1. Introduction
Multiple Sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the CNS, characterized by inflammatory demyelination, axonal damage and neuron loss [
1]. In 2020, an estimated 2.8 million people lived with MS worldwide, over 400,000 in the U.S. alone. Approximately 75% of people affected were women. MS prevalence has increased in every world region since 2013 [
2], the reasons for which are not fully understood.
MS lesions can appear throughout the CNS, which is why this demyelinating disease presents with a range of syndromes, including limb weakness and sensory loss, ataxia caused by cerebellar lesions, monocular vision loss due to optic neuritis, and/or double vision caused by brain-stem dysfunction [
3]. While approximately 10-15% of patients show a progressive clinical course of the disease from the onset, approximately 85% of patients show the relapsing-remitting form of MS (RRMS) but secondary progressive MS (SPMS) typically develops in many of these patients after 10 to 20 years, ultimately resulting in impaired mobility and cognition [
4]. Patients with MS, on average, have shortened life expectancies by 7–14 years [
5,
6].
A growing number of disease-modifying therapies are available that can reduce morbidity, especially when started early in the course of the disease [
7]. However, the etiology of MS remains incompletely understood and to this day there is no cure for MS or effective treatments for progressive forms of the disease. Thus, there is a compelling need for novel therapeutics that target mechanisms underlying progressive MS, the neurodegenerative components of this disease as well as CNS repair mechanisms [
1]
Of the three established animal models used by the scientific community, to study MS, a) viral models, including Theiler’s murine encephalomyelitis (TMEV), b) disease models that are induced by toxic chemicals such as cuprizone, and c) experimental autoimmune encephalomyelitis (EAE) and variations thereof [
8,
9]. All these models mimic similar features of MS, but the underlying molecular mechanism and pathological features vary dramatically. The most common animal model used is the EAE model, which is particularly useful for the study of neuroinflammatory pathways and is commonly as a “proof-of-principle”model to test novel drugs and treatments [
10].
23-Hydroxy ursolic acid (23-OH UA) is a naturally occurring triterpenoid found in the leaves of
Lagerstroemia speciosa or giant crepe-myrtle native to South East Asia, and leaves and twigs of
Juglans sinensis, a walnut tree found in East Asia [
11]. 23-OH UA is structurally closely related to ursolic acid (UA), a well-studied natural compound with numerous purported health benefits found in herbs and the peels of fruit. In an established mouse model of human atherosclerosis, we showed that both dietary 23-OH UA and UA have potent atheroprotective and anti-obesogenic activity in mice and protect blood monocytes against nutrient-stress-induced dysfunction [
12]. Despite similar mechanisms of action, dietary 23-OH UA is significantly more effective in preventing atherosclerotic lesion formation than UA. In a recent follow-up study using a mouse model of diet-induced obesity, we confirmed the potent anti-obesogenic properties of 23-OH UA and demonstrated that 23-OH UA also improves glucose tolerance, prevents hyperleptinemia, restores blood monocyte function, and suppresses the recruitment of monocyte-derived macrophages into adipose tissues in these mice. The mechanisms underlying the benefits of 23-OH UA appear to involve the conversion or “reprogramming” of macrophages into a unique transcriptionally hyperactive phenotype. Based on targeted gene profiling data, this macrophage phenotype appears to be highly resistant to oxidative stress, equipped with high metabolic and fuel flexibility and have potent anti-inflammatory activity [
12]. Inflammatory macrophages, dendritic cells, and microglia critically contribute to the induction and propagation of neuroinflammation. Therefore, we examined in this study whether dietary supplementation with 23-OH UA exerts similar anti-inflammatory effects on CNS innate immune cells and whether 23-OH UA protects mice against experimental autoimmune encephalomyelitis (EAE) in a mouse model of human MS [
10].
2. Materials and Methods
2.1. Animals
Female C57BL/6J (stock number 000664) were obtained from Jackson Laboratory. All mice were maintained in colony cages on a 12 h light/12 h dark cycle and fed a normal mouse laboratory diet unless otherwise stated. Twenty 6-week-old female C57BL/6 mice were randomized into two groups of ten mice. One group was fed a defined low-calorie maintenance diet (MD, AIN-93G, BioServ) the other an MD supplemented by BioServ with 0.2 % wgt/wgt 23-OH UA for 5 weeks prior to immunization and throughout the 30 days postimmunization period. 23-OH UA was custom-synthesized by WuXi App Teck (China) from Asiatic acid according to a 6-step strategy developed by Bore et al. [
11]. The structure was confirmed by MS and NMR. The purity of the final product was determined by HPLC and LCMS and was > 99%. The pure compound was sent to Bioserv, where it was added to the AIN-93G powder prior to generating pellets. Diets and water were provided ad lib. All experiments were conducted in accordance with animal protocols approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee.
2.2. EAE Induction
After 5 weeks (day 0) on MD or MD supplemented with 23-OH UA, EAE was actively induced by subcutaneous injection with 100 mg of mouse MOG
35-55 emulsified in 50 µL of complete Freund’s adjuvant (CFA) containing 5 mg/ml
Mycobacterium tuberculosis H37Ra, as described previously [
13]. 200 ng of Bordetella pertussis toxin was administered intraperitoneally (i.p.) on day 0 and day 2 after immunization. Mice were weighed and clinically scored daily for 30 days. On day 30, splenocytes were isolated and plated on ELISPOT plates pre-coated with anti-IL-17 or anti-IFN-g antibodies, restimulated with MOG
35-55 and subsequently stained and analyzed using a Series 2 ImmunoSpot analyzer.
2.3. EAE Disease and Ataxia Scoring
EAE disease was scored as previously described on a scale of 0-5 (0: no clinical disease; 1: flaccid tail; 2: partial hind limb paralysis; 3: total hind limb paralysis; 4: front and hind limb paralysis; 5: moribund or dead) [
13]. Ataxia was assessed and scored as described on a scale of 0 – 3 using the following four tests: ledge test (0: balanced and graceful; 1: cannot use hind legs well, slipping but balanced; 2: legs shaking significantly, not graceful), hindlimb clasping (0: hind legs move outward; 1: 50% of the 10 s one hind limb is moved inward; 2: 50% of the 10 s both hind limbs are moved inward; 3: >50% of the 10 s both hind limbs are near the abdomen), gait (0: normal movement; 1: slight tremor or waddle during movement; 2: hind legs are shaking during movement, severe tremor; 3: hind legs are dragging and abdomen near the floor); kyphosis (0: flat back; 1: initial curvature of back but able to flatten while moving; 2: consistent mild curvature of the back; 3: consistent mild curvature of the back) [
13]. The ataxia score was calculated as the sum of scores from all four tests. Four mice died in the control group and three in the group of mice fed a 23-OH UA-supplemented diet. All mice died between day 13 and 16. To prevent data bias, dead mice were assigned the maximum score in each category and a 30% weight loss for the remaining days of the study.
2.4. Cytokine ELISPOT Assay
Cytokine ELISPOT assay was performed and spots analyzed as described previously (18, 19). In brief, ELISPOT plates (Millipore, Billerica, MA, USA) were precoated with anti-mouse-IFN-γ mAb (eBioscience, AN-18) and anti-mouse-IL-17 mAb (Bio X Cell; 17F3). Splenocytes (5 × 105 cells/well) were restimulated with MOG35–55 peptide in HL-1 medium (Lonza) at 37°C, 5% CO2 for 24 h. Biotinylated anti-mouse-IFN-γ mAb (eBioscience; R4-6A2) and anti-mouse-IL-17 mAb (BioLegend, TC11-8H4) were then added overnight at 4°C, followed by incubation with streptavidin alkaline phosphatase (Invitrogen, Waltham, MA, USA) for 2 h at room temperature and developing with BCIP/NBT Phosphatase Substrate (KPL, Gaithersburg, MD, USA). After plate developing, image analysis of spots was performed on a Series 2 ImmunoSpot analyzer (Cellular Technology Limited, Cleveland, OH, USA). Results for antigen-specific spot-forming cells were normalized with a negative control containing peptide-free media. All measurements were performed in triplicate.
2.5. Liquid Chromatography-Mass Spectrometry
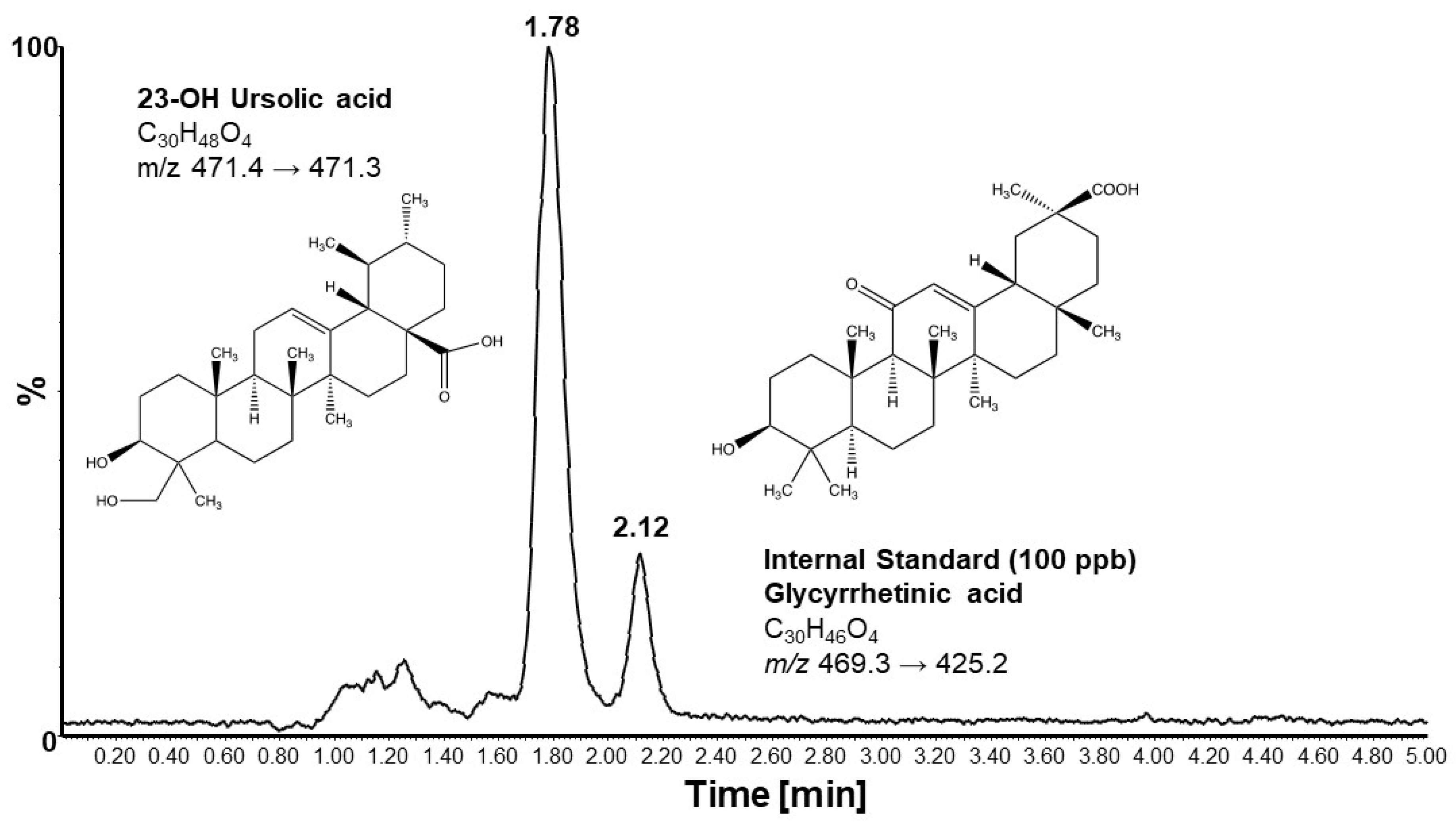
23-OH UA and glycyrrhetinic acid (GA, the internal standard) stock solutions were prepared in methanol to give a final concentration of 1.0 mg/ml. From these, 2 ppm working stock solutions were prepared by dilution in methanol. The GA working solution (10 ppm) was prepared in methanol from the 1.0 mg/mL stock solution. All solutions were kept at 4 °C and brought to room temperature prior to use.
The volume of commercial plasma (Sigma P9275) required for the analysis was spiked with GA to a final concentration of 100 ppb. Calibration standard solutions with concentrations of 0, 10, 20, 50, 100, 200, 350 and 500 ppm were prepared by spiking the required volume of the 2 ppm 23-OH UA stock solution into 50 ml volumes of the plasma containing 100 ppb GA. For samples, 50 ml of plasma was mixed with 50 ml of plasma containing 100 ppb GA. To all calibration standards and samples, 0.5 ml of ethyl acetate was added, vortexed for approximately 10 s, allowed to sit for 5 min, and vortexed again for 10 s. This was followed by centrifugation for 10 min at 12, 000 rpm. 400 ml of the ethyl acetate layer was carefully pipetted into clean microcentrifuge tubes and evaporated to dryness in a Speedvac. Calibration standards and samples were reconstituted in 50 ml of methanol and transferred into injection vials for LC-MS/MS analysis.
Chromatographic separations and mass spectrometry analyses were performed on an Acquity UPLC system directly coupled to an Acquity triple quadrupole detector (Waters Corp., Milford, MA, USA). For chromatography, isocratic elution was performed using a mixture of 80% acetonitrile and 20% water containing 10 mM ammonium formate. Separation was achieved at a constant flow rate of 0.15 mL/min using a BDS Hypersil C18 column (50 x 2.1 mm, 5 μm particle size) from Thermo Electron Corporation with a constant column temperature of 40 °C. For each analysis, 5 μl of sample was injected onto the column. Mass spectrometry analysis was conducted in negative ionization mode using multiple reaction monitoring (MRM) with capillary, cone and extraction potentials maintained at 1.5 kV, 80 V, and 3 V, respectively. The source block temperature was 110 °C and the desolvation temperature was 350 °C with a desolvation gas flow of 600 l/h. The cone gas flow rate was 10 l/h. Quantitation of 23-OH UA was conducted in multiple reaction monitoring (MRM) mode using the transition m/z 471.40/471.30 to monitor the [M–H+]–. The transition m/z 469.3/469.2 was used to monitor the internal standard, GA. Collision energy (CE) was set at 20 eV. Analyses of standards and samples were done in triplicate and peak areas used for quantitation. MassLynx v.4.1 software was used for data acquisition and analysis (Waters Corp., Milford, MA, USA).
2.6. Statistical Analyses
Mean values between the two experimental groups were compared by unpaired two-tailed Student t-test (SigmaPlot 15). Normality was tested with the Shapiro-Wilk test. Disease onset was determined for both EAE disease score and the combined Ataxia scores using one-way ANOVA on repeated measures and the Holm-Sidak test to determine on which day mean scores were significantly elevated (P > 0.05), setting values at day 0 as the control group. Unless stated otherwise, data are expressed as mean ± standard error of the mean (SEM). P<0.05 was set as the statistical significance level.
3. Results
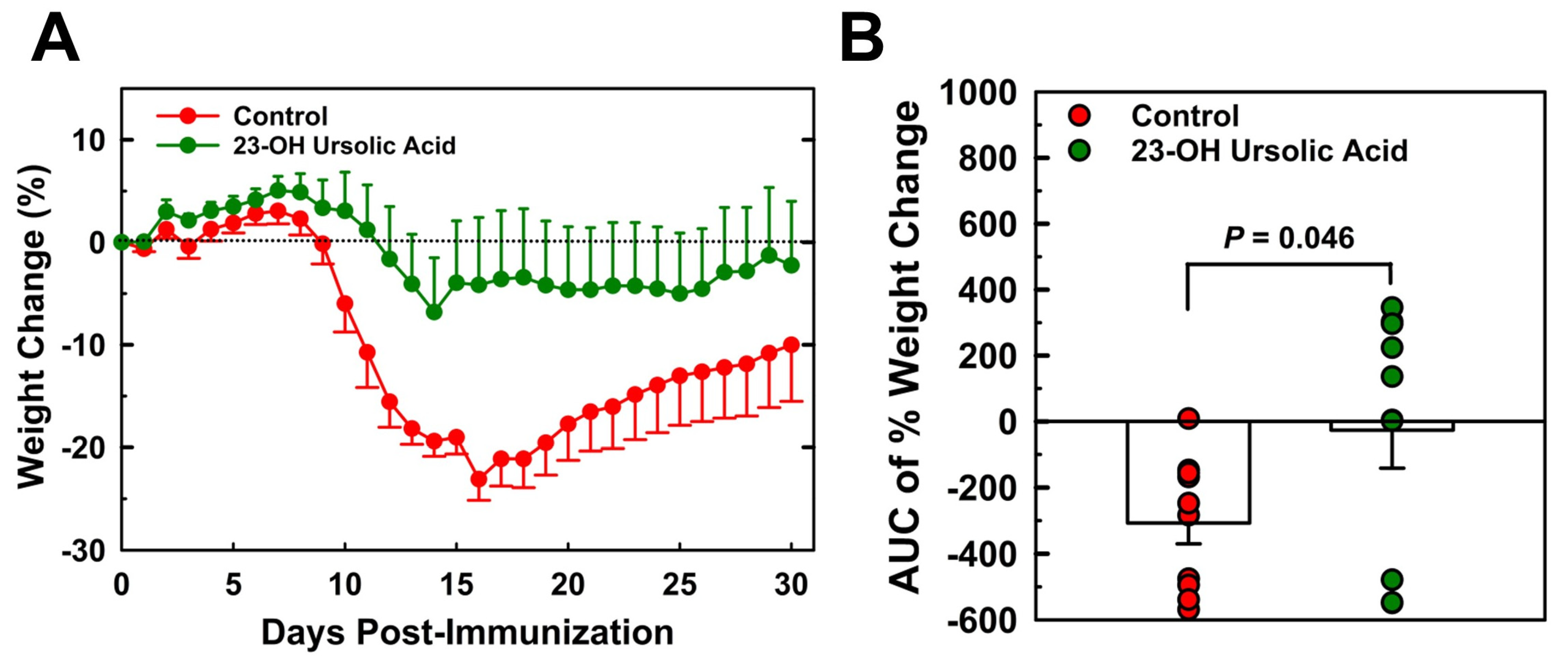
3.1. Dietary 23-OH UA prevents weight loss associated with EAE
Mice maintained on an MD and immunized with MOG
35-55 began losing weight on day 9 post-immunization (
Figure 1A). Weight loss peaked on day 16, on average at 23% of original weight before slowly recovering. By day 30, the mean body weight was still 10% below the original weight. Mice that received 23-OH UA in their diets on average were able to maintain their body weight despite the immunization (
Figure 1B). Plasma levels of 23-OH UA were assessed by LCMS (
Figure 2). The mean plasma concentration of 23-OH UA reached 7.4 ± 4.7 mM by day 30. As expected, no 23-OH UA was detected in the plasma samples from mice that received MD without 23-OH UA supplementation.
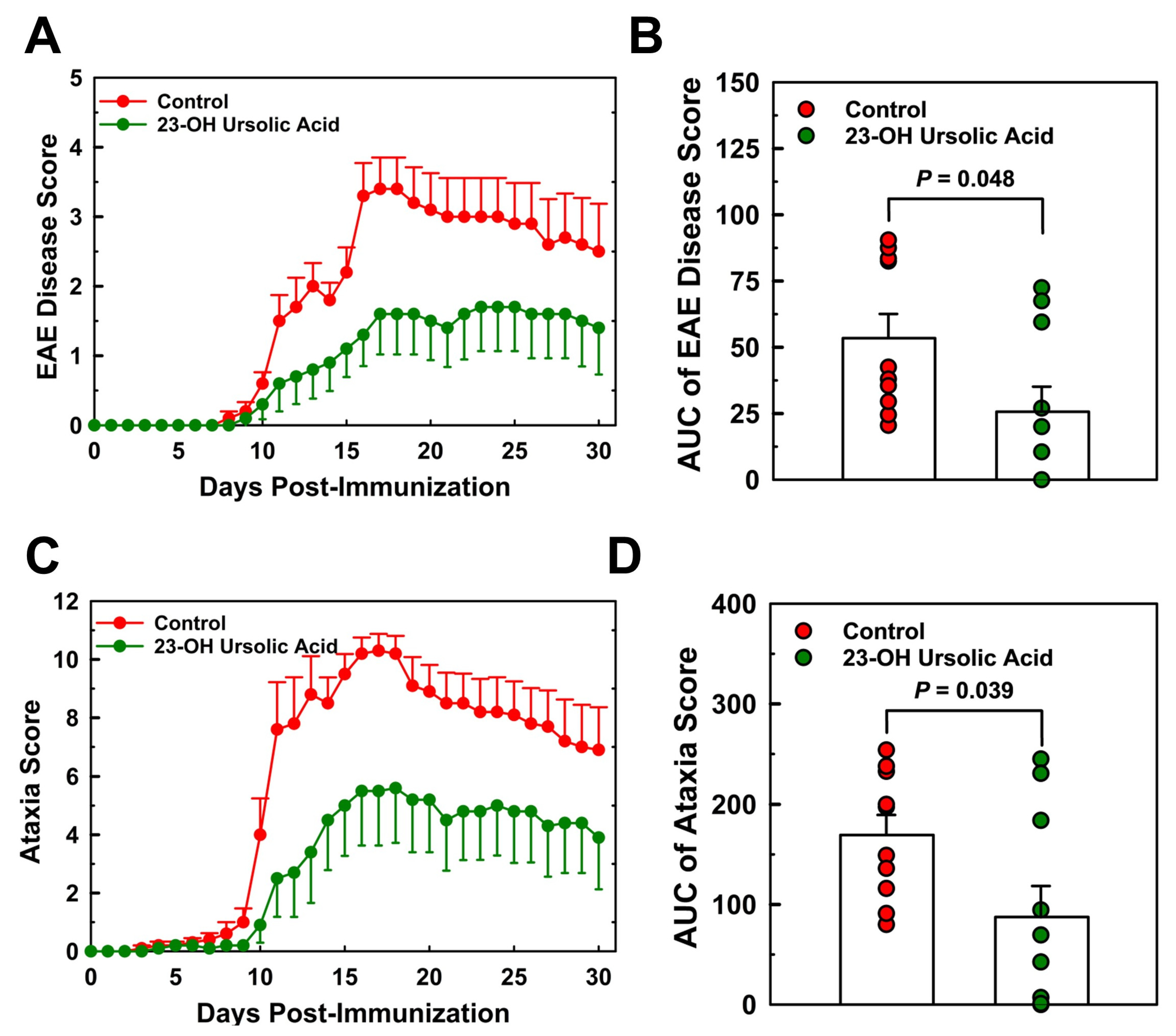
3.2. Dietary 23-OH UA reduces EAE disease and ataxia severity
MOG
35-55 immunized EAE mice maintained on an MD began showing signs of symptoms around day 8 post-immunization (
Figure 3A). Disease severity increased rapidly thereafter and peaked on day 17 at a mean score of 3.4. By day 30, the mean EAE disease score had decreased to 2.6. The rapid increase in EAE disease score was mirrored by the combined ataxia score, although the onset of visual physical impairment based on the criteria for the ataxia score was already observed around day 8 post-immunization (
Figure 3C).
Ataxia scores for the MD-fed control mice peaked at day 17 at a mean score of 10.3 and decreased to 7.0 at day 30. Feeding mice 23-OH UA protected mice from EAE and reduced EAE disease severity by 52% (
Figure 3B) and dramatically improved the overall ataxia score by 48% (
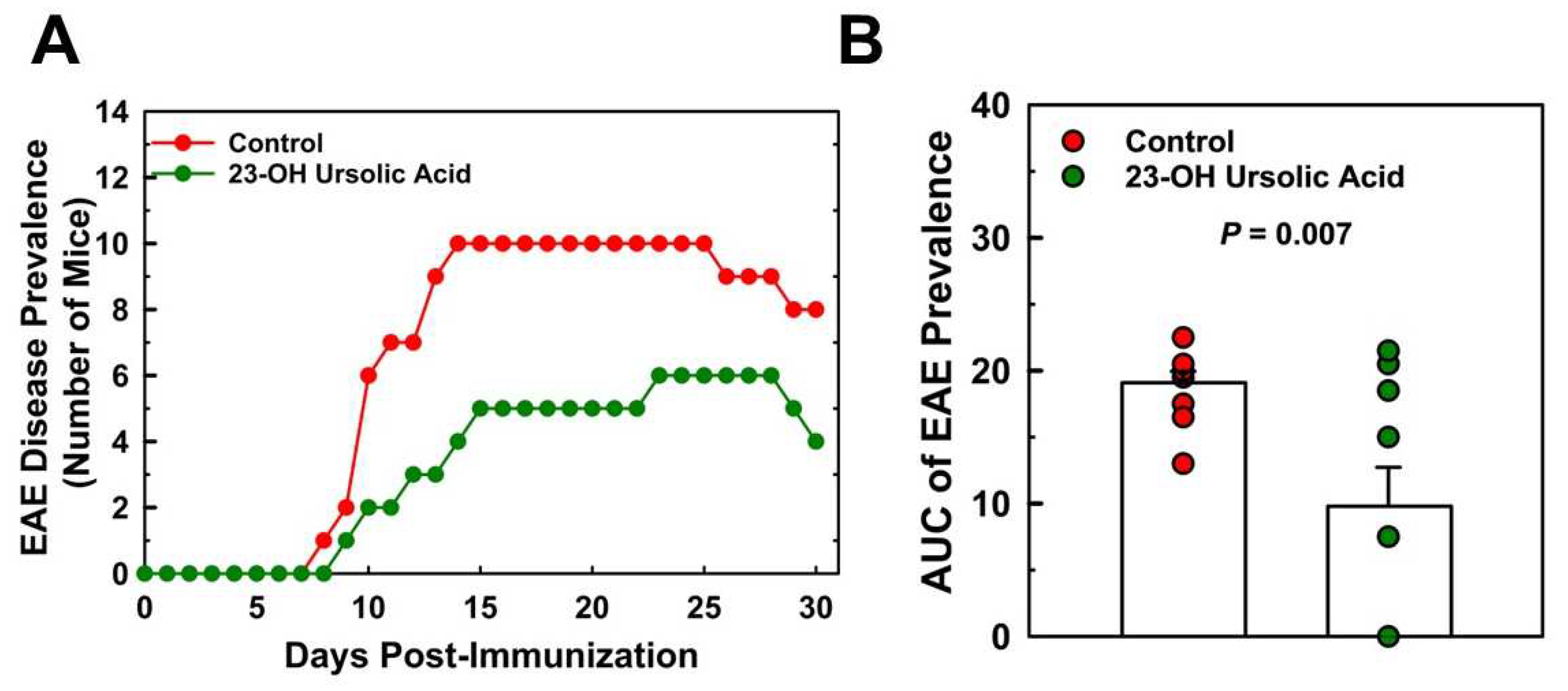
Figure 3D). Prevalence of EAE was reduced in 23-OH UA fed mice, in line with the effect of 23-OH UA on disease severity (
Figure 4A). The overall reduction in prevalence was 49% (
Figure 4B). Of note, dietary 23-OH UA also delayed disease onset (
Figure 3A+C). Initial increases in EAE disease scores were delayed by 5 days, from day 11 to day 16 post-immunization, whereas the first noted changes in ataxia were delayed by four days, from day 10 to day 14 post-immunization, While the reason for this delay is unclear at this time, one possibility is that 23-OH UA feeding may have affected immunization of the mice.
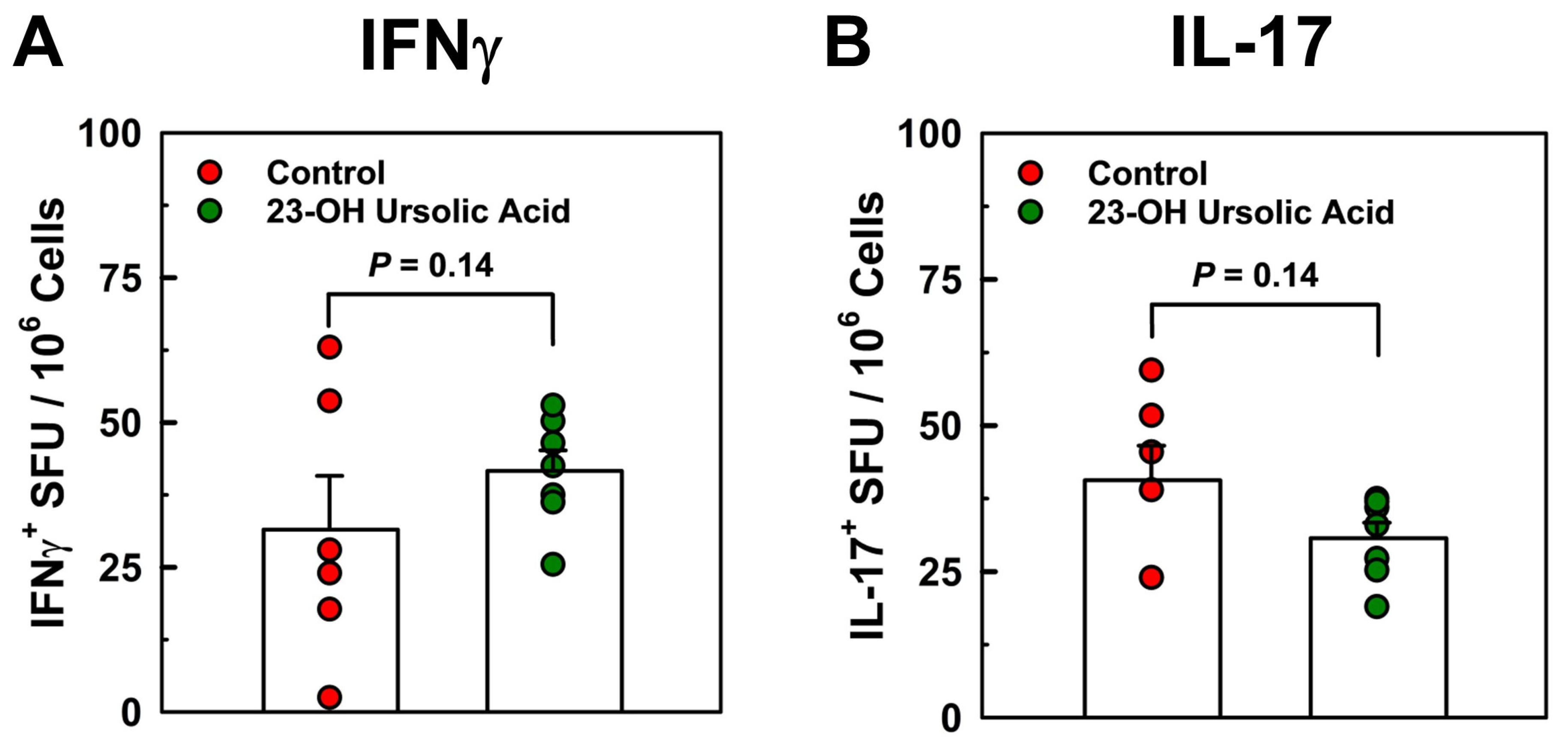
3.3. Dietary 23-OH UA does not affect peripheral neuroantigen-specific T cell responses
To address potential mechanisms underlying the potent protective effects of dietary 23-OH UA, we analyzed T cell responses in splenocytes in recall experiments with MOG
35-55 peptide by cytokine ELISPOT assays. We observed no effect of dietary 23-OH UA on Th17 or Th1 antigen-specific T cell frequency in splenocytes isolated on day 30 post-immunization and recalled with MOG
35-55 peptide (
Figure 5), suggesting that 23-OH UA does not appear to target peripheral T-cell responses.
4. Discussion
Here we demonstrate that dietary supplementation with 23-OH UA potently protects mice against EAE in a preclinical model of human MS [
10]. Ataxia and EAE disease severity and incidence were suppressed approximately 50%, and EAE-associated weight loss was prevented. Surprisingly, dietary 23-OH UA did not appear to affect peripheral T-cell responses in EAE mice, generally considered a myelin-specific T cell-mediated neuroinflammatory autoimmune disease [
14,
15,
16]. Conceivably, the supplement may exert its effects on autoreactive T cells within the CNS, or more likely, modulate the pathogenic functions of other cell types in the CNS. We reported previously that dietary 23-OH UA protects mice against diet-induced obesity adipose tissue inflammation [
17]. Of note, we monitored the mice for signs of toxicity of the supplement. We found no increase in liver toxicity as plasma AST and ALT activity levels remained unchanged [
17]. We also found no changes in blood cell counts or any other adverse effects of 23-OH UA in either of our two published studies on 23-OH UA [
12,
17]. We went on to demonstrate that 23-OH UA exerts these anti-obesogenic properties through multiple mechanisms, which include improving glucose tolerance, reversing hyperleptinemia and by protecting blood monocytes against nutrient stress-induced dysfunction [
17]. By maintaining monocyte function in high-fat diet fed mice, 23-OH UA reduced the recruitment of monocyte-derived macrophage into the adipose tissue, thereby limiting adipose tissue inflammation. It is possible that dietary 23-OH UA has a similar effect in EAE mice, limiting the recruitment of monocyte-derived macrophages to sites of inflammation within the brain and restricting their disease contribution. MDM are considered the primary cell type involved in demyelination during EAE [
18,
19,
20], suggesting that 23-OH UA’s inhibitory effects on MDM recruitment may both dampen neuroinflammation and prevent demyelination at sites of MDM accumulation.
A second mechanism underlying the anti-obesogenic and anti-inflammatory properties of 23-OH UA involves the reprogramming of macrophages by 23-OH UA into a transcriptionally hyperactive phenotype with anti-inflammatory and potentially inflammation resolving properties [
17]. Bone marrow-derived macrophages (BMDM) isolated from mice fed a high calorie diet (HCD) supplemented with 23-OH UA showed a 39-fold increase in IL-10 mRNA expression. In addition to IL-10, we observed an increase in Nrf2 and the Nrf2-dependent genes, including Gclc, Gclm, Gr, Grx1, Grx2, Gsta3 involved in glutathione synthesis and homeostasis as well as the antioxidant enzymes Cat, Gpx1, Gpx4, gpx7 and Prdx1, 3, 4, 4 and 6, suggesting this macrophage phenotype is well-protected against oxidative stress and may act as a sink for ROS and RNS in the microenvironment of sites of inflammation, reducing oxidative damage.
Even more interesting was the finding that PPARg-regulated genes were upregulated in these reprogramed BMDM, including CD36, LPL, FABP4 and GLUT4 [
17]. Zhang and colleagues reported that ursolic acid, a structural analogue to 23-OH UA, also reduces the severity of EAE by acting as a PPARg agonist and upregulating promyelinating factor CNTF in astrocytes and promoting oligodendrocyte maturation and remyelination [
21]. In a mouse model of human atherogenesis, we showed that HCD-supplementation with either ursolic acid or 23-OH UA inhibited dyslipidemia-induced monocyte priming and dysfunction, reduced weight gain and protected these mice against atherosclerosis [
12]. 23-OH UA, however, was significantly more potent than ursolic acid, possibly due to 23-OH UA’s greater predicted solubility. In this mouse model of atherosclerosis both compounds appear to act through the same mechanisms, i.e. by preventing oxidation and the subsequent inactivation and degradation of MKP-1 [
22,
23], a master regulator of monocyte and macrophage function. Based on the molecular docking model provided by Zhang et al. showing ursolic acid binding to PPARg [
21], 23-OH UA should not only fit into the same binding pocket but would benefit from an additional hydrogen bridge with Glu291, suggesting that the protective properties of 23-OH UA in EAE, like ursolic acid, may also be mediated by PPARg. The induction of PPARg-dependent genes in BMDM isolated from C57BL/6 mice maintained on a HFD supplemented with 23-OH UA supports this hypothesis [
17].
In summary, we provide evidence in a preclinical animal model of MS that 23-OH UA could be an effective oral supplement well-suited as an adjunct therapy for reducing development of acute episodes of relapsing-remitting MS and potentially slowing disease progression. Nevertheless, further studies are needed to determine the safety and efficacy of the supplement in MS patients. 23-OH UA’s ability to potently induce Nrf2-dependent antioxidant systems and maintain redox homeostasis combined with its ability to activate PPARg-dependent signaling in immune cells – and very likely other tissues – may account for the broad range of health benefits reported for 23-OH UA. Based on these findings, we propose that 23-OH UA may be well-suited as a dietary supplement for the management of MS and the prevention of chronic inflammatory disease such as atherosclerosis, obesity and diabetes.
Author Contributions
M.T.M., W.P.G. and C.C.H performed experiments and contributed significantly to the data analysis. R.A. and T.G. F. provided funding, designed the experiments, wrote the manuscript, generated the figures. All authors contributed to the editing of the manuscript.
Funding
This project was supported by institutional support to R.A. and with funding from grant NS117742 from the National Institute of Health (T.G.F.), and support from the Jesse H. & Mary Jones Gibbs Endowed Chair.
Institutional Review Board Statement
All experiments were conducted in accordance with animal protocols approved by the University of Texas at San Antonio Institutional Animal Care and Use Committee.
Acknowledgments
Measurements of plasma levels of 23-hydroxy ursolic acid were performed by the UTSA Mass Spectrometry & Proteomics Core Facility (W.P.G.)
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Correale J, Gaitan MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017, 140, 527–546.
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kingwell E, van der Kop M, Zhao Y, Shirani A, Zhu F, Oger J, Tremlett H. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012, 83, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Mortality in patients with multiple sclerosis. Neurology. 2013, 81, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Buron MD, Chalmer TA, Sellebjerg F, Barzinji I, Danny B, Christensen JR, Christensen MK, Hansen V, Illes Z, Jensen HB, Kant M, Papp V, Petersen T, Prakash S, Rasmussen PV, Schafer J, Theodorsdottir A, Weglewski A, Sorensen PS, Magyari M. Initial high-efficacy disease-modifying therapy in multiple sclerosis: A nationwide cohort study. Neurology. 2020, 95, e1041–e1051. [Google Scholar]
- van der Star BJ, Vogel DY, Kipp M, Puentes F, Baker D, Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012, 11, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Pachner AR. Experimental models of multiple sclerosis. Curr Opin Neurol. 2011, 24, 291–299. [CrossRef] [PubMed]
- Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014.
- Bore L, Honda T, Gribble GW. Partial synthesis of 23-hydroxyursolic acid isolated from medicinal plants of the Rubiaceae family. Nat Prod Lett. 2002, 16, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Nguyen HN, Ahn YJ, Medina EA, Asmis R. Dietary 23-hydroxy ursolic acid protects against atherosclerosis and obesity by preventing dyslipidemia-induced monocyte priming and dysfunction. Atherosclerosis. 2018, 275, 333–341. [Google Scholar] [CrossRef]
- Raphael I, Gomez-Rivera F, Raphael RA, Robinson RR, Nalawade S, Forsthuber TG. TNFR2 limits proinflammatory astrocyte functions during EAE induced by pathogenic DR2b-restricted T cells. JCI Insight. 2019, 4.
- Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Dong Y, Yong VW. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. 2019, 15, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2016, 15, 198–209. [Google Scholar] [CrossRef]
- Ahn YJ, Wang L, Foster S, Asmis R. Dietary 23-hydroxy ursolic acid protects against diet-induced weight gain and hyperglycemia by protecting monocytes and macrophages against nutrient stress-triggered reprogramming and dysfunction and preventing adipose tissue inflammation. J Nutr Biochem. 2020, 86, 108483. [Google Scholar]
- Epstein LG, Prineas JW, Raine CS. Attachment of myelin to coated pits on macrophages in experimental allergic encephalomyelitis. J Neurol Sci. 1983, 61, 341–348. [Google Scholar] [CrossRef]
- Bruck W, Sommermeier N, Bergmann M, Zettl U, Goebel HH, Kretzschmar HA, Lassmann H. Macrophages in multiple sclerosis. Immunobiology. 1996, 195, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Prineas JW, Parratt JDE. Multiple Sclerosis: Microglia, Monocytes, and Macrophage-Mediated Demyelination. J Neuropathol Exp Neurol. 2021, 80, 975–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang Y, Li X, Ciric B, Curtis MT, Chen WJ, Rostami A, Zhang GX. A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc Natl Acad Sci U S A. 2020, 117, 9082–9093. [Google Scholar] [CrossRef] [PubMed]
- Kim HS, Tavakoli S, Piefer LA, Nguyen HN, Asmis R. Monocytic MKP-1 is a Sensor of the Metabolic Environment and Regulates Function and Phenotypic Fate of Monocyte-Derived Macrophages in Atherosclerosis. Scientific Reports. 2016, 6, 34223. [Google Scholar] [CrossRef] [PubMed]
- Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, E2803–E2812. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).