Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Definition and Classification of Algae in Recreational Waters
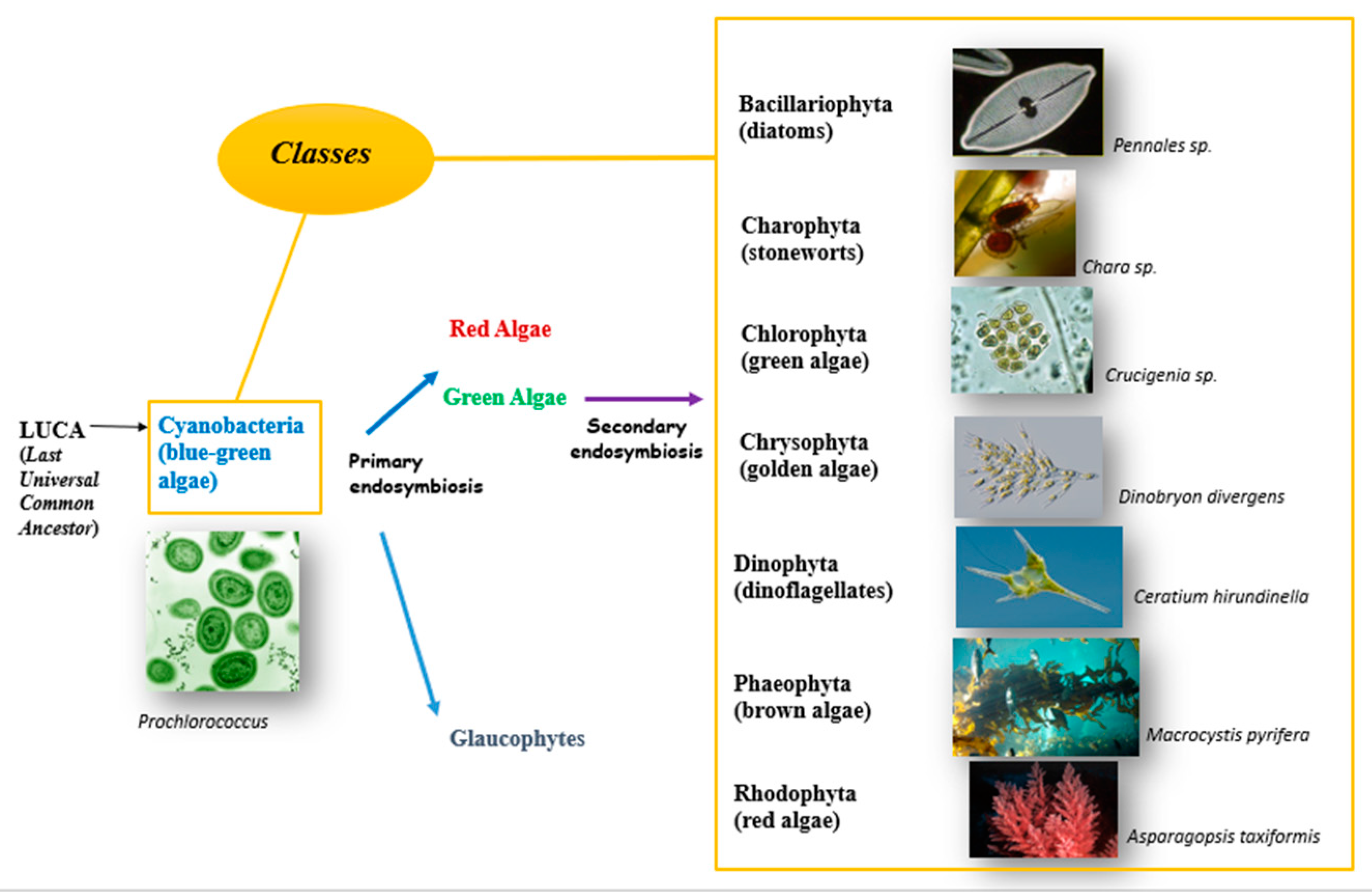
2.1. Evolution of Eukaryotic Photosynthetic Algae
2.2. Algal Taxonomy and Phylogeny
2.3. Classification of Algae
2.2. Algae in Recreational Waters
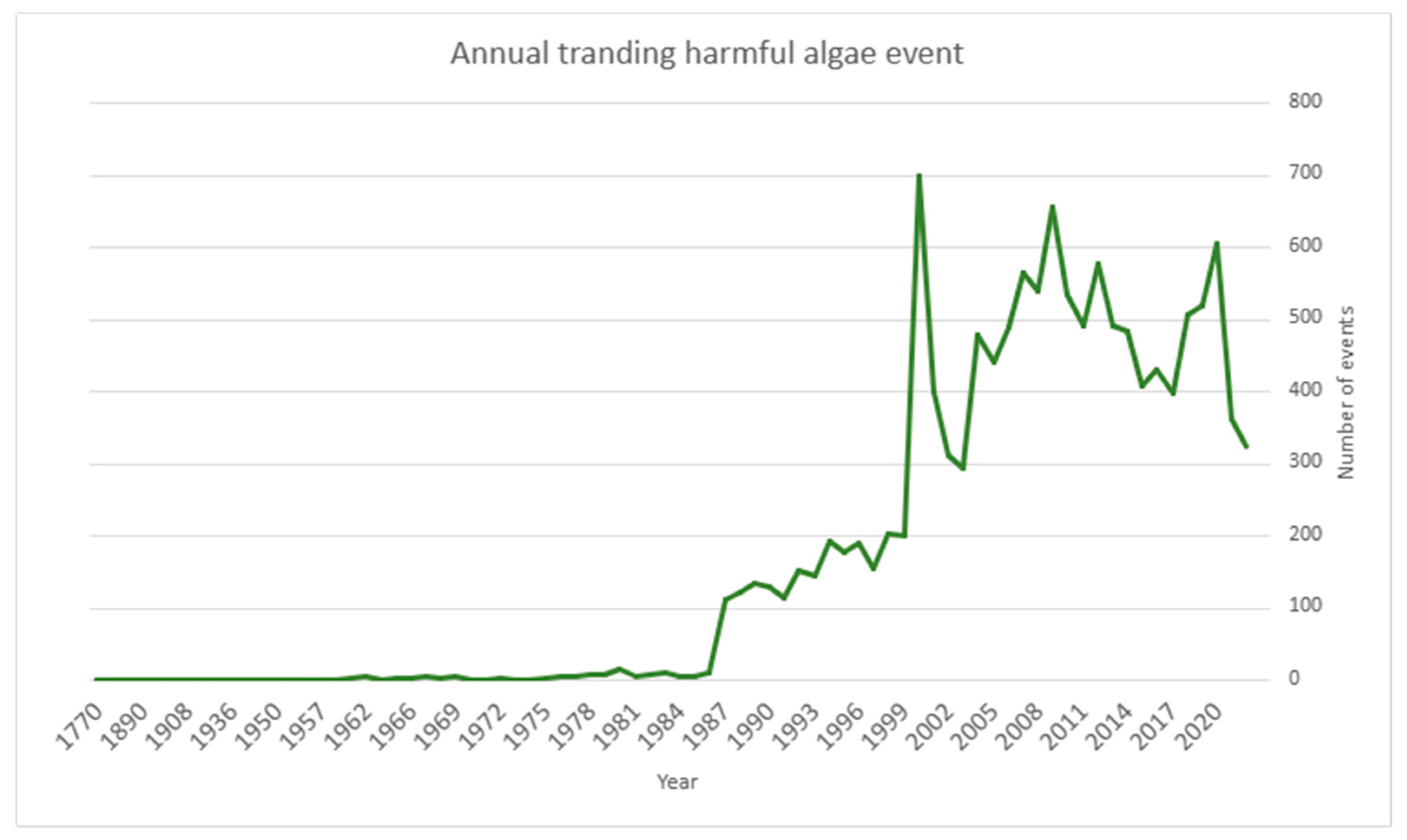
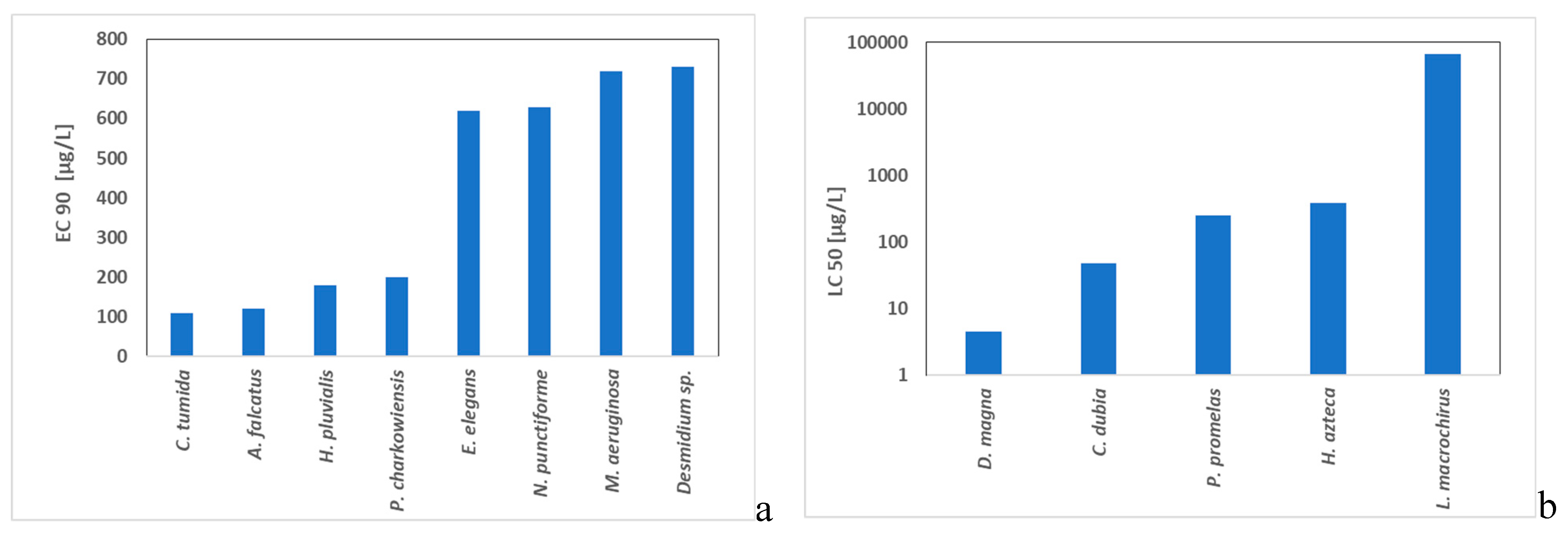
3. Environmental Epidemiology and Ecotoxicity
3.1. Ecotoxicity of Algal Blooms and Related Toxins
3.2. Environmental Epidemiology of HABs, Algal and Cyanobacterial Toxins
| Toxins procuding genera | Toxin category | Toxin classification (based on effects on humans and animals) | WHO guideline value in recreational watera | References |
|---|---|---|---|---|
| Anabaenopsis, Aphanizomenon, Dolichospermum (formerly, Ananbaena), Mycrocystis, Oscillatoria, Phormidium, Planktothrix | Microcystins | Hepatotoxins | 24 µg/L | [70,71] |
| Nodularia, Nostoc | Nodularins | Not established | [70,71,72] | |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Lyngbya, Oscillatoria, Raphidopsis, Umezakia | Cylindrospermopsin | Cytotoxins | 6 µg/L | [70,73] |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Oscillatoria | Anatoxins | Neurotoxins | 60 µg/L | [74,75] |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Lyngbya, Planktothrix Raphidiopsis | Saxitoxins | 30 µg/L | [70,76,77] | |
| Aphanizomenon, Dolichospermum (formerly, Ananbaena), Mycrocistis, Nodularia, Nostoc | b-Methylamino L-Alanine (BMAA) | Not established | [78,79] | |
| Anacystis, Dolichospermum (formerly, Ananbaena), Microcystis, Oscillatoria, Schizothrix, Synechococcus | Lypopolysaccharides | Dermatoxins | Not established | [80,81] |
| Lyngbya | Lyngbyatoxins | Not established | [82,83] | |
| Lyngbya, Oscillatoria, Schizothrix | Aplysiatoxins | Not established | [84] |
3.3. Algae and Cyanobacteria in Spa and Thermal Spring Water Sources
4. Clinical Epidemiology of HABs, Algal and Cyanobacterial Toxins
5. Anti-Algae and Treatments
5.1. Quaternary Ammonium Compounds (QAC)
5.2. Metallic Pool Algaecides
5.3. Algae Removal
6. Detection Methods of Algae
7. Risk Assessment, Laws and Water Safety Plan
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- World Health Organization; 2003. Guidelines on recreational water quality: Volume 1 Coastal and fresh waters. WHO, Geneva.
- Summers, E. J.; Ryder, J. L. A critical review of operational strategies for the management of harmful algal blooms (HABs) in inland reservoirs. J. Environ. Manag. 2023, 330, 117141. [Google Scholar] [CrossRef] [PubMed]
- Varga, C. To treat or not to treat? Misbeliefs in spa water disinfection. Int J Biometeorol 2019, 63, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, S.; Kalkan, E.; Nadaroglu, H. Removal of algae from thermal mud pool: A case study in Koprukoy (Erzurum, Northeast (NE) Turkey) thermal spring area. Bull. Chem. Soc. Ethiop. 2022, 36, 545–553. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Wang, D. Influence of allochthonous organic matters on algae removal: Organic removal and floc characteristics. Colloids Surf A Physicochem Eng Asp 2019, 583, 123995. [Google Scholar] [CrossRef]
- Qi, J.; Lan, H.C.; Miao, S.Y.; Xu, Q.; Liu, R.; Liu, H.; Qu, J. KMnO4-Fe(II) pretreatment to enhance Microcystis aeruginosa removal by aluminum coagulation: Does it work after long distance transportation. Water Res 2010, 44, 3617–3624. [Google Scholar] [CrossRef]
- Gilmour, D.J. Diversity of algae and their biotechnological potential. Adv. Microb. Physiol. 2023, 82. [Google Scholar] [CrossRef]
- Cohen, P.A.; Kodner, R.B. The earliest history of eukaryotic life: Uncovering an evolutionary story through the integration of biological and geological data. Trends Ecol. Evol. 2022, 37, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, N.J. Bangiomorpha pubescens n. gen., n. sp.: Implication for the evolution of sex, multicellularity and the mesoproterozoic/neoproterozoic radiation of eukaryotes. J. Paleontol. 2011, 26, 1–20. [Google Scholar]
- Brocks, J.J. The transition from a cyanobacterial to alga world and the emergence of animals. Emerg. Top. Life Sci. 2018, 2, 181–190. [Google Scholar] [CrossRef]
- Adl, S. M.; Simpson, A. G.; Lane, C. E.; Lukeš, J.; Bass, D.; Bowser, S. S.; Brown, M. W.; Burki, F.; Dunthorn, M.; Hampl, V.; Heiss, A.; Hoppenrath, M.; Lara, E.; Le Gall, L.; Lynn, D. H.; McManus, H.; Mitchell, E. A.; Mozley-Stanridge, S. E.; Parfrey, L. W.; Pawlowski, J.; Spiegel, F. W. Revis. Classif. Eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [CrossRef]
- Bolton, J.J. What is aquatic botany? - And why algae are plants: The importance of non-taxonomic terms for groups of organisms. Aquat. Bot. 2016, 132, 1–4. [Google Scholar] [CrossRef]
- Ponce-Toledo, R.I.; Lopez-Garcia, P.; Moreira, D. Horizontal and endosymbiotic gene transfer in early plastid evolution. New Phytol 2019, 224, 618–624. [Google Scholar] [CrossRef]
- Adl, S. M.; Bass, D.; Lane, C. E.; Lukeš, J.; Schoch, C. L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M. W.; Burki, F,; Cárdenas, P. ; Čepička, I.; Chistyakova, L.; Del Campo, J.; Dunthorn, M.; Edvardsen, B.; Eglit, Y.; Guillou, L.; Hampl, V.; Heiss, A. A.; Zhang, Q. Revisions to the classification, nomenclature and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The kingdom chromista: Origin and systematics. Prog. Phycol. Res. Biopress Bristol. 1986, 309–347. [Google Scholar]
- Javed, M.R.; Bilal, M.J.; Mehmood, M.A.; Nashat, N. Microalgae as a Feedstock for Biofuel Production: Current Status and Future Prospects. Energy Res. Dev. 2019, 3. [Google Scholar]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae - A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94. [Google Scholar] [CrossRef]
- Nation Museum of Natural Hystory. The classification. Available online: https://naturalhistory.si.edu/research/botany/research/algae/algae-classification (accessed on 20 December 2023).
- Barsani, L.; and Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology. Third edition. (2023). By CRC Press.
- Fritsch, F.E. The structure and reproduction of algae, vol I. 1935. Cambridge University Press, London.
- Ghosh, A.; Kiran, B. Carbon Concentration in Algae: Reducing CO2 From Exhaust Gas. Trends Biotechnol. 2017, 35, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Ambika, H.D. Positive and negative environmental impacts on algae. Algae Mater. 2023, 343–353. [Google Scholar] [CrossRef]
- World Health Organization; 2021a. Guidelines on Recreational Water Quality: Volume 1 Coastal and Fresh Waters. WHO, Geneva.
- Centers for Disease Control and Prevention, 2023. Available online: https://www.cdc.gov/habs/be-aware-habs.html (accessed on 7 November 2023).
- Dallas, H. Water temperature and riverine ecosystems: An overview of knowledge and approaches for assessing biotic responses, with special reference to South Africa. Water SA 2008, 34, 393–404. [Google Scholar] [CrossRef]
- Sompong, U.; Hawkins, P.R.; Besley, C.; Peerapornpisal, Y. The distribution of cyanobacteria across physical and chemical gradients in hot springs in northern Thailand. FEMS Microbiol Ecol 2005, 52, 365–376. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.H.; Kwon, E.E. (2017). The role of algae and cyanobacteria in the production and release of odorants in water. Environmental pollution (Barking, Essex: 1987) 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, M. The distribution of algae and insects in hot spring thermal gradients at Waimangu, New Zealand. N. Z. J. Mar. Freshw. Res 1969, 3, 459–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Whalen, J.K.; Cai, C.; Shan, K.; Zhou, H. Cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A nonnegligible chronic health and ecological hazard. Water Res 2023, 233, 119807. [Google Scholar] [CrossRef] [PubMed]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria e ecology, toxin production and risk management. Water Res 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Kurmayer, R.; Azevedo, S.M.F.O.; Wood, S.A.; Chorus, I.; Welker, M. Understanding the occurrence of cyanobacteria and cyanotoxins. Toxic Cyanobacteria Water 2021, 213–294. [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Harmful Algal Bloom (HAB) Associated Illness. May 27, 2016. Available online: http://www.cdc.gov/habs/general.html (accessed on 7 November 2023).
- Environmental Protection Agency, 2022. Available online: https://www.epa.gov/cyanohabs/learn-aboutcyanobacteria-and-cyanotoxins (accessed on 13 November 2023).
- Chorus, I. and Welker M. 2021 Toxic Cyanobacteria in Water - A guide to their public health consequences, monitoring and management. 2nd Edition. CRC Press, Boca Raton, FL. on behalf of the World Health Organization, Geneva. Taylor & Francis.
- Chatterjee, S.; More, M. Cyanobacterial Harmful Algal Bloom Toxin Microcystin and Increased Vibrio Occurrence as Climate-Change-Induced Biological Co-Stressors: Exposure and Disease Outcomes via Their Interaction with Gut-Liver-Brain Axis. Toxins (Basel) 2023, 15, 2. [Google Scholar] [CrossRef]
- Olker, J.; Banerji, A.; Benesh, K.; Kinziger, B.; Scott, T.; Karschnik, T.; Frisch, J.; Feist, T.; Pilli, A.; Hoff, D. Evidence Map for Ecological Toxicity of Cyanotoxins using ECOTOXicology Knowledgebase Systematic Protocols. Science Inventory 2023.
- Mehinto, A.C. , Smith, J., Wenger, E., Stanton, B., Linville, R., Brooks, B.W., Sutula, M.A., Howard, M.D.A. Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats - Evaluating the basis for developing thresholds protective of aquatic life in the United States. Sci Total Env. 2021, 795, 148864. [Google Scholar] [CrossRef]
- Shahmohamadloo R., S. , Poirier D. G., Almirall X. O., Bhavsar S. P., Sibley P. K. Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicol. Environ. Saf. 2020, 188, 109945. [Google Scholar] [CrossRef]
- Smutná, M. , Babica P., Jarque S., Hilscherová K., Maršálek B., Haeba M., Bláha L. Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon 2014, 79, 11–18. [Google Scholar] [CrossRef]
- Davis, T.W.; Koch, F.; Marcoval, M.A.; Wilhelm, S.W.; Gobler, C.J. Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 2020, 15, 26–35. [Google Scholar] [CrossRef]
- Palíková, M.; Krejcí, R.; Hilscherová, K.; Babica, P.; Navrátil, S.; Kopp, R.; Bláha, L. Effect of different cyanobacterial biomasses and their fractions with variable microcystin content on embryonal development of carp (Cyprinus carpio L.). Aquat Toxicol. 2007, 81, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Kasianchuk, N.; Siemens, E.; Henao, E.; Rzymski, P. A Review of Common Cyanotoxins and Their Effects on Fish. Toxics 2023, 11, 118. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Wu, J.L.; Liu, W.X.; Wen, C.G.; Qian, G.M.; Hu, B.Q.; Jian, S.Q.; Yang, G.; Dong, J. Effect of microcystin on the expression of Nrf2 and its downstream antioxidant genes from Cristaria plicata. Aquat. Toxicol. 2020, 225, 105526. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Pekar, H.; Oskarsson, A. Microcystins activate nuclear factor erythroid 2-related factor 2 (Nrf2) in human liver cells in vitro—Implications for an oxidative stress induction by microcystins. Toxicon 2017, 126, 47–50. [Google Scholar] [CrossRef]
- Shaw, G.R.; Seawright, A.A.; Moore, M.R.; Lam, P.K.S. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicological activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef]
- Shu, Y.; Jiang, H.; Yuen, C.N.T.; Wang, W.; He, J.; Zhang, H.; Liu, G.; Wei, L.; Chen, L.; Wu, H. Microcystin-leucine arginine induces skin barrier damage and reduces resistance to pathogenic bacteria in Lithobates catesbeianus tadpoles. Ecotoxicol. Environ. Saf. 2022, 238, 113584. [Google Scholar] [CrossRef]
- Xia, H.; Song, T.; Wang, L.; Jiang, L.; Zhou, Q.; Wang, W.; Liu, L.; Yang, P.; Zhang, X. Effects of dietary toxic cyanobacteria and ammonia exposure on immune function of blunt snout bream (Megalabrama amblycephala). Fish Shellfish Immun. 2018, 78, 383–391. [Google Scholar] [CrossRef]
- Qiao, Q.; Liang, H.; Zhang, X. Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 2013, 90, 1167–1176. [Google Scholar] [CrossRef]
- Patiño, R.; Christensen, V.G.; Graham, J.L.; Rogosch, J.S.; Rosen, B.H. Toxic Algae in Inland Waters of the Conterminous United States—A Review and Synthesis. Water 2023, 15, 2808. [Google Scholar] [CrossRef]
- Rattner, B.A.; Wazniak, C.E.; Lankton, J.S.; McGowan, P.C.; Drovetski, S.V.; Egerton, T.A. Review of harmful algal bloom effects on birds with implications for avian wildlife in the Chesapeake Bay region. Harmful Algae. 2022, 120, 102319. [Google Scholar] [CrossRef]
- EU Council, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=ES (accessed on 14 November 2023).
- Li, J.; Parkefelt, L.; Persson, K. M.; Pekar, H. Improving cyanobacteria and cyanotoxin monitoring in surface waters for drinking water supply. J. Water Secur. 2017, 3. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S. , Lettieri, T. Algal bloom and its economic impact. European Commission, JRC Technical Reports. 2016.
- World Health Organization, 2017. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. World Health Organization, Geneva.
- IARC, 2010. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. World Health Organization; International Agency for Research on Cancer; Lyon, France: 2010.
- Hurtado, I.; Pouget, L.; Fernández, S.; Cascales, P. Monitoring and forecasting cyanobacteria risk for a drinking water plant in Spain. Water Supply 2022, 22(7), 6296–6307. [Google Scholar] [CrossRef]
- Roberts, V.A.; Vigar, M.; Backer, L.; et al. Surveillance for Harmful Algal Bloom Events and Associated Human and Animal Illnesses — One Health Harmful Algal Bloom System, United States, 2016–2018. MMWR Morb Mortal Wkly Rep 2020, 69, 1889–1894. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), 2023. Summary Report – One Health Harmful Algal Bloom System (OHHABS), United States, 2021. Atlanta, Georgia: U.S. Department of Health and Human Services, CDC, 2023. Available online: https://www.cdc.gov/habs/data/2021-ohhabs-data-summary.html (accessed on 11 November 2023).
- Harmful Algae Event Database, 2023. Available online: http://haedat.iode.org/ (accessed on 13 November 2023).
- Tito, J.C.R.; Luna, L.M.G.; Noppe, W.N.; Hubert, I.A. First Report on Microcystin-LR Occurrence in Water Reservoirs of Eastern Cuba, and Environmental Trigger Factors. Toxins 2022, 14, 209. [Google Scholar] [CrossRef]
- Oliveira, E.D.C.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapa, Brazil. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Banack, S.A. , Dargham, S.R., Richer, R.A. Cyanobacteria and cyanotoxins are present in drinking water impoundments and groundwater wells in desert environments. Toxicon 2016, 114, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Douma, M.; Ouahid, Y.; del Campo, F.F.; Loudiki, M.; Mouhri, K.; Oudra, B. Identification and quantification of cyanobacterial toxins (microcystins) in two Moroccan drinking-water reservoirs (Mansour Eddahbi, Almassira). Environ. Monit. Assess. 2010, 160, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Gucci, P.M.B.; Pierdominici, E.; Sestili, P.; Joppolo, A.; Volterra, L. Anatoxin-a and previously unknown toxin in Anabaena planctonica from blooms found in Lake Mulargia (Italy). Toxicon 1994, 32, 369–73. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Gucci, P.M.B.; Pierdominici, E.; Sestili, P.; Joppolo, A.; Volterra, L. Production of microcystinlike toxins in different freshwater species of Oscillatoria. Toxicon 1992, 32, 1307–11. [Google Scholar] [CrossRef] [PubMed]
- Messineo V; Bogialli S; Melchiorre S; Sechi N; Luglié A; Casiddu P; Mariani MA; Padedda BM; Di Corcia A; Mazza R; Carloni E; Bruno M. Cyanobacterial toxins in Italian freshwaters. Limnologica 2009, 39, 95–106. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità, 2011. Rapporti ISTISAN 11/35 Pt.1. Cyanobacteria in water for human consumption. State of knowledge for risk assessment. Volume 1. Edited by Luca Lucentini and Massimo Ottaviani for “National Group for cyanobacteria risk management in water for human consumption” 2011, xxii, 165 p.
- S-3 EUROHAB Project, 2023. Sentinel-3 satellite products for detecting Eutrophication and Harmful Algal Bloom events in the French-English Channel. European Union. Official project website Available to: https://www.s3eurohab.eu/ (accessed on 14 November 2023). (accessed on 14 November 2023).
- Boopathi, T. and J.S. Ki, Impact of environmental factors on the regulation of cyanotoxin production. Toxins (Basel), 2014, 6, 1951–78. [Google Scholar] [CrossRef]
- Pearson, L. , et al., On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar Drugs 2010, 8, 1650–80. [Google Scholar] [CrossRef]
- Weirich, C.A. and T.R. Miller. Freshwater harmful algal blooms: toxins and children's health. Curr Probl Pediatr Adolesc Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R. and I.R. Falconer, Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Env. Toxicol 2003, 18, 94–103. [Google Scholar] [CrossRef]
- Astrachan, N.B.; Archer, B.G.; Hilbelink, D.R. Evaluation of the subacute toxicity and teratogenicity of anatoxin-a. Toxicon 1980, 18, 684-8. [Google Scholar] [CrossRef]
- Bumke-Vogt, C.; Mailahn, W.; Chorus, I. Anatoxin-a and neurotoxic cyanobacteria in German lakes and reservoirs. Environ. Toxicol. 1999, 14, 117–125. [Google Scholar] [CrossRef]
- Van Apeldoorn, M.E. , et al. Toxins of cyanobacteria. Mol Nutr Food Res 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Strichartz, G. Structural determinants of the affinity of saxitoxin for neuronal sodium channels. Electrophysiological studies on frog peripheral nerve. J Gen Physiol 1984, 84, 281–305. [Google Scholar] [CrossRef]
- Holtcamp, W. The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease? Env. Health Perspect 2012, 120, A110–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L. , et al., Quantification of neurotoxin BMAA (beta-N-methylamino-Lalanine) in seafood from Swedish markets. Sci Rep 2014, 4, 6931. [Google Scholar] [CrossRef] [PubMed]
- Torokne, A.; Palovics, A. ; M. Bankine. Allergenic (sensitization, skin and eye irritation) effects of freshwater cyanobacteria--experimental evidence. Env. Toxicol 2001, 16, 512-6. [Google Scholar] [CrossRef] [PubMed]
- Blahova, L. , et al., The isolation and characterization of lipopolysaccharides from Microcystis aeruginosa, a prominent toxic water bloom forming cyanobacteria. Toxicon 2013, 76, 187–96. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.T.; Webb, P.M.; Shaw, G.R. The toxins of Lyngbya majuscule and their human and ecological health effects. Environ. Int. 2001, 27, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Arthur, K. , et al., The exposure of green turtles (Chelonia mydas) to tumour promoting compounds produced by the cyanobacterium Lyngbya majuscula and their potential role in the aetiology of fibropapillomatosis. Harmful Algae 2008, 7, 114–125. [Google Scholar] [CrossRef]
- Churro, C.; Dias, E.; Valrio, E. Risk Assessment of Cyanobacteria and Cyanotoxins, the Particularities and Challenges of Planktothrix spp. Monitoring. 2012.
- Gere, D.; Róka, E.; Záray, G.; Vargha, M. Disinfection of Therapeutic Spa Waters: Applicability of Sodium Hypochlorite and Hydrogen Peroxide-Based Disinfectants. Water 2022, 14, 690. [Google Scholar] [CrossRef]
- World Health Organization. 2013. WHO traditional medicine strategy: 2014-2023. World Health Organization. ISBN: 9789241506090.
- Balegová, J. The mineral spa of Rudnok as described by staff colonel Henrik Mayer. Comm. Hist. Art. Med. 2008, 54, 87–92. [Google Scholar]
- World Health Organization, 2006. Available online: http://apps.who.int/iris/bitstream/10665/43336/1/9241546808_eng.pdf (accessed on 6 November 2023).
- Health and Safety Executive, 2014. Available online: www.hse.gov.uk/pubns/books/hsg282.htm (accessed on 6 November 2023).
- EU Council, 2020. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=ES. (accessed on November 7th, 2023).
- Ministero della Salute. 2012. Decreto 7 febbraio 2012, n. 25 Disposizioni tecniche concernenti apparecchiature finalizzate al trattamento dell'acqua destinata al consumo umano. (12G0044) (GU Serie Generale n.69 del 22-03-2012) Available to: https://shorturl.at/gDM35. (Accessed on November 5th, 2023). 5 November.
- Regione Toscana, 2004. Legge Regionale 27 luglio 2004, n. 38 Norme per la disciplina della ricerca, della coltivazione e dell'utilizzazione delle acque minerali, di sorgente e termali. (GU 3a Serie Speciale Regioni n.45 del 13/11/2004) Available to: https://www.edizionieuropee.it/law/html/208/to4_04_012.html. (Accessed on November 5th, 2023). 5 November 2023.
- Regione Campania, 2008. Legge Regionale n. 8 del 29 luglio 2008. “Disciplina della ricerca ed utilizzazione delle acque minerali e termali, delle risorse geotermiche e delle acque di sorgente”. Available to: https://www.regione.campania.it/normativa/userFile/documents/attachments/1953_8-2008Storico.pdf (Accessed on November 5th, 2023). 5 November.
- Sevillano, D.; Romero-Lasta, C.I.; Alou, L.; Gonzáles, N. Impact of the biotic and abiotic components of low mineralized natural mineral waters on the growth of pathogenic bacteria of human origin: a key to self-control of spa water quality. J. Hydrol 2018, 566, 227–234. [Google Scholar] [CrossRef]
- Margarucci, L. M.; Romano Spica, V.; Gianfranceschi, G.; Valeriani, F. Untouchability of natural spa waters: Perspectives for treatments within a personalized water safety plan. Environ. Int. 2019, 133(Pt A), 105095. [Google Scholar] [CrossRef]
- Valeriani, F.; Gianfranceschi, G.; Romano Spica, V. The microbiota as a candidate biomarker for SPA pools and SPA thermal spring stability after seismic events. Environ. Int. 2020, 137, 105595. [Google Scholar] [CrossRef] [PubMed]
- Giampaoli, S.; Valeriani, F.; Gianfranceschi, G.; Vitali, M.; Delfini, M.; Festa, M.; Bottari, E.; Romano Spica, V. Hydrogen sulfide in thermal spring waters and its action on bacteria of human origin. Microchem. J. 2013, 108, 210–214. [Google Scholar] [CrossRef]
- Vachee, A.; Vincent, P.; Struijk, C.B.; Mossel, D.A.A.; Leclerc, H. Study of the fate of the autochtonous bacterial flora of still mineral waters by analysis of restriction fragment length polymorphism of genes coding for rRNA. Syst. Appl. Microbiol. 1997, 20, 492–503. [Google Scholar] [CrossRef]
- Lu, J.; Struewing, I.; Vereen, E.; Kirby, A.; Levy, K.; Moe, C.; Ashbolt, N. Molecular detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. J Appl Microbiol 2016, 120, 509–521. [Google Scholar] [CrossRef]
- Giorgio, A.; Carraturo, F.; Aliberti, F.; De Bonis, S.; Libralato, G.; Morra, M.; Guida, M. Characterization of microflora composition and antimicrobial activity of algal extracts from Italian thermal muds. J. Nat. Sci. Biol. Med. 2018, 9. [Google Scholar]
- Kalkan, E. Algae potential of the Delicermik thermal spring area (Köprüköy-Erzurum, NE Turkey). Int. J. Lat. Technol. Eng. Manag. Appl. Sci. 2019, 8, 98–101. [Google Scholar]
- Pabuccu, K. A research on Köprüköy-Deliçermik algal flora. Master Thesis, Graduate School of Natural and Applied Sciences, Atatürk University, Turkey, 1993.
- Gerwick, L; Gerwick, W.H.; Coates, R.C.; Engene, N.; Grindberg, R.V.; Jones A.C., et al.; et al. Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe 2008, 3, 277–284. [Google Scholar] [CrossRef]
- Sivonen, K; Börner, T. Bioactive compounds produced by cyanobacteria. In: The Cyanobacteria: Molecular Biology, Genomics and Evolution. Norfolk: Caister Acad. Press 2008, 15997.
- Costa, M.; Garcia, M.; Costa Rodrigues, J.; Costa, M.S.; Ribeiro, M.J.; Fernandes, M.H.; et al. Exploring bioactive properties of marine cyanobacteria isolated from the Portuguese coast: High potential as a source of anticancer compounds. Mar Drugs 2013, 12, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Surakka, A. , Sihvonen, L.M., Lehtimäki, J.M., Wahlsten, M., Vuorela, P., Sivonen, K., et al. Benthic cyanobacteria from the Baltic sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and a apoptosis inducing phormidium strain. Env. Toxicol 2005, 20, 285–92. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O.; et al. Novel extracellular diterpenoids with biological activity from the Cyanobacterium nostoc commune. J Nat Prod 2000, 63, 339–43. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.A.; Metera, K.L.; Kumar, J.R.; Watson, L.V.; Girouard, G.S.; Guan, Y., et al.; et al. Immunostimulatory principles from Chlorella pyrenoidosa – Part 1: Isolation and biological assessment in vitro. Phytomedicine 2007, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Kido, N. ; Sugiyama, T; Yokochi, T. Antiviral activity of acidic polysaccharides from Coccomyxa gloeobotrydiformi, a green alga, against an in vitro human influenza A virus infection. Immunopharmacol Immunotoxicol 2013, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kust, A.; Urajová, P.; Hrouzek, P.; Čapková, K.; Štenclová, L.; Řeháková, K.; Kozlíková-Zapomělová, E.; Lepšová-Skácelová, O.; Lukešová, A.; Mareš, J. ; A new microcystin producing Nostoc strain discovered in broad toxicological screening of non-planktic Nostocaceae (cyanobacteria). Toxicon 2018, 150, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Otero, P.; Alfonso, A.; Ramos, V.; Vasconcelos, V.; Aráoz, R.; Botana, L.M. Detection of anatoxin-a and three analogs in Anabaena spp. cultures: new fluorescence polarization assay and toxin profile by LC-MS/MS. Toxins 2014, 6, 402–415. [Google Scholar] [CrossRef]
- Stoyneva, M. Survey on green algae of Bulgarian thermal springs. Biol. Bratisl. - Sect. Bot. 2003, 58, 563–574. [Google Scholar]
- Lai, G.G.; Wetzel, C.E.; Ector, L.; Lugliè, A.; Padedda, B.M. Composition, structure, and distribution of diatom assemblages in Mediterranean thermal spring ecotones affected by natural and human disturbances. Aquat Sci 2023, 85, 55. [Google Scholar] [CrossRef]
- Ulcay, S.; Öztürk, M.; Kurt, O. Algae Flora of Germencik-Alangüllü (Aydın, Turkey) Thermal Water. Celal Bayar Univ. J. Sci. 2017, 13, 601–608. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Süßwasserflora von Mitteleuropa. Cyanoprokaryota; 2nd Part: Oscil. 2005, 19, 759.
- Veen, A.; Hof, C.H.J.; Kouwets, F.A.C.; Berkhout, T. Rijkswaterstaat Waterdienst, Informatiehuis Water [Taxa Watermanagement the Netherlands (TWN)] Available to: http://ipt.nlbif.nl/ipt/resource?r=checklist-twn. (Accessed November 7th, 2023). 7 November.
- Anagnostidis, K. Untersuchungen über die Cyanophyceen einiger Thermen in Griechenland. Institute Systematic Botany & Pflanzengeorge University. Thessalon. 1961, 7, 1–322.
- Shanab, M.M.A. Algal flora of Ain Helwan I. Algae of the Worm Spring. Egypt. J. Phycol. 2006, 7, 209–231. [Google Scholar] [CrossRef]
- Gupta, R. K. Mini review: Thermal algae of India with special reference to Jharkhand. Biospectra 2017, 12, 15–20. [Google Scholar]
- Varga, C. Water hygiene and toxicology: actual problems and new research trends in Hungary. Acta Biol Debr Oecol Hung 2012, 29, 9–120. [Google Scholar]
- Antunes, J.T.; Leao, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front Microbiol 2015, 6, 473. [Google Scholar] [CrossRef]
- Simon, N.; Cras, A. L.; Foulon, E.; Lemée, R. Diversity and evolution of marine phytoplankton. Comptes rendus biologies 2009, 332, 159–170. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Experimental immunologyFirst report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 37, 314–317. [Google Scholar] [CrossRef]
- Kouakou CRC, Poder TG. Economic impact of harmful algal blooms on human health: a systematic review. J Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef]
- Moore, S. K.; Trainer, V. L.; Mantua, N. J.; Parker, M. S.; Laws, E. A.; Backer, L.C.; Fleming, L.E. . Impacts of climate variability and future climate change on harmful algal blooms and human health. Environmental health : a global access science source 2008, 7, S4. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): a reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arh. Za Hig. Rada I Toksikol. 2013, 64, 119–130. [Google Scholar] [CrossRef]
- Ananya; Kamal, A.; Iffat, A. Cyanobacteria “the blue green algae” and its novel applications: A brief review. International J. Innov. Appl. Stud. 2014, 7, 251–261. [Google Scholar]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Codd, G.A.; Steffensen, D.A. Toxic blooms of cyanobacteria in Lake Alexandrina, South Australia - Learning from history. Mar. Freshw. Res. 1994, 45. [Google Scholar] [CrossRef]
- Dillenberg, H.O.; Dehnel, M.K. Toxic waterbloom in Saskatchewan, 1959. Can Med Assoc J 1960, 83, 1151–4. [Google Scholar] [PubMed]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Env. Toxicol 2003, 18, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.R.; Runnegar, M.T. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl Env. Microbiol 1985, 50, 1292–5. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, B.; Barlow, T. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Teixeira, M. da G.; Costa, M. da C. Gastroenteritis epidemic in the area of the Itaparica Dam, Bahia, Brazil. Bull Pan Am Health Organ 1993, 27, 244-53. [Google Scholar]
- Azevedo, S.M.; Carmichael, W.W. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 441-6, 181–182. [Google Scholar] [CrossRef]
- Backer, L.C.; Fleming, L.E. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.M. Epidemiology of recreational exposure to freshwater cyanobacteria – an international prospective cohort study. BMC Public Health 2006, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.A.; Fleming, L.E. Florida Red Tide Toxins (Brevetoxins) and Longitudinal Respiratory Effects in Asthmatics. Harmful Algae 2011, 10, 744–748. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fleming, L.E. Aerosolized red tide toxins (brevetoxins) and asthma: Continued health effects after 1 hour beach exposure. Harmful Algae 2011, 10, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Aifeng, L.; Jinggang, M. Toxins in mussels (Mytilus galloprovincialis) associated with diarrhetic shellfish poisoning episodes in China. Toxicon 2012, 60, 420–425. [Google Scholar]
- Giannuzzi, L.; Sedan, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, B.; Gervais, M.C. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci Total Env. 2014, 1, 397–403. [Google Scholar] [CrossRef]
- Trevino-Garrison, I.; DeMent, J. Human illnesses and animal deaths associated with freshwater harmful algal blooms-Kansas. Toxins 2015, 7, 353–66. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Pavaux, A. Environmental, human health and socioeconomic impacts of Ostreopsis spp. Blooms in the NW Mediterranean. Blooms in the NW Mediterranean. Harmful Algae 2022, 119. [Google Scholar] [CrossRef]
- Veldee, M.V. ; An Epidemiological Study of Suspected Water-borne Gastroenteritis. Am J Public Health Nations Health 1931, 21, 1227–35. [Google Scholar] [CrossRef]
- Backer, L.C.; Kirkpatrick, B. Occupational exposure to aerosolized brevetoxins during Florida red tide events: effects on a healthy worker population. Env. Health Perspect 2005, 113, 644–9. [Google Scholar] [CrossRef]
- Jiye, L.; So-Young, W. Dynamic calibration of phytoplankton blooms using the modified SWAT model. J. Clean. Prod. 2022, 343. [Google Scholar]
- Figgatt, M.; Muscatiello, N. Harmful Algal Bloom-Associated Illness Surveillance: Lessons From Reported Hospital Visits in New York, 2008-2014. Am J Public Health 2016, 106, 440–2. [Google Scholar] [CrossRef]
- Wu, J.; Hilborn, E.D. Acute health effects associated with satellite-determined cyanobacterial blooms in a drinking water source in Massachusetts. Env. Health 2021, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.D.; Roberts, V.A. Centers for Disease Control and Prevention (CDC). Algal bloom-associated disease outbreaks among users of freshwater lakes--United States, 2009-2010. MMWR Morb Mortal Wkly Rep 2014, 63, 11-5. [Google Scholar] [PubMed]
- Roberts, V.A.; Vigar, M. Surveillance for Harmful Algal Bloom Events and Associated Human and Animal Illnesses - One Health Harmful Algal Bloom System, United States, 2016-2018. MMWR Morb Mortal Wkly Rep 2020, 69, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Carmichael, W. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Li, Y. Quantifying Karenia brevis bloom severity and respiratory irritation impact along the shoreline of Southwest Florida. PLoS One 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.E.; Friedman, M.A. Neurological illnesses associated with Florida red tide (Karenia brevis) blooms. Harmful Algae 2019, 82, 73–81. [Google Scholar] [CrossRef]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemée, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; de Haro, L. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin Toxicol 2010, 48, 839–44. [Google Scholar] [CrossRef]
- Lavery, A.; Backer, L.; Daniel, J. Evaluation of Electronic Health Records to Monitor Illness From Harmful Algal Bloom Exposure in the United States. J Env. Health 2021, 839, 8–14. [Google Scholar]
- Abdullah, L.; Ferguson, S.; Niedospial, D.; Patterson, D.; Oberlin, S.; Nkiliza, A.; Bartenfelder, G.; Hahn-Townsend, C.; Parks, M.; Crawford, F.; Reich, A.; Keegan, A.; Kirkpatrick, B.; Mullan, M. Exposure-response relationship between K. brevis blooms and reporting of upper respiratory and neurotoxin-associated symptoms. Harmful Algae 2022, 117. [Google Scholar] [CrossRef]
- Mchau, G.J.; Makule, E.; Machunda, R.; Gong, Y.Y.; Kimanya, M. Harmful algal bloom and associated health risks among users of Lake Victoria freshwater: Ukerewe Island, Tanzania. J. Water Health 2019, 17, 826–836. [Google Scholar] [CrossRef]
- Vila, M.; Abós-Herràndiz, R.; Isern-Fontanet, J.; Alvarez, J.; Berdalet, E. Establishing the link between Ostreopsis cf. Ovata blooms and human health impacts using ecology and epidemiology. Ovata blooms and human health impacts using ecology and epidemiology. Sci. Mar. 2016, 80, 107–115. [Google Scholar] [CrossRef]
- Lavery, A.M.; Backer, L.C.; Roberts, V.A.; DeVies, J.; Daniel, J. ; (). Evaluation of Syndromic Surveillance Data for Studying Harmful Algal Bloom-Associated Illnesses - United States, 2017-2019. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1191–1194. [Google Scholar] [CrossRef]
- Illoul, H.; Hernández, F.R.; Vila, M.; Adjas, N.; Younes, A.A.; Bournissa, M.; Koroghli, A.; Marouf, N.; Rabia, S.; Ameur, F.L.K. The genus Ostreopsis along the Algerian coastal waters (SW Mediterranean Sea) associated with a human respiratory intoxication episode. Cryptogam. Algol. 2012, 2, 209–216. [Google Scholar] [CrossRef]
- Figgatt, M.; Hyde, J.; Dziewulski, D.; Wiegert, E.; Kishbaugh, S.; Zelin, G.; Wilson, L. Harmful Algal Bloom-Associated Illnesses in Humans and Dogs Identified Through a Pilot Surveillance System - New York, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 1182–1184. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Dalpra, D.; Tamer, R.; Zaias, J.; Cheng, Y.S.; Pierce, R.; Naar, J.; Abraham, W. Clark, R.; Zhou, Y.; Henry, M.S.; Johnson, D.; Van De Bogart, G.; Bossart, G.D.; Harrington, M.; Baden, D.G. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Env. Health Perspect 2005, 113, 650-7. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Bean, J.A.; Kirkpatrick, B.; Cheng, Y.S.; Pierce, R.; Naar, J.; Nierenberg, K.; Backer, L.C.; Wanner, A.; Reich, A.; Zhou, Y.; Watkins, S.; Henry, M.; Zaias, J.; Abraham, W.M.; Benson, J.; Cassedy, A.; Hollenbeck, J.; Kirkpatrick, G.; Clarke, T.; Baden, D.G. Exposure and effect assessment of aerosolized red tide toxins (brevetoxins) and asthma. Environ. Health Perspect. 2009, 117, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Jin, D.; Beet, A.; Kirkpatrick, B.; Reich, A.; Ullmann, S.; Fleming, L.E.; Kirkpatrick, G. The human health effects of Florida red tide (FRT) blooms: an expanded analysis. Environ. Int. 2014, 68, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Jin, D.; Polansky, L.Y.; Kirkpatrick, B.; Kirkpatrick, G.; Fleming, L.E.; Reich, A.; Watkins, S.M.; Ullmann, S.G.; Backer, L.C. The costs of respiratory illnesses arising from Florida gulf coast Karenia brevis blooms. Environ. Health Perspect. 2009, 117, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; Baden, D.G. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar] [CrossRef]
- Chomérat, N.; Antajan, E.; Auby, I.; Bilien, G.; Carpentier, L.; Casamajor, M.-N.d.; Ganthy, F.; Hervé, F.; Labadie, M.; Méteigner, C.; et al. First Characterization of Ostreopsis cf. ovata (Dinophyceae) and Detection of Ovatoxins during a Multispecific and Toxic Ostreopsis Bloom on French Atlantic Coast. Mar. Drugs 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Bean, J.A.; Fleming, L.E.; Kirkpatrick, G.; Grief, L.; Nierenberg, K.; Reich, A.; Watkins, S.; Naar, J. Gastrointestinal Emergency Room Admissions and Florida Red Tide Blooms. Harmful Algae 2010, 9, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Masó, M.; Sampedro, N.; et al. The genus Ostreopsis in recreational waters of the Catalan Coast and Balearic Islands (NW Mediterranean Sea): is this the origin of human respiratory difficulties? ISSHA IOC UNESCO 2008, 334–336. [Google Scholar]
- Gallitelli, M. , Ungaro, N. Respiratory illness as a reaction to tropical algal blooms occurring in a temperate climate. JAMA 2005, 293, 2599–2600. [Google Scholar]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G. ; Collaborative Group for the Ligurian Syndromic Algal Surveillance. Ostreopsis ovata and human health: epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005-06, in north-west Italy. Euro Surveill 2007, 12. [Google Scholar]
- Milian, A.; Nierenberg, K.; Fleming, L.E.; Bean, J.A.; Wanner, A.; Reich, A.; Backer, L.C.; Jayroe, D.; Kirkpatrick, B. Reported respiratory symptom intensity in asthmatics during exposure to aerosolized Florida red tide toxins. J. Asthma: Off. J. Assoc. Care Asthma 2007, 44, 583–587. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Reich, A.; Zaias, J.; Cheng, Y.S.; Pierce, R.; Naar, J.; Abraham, W.M.; Baden, D.G. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest 2007, 131, 187–194. [Google Scholar] [CrossRef]
- Todd, E.C.D. Preliminary estimates of costs of foodborne disease in the United States. J. Food Prot. 1989, 52, 595–601. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H. A.; Johnson, L.; Franco, O. H.; Butterworth, A. S.; Forouhi, N. G.; Thompson, S. G. , Khaw, K. T., Mozaffarian, D., Danesh, J., & Di Angelantonio, E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Annals of internal medicine 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Carter, R. A A.; Joll, C. A. Occurrence and formation of disinfection by-products in the swimming pool environment: A critical review. J. Environ. Sci. (China) 2017, 58, 19–50. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sahin, O.; Sekine, R.; Stewart, R.A. Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach. Water 2022, 14, 2062. [Google Scholar] [CrossRef]
- PWTAG - Pool Water Treatment Advisory Group ( (2009). Swimming Pool Water: Treatment and Quality Standards for Pools and Spas. BCPublications.
- Bélanger, A. S.; Brouard, J. S.; Charlebois, P.; Otis, C.; Lemieux, C.; Turmel, M. Distinctive architecture of the chloroplast genome in the chlorophycean green alga Stigeoclonium helveticum. Mol. Genet. Genom. :MGG 2006, 276, 464–477. [Google Scholar] [CrossRef]
- Martić, A.; Čižmek, L.; Ul'yanovskii, N. V.; Paradžik, T.; Perković, L.; Matijević, G.; Vujović, T.; Baković, M.; Babić, S.; Kosyakov, D. S.; Trebše, P.; Čož-Rakovac, R. ; & Čož-Rakovac, R. Intra-Species Variations of Bioactive Compounds of Two Dictyota Species from the Adriatic Sea: Antioxidant, Antimicrobial, Dermatological, Dietary, and Neuroprotective Potential. Antioxid. (Basel Switz.) 2023, 12, 857. [Google Scholar] [CrossRef]
- Puetz, J.D. Swimming Pool Water Chemistry. The Care and Treatment of Swimming Pool Water; Arch Chemicals, Inc., Now a Part of Lonza: Norwalk, CT, USA, 2013.
- Bures, F. Quaternary ammonium compounds: simple in structure, complex in application. Top. Curr. Chem. (Cham) 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Zhang, H.; Wang, N.; Ma, B.; Liu, X.; Niu, L.; Yang, F.; Xu, Y.; Zhang, X. Effects of copper sulfate algaecide on the cell growth, physiological characteristics, the metabolic activity of Microcystis aeruginosa and raw water application. J. Hazard. Mater. 2023, 445, 130604. [Google Scholar] [CrossRef] [PubMed]
- Juergensen, L.; Busnarda, J.; Caux, P.Y.; Kent, R.A. Fate, behavior, and aquatic toxicity of the fungicide DDAC in the Canadian environment. Environ. Toxicol 2000, 15, 174–200. [Google Scholar] [CrossRef]
- Frank, T. Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC). US Environmental Protection Agency Office of Prevention, Pesticides, and Toxic Substances, Report, 2006, 10–44.
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Alampi, R.; Vazzana, I.; Felice, M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol 2006, 180, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Michalek, K.; Ventura, A.; Sanders, T. Mytilus hybridisation and impact on aquaculture: A minireview. Mar. Genom. 2016, 27, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Moore and Kellerman. U. S. Dept. Agri. Bureau Plant Industry, Bull. No. 1994, 64, I904.
- Bishop, W.M.; Johnson, B.M.; Rodgers Jr, J. H. Comparative responses of target and nontarget species to exposures of a copper-based algaecide. J Aquat Plant Manag 2014, 52, 65–70. [Google Scholar]
- Kang, L.; Mucci, M.; Fang, J.; Lürling, M. New is not always better: Toxicity of novel copper based algaecides to Daphnia magna. Ecotoxicol. Environ. Saf. 2022, 241, 113817. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Ren, B.; Zhang, Z.; Deng, X.; Yin, W.; Yang, S. Algae removal characteristics of the ultrasonic radiation enhanced drinking water treatment process. J. Water Process Eng. 2023, 55, 104154. [Google Scholar] [CrossRef]
- Sheng, D.; Bu, L.; Zhu, S.; Wu, R.; Shi, Z.; Zhou, S. Pre-oxidation coupled with charged covalent organic framework membranes for highly efficient removal of organic chloramines precursors in algae-containing water treatment. Chemosphere 2023, 333, 138982. [Google Scholar] [CrossRef]
- Zhang, Y. , Luo, P., Zhao, S., Kang, S., Wang, P., Zhou, M., Lyu, J. Control and remediation methods for eutrophic lakes in the past 30 years. Control and remediation methods for eutrophic lakes in the past 30 years. Water Sci. Technol. 2020, 81, 1099–1113. [Google Scholar]
- Rondeau, V.; Commenges, D.; Jacqmin-Gadda, H.; Dartigues, J.F. Relation between aluminum concentrations in drinking water and Alzheimer's disease: an 8-year follow-up study. Am. J. Epidemiol. 2000, 152, 59–66. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Tazart, Z.; Enaime, G.; Douma, M.; Ounas, A.; Loudiki, M. Nature-based coagulants for drinking water treatment: An ecotoxicological overview. Water Environ. Res. 2022, 94, e10782. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Douma, M.; Ounas, A.; Yaacoubi, A.; Lofrano, G.; Loudiki, M. A review of plant-based coagulants for turbidity and cyanobacteria blooms removal. Environ. Sci. Pollut. Res. 2022, 29, 42601–42615. [Google Scholar] [CrossRef] [PubMed]
- El Bouaidi, W.; Essalhi, S.; Douma, M.; Tazart, Z.; Ounas, A.; Enaime, G. ; Yaacoubi, A;, Loudiki, M. Evaluation of the potentiality of Vicia faba and Opuntia ficus indica as eco-friendly coagulants to mitigate Microcystis aeruginosa blooms. Desalin WATER Treat 2020, 196, 198–213. [Google Scholar] [CrossRef]
- Ma, J.; Jia, B.; Li, S.; Kong, Y.; Nie, Y.; Zhang, H.; Gao, T. Enhanced coagulation of covalent composite coagulant with potassium permanganate oxidation for algae laden water treatment: Algae and extracellular organic matter removal. Chem. Eng. J. Adv. 2023, 13, 100427. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Y.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, L.; Ren, L. Photo-Fenton self-cleaning carbon fibers membrane supported with Zr-MOF@ Fe2O3 for effective phosphate removal from algae-rich water. Chemosphere 2023, 323, 138175. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Y.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, L.; Ren, L. Photo-Fenton self-cleaning carbon fibers membrane supported with Zr-MOF@ Fe2O3 for effective phosphate removal from algae-rich water. Chemosphere 2023, 323, 138175. [Google Scholar] [CrossRef]
- Barkallah, M.; Elleuch, J.; Smith, K.F.; Chaari, S.; Ben Neila, I.; Fendri, I.; Michaud, P.; Abdelkafi, S. Development and application of a real-time PCR assay for the sensitive detection of diarrheic toxin producer Prorocentrum lima. J. Microbiol. Meth. 2020, 178, 106081. [Google Scholar] [CrossRef]
- Chin Chwan Chuong, J.J.; Rahman, M.; Ibrahim, N.; Heng, L.Y.; Tan, L.L.; Ahmad, A. Harmful Microalgae Detection: Biosensors versus Some Conventional Methods. Sens. (Basel) 2022, 22, 3144. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Wang, Y.; Chen, G. A review of the current and emerging detection methods of marine harmful microalgae. Sci Total Environ. 2022, 815, 152913. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Kim, M.; Lee, W.H. A novel method for cell counting of microcystis colonies in water resources using a digital imaging flow cytometer and microscope. Environ. Eng. Res. 2019, 24, 397–403. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto António, D.; Loo, R.; Lettieri, T. Cyanotoxins: methods and approaches for their analysis and detection. Jt. Res. Cent. (JRC) Tech. Rep. Eur. Comm. 2017. [Google Scholar]
- Ghosh, P.; Mukherji, S. Fate, detection technologies and toxicity of heterocyclic PAHs in the aquatic and soil environments. Sci Total Env. 2023, 892, 164499. [Google Scholar] [CrossRef] [PubMed]
- Jing-Yan, L.; Li-Hua, Z.; Zhen-Hui, R. Recent application of spectroscopy for the detection of microalgae life information: A review. Applied Spectroscopy Reviews 55, 1, 26–59. [Google Scholar]
- Murdock, J. N.; Wetzel, D. L. FT-IR Microspectroscopy Enhances Biological and Ecological Analysis of Algae. Appl. Spectrosc. Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Saleem, F.; Jiang, J.L.; Atrache, R.; Paschos, A.; Edge, T.A.; Schellhorn, H.E. Cyanobacterial Algal Bloom Monitoring: Molecular Methods and Technologies for Freshwater Ecosystems. Microorganisms 2023, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Jiang, J.L.; Atrache, R.; Paschos, A.; Edge, T.A.; Schellhorn, H.E. Cyanobacterial Algal Bloom Monitoring: Molecular Methods and Technologies for Freshwater Ecosystems. Microorganisms 2023, 11, 851. [Google Scholar] [CrossRef]
- Jin, C.; Mesquita, M.M.F.; Deglint, J.L.; Emelko, M.B.; Wong, A. Quantification of Cyanobacterial Cells via a Novel Imaging-Driven Technique with an Integrated Fluorescence Signature. Sci. Rep. 2018, 8, 9055. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Determination of Cyanotoxins in Drinking and Ambient Freshwaters. (Accessed on December 15th, 2023). Available to: https://www.epa.gov/cyanohabs/determination-cyanotoxins-drinking-and-ambient-freshwaters. 15 December.
- Islam, M.S.; Aryasomayajula, A. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines. Micromachines. Selvaganapathy P.R 2017, 8, 83. [Google Scholar] [CrossRef]
- Pandur, Ž.; Dular, M.; Kostanjšek, R.; Stopar, D. Bacterial Cell Wall Material Properties Determine E. coli Resistance to Sonolysis. coli Resistance to Sonolysis. Ultrason. Sonochem 2022, 83, 105919. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Audet, J. Current Techniques for Single-Cell Lysis. J. R. Soc. Interface 2008, 5, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Danaeifar, M. New Horizons in Developing Cell Lysis Methods: A Review. Biotechnol. Bioeng. 2022, 119, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, K.K.; Gates, C.; Lee, T.; Cameron, J.C. Proximity-Based Proteomics Reveals the Thylakoid Lumen Proteome in the Cyanobacterium Synechococcus Sp. PCC 7002. PCC 7002. Photosynth. Res. 2021, 147, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.K.; Evitt, N.H.; Swartz, J.R. Chemical Lysis of Cyanobacteria. J. Biol. Eng. 2015, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Vallaeys, T.; Hendrickx, L.; Natalie, L.; Wilmotte, A. An Efficient DNA Isolation Protocol for Filamentous Cyanobacteria of the Genus Arthrospira. J. Microbiol. Methods 2010, 80, 148–154. [Google Scholar] [CrossRef]
- Qian, S.S.; Chaffin, J.D.; DuFour, M.R; Sherman, J.J.; Golnick, P.C.; Collier, C.D.; Nummer, S.A.; Margida, M.G. Quantifying and Reducing Uncertainty in Estimated Microcystin Concentrations from the ELISA Method. Environ. Sci. Technol. 2015, 49, 14221–14229. [Google Scholar] [CrossRef]
- 225. Fischer,W.J.; Garthwaite, I.; Miles, C.O.; Ross, K.M.; Aggen ,J.B.; Chamberlin, A.R.; Towers, N.R.; Dietrich, D.R. Congener-Independent Immunoassay for Microcystins and Nodularins. Environ. Sci. Technol. 2001, 35, 4849–4856.
- Shoemaker, J.A.; Tettenhorst, D.R.; de la Cruz, A. Single Laboratory Validated Method for Determination of Microcystins and Nodularin in Ambient Freshwaters by Solid Phase Extraction and Liquid Chromatography/ Tandem Mass Spectrometry (LC/MS/MS) (Accessed on December 16th 2023). Available to: https://www.epa.gov/sites/default/files/2017-11/documents/microcystin_method_for_ambient_water_nov_2017.pdf. 16 December.
- Zaffiro, A.; Rosenblum, L.; Wendelken, S.C. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay (Accessed on th 2023). Available to: https://www.epa.gov/sites/default/files/2016-09/documents/method-546-determination-total-microcystins-nodularins-drinking-water-ambient-water-adda-enzyme-linked-immunosorbent-assay.pdf. 16 December.
- He, X.; Stanford, B.D.; Adams, C.; Rosenfeldt, E.J.; Wert, E.C. Varied Influence of Microcystin Structural Difference on ELISA Cross-Reactivity and Chlorination Efficiency of Congener Mixtures. Water Res. 2017, 126, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, D.O.; Holland, P.; Sprosen, J. Method for Detecting Classes of Microcystins by Combination of Protein Phosphatase Inhibition Assay and ELISA: Comparison with LC-MS. Toxicon 2005, 45, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.; McNamee, S. E.; Boots, B.; Cimarelli, L.; Guillebault, D.; Helmi, K.; Marcheggiani, S.; Panaiotov, S.; Breitenbach, U.; Akçaalan, R.; Medlin, L. K.; Kittler, K.; Elliott, C. T.; Campbell, K. A Validated UPLC-MS/MS Method for the Surveillance of Ten Aquatic Biotoxins in European Brackish and Freshwater Systems. Harmful Algae 2016, 55, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O. Separation Techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar]
- 232. Kumar, P; Rautela, A.; Kesari, V.; Szlag, D.; Westrick, J.; Kumar, S. Recent Developments in the Methods of Quantitative Analysis of Microcystins. J. Biochem. Mol. Toxicol. 2020, 34, e22582. [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Barón-Sola, Á.; Campo, F.F.d.; Sanz-Alférez, S. Dynamics of Cylindrospermopsin Production and Toxin Gene Expression in Aphanizomenon Ovalisporum. Adv. Microbiol. 2016, 6, 381–390. [Google Scholar] [CrossRef]
- Jungblut, A.-D.; Neilan, B.A. Molecular Identification and Evolution of the Cyclic Peptide Hepatotoxins, Microcystin and Nodularin, Synthetase Genes in Three Orders of Cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef]
- Ngwa, F.; Madramootoo, C.; Jabaji, S. Monitoring Toxigenic Microcystis Strains in the Missisquoi Bay, Quebec, by PCR Targeting Multiple Toxic Gene Loci. Environ. Toxicol. 2014, 29, 440–451. [Google Scholar] [CrossRef]
- Furukawa, K.; Noda, N.; Tsuneda, S.; Saito, T.; Itayama, T.; Inamori, Y. Highly Sensitive Real-Time PCR Assay for Quantification of Toxic Cyanobacteria Based on Microcystin Synthetase A Gene. J. Biosci. Bioeng. 2006, 102, 90–96. [Google Scholar] [CrossRef]
- Gupta, R.S.; Mathews, D.W. Signature Proteins for the Major Clades of Cyanobacteria. BMC Evol. Biol. 2010, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Impact of Genomics on Clarifying the Evolutionary Relationships amongst Mycobacteria: Identification of Molecular Signatures Specific for the Tuberculosis-Complex of Bacteria with Potential Applications for Novel Diagnostics and Therapeutics. High-Throughput 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, C.; Struewing, I.; Li, X.; Allen, J.; Lu, J. Cyanotoxin-Encoding Genes as Powerful Predictors of Cyanotoxin Production during Harmful Cyanobacterial Blooms in an Inland Freshwater Lake: Evaluating a Novel Early-Warning System. Sci. Total Environ. 2022, 830, 154568. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Struewing, I.; Wymer, L.; Tettenhorst, D.R.; Shoemaker, J.; Allen, J. Use of qPCR and RT-qPCR for Monitoring Variations of Microcystin Producers and as an Early Warning System to Predict Toxin Production in an Ohio Inland Lake. Water Res. 2020, 170, 115262. [Google Scholar] [CrossRef] [PubMed]
- Crevecoeur, S; Edge, T.A.; Watson, L.C.; Watson, S.B.; Greer, C.W.; Ciborowski, J.J.H.; Diep, N.; Dove, A.; Drouillard, K.G.; Frenken, T.; McKay, R.M.; Zastepa, A.; Comte, J. Spatio-Temporal Connectivity of the Aquatic Microbiome Associated with Cyanobacterial Blooms along a Great Lake Riverine-Lacustrine Continuum. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Pillet, F.; Dague, E.; Ilić, J.P.; Ružić, I.; Rols, M.-P.; DeNardis, N.I. Changes in Nanomechanical Properties and Adhesion Dynamics of Algal Cells during Their Growth. Bioelectrochem. Amst. Neth. 2019, 127, 154–162. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Marcuello, C.; Lostao, A.; Calvo-Begueria, L.; Velazquez-Campoy, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.-L. Microcystin-LR Binds Iron, and Iron Promotes Self-Assembly. Environ. Sci. Technol. 2017, 51, 4841–4850. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Zhao, Y.-N.; Zhang, X.; Wei, X.; Chen, M.-L.; Chen, X.-W. Biochemical Analysis Based on Optical Detection Integrated Microfluidic Chip. TrAC Trends Anal. Chem. 2023, 158, 116865. [Google Scholar] [CrossRef]
- Knob, R.; Hanson, R.L.; Tateoka, O.B.; Wood, R.L.; Guerrero-Arguero, I.; Robison, R.A.; Pitt, W.G.; Woolley, A.T. Sequence-Specific Sepsis-Related DNA Capture and Fluorescent Labeling in Monoliths Prepared by Single-Step Photopolymerization in Microfluidic Devices. J. Chromatogr. A. 2018, 1562, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mao, S.; Li, W.; Lin, J.-M. Microfluidic Devices in the Fast-Growing Domain of Single-Cell Analysis. Chem.—Eur. J. 2018, 24, 15398–15420. [Google Scholar] [CrossRef]
- Gaur, A.; Pant, G.; Jalal, A.S. Computer-Aided Cyanobacterial Harmful Algae Blooms (CyanoHABs) Studies Based on Fused Artificial Intelligence (AI) Models. Algal Res. 2022, 67, 102842. [Google Scholar] [CrossRef]
- Baek, S.-S.; Pyo, J.; Pachepsky, Y. , Park, Y., Ligaray, M., Ahn, C.-Y.; Kim, Y.-H.; Ahn Chun, J.; Hwa Cho, K. Identification and Enumeration of Cyanobacteria Species Using a Deep Neural Network. Identification and Enumeration of Cyanobacteria Species Using a Deep Neural Network. Ecol. Indic. 2020, 115, 106395. [Google Scholar] [CrossRef]
- Hennon, G.M.M.; Dyhrman, S.T. Progress and promise of omics for predicting the impacts of climate change on harmful algal blooms. Harmful Algae 2020, 91, 101587. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Yang, X.; Zhang, Y.; Zhang, L.; Ren, H.; Wu, B.; Ye, L. A Review of the Application of Machine Learning in Water Quality Evaluation. Eco-Environ. Health 2022, 1, 107–107. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Kibuye, F.A.; Ajjampur, S.; Glover, C.M. , Hofmann, R.; Gaget,V.; Owen, C.; Wert, E.C.; Zamyadi, A. State of Knowledge on Early Warning Tools for Cyanobacteria Detection. State of Knowledge on Early Warning Tools for Cyanobacteria Detection. Ecol. Indic. 2021, 133, 108442. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Guidelines for safe recreational water environments: swimming pool and similar environment. Geneva: WHO 2006.
- Ibelings BW, Kurmayer R, Azevedo SMFO, Wood SA, Chorus I.; Welker M. Understanding the occurrence of cyanobacteria and cyanotoxins. In: Chorus I, Welker M, editors. Toxic cyanobacteria in water, second edition. Geneva: World Health Organization 2021.
- Burch, M.; Brookes, J., Chorus, I. Assessing and controlling the risk of cyanobacterial blooms: waterbody conditions. In: Chorus I, Welker M, editors. Toxic cyanobacteria in water, second edition. Geneva: World Health Organization 2021.
| Author | Year | Country | Types of Recreational Water | Kingdom | Exposure route | Population | Outcome syndrome |
|---|---|---|---|---|---|---|---|
| Codd, G.A. 1994 ]130] |
1878 | Australia | Lake water | Cyanobacteria Nodularia spumigena | Ingestion | Several hundred livestock deaths; 1 human |
Undescribed illness in human |
| Dillenberg, H.O. 1960 [131] |
1959 | Saskatchewan, Canada | Lake water | Cyanobacteria Anabaena circinalis |
Ingestion | Multiple livestock, fish, geese, dogs died >2 human events |
Gastrointestinal illness |
| Griffiths 2003 [132] |
1979 | Palm Island, Australia | Potable water reservoir | Cyanobacterium Cylindrospermopsis raciborskii | - | 140 children and 10 adults hospitalized |
Hepatoenteritis, acute kidney disease and liver failure |
| Hawkins 1985 [133] |
1979 | Australia | Fresh water | Cylindrospermopsis raciborskii | 138 people | Hepatoenteritis | |
| Jeffery 2004 [134] |
1987 | Canada | Sea water | Pseudo-nitzschia spp. Nitzschia spp. |
Ingestion | 150 people with 19 hospitalization and 4 deaths | Gastrointestinal effects and/or neurological signs |
| Texeira 1993 [135] |
1988 | Brazil | Fresh water | Chlorophyll-a | Drinkingwater from the Itaparicareservoir | 88 human fatalities and 2000 people poisoned |
|
| Azevedo 2002 [136] |
1996 | Brazil | In the filtration system | Cyanobacterium | Dyalisis | 116 human cases | Headache, blurred vision, eye pain, nausea,and vomiting. Of these, 100 patients developed acute liver failure and more than half of them died |
| Backer 2003 [137] |
1999 | Sarasota and Jacksonville, Florida, USA |
Sea water | Karenia brevis | Inalation | 129 adults (≥18 years) who spent time (10 min to ∼8 h; average of 71 min) on beaches during February and October 1999 | Upper and lower respiratory irritation |
| Stewart 2006 [138] |
2006 | Southern Queensland and the Myall Lakes area of New South Wales, Australia; Northeast and central Florida, USA | Lake water | Phytoplankton | Ingestion | 1331 study participants (n = 216 in Florida) comprised of adults and children |
Difficulty breathing, dry and productive cough, runny nose, unusual sneezing sore throat, wheezy breathing, cutaneous, fever |
| Bean 2011 [139] |
2011 | Sarasota, Florida, USA | Sea water | Karenia brevis | Inalation | 125 asthmatic patients residents of the Sarasota, Florida for ≥6 months | |
| Kirkpatrick 2011 [140] |
2011 | Sarasota, Florida, USA | Sea water | Inalation | 52 participants (≥12 years) with self-report of physician-diagnosedasthma |
Increased symptoms of asthma and suppressed respiratory function, suppression for several days after 1 h of exposure to the brevetoxin aerosols | |
| Li 2012 [141] |
2011 | China | Sea water | Amphidoma languida, Azadinium spinosum, Protoceratium reticulatum, Lingulodinium polyedrum, Gonyaulax spinifera, Dinophysis spp, Prorocentrum lima, Dinophysis spp | Consumption of contaminated shellfish | More than 200 people | Diarrheic shellfish poisoning: diarrhea, nausea, vomiting, and abdominal pain |
| Giannuzzi 2011 [142] |
2011 | Uruguay | Fresh water | Cyanobacteria | Drinking water | Human acute intoxication |
Intoxication |
| Levesque 2014 [143] |
2014 | Quebec province, Canada | Lake water | Cyanobacteria | 267 families consisting of 466 subjects |
Gastrointestinal symptoms | |
| Trevino-Garrison 2015 [144] |
2015 | Kansas, USA | Lake water | Cyanobacteria | Direct contact, 100% (7/7), ingestion, 43% (3/7), inhalation, 14% (1/7). The median time from exposure to symptom onset was 24 h, with a range of 3–48 h | 13 humans. Seven confirmed illnesses, median age 40 years | Nausea, vomiting, diarrhea, cough, sore throat, rash and liver damage |
| Berdalet 2022 [145] |
2022 | Sant Andreu de Llavaneres, Catalonia, Spain |
Sea water | Ostreopsis | 16 staff of a nearby indoor-outdoor restaurant directly exposed to marine aerosols at least 8 h daily |
Malaise, runny nose, sore eyes, headaches | |
| Veldee 1931 ]146] |
1930–1931 | United States | River water | Cyanobacteria Anabaena flos-aquae |
drinking water | > 6 events | Gastrointestinal illness |
| Backer 2005 [147] |
2001 and 2003 | Sarasota and Manatee, Florida, USA |
Sea water | Exposure to aerosolized brevetoxins |
28 healthy lifeguards (≥18 years) who are occupationally exposed to red tide toxins during daily work activities | Upper respiratory symptoms and headache | |
| Lee 2022 [148] |
2005–2017 | South Korea | Fresh water | Neurodegenerative disease outcomes including motor neuron disease, Alzheimer’s disease, and Parkinson’s disease | |||
| Figgatt 2016 [149] |
2008 - 2014 | New York, USA | Fresh water | 228 hospital visits: 5.3% of them were hospitalized | |||
| Wu 2021 [150] |
2008–2011 | Greater Boston area, Massachusetts, USA | Fresh water | Cyanobacteria | Human patients who visited the emergency rooms of approximately 70 Massachusetts hospitals | Gastrointestinal, respiratory and dermal illnesses | |
| Hilborn 2014 [151] |
2009–2010 | USA | Lake water | Cyanobacteria | Recreational activities | 41 cases | Gastrointestinal, general, dermatologic, eye/ear, neurologic, respiratory symptoms |
| Roberts 2020 [152] |
2016–2018 | USA | 366 fresh water (94%), 1 brackish, 22 unknown (6%) | Cyanobacteria, Dinoflagellates, Raphidophyceans, Diatoms | Exposure: Public outdoor area 235 (60%), Beach 63 (16%), Private residence 53 (14%), Other 57 (15%), Park 27 (7%), Unknown 22 (6%) | 389 human cases: 341 probable (88%), 14 confirmed, 34 suspected, 153 were < 18 years | Gastrointestinal 262 (67%), generalized 169 (43%), dermatologic 104 (27%), ear, nose, or throat 62 (16%), neurologic 56 (14%), cardiopulmonary 41 (11%), ophthalmologic 30 (8%), other 30 (8%), musculoskeletal 13 (3%), genitourinary 6 (2%), unknown 9 (2%). No deaths were reported |
| Backer 2008 [153] |
3 days in August of 2006 | Unspecified lakes, USA | Lake water | Microcystisaeruginosa | 97 participants | Respiratory (sore throat; congestion; cough; throat irritation; eye irritation; other), dermatologic (itchy skin, red skin, hives, skin irritation, rash, other); other (earache, agitation, headache, abdominal pain, diarrhoea) | |
| Backer 2010 [154] |
August 2007 | Two lakes in Siskiyou County, California, USA |
Lake water | Respiratory (sore throat; congestion; cough; throat irritation; eye irritation; other), dermatologic (itchy skin, red skin, hives, skin irritation, rash, other); other (earache, agitation, headache, abdominal pain, diarrhea) | |||
| Stumpf 2022 [155] |
August 2006–January 2019 |
Sarasota and Manatee Counties, Florida, USA |
Sea water | Volunteers | Respiratory irritation conditions | ||
| Diaz 2019 [156] |
Between 2005 and 2009 | 6 counties in Southwest Florida, USA |
Sea water | Karenia brevis | Emergency department (ED) visitsin patients ≥55 years | Headache | |
| Tichadou 2010 [157] |
Between 2006 and 2009 | French Mediterranean coast | Sea water | French Mediterranean Ostreopsis | 47 patients | General flu-like symptoms, including headache, joint pain, vertigo, fever, fatigue, and diarrhea | |
| Lavery 2021 [158] |
Between January 2009 and April 2019 | USA | Lake water | 380 records in the Commercial database and 178 records in the Medicaid database | Cough, allergy, other malaise and fatigue, headache, routine general medical examination, shortness of breath, hypertension, acute pharyngitis, and acute upper respiratory infection | ||
| Abdullah 2022 [159] |
Between June 2019 and August 2021 |
5 counties in Southwest Florida, USA |
Sea water | Karenia brevis | Exposure to aerosolizedbrevetoxins | 258 participants | Respiratory tract symptoms (coughing, sneezing, sore throat,nasal congestion); neurological (headaches and dizziness); andsystemic outcomes (fever, chills, nausea) |
| Mchau 2019 [160] |
December 2018 | Ukerewe Island, Tanzania |
Lake water | Phytoplankton Chlorophyceae, Cyanophyceae, Bacillariophyceae and Dinophyceae | 134 subjects (31%) used lake water, 229 (53%) used well water and 69 (16%) used treated supplied pipe water as their main source of water for drinking and other domestic uses |
432 subjects > 18 years (mean age 42) | Vomiting, diarrhea, skin irritation, eye irritation, throat irritation and stomach upset |
| Vila 2016 [161] |
From June toDecember 2013 | Sant Andreu de Llavaneres,Catalonia, Spain | Sea water | Ostreopsis | 16 staff of a nearby indoor-outdoor restaurant directly exposed to marine aerosols at least 8 h daily |
Eye irritation, nose irritation, rhinorrhoea, and general malaise | |
| Lavery 2021 [162] |
January 1, 2017–December 31, 2019 | USA | Lake water | Unspecified Cyanobacteria | Inhalation, ingestionor skin contact | Patients aged 18–44 years (37%) and 45–64 years (30%) | 133 respiratory (41%), 44 gastrointestinal (14%), 33 neurologic (10%), and 27 dermatologic (8%) |
| Illoul 2012 [163] |
July 2009 | Beaches in Western area ofAlgiers Wilaya, Algeria | Sea water | Ostreopsis | 300 patients | Fever, rhinitis, conjunctivitis, cough and skin irritations | |
| Figgatt M. 2015 [164] |
June–September 2015 | New York, USA | Fresh waters | Unspecified Cyanobacteria | Recreational activities included swimming (28 patients; 88%); wakeboarding, jet skiing, waterskiing or tubing (seven; 22%); boating (seven; 22%); wading (six; 19%); and using personalwatercraft (four; 13%) |
32 human cases, median age 24 years (2–63 years) |
Skin problems, cough, abdominal pain, diarrhea, nausea, vomiting and other symptoms (e.g. chills, muscle aches, or watery eyes), fatigue/general weakness, Sore throat, Neuroglogic (e.g., headache or seizure); no hospitalizations or deaths were reported |
| Fleming 2005 [165] |
March 2003 | Sarasota, Florida, USA | Sea water | Exposure to aerosolizedbrevetoxins during red tide events | 59 persons (≥12 years) with physician-diagnosed asthma |
Respiratory symptoms and respiratory impairment |
|
| Fleming 2009 [166] |
March 2005 and September 2006 | Sarasota, Florida, USA | Sea water | Exposure to aerosolized brevetoxins |
87 persons (≥12 years) with physician-diagnosed asthma |
Respiratory symptmos like cough, wheezing, shortness of breath and other like throat irritation, nasal congestion, eye irritation, headache, itchy skin, diarrhoea |
|
| Hoagland 2014 [167] |
Monthly emergency department (ED) data 2005–2009 and monthly hospital inpatient data from 1999 to 2009 | 6 counties in Southwest Florida, USA |
Sea water | Karenia brevis | Emergency department (ED) visits in patients ≥55 years |
Adverse respiratory and digestivehealth effects | |
| Hoagland 2009 [168] |
October 2001–September 2006 | Sarasota, Florida, USA | Sea water | Karenia brevis | Exposure to aerosolized brevetoxins |
Daily emergency department (ED) visits |
Pneumonia, bronchitis, asthma, upper airway disease UAD |
| Kirkpatrick 2006 [169] |
October 1–December 31, 2001 (red tide period) and October 1–December31, 2002 (non-red tide period) |
Sarasota, Florida, USA | Sea water | Red tide | Emergency room (ER) visits | Pneumonia 31%, bronchitis 56%, asthma 44%, and upper airway disease 64% |
|
| Chomerat 2022 [170] |
September 2020 and 2021 | Several beaches on the French Basque coast |
Sea water | Ostreopsis | Direct contact with water and/or after inhalation of aerosols | 674 confirmed cases | Difficulty breathing, irritations, headaches, dry cough, nose irritation, eye irritation and/or dermatitis, and general malaise |
| Kirkpatrick 2010 [171] |
September 1–December 31, 2001, and thenon-bloom period during the fall of 2002, September 1–December 31, 2002 |
Sarasota, Florida, USA | Sea water | Exposure to aerosolized brevetoxins |
Number of emergency room (ER) admissions during the Florida red tide bloom |
Gastritis, duodenitis, and non-infectious enteritis and colitis (acute, chronic, unspecified, and other) |
|
| Vila 2008 [172] |
Summer 2004 | Sant Andreu de Llavaneres, Catalonia, Spain |
Sea water | 62 people | Rhinorrhea (74.2%), nose irritation (66.1%), throat irritation (62.9%), coughing (59.7%), expectoration (51.6%), eye irritation (41.4%) and migraine (40.3%) | ||
| Galitelli 2006 [173] |
Summers of 2003 and 2004 |
Coasts of Bari, Italy | Sea water | Ostreopsisgenus | Recreational or working activities on the beach |
28 patients ( 1 child; mean age, 38.6 years) |
Rhinorrhoea 100%; cough 43%; fever 14%; and bronchoconstriction with mild dyspnoea and wheezes 43%. Conjunctivitis 11% |
| Bresciani & Durando 2007 [174] |
Summers of 2005 and 2006 | Genova and La Spezia, Italy |
Sea water | Ostreopsis ovata | 209 patients (mean age 35.9 ± 20.1 years, range 1–89 years). 43 patients seeking medical help at the emergency departments needed hospitalization | Fever (63.6%), sore throat (50.2%), cough (40.2%), and dyspnoea (38.8%) | |
| Milian 2007 [175] |
Unexposed period: March 2003 and March 2005; exposed period: January 2003, May 2004 October 2004 |
Sarasota, Florida, USA | Sea water | Exposure to aerosolized brevetoxins |
97 persons (≥12 years) with physician-diagnosed asthma |
Respiratory symptoms like eye and/or throat irritation; nasal congestion; cough; wheeze; chest tightness |
|
| Fleming 2007 [176] |
Unexposed period: March 2003 and March 2005; exposed period: January 2003, May 2004 October 2004 |
Sarasota, Florida, USA | Sea water | Exposure to aerosolized brevetoxins |
97 persons (≥12 years) with physician-diagnosed asthma |
Respiratory symptoms like eye and/or throat irritation; nasal congestion; cough; wheeze; chest tightness |
| QAC | Formula | CAS-Number | Molecular weight |
Half-life | Log Koc | Log Dow/Kow |
| ATMACs | CH3(CH3)7-17 N(CH3)3Cl | C8:10108-86-8 C10: 10108-86-9 C12: 112-00-5 C14: 4574-04-3 C16: 112-02-7 C18: 112-03-8 |
C8: 207.8 C10: 235.8 C12: 263.9 C14: 291.9 C16: 320.0 C18: 348.0 |
- | C8: 2.78 C10: 3.30 C12: 3.82 C14: 4.34 C16: 4.36 C18: 5.38 |
C8: -1.05 C10: -0.189 C12: 0.857 C14: 1.77 C16: 2.43 C18: 3.25 |
| DDACs | [CH3(CH3)7-17]2 N(CH3)2Cl | C8: 5538-94-3 C10: 7173-51-5 C12: 3401-74-9 C14: 10108-91-5 C16: 1812-53-9 C18:107-64-2 |
C8: 306.0 C10: 362.1 C12: 418.2 C14: 474.3 C16: 530.4 C18: 586.5 |
180 days in river water (EPA, 2017) 1048 days in soil [189] |
C8:4.64 C10: 5.68 C12: 6.73 C14: 7.71 C16: 8.81 C18: 9.86 |
C8: 1.57 C10: 2.59 C12: 4.31 C14: 6.25 C16: 9.98 C18: 9.52 |
| BACs | CH3(CH3)5-17N(Cl) (CH3)2 CH2 C6H5 | C6: 22559-57-5 C8: 959-55-7 C10: 965-32-2 C12: 139-07-1 C14: 139-08-2 C16: 122-18-9 C18: 122-19-0 |
C6: 255.8 C8: 283.9 C10: 311.9 C12: 340.0 C14: 368.0 C16: 396.1 C18: 424.1 |
379 days (in water at pH 9) [190] |
C6: 3.87 C8: 4.39 C10: 4.91 C12: 5.43 C14: 5.96 C16: 6.48 C18: 7.00 |
C6: -0.763 C8: 0.233 C10: 1.31 C12: 2.10 C14: 2.78 C16: 3.54 C18: 4.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





