences in the parameters above, the percent from predicted values were analyzed. In group II, the percent of peak VO
2 predicted, aerobic threshold predicted percent, and percent of O
2/HR predicted were significantly lower in younger patients in comparison with patients over 60 years. Minute ventilation (VE/VCO
2) on peak exercise did not differ between groups, whereas VE/VCO
2 on aerobic threshold was lower in younger patients compared with patients over 60 years. Breathing reserve was significantly lower in patients over 60 years (
Table 1).
3.2. Echocardiography data
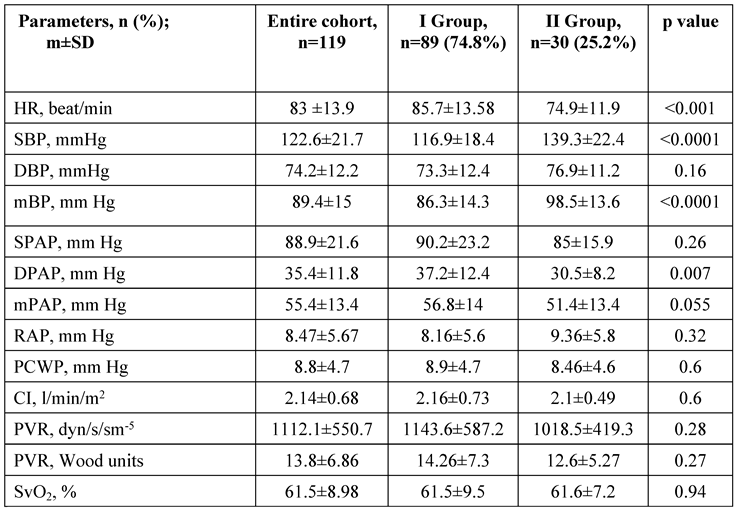
Left atrium (LA) volume index and LV end-diastolic dimension were larger in group II patients (≥ 60 years old), when compared with patients <60 years. No significant difference in the sizes of the right heart and function was registered between the two groups. A trend towards a higher right-to-left ventricular end-diastolic dimension ratio (RV/LV ratio) was found in patients <60 years old in comparison with patients ≥60 years old (Table 2, online supplements). No differences were registered in stroke volume derived from echocardiography and the LV ejection fraction between the two groups.
A lower median LA volume index was found in deceased patients: 21.5 [18.0; 26.0] vs 25.5 ml/m2 [22.0; 34.1], p=0.001, and in patients with cardiac index less than 2.0 l/min/m2: 24.0 [19.0; 28.0] vs 27.0; [23.0; 35.0] ml/m2, p=0.01. A smaller mean LA diameter was registered in patients with syncope (34.4±6.7 vs 38.2±7.3 mm, p=0.003).
In patients with PAH III-IV FC (WHO), the LV end-diastolic dimension (62.7±19.4 vs 80.7±23.3 mm, p<0.001) and LV stroke volume were smaller than in I-II FC patients (41.3± 13.5 vs 54.1±14.4 ml, p<0.0001). A smaller LV end-diastolic dimension was associated with a cardiac index less than 2.0 l/min/m2 (p<0.001) and NT-proBNP>1400 pg/ml (p<0.0001).
3.3. Hemodynamic data
Patients in group I exhibited a significantly lower mean blood pressure, higher heart rate, and mean PAP in comparison with group II patients (
Table 3). A negative correlation was revealed between the age and SatO
2 (ρ=-0.39, p<0.001) and with the Tiffno index (ρ=-0.26, p<0.01). The presence of COPD was associated with a tendency to lower SatO
2 (92.4±4.3 vs 94.6±3.7%, t-test, p=0.06) in the entire cohort of patients.
Patients with LHD phenotype had significantly higher mean blood pressure (95.2±13.4 vs 85.3±14.8 mm Hg, p=0.005), and lower PVR (PVR: 11.8±4.3 vs 15.2±7.9 WU, p=0.0087), when compared with patients without LHD.
Obesity was associated with a lower PVR index (6.1±2.5 vs 9.15±4.9 WU/m2; p<0.001) which was supported by the inverse correlation between the body mass index and PVR (ρ=-0.26; p=0.003). The presence of obesity and smoking history were associated with lower SatO2 (p=0.01; p=0.01, respectively).
3.4. Laboratory data
Estimated GFR was markedly decreased in group II patients in comparison with group I patients, whereas no significant difference was registered in hemoglobin, NT-proBNP, creatinine, bilirubin, and uric acid concentrations between the two groups (
Table 1). Estimated GFR was lower in patients with hypertension in comparison with patients without hypertension (72.6±22.9 vs 83.2±23.8 ml/min/1.73 m
2, p=0.01). A positive correlation was detected between the age and uric acid (ρ=0.30, p=0.006), creatinine (ρ=0.22, p=0.017), and NT-proBNP levels in serum (ρ=0.17, p=0.06). The inverse correlation between the eGFR and NT-proBNP level in serum was just about the statistical value of confidence (ρ=-0.18, p=0.05) in the entire cohort of patients.
3.5. Risk stratification
3.5.1. Entire IPAH group (n=119).
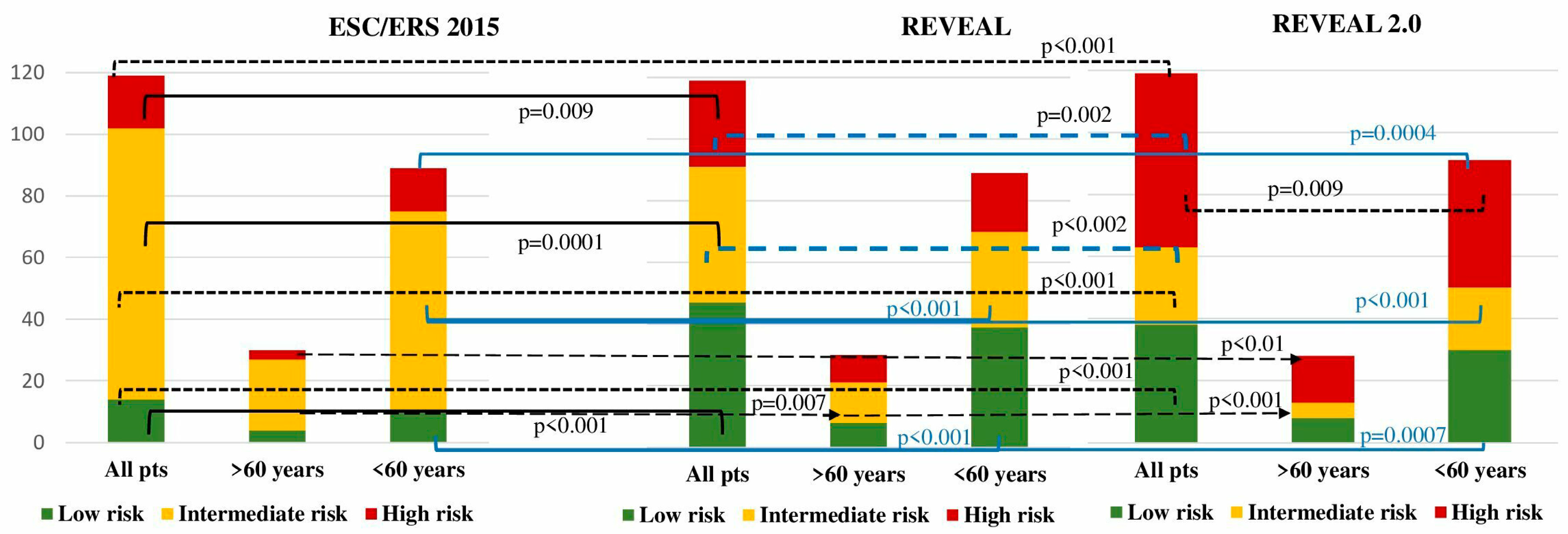
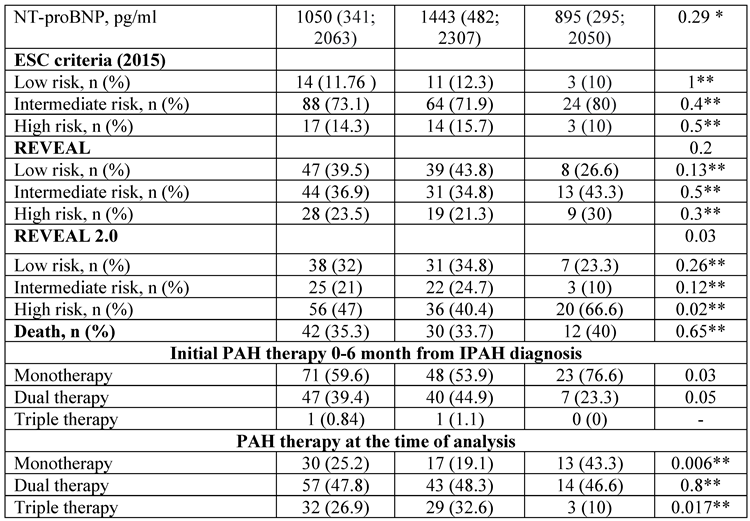
Patients were stratified according to the ESC/ERS 2015, REVEAL and REVEAL 2.0 risk stratification scales. Considerable differences were registered in between the risk stratification scales when applied to the entire cohort of patients. Significantly more high-risk patients were observed using the REVEAL 2.0 compared to the ESC/ERS risk scale (47 vs 14.3%, p<0.001), while with the REVEAL scale, 23.5% patients were appraised as a high risk (p=0.009 compared to the ESC/ERS). Similar inconsistencies were observed regarding the low-risk and intermediate-risk numbers of patients in between ESC/ERS and REVEAL (
Figure 1). Intermediate-risk patients in the entire IPAH population were the predominant group according to the ESC/ERS scale in comparison with the REVEAL and REVEAL 2.0 scales (73.1 vs 36.9 vs 21%; p<0.001; p<0.0001, respectively). The REVEAL and REVEAL 2.0 scales corresponded with each other in low-risk patient stratification (39.5 vs 32%, p=0.14), whereas high-risk patients were more prevalent with REVEAL 2.0 score (47 vs. 25.3%, p=0.002). More patients with intermediate- risk were stratified with a REVEAL score in comparison with REVEAL 2.0 (36.9 vs 21%, p=0.01).
3.5.2. Group I (under 60 years)
A significant difference in low-risk patient numbers was revealed in comparison between ESC/ERS and REVEAL (12.3 vs. 43.8%, p<0.001), and REVEAL 2.0 scales (12.3 vs. 34.8%, p=0.0007). According to the ESC/ERS, the majority of patients were intermediate-risk in comparison with REVEAL (71.9 vs. 34.8%, p<0.001) and REVEAL 2.0 scales (71.9 vs. 24.7%, p<0.001). The lowest proportion of high-risk patients was stratified with ESC/ERS in comparison with REVEAL (15.7 vs. 21.3%, p=0.4) and REVEAL 2.0 (15.7 vs. 40.4%, p=0.0004). No differences were registered between REVEAL and REVEAL 2.0 in stratification of low- (p=0.2) and intermediate-risk patients (p=0.18). A considerably elevated proportion of high-risk patients was stratified according to REVEAL 2.0 compared with REVEAL (40.4 vs. 21.3%, p=0.009) (
Table 1,
Figure 1).
3.5.3. Group II (over 60 years)
No difference in low-risk patients was observed in between ESC/ERS and REVEAL (10 vs. 26.6%, p=0.2), and REVEAL 2.0 systems (10 vs. 23.3%, p=0.2). The intermediate risk was allocated to the majority of patients with the ESC/ERS scale in comparison with REVEAL (80 vs. 43.3%, p=0.007) and REVEAL 2.0 (80 vs. 10%, p<0.001). High risk was attributed to the minority of patients according to the ESC/ERS scale compared to REVEAL (10 vs. 30%, p=0.1) and REVEAL 2.0 (10 vs. 66.6%, p<0.001). The numbers of the low-risk group did not differ between REVEAL and REVEAL 2.0 scores (p=1). Whereas, a significant difference was registered between REVEAL and REVEAL 2.0 scores in intermediate (p=0.007) and high-risk (p=0.009) groups.
No differences in risk were observed between group I and group II using ESC/ERS and REVEAL. The only REVEAL 2.0 exhibited an increased percentage of high-risk patients in group II when compared with patients <60 years (60.6 vs. 40.4%, p=0.02) (
Table 1,
Figure 1).
3.6. H2FpEF-score
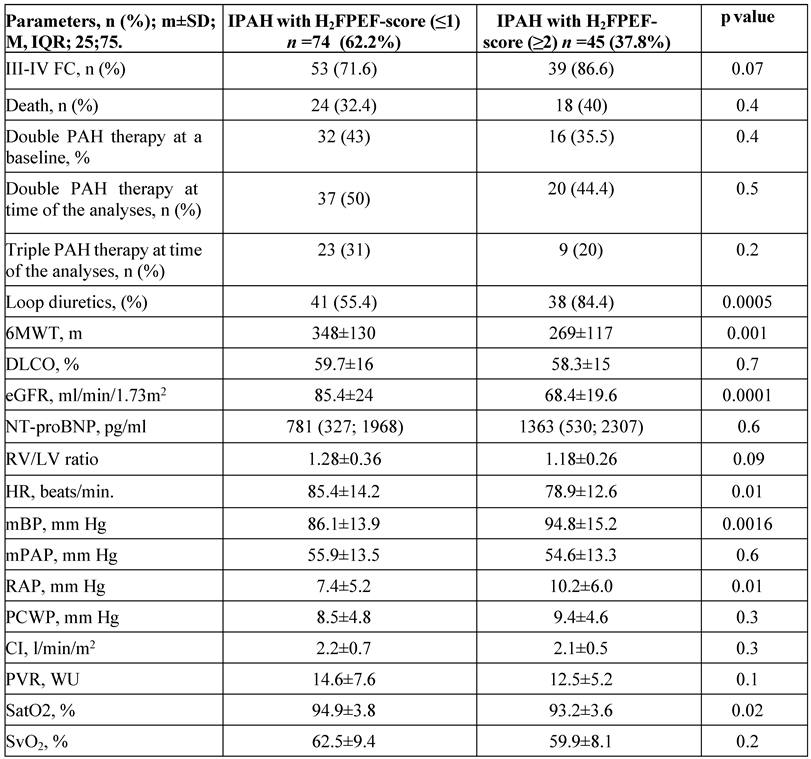
H2FpEF-score ≤1 was registered in 74 patients (62.2%), and H2FpEF score ≥2 in 45 patients (37.8%) in the entire cohort. Patients with H2FpEF score ≥2 had significantly lower 6MWT distance, eGFR, and SatO
2, whereas mean BP and right atrial pressure (RAP) were significantly elevated in comparison with patients with H2FpEF score≤1. H2FpEF score ≥2 was more prevalent in elderly patients in comparison with the younger group (51 vs. 9.5%, p=0.0001). No significant differences in PCWP, cardiac index, and PVR were observed between the H2FpEF scores in the entire IPAH cohort. We didn’t reveal a significant difference in NT-proBNP and the number of PAH medications prescribed to the patients by the H2FpEF score in the entire cohort of patients. Loop diuretics were used more often in patients with H2FpEF score≥2 in comparison with H2FpEF score≤1 (
Table 4). In group II (≥60 years old), the number of patients with H2FpEF score ≤1 was very limited (n=7, 23.3%) failing to demonstrate the true differences with H2FpEF score ≥2 patients.
3.7. Survival analysis
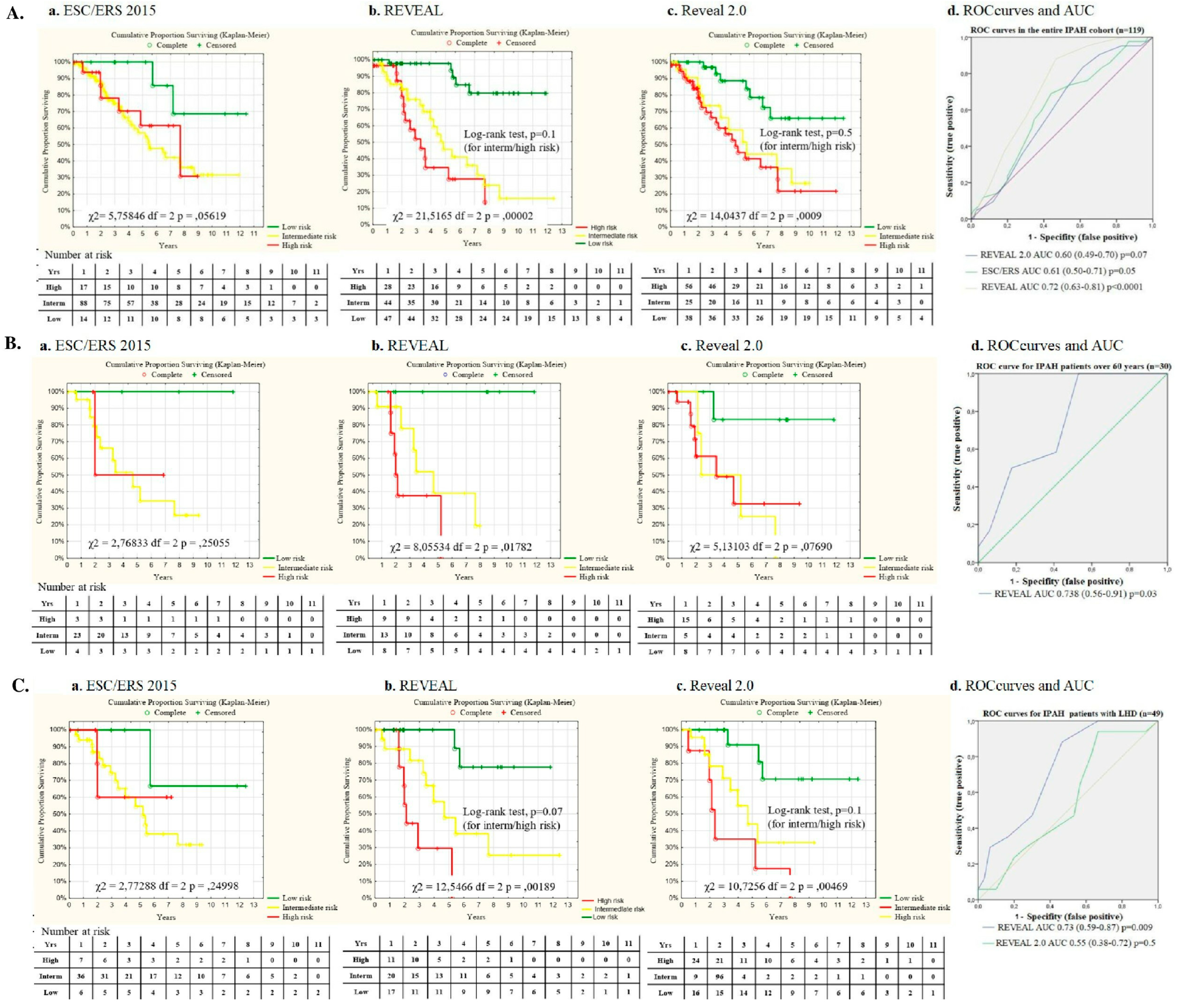
In the entire cohort (n=116), all-cause mortality was 35.3% (n=42). The main cause of death was PAH progression in 76% (n=32), sudden death was reported in 7 cases (16.6%), pneumonia in 1 patient (2.4%), pneumothorax during RHC in 1 patient (2.4%), and one case (2.4%) of severe gastric bleeding. For the entire cohort, the Kaplan-Meier estimated survival rates for 1, 2, 3, 5, 7, and 10 years after diagnosis were 95%, 88.6%, 78.5%, 61.7%, 48.5% and 33.7%, respectively. Mortality rates did not differ between the two groups (
Table 1) as well as survival rates (Figure 2). No difference in survival was registered in patients with (n=49) and without LHD comorbidities (n=70) (Log-rank test, p=0.65). No significant difference in survival was observed in the entire IPAH cohort in different H2FpEF-score groups (Log-rank test, p=0.2).
Figure 2. Kaplan-Meier survival curves: A. Survival in the entire IPAH patients cohort; B. Survival in group I (under 60 years) and group II (over 60 years); C. Survival in the entire IPAH cohort of patients (n=119) by the initial PAH therapy; D. Survival in entire IPAH cohort with PAH therapy at the time of analyses.
The REVEAL score exhibited the strongest predictive value between low, intermediate and high-risk patient survival in comparison with REVEAL 2.0 and the ESC/ERS score in the entire IPAH patients cohort (
Figure 3A, 3D). Nevertheless, no significant discrimination was observed in survival in IPAH patients with intermediate and high risk at baseline.
In group II patients the best predictive value for survival was observed using REVEAL risk score, whereas survival prediction did not reach statistical significance with REVEAL 2.0 and ESC/ERS score (
Figure 3 B, D).
The REVEAL score reliably predicted survival in patients with LHD comorbidities (n=49), whereas the predictive value for the REVEAL 2.0 score was statistically insignificant, as well as the ESC/ERS score (χ
2 =2.7, p=0.2) (
Figure 3C, D).
3.8. PAH-specific therapy
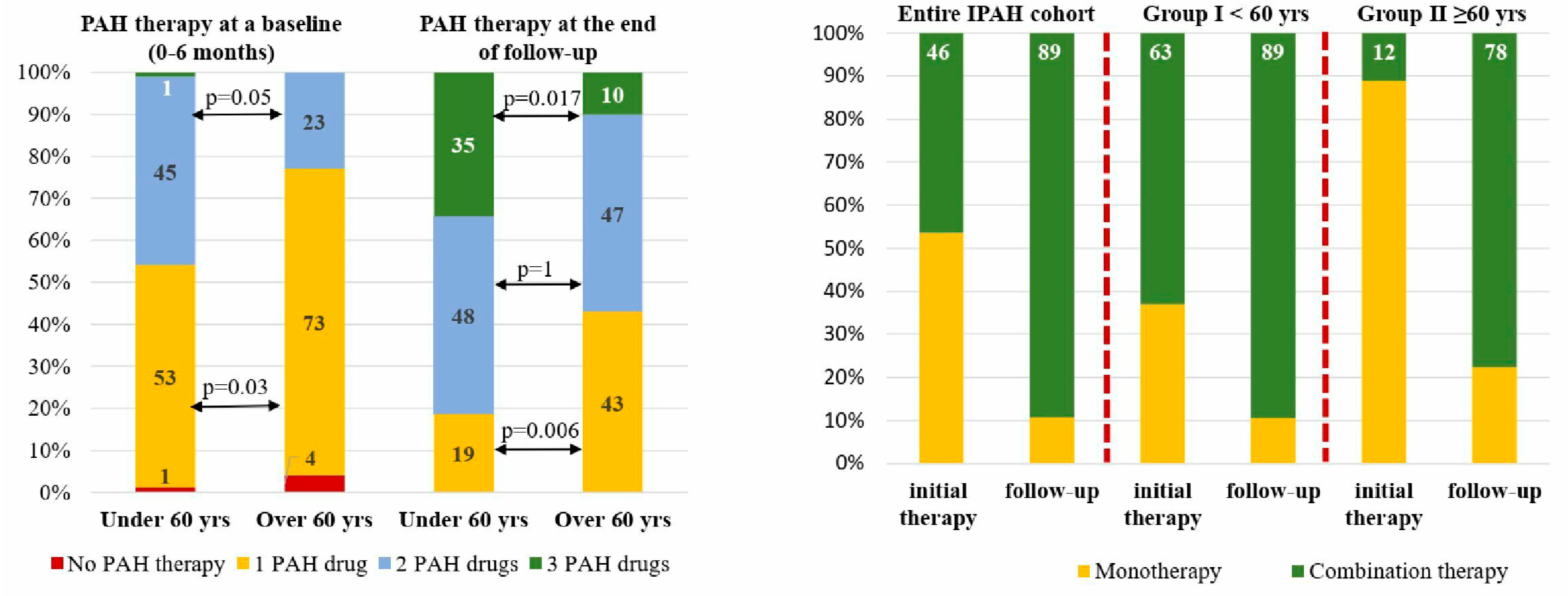
Almost all patients had been receiving PAH-specific therapy within the first 6 months since the diagnosis of IPAH was confirmed. The majority of patients started with monotherapy 59.6% (n=71), 39.4% (n=47) with double combination therapy and only one patient (0.84%) with triple combination PAH therapy. Elderly patients were significantly less likely to receive initial combination therapy in comparison with group I patients (χ
2=4.8, p=0.028). Sildenafil was the drug most used in mono- and combination PAH therapy. At the time of the analysis, the difference in the number of PAH drugs was even more pronounced than at baseline with more numbers for younger patients in group I compared to the group II patients (χ
2=9.49, p=0.008). The observed difference in PAH drug number was particularly evident in young patients for triple combination therapy (χ
2=5.82; p=0.016). No statistical difference was observed in double-combination PAH therapy between the two groups at the time of analysis (48.3 vs. 46.6% in group II, p=0.1). Near half (43.3%) of the patients in group II receive monotherapy in comparison with only 19.1% of patients in group I (χ
2=6.98; p=0.008) (
Table 1,
Figure 4).
Twenty-eight patients were stratified as high-risk according to the REVEAL score in the entire population, 19 patients in group I and 9 patients in group II. Initial combination therapy had been started in 13 patients (46.4%) in the entire group, besides those 12 patients (63%) were in group I and only 1 patient (11.1%) in group II (p=0.015). During the follow-up the number of patients receiving combination PAH therapy increased up to 24 patients (89.3%) predominantly in group II (n=7, 77.7%, p=0.01) and minority in group I (n=17, 89.5%, p=0.2) (
Figure 4).
No significant differences in survival were registered in patients with mono- or combination PAH-specific therapy at a baseline and at the time of analysis in the entire IPAH cohort (Figure 2 C, D). The same observation was registered in group II (p=0.6) as well as in IPAH patients with LHD (p=0.8).
Seventy-two patients were stratified as intermediate and high-risk according to the REVEAL score in the entire IPAH cohort. No significant correlations were registered between the initial number of PAH drugs and survival for the intermediate (p=0.9) and high-risk group (p=0.2). Inhaled iloprost was used as a third PAH drug only in 22 patients, predominantly in group I (n=20).
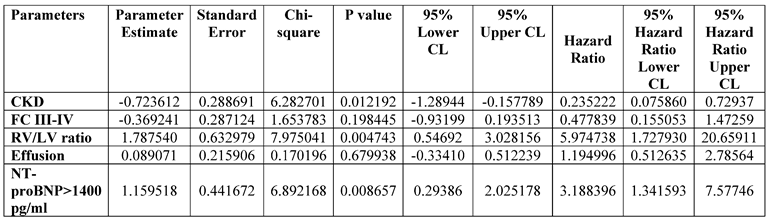
3.9. Factors associated with death
Demographics, symptoms, comorbidity, H2FpEF score, 6MWT distance, CPET parameters, echocardiographic and RHC parameters, lung function testing including DLCO, laboratory variables were analyzed in association with death in the entire cohort of IPAH patients using univariate Cox regression analysis with subsequent selection of factors that had a statistically significant association with mortality, the highest level of risk ratio and well established according to the previous studies in IPAH patients [
5,
14] (Table 5, on-line supplements). Chronic kidney disease presence, the RV/LV ratio, and NT-proBNP >1400 pg/ml were independently associated with death in the entire cohort of IPAH patients (
Table 6). Cardiac index and mixed venous oxygen saturation were eliminated from the analyses due to the tight interrelationship with each other and NT-proBNP.
4. Discussion
The main findings of our study are the evidence of a high discrepancy in risk stratification between ESC/ERS 2015, REVEAL, and REVEAL 2.0 scores and insufficient relevance of the scales for patients over 60 years and patients with comorbidities. The absence of differences in survival between IPAH patients under and over 60 years is an unexpected finding.
No risk stratification scales discriminated the actual risk of mortality between the intermediate and high-risk patients in the entire IPAH population and the elderly group. The REVEAL score exhibited better discrimination of the intermediate and high-risk groups in IPAH patients with LHD than either REVEAL 2.0. The predictive value for REVEAL 2.0 and REVEAL in the entire IPAH population and patients with LHD was in concordance with the other studies [
15,
16,
17]. ESC/ERS 2015 risk stratification scale provided the highest proportion of intermediate-risk patients in all groups and underestimated the number of high-risk patients, which corresponded with other large registry-based studies [
18]. The largest number of high-risk patients was stratified using the REVEAL 2.0 scale in the younger and elderly cohorts, as well as in the entire IPAH population in our study. That might be related to the significant contribution of chronic kidney disease presence [
19,
20], and severely impaired mobility in elderly patients with comorbidities [
21]. However, the decreased glomerular filtration rate is not specific to PAH, but it is strongly associated with age, arterial hypertension, and diabetes mellitus presence in the general population. Chronic kidney disease, NTproBNP≥1400 pg/ml, and the RV/LV ratio appear to independently predict death in the entire population of patients in our study. Indeed, NT-proBNP demonstrates tight correlations with the major clinical, echocardiography, and hemodynamic determinants of PAH severity [
22,
23,
24] and exhibits high predictive value in serial evaluations for long-term survival [
25]. Elevated NT-proBNP level seems to overestimate the PAH risk in elderly patients with decreased eGFR [
26].
The absence of constant survival predictors, like etiology of PAH, age, gender, and comorbidity remains the main drawback of the ESC/ERS risk scale. Cardiac index, stroke volume index, and mixed venous oxygen saturation, used in ESC/ERS score, are closely interrelated with each other and could add points and overlap the effect of other indicators. REVEAL and REVEAL 2.0 scales use PVR for the assessment of pulmonary vascular disease severity and prognosis. However, patients with IPAH and PVR <5 WU or >32 WU are quite rarely seen in clinical practice. Moreover, the variability of the right ventricle-pulmonary artery uncoupling has been well defined, therefore, PVR might not reflect prognosis in all cases. A low number of patients with a PVR value of <5 WU (n=11) and the predicted DLCO>80% (n=6) could be additional reasons for the elevated amount of high-risk patients according to the REVEAL 2.0 score in our study. The implementation of cardiac visualization with magnetic resonance imaging (MRI) in the ESC/ERS 2022 scale might significantly improve early diagnostics of the right heart disadaptation and therefore, facilitate the discrimination between low- and intermediate-risk patients. In our study, the RV/LV ratio was determined as an independent predictor for survival in the entire population. A positive correlation was established between the RV/LV ratio and pulmonary artery pressures, and PVR in previous studies [
27,
28,
29]. Elderly patients and patients with H2FpEF≥2 are characterized by a smaller RV/LV ratio, possibly contributing to a phenotype of PH associated with HFpEF or vice versa with IPAH in elderly patients.
We have not found a significant association between the 6MWT distance and survival in the entire group. A low 6MWT distance and higher FC (WHO) could reflect at least more prevalent comorbidity (hypertension, COPD, obesity, arthritis) and low physical fitness in the elderly population, and therefore, overestimate the severity of IPAH. In this issue, CPET is suggested to clarify the mechanisms of exercise intolerance [
30]. The excellent correlations of cardiopulmonary parameters with invasive hemodynamics make CPET extremely promising and desirable for non-invasive assessment of PAH severity and prognosis. The absolute values of peak oxygen consumption and peak minute ventilation per unit of carbon dioxide have been included in the risk stratification proposed by the ESC/ERS in 2015. Unless those parameters have not been widely used due to the low concordance with 6MWT and the interpretation discrepancies between the absolute and predicted values [
31]. We observed the comparable absolute values of peak VO
2, oxygen pulse, and CO
2 ventilatory equivalent in two groups, while the predicted values of peak VO
2, aerobic threshold, and O
2/HR were significantly lower in younger patients in comparison with patients over 60 years. This observation suggests a greater compromise of the cardiorespiratory system in young patients. Validation of predictable absolute values for age and sex can significantly improve the relevance of cardiorespiratory testing for risk stratification [
32,
33].
At present, there is no specific tool for risk assessment in IPAH with comorbidities. The implementation of IPAH phenotype clustering might be a more effective approach in terms of disease course prediction and choosing the number of PAH drugs for initial therapy [
34]. Four distinct IPAH clusters were determined by R. Badagliacca et al. (2020) in 252 prevalent patients based on age, gender, right heart remodeling on echocardiography, invasive hemodynamics, and CPET. However, this approach did not take into account comorbidity which could unpredictably modify the disease course. M. Hoeper et al. (2020, 2022) propose d an alternative three-cluster approach in IPAH phenotyping with consideration of age, gender, smoking history, and diffusion capacity for carbon monoxide. The authors particularly focused on lung disease phenotype identification as it exhibits poor treatment response and the worst prognosis comparable with group III PH [
35,
36]. Bayesian network modeling in the PHORA calculator might be a new promising approach for individual survival calculation using simultaneous assessment and interrelationship of weighting demography, clinical, imaging, laboratory, invasive hemodynamics, and exercise testing parameters [
37]. The implementation of certain comorbidities or main indices of comorbidities might strengthen the PHORA risk estimation.
The challenge in the differential diagnosis of IPAH and PH associated with HFpEF in elderly patients with comorbidity remains evident [
21,
38]. In our study, the mean age of the IPAH population was 45 years, which was comparable with the first NIH registry [
39]. Patients over 60 years represented 25.2% of the entire population compared to 63% of patients over 65 years in the COMPERA registry [
6,
7]. The provocative tests with fluid loading or exercise challenge during the RHC were not part of routine clinical practice at the time of the majority of PAH registries [
6,
7,
15,
18] and our study conductance. We deliberately excluded patients with clinically significant LHD, unreliable PCWP and assessed the differences in IPAH patients depending on H2FpEF score. We have not seen any differences in PVR, cardiac index and PWCP between young and elderly patients, as well as between groups with H2FpEF ≥2 and H2FpEF≤1. PCWP was almost the same for both groups (8.46±4.6 vs 8.9±4.7 mmHg, p=0.6) and was lower compared with PCWP obtained from COMPERA and Swiss registries (10±3 and 12±7 mmHg, respectively) and N. Öcal et al (2022) data [
7,
31,
41]. The majority of patients with H2FPEF score ≥2 are treated with loop diuretics that could reduce PCWP. In those cases, fluid loading might not be sufficient for PCWP elevation up to 18-20 mmHg [
42]. Exercise testing with PCWP registration might be a more relevant physiological provocative test for HFpEF unmasking [
43,
44]. Another issue is the PVR cut-off for pulmonary vascular disease definition in elderly patients [
45,
46,
47]. PVR was similar in young and elderly IPAH patients in our study (13.8±6.86 and 12.6±5.27 WU, respectively) and was comparable with PVR value in young typical IPAH patients in NIH, ASPIRE, REVEAL, and French registers. Whereas, PVR in COMPERA and Swiss registries was lower (9.6±5.5; 8.5±5.2 WU, respectively) [
7,
40]. In the Italian PATRIARCA Registry PVR was 5.56 ± 3.31 WU in patients with PH associated with HFpEF [
50], which was much lower in comparison with IPAH patients with H2FpEF≥2 in our study (12.5±5.2 WU), respectively. Therefore, we could suggest that elderly patients even with H2FpEF≥2 in our study represented IPAH.
Observed in our study cumulative years survival rates from 1 to 10 years of follow-up are comparable with other registries, as well as the rate of the initial combined therapy prescription (46%) [
6,
35,
50,
51]. We have to emphasize, that parenteral prostanoids are still not available. The high rate of PAH therapy escalation (from 46 to 89%) might partly explain the relatively high survival in the entire cohort.
The most striking result of our study was a similar survival rate in elderly and younger IPAH patients even with limited use of initial combination PAH therapy in elderly patients (12%) in comparison with younger patients (63%). These data are in line with the current ESC/ERS guideline 2022, which proposed the initial monotherapy for IPAH patients with LHD or lung disease comorbidity despite the baseline risk stratification. No correlations were registered between the initial number of PAH drugs and the survival in groups and the entire population. Similar results were obtained by B. Stubbe et al. (2021) in the analysis of monotherapy prescription in atypical IPAH and PAH group I patients from 4 PAH referral centers in Germany [
52]. Comparable survival in young and elderly patients in our study might be attributed to the absence of patients with severe lung, left heart diseases, or pathology extremely diminishing life expectancy, as well as a high rate of PAH therapy escalation in elderly patients (from 12 to 56.6%) during follow-up. The escalation rate in our study is much higher in comparison to the M. Hoeper et al. study (2022), where only 28% of patients with LHD comorbidities were allocated to the combined PAH therapy on follow-up [
35]. Even though treatment goals were not met in the majority of cases in the elderly population of IPAH patients with comorbidities in the COMPERA registry and AMBITION study [
35,
53], treatment escalation might improve outcomes in some cases.
No statistically significant difference in survival among patients with different H2FpEF score groups has been found. These results may greatly depend on the small number of patients with high H2pEF in our study. A. Kianzad et al. (2022) revealed that IPAH patients with certain hemodynamic profiles with low cardiac index and PCWP, high mean PAP exhibited similar responses with PAH therapy even in IPAH patients with high H2FPEF-score in comparison with IPAH patients with low H2FPEF-score [
54]. The results of our study confirm the feasibility of the initial monotherapy in elderly IPAH patients with comorbidities. Nevertheless, the presence of hemodynamic and heart remodeling features of high PAH risk could be a justification for sequential combination therapy. Unfavorable PAH phenotype with early onset of the pulmonary vascular disease, and fast progression despite the number of PAH drugs used might be a reason for unmet expectations regarding the observed survival rate in young IPAH patients [
55,
56].
Taking into account the number of reasons for pulmonary vasculature and heart remodeling with aging [
57,
58], IPAH identification remains an exciting diagnostic dilemma. Sophisticated diagnostic workup with hemodynamic testing, weighting of hemodynamic parameters and clinical presentation, comorbidity, right and left heart remodeling, and laboratory markers are warranted in the elderly population for true IPAH diagnosis. Large-scale, long-term observational studies are needed to achieve personalized IPAH description and PAH strategy choice in IPAH patients using a multimodal approach and genetic studies [
59].