Submitted:
26 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
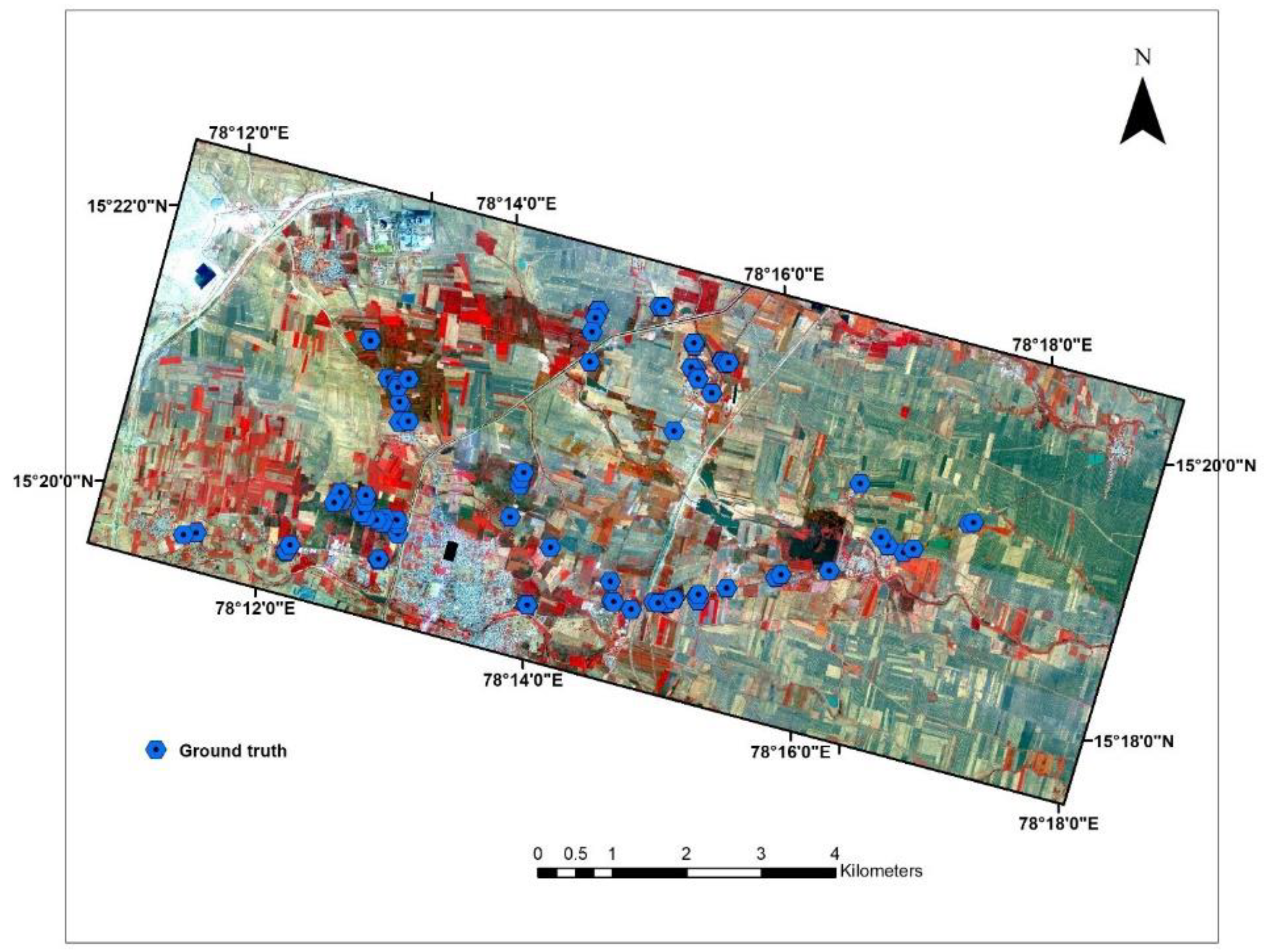
2.2. Sampling Sites and Field Data Collection
2.3. Sampling of LAI
2.4. Canopy Spectral Reflectance Measurements
2.5. AVIRIS-NG Airborne Data Acquisition
2.6. Data Processing
2.7. Data Analysis
3. Results
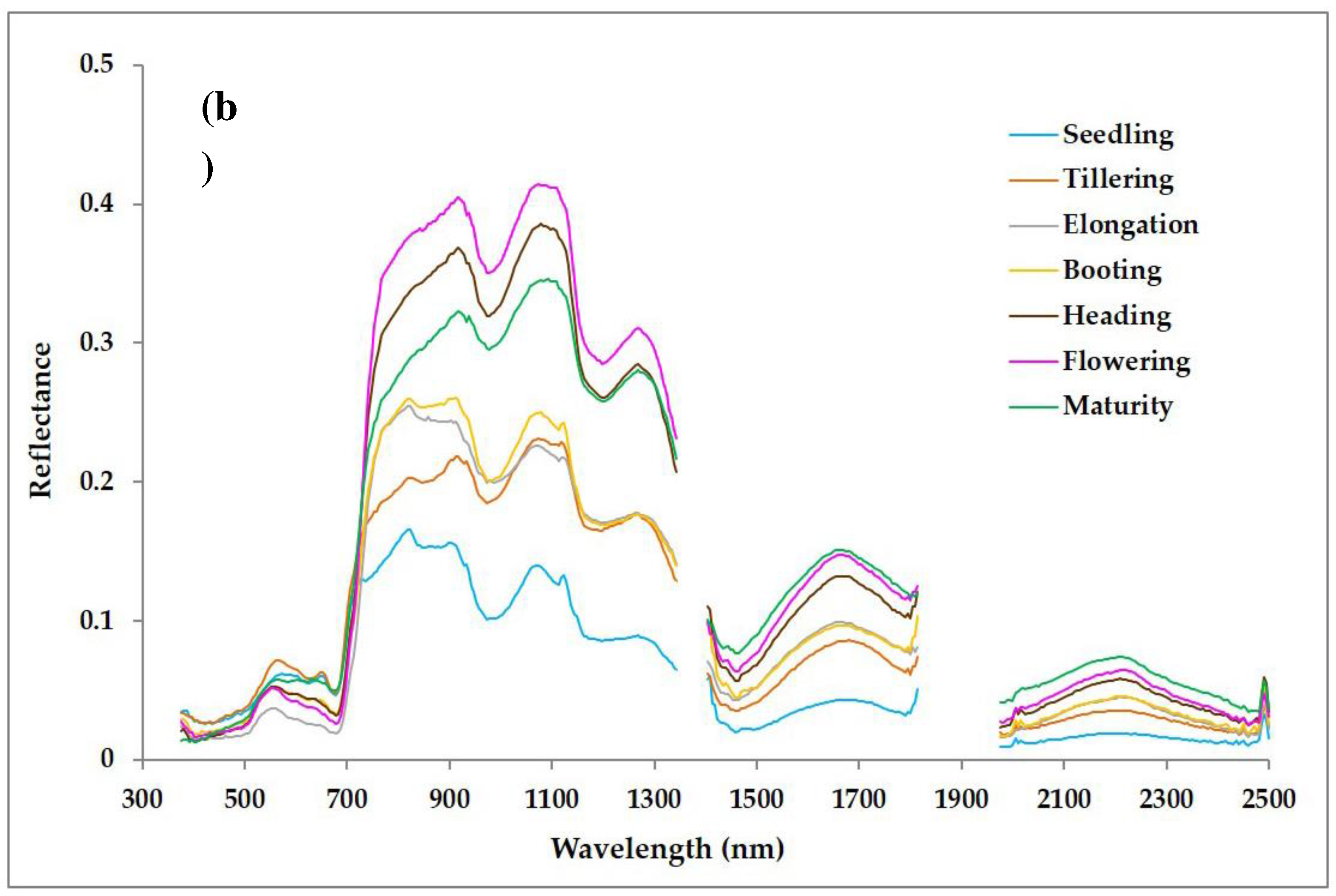
3.1. Reflectance Spectra of Rice Canopy from Hand held Hyperspectral Radiometry
3.2. Reflectance Spectra of Rice Canopy from AVIRIS-NG
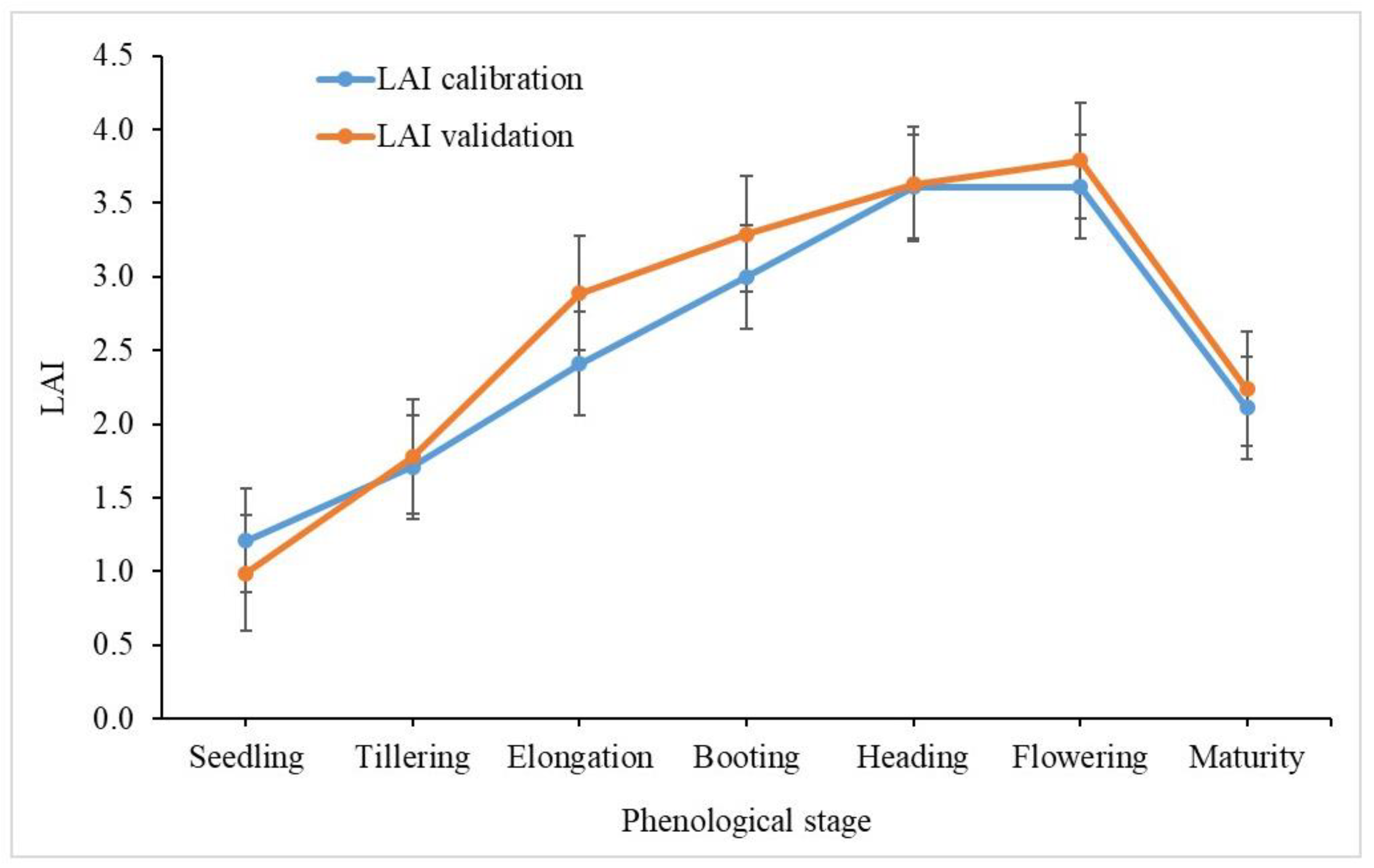
3.3. Rice LAI at Different Phenological Stages
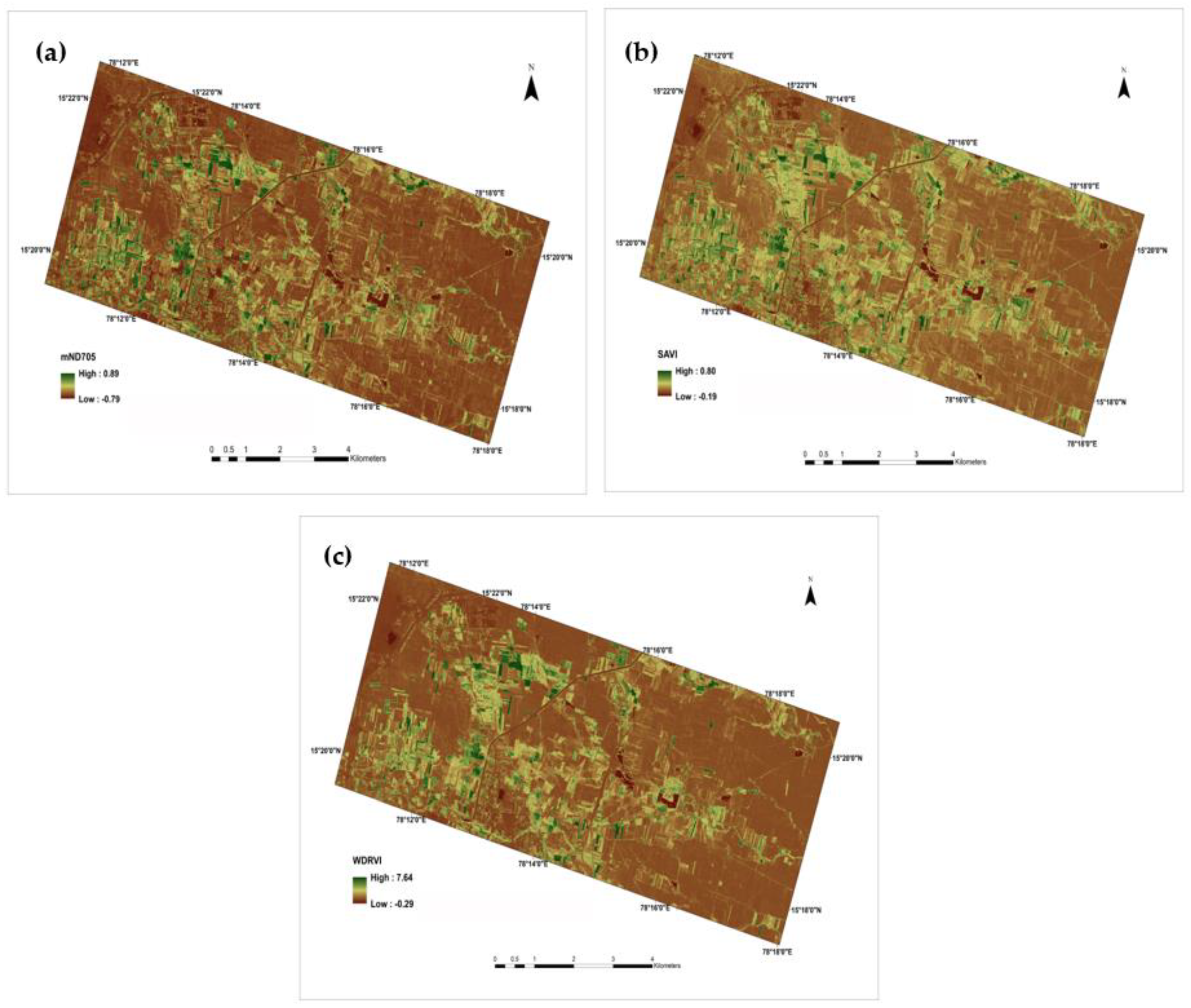
3.4. Relationship of Vegetation Indices to Rice Phenological Stages
3.5. Evaluation of Vegetation Indices for Rice LAI Estimation
3.6. Validation of VIs for Estimation of LAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix
References
- World Rice Production 2022/2023. Available online: http://www.worldagriculturalproduction. com/crops/rice.aspx/ (accessed on 21 September 2022).
- Thenkabail, P.S.; Smith, R.B.; Pauw, E.D. Hyperspectral vegetation indices and their relationship with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 152-182.
- Atzberger, C.; Darvishzadeh, R.; Immitzer, M.; Schlerf, M.; Skidmore, A.; Le Maire, G. Comparative analysis of different retrieval methods for mapping grassland leaf area index using airborne imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 19-31. [CrossRef]
- Prabhakar, M.; Gopinath, K.A.; Kumar, N.R.; Thirupathi, M.; Sravan, U.S.; Kumar, G.S.; Siva, G.S.; Meghalakshmi, G.; Vennila, S. Detecting the invasive fall armyworm pest incidence in farm fields of southern India using Sentinel-2A satellite data. Geocarto Int. 2021, 37, 3801-3816. [CrossRef]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation, 7th ed.; John Wiley Sons: New Jersey, United States, 2015.
- Datt, B. Remote sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and total Carotenoid content in Eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111-121.
- Chen, J.M.; Black, T. Defining leaf area index for non-flat leaves. Plant, Cell Environ. 1992, 15, 421-429. [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in-situ leaf area index determination: Part, I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19-35.
- Tao, H.; Feng, H.; Xu, L.; Miao, M.; Long, H.; Yue, J.; Li, Z.; Yang, G.; Yang, X.; Fan, L. Estimation of crop growth parameters using UAV-based hyperspectral remote sensing data. Sensors 2020, 20, 1296. [CrossRef]
- Chen, Y.; Zhang, Z.; Tao, F. Improving regional winter wheat yield estimation through assimilation of phenology and leaf area index from remote sensing data. Eur. J. Agron. 2018, 101, 163-173. [CrossRef]
- Srinet, R.; Nandy, S.; Patel, N. Estimating leaf area index and light extinction coefficient using Random Forest regression algorithm in a tropical moist deciduous forest, India. Ecol. Inform. 2019, 52, 94-102. [CrossRef]
- Walthall, C.; Dulaney, W.; Anderson, M.; Norman, J.; Fang, H.; Liang, S. A comparison of empirical and neural network approaches for estimating corn and soybean leaf area index from Landsat ETM+ imagery. Remote Sens. Environ. 2004, 92, 465–474. [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352.
- He, L.; Ren, X.; Wang, Y.; Liu, B.; Zhang, H.; Liu, W.; Feng, W.; Guo, T. Comparing methods for estimating leaf area index by multi-angular remote sensing in winter wheat. Sci. Rep. 2020, 10, 13943. [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [CrossRef]
- Roujean, J.; Breon, F. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [CrossRef]
- Ma, B.L.; Dwyer, L.M.; Costa, C.; Cober, E.R.; Morrison, M.J. Early prediction of soybean yield from canopy reflectance measurements. Agron. J. 2001, 93, 1227–1234. [CrossRef]
- Clevers, J.; de Jong, S.M.; Epema, G.F.; van der Meer, F.D.; Bakker, W.H.; Skidmore, A.K.; Scholte, K.H. Derivation of the red edge index using the MERIS standard band setting. Int. J. Remote Sens. 2002, 23, 3169–3184. [CrossRef]
- Vina, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A review study. Remote Sens. 2018, 10, 85.
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Pearson Education India: Noida, Uttar Pradesh, India, 2009.
- Singh, P.; Srivastava, P.K.; Mall, R.K.; Bhattacharya, B.K.; Prasad, R. A hyperspectral R based leaf area index estimator: Model development and implementation using AVIRIS-NG. Geocarto Int. 2022, 37, 12792-12809. [CrossRef]
- Wang, Y.; Zhang, K.; Tang, C.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Estimation of rice growth parameters based on linear mixed-effect model using multispectral images from fixed-wing unmanned aerial vehicles. Remote Sens. 2019, 11, 1371. [CrossRef]
- Zheng, H.; Cheng, T.; Zhou, M.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Improved estimation of rice aboveground biomass combining textural and spectral analysis of UAV imagery. Precis. Agric. 2019, 20, 611-629.
- Ryu, C.; Suguri, M.; Umeda, M. Model for predicting the nitrogen content of rice at panicle initiation stage using data from airborne hyperspectral remote sensing. Biosyst. Eng. 2009, 104, 465-475. [CrossRef]
- Nakanishi, T.; Imai, Y.; Morita, T.; Akamatsu, Y.; Odagawa, S.; Takeda, T.; Kashimura, O. Evaluation of wheat growth monitoring methods based on hyperspectral data of later grain filling and heading stages in Western Australia. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, 39, 295-300. [CrossRef]
- Delalieux, S.; Auwerkerken, A.; Verstraeten, W.W.; Somers, B.; Valcke, R.; Lhermitte, S.; Keulemans, J.; Coppin, P. Hyperspectral reflectance and fluorescence imaging to detect scab induced stress in apple leaves. Remote Sens. 2009, 1, 858-874.
- Technical Guide, A: (CO), 1999.
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red-edge spectral measurements from Sugar Maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575.
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote. Sens. 1996, 22, 229–242. [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 331–354. [CrossRef]
- LeMaire, G.; François, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Remote Sens. 1997, 18, 2691–2697. [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107.
- Birth, G.S.; McVey, G. Measuring the color of growing turf with a reflectance spectrophotometer. Agron. J. 1968, 60, 640-643. [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the vernal advancements and retrogradation of natural vegetation. In NASA/GSFC, Final Report, Greenbelt, MD, USA, 1974; pp. 1–137.
- Jordan, C.F. Derivation of leaf area index from quality of light on the forest floor. Ecology 1969, 50, 663–666.
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195-213. [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [CrossRef]
- Le Maire, G., Francois, C., Soudani, K., Berveiller, D., Pontailler, J.Y., Breda, N., Genet, H., Davi, H.; Dufrene, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846-3864.
- Gong, P.; Pu, R.; Biging, G.S.; Larrieu, M.R. Estimation of forest leaf area index using vegetation indices derived from Hyperion hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1355-1362. [CrossRef]
- Penuelas, J.; Filella, I. Reflectance assessment of mite effects on apple trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Lilella, I. Estimation of plant water content by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875.
- Merton, R.; Huntington, J. Early simulation results of the ARIES-1 satellite sensor for multi-temporal vegetation research derived from AVIRIS. In Proceedings of the eighth annual JPL airborne earth science workshop, Pasadena, CA, USA, February 1999; pp. 9-11.
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.W.; Keulemans, W.; Coppin, P. A near-infrared narrow-waveband ratio to determine Leaf Area Index in orchards. Remote Sens. Environ. 2008, 112, 3762-3772. [CrossRef]
- Gu, Y.; Brown, J.F.; Verdin, J.P.; Wardlow, B. A five-year analysis of MODIS NDVI and NDWI for grassland drought assessment over the central Great Plains of the United States. Geophys. Res. Lett. 2007, 34, L06407. [CrossRef]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influence of soil salinity, growth form, and leaf moisture on the spectral radiance of Spartina alterniflora canopies. Photogram. Eng. Remote Sens. 1983, 49, 77–83.
- Guyot, G.; Guyon, D.; Riom, J. Factors affecting the spectral response of forest canopies: A review. Geocarto Int. 1988, 4, 3-18. [CrossRef]
- Peng, Y.; Gitelson, A.A.; Keydan, G.; Rundquist, D.C.; Moses, W. Remote estimation of gross primary production in maize and support for a new paradigm based on total crop chlorophyll content. Remote Sens. Environ. 2011, 115, 978-989. [CrossRef]
- Govaerts, Y.M.; Verstraete, M.M.; Pinty, B.; Gobron, N. Designing optimal spectral indices: A feasibility and proof of concept study. Int. J. Remote Sens. 1999, 20, 1853-1873. [CrossRef]
- SAS Institute Inc. SAS OnlineDocVR 9.2. Cary (NC): 2009 [accessed on 20 June 2023]. http://support.sas.com/documentation.
- Liu, Z.; Huang, W.; Mao, G.; Li, C.; Xu, X.; Ding, X.; Shi, J.; Zhou, B. Estimating foliar pigment concentration of rice crop using integrated hyperspectral index. In Computer and Computing Technologies in Agriculture V: CCTA 2011. IFIP Advances in Information and Communication Technology. Beijing, China, October 29-31; D. Li, Y. Chen Eds. Springer: Berlin, Heidelberg, Germany, 2011, 264-274. [CrossRef]
- Wu, L.; Yuan, S.; Huang, L.; Sun, F.; Zhu, G.; Li, G.; Fahad, S.; Peng, S.; Wang, F. Physiological mechanisms underlying the high-grain yield and high-nitrogen use efficiency of elite rice varieties under a low rate of nitrogen application in China. Front. Plant Sci. 2016, 7, 1024.
- Carvalho, S.; Van der Putten, W.H.; Hol, W.H.G. The potential of hyperspectral patterns of winter wheat to detect changes in soil microbial community composition. Front. Plant Sci. 2016, 7, 759. [CrossRef]
- Lima, I.P.; Jorge, R.G.; de Lima, J.L.M.P. Remote sensing monitoring of rice fields: Towards assessing water saving irrigation management practices. Front. Remote Sens. 2021, 2, 762093.
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages. Front. Plant Sci. 2017, 8, 820. [CrossRef]
- Kawamura, K.; Ikeura, H.; Phongchanmaixay, S.; Khanthavong, P. Canopy hyperspectral sensing of paddy fields at the booting stage and PLS regression can assess grain yield. Remote Sens. 2018, 10, 1249. [CrossRef]
- Gausman, H.W.; Allen, W.A.; Cardenas, R. Reflectance of cotton leaves and their structure. Remote Sens. Environ. 1969, 1, 19–22.
- Woolley, J.T. Reflectance and transmittance of light by leaves. Plant Physiol. 1970, 47, 656–662. [CrossRef]
- Feng, W.; Guo, B.B.; Wang, Z.J.; He, L.; Song, X.; Wang, Y.H.; Guo, T.C. Measuring leaf nitrogen concentration in winter wheat using double-peak spectral reflection remote sensing data. Field Crop Res. 2014, 159, 43–52. [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crop Res. 2014, 155, 213–223.
- Peng, Y.; Nguy-Robertson, A.; Arkebauer, T.; Gitelson, A.A. Assessment of canopy chlorophyll content retrieval in maize and soybean: Implications of hysteresis on the development of generic algorithms. Remote Sens. 2017, 9, 226.
- Dong, T.; Liu, J.; Shang, J.; Qian, B.; Ma, B.; Kovacs, J.M.; Walters, D.; Jiao, X.; Geng, X.; Shi, Y. Assessment of red-edge vegetation indices for crop leaf area index estimation. Remote Sens. Environ. 2019, 222, 133-143. [CrossRef]
- Chen, J. Gu, S. Shen, M. Tang, Y. Matsushita, B. Estimating aboveground biomass of grassland having a high canopy cover: An exploratory analysis of in-situ hyperspectral data. Int. J. Remote Sens. 2009, 30, 6497-6517.
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Biophysical and biochemical characterization and plant species studies, 2nd ed.; CRC Press: Florida, United States, 2018.
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.K.; Abkar, A.A. Leaf Area Index derivation from hyperspectral vegetation indices and the red edge position. Int. J. Remote Sens. 2009, 30, 6199–6218.
- Herrmann, I. Pimstein, A. Karnieli, A. Cohen, Y. Alchanatis, V. Bonfil, D.J. LAI assessment of wheat and potato crops by VENµS and Sentimel-2 bands. Remote Sens Environ. 2011, 115, 2141-2151.
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387.
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221.
- He, J.; Zhang, N.; Su, X.; Lu, J.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimating leaf area index with a new vegetation index considering the influence of rice panicles. Remote Sens. 2019, 11, 1809. [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165-173.
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192, 140–148. [CrossRef]
- Kira, O.; Nguy-Robertson, A.L.; Arkebauer, T.J.; Linker, R.; Gitelson, A.A. Toward generic models for green LAI estimation in maize and soybean: Satellite observations. Remote Sens. 2017, 9, 318. [CrossRef]
- Kattenborn, T.; Fassnacht, F.E.; Schmidtlein, S. Differentiating plant functional types using reflectance: Which traits make the difference? Remote. Sens. Ecol. Conserv. 2019, 5, 5-19.



| Crop growth stage |
Field samples considered for calibration | Field samples considered for validation |
|---|---|---|
| Seedling | 14 | 12 |
| Tillering | 19 | 12 |
| Elongation | 9 | 7 |
| Booting | 11 | 7 |
| Heading | 10 | 6 |
| Flowering | 8 | 5 |
| Maturity | 7 | 5 |
| Total | 78 | 54 |
| Spectral Vegetation Index | Formula | Reference |
|---|---|---|
| VOG 1 | [30] | |
| MTCI | [31] | |
| VOG 2 | [30] | |
| MSR | [32] | |
| mND705 | [33] | |
| DD | [34] | |
| GNDVI | [35] | |
| OSAVI | [36] | |
| RDVI | [17] | |
| SR | [37] | |
| MTVI 2 | [13] | |
| SAVI | [15] | |
| NDVI | [38] | |
| RVI | [39] | |
| EVI 1 | [40] | |
| DVI | [39] | |
| PRI | [41] | |
| TVI | [16] | |
| DDn | [42] | |
| MSR705 | [33] | |
| MNLI | [43] | |
| SIPI | [44] | |
| WI | [45] | |
| RVSI | [46] | |
| Slaidi | where S=5 | [47] |
| NDWI | [48] | |
| NDII | [49] | |
| REP | [50] | |
| WDRVI | [51] |
| Crop growth stage | Number of fields surveyed | LAI | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | P value | CV | ||
| Seedling | 14 | 1.21 ± 0.45 | 0.54 | 2.20 | 0.59 | 37.50 |
| Tillering | 19 | 1.71 ± 0.54 | 0.75 | 2.60 | 0.68 | 31.66 |
| Elongation | 9 | 2.41 ± 0.53 | 1.60 | 3.00 | 0.12 | 21.79 |
| Booting | 11 | 3.00 ± 0.82 | 1.70 | 4.00 | 0.04 | 27.24 |
| Heading | 10 | 3.61 ± 0.47 | 3.10 | 4.30 | 0.15 | 13.00 |
| Flowering | 8 | 3.61 ± 0.45 | 2.80 | 4.10 | 0.42 | 12.49 |
| Maturity | 7 | 2.11 ± 0.35 | 1.60 | 2.50 | 0.38 | 16.48 |
| VI | Seedling | Tillering | Elongation | Booting | Heading | Flowering | Maturity |
|---|---|---|---|---|---|---|---|
| WI | 1.80 ± 0.93 | 1.63 ± 0.69 | 1.24 ± 0.19 | 1.22 ± 0.18 | 1.18 ± 0.02 | 1.13 ± 0.03 | 1.07 ± 0.07 |
| NDWI | 0.26 ± 0.28 | 0.26 ± 0.20 | 0.14 ± 0.11 | 0.14 ± 0.08 | 0.15 ± 0.01 | 0.11 ± 0.03 | 0.04 ± 0.07 |
| NDII | 0.56 ± 0.20 | 0.54 ± 0.17 | 0.43 ± 0.11 | 0.44 ± 0.06 | 0.51 ± 0.01 | 0.46 ± 0.04 | 0.29 ± 0.14 |
| SLAIDI | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| NDVI | 0.54 ± 0.14 | 0.64 ± 0.08 | 0.80 ± 0.08 | 0.82 ± 0.08 | 0.91 ± 0.02 | 0.84 ± 0.03 | 0.53 ± 0.21 |
| OSAVI | 0.38 ± 0.10 | 0.44 ± 0.07 | 0.58 ± 0.07 | 0.62 ± 0.08 | 0.74 ± 0.04 | 0.67 ± 0.04 | 0.43 ± 0.15 |
| GNDVI | 0.41 ± 0.09 | 0.49 ± 0.07 | 0.64 ± 0.08 | 0.67 ± 0.09 | 0.78 ± 0.02 | 0.71 ± 0.03 | 0.53 ± 0.11 |
| RVSI | -0.01 ± 0.00 | -0.01 ± 0.00 | -0.01 ± 0.00 | -0.01 ± 0.01 | 0.01 ± 0.00 | -0.00 ± 0.00 | -0.01 ± 0.00 |
| REP | 735.60 ± 64.56 | 728.59 ± 8.77 | 725.35 ± 1.64 | 725.43 ± 2.45 | 729.34 ± 0.54 | 727.22 ± 1.09 | 742.50 ± 16.60 |
| SR | 3.78 ± 1.43 | 4.89 ± 1.31 | 10.64 ± 4.44 | 12.01 ± 4.70 | 20.79 ± 3.97 | 11.82 ± 2.88 | 4.28 ± 2.68 |
| RDVI | 0.27 ± 0.07 | 0.31 ± 0.05 | 0.42 ± 0.06 | 0.45 ± 0.06 | 0.56 ± 0.04 | 0.50 ± 0.03 | 0.33 ± 0.11 |
| SAVI | 0.82 ± 0.21 | 0.97 ± 0.13 | 1.20 ± 0.12 | 1.23 ± 0.12 | 1.36 ± 0.03 | 1.26 ± 0.05 | 0.80 ± 0.32 |
| MSR | 0.91 ± 0.37 | 1.19 ± 0.30 | 2.19 ± 0.70 | 2.40 ± 0.70 | 3.54 ± 0.43 | 2.42 ± 0.41 | 0.98 ± 0.65 |
| TVI | 7.15 ± 2.86 | 8.00 ± 2.18 | 12.01 ± 2.47 | 13.69 ± 2.33 | 17.95 ± 2.07 | 15.94 ± 1.54 | 9.75 ± 4.18 |
| MNLI | -0.41 ± 0.05 | -0.40 ± 0.05 | -0.47 ± 0.05 | -0.50 ± 0.04 | -0.60 ± 0.04 | -0.57 ± 0.03 | -0.51 ± 0.04 |
| MTCI | 0.58 ± 0.32 | 1.27 ± 0.49 | 2.80 ± 0.84 | 3.32 ± 1.33 | 6.26 ± 0.54 | 3.80 ± 0.83 | 1.69 ± 0.76 |
| MTVI2 | 0.17 ± 0.06 | 0.20 ± 0.05 | 0.30 ± 0.06 | 0.34 ± 0.06 | 0.47 ± 0.06 | 0.40 ± 0.04 | 0.21 ± 0.11 |
| PLS | 1.40 ± 0.42 | 1.53 ± 0.33 | 2.07 ± 0.49 | 2.41 ± 0.38 | 3.26 ± 0.31 | 2.74 ± 0.37 | 1.99 ± 0.46 |
| RVI | 3.83 ± 1.49 | 4.84 ± 1.22 | 10.83 ± 4.54 | 12.71 ± 5.72 | 21.75 ± 3.91 | 11.95 ± 2.90 | 4.30 ± 2.73 |
| DVI | 0.14 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.05 | 0.25 ± 0.04 | 0.35 ± 0.04 | 0.31 ± 0.03 | 0.21 ± 0.06 |
| PRI | 0.13 ± 0.03 | 0.09 ± 0.02 | 0.05 ± 0.03 | 0.04 ± 0.03 | 0.00 ± 0.01 | 0.04 ± 0.02 | 0.09 ± 0.02 |
| WDRVI | 0.50 ± 0.19 | 0.64 ± 0.14 | 0.98 ± 0.20 | 1.03 ± 0.20 | 1.27 ± 0.06 | 1.06 ± 0.10 | 0.51 ± 0.31 |
| VOG1 | 1.06 ± 0.09 | 1.19 ± 0.12 | 1.53 ± 0.19 | 1.62 ± 0.25 | 2.11 ± 0.09 | 1.72 ± 0.15 | 1.26 ± 0.19 |
| mND705 | 0.16 ± 0.10 | 0.31 ± 0.10 | 0.52 ± 0.09 | 0.56 ± 0.11 | 0.74 ± 0.02 | 0.60 ± 0.07 | 0.29 ± 0.16 |
| MSR705 | 0.71 ± 0.09 | 0.74 ± 0.05 | 0.86 ± 0.05 | 0.86 ± 0.06 | 0.91 ± 0.01 | 0.88 ± 0.02 | 0.79 ± 0.10 |
| SIPI | 1.27 ± 0.21 | 1.13 ± 0.08 | 1.05 ± 0.03 | 1.04 ± 0.05 | 1.01 ± 0.01 | 1.04 ± 0.02 | 1.41 ± 0.30 |
| DDN | -0.20 ± 0.04 | -0.21 ± 0.05 | -0.32 ± 0.07 | -0.37 ± 0.07 | -0.54 ± 0.06 | -0.46 ± 0.05 | -0.41 ± 0.02 |
| DD | -0.05 ± 0.01 | -0.01 ± 0.02 | 0.04 ± 0.03 | 0.06 ± 0.04 | 0.13 ± 0.02 | 0.08 ± 0.02 | 0.00 ± 0.04 |
| VOG2 | -0.02 ± 0.01 | -0.04 ± 0.02 | -0.11 ± 0.04 | -0.13 ± 0.07 | -0.28 ± 0.03 | -0.16 ± 0.04 | -0.05 ± 0.04 |
| VI | Seedling | VI | Tillering | VI | Elongation | VI | Booting | VI | Heading | VI | Flowering | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||||||
| SR | 0.66*** | 0.28 | VOG2 | 0.52*** | 0.38 | REP | 0.74** | 0.29 | WDRVI | 0.67** | 0.49 | NDII | 0.10ns | 0.47 | PRI | 0.44ns | 0.37 |
| MSR | 0.65*** | 0.28 | PRI | 0.47** | 0.41 | MSR705 | 0.70** | 0.31 | NDVI | 0.65** | 0.52 | WI | 0.08ns | 0.48 | REP | 0.36ns | 0.39 |
| RVI | 0.65*** | 0.28 | mND705 | 0.44** | 0.42 | NDVI | 0.69** | 0.31 | SAVI | 0.65** | 0.51 | SIPI | 0.08ns | 0.48 | mND705 | 0.36ns | 0.39 |
| WDRVI | 0.64*** | 0.28 | NDVI | 0.42** | 0.42 | SAVI | 0.69** | 0.31 | MSR | 0.64** | 0.52 | MSR705 | 0.07ns | 0.48 | SIPI | 0.35ns | 0.39 |
| SAVI | 0.60** | 0.29 | SAVI | 0.42** | 0.42 | WDRVI | 0.68** | 0.32 | SIPI | 0.62** | 0.53 | RVSI | 0.05ns | 0.48 | MTCI | 0.33ns | 0.40 |
| NDVI | 0.59** | 0.30 | WDRVI | 0.42** | 0.42 | MSR | 0.66** | 0.33 | MSR705 | 0.60** | 0.55 | REP | 0.03ns | 0.49 | VOG2 | 0.32ns | 0.40 |
| RVSI | 0.59** | 0.30 | MSR | 0.41** | 0.42 | SR | 0.65** | 0.33 | SR | 0.59** | 0.55 | SR | 0.02ns | 0.49 | RVSI | 0.26ns | 0.42 |
| OSAVI | 0.57** | 0.31 | RVI | 0.41** | 0.43 | SIPI | 0.64* | 0.34 | mND705 | 0.59** | 0.55 | MSR | 0.01ns | 0.50 | VOG | 0.25ns | 0.42 |
| RDVI | 0.56** | 0.31 | SIPI | 0.41** | 0.43 | RVI | 0.60* | 0.36 | OSAVI | 0.58** | 0.56 | TVI | 0.01ns | 0.50 | NDVI | 0.24ns | 0.42 |
| GNDVI | 0.55** | 0.32 | SR | 0.40** | 0.43 | GNDVI | 0.58* | 0.37 | GNDVI | 0.54* | 0.58 | MNLI | 0.01ns | 0.50 | SR | 0.24ns | 0.42 |
| MTVI2 | 0.55** | 0.32 | VOG | 0.40** | 0.43 | mND705 | 0.57* | 0.37 | RDVI | 0.54* | 0.58 | PLS | 0.01ns | 0.50 | SAVI | 0.24ns | 0.42 |
| TVI | 0.53** | 0.32 | MTCI | 0.38** | 0.44 | PRI | 0.55* | 0.38 | MTVI2 | 0.53* | 0.59 | RVI | 0.01ns | 0.49 | MSR | 0.24ns | 0.42 |
| mND705 | 0.52** | 0.32 | GNDVI | 0.32* | 0.46 | VOG | 0.54* | 0.38 | RVI | 0.53* | 0.59 | DVI | 0.01ns | 0.50 | WDRVI | 0.24ns | 0.42 |
| PLS | 0.48** | 0.34 | DD | 0.30* | 0.46 | MTCI | 0.51* | 0.39 | DD | 0.52* | 0.60 | mND705 | 0.01ns | 0.50 | DD | 0.24ns | 0.43 |
| Crop growth stage | Number of fields | LAI | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | P value | CV (%) | ||
| Seedling | 12 | 0.99 ± 0.23 | 0.60 | 1.31 | 0.33 | 23.51 |
| Tillering | 12 | 1.78 ± 0.40 | 1.20 | 2.42 | 0.42 | 22.41 |
| Elongation | 7 | 2.89 ± 0.40 | 2.50 | 3.60 | 0.29 | 13.80 |
| Booting | 7 | 3.29 ± 0.58 | 2.50 | 4.10 | 0.88 | 17.70 |
| Heading | 6 | 3.63 ± 0.35 | 3.20 | 4.20 | 0.92 | 9.64 |
| Flowering | 5 | 3.79 ± 0.57 | 2.90 | 4.50 | 0.42 | 15.11 |
| Maturity | 5 | 2.24 ± 0.68 | 1.50 | 3.10 | 0.53 | 30.38 |
| VI | Seedling | Tillering | Elongation | Booting | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| mND705 | 0.52** | 0.17 | 0.42* | 0.32 | 0.88** | 0.15 | 0.51ns | 0.45 |
| SR | 0.50** | 0.17 | 0.40* | 0.32 | 0.66* | 0.26 | 0.10ns | 0.60 |
| MSR | 0.51** | 0.17 | 0.48* | 0.30 | 0.67* | 0.25 | 0.42ns | 0.49 |
| RVI | 0.51** | 0.17 | 0.38* | 0.33 | 0.71* | 0.24 | 0.10ns | 0.60 |
| WDRVI | 0.47* | 0.18 | 0.37* | 0.33 | 0.76* | 0.22 | 0.62* | 0.39 |
| SAVI | 0.49* | 0.17 | 0.56** | 0.28 | 0.63* | 0.27 | 0.59* | 0.41 |
| NDVI | 0.45* | 0.18 | 0.34* | 0.34 | 0.73* | 0.23 | 0.54ns | 0.43 |
| GNDVI | 0.49* | 0.17 | 0.45* | 0.31 | 0.83** | 0.18 | 0.50ns | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





