Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Techniques
2.3. Clinical Investigations
2.4. Statistical Analysis
3. Results
3.1. Comparison of Clinical Results Between the Iron and Control Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larocque, B.J.; Gilbert, K.; Brien, W.F. Prospective validation of a point score system for predicting blood transfusion following hip or knee replacement. Transfusion 1998, 38, 932–937. [Google Scholar] [CrossRef]
- Kim, M.S.; Koh, I.J.; Choi, K.Y.; Yang, S.C.; In, Y. Efficacy and Safety of Intravenous Ferric Carboxymaltose in Patients with Postoperative Anemia Following Same-Day Bilateral Total Knee Arthroplasty: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 1457. [Google Scholar] [CrossRef]
- Rosencher, N.; Kerkkamp, H.E.; Macheras, G.; et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003, 43, 459–469. [Google Scholar] [CrossRef]
- Shander, A. Emerging risks and outcomes of blood transfusion in surgery. Semin. Hematol. 2004, 41, 117–124. [Google Scholar] [CrossRef]
- Della Valle, C.J.; Buvanendran, A.; Mont, M.A.; Callaghan, J.J. Contemporary Blood Conservation in Hip and Knee Arthroplasty: Tranexamic Acid is an Important Piece of the Puzzle! J. Arthroplasty 2018, 33, 3063–3064. [Google Scholar] [CrossRef]
- Mueller, M.M.; Van Remoortel, H.; Meybohm, P.; et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. Jama 2019, 321, 983–997. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef]
- Scott, L.J. Ferric Carboxymaltose: A Review in Iron Deficiency. Drugs 2018, 78, 479–493. [Google Scholar] [CrossRef]
- Keating, G.M. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef]
- Sibanda, N.; Copley, L.P.; Lewsey, J.D.; et al. Revision rates after primary hip and knee replacement in England between 2003 and 2006. PLoS Med 2008, 5, e179. [Google Scholar] [CrossRef]
- Kim, T.W.; Kang, S.B.; Chang, C.B.; Moon, S.Y.; Lee, Y.K.; Koo, K.H. Current Trends and Projected Burden of Primary and Revision Total Knee Arthroplasty in Korea Between 2010 and 2030. J Arthroplasty 2021, 36, 93–101. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Bozic, K.J.; Katz, P.; Cisternas, M.; Ono, L.; Ries, M.D.; Showstack, J. Hospital resource utilization for primary and revision total hip arthroplasty. J. Bone Joint Surg. Am. 2005, 87, 570–576. [Google Scholar] [CrossRef]
- van Iperen, C.E.; Kraaijenhagen, R.J.; Biesma, D.H.; Beguin, Y.; Marx, J.J.; van de Wiel, A. Iron metabolism and erythropoiesis after surgery. Br. J. Surg. 1998, 85, 41–45. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Bisbe, E.; Moltó, L.; Arroyo, R.; Muniesa, J.M.; Tejero, M. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. Br. J. Anaesth. 2014, 113, 402–409. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, T.Y.; Kim, H.J.; Ro, Y.J.; Jang, H.Y.; Koh, W.U. The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Spahn, D.R. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010, 113, 482–495. [Google Scholar] [CrossRef]
- Maniar, A.R.; Mishra, A.; Sanghavi, N.; Maniar, R.N. Does Postoperative Intravenous Ferric Carboxymaltose Hasten the Recovery of Hemoglobin in Patients Post Total Knee Arthroplasty? J. Arthroplasty 2022, 37, S155–s8. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Noveck, H.; Berlin, J.A.; Gould, S.A. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 2002, 42, 812–818. [Google Scholar] [CrossRef]
- Muñoz, M.; Breymann, C.; García-Erce, J.A.; Gómez-Ramírez, S.; Comin, J.; Bisbe, E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008, 94, 172–183. [Google Scholar] [CrossRef]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.B.; Gwam, C.U.; Naziri, Q.; et al. Are Allogeneic Transfusions Decreasing in Total Knee Arthroplasty Patients? National Inpatient Sample 2009-2013. J Arthroplasty 2018, 33, 1705–1712. [Google Scholar] [CrossRef]
- Gombotz, H.; Rehak, P.H.; Shander, A.; Hofmann, A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion 2014, 54, 2646–2657. [Google Scholar] [CrossRef]
- Hart, A.; Khalil, J.A.; Carli, A.; Huk, O.; Zukor, D.; Antoniou, J. Blood transfusion in primary total hip and knee arthroplasty. Incidence, risk factors, and thirty-day complication rates. J. Bone Joint Surg. Am. 2014, 96, 1945–1951. [Google Scholar] [CrossRef]
- D'Amato, T.; Kon, E.; Martorelli, F.; et al. Effect of intravenous ferric carboxymaltose supplementation in non-anaemic iron deficient patients undergoing hip and knee arthroplasty. J. Biol. Regul. Homeost. Agents 2020, 34, 69–77, Congress of the Italian Orthopaedic Research Society. [Google Scholar] [PubMed]
- Jeong, J.H.; Chang, M.J.; Kang, S.B.; Park, H.J.; Lee, K.H.; Chang, C.B. Postoperative Intravenous Iron Supplementation Does Not Improve Hemoglobin Level and Transfusion Rate Following Staged Bilateral Total Knee Arthroplasty. J. Arthroplasty 2020, 35, 2444–2450. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Hyppa, A.; Chuang, A.; et al. A Prospective Randomised Controlled Trial of a Single Intravenous Infusion of Ferric Carboxymaltose vs Single Intravenous Iron Polymaltose or Daily Oral Ferrous Sulphate in the Treatment of Iron Deficiency Anaemia in Pregnancy. Semin. Hematol. 2018, 55, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Farley, K.X.; Anastasio, A.T.; Premkumar, A.; Boden, S.D.; Gottschalk, M.B.; Bradbury, T.L. The Influence of Modifiable, Postoperative Patient Variables on the Length of Stay After Total Hip Arthroplasty. J. Arthroplasty 2019, 34, 901–906. [Google Scholar] [CrossRef]
- Cuenca, J.; García-Erce, J.A.; Martínez, F.; Cardona, R.; Pérez-Serrano, L.; Muñoz, M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int. J. Surg. 2007, 5, 89–94. [Google Scholar] [CrossRef]
| Iron group (n = 41) | Control group (n = 41) | P Value† | |
| Age (yr) | 71.3 ± 7.2 | 71.5 ± 8.3 | 0.932 |
| Women (no. [%]) | 29 (70.7) | 24 (58.5) | 0.248 |
| BMI (kg/m2) | 25.2 ± 3.6 | 26.5 ± 4.2 | 0.132 |
| ASA status | 0.219 | ||
| 1 or 2 | 32 (78.0) | 27 (65.8) | |
| ≥3 | 9 (22.0) | 14 (34.2) | |
| Cause of revision TKA (no. [%]) | 0.553 | ||
| Septic | 17 (41.5) | 15 (36.6) | |
| Aseptic | 17 (41.5) | 11 (26.8) | |
| Cause of revision THA (no. [%]) | 0.654 | ||
| Septic | 2 (4.9) | 3 (7.3) | |
| Aseptic | 5 (12.1) | 12 (29.3) | |
| Operative time (min) | 132.9 ± 27.0 | 130.9 ± 37.9 | 0.777 |
| Comorbidities (no. [%]) | |||
| Diabetes mellitus | 14 (34.1) | 11 (26.8) | 0.472 |
| Hypertension | 28 (68.2) | 30 (73.1) | 0.627 |
| Heart disease | 10 (24.3) | 8 (19.5) | 0.594 |
| Cerebrovascular disease | 7 (17.0) | 2 (4.9) | 0.077 |
| Bleeding tendency* (no. [%]) | |||
| Aspirin therapy | 10 (14.3) | 8 (19.5) | 0.594 |
| Clopidogrel | 3 (7.3) | 3 (7.3) | 0.999 |
| Total set | Anemic patients* | |||||||
| Iron (n = 41) |
Control (n = 41) |
P Value† | Iron (n = 26) |
Control (n = 27) |
P Value† | |||
| Absolute Hb (g/dL) | ||||||||
| 2 wks preop | 12.6 ± 1.5 | 12.8 ± 1.3 | 0.433 | 11.7 ± 1.1 | 12.1 ± 0.6 | 0.144 | ||
| Immediate postop | 11.4 ± 1.3 | 11.4 ± 1.2 | 0.986 | 11.0 ± 1.2 | 10.9 ± 0.8 | 0.742 | ||
| POD 1 | 10.3 ± 1.2 | 10.2 ± 1.3 | 0.986 | 9.8 ± 1.0 | 9.7 ± 0.9 | 0.547 | ||
| POD 3 | 9.5 ± 1.2 | 9.1 ± 1.4 | 0.355 | 9.1 ± 1.1 | 8.8 ± 1.3 | 0.436 | ||
| 2 wks postop | 10.5 ± 1.4 | 10.5 ± 1.2 | 0.825 | 10.2 ± 1.2 | 10.0 ± 0.9 | 0.789 | ||
| 4 wks postop | 12.0 ± 1.4 | 11.7 ± 1.1 | 0.720 | 11.3 ± 1.0 | 11.2 ± 0.8 | 0.834 | ||
| 3 mo postop | 12.5 ± 1.6 | 12.4 ± 1.1 | 0.813 | 12.0 ± 1.3 | 12.0 ± 0.8 | 0.574 | ||
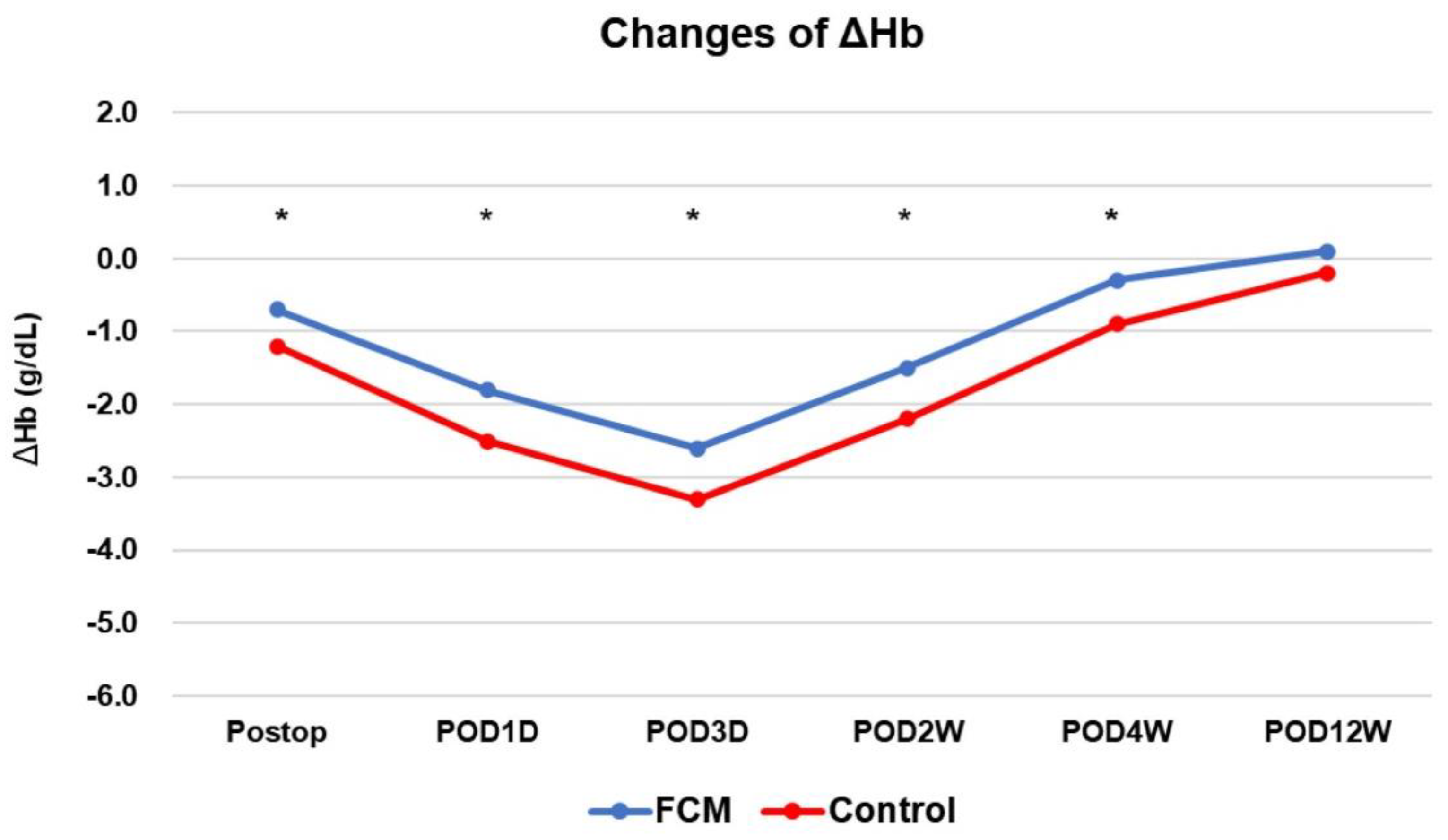
| Change in Hb (g/dL) | ||||||||
| Preop to immediate postop | −1.1 ± 1.0 | −1.4 ± 0.9 | 0.287 | −0.7 ± 0.6 | −1.2 ± 0.8 | 0.024 | ||
| Preop to POD 1 | −2.3 ± 1.0 | −2.6 ± 1.1 | 0.312 | −1.8 ± 0.8 | −2.4 ± 0.9 | 0.034 | ||
| Preop to POD 3 | −3.1 ± 1.3 | −3.7 ± 1.3 | 0.076 | −2.6 ± 0.9 | −3.3 ± 1.2 | 0.046 | ||
| Preop to 2 wks postop | −2.0 ± 1.1 | −2.6 ± 1.0 | 0.073 | −1.5 ± 0.9 | −2.2 ± 0.6 | 0.025 | ||
| Preop to 4 wks postop | −0.6 ± 1.0 | −1.1 ± 0.9 | 0.052 | −0.3 ± 0.7 | −0.9 ± 0.8 | 0.040 | ||
| Preop to 3 mo postop | 0.0 ± 0.8 | −0.2 ± 0.9 | 0.318 | 0.1 ± 0.8 | −0.2 ± 0.8 | 0.256 | ||
| Transfusion rate (n, [%]) | 5 (12.1) | 8 (19.5) | 0.364 | 3 (11.1) | 5(12.1) | 0.141 | ||
| Iron group (n=41) | Control group (n=41) | P Value* | |
| Serum ferritin (ng/dL) | |||
| 2 wks preop | 164.2 ± 182.5 | 104.0 ± 67.1 | 0.100 |
| POD 1 | 1209.6 ± 520.7 | 206.7 ± 113.0 | <0.001 |
| POD 3 | 1200.2 ± 531.7 | 283.2 ± 184.6 | <0.001 |
| 4 wks postop | 603.4 ± 259.0 | 156.0 ± 98.0 | <0.001 |
| 3 mo postop | 352.0 ± 155.3 | 60.5 ± 53.7 | <0.001 |
| TIBC (ug/dL) | |||
| 2 wks preop | 311.4 ± 58.8 | 323.2 ± 44.9 | 0.329 |
| POD 1 | 214.2 ± 35.0 | 244.0 ± 44.0 | 0.002 |
| POD 3 | 189.7 ± 36.2 | 236.0 ± 137.4 | 0.051 |
| 4 wks postop | 251.9 ± 38.8 | 309.0 ± 55.6 | <0.001 |
| 3 mo postop | 272.7 ± 52.6 | 340.8 ± 52.9 | <0.001 |
| Iron (ug/dL) | |||
| 2 wks preop | 86.1 ± 34.1 | 81.0 ± 33.4 | 0.523 |
| POD 1 | 30.5 ± 12.6 | 32.8 ± 30.9 | 0.677 |
| POD 3 | 35.8 ± 13.7 | 47.8 ± 138.7 | 0.603 |
| 4 wks postop | 80.1 ± 44.9 | 66.1 ± 23.4 | 0.115 |
| 3 mo postop | 93.6 ± 27.0 | 69.6 ± 30.4 | 0.002 |
| TSAT (%) | |||
| 2 weeks preop | 29.0 ± 14.4 | 25.0 ± 9.6 | 0.169 |
| POD 1 | 14.8 ± 6.8 | 13.2 ± 10.4 | 0.434 |
| POD 3 | 19.3 ± 7.4 | 14.1 ± 12.0 | 0.034 |
| 4 wks postop | 32.1 ± 19.1 | 21.9 ± 8.0 | 0.007 |
| 3 mo postop | 35.5 ± 13.1 | 20.9 ± 9.6 | <0.001 |
| Iron group (n = 41) | Control group (n = 41) | P Value* | |
| EQ-5D-1 Mobility | |||
| Preop | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.323 |
| 4 wks postop | 1.9 ± 0.2 | 1.8 ± 0.3 | 0.751 |
| 3 mo postop | 1.6 ± 0.5 | 1.5 ± 0.5 | 0.628 |
| EQ-5D-2 Self Care | |||
| Preop | 1.6 ± 0.4 | 1.8 ± 0.5 | 0154 |
| 4 wks postop | 1.8 ± 0.3 | 1.7 ± 0.5 | 0.182 |
| 3 mo postop | 1.2 ± 0.4 | 1.3 ± 0.4 | 0.612 |
| EQ-5D-3 Usual Activities | |||
| Preop | 1.9 ± 0.2 | 2.0 ± 0.1 | 0.570 |
| 4 wks postop | 1.9 ± 0.1 | 1.8 ± 0.3 | 0.266 |
| 3 mo postop | 1.5 ± 0.5 | 1.3± 0.4 | 0.179 |
| EQ-5D-4 Pain/Discomfort | |||
| Preop | 2.8 ± 0.4 | 2.9 ± 0.2 | 0.145 |
| 4 wks postop | 1.9 ± 0.2 | 1.8 ± 0.3 | 0.751 |
| 3 mo postop | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.734 |
| EQ-5D-5 Anxiety/Depression | |||
| Preop | 1.6 ± 0.5 | 1.6 ± 0.4 | 0.831 |
| 4 wks postop | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.769 |
| 3 mo postop | 1.4 ± 0.4 | 1.4 ± 0.5 | 0.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





