Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General
2.2. Fly Stocks
2.3. Drugs
2.4. Climbing Assay
2.5. Survival Curves
2.6. Immunohistochemistry
2.7. Electron Microscopy Analysis
2.8. Statistics
3. Results
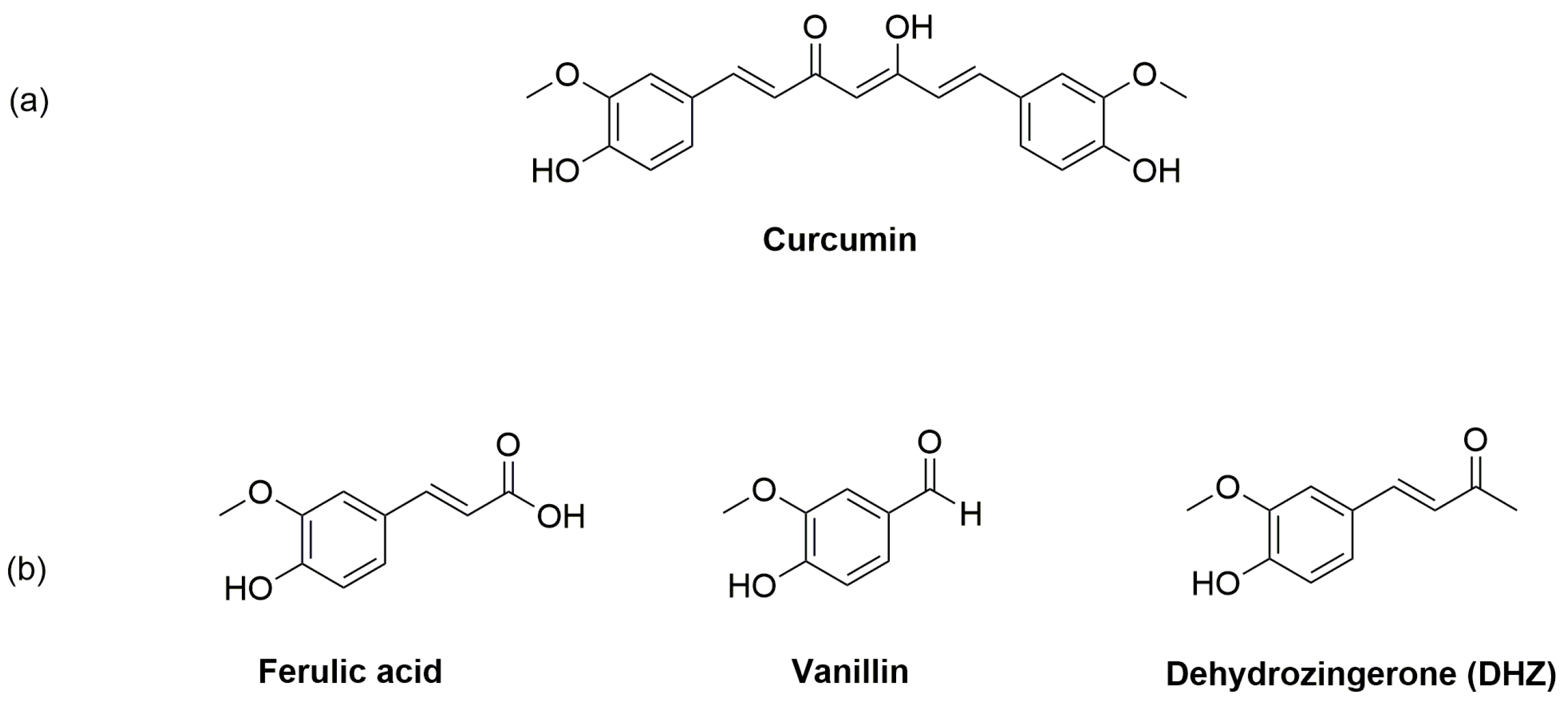
3.1. Chemistry
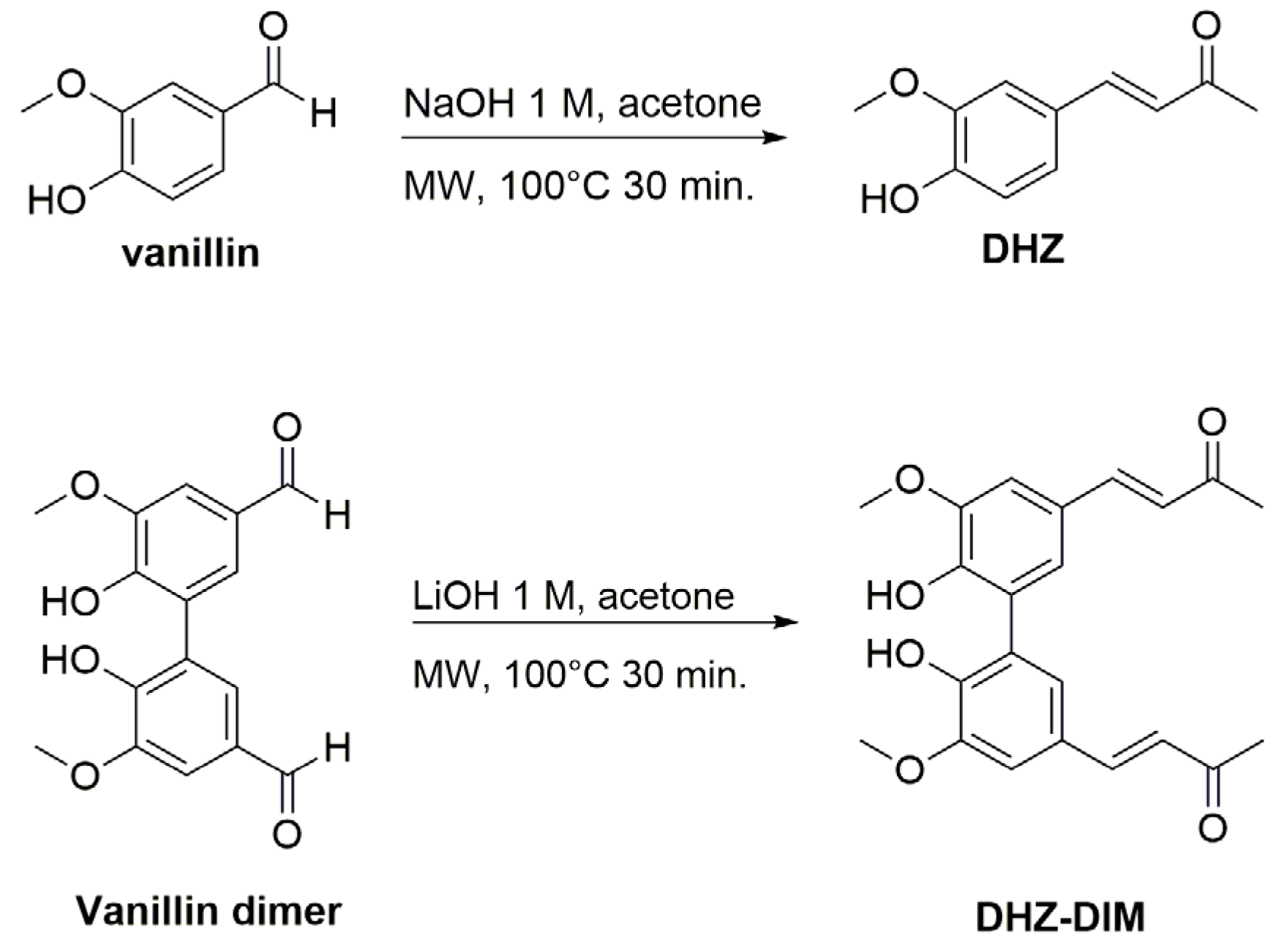
3.1.1. Synthetic Procedures
[(E)-4-(4-hydroxy-3-methoxyphenyl) but-3-en-2-one] (DHZ)
[(3E,3′E)-4,4′-(6,6′-dihydroxy-5,5′-dimethoxy-[1,1′-biphenyl]-3,3′-diyl) bis(but-3-en-2-one)] (DHZ-DIM)
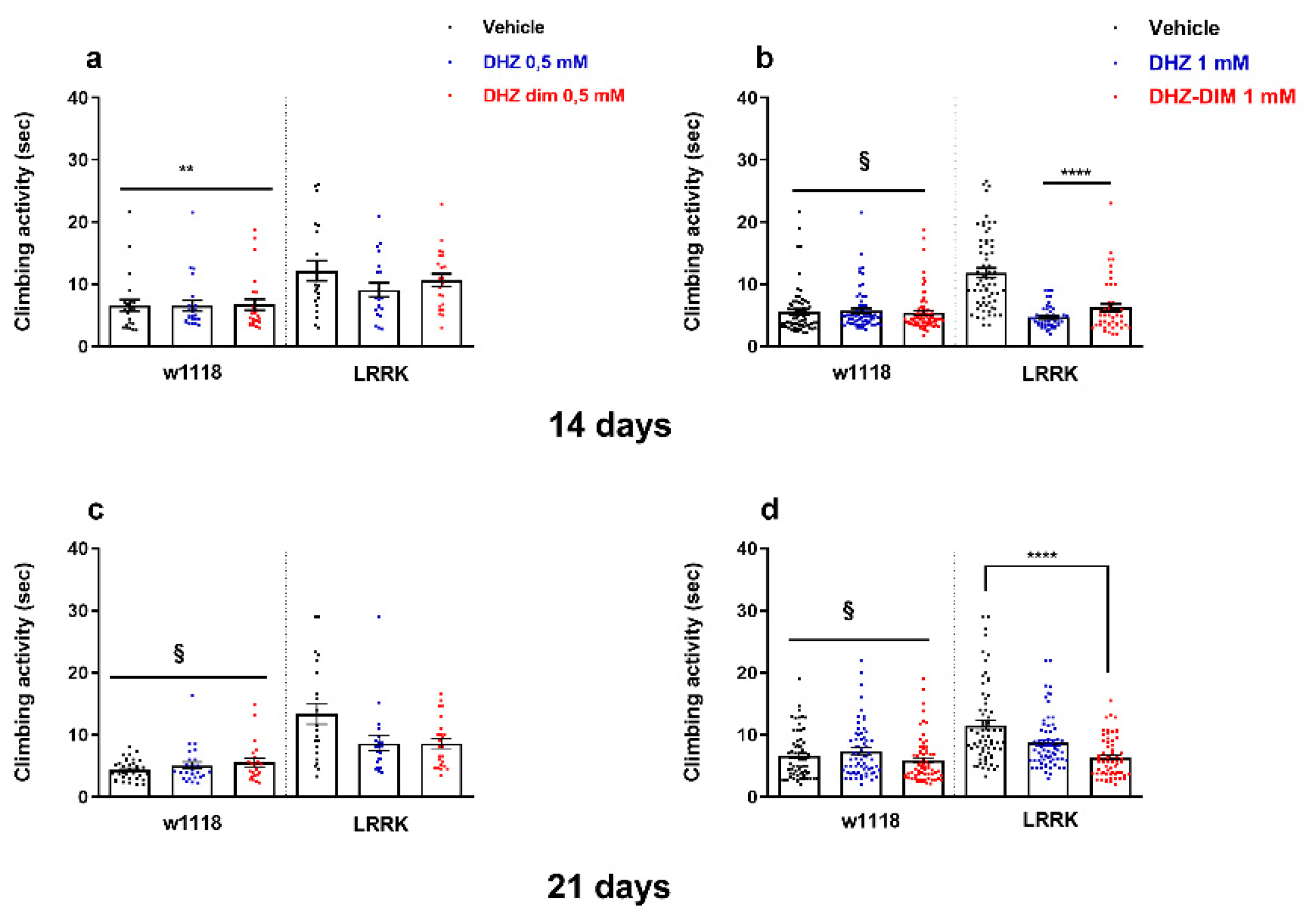
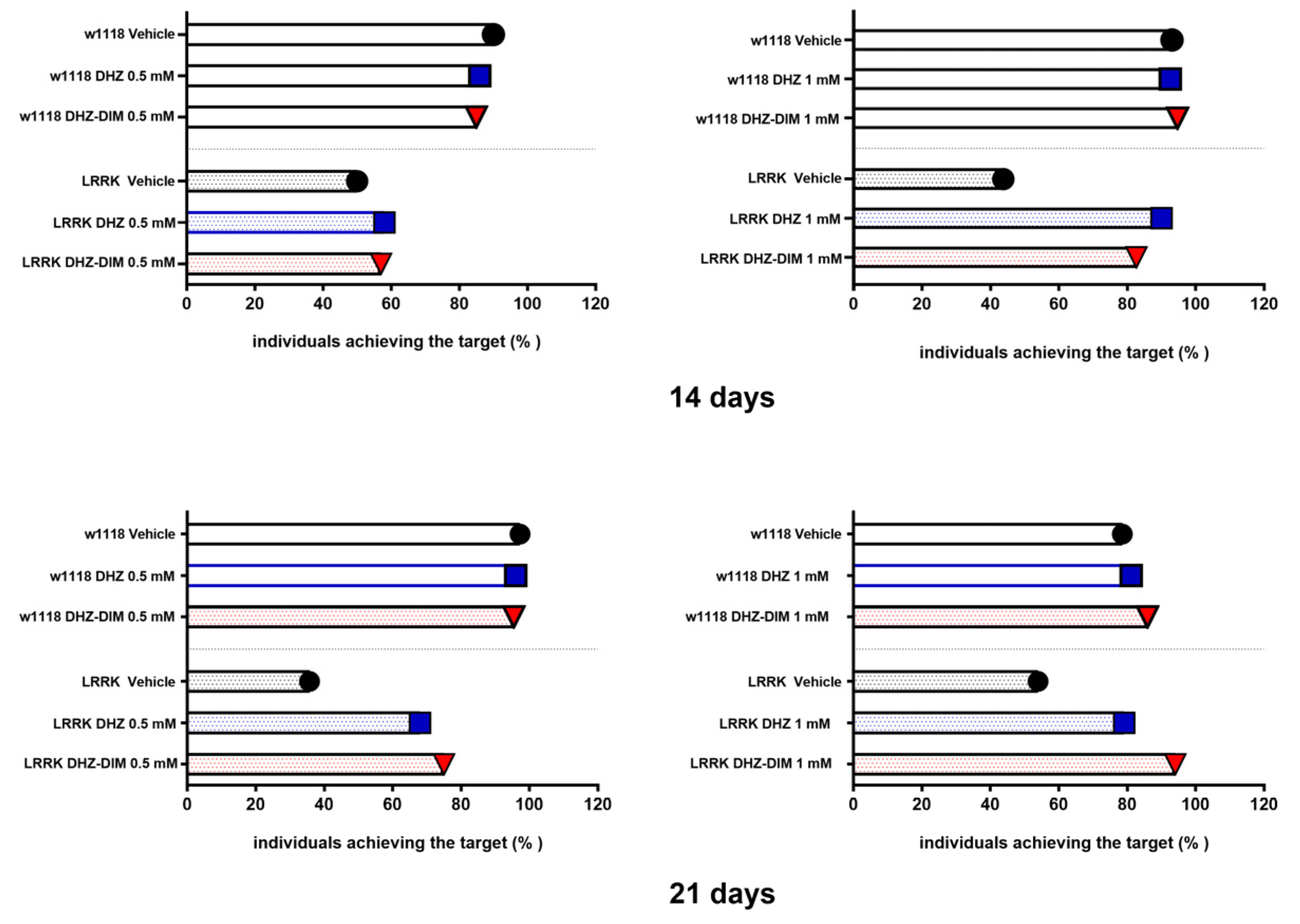
3.2. DHZ and DHZ-DIM Prevent the Motor Impairment in LRRK: Climbing Test
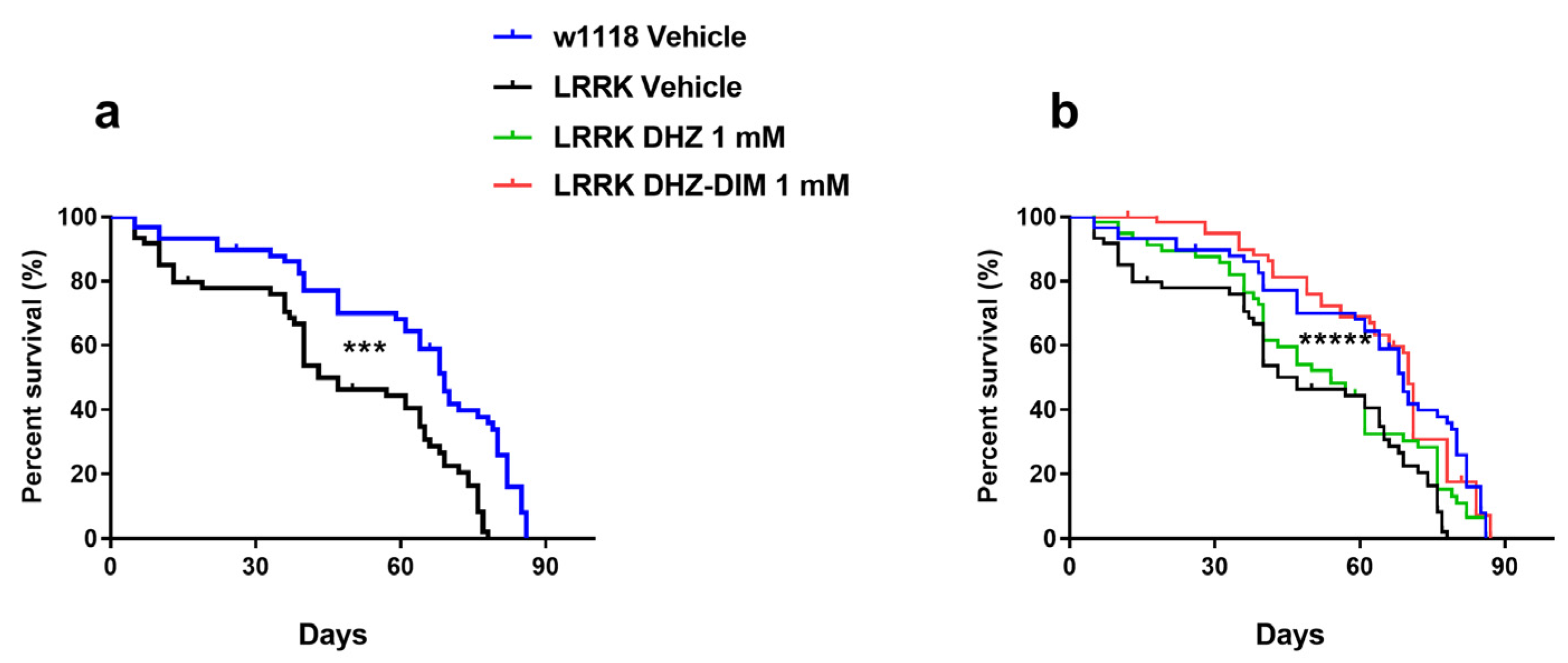
3.3. DHZ and DHZ-DIM Extended Longevity
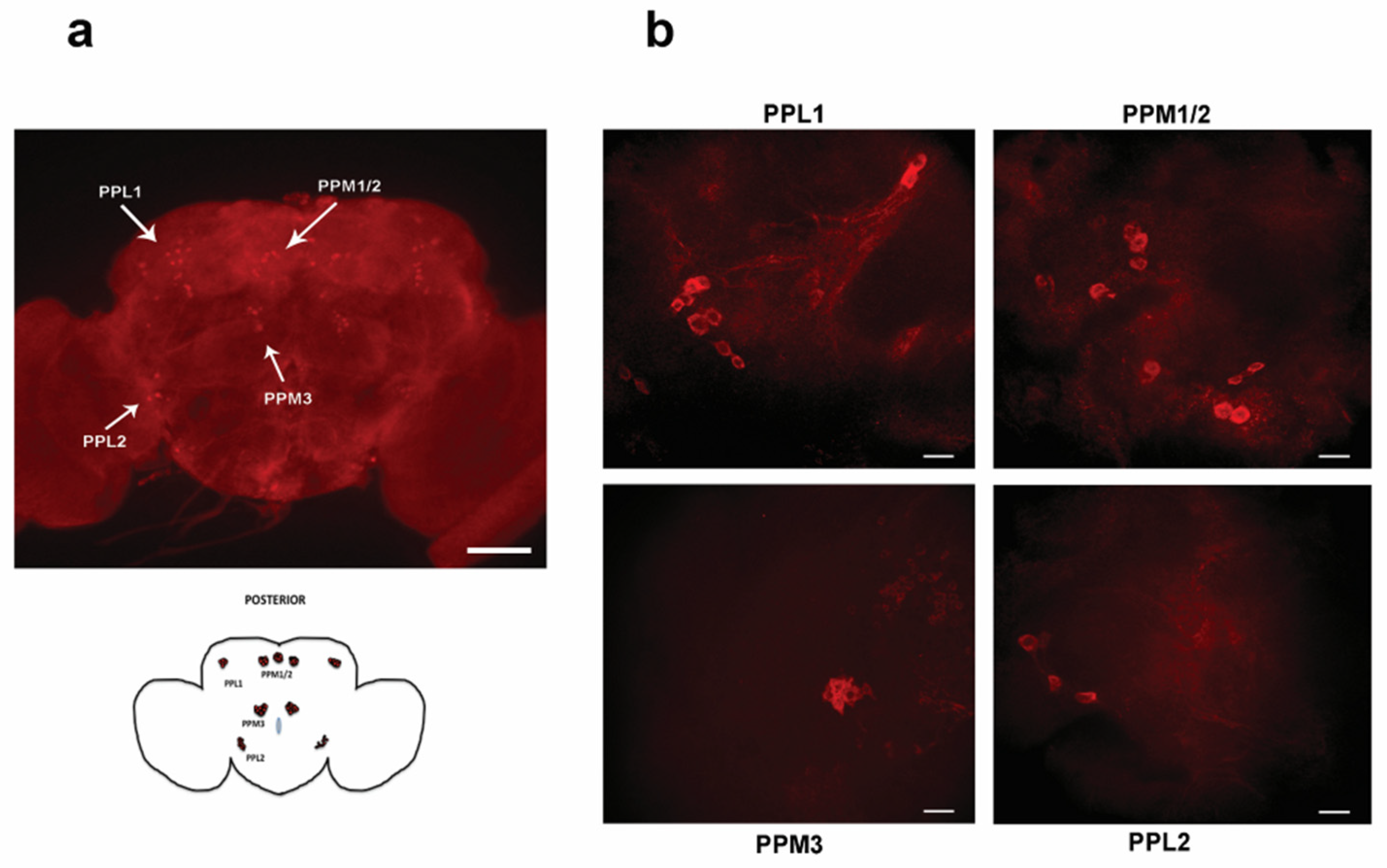
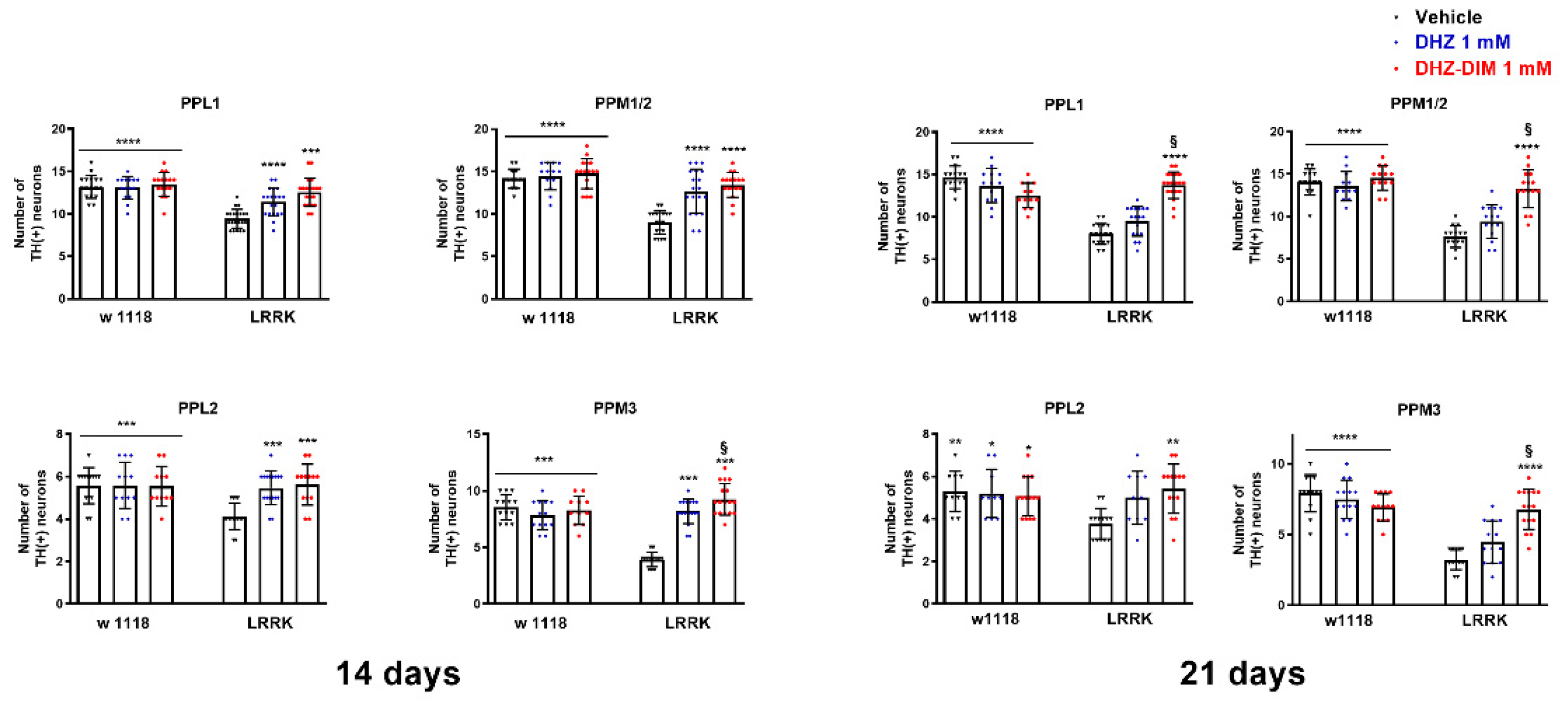
3.4. DHZ and DHZ-DIM Prevent the Loss of Dopaminergic Neurons
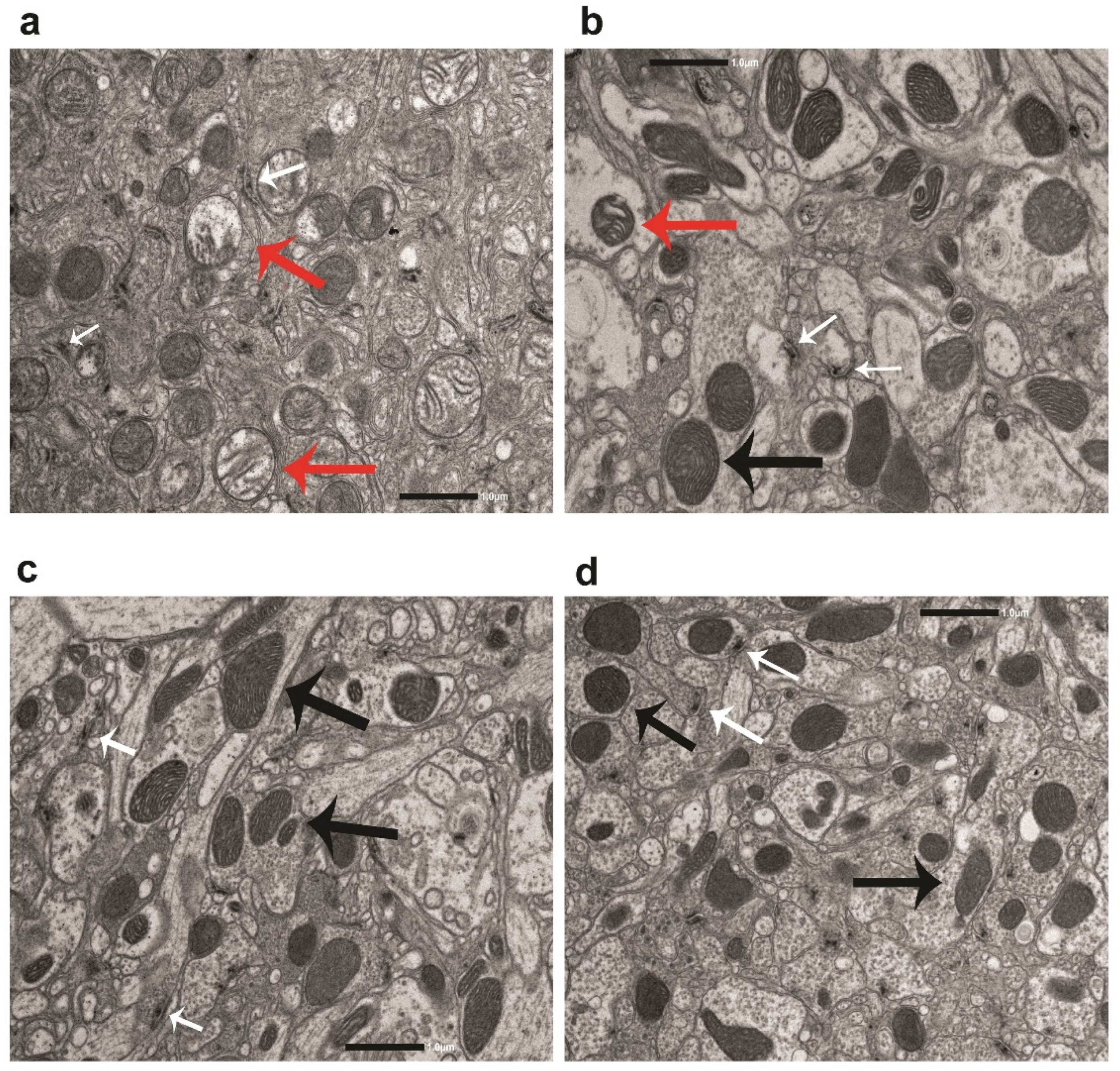
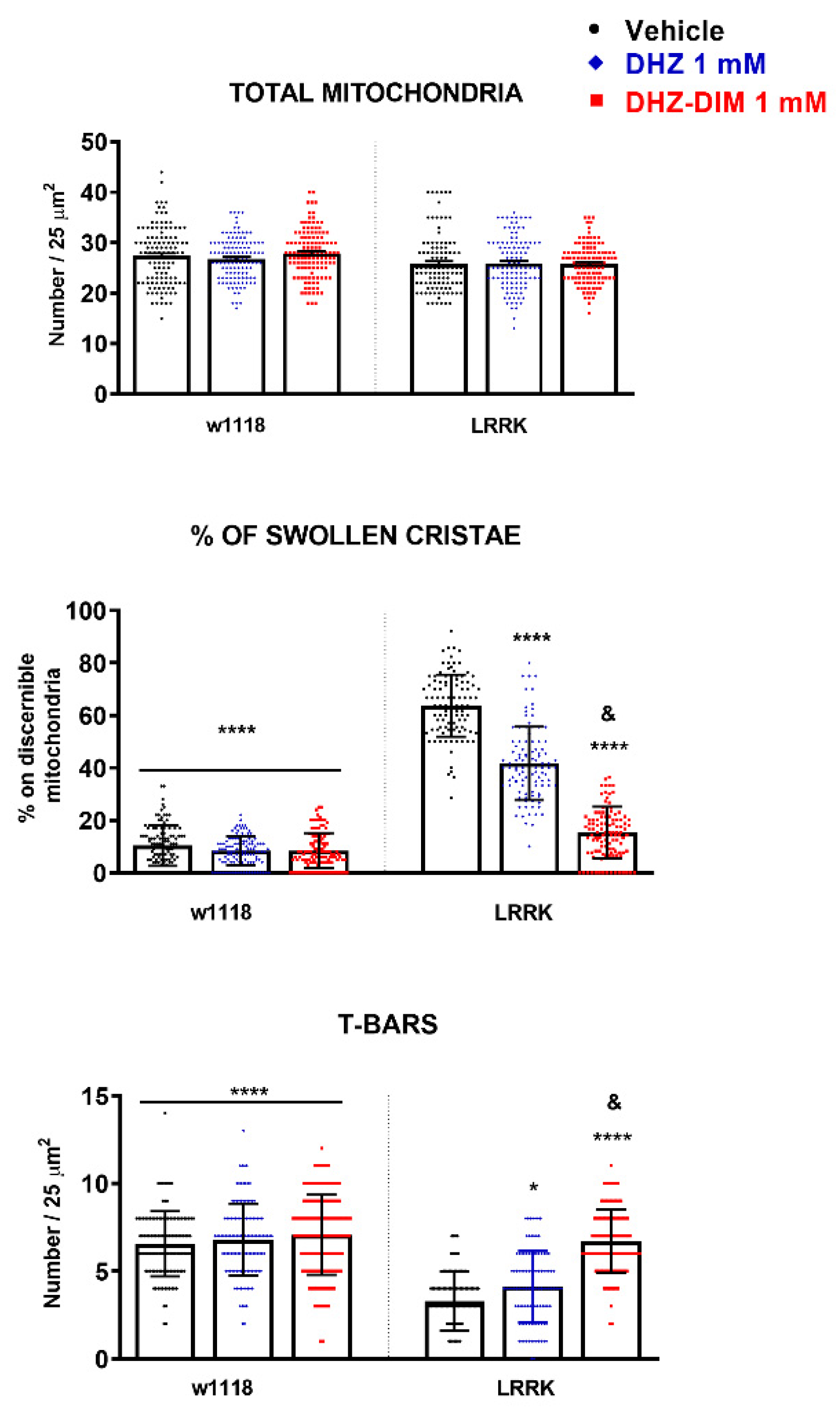
3.5. DHZ-DIM is More Effective than Monomer in Preventing the Mitochondrial Damage and the Loss of T-Bars in LRRK2 Drosophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial Clinical Manifestations of Parkinson’s Disease: Features and Pathophysiological Mechanisms. Lancet Neurol 2009, 8, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s Disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Odin, P. The Challenge of Non-Motor Symptoms in Parkinson’s Disease. Prog Brain Res 2010, 184, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr Neurol Neurosci Rep 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front Neuroanat 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.N.; Hastings, T.G. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease and Monogenic Parkinsonism. Neurobiol Dis 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit Rev Food Sci Nutr 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin Exp Pharma Physio 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple Biological Activities of Curcumin: A Short Review. Life Sciences 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol Sci 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of Curcumin in Brain Disorders. Biofactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J Pharm Biomed Anal 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Parihar, V.K.; Dhawan, J.; Kumar, S.; Manjula, S.N.; Subramanian, G.; Unnikrishnan, M.K.; Rao, C.M. Free Radical Scavenging and Radioprotective Activity of Dehydrozingerone against Whole Body Gamma Irradiation in Swiss Albino Mice. Chem Biol Interact 2007, 170, 49–58. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.M.; Lee, E.S.; Kim, N.; Lee, J.O.; Lee, H.J.; Park, N.Y.; Jo, J.Y.; Ham, B.Y.; Han, S.H.; et al. Dehydrozingerone Exerts Beneficial Metabolic Effects in High-Fat Diet-Induced Obese Mice via AMPK Activation in Skeletal Muscle. J Cell Mol Med 2015, 19, 620–629. [Google Scholar] [CrossRef]
- Dettori, M.A.; Pisano, M.; Rozzo, C.; Delogu, G.; Fabbri, D. Synthesis of Hydroxylated Biphenyl Derivatives Bearing an Alpha,Beta-Unsaturated Ketone as a Lead Structure for the Development of Drug Candidates against Malignant Melanoma. CHEMMEDCHEM 2021, 16, 1022–1033. [Google Scholar] [CrossRef]
- Hampannavar, G.A.; Karpoormath, R.; Palkar, M.B.; Shaikh, M.S. An Appraisal on Recent Medicinal Perspective of Curcumin Degradant: Dehydrozingerone (DZG). Bioorganic & Medicinal Chemistry 2016, 24, 501–520. [Google Scholar] [CrossRef]
- Moorkoth, S.; Prathyusha, N.S.; Manandhar, S.; Xue, Y.; Sankhe, R.; Pai, K.S.R.; Kumar, N. Antidepressant-like Effect of Dehydrozingerone from Zingiber Officinale by Elevating Monoamines in Brain: In Silico and in Vivo Studies. Pharmacol. Rep 2021, 73, 1273–1286. [Google Scholar] [CrossRef]
- Lee, E.S.; Kang, J.S.; Kim, H.M.; Kim, S.J.; Kim, N.; Lee, J.O.; Kim, H.S.; Lee, E.Y.; Chung, C.H. Dehydrozingerone Inhibits Renal Lipotoxicity in High-fat Diet–Induced Obese Mice. J Cell Mol Med 2021, 25, 8725–8733. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Cheruku, S.P.; Rao, V.; Vibhavari, R.J.A.; Sumalatha, S.; Gourishetti, K.; Rao, C.M.; Kumar, N. Dehydrozingerone Protects Temozolomide-Induced Cognitive Impairment in Normal and C6 Glioma Rats besides Enhancing Its Anticancer Potential. 3 Biotech 2020, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Tirunavalli, S.K.; Gourishetti, K.; Kotipalli, R.S.S.; Kuncha, M.; Kathirvel, M.; Kaur, R.; Jerald, M.K.; Sistla, R.; Andugulapati, S.B. Dehydrozingerone Ameliorates Lipopolysaccharide Induced Acute Respiratory Distress Syndrome by Inhibiting Cytokine Storm, Oxidative Stress via Modulating the MAPK/NF-κB Pathway. Phytomedicine 2021, 92, 153729. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Wen, C.; Yan, Z.; Olatunji, O.J.; Yin, Z. Dehydrozingerone Alleviates Hyperalgesia, Oxidative Stress and Inflammatory Factors in Complete Freund’s Adjuvant-Induced Arthritic Rats. Drug Des Devel Ther 2022, 16, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic Potential of Ginger and Its Compounds in Age-Related Neurological Disorders. Pharmacol Ther 2018, 182, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, P.J.; Bures, M.; Praestgaard, J.; Fesik, S.W. Privileged Molecules for Protein Binding Identified from NMR-Based Screening. J Med Chem 2000, 43, 3443–3447. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef]
- Pisano, M.; Pagnan, G.; Dettori, M.A.; Cossu, S.; Caffa, I.; Sassu, I.; Emionite, L.; Fabbri, D.; Cilli, M.; Pastorino, F.; et al. Enhanced Anti-Tumor Activity of a New Curcumin-Related Compound against Melanoma and Neuroblastoma Cells. Molecular Cancer 2010, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Janiak, M.; Amarowicz, R. Protective Effects of Equimolar Mixtures of Monomer and Dimer of Dehydrozingerone with α-Tocopherol and/or Ascorbyl Palmitate during Bulk Lipid Autoxidation. Food Chemistry 2014, 157, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Profumo, E.; Buttari, B.; D’Arcangelo, D.; Tinaburri, L.; Dettori, M.A.; Fabbri, D.; Delogu, G.; Riganò, R. The Nutraceutical Dehydrozingerone and Its Dimer Counteract Inflammation- and Oxidative Stress-Induced Dysfunction of In Vitro Cultured Human Endothelial Cells: A Novel Perspective for the Prevention and Therapy of Atherosclerosis. Oxid Med Cell Longev 2016, 2016, 1246485. [Google Scholar] [CrossRef]
- Marchiani, A.; Mammi, S.; Siligardi, G.; Hussain, R.; Tessari, I.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sanna, D.; et al. Small Molecules Interacting with α-Synuclein: Antiaggregating and Cytoprotective Properties. Amino Acids 2013, 45, 327–338. [Google Scholar] [CrossRef]
- Kumari, U.; Tan, E.K. LRRK2 in Parkinson’s Disease: Genetic and Clinical Studies from Patients. FEBS J 2009, 276, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.G.; Reed, X.; Singleton, A.B. Genetics in Parkinson Disease: Mendelian versus Non-Mendelian Inheritance. J Neurochem 2016, 139 Suppl 1, 59–74. [Google Scholar] [CrossRef]
- Trinh, J.; Zeldenrust, F.M.J.; Huang, J.; Kasten, M.; Schaake, S.; Petkovic, S.; Madoev, H.; Grünewald, A.; Almuammar, S.; König, I.R.; et al. Genotype-Phenotype Relations for the Parkinson’s Disease Genes SNCA, LRRK2, VPS35: MDSGene Systematic Review. Mov Disord 2018, 33, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Biskup, S.; Moore, D.J.; Celsi, F.; Higashi, S.; West, A.B.; Andrabi, S.A.; Kurkinen, K.; Yu, S.-W.; Savitt, J.M.; Waldvogel, H.J.; et al. Localization of LRRK2 to Membranous and Vesicular Structures in Mammalian Brain. Ann Neurol 2006, 60, 557–569. [Google Scholar] [CrossRef]
- Taymans, J.-M.; Van den Haute, C.; Baekelandt, V. Distribution of PINK1 and LRRK2 in Rat and Mouse Brain. J Neurochem 2006, 98, 951–961. [Google Scholar] [CrossRef]
- Wallings, R.; Manzoni, C.; Bandopadhyay, R. Cellular Processes Associated with LRRK2 Function and Dysfunction. FEBS J 2015, 282, 2806–2826. [Google Scholar] [CrossRef]
- Price, A.; Manzoni, C.; Cookson, M.R.; Lewis, P.A. The LRRK2 Signalling System. Cell Tissue Res 2018, 373, 39–50. [Google Scholar] [CrossRef]
- Mills, R.D.; Mulhern, T.D.; Cheng, H.-C.; Culvenor, J.G. Analysis of LRRK2 Accessory Repeat Domains: Prediction of Repeat Length, Number and Sites of Parkinson’s Disease Mutations. Biochem Soc Trans 2012, 40, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Schweitzer, K.J.; Leitner, P.; Zimprich, A.; Lichtner, P.; Belcredi, P.; Brüssel, T.; Schulte, C.; Maass, S.; Nägele, T.; et al. Type and Frequency of Mutations in the LRRK2 Gene in Familial and Sporadic Parkinson’s Disease*. Brain 2005, 128, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Schapira, A.H. Uniting Chinese across Asia: The LRRK2 Gly2385Arg Risk Variant. Eur J Neurol 2008, 15, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, I.N.; Kaganovich, A.; Hauser, D.N.; Beylina, A.; Chia, R.; Ding, J.; Maric, D.; Jaffe, H.; Cookson, M.R. The G2385R Variant of Leucine-Rich Repeat Kinase 2 Associated with Parkinson’s Disease Is a Partial Loss-of-Function Mutation. Biochem J 2012, 446, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Peng, R.; Wu, Y.R.; Wu, R.M.; Wu-Chou, Y.H.; Tan, L.C.; An, X.K.; Chen, C.M.; Fook-Chong, S.; Lu, C.S. LRRK2 G2385R Modulates Age at Onset in Parkinson’s Disease: A Multi-Center Pooled Analysis. Am J Med Genet B Neuropsychiatr Genet 2009, 150B, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ross, O.A.; Soto-Ortolaza, A.I.; Heckman, M.G.; Aasly, J.O.; Abahuni, N.; Annesi, G.; Bacon, J.A.; Bardien, S.; Bozi, M.; Brice, A.; et al. Association of LRRK2 Exonic Variants with Susceptibility to Parkinson’s Disease: A Case-Control Study. Lancet Neurol 2011, 10, 898–908. [Google Scholar] [CrossRef]
- Carrion, M.D.P.; Marsicano, S.; Daniele, F.; Marte, A.; Pischedda, F.; Di Cairano, E.; Piovesana, E.; von Zweydorf, F.; Kremmer, E.; Gloeckner, C.J.; et al. The LRRK2 G2385R Variant Is a Partial Loss-of-Function Mutation That Affects Synaptic Vesicle Trafficking through Altered Protein Interactions. Sci Rep 2017, 7, 5377. [Google Scholar] [CrossRef]
- Bilen, J.; Bonini, N.M. Drosophila as a Model for Human Neurodegenerative Disease. Annu Rev Genet 2005, 39, 153–171. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences in Drosophila Melanogaster. Genome Res 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Aryal, B.; Lee, Y. Disease Model Organism for Parkinson Disease: Drosophila Melanogaster. BMB Rep 2019, 52, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Imai, Y.; Gehrke, S.; Liu, S.; Lu, B. The Synaptic Function of LRRK2. Biochem Soc Trans 2012, 40, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, V.L.; Whitworth, A.J. Mechanisms of Parkinson’s Disease: Lessons from Drosophila. Curr Top Dev Biol 2017, 121, 173–200. [Google Scholar] [CrossRef]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Functional and Morphological Correlates in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Drug Effects of Withania Somnifera (Dunal) Administration. PLoS One 2016, 11, e0146140. [Google Scholar] [CrossRef]
- Casu, M.A.; Mocci, I.; Isola, R.; Pisanu, A.; Boi, L.; Mulas, G.; Greig, N.H.; Setzu, M.D.; Carta, A.R. Neuroprotection by the Immunomodulatory Drug Pomalidomide in the Drosophila LRRK2WD40 Genetic Model of Parkinson’s Disease. Front Aging Neurosci 2020, 12, 31. [Google Scholar] [CrossRef]
- Diana, A.; Collu, M.; Casu, M.A.; Mocci, I.; Aguilar-Santelises, M.; Setzu, M.D. Improvements of Motor Performances in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Effects of Dialyzed Leucocyte Extracts from Human Serum. Brain Sci 2020, 10, 45. [Google Scholar] [CrossRef]
- Mocci, I.; Casu, M.A.; Sogos, V.; Liscia, A.; Angius, R.; Cadeddu, F.; Fanti, M.; Muroni, P.; Talani, G.; Diana, A.; et al. Effects of Memantine on Mania-like Phenotypes Exhibited by Drosophila Shaker Mutants. CNS Neurosci Ther 2023, 29, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Monastirioti, M. Biogenic Amine Systems in the Fruit Fly Drosophila Melanogaster. Microsc Res Tech 1999, 45, 106–121. [Google Scholar] [CrossRef]
- Lima, S.Q.; Miesenböck, G. Remote Control of Behavior through Genetically Targeted Photostimulation of Neurons. Cell 2005, 121, 141–152. [Google Scholar] [CrossRef]
- Zars, T. Behavioral Functions of the Insect Mushroom Bodies. Curr Opin Neurobiol 2000, 10, 790–795. [Google Scholar] [CrossRef]
- Quintero-Espinosa, D.A.; Sanchez-Hernandez, S.; Velez-Pardo, C.; Martin, F.; Jimenez-Del-Rio, M. LRRK2 Knockout Confers Resistance in HEK-293 Cells to Rotenone-Induced Oxidative Stress, Mitochondrial Damage, and Apoptosis. Int J Mol Sci 2023, 24, 10474. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, M.; Nichols, R.J.; Deak, M.; Campbell, D.G.; Gillardon, F.; Knebel, A.; Alessi, D.R. LRRK2 Phosphorylates Moesin at Threonine-558: Characterization of How Parkinson’s Disease Mutants Affect Kinase Activity. Biochem J 2007, 405, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.D.; Peng, Y.; Ho, C.C.-Y.; Rideout, H.J.; Petrey, D.; Liu, P.; Dauer, W.T. The WD40 Domain Is Required for LRRK2 Neurotoxicity. PLOS ONE 2009, 4, e8463. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Zhao, Y.; Skipper, L.; Tan, M.G.; Di Fonzo, A.; Sun, L.; Fook-Chong, S.; Tang, S.; Chua, E.; Yuen, Y.; et al. The LRRK2 Gly2385Arg Variant Is Associated with Parkinson’s Disease: Genetic and Functional Evidence. Hum Genet 2007, 120, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Rodriguez-Sabate, C.; Morales, I.; Sanchez, A.; Sabate, M. Parkinson’s Disease as a Result of Aging. Aging Cell 2015, 14, 293–308. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Przedborski, S. Oxidative Stress in Parkinson’s Disease: A Mechanism of Pathogenic and Therapeutic Significance. Ann N Y Acad Sci 2008, 1147, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Beal, M.F. Mitochondrial Biology and Oxidative Stress in Parkinson Disease Pathogenesis. Nat Clin Pract Neurol 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Cook C, Petrucelli L, 2009 A Critical Evaluation of the Ubiquitin-Proteasome System in Parkinson’s Disease. Biochim Biophys Acta 1792, 664–675.
- Pan, T.; Kondo, S.; Le, W.; Jankovic, J. The Role of Autophagy-Lysosome Pathway in Neurodegeneration Associated with Parkinson’s Disease. Brain 2008, 131, 1969–1978. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.K.; Koleva, L.; Kancheva, V.D.; Delogu, G. Comparative Study of Antioxidant Potential of Curcumin and Its Degradation Products–Vanillin, Ferulic Acid and Dehydrozingerone. Bulg Chem Commun, 2018, 50, 158–163. [Google Scholar]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Dmp53, Basket and drICE Gene Knockdown and Polyphenol Gallic Acid Increase Life Span and Locomotor Activity in a Drosophila Parkinson’s Disease Model. Genet Mol Biol 2013, 36, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective Effects of Methanolic Extract of Avocado Persea Americana (Var. Colinred) Peel on Paraquat-Induced Locomotor Impairment, Lipid Peroxidation and Shortage of Life Span in Transgenic Knockdown Parkin Drosophila Melanogaster. Neurochem Res 2019, 44, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of Curcumin on Lifespan, Activity Pattern, Oxidative Stress, and Apoptosis in the Brains of Transgenic Drosophila Model of Parkinson’s Disease. Biomed Res Int 2014, 2014, 606928. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Youdim, M.B.H. Catechin Polyphenols: Neurodegeneration and Neuroprotection in Neurodegenerative Diseases. Free Radic Biol Med 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.K.; Chung, K.K.K. Oxidative and Nitrosative Stress in Parkinson’s Disease. Biochim Biophys Acta 2009, 1792, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, T.; Liu, Z.; Arbez, N.; Yan, J.; Moran, T.H.; Ross, C.A.; Smith, W.W. LRRK2 Kinase Activity Mediates Toxic Interactions between Genetic Mutation and Oxidative Stress in a Drosophila Model: Suppression by Curcumin. Neurobiol Dis 2012, 47, 385–392. [Google Scholar] [CrossRef]
- Reale, M.; Pesce, M.; Priyadarshini, M.; Kamal, M.A.; Patruno, A. Mitochondria as an Easy Target to Oxidative Stress Events in Parkinson’s Disease. CNS Neurol Disord Drug Targets 2012, 11, 430–438. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.H.; Fujioka, H.; Liu, J.; Wilson-Delfosse, A.; Chen, S.G.; Perry, G.; Casadesus, G.; Zhu, X. LRRK2 Regulates Mitochondrial Dynamics and Function through Direct Interaction with DLP1. Hum Mol Genet 2012, 21, 1931–1944. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Shaltouki, A.; Gonzalez, A.E.; Bettencourt da Cruz, A.; Burbulla, L.F.; St Lawrence, E.; Schüle, B.; Krainc, D.; Palmer, T.D.; Wang, X. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell 2016, 19, 709–724. [Google Scholar] [CrossRef]
- Mancini, A.; Mazzocchetti, P.; Sciaccaluga, M.; Megaro, A.; Bellingacci, L.; Beccano-Kelly, D.A.; Di Filippo, M.; Tozzi, A.; Calabresi, P. From Synaptic Dysfunction to Neuroprotective Strategies in Genetic Parkinson’s Disease: Lessons From LRRK2. Front Cell Neurosci 2020, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Angeles, D.C.; Ho, P.; Chua, L.L.; Wang, C.; Yap, Y.W.; Ng, C.; Zhou, Z. dong; Lim, K.-L.; Wszolek, Z.K.; Wang, H.Y.; et al. Thiol Peroxidases Ameliorate LRRK2 Mutant-Induced Mitochondrial and Dopaminergic Neuronal Degeneration in Drosophila. Hum Mol Genet 2014, 23, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-Targeted Antioxidants for Treatment of Parkinson’s Disease: Preclinical and Clinical Outcomes. Biochim Biophys Acta 2014, 1842, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Liu, J.; Ao, N.; Yan, B.; Shen, W.; Wang, X.; Li, X.; Luo, C.; Liu, J. Combined R-α–Lipoic Acid and Acetyl-L-Carnitine Exerts Efficient Preventative Effects in a Cellular Model of Parkinson’s Disease. J Cell Mol Med 2010, 14, 215–225. [Google Scholar] [CrossRef]
- Abdin, A.A.; Sarhan, N.I. Intervention of Mitochondrial Dysfunction-Oxidative Stress-Dependent Apoptosis as a Possible Neuroprotective Mechanism of α-Lipoic Acid against Rotenone-Induced Parkinsonism and L-Dopa Toxicity. Neurosci Res 2011, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective Effects of Hesperidin, a Plant Flavanone, on Rotenone-Induced Oxidative Stress and Apoptosis in a Cellular Model for Parkinson’s Disease. Oxid Med Cell Longev 2013, 2013, 102741. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yu, H.-T.; Pu, X.-P.; Du, G.-H. Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and up-Regulation of DJ-1 Protein Expression. Molecules 2013, 18, 14726–14738. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective Effect of Lycopene on Oxidative Stress and Cognitive Decline in Rotenone Induced Model of Parkinson’s Disease. Neurochem Res 2011, 36, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.A.; Smith, W.W. Curcumin Protects against A53T Alpha-Synuclein-Induced Toxicity in a PC12 Inducible Cell Model for Parkinsonism. Pharmacol Res 2011, 63, 439–444. [Google Scholar] [CrossRef]
- Parkinson Study Group QE3 Investigators; Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; et al. A Randomized Clinical Trial of High-Dosage Coenzyme Q10 in Early Parkinson Disease: No Evidence of Benefit. JAMA Neurol 2014, 71, 543–552. [CrossRef]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators; Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; et al. Effect of Creatine Monohydrate on Clinical Progression in Patients with Parkinson Disease: A Randomized Clinical Trial. JAMA 2015, 313, 584–593. [CrossRef]
- Fouquet, W.; Owald, D.; Wichmann, C.; Mertel, S.; Depner, H.; Dyba, M.; Hallermann, S.; Kittel, R.J.; Eimer, S.; Sigrist, S.J. Maturation of Active Zone Assembly by Drosophila Bruchpilot. J Cell Biol 2009, 186, 129–145. [Google Scholar] [CrossRef]
- Piccoli, G.; Onofri, F.; Cirnaru, M.D.; Kaiser, C.J.O.; Jagtap, P.; Kastenmüller, A.; Pischedda, F.; Marte, A.; von Zweydorf, F.; Vogt, A.; et al. Leucine-Rich Repeat Kinase 2 Binds to Neuronal Vesicles through Protein Interactions Mediated by Its C-Terminal WD40 Domain. Mol Cell Biol 2014, 34, 2147–2161. [Google Scholar] [CrossRef]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The Role of Ca2+ Signaling in Parkinson’s Disease. Disease Models & Mechanisms 2017, 10, 519–535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




