Introduction
The stems of plants can be classified into different types based on their growth patterns, including erect stems, twining stems, climbing stems, and creeping stems, etc. Many plant species have twining stems, such as morning glories and beans. Like all spiral structures, twining stems have two different chiralities: left-handed (clockwise) and right-handed (counterclockwise). Among the existing plant species, right-handed twining stems are far more common than left-handed ones, with the proportion of right-handed twining stems exceeding 90% [
1]. The chirality of twining stems has always been a fascinating topic. There is a popular hypothesis about the origin of twining stem chirality [
2,
3,
4], suggesting that left-handed and right-handed twining stems originated in the northern and southern hemispheres, respectively, due to the influence of the Earth's rotation. However, this hypothesis is obviously incorrect because: (1) neither the Coriolis force nor the change in the direction of the sun is sufficient to distort stem growth; (2) acquired phenotypes cannot be written into genes; (3) species within the same family or genus often have different twining directions, which also does not support the separate origins in the northern and southern hemispheres. This question has also been discussed previously [
1].
Many people believe that the chirality of twining stems in a given species is fixed, but this is not the case. The chirality of twining stems can be variable in certain species. A few studies have briefly mentioned this phenomenon. For instance, in Hashimoto's 2002 paper, it was mentioned that “Rare exceptions with random handedness are found in climbing pteridophytes belonging to the genus
Lygodium—although the climbing organ of these plants is not a stem, as in other climbing plants, but is an indeterminately growing leaf.” [
2]. In 2014, Hu et al. discussed the twining stems of
Mikania micrantha, noting that they exhibit two directions [
5]. Through observations, we have found that twining stems with variable handedness are not rare. Given the lack of in-depth research and the tendency to overlook this phenomenon, we have decided to provide a more detailed investigation here. In this article, we refer to twining stems with only one chirality as "fixed-chirality twining stem" (FCTS), while twining stems with variable chirality are referred to as "variable-chirality twining stem" (VCTS). These two types of stem can also be distinguished by the terms “unidirectional twining stem” (UDTS) and “bidirectional twining stem” (BDTS). The contrast between VCTS and FCTS was visually shown in
Figure 1.
Results
1. The twining direction of the stem in Mikania micrantha is variable.
Mikania micrantha, also known as the mile-a-minute weed (薇甘菊, Weiganju in Chinese), is a species of the Asteraceae family native to tropical America. In recent decades, it has become a serious invasive plant in southern China [
6].
The stems of
M. micrantha can take two forms: trailing (creeping) and twining. As described previously [
5], the twining stems of
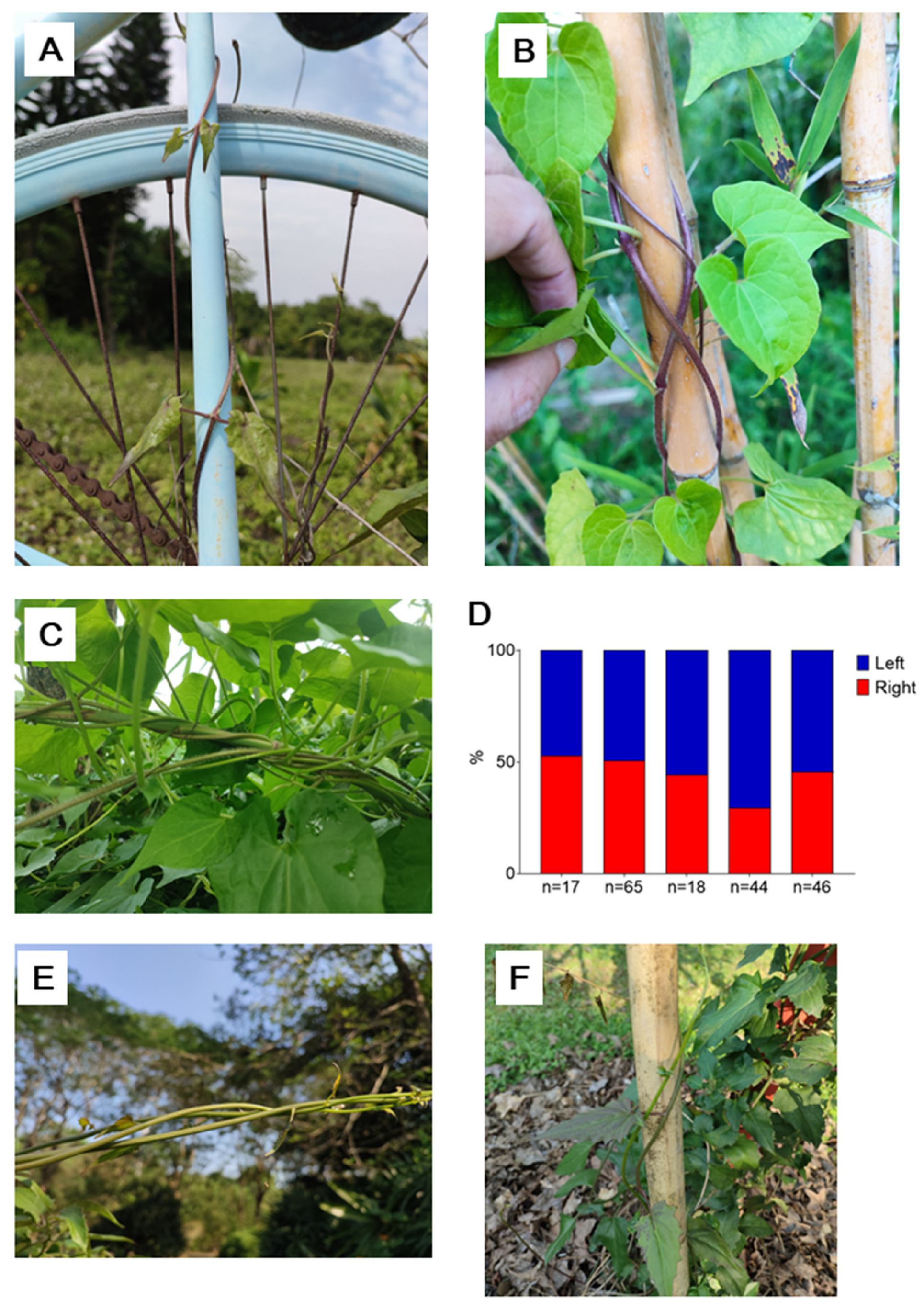
M. micrantha exhibit both left-handed and right-handed chiralities (
Figure 1F;
Figure 2A-C). Different branches of the same plant can have different chirality, and even within the growth process of a single branch, the chirality can change (Supplementary video 1). However, it seems more common for the twining direction to change from left to right rather than from right to left (data not shown). Based on the survey of the numbers of the two stem chiralities of the
M. micrantha in Guangzhou, China, the proportion of left-handed lianas are slightly more than 50%, but not significant (
Figure 2D, P=0.11).
The Asteraceae family comprises a vast number of plant species, and although the proportion of twining stems is not high, there are still a lot of species with twining stems within this family. For instance, the variegated wax ivy (
Senecio macroglossus DC.) exhibits a fixed right-handed twining stem. Additionally, we have recently observed that
Pseudogynoxys chenopodioides also possesses VCTS (
Figure 2E and F), suggesting that VCTS may be relatively common within the Asteraceae family.
2. The twining direction of the stem in Pleuropterus multiflorus is variable.
Pleuropterus multiflorus (Thunb.) Nakai (
Fallopia multiflora, 何首乌,Heshouwu in Chinese)is a species of the Polygonaceae family and is an important traditional Chinese medicinal herb known for its root and stem. It is widely distributed throughout China and its genome has been sequenced and assembled recently [
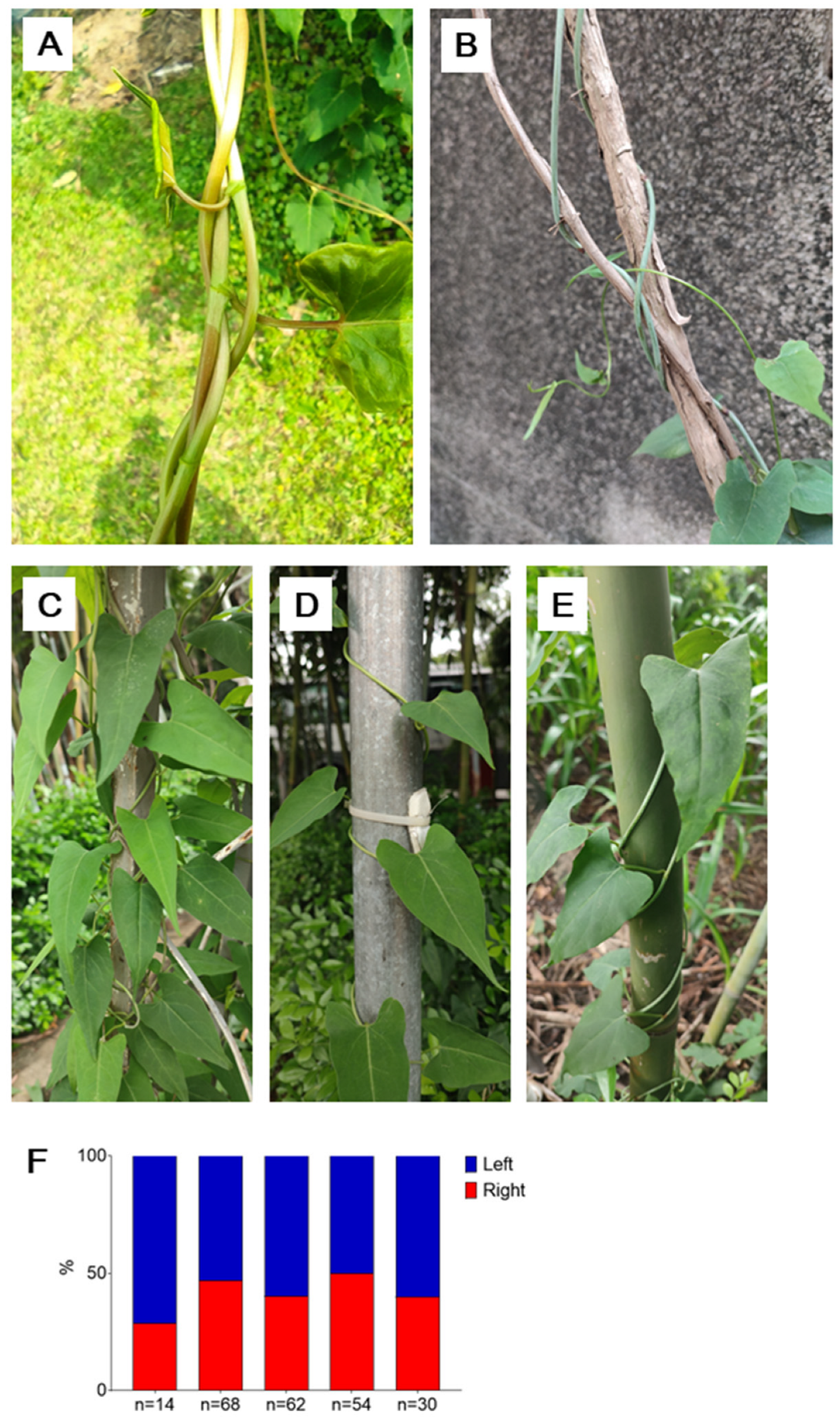
7]. Some popular literature in Chinese mentions that Heshouwu has twining stems with both chiralities, but there have been no formal reports on this. We have observed and recorded the proportion of the two chiralities in Heshouwu (
Figure 3). Additionally, we have observed that the twining direction can change midway in a single stem (supplementary video 2). Currently, we only know that Heshouwu, as a member of the Polygonaceae family, exhibits variable direction twining stems (VCTS), and further observation is needed to determine if there are other members of VCTS in the family.
Similar to the case in
M. micrantha, none of the two chiralities is dominant in the stems of
P. multiflorus (
Figure 3E, P=0.06).
3. The stems of the Hedyotis hedyotidea exhibit two twining directions
Hedyotis hedyotidea (DC.) Merr. (牛白藤, Niubaiteng in Chinese) is a species of the Rubiaceae family, characterized as a shrub or twining lianas, which is also used as Chinese herb medicine [
8]. The majority of its twining stems are right-handed, but there are also left-handed ones (
Figure 4). The Rubiaceae family comprises numerous species, mostly distributed in tropical and subtropical regions. The composition of twining stems within the family is complex. For example,
Paederia foetida and
Mussaenda pubescens exhibit fixed left-handed twining, while
Morinda parvifolia and
Morinda umbellata exhibit fixed right-handed twining.
4. Some members of the Codonopsis genus of the Campanulaceae family exhibit two twining directions.
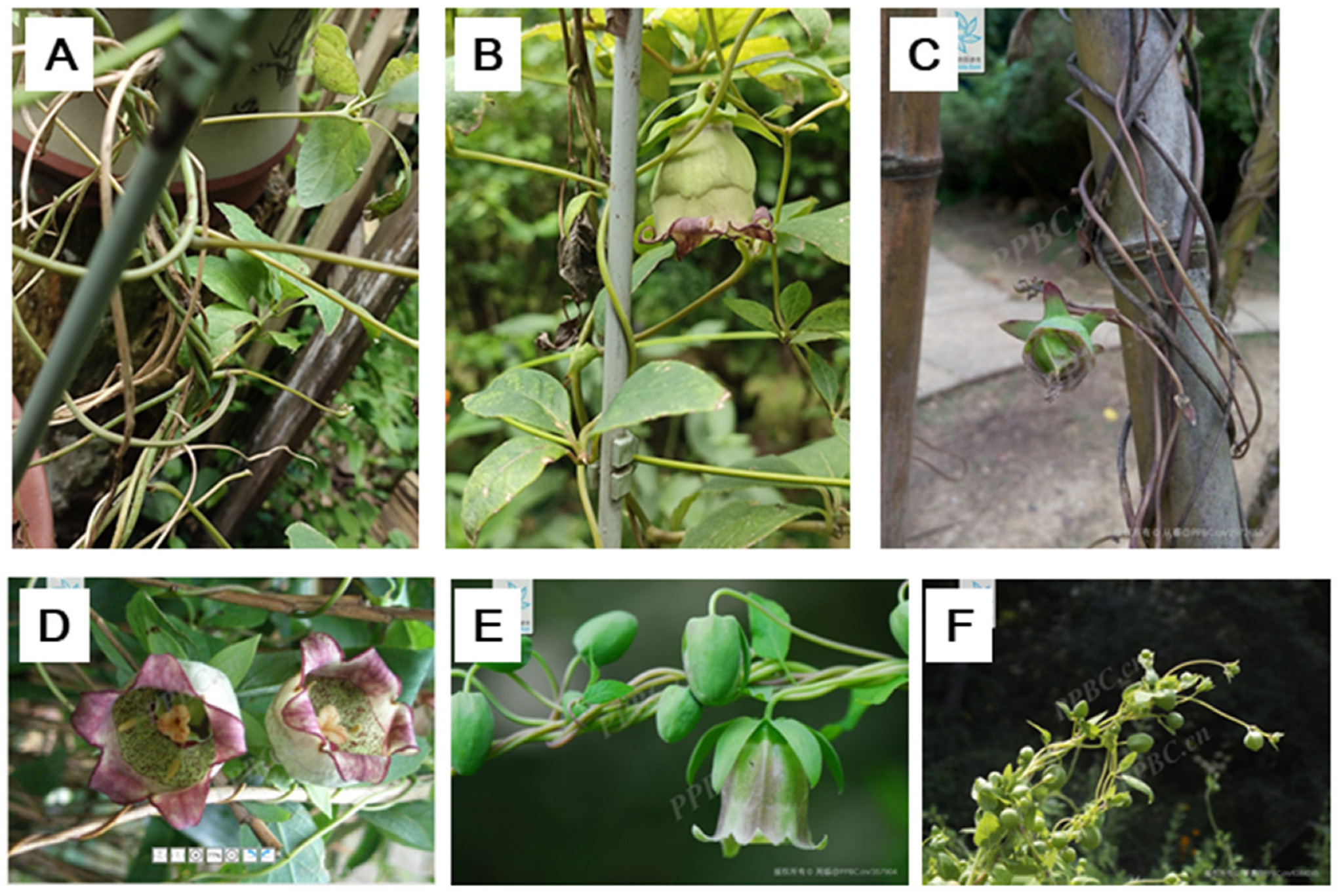
Codonopsis pilosula and
Codonopsis lanceolata, both known as Dangshen (党参), are well-known traditional medicinal herbs [
9] and they also have VCTS (
Figure 5). We have searched for images on the Plant Plus of China website (PPBC) and found that
Codonopsis henryi and
Codonopsis tubulosa, known as Chuan'e Dangshen (川鄂党参) and Guanhua Dangshen (管花党参), respectively, also exhibit VCTS. Therefore, it is possible that most or all twining members of the
Codonopsis genus have VCTS.
5. Dendrotrophe varians exhibit two twining directions.
Dendrotrophe varians (Blume) Miquel (寄生藤, Jishengteng in Chinese) is a hemiparasitic plant with large woody vines belonging to the Santalaceae family [
10]. It is currently the only known VCTS of large woody vines/lianas (
Figure 6). Since plants in the Santalaceae family are not very common, it is currently unknown if there are other members of VCTS in the family. We have also observed another suspected large woody vine VCTS in Guangdong province, China; but the quantity of that plant is very limited, and there is no definitive conclusion yet.
6. The stem of Aconitum volubile has two twining directions
Aconitum volubile Pall. ex Koeue, also known as Twining Monkshood (蔓乌头, Manwutou in Chinese), is a member of the
Aconitum genus in the Ranunculaceae family. Many species of
Aconitum genus were also used as traditional Chinese herb medicine [
11]. While most members of the
Aconitum genus have erect stems, there are a few, such as
A. volubile, that have twining stems. The twining stems of
A. volubile also exhibit both left-handed and right-handed chiralities (
Figure 7).
7. The lianas of Clerodendrum thomsoniae has two twining directions
Clerodendrum thomsoniae Balf. f., known as Bleeding Heart Vine (龙吐珠, Longtuzhu in Chinese), is a twining lianas in the Lamiaceae family. Although it is primarily a climbing vine, it can also exhibit slight twining. The twining of
C. thomsoniae has a relatively long pitch, and the direction can be both left-handed and right-handed (
Figure 8).
8. The growth pattern of leaf rachises in the Lygodium genus is similar to twining stems, and the twining direction is variable
So far, the VCTS we have found are all belong to the angiosperms, specifically the eudicots. We have not yet found this phenomenon in monocots, basal angiosperms, magnoliids, or gymnosperms. However, it is interesting to note that similar phenomena exist in ferns [
2]. In the
Lygodium genus (海金沙, Haijinsha in Chinese) of the Lygodiaceae family, the leaf rachises can grow indefinitely and twine around support structures, resembling stems in flowering plants. We have examined
L. japonicum,
L. microphyllum (
Figure 9), and
L. flexuosum and found that their leaf rachises exhibit both left-handed and right-handed twining directions. The right-handed leaves were significantly more than the left-handed ones (
Figure 9F, P=5.8E-11). The left-handed leaves are less than 20%, rare but still easy to encounter.
We speculate that members of the Lygodium genus may all have two twining directions. Although they twine with their leaf rachises rather than stems, the similar growth pattern suggests that they might have similar regulatory mechanisms. Additionally, ferns as an evolutionary outgroup can provide insights into the evolution of twining stems in seed plants.
9. The relation between twining chirality and taxa
Previous study has shown that there was no clear relation between the twining direction and phylogeny at the family level [
12], and may have some relation at the genus level [
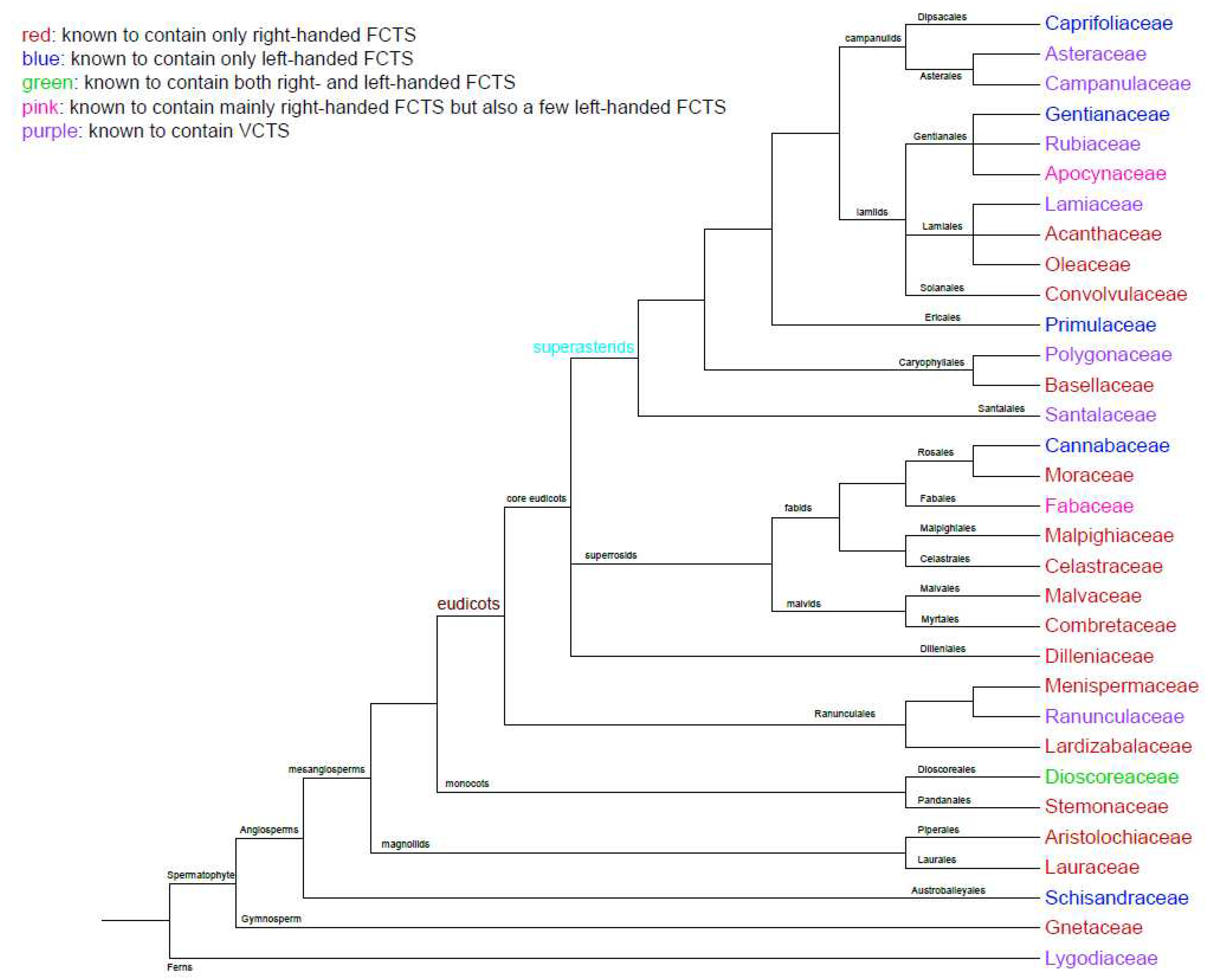
13]. Here, to examine the evolutionary relationships of known taxa with VCTS, we constructed a phylogenetic tree that includes the families of twining stem plants commonly found in China (
Figure 10).
As previously described, the majority of twining stems possess right-handed chirality. Most known families have exclusively right-handed FCTS (red fonts). These families can be divided into two categories: one with numerous twining stem members, such as the Convolvulaceae and Menispermaceae, all of which exhibit right-handed twining; and another category where twining stem members are few, but all known members exhibit right-handed twining.
There are very few families known to have exclusively left-handed FCTS. The most certain examples are the Cannabaceae (Humulus genus), Caprifoliaceae, and Schisandraceae (blue fonts).
Some families have members that exhibit both left-handed and right-handed twining, without a clear dominance. For example, in the Rubiaceae family, both left-handed and right-handed twining, as well as VCTS, can be found. The Dioscoreaceae family (known for Dioscorea genus) is also interesting, as it has both left-handed and right-handed members (green fonts). For instance, D. polystachya, D. alata, and D. cirrhosa exhibit right-handed twining, while D. nipponica, D. bulbifera, and D. esculenta are left-handed, with no predominance shown in either of the chiralities.
There are also some families where the majority of twiners are right-handed, but a few are left-handed (pink fonts). For example, in the Papilionoideae subfamily of Fabaceae family, the genera Glycine, Vigna, Phaseolus, Lablab, and Mucuna exhibit right-handed twining, while Wisteriopsis exhibits left-handed twining (Supp. Figure 1). Additionally, the genus Westeria is predominantly right-handed, but W. floribunda, a member of this family, exhibits left-handed twining. In the Apocynaceae family, most twining stems are right-handed, but the twining stem of Strophanthus divaricatus is left-handed (Supp. Figure 2).
From the phylogenetic tree, it can be observed that known VCTS (purple fonts) are all found in eudicots (labeled with brown color). Furthermore, within the eudicots, both left-handed and VCTS are slightly more abundant in the Superasterids (labeled with cyan color), suggesting a higher degree of twining direction uncertainty within this clade, although the trend is not strongly pronounced.
Twining stems are rare in monocots, with the Dioscoreaceae and Stemonaceae being the most common examples. Among gymnosperms, the only known genus with twining stems is Gnetum, and all known species within it exhibit right-handed twining (Supp. Figure 3).
Discussion
We have reported on several species (genera) of eight families with VCTS that can be observed in China, including seven species (genera) from angiosperms and one genus from ferns (twining leaf rachis). These reports are based on our observations over the past two years mainly in Guangdong province, China. Our study suggests that VCTS is not a rare phenomenon in plant twiners. Due to the limited attention and research in this field, the actual number of species with VCTS in nature is likely much greater than currently known, awaiting further discoveries. For example, we have seen instances where Heshouwu has been described as both left-handed, right-handed, and bidirectional in Chinese internet sources. If an observer assumes that all twining stem chirality is fixed, they are more likely to overlook the phenomenon of variable chirality and instead consider the predominant chirality or the initially observed chirality as the characteristic of that twiner. Therefore, VCTS should be recognized and accepted as a concept to avoid certain misconceptions in the study of twining stems.
The widespread distribution of twining stems in angiosperms suggests that twining stems have evolved independently for multiple times and may require only a few genetic mutations. The handedness of twining stems does not show a clear relationship with phylogenetic groups, with only some weak trends. For example, the previously mentioned members of the superasterids have a higher degree of uncertainty in twining direction.
As for why right-handed twining stems are far more abundant than left-handed ones, the reason is still unknown, just like the number of right-handed snails far surpasses that of left-handed snails. The genes controlling the chirality of plant twining stems have not been reported. VCTS could provide a good model for studying this issue, as the genomes of the same plant are identical, and differences in chirality should be determined by gene expression. Thus, there is hope that key genes governing chirality can be identified through transcriptomic studies.
Furthermore, the genera Wisteria and Wisteriopsis in the Fabaceae family, as well as the genus Dioscorea in the Dioscoreaceae family, may also serve as good models for studying twining stems. Additionally, comparing twining and non-twining members within the same genus, such as Ipomoea batatas (sweet potato) and I. triloba, or the honeysuckle Lonicera japonica (金银花,Jinyinhua in Chinese) and L. maackii, can help in identifying the key genes controlling twining.
In conclusion, we hope that our article can provide new insights into the study of twining stems and stimulate progress in related fields.
Finally, this article is based on the information currently available to us. As our understanding deepens, certain conclusions are bound to change, such as the discovery of additional VCTS or the realization that certain genera or families, believed to have only one twining handedness, actually exhibit both. We also warmly welcome readers to provide feedback on any errors or areas that need improvement, as it will contribute to the continuous refinement of our knowledge regarding twining stems.
Materials and Methods
The Aconitum volubile stems were observed in Xiaoxing’an Mountains, Heilongjiang province, China. The stems of Codonopsis genus were searched from Plant Plus of China website (iplant.cn). The rest of the observations were all performed in Gongdong province, China.
The phylogenetic tree was constructed based on the APG IV System and customized at iTol (
https://itol.embl.de/) on line.
The differences between the proportions of certain chiralities and 50% were tested using chi-square test, and P<0.05 is considered significant.
References
- Will Edwards ATM. The global trend in plant twining direction. Global Ecology and Biogeography. 2007;16:795–800.
- Takashi Hashimoto. Molecular genetic analysis of left-right handedness in plants. Philosophical Transactions of the Royal Society B. 2002;357:799–808. [CrossRef]
- Goriely A, Neukirch S. Mechanics of Climbing and Attachment in Twining Plants. Phys Rev Lett. 2006;97:184302. [CrossRef]
- Bowling AJ, Vaughn KC. Gelatinous fibers are widespread in coiling tendrils and twining vines. American Journal of Botany. 2009;96:719–27. [CrossRef]
- Liang Hu ML. Climbing capacity of the invasive vine Mikania micrantha Kunth on vertical artificial poles. Biological invasions. 2014;16:295–302.
- Liu B, Yan J, Li W, Yin L, Li P, Yu H, et al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nature communications. 2020;11. [CrossRef]
- Zhao Y, Yang Z, Zhang Z, Yin M, Chu S, Tong Z, et al. The first chromosome-level Fallopia multiflora genome assembly provides insights into stilbene biosynthesis. Horticulture Research. 2023;10. [CrossRef]
- Zhou Y, Weng X, Dou R, Tan X, Zhang T, Fang J, et al. Betulin from Hedyotis hedyotidea ameliorates concanavalin A-induced and T cell-mediated autoimmune hepatitis in mice. Acta Pharmacologica Sinica. 2016;38:201–10. [CrossRef]
- Yue J, Xiao Y, Chen W. Insights into Genus Codonopsis: From past Achievements to Future Perspectives. Critical Reviews in Analytical Chemistry. 2023;0:1–32. [CrossRef]
- Shin HW, Lee NS. Understanding plastome evolution in Hemiparasitic Santalales: Complete chloroplast genomes of three species, Dendrotrophe varians, Helixanthera parasitica, and Macrosolen cochinchinensis. PLoS One. 2018;13:e0200293.
- Kakkar RA, Haneen MA, Parida AC, Sharma G. The known, unknown, and the intriguing about members of a critically endangered traditional medicinal plant genus Aconitum. Front Plant Sci. 2023;14:1139215. [CrossRef]
- Robyn J. Burnham CR-M. Phylogenetic Influence on Twining Chirality in Lianas from Amazonian Peru. Annals of the Missouri Botanical Garden. 2011;98:196–205.
- GongHua L, HaiXin C, YongAn L, JianPing S. Phylogenetic analysis of chirality of twining plants. Agricultural Science & Technology - Hunan. 2010;11:34–8.
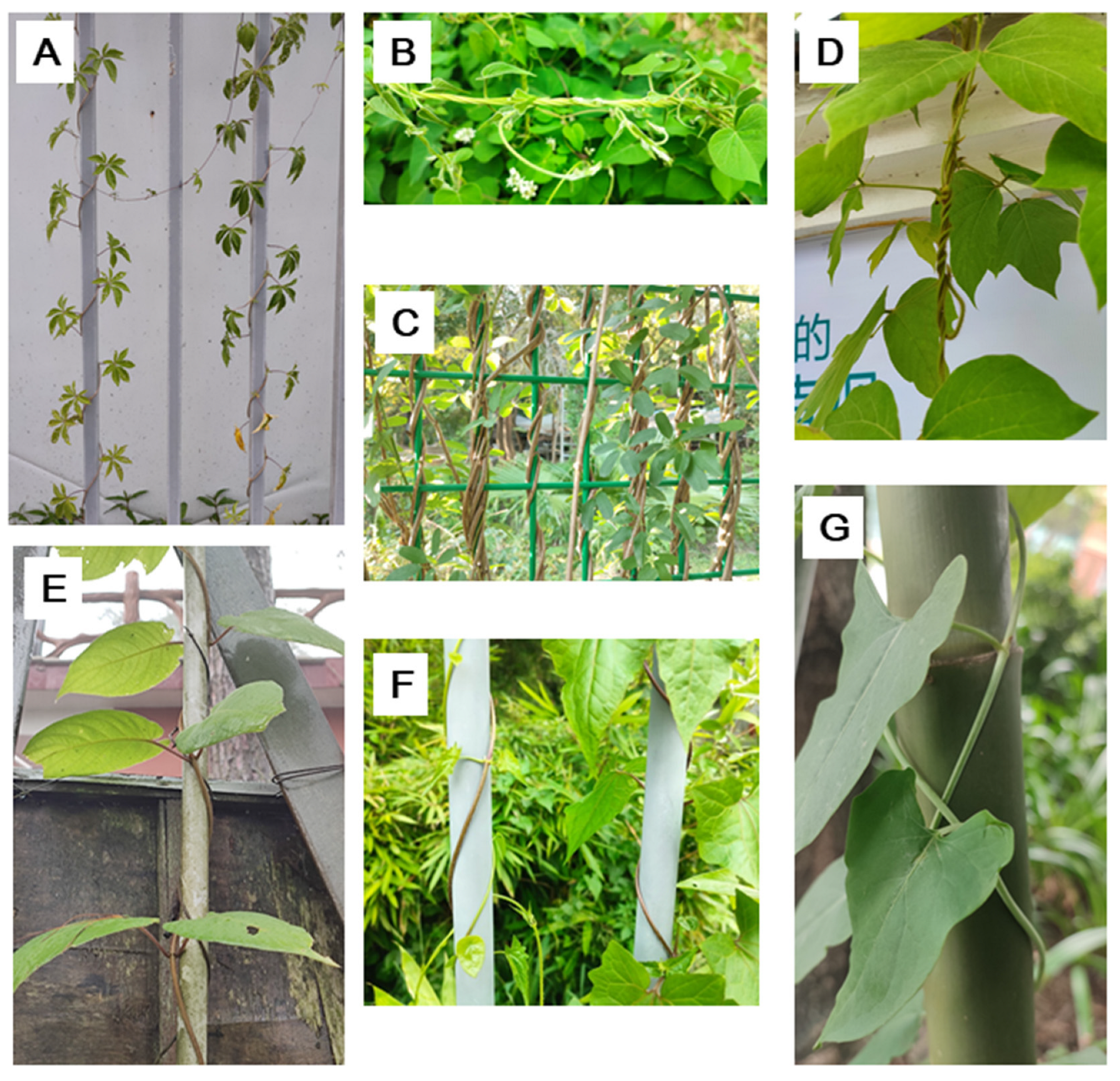
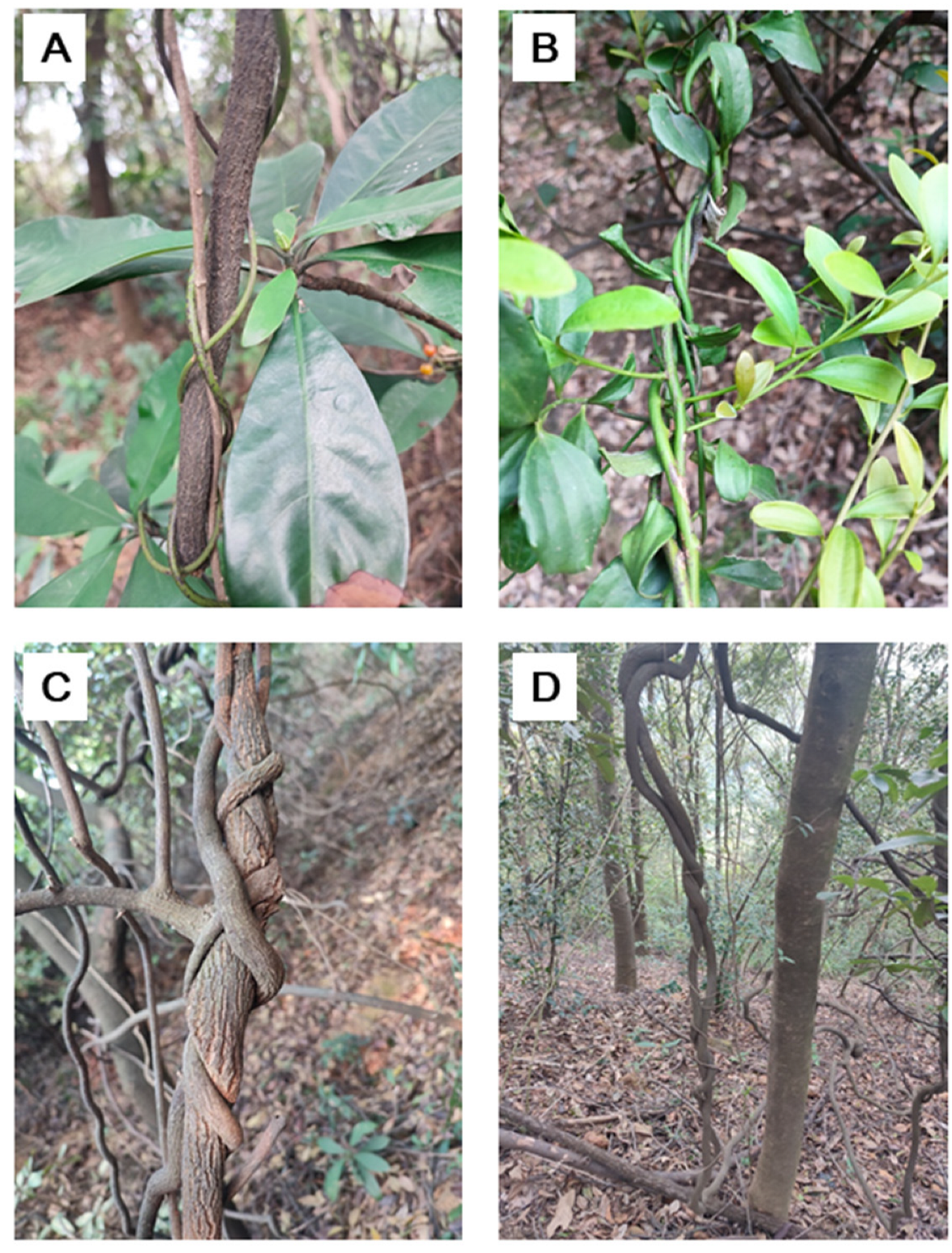
Figure 1.
Examples for fixed chirality twining stem (FCTS) and variable chirality twining stem (VCTS). A, right-handed FCTS of Ipomoea cairica, an invasive plant in south China; B, right-handed FCTS of Cynanchum rostellatum; C, right-handed FCTS of Akebia quinata; D, right-handed FCTS of Pueraria montana; E, left-handed FCTS of Paederia foetida; F, VCTS of Mikania micrantha, with a contrast of the right-handed FCTS of Stephania longa; G, VCTS of Pleuropterus multiflorus.
Figure 1.
Examples for fixed chirality twining stem (FCTS) and variable chirality twining stem (VCTS). A, right-handed FCTS of Ipomoea cairica, an invasive plant in south China; B, right-handed FCTS of Cynanchum rostellatum; C, right-handed FCTS of Akebia quinata; D, right-handed FCTS of Pueraria montana; E, left-handed FCTS of Paederia foetida; F, VCTS of Mikania micrantha, with a contrast of the right-handed FCTS of Stephania longa; G, VCTS of Pleuropterus multiflorus.
Figure 2.
The stem twining chirality of M. micrantha and Pseudogynoxys chenopodioides. A-C, the two twining chiralities of the stems of M. micrantha; D, the proportion of the two chiralities in M. micrantha, each column representing one observation of one time and one place; E and F, the two twining chiralities of the stems of P. chenopodioides.
Figure 2.
The stem twining chirality of M. micrantha and Pseudogynoxys chenopodioides. A-C, the two twining chiralities of the stems of M. micrantha; D, the proportion of the two chiralities in M. micrantha, each column representing one observation of one time and one place; E and F, the two twining chiralities of the stems of P. chenopodioides.
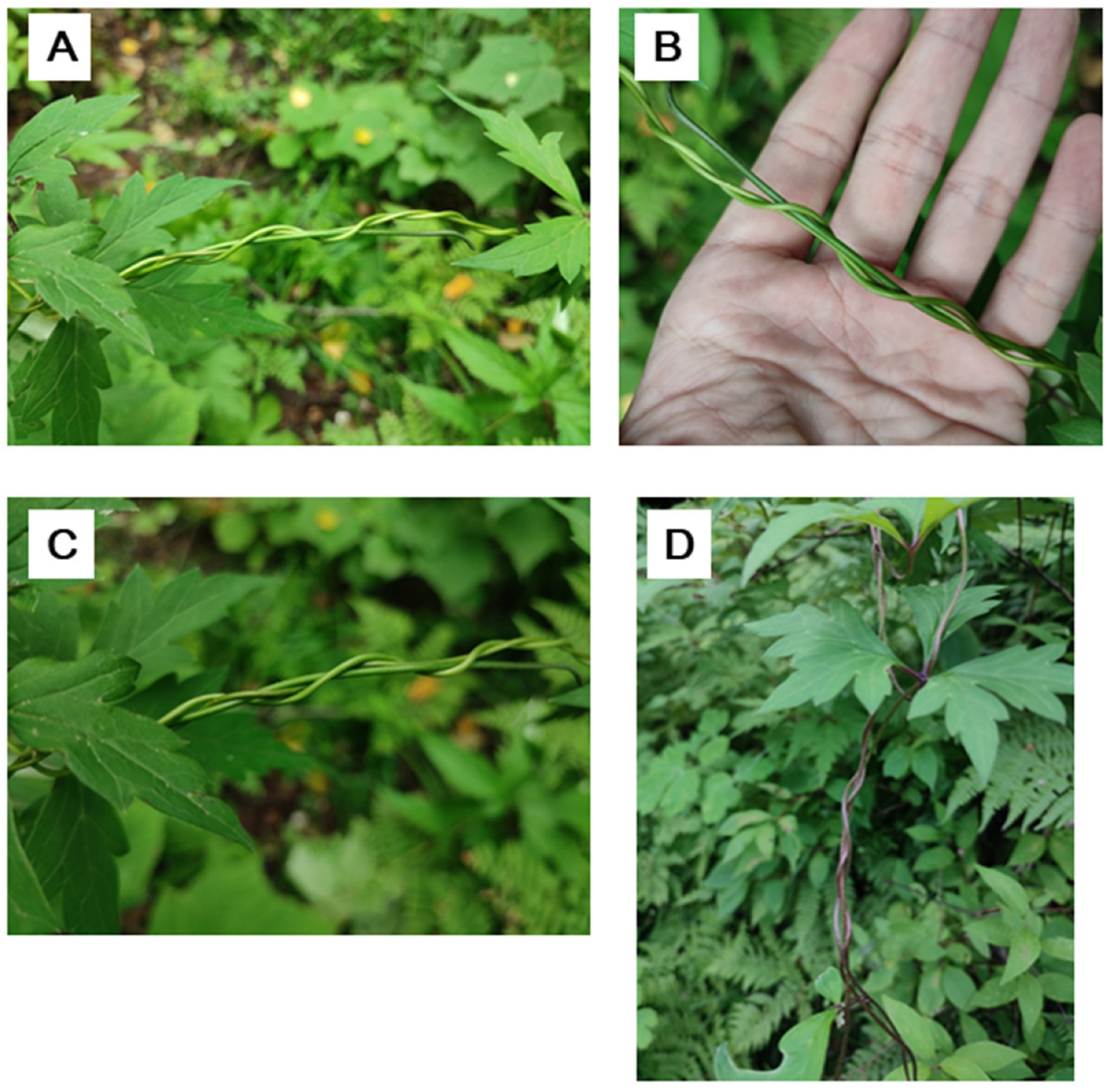
Figure 3.
The stem twining chirality of P. multiflorus. A-E, the two twining chiralities of the stems of P. multiflorus; F, the proportion of the two chiralities in P. multiflorus, each column representing one observation of one time and one place.
Figure 3.
The stem twining chirality of P. multiflorus. A-E, the two twining chiralities of the stems of P. multiflorus; F, the proportion of the two chiralities in P. multiflorus, each column representing one observation of one time and one place.
Figure 4.
The stem twining chirality of Hedyotis hedyotidea.
Figure 4.
The stem twining chirality of Hedyotis hedyotidea.
Figure 5.
The stem twining chirality in the Codonopsis genus. A-D, C. lanceolate; E and F, C. pilosula. the photos of C-F were cited from PPBC.
Figure 5.
The stem twining chirality in the Codonopsis genus. A-D, C. lanceolate; E and F, C. pilosula. the photos of C-F were cited from PPBC.
Figure 6.
The stem twining chirality of Dendrotrophe varians.The big leaves in A is from plant of Rubiaceae, not D. varians.
Figure 6.
The stem twining chirality of Dendrotrophe varians.The big leaves in A is from plant of Rubiaceae, not D. varians.
Figure 7.
The stem twining chirality of Aconitum volubile.
Figure 7.
The stem twining chirality of Aconitum volubile.
Figure 8.
The stem twining chirality of Clerodendrum thomsoniae.
Figure 8.
The stem twining chirality of Clerodendrum thomsoniae.
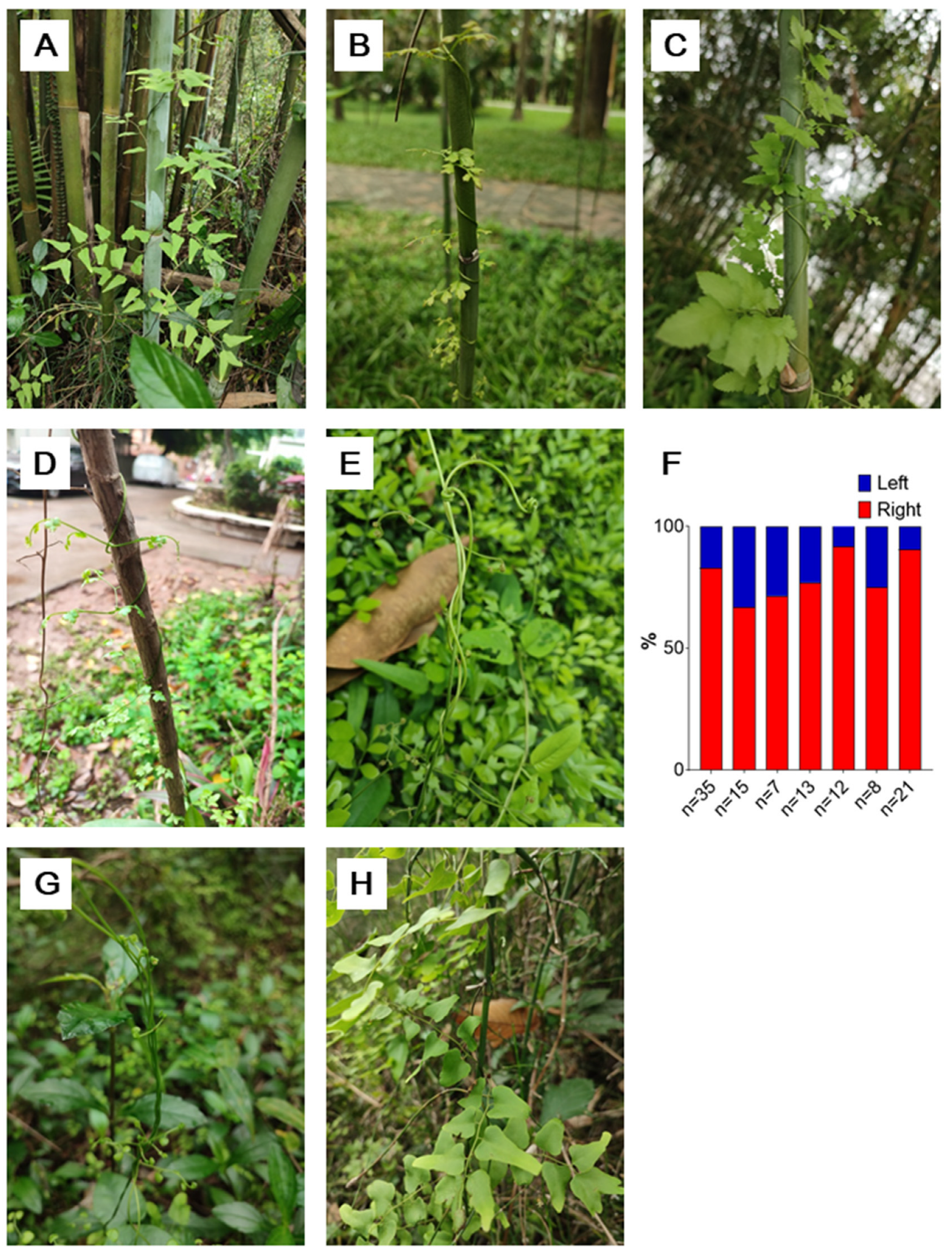
Figure 9.
The leaf rachis twining chirality in the Lygodium genus. A-E, the two twining chiralities of the leaf rachises of L. japonicum, F, the proportion of the two chiralities in L. japonicum, each column representing one observation of one time and one place; G and H, the two twining chiralities of the leaf rachises of L. microphyllum. .
Figure 9.
The leaf rachis twining chirality in the Lygodium genus. A-E, the two twining chiralities of the leaf rachises of L. japonicum, F, the proportion of the two chiralities in L. japonicum, each column representing one observation of one time and one place; G and H, the two twining chiralities of the leaf rachises of L. microphyllum. .
Figure 10.
The phylogenetic relationship of the families of commonly found twining stem plants in China. The tree was constructed based on the APG IV System, only showing the relationship of taxa, and the length of the branches doesn’t represent distance or time.
Figure 10.
The phylogenetic relationship of the families of commonly found twining stem plants in China. The tree was constructed based on the APG IV System, only showing the relationship of taxa, and the length of the branches doesn’t represent distance or time.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).