1. Introduction
In marine and coastal ecosystems one of the main pollutants are heavy metals (HMs), which are chemical elements whose density is greater than 5 g/cm³. Some of the most common HMs in aquatic environments include cadmium (Cd), lead (Pb), arsenic (As), zinc (Zn), copper (Cu) and mercury (Hg). HMs can enter the marine environment through various sources, including natural sources and anthropogenic activities [
1,
2]. Agricultural, industrial and domestic activities are considered anthropogenic and their increase is associated as one of the main causes of progressive pollution of water resources [
3]. HMs can also enter the sediment from water bodies with a progressive increase in their concentrations over time. The physical and chemical characteristics of sediments such as texture, organic composition and pH can affect the bioavailability and toxicity of HMs in marine ecosystems [
4]. Their diffusion phenomenon in the aquatic environment is relatively low, however, a large amount of HMs can be transferred or mobilized from sediments to surface waters, representing a continuous ecological threat to species inhabiting sediments and water bodies [
5]. The persistent, toxic and bioaccumulative properties of HMs induce a series of pathologies such as liver, kidney and muscle dysfunctions, which alter the growth, development, metabolism, behavior and adaptation of marine species [
6].
If benthic fauna is contaminated by HMs, it generates a negative impact on the survival and repopulation of its consumers in the trophic chain, which is significant. In addition, the supply of micronutrients and macronutrients from marine resources, such as mollusks and fish, is essential for human health, so their entry will be linked to biomagnification of HMs [
7]. As top predators, humans can come into contact with HMs by consuming species that live in contaminated sediments, such as bivalve mollusks, which are potentially bioaccumulating species of these elements in high concentrations [
8].
Bioaccumulation of cadmium (Cd) in the body causes damage to the liver, testes, kidneys and bones. Its chronic accumulation causes a reduction in the reabsorption of nutrients and low molecular weight proteins [
9]. Reported health effects of Pb exposure are cardiovascular disease, idiopathic intellectual disability, and idiopathic intellectual development [
10]. Hexavalent chromium (Cr VI) is reduced to trivalent chromium (Cr III) by a biochemical process that occurs after absorption into the body; chromium reduction leads to DNA damage causing histological damage [
11]. A common pathology is skin hypersensitivity, which is caused by Ni allergy that develops dermatitis [
12]. Aluminum (Al) causes numerous diseases such as Alzheimer’s disease, pulmonary fibrosis and generates acute neurotoxicity. In humans, certain types of cancer and coronary heart disease are correlated with high levels of exposure [
13]. One way of biomonitoring HMs contamination is by quantifying these metals in aquatic organisms [
14]. Bivalve molluscs have been used as bioindicators of HMs for several decades, because they are highly sensitive and specific species, and have a high bioaccumulation capacity. The concentration of HMs in the tissues of bivalve molluscs reflects the presence and availability of these contaminants in their environment. Therefore, assessment of the abundance of HMs in the tissues of bivalve molluscs can be used to identify spatial and temporal trends in HMs contamination [
7].
Anadara tuberculosa is a filter-feeding bivalve mollusk endemic to American Pacific mangroves. It develops mainly in muddy substrates, especially clays and clayey silts [
15]. Due to its ability to accumulate heavy metals, A. tuberculosa has been proposed as a prospective biomarker for biomonitoring metal pollution in coastal mangrove ecosystems.
The Gulf of Guayaquil (GG), the largest estuarine ecosystem on the Pacific coast of South America, is home to a large number of aquatic species, including
A. tuberculosa. However, research in recent years indicates that the GG is experiencing increasing pollution from anthropogenic activities, such as industry, agriculture, fishing, aquaculture and urbanization [
16]. This pollution represents a risk to the health of ecosystems and the human communities that depend on them [
17].
The present study focuses on the quantification by inductively coupled plasma optical emission spectroscopy (ICP-OES) of the heavy metals cadmium (Cd), lead (Pb), chromium (Cr), nickel (Ni), copper (Cu) and Zinc (Zn) in bivalve mollusks of the Anadara tuberculosa species known as "concha negra" or "concha prieta" in three sectors of the Gulf of Guayaquil (GG) in order to identify the degree of bioaccumulation of heavy metals in the species.
2. Materials and Methods
2.1. Study Area
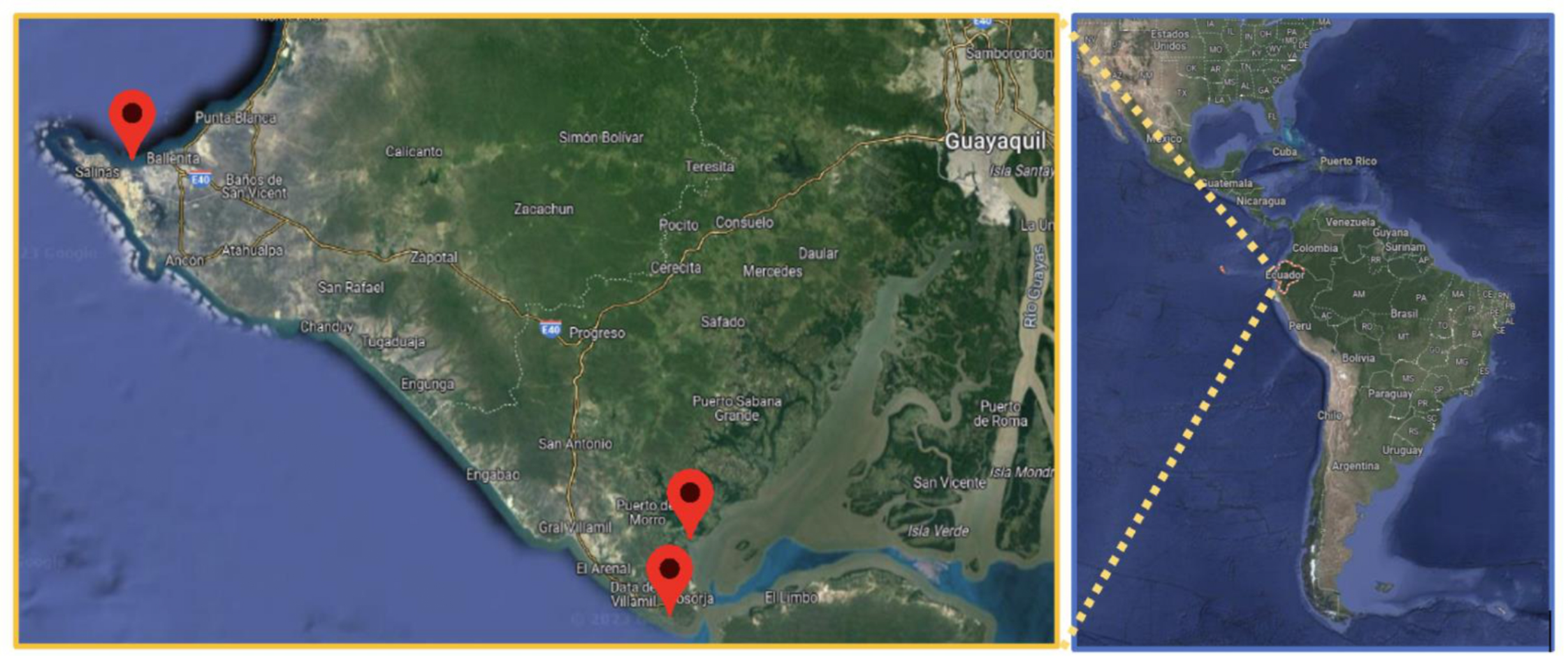
Figure 1.
Section of the map of Ecuador with the location of the three A. tuberculosa sampling sectors using Google Earth.
Figure 1.
Section of the map of Ecuador with the location of the three A. tuberculosa sampling sectors using Google Earth.
The Gulf of Guayaquil (GG) has an area of 13.711 Km
2 of the Ecuadorian continental shelf [
18]. The GG is located in Ecuador between the provinces of Guayas and El Oro 81º00’00" W and 03º23’34" S. It has a tropical climate with two seasons, a rainy season from December to May and a dry season from June to November [
17]. The GG is home to Guayaquil, a rapidly growing city and represents 80% of Ecuador’s total mangrove area [
19].
2.2. Sampling
In February 2023, 33 samples of A. tuberculosa were randomly collected from 3 sectors of the Gulf of Guayaquil: Puerto El Morro (n=11), General Villamil Playas (n=11) and La Libertad (n=11). Samples of concha prieta intended for direct consumption by the inhabitants of the sector were collected at seafood markets. The samples were stored in a polyethylene bag with a zip closure and a temperature of 4 ºC. The collected samples were taken to the laboratory for processing.
2.3. Sample Preparation and Analysis
During sample processing, each sample was washed externally with deionized water (DDW). Then, the contents of the mollusk were extracted and the moisture was removed until constant weight in an oven. The dried samples were crushed in a laboratory manual mortar and pestle and were individually packed in polyethylene bags. For sample digestion, the microwave acid digestion technique was used in the digester (Mars 6, CEM). Approximately 0.5 g of the sample was weighed and 10 mL of ultrapure grade nitric acid was added in a Teflon digestion vessel and heated for 15 min at a temperature of 200 °C. After cooling, filtration was performed and brought to a volume of 50 mL. Finally, for the quantification of heavy metal concentration of the studied elements in solution were determined using inductively coupled plasma optical emission spectrometer (ICP-OES;iCAP 7400 Duo, Thermo Scientific, Waltham,MA,USA).
2.4. Quality Assurance and Quality Control (QA/QC)
Duplicate samples and reagent blanks were used in the study. The value for the calibration curves of each element was greater than 0.999.
2.5. Statiscal Analysis
Descriptive statistics of the data were calculated with SPSS 26 (IBM, Armonk, NY, USA), and Minitab 19 was used for the graphs. To determine if there is a significant difference with the permissible limit values for heavy metals, a t-test was used, with a confidence level of 95 %. Rstudio, version 4.3.2 (2023-10-31 ucrt) was used to elaborate the mean diagram of the interaction of variables.
3. Results and Discussion
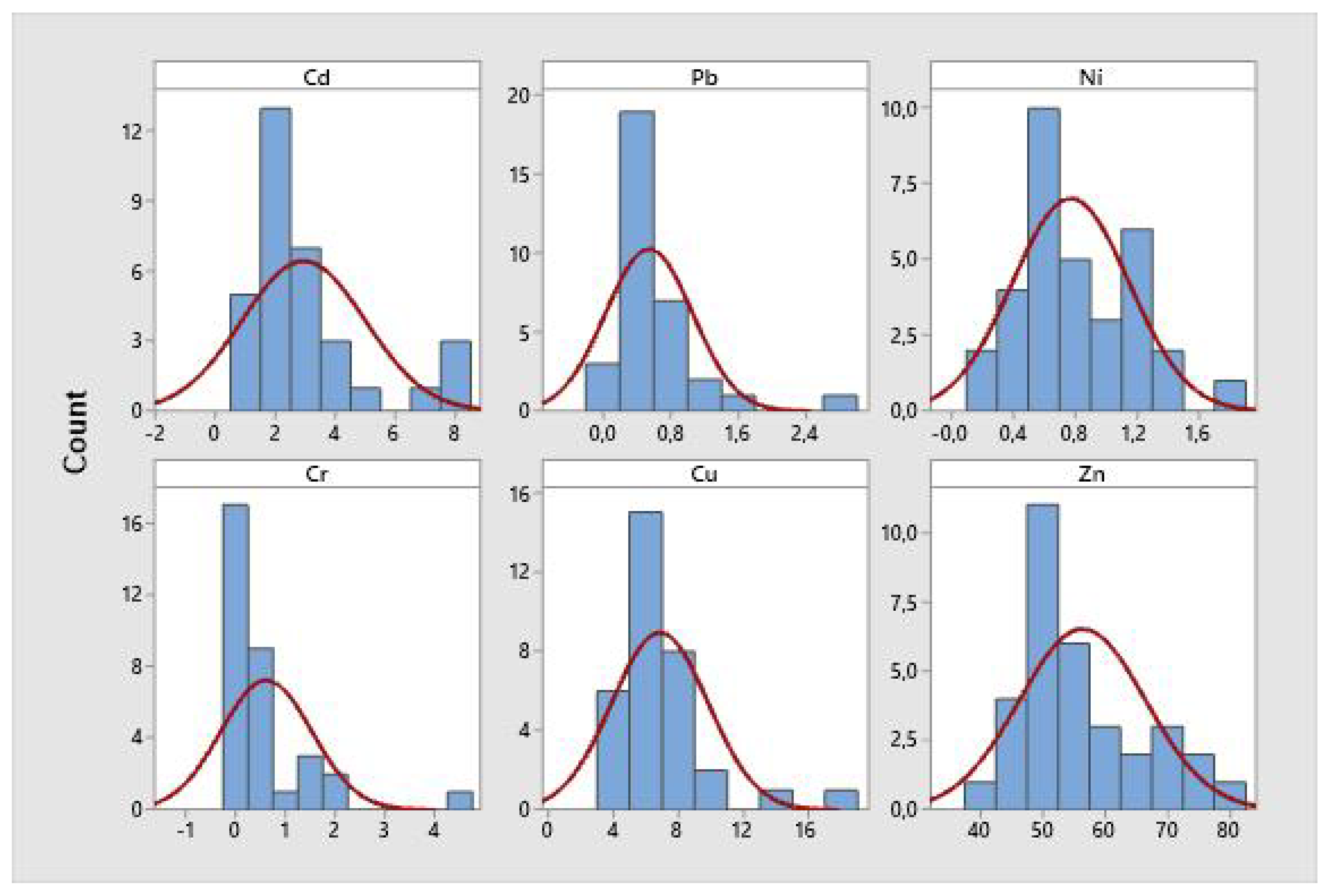
3.1. Concentrations of Cd, Pb, Cr, Ni, Cu and Zn.
The concentrations of Cd, Pb, Cr, Ni, Cu and Zn in samples of
A. tuberculosa concha prieta are presented in
Table 1 and
Figure 2. The maximum allowable concentration of the heavy metals Cd and Pb in bivalve molluscs established by the European Union [
20] was used for comparison with this study. For the heavy metals Cr, Ni, Cu and Zn, the daily intake limit issued by the National Institutes of Health (NHI) and the Spanish Agency for Food Safety and Nutrition (AESAN) was recognized.
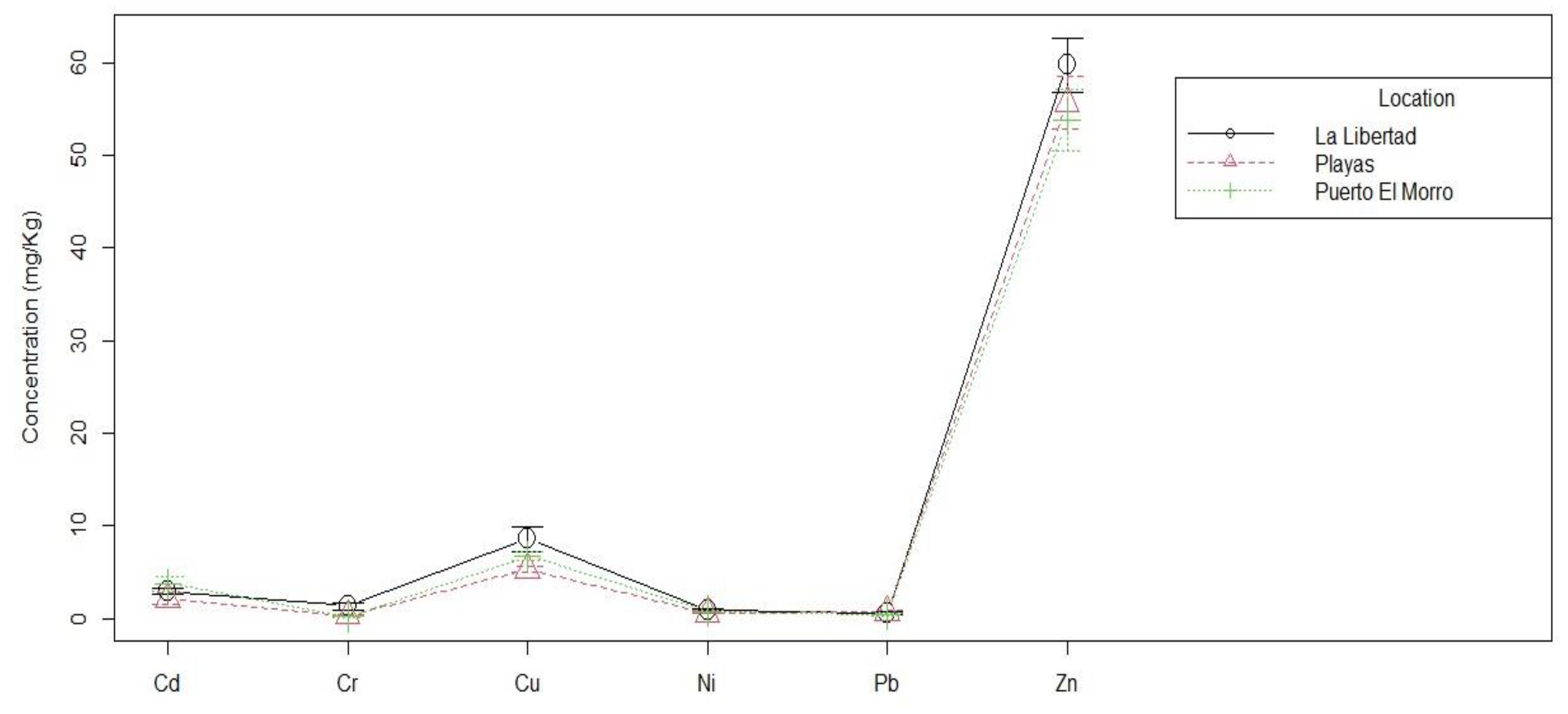
Tukey’s test was performed to contrast which pairs of means are significantly different. Considering the p-values, no significant differences were found between the means of the variable concentration of metals associated with the locations.
We performed Tukey’s test to contrast which pairs of means are significantly different. Taking into account the p-values obtained, for the usual , significant differences between the means of the variable means of metals associated with the concentration of the Cu-Cd groups, as well as Zn-Cd, Cu-Cr, Zn-Cr, Zn-Cr, Ni-Cu, Pb-Cu, Zn-Cu, Zn-Ni and also Zn-Pb are appreciated.
3.2. Cadmium
The mean Cd concentration in the A. tuberculosa bivalve samples from the GG was 2.93 ± 2.04 mg/kg (range: 0.50-8.20 mg/kg)
Table 1. This value exceeds the European Union (EU) maximum permitted limit of 1.0 mg/kg, established in Regulation (EC) No. 1881/2006 [
20].
Of the 33 samples of A. tuberculosa analyzed, 93.9% exceeded the permitted limit for Cd. In the Puerto el Morro sector, the mean Cd concentration (3.75 mg/kg) was almost four times the permitted limit. The average Cd concentrations in General Villamil Playas (2.14 mg/kg) and La Libertad (2.90 mg/kg) were also more than 200% higher than the permitted limit. These results coincide with the range of Cd concentration reported [
16] in A. tuberculosa sampled in the GG (1.40-3.97 ppm).
In
Figure 3, it is observed that the mean cadmium (Cd) concentrations in the three sampled sites are similar. This result is consistent with reports [
21], which indicate that Cd contamination is mostly of geogenic origin. However, the indiscriminate use of agricultural inputs and their residues from crop runoff also contribute to these high levels of Cd. Therefore, it is necessary to continue monitoring the content of this metal and to look for mitigation alternatives.
3.3. Lead
The mean concentration of Pb in the bivalve
A. tuberculosa was 0.54 ± 0.51 mg/Kg
Table 1, which is below the permissible limit of 1.5 mg/Kg established by the European Union in Regulation (EC) No. 1881/2006 [
20]. The average concentrations of the three sampled sectors (General Villamil Playas, La Libertad and Puerto El Morro) comply with this limit.
The General Villamil Playas sector presented the highest Pb concentration (0.68 ± 0.75 mg/Kg), followed by La Libertad (0.56 mg/Kg) and Puerto El Morro (0.39 mg/Kg). Only 3% of the samples exceeded the permissible limit, with values almost double this limit. These results coincide with the Pb concentration range reported in A. tuberculosa sampled in the Gulf of Guayaquil (0.11-7.52 ppm).
Figure 3 shows that the average concentrations of lead (Pb) in the three sampled sites are similar. According to the research [
22], the combustion of gasoline from boats used in fishing activities is one of the main anthropogenic sources of Pb in landing areas of artisanal docks in Ecuador and a possible source of Pb in the sectors sampled. Although Pb concentrations are below the limits proposed by the European Union, a higher Pb content can be observed in Playas and La Libertad, since they are fishing ports that harbor vessels a few kilometers from their shores on a daily basis. Unlike Puerto El Morro, a protected area and wildlife refuge since 2011, so there is greater control and surveillance of both fishing and ecotourism.
3.4. Chromium
The mean Cr concentration in the
A. tuberculosa samples is 0.6121 ± 0.15 mg/Kg (range: 0.0-4.50 mg/Kg)
Table 1. This value is similar to that reported. The highest mean concentration among the sectors evaluated corresponds to La Libertad (1.34 mg/Kg). Although maximum tolerable intakes or maximum acceptable limits for this metal have not yet been established in Ecuador, other institutions such as the NHI recommend a lower intake of 35
g/day for adult men and 25
g/day for adult women considering an age of 19 to 50 years [
23]. Also, the US EPA has established maximum oral reference doses of 1.5 mg/Kg per day for Cr (III) [
24]. Only 9% of the data are above 1.5 mg/kg and correspond to data from La Libertad, of which 3% are three times the maximum limit suggested by the US EPA. One of the main sources of chromium is the manufacturing processes of tanneries and industrial electroplating.
It is expected that these data will help to improve future monitoring and analysis of industrial impact. It is proposed to identify the industries surrounding the sampling sector. This will allow us to characterize their effluents and evaluate their impact on chromium concentrations in La Libertad’s water bodies, which could be affected by chromium residues.
3.5. Nickel
The mean concentration of Ni in the
A. tuberculosa samples is 0.77 mg/Kg ±0.37 with a range: 0.20 - 1.70 mg/Kg
Table 1. The lowest mean quantified in the study corresponds to the General Villamil Playas sector mean 0.57 ± 0.21 mg/Kg (range: 0.30-1.10 mg/Kg), while the sector with the highest mean 0.93 ± 0.24 mg/Kg (range: 0.60-1.20 mg/Kg).
Although in Ecuador there are no defined limits for nickel in bivalves, the Spanish Agency for Food Safety and Nutrition (AESAN), recommends a maximum average nickel intake of 150-900
g/day [
25]. In addition, Brazilian legislation on heavy metals establishes a tolerance of 5 mg/kg of nickel in food [
26].
In contrast to other mangrove areas in Ecuador, such as Estero Salado, where nickel concentrations of up to 60.14 mg/kg have been recorded, the results of nickel analyses in the three sectors studied suggest that nickel concentrations are within the permissible intake limits established by the health authorities cited above. This is positive, since the communities in these sectors depend on fishing as a source of food.
However, it is important to note that excessive consumption of nickel can have adverse health effects, such as allergies, dermatitis, respiratory problems and even cancer. Therefore, it is recommended that people living in these areas limit their consumption of bivalves to once a week, and choose those that have undergone quality controls.
3.6. Copper
The mean Cu concentration in the
A. tuberculosa samples was 6.86 ± 0.51 mg/Kg with a range: 4.10-18.20 mg/Kg
Table 1. According to FAO (1982) a daily intake of 2-3 mg of copper through the diet is sufficient to meet the nutritional needs of adults, establishing a maximum tolerable intake of 0.5 mg Cu/kg body weight per day[34]. Other sources suggest an upper daily intake limit of 10 mg [
23]. The copper levels reported in the three sectors can be considered acceptable for the consumption habits of the region. Furthermore, these results are much lower than those reported in the Estero Salado, Guayas province, found copper concentrations of up to 204.1 mg/Kg.
However, in
Figure 3, it is observed that the letters of the Tukey statistical method indicate significant differences between the means of copper concentrations and the other metals. This means that it is likely that these differences are not due to chance, but are due to real factors, such as environmental pollution, industrial activity or soil geology.
In the same figure, it is observed that copper concentrations are higher in La Libertad (8.5636 ± 4.27 mg/Kg; range 4.20-18.20 mg/Kg) than in Puerto El Morro and General Villamil Playas (5.30 ± 1.02 mg/Kg; range: 4.10-7.50 mg/Kg). This suggests that La Libertad may be more contaminated with copper than the other two locations. To determine the cause of differences in copper concentrations, a more detailed analysis is needed. This may include studying the sources of contamination in the area, soil and water conditions, and industrial activity.
3.7. Zinc
The mean Zn concentration in the
A. tuberculosa samples was 56.38 ± 1.75 mg/Kg (range: 81.60-40.00 mg/Kg). According to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) the average daily intake of Zn should not exceed 20 mg/day for adults or a maximum tolerable daily intake of 0.3-1 mg/Kg body weight [
27].
Compared to other metals, Zn is present in higher amounts in the tissues of A. tuberculosa. These results are consistent with those obtained, who also observed elevated Zn concentrations in bivalves of the genera Anadara, Perna, Crassostrea, Meretrix and Amusium [
28].
These elevated Zn concentrations could be due to several causes. First, Zn is a natural constituent of bivalve tissues, and is present in considerable concentrations [
27]. Zn is a trace element necessary for the proper functioning of living organisms but in quantities lower than those reported in
Table 1. Therefore, its high concentrations in the tissues of this bivalve may be due to a greater facility to uptake and bioaccumulate Zn from the sediment or water. To confirm this hypothesis, bioavailable zinc content in sediment and water should be evaluated and correlated with zinc content in bivalve tissues in longitudinal studies.
Figure 3 also shows significant differences between the means of Zn concentrations and the other metals. Zn concentrations are higher in La Libertad (59.73 ±9.57 mg/Kg; range: 45.70-75.30 mg/Kg) and lower in Puerto El Morro (53.78 ± 11.10 mg/Kg; range: 40.00 - 81.60 mg/Kg). This could suggest that the higher Zn levels in La Libertad may be the result of some type of contamination and not only due to its natural content. To determine the causes, further analysis is needed considering sources of contamination in the area, soil and water conditions, and industrial activity.
4. Conclusions
From a local public health point of view, the mean concentrations of the metals Pb, Cr, Ni, Cu and Zn are below the maximum levels allowed by the EU, US EPA, AESAN, IHN, FAO and JECFA, respectively. However, the average concentration of Cd is twice the permitted limits suggested by the EU in bivalves. In order not to exceed the admissible daily intake of Cd proposed by AESAN, it is recommended to limit the consumption of this food to 0.36 Cd/Kg body weight which corresponds to a weekly dietary intake of 2.52 Cd/Kg body weight. Based on the Cd averages in the three sectors, consumption of A. tuberculosa from General Villamil Playas is recommended, followed by La Libertad and lastly those from Puerto El Morro. It is important to note that these recommendations apply to the bivalves analyzed in this study. It is possible that Cd concentrations in other bivalves from the same area may be different. Therefore, it is recommended that persons consuming bivalves consult with local health authorities for specific information on safe consumption limits. The differences in mean Cu and Zn concentrations for the other metals among the three sectors suggest that the causes of contamination may be different. To determine the cause of these differences, a more detailed analysis including sources of contamination in the area, effluents, soil and water conditions, and industrial activity is recommended.
According to several studies reviewed in this research, bivalves employ regulatory and detoxification processes that can modify their accumulation of metals. Therefore, it is recommended to evaluate the concentrations of these metals in bivalve tissues from early to adult stages, and correlate it with their content in sediment and water. On the other hand, it is recommended to evaluate processing techniques such as cooking and washing to determine if they reduce their concentrations. Finally, this study highlights the importance of periodic food quality controls, especially in areas with a high risk of heavy metal contamination.
Author Contributions
Conceptualization, K.C., A.P. and J.B.; methodology, K.C., A.P. and J.B.; validation, K.C., A.P. and J.B.; formal analysis, K.C., A.P. and J.B.; investigation, K.C., A.P. and J.B.; resources, K.C., A.P. and J.B.; data curation, K.C., A.P. and J.B.; writing—original draft preparation, K.C., A.P. and J.B.; writing—review and editing, K.C., A.P. and J.B.; visualization, K.C., A.P. and J.B.; supervision, K.C., A.P. and J.B.; project administration, K.C.; funding acquisition, K.C., A.P. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Politécnica Salesiana-Ecuador Sede Guayaquil, through a project of the GIAB research group.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Funding was provided by the Universidad Politécnica Salesiana Sede Guayaquil. We would like to thank the eighth level Biotechnology Engineering students of period 62 for helping in sample collection.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in the manuscript.
References
- Tanhan, P.; Lansubsakul, N.; Phaochoosak, N.; Sirinupong, P.; Yeesin, P.; Imsilp, K. Human Health Risk Assessment of Heavy Metal Concentration in Seafood Collected from Pattani Bay, Thailand. Toxics 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, X.; Wang, L.; Han, J.; Katuwal, H.B.; Jiao, S.; Qiu, G. Human Dietary Exposure to Heavy Metals via Rice in Nepal. International Journal of Environmental Research and Public Health 2023, 20, 4134. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, T.; Kargın, D.; Çoğun, H.Y.; Temiz, Ö.; Varkal, H.S.; Kargın, F. Accumulation and health risk assessment of heavy metals in tissues of the shrimp and fish species from the Yumurtalik coast of Iskenderun Gulf, Turkey. Heliyon 2019, 5, e02131. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zheng, S.; Wan, T.; Wang, L.; Yang, Q.; Zhang, J. Nematode as a biomonitoring model for evaluating ecological risks of heavy metals in sediments from an urban river. Ecological Indicators 2023, 147, 110013. [Google Scholar] [CrossRef]
- Márquez, A.; Senior, W.; Martínez, G.; Castañeda, J.; González, Á. CONCENTRACIONES DE METALES EN SEDIMENTOS Y TEJIDOS MUSCULARES DE ALGUNOS PECES DE LA LAGUNA DE CASTILLERO, VENEZUELA. Metals Concentration in Sediments and Muscular Tissues of Some Fish from the Castillero Lagoon, Venezuela 2008. XVIII, 121–133.
- Ahmed, M.I.; Ambali, A.; Karshima, S.N.; Mohammed, K.M. Heavy metal concentrations, water quality and health risk assessment of freshwater fish from the Lake Chad basin. Limnologica 2023, 103, 126135. [Google Scholar] [CrossRef]
- Anagha, B.; Athira, P.S.; Anisha, P.; Charles, P.E.; Anandkumar, A.; Rajaram, R. Biomonitoring of heavy metals accumulation in molluscs and echinoderms collected from southern coastal India. Marine Pollution Bulletin 2022, 184, 114169. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jeon, H.; Shin, H.S. Risk Assessment and Determination of Arsenic and Heavy Metals in Fishery Products in Korea. Foods 2023, 12, 3750. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yue, C.; Sun, Q.; Wang, Y.; Gong, Z.; Zhang, K.; Da, J.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. Cadmium exposure exacerbates kidney damage by inhibiting autophagy in diabetic rats. Ecotoxicology and Environmental Safety 2023, 267, 115674. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: a health impact and economic modelling analysis. The Lancet Planetary Health 2023, 7, e831–e840. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, J.C. Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicology and Environmental Safety 2016, 125, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nibe-Shirakihara, Y.; Tamura, A.; Aonuma, E.; Arakawa, S.; Otsubo, K.; Nemoto, Y.; Nagaishi, T.; Tsuchiya, K.; Shimizu, S.; Ma, A.; Watanabe, M.; Uo, M.; Okamoto, R.; Oshima, S. Nickel particles are present in Crohn’s disease tissue and exacerbate intestinal inflammation in IBD susceptible mice. Biochemical and Biophysical Research Communications 2022, 592, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Aluminum. Encyclopedia of Toxicology 2024, pp. 329–334. [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Spatial Variation of Metallic Contamination and Its Ecological Risk in Sediment and Freshwater Mollusk: Melanoides tuberculata (Müller, 1774) (Gastropoda: Thiaridae). [CrossRef]
- Prado-Carpio, E.C.; Martínez-Soto.; Moisés E.; Rodríguez-Monroy.; Carlos.; Quiñonez-Cabeza.; Manuel.; Olivo-Garrido, M.L. Biología, productividad y atributos comerciales del molusco bivalvo «concha prieta» (Anadara tuberculosa) Biology, productivity and commercial attributes of the «black ark Shell» bivalve mollusk (Anadara tuberculosa). Pag, 12. [CrossRef]
- Navarrete-Forero, G.; Morales Baren, L.; Dominguez-Granda, L.; Pontón Cevallos, J.; Marín Jarrín, J.R. CONTAMINACIÓN POR METALES PESADOS EN EL GOLFO DE GUAYAQUIL: INCLUSO DATOS LIMITADOS REFLEJAN IMPACTOS AMBIENTALES DE LAS ACTIVIDADES ANTRÓPICAS. Revista internacional de contaminación ambiental 2019, 35, 731–755. [Google Scholar] [CrossRef]
- Pontón-Cevallos, J.; Marín Jarrín, J.R.; Rosado-Moncayo, A.M.; Bonifaz, M.J.; Quiroga, M.d.M.; Espinoza, M.E.; Borbor-Córdova, M.J.; Pozo-Cajas, M.; Goethals, P.L.; Domínguez-Granda, L.E. Spatio-temporal variability of Brachyura larval assemblages in mangroves of the Gulf of Guayaquil’s inner estuary. Regional Studies in Marine Science 2021, 41, 101601. [Google Scholar] [CrossRef]
- Pinto, E.B.; Slowey, N.C. Stable isotope evidence for the origins of waters in the Guayas estuary and Gulf of Guayaquil. Estuarine, Coastal and Shelf Science 2021, 250, 107151. [Google Scholar] [CrossRef]
- Calle, P.; Monserrate, L.; Medina, F.; Calle Delgado, M.; Tirapé, A.; Montiel, M.; Ruiz Barzola, O.; Cadena, O.A.; Dominguez, G.A.; Alava, J.J. Mercury assessment, macrobenthos diversity and environmental quality conditions in the Salado Estuary (Gulf of Guayaquil, Ecuador) impacted by anthropogenic influences. Marine Pollution Bulletin 2018, 136, 365–373. [Google Scholar] [CrossRef] [PubMed]
- CE. REGLAMENTO (CE) N o 1881/2006 DE LA COMISIÓN. Technical report.
- Flores, E.; Pozo, W.; Pernía, B.; Sánchez, W. Niveles de cadmio en atún fresco y enlatado para consumo humano en Ecuador. MASKANA 2018, 9, 35–40. [Google Scholar] [CrossRef]
- Collaguazo, N.Y.; Armijos, H.A.; Loja, G.M. Cuantificación de metales pesados en Anadara tuberculosa(Mollusca:bivalvia) del estero Huaylá de Puerto Bolívar, por espectrofotometría de absorción atómica. // Quantification of heavy metals in Anadara tuberculosa, (Mollusca: bivalvia) from the Huaylá e. CIENCIA UNEMI 2017, 10, 1–10. [Google Scholar] [CrossRef]
- National Institutes of Health. Cobre - Datos en español, 2022.
- USEPA. Chromium(III), insoluble salts (CASRN 16065-83-1) | IRIS | US EPA, 2021.
- AESAN. Niquel.
- para la transición ecológica y el reto demográfico, M. Metales pesados, 1989.
- FAO.; WHO. JOINT FAO / WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON CONTAMINANTS IN FOODS WORKING DOCUMENT FOR INFORMATION AND USE IN DISCUSSIONS RELATED TO CONTAMINANTS AND TOXINS IN THE GSCTFF.
- Kadmium, T.; Zink Di Dalam Kerang, D.; Anadara, .; Selangor, K.; Sabarina, M..; Yunus, M.; Hamzah, Z.; Azlin, N.; Ariffin, N.; Muslim, M.B. CADMIUM, CHROMIUM, COPPER, LEAD, FERUM AND ZINC LEVELS IN THE COCKLES (Anadara granosa) FROM KUALA SELANGOR, MALAYSIA. The Malaysian Journal of Analytical Sciences 2014, 18, 514–521.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).