Introduction
Francis Click wrote “wobble hypothesis” in 1966 [
1], and explained wobble base parings between inosine nucleoside (hypoxanthine as a base) of tRNA anticodon and codon bases, after the tRNA
Ala structure from brewery yeast was reported to have inosine [
2] and was located in the first position of the anticodon, wobble position [
3]. Subsequent studies demonstrated that utilization of inosine in the wobble position is ubiquitous, especially in eukaryotes, but not conserved in all applicable tRNAs of organisms. Since then, tRNA modifications, aminoacylation of tRNAs, the ribosomes and translation machinery were intensively studied revealing aspects of the translation systems.
As tRNA genome databases and analysis programs have been established in current genome era, starting from the tRNA genomic genes, reconsideration of codon-anticodon pairings should be useful to deepen understanding of translation of the genes. Here I propose a concept of “decoding tables” for genetic code as a conceptual framework. The decoding table depict codon-tRNA anticodon pairings, based on tRNA genomic gene information (tDNA)[
4,
5] and experimentally elucidated general features of tRNA. The use of wobble position pairings is specific to individual organisms. Among a lot of tRNA modifications, the decoding tables selectively include modifications at the anticodon site that affect selection of the pairing codon. The tables have limitations: 1) tRNA modifications that do not influence codon selection are not included, and 2) contextual effects are not considered. Despite these limitations, I believe that the decoding tables are crucial for understanding the codon-tRNA anticodon pairings in functional terms. Creatures often employ idiosyncratic system and possess complex compensatory mechanisms to survive and react to diverse situations and stresses. Given the intricate nature of those studies, achieving complete understanding may remain elusive, underscoring the importance of presenting current knowledge concisely.
2. tRNA repertoires
Genome analyses have revealed variations in the number of tRNA genes and distinct tRNA repertoires among individual organisms. The tRNA repertoire refers to the number of tRNA genes defined by anticodons. For example, the
Saccharomyces cerevisiae genome has 275 tRNA genes, constituting a 41 tRNA repertoire with different anticodons, to decode 61 sense codons, except for termination codons (GtRNAdb,
http://gtrnadb.ucsc.edu) (SGD,
https://www.yeastgenome.org)[
4,
6]. The number of tRNA genes varies substantially across organisms, ranging from fewer than 35 (e,g.,
Ureplasma urealyticum, and
Borrelia. burgdorferi) to more than 3000 (e,g.,
Xenopus tropicalis, and
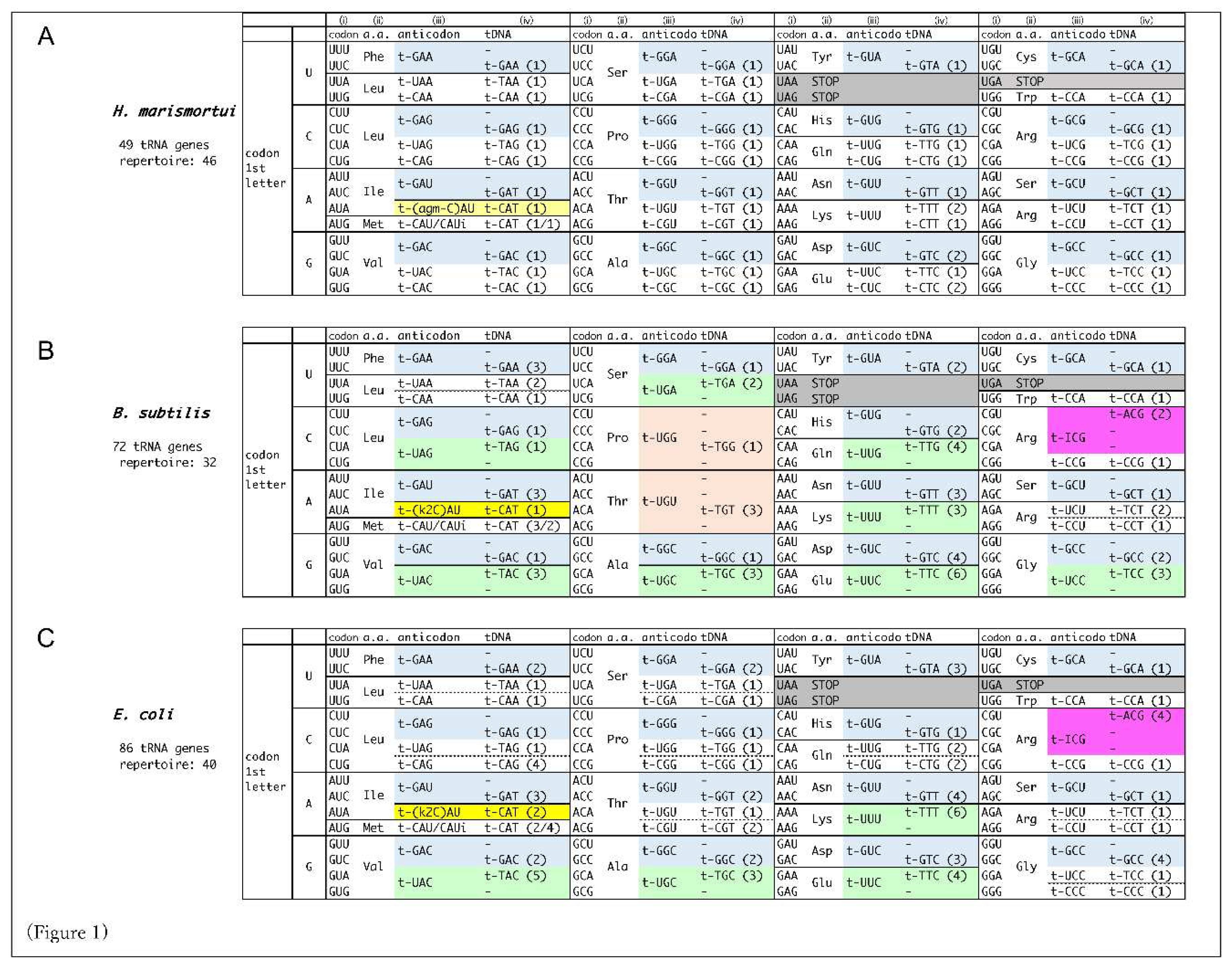
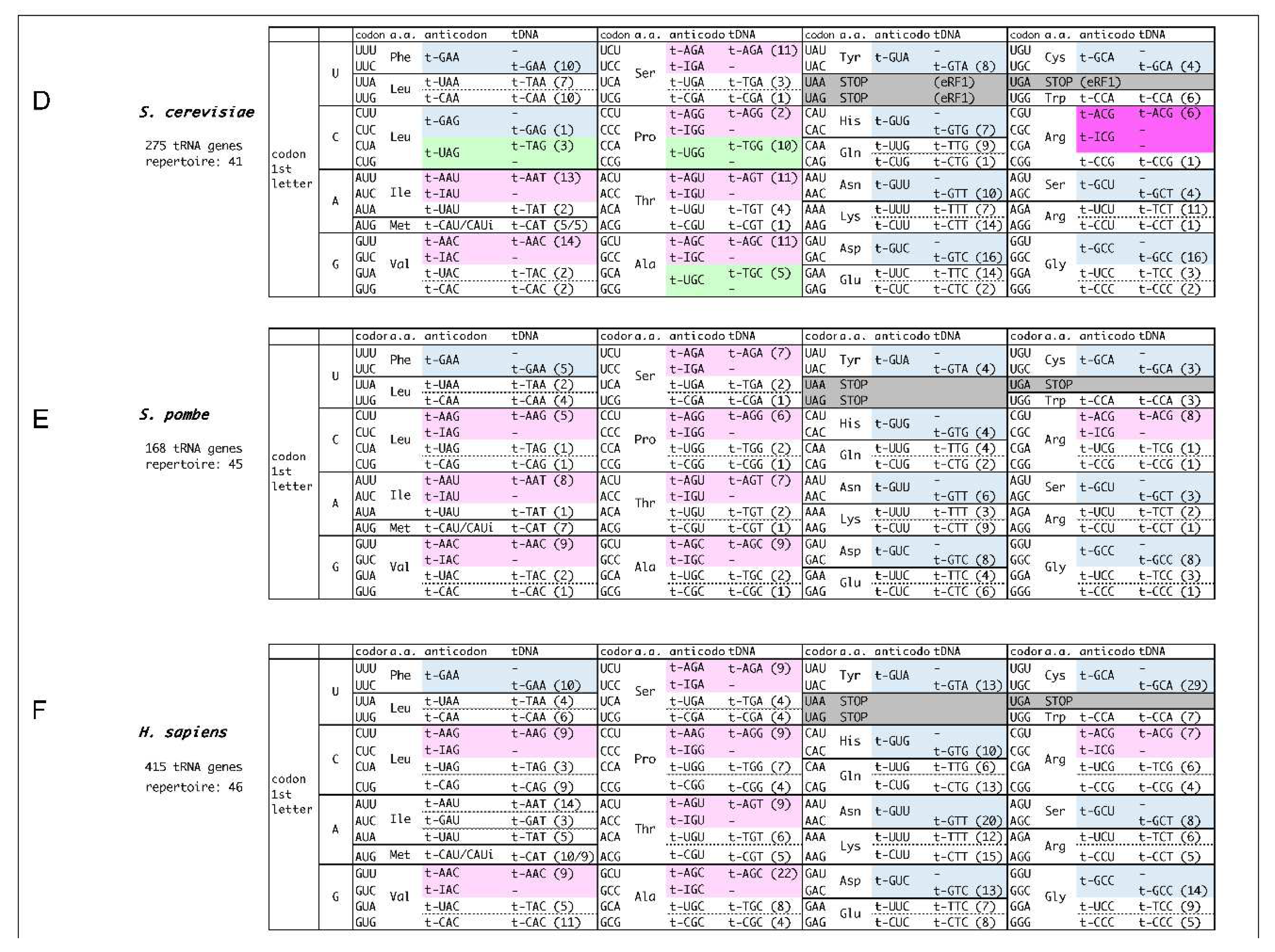
Danio rerio). The number of tRNA repertoire typically falls within 32 to 54. No organism has a 61 tRNA repertoire corresponding to 61 sense codons, i.e., any of tRNA repertoire should decipher more than one codon. Considering the different tRNA repertoires among organisms, we propose the concept of “decoding tables” that describe the codon-anticodon pairings in each organism, comparatively showing the features of codon-anticodon pairings among organisms (
Figure 1). The heart of the decoding table is wobble base-pairings [
1], that is allowed non-Watson-Crick pairing between the 3
rd base of the mRNA codon and the 1
st base of the tRNA anticodon (position 34 of tRNA). Although tRNAs are heavily modified[
7,
8,
9], the tables include only major modifications at anticodons known to change codon specificity. The wobble position inosine nucleoside (hypoxanthine as the base) edited from adenine is essential in the decoding, having pairing ability with C-ending codons in addition to U-ending codons [
10], in eukaryotes. Lysidine and agmatidine modified from cytidine, in bacteria and in archaea respectively, play a specific role in deciphering AUA codon as isoleucine, distinguishing from AUG methionine codon and AUG initiation codon[
11,
12]. Eukaryotic mitochondrial tRNAs are not included in this report.
3. Basis of the decoding tables
The decoding tables for six organisms from three domains of life are depicted in
Figure 1. The rules implemented to depict the decoding tables are as follows:
A set of decoding information comprises (i) codons, (ii) amino acids, (iii) tRNA anticodons, and (iv) tDNA anticodons, described in this order within the boxes. The number of corresponding tRNA genes with the anticodon is shown in the parentheses within (iv). In short, (i) and (iii) represent the codon-anticodon pairing.
tDNA anticodons (iv) are essentially derived from the tRNA genome database [
4], and placed by appropriate codon-anticodon pairings. When a codon (i) forms complete Watson-Crick base pairs with a tDNA anticodon (iv) (either adenine (A)- thymine (T) or guanine (G)- cytosine (C)), the tRNA anticodon (iii) is corresponding RNA anticodon to the tDNA anticodon.
-
When a codon does not make perfect match pairing with any of tDNA anticodons, potential wobble-pairing is introduced into the 1st base of the anticodon (position 34 of tRNA) (iii) from the tDNA anticodon (iv).
- 3-1)
The anticodon 1
st G is assumed to pair with the codon 3
rd U, in addition to 3
rd C (shown in light blue in
Figure 1), as G-U pairing in secondary structure is often observed in RNA (shown in pale blue). [
13,
14,
15]
- 3-2)
The G-U pairing allows the anticodon 1st U to pair with the codon 3rd G, in addition to 3rd A (shown in light green).
- 3-3)
In some codon boxes (codons that share 1
st and 2
nd bases), only one tRNA repertoire with anticodon 1
st U is assigned. In these cases, superwobbling, which describes the anticodons with 1
st U can pair with any of codon 3
rd base, is implemented (shown in orange). Superwobbling has been evidenced in some organisms [
16,
17].
- 3-4)
Where the anticodon 1
st G is not assigned but 1
st A in codon boxes, inosine (I) is implemented to pair with both the codon 3
rd C and 3
rd U (shown in pale pink). In special cases of tRNA
Arg (ICG), the anticodon 1
st I pairs with the codon 3
rd A in addition to 3
rd C and U, and the type of pairing is called A-I wobble, as is observed in
Escherichia coli and
S. cerevisiae (shown in pink) [
18]. Intriguingly, the anticodon 1
st inosine does not necessarily pair with U, allowing the possibility of the precursor tRNA with anticodon 1
st A to be utilized. In this meaning, the extent of usage of the anticodon 1
st inosine containing tRNAs (i-tRNAs) is yet to be determined, though i-tRNAs are definitely necessary to decipher C-ending codons in the boxes.
In prokaryotes and archaea, lysidine(k2) and agmatidine(agm) modifications of the anticodon 1
st C of CAU are used to decipher AUA codon, respectively, according to previous reports. These k2-CAU and amg-CAU are deciphered by tRNA
Ile as isoleucine, distinguishing AUG codon for methionine (shown yellow) [
11,
12].
Variant genetic codes should be considered for organisms in use [
19,
20], however, these organisms are not included in
Figure 1.
Following the scheme described above, a decoding table can be constructed for any organisms using the genome tRNA database (or genome sequence data and a tRNA gene finding program), as a concise summary. The decoding tables described in
Figure 1 are illustrative models. The anticodon defining procedure ensures no inconsistency with previous reports in relation to codon- anticodon pairings. The accuracy of the decoding tables largely relies on tRNA genome database, i.e., the tRNA-finding algorithm, which may not be entirely precise, especially in higher eukaryotes with complex genome structures.
4. Comparative wobble pairing usage
The comparative decoding tables for the organisms, archaeal
Haloarcula maristortui, bacterial
Bacillus subtilis and
Escherichia coli, eukaryotic
Saccharomyces cerevisiae,
Schizosaccharomyces pombe, and
Homo sapiens, are depicted in
Figure 1. Wobble usage is denoted by colour, revealing domain-specific tendencies and additional organism-specificities. Inosine-containing i-tRNAs are one in
E. coli, seven in
S. cerevisiae, eight in
S. pombe, and seven in
H. sapiens. The i-tRNAs are predominantly utilized in eukaryotes [
21,
22]. The inosines in i-tRNAs are edited from adenines by the oxidative deamination via the Tad2/Tad3 complex in
S. cerevisiae, ADAT2/ADAT3 in
H. sapiens, and TadA in
E. coli [
10,
23,
24]. The inosine adapts a guanine-like structure for base pairing, and read as G in canonical nucleotide sequencing [
10,
18]. The tRNA
Arg(ICG) pairs with U, C and A, that may be contributed tautomerism in the decoding. The type of pairings is restricted to bacteria and a few yeasts, and is the only one i-tRNA in bacteria. The other i-tRNAs are used only in eukaryotes.
Uracil plays a substantial role in wobble pairing, demonstrating the widest pairing capacity [
16,
21]. In
B subtilis, tRNA
Pro and tRNA
Thr with wobble position U correspond to four codons (shown in pale orange), which are all utilized according to the codon usage of
B. subtilis (Codon Usage Database:
https://www.kazusa.or.jp/codon). The type of 4-codon-decoding tRNAs are restricted to the codon boxes in which amino acids are not defined by the codon’s 3
rd base, and are observed in certain bacteria. In the pairings, probably unmodified or lightly modified tRNA wobble U likely pairs with any of four bases with varying levels of efficiency. On the other hand, anticodon 1
st U is variously modified depending on tRNAs (modomics,
https://iimcb.genesilico.pl/modomics)[
8]. Examples of such modifications for
E. coli and
S. cerevisiae are shown in
Figures S1 and
Figure S2. tRNA anticodon modifications, other than inosine, that affect decoding appears to have been introduced to distinguish codons, thereby increasing codon-anticodon specificity.
Although the decoding tables do not indicate decoding efficiency in the ribosome, they reveal intriguing deviations in translation systems among species and the evolution of tRNA repertoires. The decoding tables may offer valuable insights into the significance of codon degeneracy, as the codon usage in mRNAs and the repertoire of tRNAs directly impact the GC content (%) of organisms, especially those with a small genome size. Based on the decoding tables, codon degeneracy appears to be defined by the supplied aminoacyl-tRNA repertoires rather than wobble codon acceptability of tRNAs, suggesting a presumed co-evolution. Furthermore, the decoding tables may provide important information for heterologous expression in genetic engineering.
5. Around initiation and termination codons
Two distinct features in the decoding tables exist around AUG codon, and around UGA stop codon. The AUG codon and UGA stop codons must have been needed to establish orthogonality to be used as initiation and stop codon, respectively. To distinguish AUA codon from AUG codon, archaea and bacteria appear to have established different tRNA anticodon 1
st C modifications, namely agmatidine and lysidine (depicted in
Figure 1A for
H. marismortui,
Figure 1B for
B. subtilis, and
Figure 1C for
E. coli). In contrast, eukaryotes somehow established specific tRNA(UAU) to read only AUA codon. In
S. cerevisiae, two anticodon uracyl bases of tRNA(UAU) are modified to pseudouridines to be distinguished (
Figure S2). The lysidine and agmatidine modifications are introduced into the pyrimidine-ring-C2 of position 34C base, conversely, most of modifications of position 34U base are introduced into the pyrimidine-ring-C5. These modifications are positioned in structurally opposite directions [
25].
As for UGA stop codon, most of organisms have established orthogonality with a specific tRNA
Trp(CCA) to decipher UGG codon exclusively. However, the A-I wobble observed in certain bacteria and yeasts to decipher CGA codon might be a remnant of the evolutionary process of distinguishing UGA codon. Bacteria utilize two peptide release factors, RF1 for UAA and UAG stop codons and RF2 for UAA and UGA stop codons [
26]. In eukaryotes, omnipotent eRF1 deciphers all three stop codons including UGA codon, which established orthogonality with UGG. Instead, CGA codon, which is one base difference with UGA at non-wobble position, is deciphered by eRF1/eRF3 complex in misdecoding level to release polypeptide under specific conditions [
27]. It is of interesting to investigate whether the A-I wobble is strategically employed in relation to translation termination. The introduction of initiation codon and stop codons into the genetic code must have been a complex process.
6. Evaluation of the decoding tables
The decoding tables show the most suitable codon-anticodon pairings deduced from the databases and experimental reports. While reports describe the overlapping of decoding codons, the decoding tables do not consider multiple tRNA anticodons for a codon. It should be reminded in relation to stop codons, such as variant genetic code, and fidelity of the decoding of sense and stop codons. While the concise decoding tables provide a clear overview, detailed information on the extent of these effects is challenging to ascertain. Reconstitution experiments in vitro face difficulties in setting up conditions, and in vivo experiments often trigger compensatory reactions, even though they surely demonstrate aspects of the decoding of genetic codes. The decoding tables effectively illustrate codon-anticodon pairings, particularly highlighting tRNA repertoires, given their foundation on a compilation of reports.
From the perspective of aminoacylation, typically only one aminoacyl-tRNA synthetase (aa-RS) is responsible for adding each amino acid to the corresponding tRNA [
28,
29]. Evaluating the decoding tables in the context of aminoacylation is crucial. Each aa-RS recognizes specific elements in its substrate tRNAs, known as identity elements [
29,
30]. Among the anticodons, positions 35 and 36 bases (2
nd and 3
rd bases of anticodons) are included in identity elements of not a few of aminoacyl-tRNAs. Additionally, position 34G is included in the identity elements of some tRNAs, such as tRNA
Cys tRNA
Tyr, confirming the anticodon 1
st G containing tRNAs are the substrates of the aa-RSs. On the contrary, A or I at tRNA position 34 are not included in identity elements. This raises an important question regarding whether position 34A, the precursor of position 34I, is used for decoding or not. It should be clarified at a general level. Answers might have been within reported data hidden to be analyzed to a certain level. Some reports indicate position 34 I/A are involved in some regulation [
31,
32], but not intensively studied.
The recognition of tRNA
Ile(k2-CAU) and tRNA
Ile(agm-CAU) by isoleucyl-tRNA synthetases is rather exceptional, and reported that modified position 34 bases are included in the identity elements of their synthetases [
29]. Thus, for these tRNAs, the indicated modifications are prerequisites for aminoacylation. It is an interesting contrast that the position 34I and 34A are not included in identity elements.
The decoding tables apply to organisms without major discrepancies. They shed light on codon-tRNA anticodon pairings in the ribosome which might be unconsciously overlooked under uniform translation system from genes to proteins. The decoding tables contribute to our understanding of tRNA repertoires and the decoding in translation in organisms, and evolution of translation systems. It is of interesting to consider the evolution of translation machinery that decipher genetic codes, essential to all organisms. The intricacies of decoding of the codons in the ribosome including related factors biogenesis, is what a contrast with resulting decoding products observed as polypeptides.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org; Figure S1 and S2.
Funding
This research received no external funding.
Acknowledgments
I thank Prof. Koichi Ito for kind discussions and comments. I thank GSFS, the University of Tokyo, for treating me as a guest scientist.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviation
i-tRNA: position 34 inosine containing tRNA
References
- Crick:F.H.C. [1966] Codon—anticodon pairing: The wobble hypothesis. Journal of Molecular Biology, 19, 548–555. [CrossRef]
- Holley,R. [1965] Structure of an alanine transfer ribonucleic acid. science, 194, 868–871. [CrossRef]
- Madison,J., Eferett,G. and Kung,H. [1966] Nucleotide sequence of a yeast tyrosine transfer RNA. science, 153, 531–534. [CrossRef]
- Lowe,T.M. and Chan,P.P. [2016] tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Research, 44, W54–W57. [CrossRef]
- Chan,P.P., Lin,B.Y., Mak,A.J. and Lowe,T.M. [2021] classification of transfer RNA genes. 49, 9077–9096. [CrossRef]
- Cherry,J.M., Hong,E.L., Amundsen,C., Balakrishnan,R., Binkley,G., Chan,E.T., Christie,K.R., Costanzo,M.C., Dwight,S.S., Engel,S.R., et al. [2012] Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Research, 40, 700–705. [CrossRef]
- Dunin-Horkawicz,S., Czerwoniec,A., Gajda,M.J., Feder,M., Grosjean,H. and Bujnicki,J.M. [2006] MODOMICS: a database of RNA modification pathways. Nucleic acids research, 34, 145–149. [CrossRef]
- Cappannini,A., Ray,A. and Mukherjee,S. [2023] MODOMICS : a database of RNA modi cations and related inf ormation . 2023 updat e.
- Grosjean,H., de Crécy-Lagard,V. and Marck,C. [2010] Deciphering synonymous codons in the three domains of life: Co-evolution with specific tRNA modification enzymes. FEBS Letters, 584, 252–264. [CrossRef]
- Gerber,A.P. and Keller,W. [1999] An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science, 286, 1146–1149. [CrossRef]
- Suzuki,T. and Miyauchi,K. [2010] Discovery and characterization of tRNAIle lysidine synthetase (TilS). FEBS Letters, 584, 272–277. [CrossRef]
- Mandal,D., Köhrer,C., Su,D., Russell,S.P., Krivos,K., Castleberry,C.M., Blum,P., Limbach,P.A., Söll,D. and RajBhandary,U.L. [2010] Agmatidine, a modified cytidine in the anticodon of archaeal tRNA Ile, base pairs with adenosine but not with guanosine. Proceedings of the National Academy of Sciences of the United States of America, 107, 2872–2877. [CrossRef]
- Volume 5 Number 11 November 1978 Nucleic Acids Research [1978] Nucleic Acids Research, 5, 4451–4462. [CrossRef]
- Demeshkina,N., Jenner,L., Westhof,E., Yusupov,M. and Yusupova,G. [2013] New structural insights into the decoding mechanism: Translation infidelity via a G·U pair with Watson-Crick geometry. FEBS Letters, 587, 1848–1857. [CrossRef]
- Ananth,P., Goldsmith,G. and Yathindra,N. [2013] An innate twist between Crick’s wobble and Watson-Crick base pairs. Rna, 19, 1038–1053. [CrossRef]
- Rogalski,M., Karcher,D. and Bock,R. [2008] Superwobbling facilitates translation with reduced tRNA sets. Nature Structural and Molecular Biology, 15, 192–198. [CrossRef]
- Lei,L. and Burton,Z.F. [2022] “Superwobbling” and tRNA-34 Wobble and tRNA-37 Anticodon Loop Modifications in Evolution and Devolution of the Genetic Code. Life, 12. [CrossRef]
- Wada,M. and Ito,K. [2023] The CGA codon decoding through tRNAArg(ICG) supply governed by Tad2/Tad3 in Saccharomyces cerevisiae. FEBS Journal, 290, 3480–3489. [CrossRef]
- Lozupone,C.A., Knight,R.D. and Landweber,L.F. [2001] The molecular basis of nuclear genetic code change in ciliates. Current Biology, 11, 65–74. [CrossRef]
- Kim,O.T.P., Sakurai,A., Saito,K., Ito,K., Ikehara,K. and Harumoto,T. [2008] Ciliates use both variant and universal genetic codes: Evidence of omnipotent eRF1s in the class Litostomatea. Gene, 417, 51–58. [CrossRef]
- Novoa,E.M., Pavon-Eternod,M., Pan,T. and Ribas De Pouplana,L. [2012] A role for tRNA modifications in genome structure and codon usage. Cell, 149, 202–213. [CrossRef]
- Rafels-Ybern,À., Torres,A.G., Camacho,N., Herencia-Ropero,A., Frigolé,H.R., Wulff,T.F., Raboteg,M., Bordons,A., Grau-Bove,X., Ruiz-Trillo,I., et al. [2019] The expansion of inosine at the wobble position of tRNAs, and its role in the evolution of proteomes. Molecular Biology and Evolution, 36, 650–662. [CrossRef]
- Ramos-Morales,E., Bayam,E., Del-Pozo-Rodríguez,J., Salinas-Giegé,T., Marek,M., Tilly,P., Wolff,P., Troesch,E., Ennifar,E., Drouard,L., et al. [2021] The structure of the mouse ADAT2/ADAT3 complex reveals the molecular basis for mammalian tRNA wobble adenosine-to-inosine deamination. Nucleic Acids Research, 49, 6529–6548. [CrossRef]
- Wolf,J., Gerber,A.P. and Keller,W. [2002] TadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO Journal, 21, 3841–3851. [CrossRef]
- Agris,P.F., Eruysal,E.R., Narendran,A., Väre,V.Y.P., Vangaveti,S. and Ranganathan,S. V. [2018] Celebrating wobble decoding: Half a century and still much is new. RNA Biology, 15, 537–553. [CrossRef]
- Ito,K., Uno,M. and Nakamura,Y. [2000] A tripeptide ‘anticodon’ deciphers stop codons in messenger RNA. Nature, 403, 680–684. [CrossRef]
- Wada,M. and Ito,K. [2019] Misdecoding of rare CGA codon by translation termination factors, eRF1/eRF3, suggests novel class of ribosome rescue pathway in S. cerevisiae. FEBS Journal, 286, 788–802. [CrossRef]
- Gomez,M.A.R. and Ibba,M. [2020] Aminoacyl-tRNA synthetases. Rna, 26, 910–936. [CrossRef]
- Gieg,R. and Eriani,G. [2023] The tRNA identity landscape for aminoacylation and beyond. Nucleic Acids Research, 51, 1528–1570. [CrossRef]
- Abelson,J. [1989] tRNA IDENTITY Jennifer Normanl / and John Abelson. Identity.
- Rubio,M.A.T., Ragone,F.L., Gaston,K.W., Ibba,M. and Alfonzo,J.D. [2006] C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei. Journal of Biological Chemistry, 281, 115–120. [CrossRef]
- Bertotti,S., Fleming,I., de los Milagros Cámara,M., Cameán,C.C., Carmona,S.J., Agüero,F., Balouz,V., Zahn,A., Di Noia,J.M., Alfonzo,J.D., et al. [2022] Characterization of ADAT2/3 molecules in Trypanosoma cruzi and regulation of mucin gene expression by tRNA editing. Biochemical Journal, 479, 561–580. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).