Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Model
2.1. Heat conduction in the heater
2.2. Heat transfer mechanisms
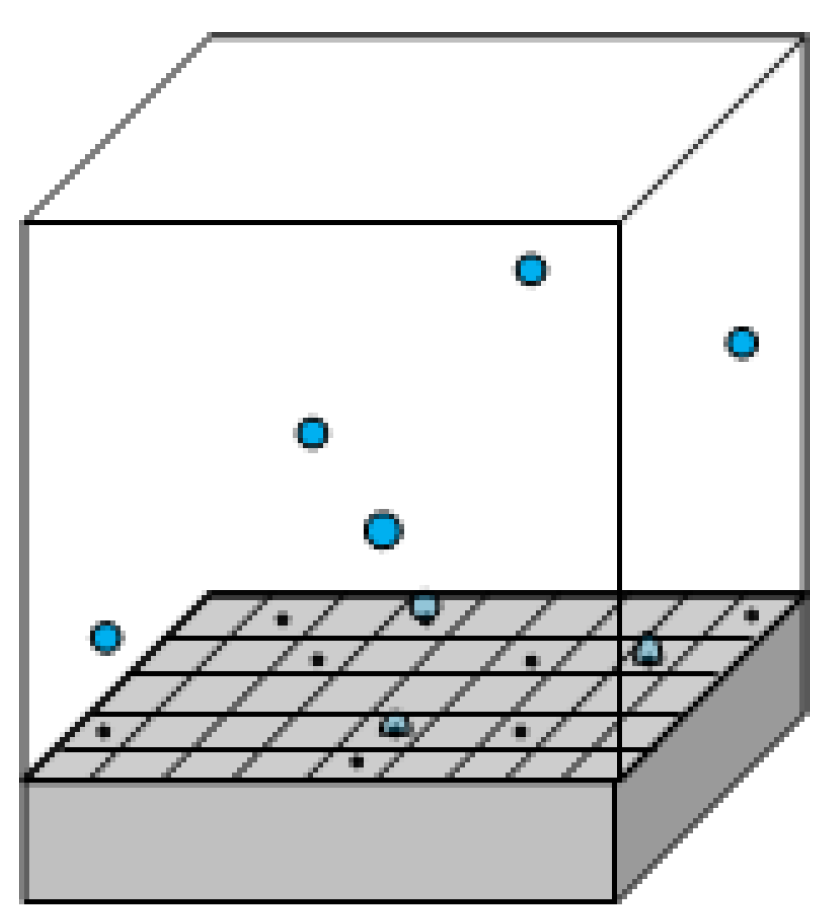
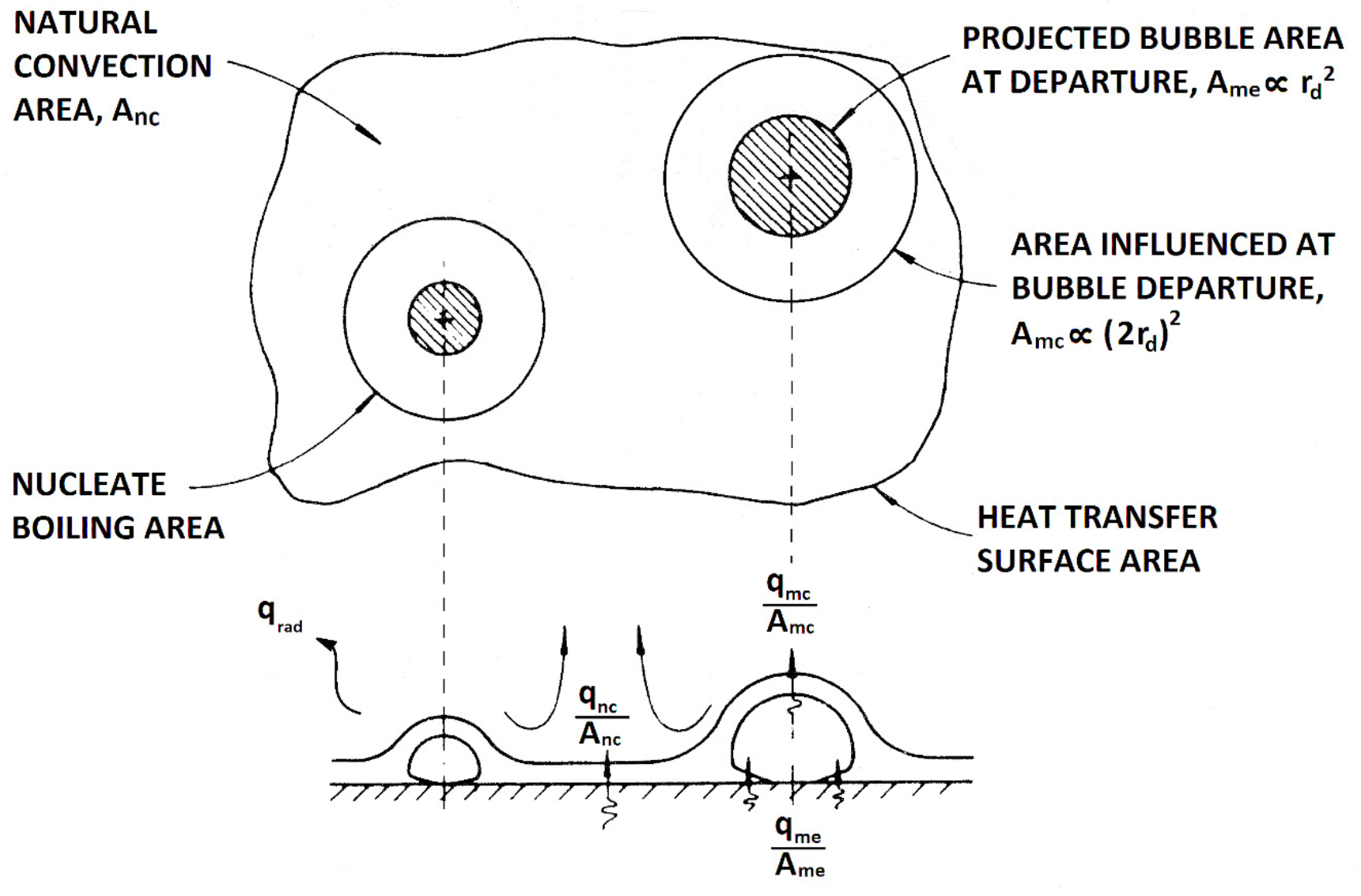
- Natural convection (): The density difference between the hot and cold fluid induces convective flows, extracting heat from the heater across its free area, .
- Microlayer evaporation, (): It is assumed that the vapor mass forming the bubble originates from the evaporation of the superheated microlayer between the heater surface and the bubble itself.
- Microconvection, (): Detaching bubbles remove the superheated liquid layer in their vicinity. Colder liquid from the bulk of the pool quenches an area , and heat is required to recover the microlayer subcooling.
- Radiation, (): Heat is transferred in the form of electromagnetic waves from the heater to the liquid.
2.2.1. Effect of the contact angle and natural convection
2.2.2. Microlayer evaporation and microconvection
2.2.3. Radiation
3. Results
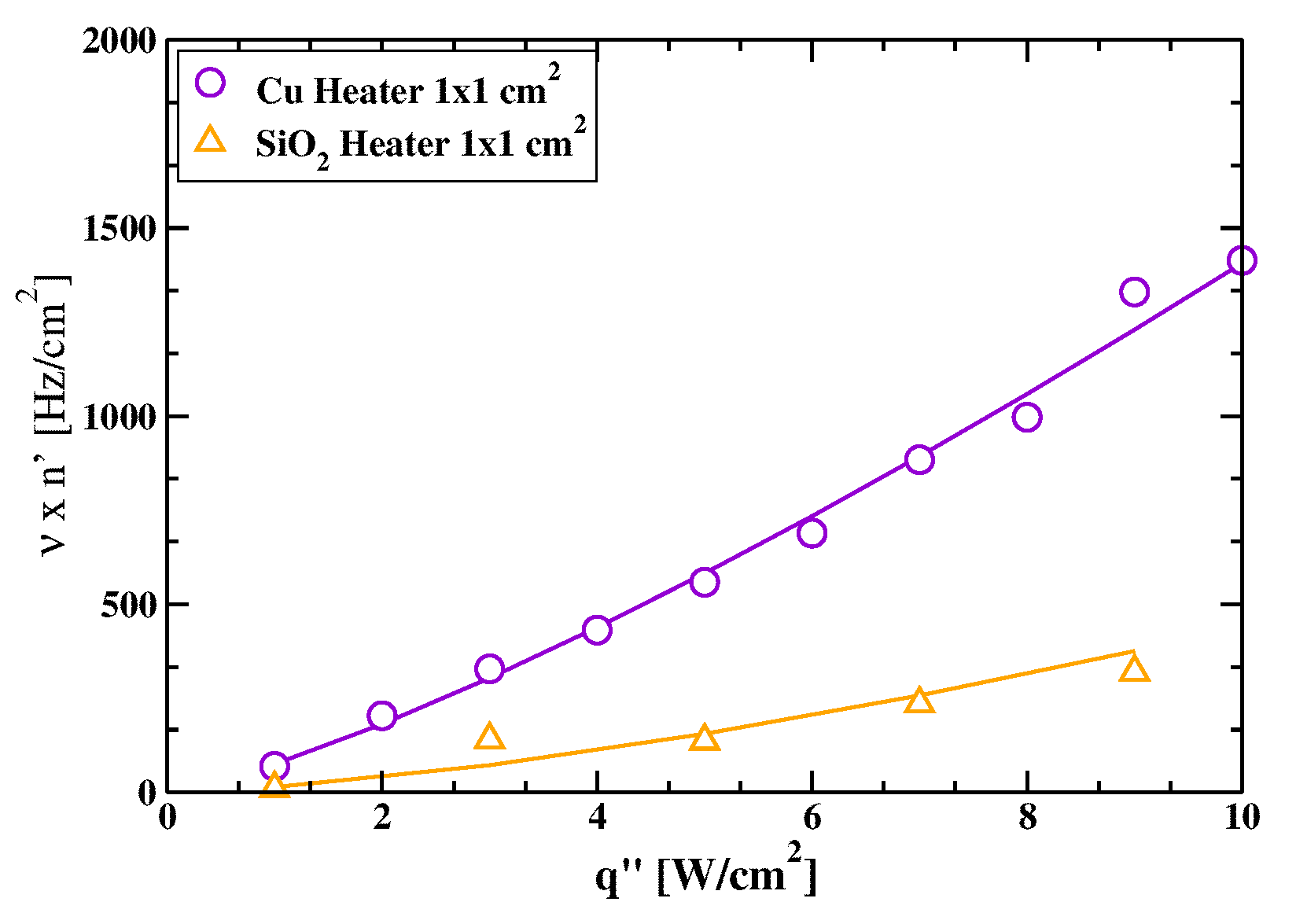
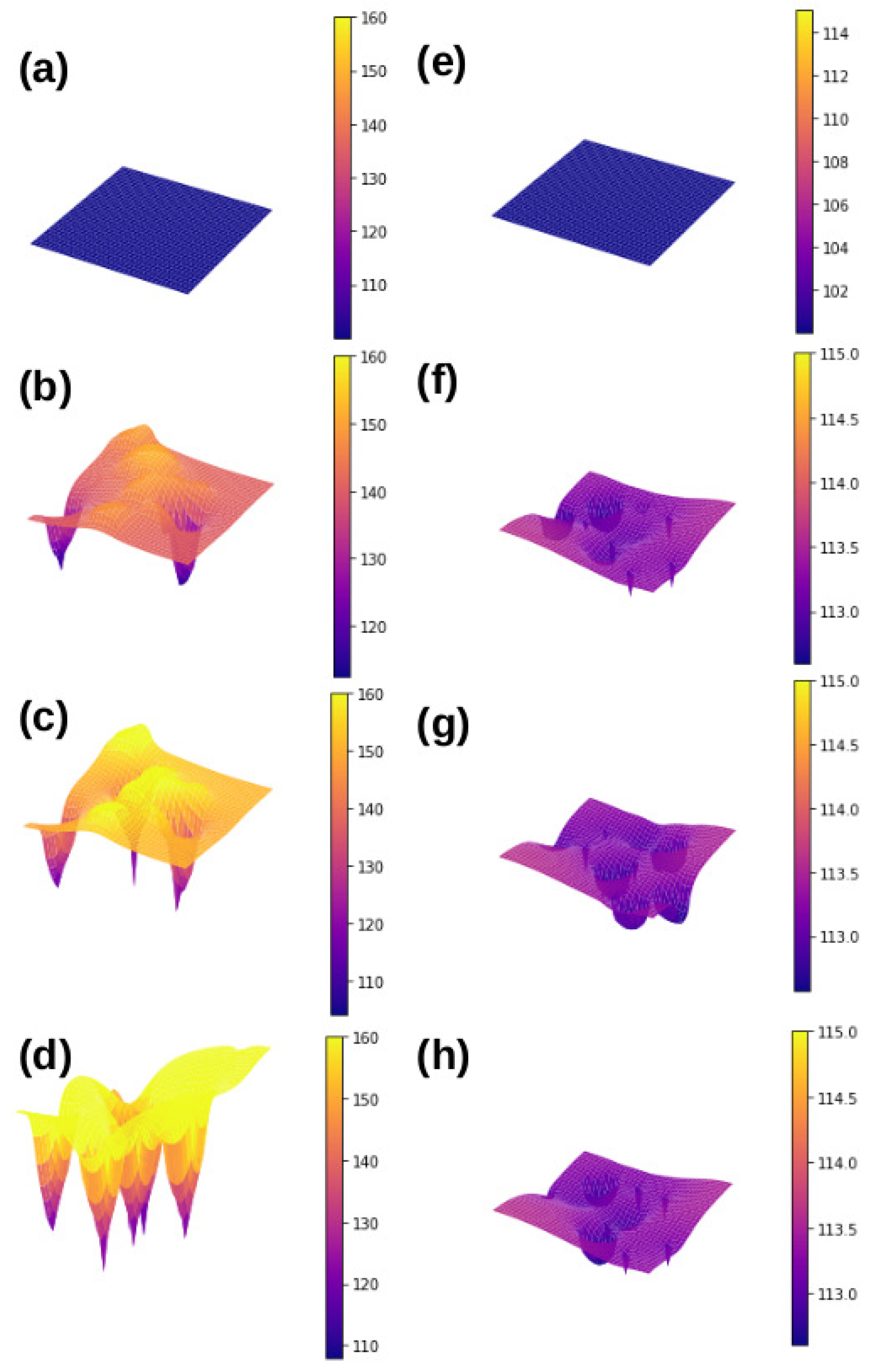
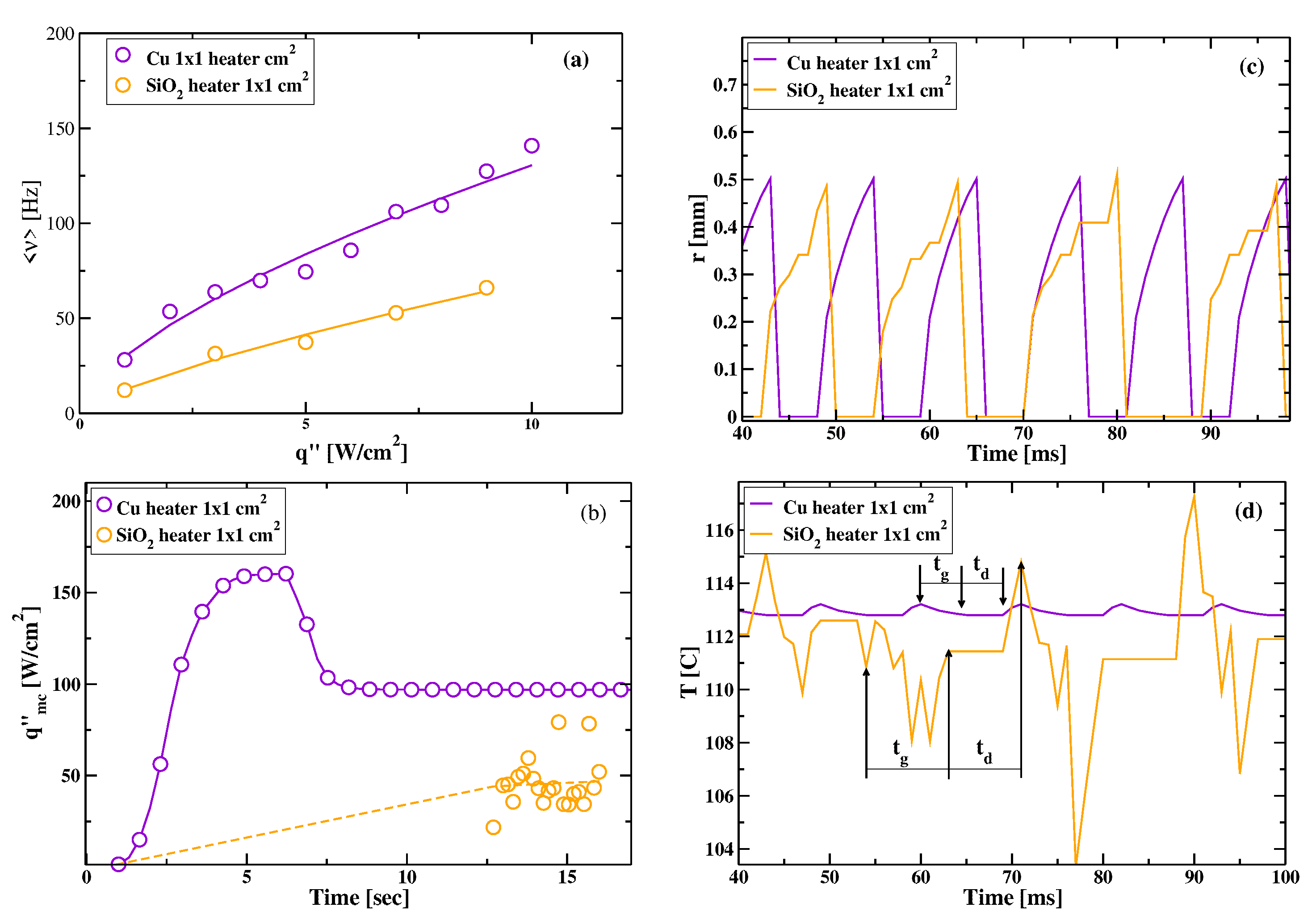
3.1. Comparative Cooling Performance of and Heaters
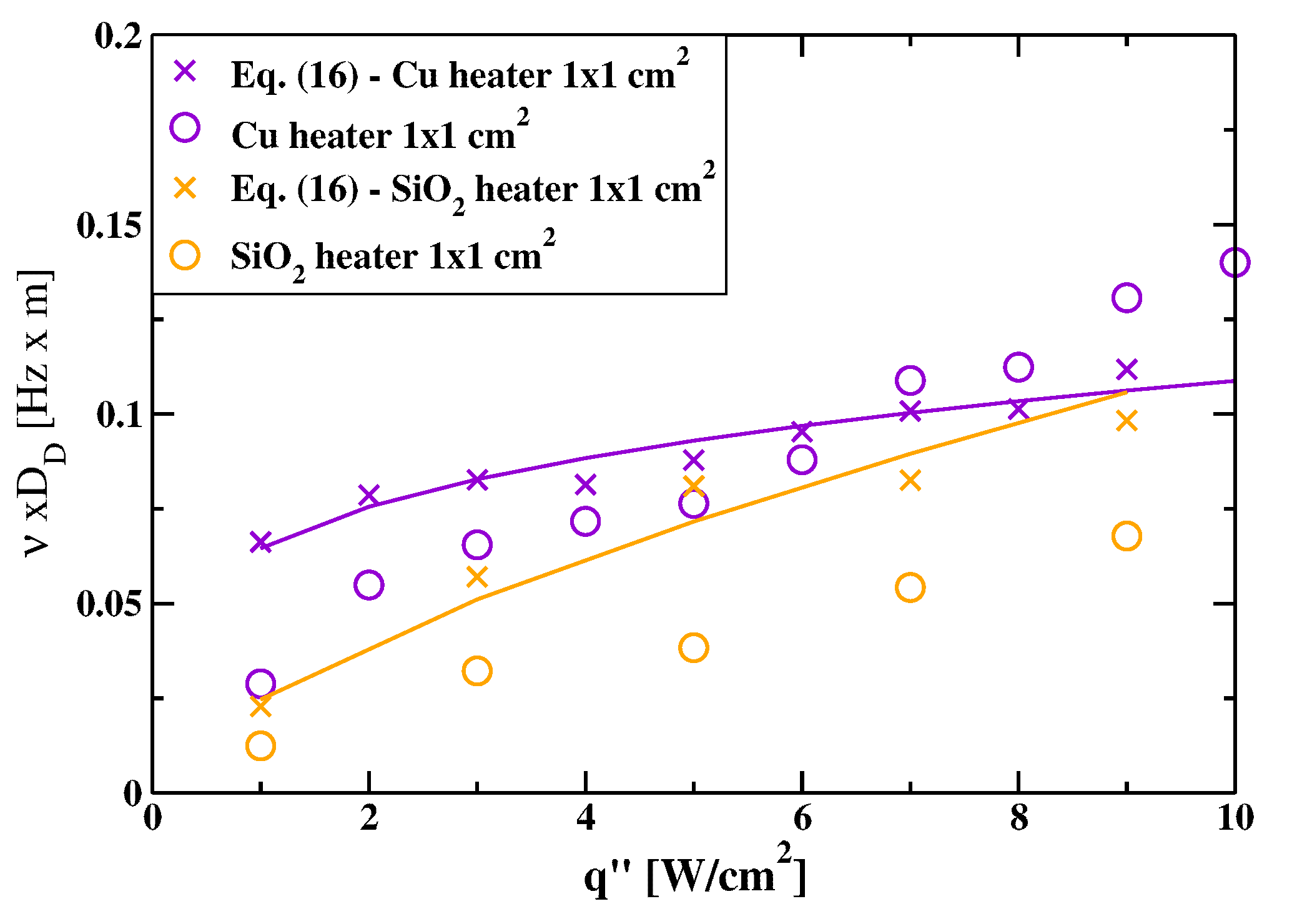
3.2. Mean Bubble Frequency and Associated Times: A Comparison between and Heaters
4. Conclusions
References
- Yeh, L. Review of heat transfer technologies in electronic equipment. Trans. ASME-P-J. Electron. Pack. 1995, 117, 333–339. [Google Scholar] [CrossRef]
- Manglik, R.M. On the advancements in boiling, two-phase flow heat transfer, and interfacial phenomena. J. Heat Transfer 2006, 128, 1237–1242. [Google Scholar] [CrossRef]
- Kandlikar, S.G. History, advances, and challenges in liquid flow and flow boiling heat transfer in microchannels: a critical review. J. Heat Transfer 2012, 134, 034001. [Google Scholar] [CrossRef]
- Bankoff, S. Entrapment of gas in the spreading of a liquid over a rough surface. AIChE J. 1958, 4, 24–26. [Google Scholar] [CrossRef]
- Cho, H.J.; Wang, E.N. Bubble nucleation, growth, and departure: A new, dynamic understanding. Int. J. Heat Mass Transf. 2019, 145, 1–8. [Google Scholar] [CrossRef]
- Yabuki, T.; Nakabeppu, O. Heat transfer mechanisms in isolated bubble boiling of water observed with MEMS sensor. Int. J. Heat Mass Transf. 2014, 76 (C), 286–297. [Google Scholar] [CrossRef]
- Goodman, J.B.; Krase, N.W. Solubility of nitrogen in water at high pressures and temperatures. Ind. Eng. Chem. 1931, 23, 401–404. [Google Scholar] [CrossRef]
- Forrest, E.; et al. . EAugmentation of nucleate boiling heat transfer and critical heat flux using nanoparticle thin-film coatings. Int. J. Heat Mass 2010, 53, 58–67. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Ali, A.F. Enhanced nucleate boiling on copper micro-porous surfaces. Int. J. Multiphase Flow 2010, 36, 780–792. [Google Scholar] [CrossRef]
- D. Ćoso, D.; Srinivasan, V.; Lu, M.C.; Chang, J.Y.; Majumdar, A. Enhanced heat transfer in biporous wicks in the thin liquid film evaporation and boiling regimes. J. Heat Transfer 2012, 134.
- Kandlikar, S. Controlling bubble motion over heated surface through evaporation momentum force to enhance pool boiling heat transfer. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Paxson, A.T.; Yage, J.L.; Gleason, K.K.; Varanasi, K.K. Stable dropwise condensation for enhancing heat transfer via the initiated chemical vapor deposition (iCVD) of grafted polymer films. Adv. Mater 2014, 26, 418–423. [Google Scholar] [CrossRef]
- Son, G.; Dhir, V.K.; Ramanujapu, N. Dynamics and heat transfer associated with a single bubble during nucleate boiling on a horizontal surface. J. Heat Transf. 1999, 121. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kandlikar, S.G. Numerical study of single bubbles with dynamic contact angle during nucleate pool boiling. Int. J. Heat Mass Transf. 2007, 50, 127–138. [Google Scholar] [CrossRef]
- Nam, Y.; Aktinol, E.; Dhir, V.K.; Ju, Y.S. Single bubble dynamics on a superhydrophilic surface with artificial nucleation sites. Int. J. Heat Mass Transf. 2011, 54, 1572–1577. [Google Scholar] [CrossRef]
- Churaev, N. The effect of adsorbed layers on van der waals forces in thin liquid films. Coll. Polym. Sci. 1975, 253, 120–126. [Google Scholar] [CrossRef]
- Chung, J.N.; Chen, T.; Maroo, S.C. A review of recent progress on nano/micro scale nucleate boiling fundamentals. Front. Heat Mass Transf. 2011, 2. [Google Scholar] [CrossRef]
- Urbano, A.; Tanguy, S.; Huber, G.; Colin, C. Direct numerical simulation of nucleate boiling in micro-layer regime. Int. J. Heat Mass Transf. 2018, 123, 1128–1137. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H. Hydrodynamic formation of a microlayer underneath a boiling bubble. Int. J. Heat Mass Transf. 2018, 120, 1229–1240. [Google Scholar] [CrossRef]
- Zou, A.; Chanana, A.; Agrawal, A.; Jr., P.C.W.; Maroo, S.C. Steady state vapor bubble in pool boiling. Scient. Rep. 2016, 6.
- Giustini, G.; Jung, S.; Kim, H.; Walker, S. Evaporative thermal resistance and its influence on microscopic bubble growth. Int. J. Heat Mass Transf. 2016, 101, 733–741. [Google Scholar] [CrossRef]
- Sato, Y.; Niceno, B. A depletable micro-layer model for nucleate pool boiling. J. Comput. Phys. 2015, 300, 20–52. [Google Scholar] [CrossRef]
- Ghazivini, M.; Hafez, M.; Ratanpara, A.; Kim, M. A review on correlations of bubble growth mechanisms and bubble dynamics parameters in nucleate boiling. J. Thermal Analysis and Calorimetry 2022, 147, 6035–6071. [Google Scholar] [CrossRef]
- Cooper, M.G. The microlayer nucleate and bubble growth in pool booling. Int. J. Heat Mass Transf. 1969, 12, 915–933. [Google Scholar] [CrossRef]
- Stralen, S.J.D.V.; Sohal, M.S.; Cole, R.; Sluyter, W.M. Bubble growth rates in pure and binary systems: Combined effect of relaxation and evaporation microlayers. Int. J. Heat Mass Transf. 1975, 18, 453–461. [Google Scholar] [CrossRef]
- Han, C.Y.; Griffith, P. The mechanism of heat transfer in nucleate pool boiling - Part I. Int. J. Heat Mass Transf. 1965, 8, 887–904. [Google Scholar]
- Podowski, M.Z.; Podowski, R.M. Mechanistic Multidimensional Modeling of Forced Convection Boiling Heat Transfer. Sci. and Tech. of Nuclear Installations 2008, 2009. [Google Scholar] [CrossRef]
- Basu, N.; Warrier, G.R.; Dhir, V.K. Wall heat flux partitioning during subcooled flow boiling: Part 1 - Model development. J Heat Transfer 2005, 127, 131–140. [Google Scholar] [CrossRef]
- Buyevich, Y.A.; Webbon, B.W. The isolated bubble regime in pool nucleate boiling. Int. J. Heat Mass Transf. 1997, 40, 365–377. [Google Scholar] [CrossRef]
- Chang, M.J.; Chow, L.C.; Chang, W.S. Improved alternating-direction implicit method for solving transient three-dimensional heat diffusion problems. Num. Heat Transf, Part B: Fund.: An Int. J. Comp Meth. 1991, 19, 69–84. [Google Scholar] [CrossRef]
- Judd, R.; Hwang, K. The isolated bubble regime in pool nucleate boiling. J. Heat Transfer 1976, 98, 623. [Google Scholar] [CrossRef]
- Marcel, C.; Clausse, A.; Frankiewicz, C.; Betz, A.; Attinger, D. Numerical investigation into the effect of surface wettability in pool boiling heat transfer with a stochastic-automata model. Int. J. Heat Mass Transf. 2017, 111, 657–665. [Google Scholar] [CrossRef]
- McAdams, W. Heat Transmission, 3 ed.; McGraw-Hill: New York, 1954; chapter 4. [Google Scholar]
- Goldstein, R.; Sparrow, E.; Jones, D. Natural Convection Mass Transfer Adjacent to Horizontal Plates. International Journal of Heat and Mass Transfer 1973, 16, 1025. [Google Scholar] [CrossRef]
- Lloyd, J.; Moran, W. Natural Convection Mass Transfer Adjacent to Horizontal Surfaces of Various Platforms. ASME Paper, 1974.
- Snyder, N.W. Summary of conference on bubble dynamics and boiling heat transfer held at the Jet Propulsion Laboratory. Jpl memo, Jet Propulsion Laboratory, California Institute of Technology, 1956.
- Bergles, A. Boiling heat transfer. Modern Developments and Advances; Elsevier: New York, 1992; chapter Elements of boiling heat transfer; pp. 389–416. [Google Scholar]
- van Stralen, S.; Cole, R. Boiling Phenomena: Physicochemical and Engineering Fundamentals and Applications; Number 1 in Boiling Phenomena: Physicochemical and Engineering Fundamentals and Applications, Hemisphere Publishing Corporation, 1979. [Google Scholar]
- Golobic, I.; Petkovsek, J.; Baselj, M.; Papez, A.; Kenning, D.B.R. Experimental determination of transient wall temperature distributions close to growing vapor bubbles. Heat and Mass Transfer 2009, 45, 857–866. [Google Scholar] [CrossRef]
- van Straken, S.; Cole, R. Bolining phenomena; McGraw-Hill: New York, 1979; Vol. 1 of Physicochemical and Engineering Fundamentals ans Applications, chapter 6; pp. 155–165. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




