Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
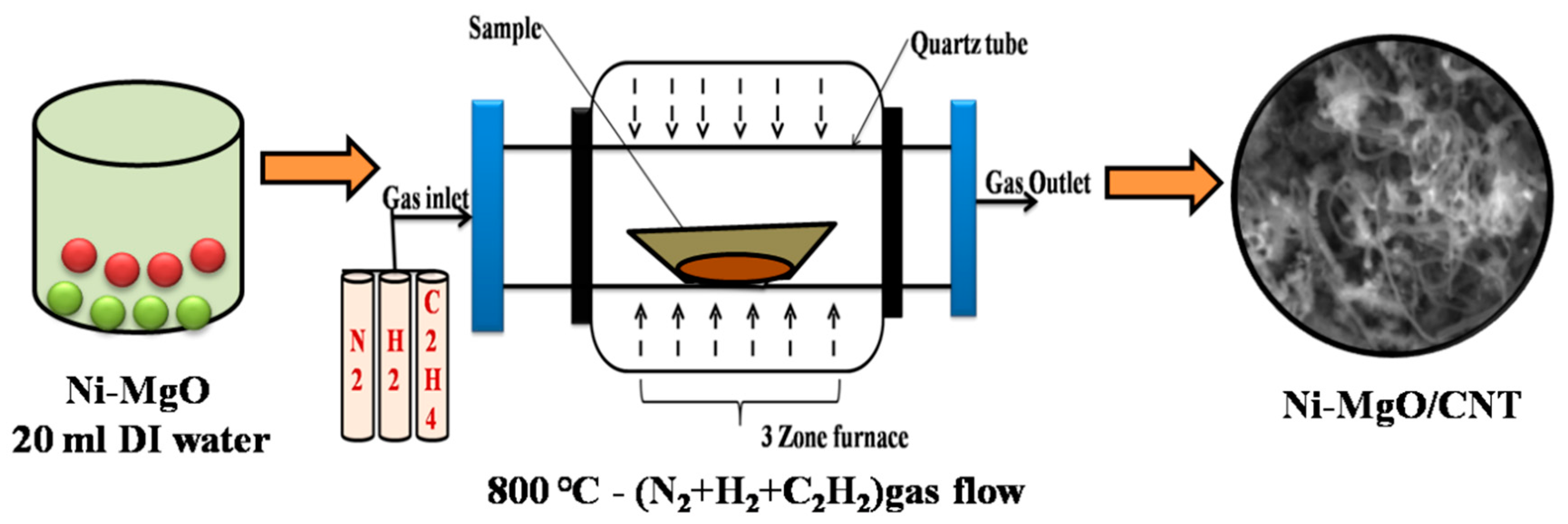
2.2. Synthesis procedure
2.3. Characterizations
3. Results and Discussion
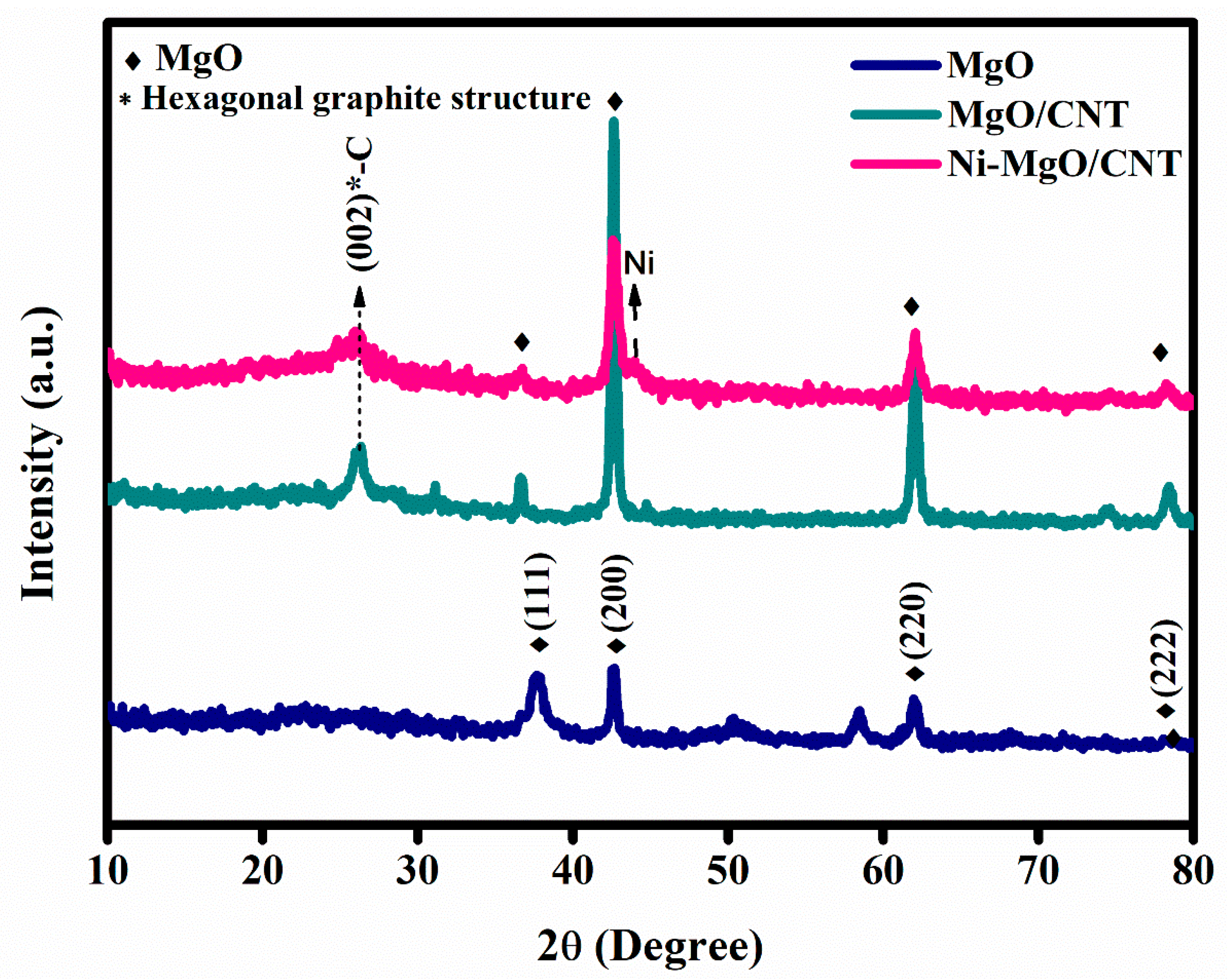
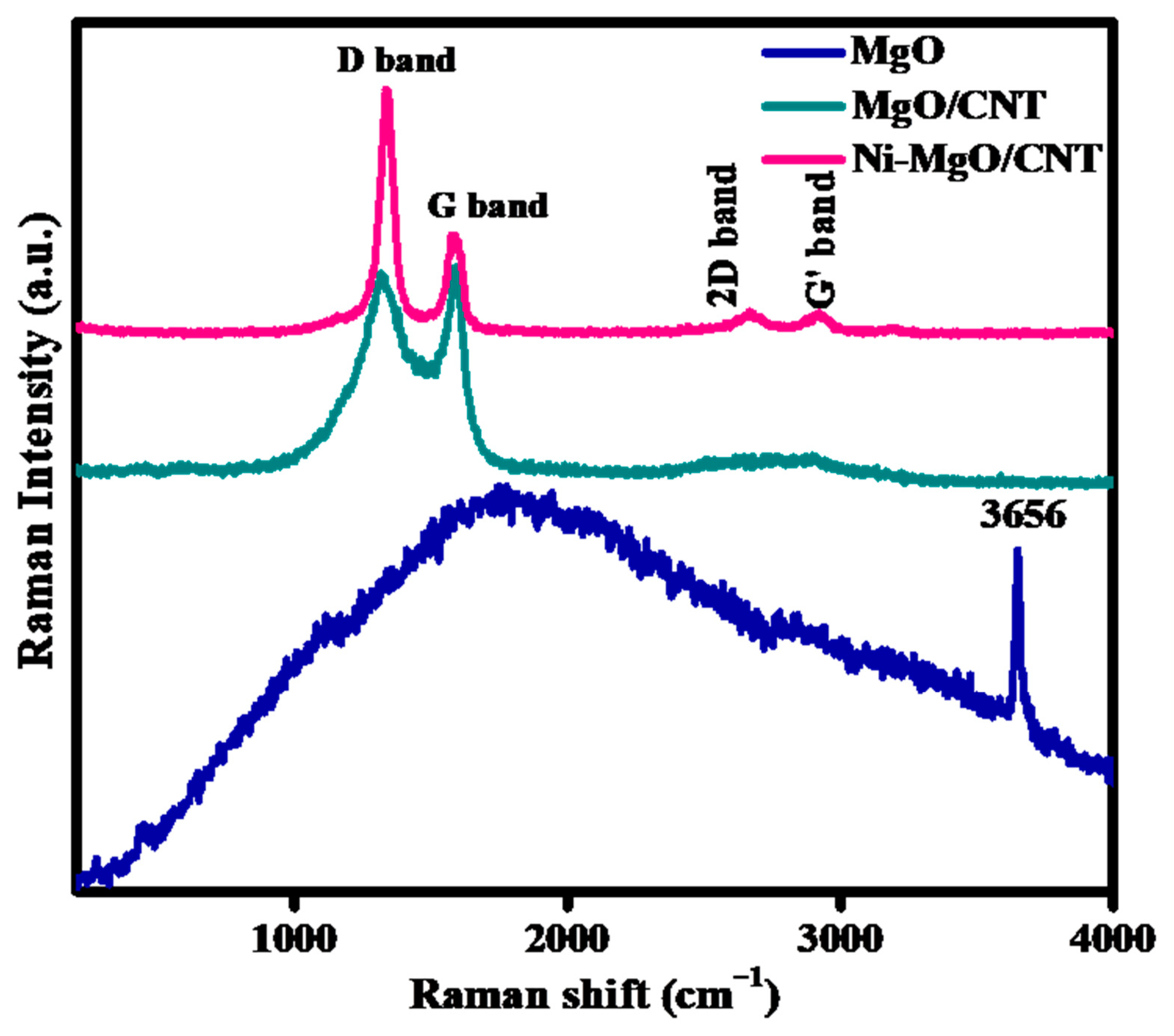
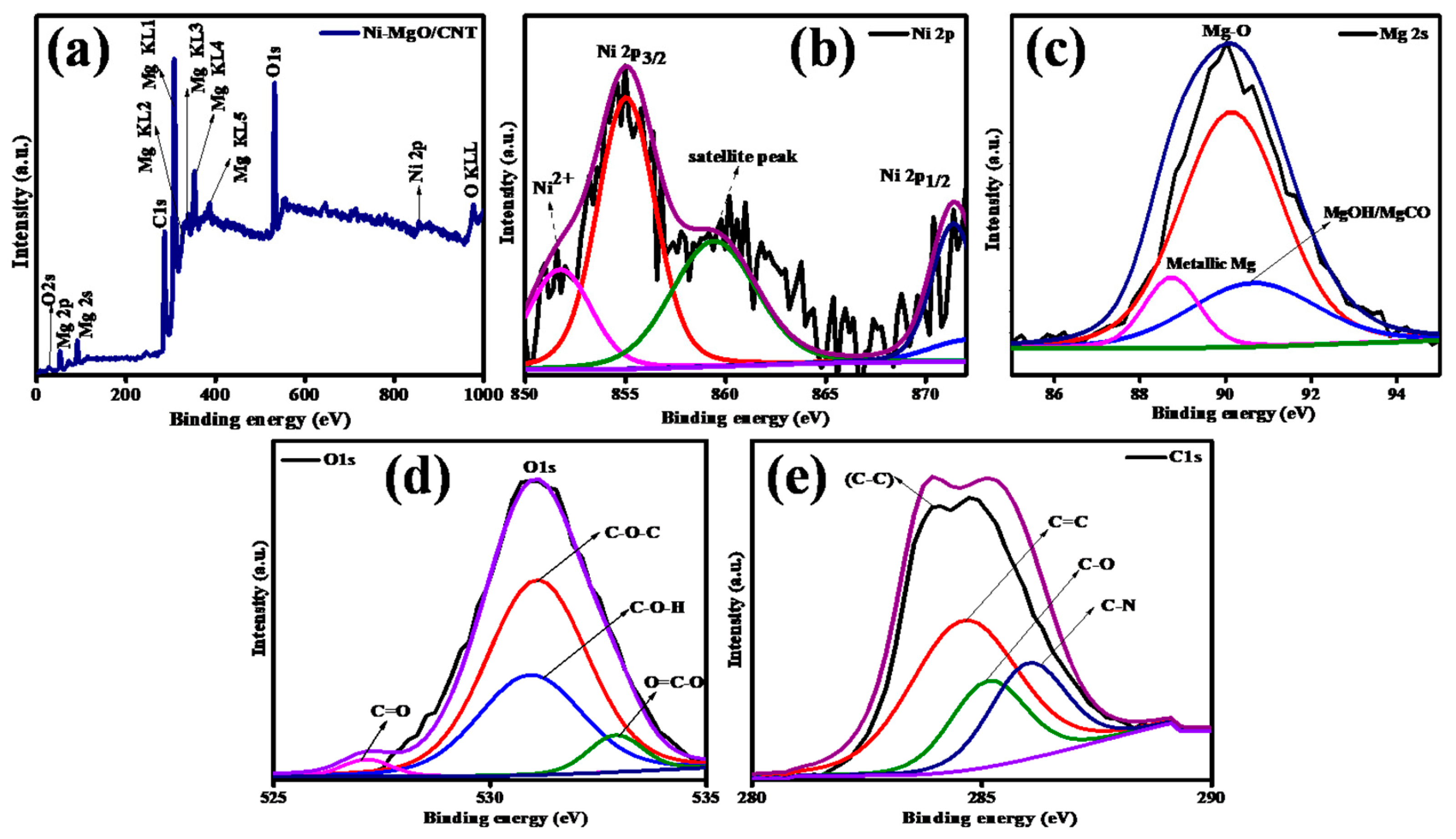
3.1. Phases and chemical structure analysis
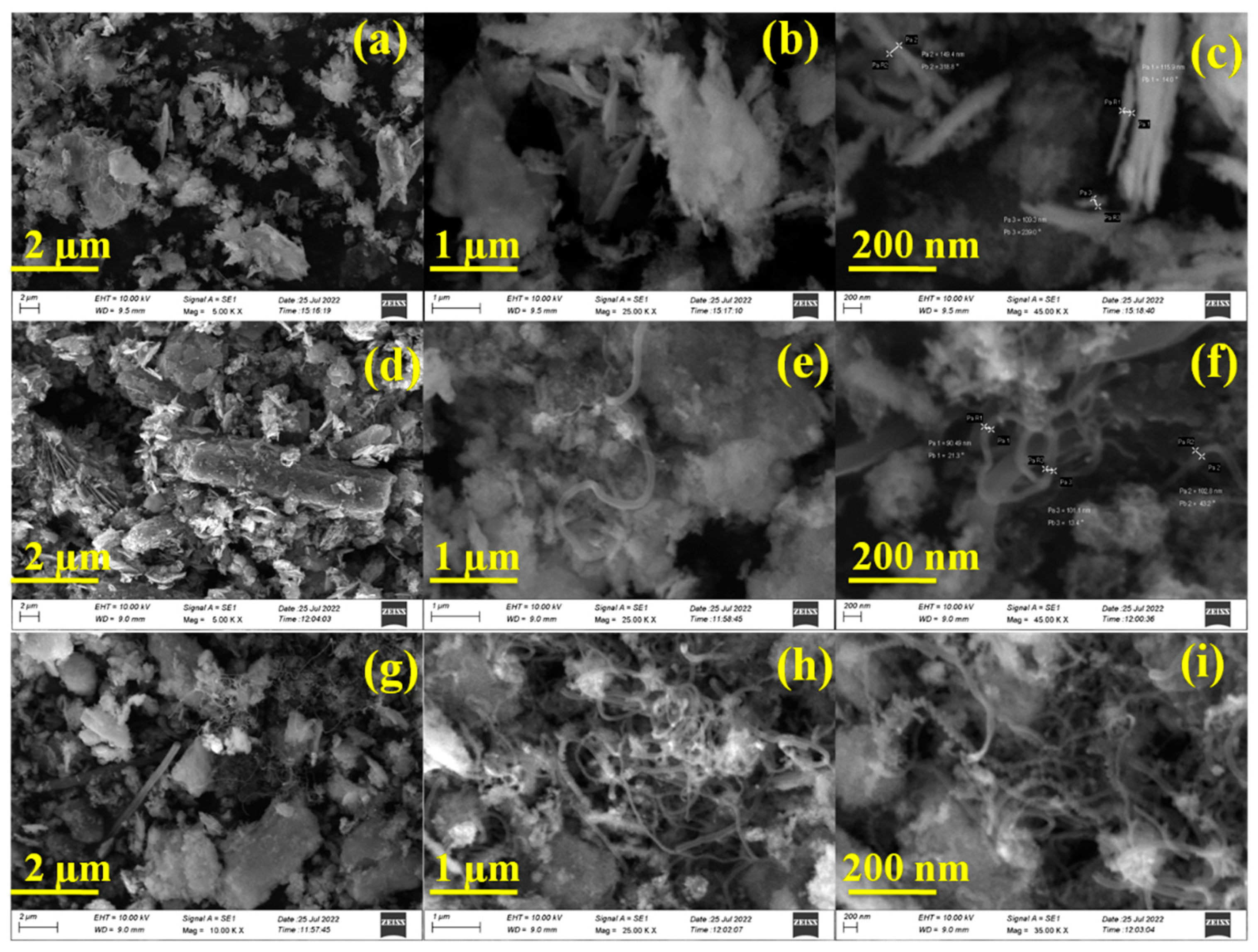
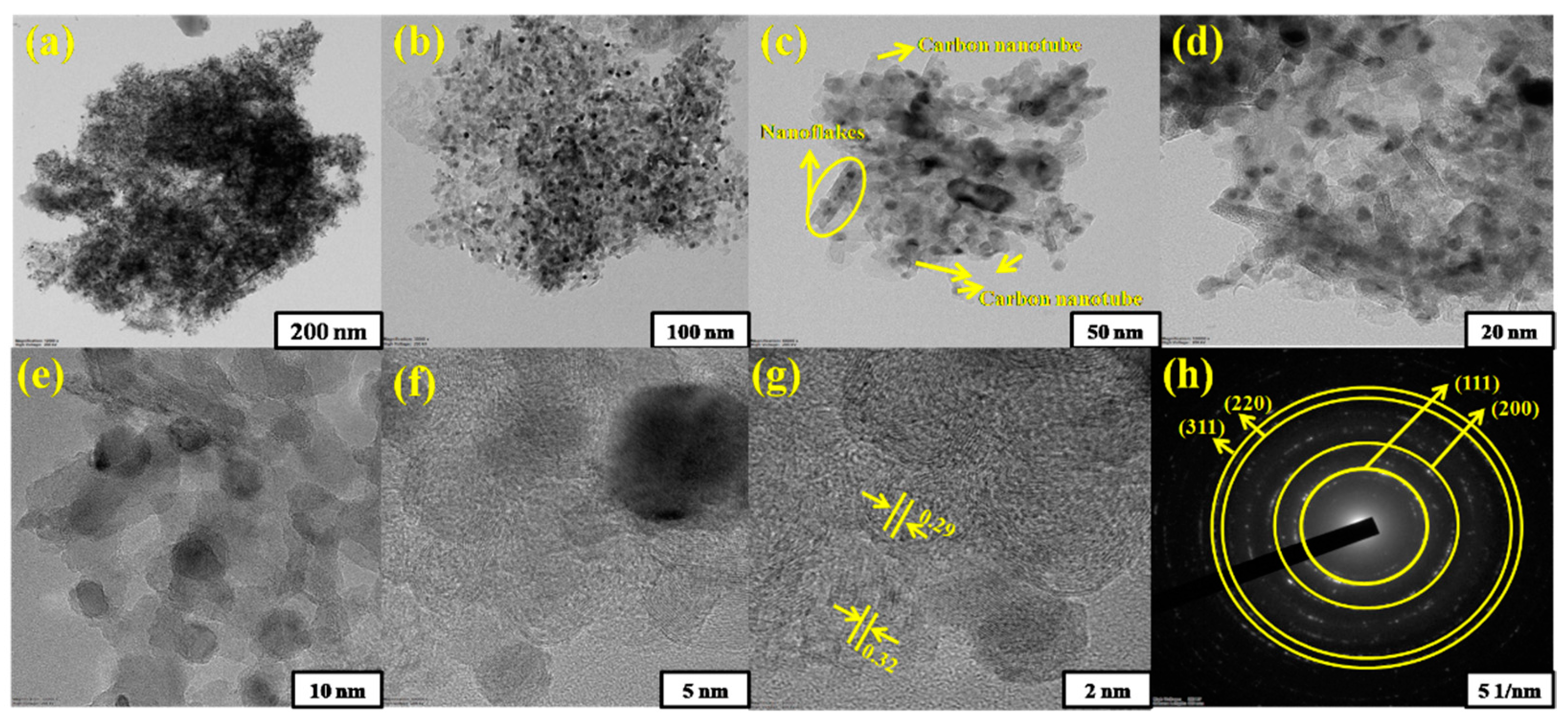
3.2. Microstructural/Nanostructural analysis
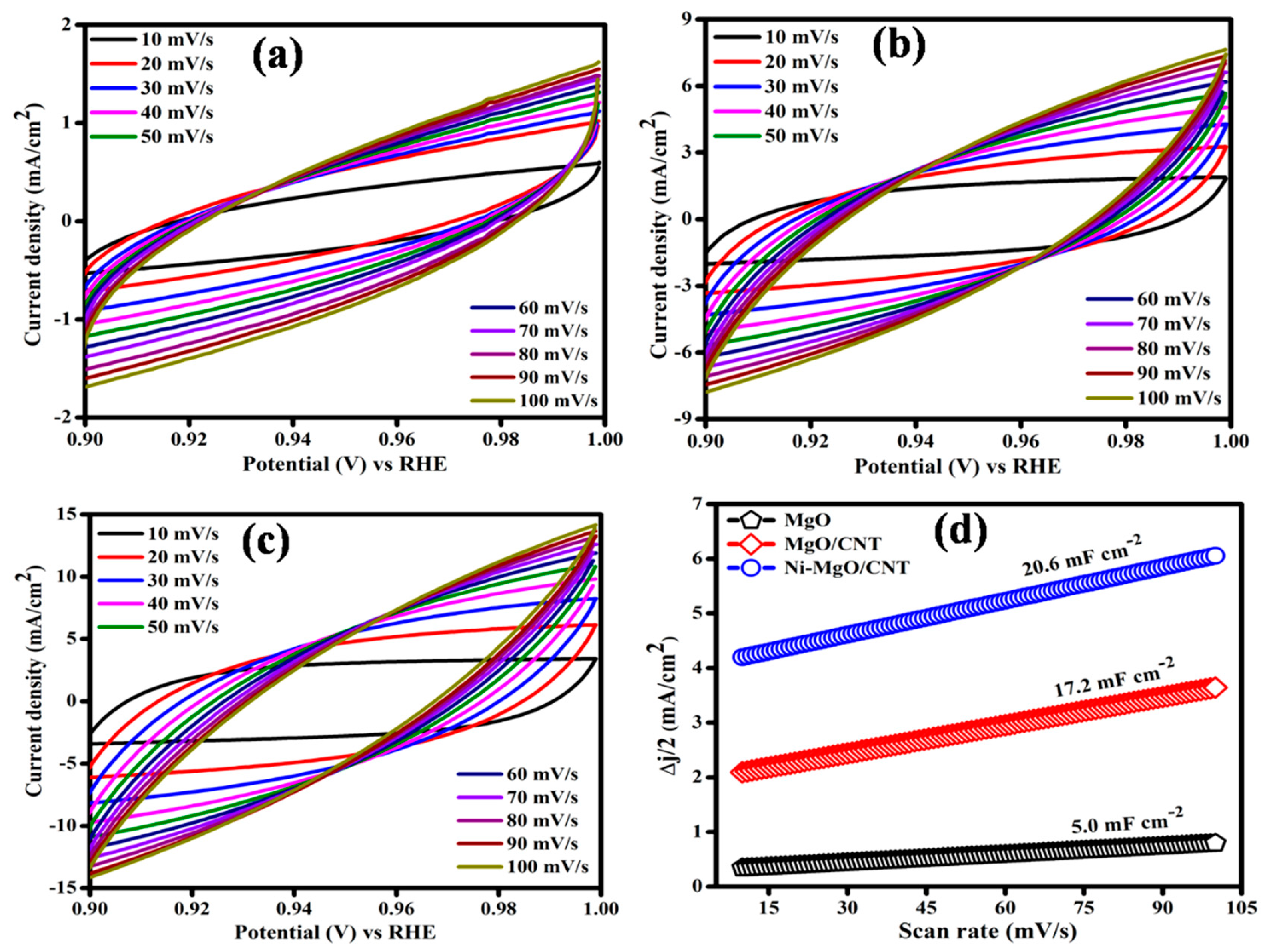
3.3. Electrocatalytic hydrogen evolution reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production–A review. Renewable and sustainable energy reviews 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: the future energy carrier. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Susmozas, A.; Iribarren, D.; Dufour, J. Life-cycle performance of indirect biomass gasification as a green alternative to steam methane reforming for hydrogen production. International Journal of hydrogen energy 2013, 38, 9961–9972. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chemical Society Reviews 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: current state and prospects. Journal of Materials Chemistry A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Son, M.-K. Key strategies on Cu2O photocathodes toward practical photoelectrochemical water splitting. Nanomaterials 2023, 13, 3142. [Google Scholar] [CrossRef]
- Yoon, S. J.; Lee, S. J.; Kim, M. H.; Park, H. A.; Kang, H. S.; Bae, S-Y.; Jeon, I-Y. Recent Tendency on Transition-Metal Phosphide Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline Media. Nanomaterials 2023, 13, 2613. [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy & Environmental Science 2014, 7, 2255–2260. [Google Scholar]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy & Environmental Science 2014, 7, 1919–1923. [Google Scholar]
- Yu, F.; Yu, L.; Mishra, I.K.; Yu, Y.; Ren, Z.F.; Zhou, H.Q. Recent developments in earth-abundant and non-noble electrocatalysts for water electrolysis. Materials Today Physics 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Advanced materials 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Wang, Q.; Zhang, X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Materials 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, L.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technology 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Abdulaziz, A. M.A.; Ahmed, S. A.F.; Naitik, P.; Salwa, B. A.; Nouf, A. B.; Ahmed, A. I.; Ahmed, Y. El.; Jehad, K. A.; Ahmed, E. A.; Anis, H. F.; Rawesh, K. Alumina-Magnesia-Supported Ni for Hydrogen Production via the Dry Reforming of Methane: A Cost-Effective Catalyst System. Nanomaterials 2023, 13, 2984. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: design, synthesis, and energy-related applications. AngewandteChemie International Edition 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Peng, L.; Zheng, X.; Li, L.; Zhang, L.; Yang, N.; Xiong, K.; Chen, H.; Li, J.; Wei, Z. Chimney effect of the interface in metal oxide/metal composite catalysts on the hydrogen evolution reaction. Applied Catalysis B: Environmental 2019, 245, 122–129. [Google Scholar] [CrossRef]
- Liu, W.; Tan, W.; He, H.; Yang, Y. Electrodeposition of self–supported Ni–Mg–La electrocatalyst on Ni foam for efficient hydrogen evolution reaction. ElectrochimicaActa 2022, 411, 140058. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Rouhaghdam, A.S. Three-dimensional porous Ni-CNT composite nanocones as high performance electrocatalysts for hydrogen evolution reaction. Journal of Electroanalytical Chemistry 2018, 829, 194–207. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Tahir, N.; Jamil, A.; Afzal, A. Design of advanced self-supported electrode by surface modification of copper foam with transition metals for efficient hydrogen evolution reaction. International Journal of Hydrogen Energy 2020, 45, 33396–33406. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, N.; Li, J.; Deng, X.; Sha, J.; He, C. Hard-template synthesis of three-dimensional interconnected carbon networks: rational design, hybridization and energy-related applications. Nano Today 2019, 29, 100796. [Google Scholar] [CrossRef]
- Vaka, M.; Walvekar, R.; Yanamadala, S. Carbon nanotubes and their composites: from synthesis to applications. Contemporary nanomaterials in material engineering applications 2021, 37–67. [Google Scholar]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Ji, Y.; Yang, J.; Fan, K.; Ma, X.; Wang, C.; Shu, R; Chen, Y. Surface engineering induced hierarchical porous Ni12P5-Ni2P polymorphs catalyst for efficient wide pH hydrogen production. Applied Catalysis B: Environmental 2021, 282, 119609. [CrossRef]
- Rao, K.G.; Ashok, C.H.; Rao, K.V.; Chakra, C.S. Structural properties of MgO nanoparticles: synthesized by co-precipitation technique. International Journal of Science and Research 2014, 3, 43–46. [Google Scholar]
- Khachatur Manukyan, V.; Christopher Shuck, E.; Mathew Cherukara, J.; Sergei Rouvimov.; Dmitry Kovalev, Y.; Alejandro Strachan.; Alexander Mukasyan, S.; Exothermic Self-Sustained Waves with Amorphous Nickel. J. Phys. Chem. C 2016, 120, 5827–5838.
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Keshavarz, M.; Kehtari, M.; Ramakrishna, S.; Berto, S. MgO-incorporated carbon nanotubes-reinforced Mg-based composites to improve mechanical, corrosion, and biological properties targeting biomedical applications. Journal of Materials Research and Technology 2022, 976–990. [Google Scholar] [CrossRef]
- Imran Ali.; Tahani Saad AlGarni.; Elena Burakova.; Alexey Tkachev.; Evgeny Tugolukov.; Tatyana Dyachkova.; Artem Rukhov.; Irina Gutnik.; Evgeny Galunin. A new approach to the economic synthesis of multi-walled carbon nanotubes using a Ni/MgO catalyst. Materials Chemistry and Physics 2021, 261, 124234. [CrossRef]
- Ghazaleh Allaedini.; Siti Masrinda Tasirin.; Payam Aminayi.; Synthesis of CNTs via chemical vapor deposition of carbon dioxide as a carbon source in the presence of NiMgO. Journal of Alloys and Compounds 2015, 647, 809–814. [CrossRef]
- Ryu, H.; Singh, B.K.; Bartwal, K.S. Synthesis and optimization of MWCNTs on Co-Ni/MgO by thermal CVD. Advances in condensed matter physics 2008. [Google Scholar] [CrossRef]
- ZHANG, Q.; Yi, L.; Ling, H.U.; Qian, W.Z.; Luo, G.H.; Fei, W. Synthesis of thin-walled carbon nanotubes from methane by changing the Ni/Mo ratio in a Ni/Mo/MgOcatalyst. New Carbon Materials 2008, 23, 319–325. [Google Scholar] [CrossRef]
- Zhou, L.P.; Ohta, K.; Kuroda, K.; Matsuishi, K.; Gao, L.; Matsumoto, T.; Nakamura, J. Catalytic functions of Mo/Ni/MgO in the synthesis of thin carbon nanotubes. The Journal of Physical Chemistry B 2005, 109, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K., Hu, J., Kobel, C., and Richards, R. Efficient Preparation and Catalytic Activity of MgO(111) Nanosheets. Angew. Chem. Int. Ed. 2006, 45, 7277–7281. [CrossRef] [PubMed]
- Taleshi, F.; Hosseini, A.A. Synthesis of uniform MgO/CNT nanorods by precipitation method. Journal of Nanostructure in Chemistry 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Ding, Y.D.; Song, G.; Zhu, X.; Chen, R.; Liao, Q. Synthesizing MgO with a high specific surface for carbon dioxide adsorption. Rsc Advances 2015, 5, 30929–30935. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Synthesis of CNTs via chemical vapor deposition of carbon dioxide as a carbon source in the presence of NiMgO. Journal of Alloys and Compounds 2015, 647, 809–814. [Google Scholar] [CrossRef]
- Ikram, M.; Inayat, T.; Haider, A.; Ul-Hamid, A.; Haider, J.; Nabgan, W.; Saeed, A.; Shahbaz, A.; Hayat, S.; Ul-Ain, K.; Butt, A.R. Graphene oxide-doped MgO nanostructures for highly efficient dye degradation and bactericidal action. Nanoscale research letters 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Yanagida, M.; Shirai, Y.; Miyano, K. Effect of different surface treatments of sputtered NiOX on the photovoltaic parameters of perovskite solar cells: a correlation study. Applied physics express 2020, 13, 025505. [Google Scholar] [CrossRef]
- Branca, C.; Frusteri, F.; Magazu, V.; Mangione, A. Characterization of carbon nanotubes by TEM and infrared spectroscopy. The Journal of Physical Chemistry B 2004, 108, 3469–3473. [Google Scholar] [CrossRef]
- Le Febvrier, A.; Jensen, J.; Eklund, P. Wet-cleaning of MgO (001): Modification of surface chemistry and effects on thin film growth investigated by x-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectroscopy. Journal of Vacuum Science & Technology A 2017, 35, 021407. [Google Scholar]
- Skorupska, M.; Kamedulski, P.; Lukaszewicz, J.P.; Ilnicka, A. The improvement of energy storage performance by sucrose-derived carbon foams via incorporating nitrogen atoms. Nanomaterials 2021, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Yumitori, S. Correlation of C1s chemical state intensities with the O1s intensity in the XPS analysis of anodically oxidized glass-like carbon samples. Journal of materials science 2000, 35, 139–146. [Google Scholar] [CrossRef]
- Dwivedi, N.; Yeo, R.J.; Satyanarayana, N.; Kundu, S.; Tripathy, S.; Bhatia, C.S. Understanding the role of nitrogen in plasma-assisted surface modification of magnetic recording media with and without ultrathin carbon overcoats. Scientific reports 2015, 5, 7772. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, B.; Chen, S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Advanced Materials 2018, 30, 1801995. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, M.; Huang, Y.; Li, J.; Zhou, X.; Ma, G.; Ren, S. One-pot synthesis of NiCoP/CNTs composites for lithium ion batteries and hydrogen evolution reaction. Ionics 2020, 26, 1771–1778. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Cui, P.; Cheng, N.; Lao, M.; Xu, X.; Dou, S.X.; Sun, W. Nickel single atom-decorated carbon nanosheets as multifunctional electrocatalyst supports toward efficient alkaline hydrogen evolution. Nano Energy 2021, 83, 105850. [Google Scholar] [CrossRef]
- Jia, L.; Du, G.; Han, D.; Wang, Y.; Zhao, W.; Su, Q.; Ding, S.; Xu, B. Magnetic electrode configuration with polypyrrole-wrapped Ni/NiFe2O4 core–shell nanospheres to boost electrocatalytic water splitting. Chemical Engineering Journal 2023, 454, 140278. [Google Scholar] [CrossRef]
- Wu, L.; Ji, L.; Wang, H.; Li, X.; Wu, X.; Zeng, S.; Li, L.; Xiao, Y.; Zhang, Q. Preparation and hydrogen evolution performance of porous Ni-Cu-Ti/CNTs-Ni electrode. Vacuum 2023, 218, 112598. [Google Scholar] [CrossRef]
- Ke, H.; Wang, J.; Yu, N.; Pu, Y.; Tan, J.; Gong, M.; Zhang, W.; Xue, Y.; Yu, F. Interface-engineered Ni/CePO4 heterostuctures for efficient electro-/photo-catalytic hydrogen evolution. Fuel 2023, 344, 127971. [Google Scholar] [CrossRef]
- Park, J.W.; Park, G.; Kim, M.; Han, M.; Jang, J.; Yamauchi, Y.; Yuliarto, B.; Krüger, P.; Kim, J.; Park, N.; Lim, H. Ni-single atom decorated mesoporous carbon electrocatalysts for hydrogen evolution reaction. Chemical Engineering Journal 2023, 468, 143733. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.; Liu, Y.; Zhang, Y.; Shi, J.; Xiong, J.; Zhou, S.; Li, J.; Fan, L.; Cai, W. Soft-template derived Ni/Mo2C hetero-sheet arrays for large current density hydrogen evolution reaction. Journal of Colloid and Interface Science 2023, 635, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, Q.; Kitiphatpiboon, N.; Zhang, J.; Feng, C.; Li, S.; Zhao, Q.; Abudula, A.; Ma, Y.; Guan, G. Heterojunction engineering of Ni3S2/NiS nanowire for electrochemical hydrogen evolution. Fuel 2023, 331, 125794. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. international journal of hydrogen energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Li, X.; Yan, W.; Fan, B.; Wang, Z. Lattice-controllable in-situ synthesis of Co-Ni-mixed sulfide/polypyrrole nanostructures on carbon paper for hydrogen evolution reaction in alkaline media. Journal of Alloys and Compounds 2023, 960, 170730. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Zhang, B.; Hu, Y.; Wang, D.Y.; Yang, J.; Pennycook, S.J. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nature communications 2014, 5, 4695. [Google Scholar] [CrossRef]
- Bredar, A.R.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Applied Energy Materials 2020, 3, 66–98. [Google Scholar] [CrossRef]

| Catalyst | Electrolyte | j (mA/cm²) | ɳ (mV) | Ref |
|---|---|---|---|---|
| Ni-N-C-250/Pt | KOH | 10 | 400 | 45 |
| Ni/NiFe2O4@PPy | KOH | 10 | 127 | 46 |
| Ni-Cu-Ti/CNTs-Ni | KOH | 100 | 140 | 47 |
| Ni/0.1CePO4 catalyst | KOH | 10 | 120 | 48 |
| Ni-MSACs | KOH | 10 | 270 | 49 |
| NS-Ni/Mo2C@NF | KOH | 100 | 151 | 50 |
| Ni3S2@NiS-250/NF | KOH | 10 | 129 | 51 |
| CNS/PHS/CP | KOH | 10 | 186 | 52 |
| Ni-MgO/CNT | KOH | 10 | 117 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





