Introduction
Malignant peritoneal mesothelioma is an uncommon primary tumour of the peritoneal lining and is the second most common type of mesothelioma[
1]; it accounts for about 30% of all malignant mesotheliomas[
2]. Other significant and well-documented types are Well-Differentiated Papillary Mesothelioma, Multicystic Mesothelioma, and Desmoplastic Mesothelioma with subtypes pure sarcomatoid mesothelioma and lymphohistiocytoid mesothelioma[
3]. Cystic mesotheliomas are rare and occur predominantly in young to middle-aged women[
4]. In this subtype (Multicystic peritoneal mesothelioma), there is no association with asbestos exposure. Typically, patients present with weight loss, anorexia, abdominal pain and/or abdominal distension, and pyrexia of unknown origin.
Case History
A 47-year-old female patient came to surgery OPD in a hospital with a complaint of abdominal pain associated with abdominal distension, nausea, significant weight loss, and constipation on/off for 2 months. They advised an X-ray abdomen and ultrasound examination of the patient, based on the findings of the X-ray and USG they suggested a contrast study of the abdomen. There is no history of occupational asbestos exposure.
X-ray Findings
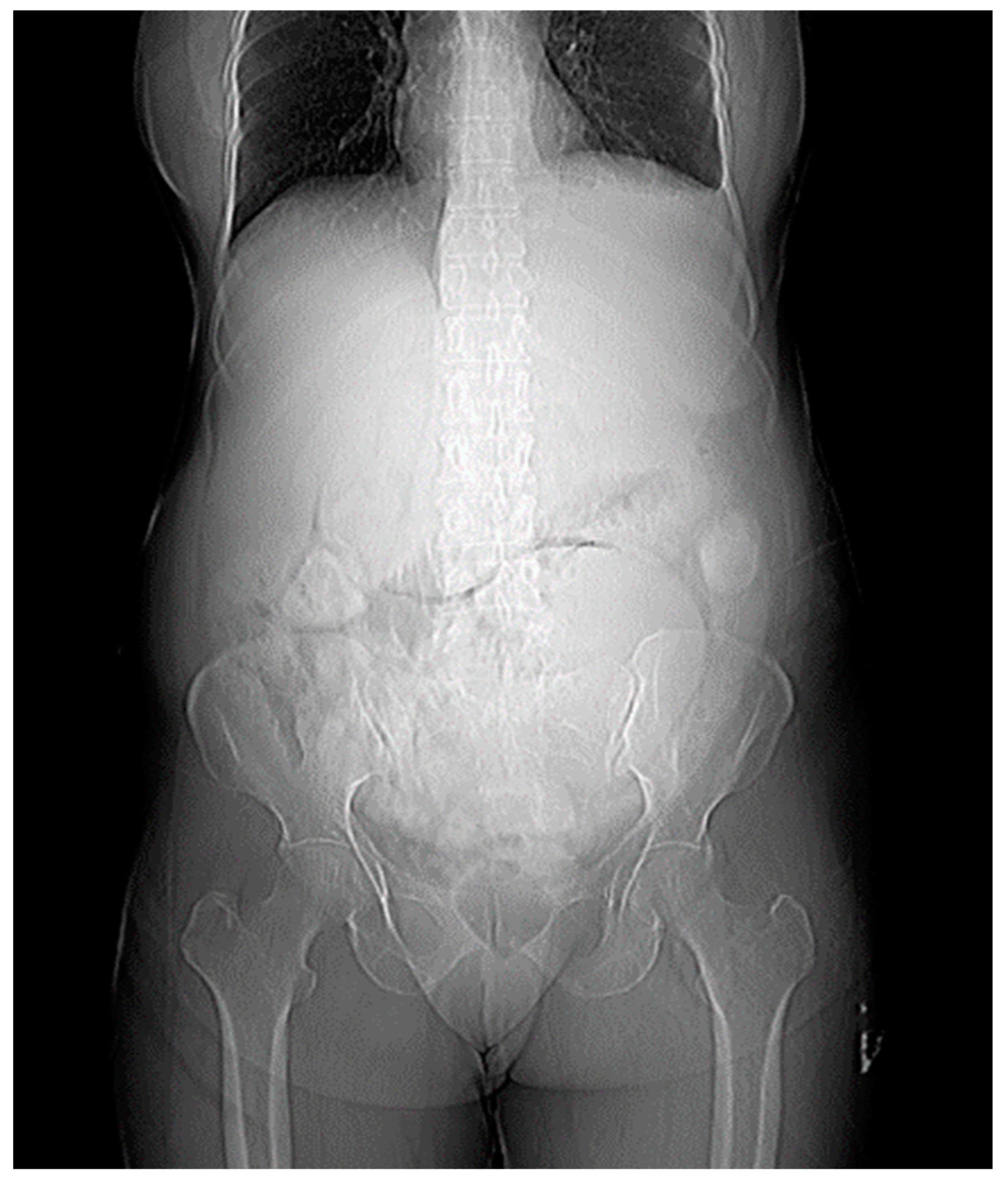
The X-ray findings reveal several significant observations. Firstly, there is a large soft tissue opacity observed in the epigastric, left hypochondriac, and left lumbar regions, causing displacement of the bowel loops in a peripheral manner. This particular opacity appears to be extensive, covering multiple abdominal regions.
Secondly, another soft tissue opacity is identified in the left iliac fossa, hypogastrium, and pelvis. The presence of this opacity in the lower left quadrant of the abdomen suggests potential implications for the structures in this area.
Furthermore, an enlargement of the hepatic shadow is noted in the X-ray images. This finding indicates an increase in the size of the liver, which may warrant further investigation into potential underlying causes.
Lastly, the X-ray reveals blunting of the left costophrenic (CP) angle. This observation suggests a reduction in the sharpness of the angle formed by the diaphragm and the ribcage on the left side, and it could be indicative of certain pathological conditions.
Ultrasound Abdomen
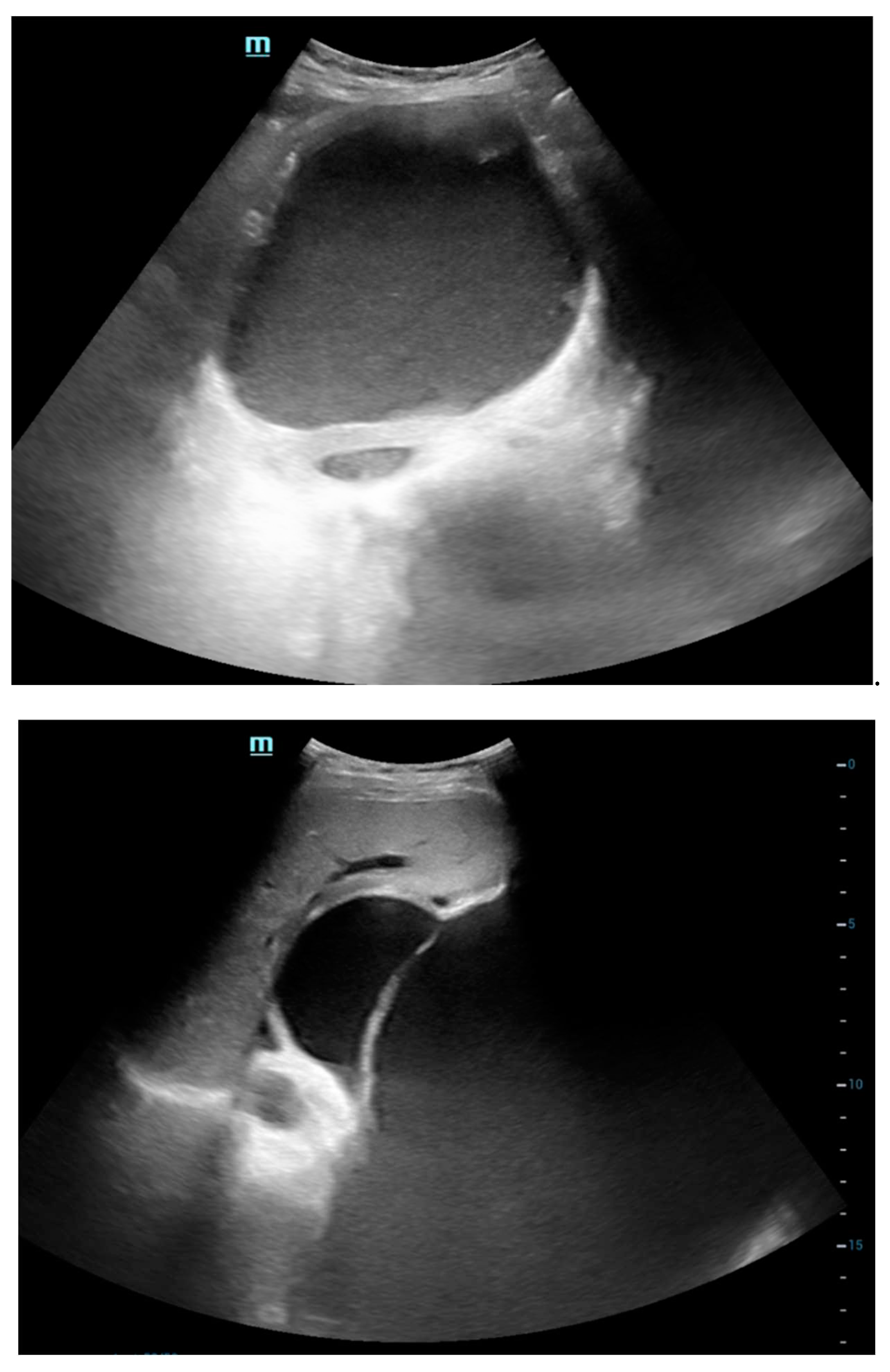
The ultrasonography (USG) findings reveal a sizable lobulated cystic lesion characterized by internal septations and dense echoes, extending throughout the abdomen and pelvis, with its upper boundary reaching the epigastric region. Notably, one of the septa in the left hypochondriac region exhibits a maximum thickness of approximately 7 mm. No solid components were discerned within the lesion. In the epigastric region, the lesion exerts pressure on and displaces the stomach in a superior direction. Additionally, the lesion causes peripheral displacement of bowel loops. These findings provide a comprehensive description of the observed abnormalities, offering valuable insights into the nature and extent of the cystic lesion.
CT Contrast Abdomen Findings
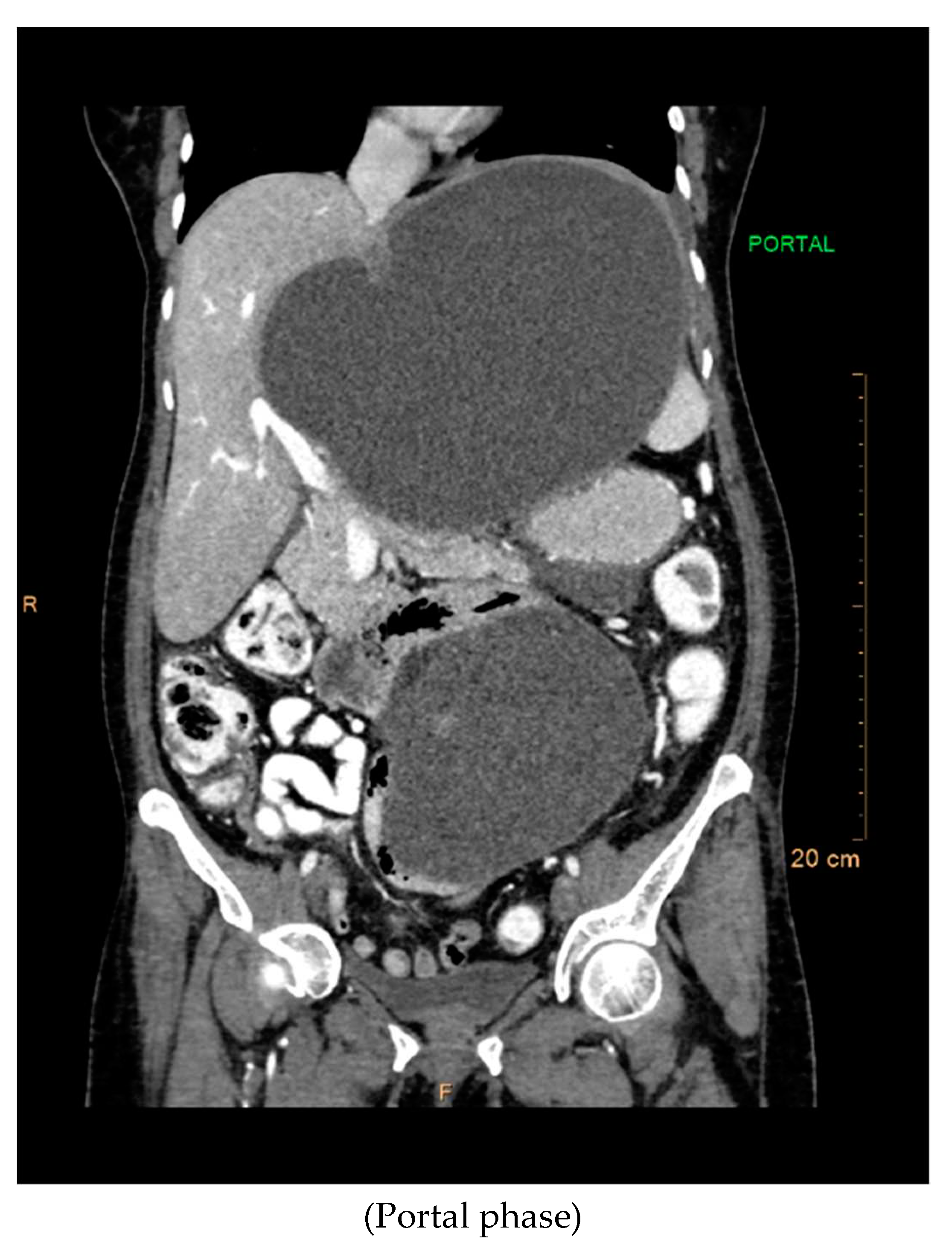
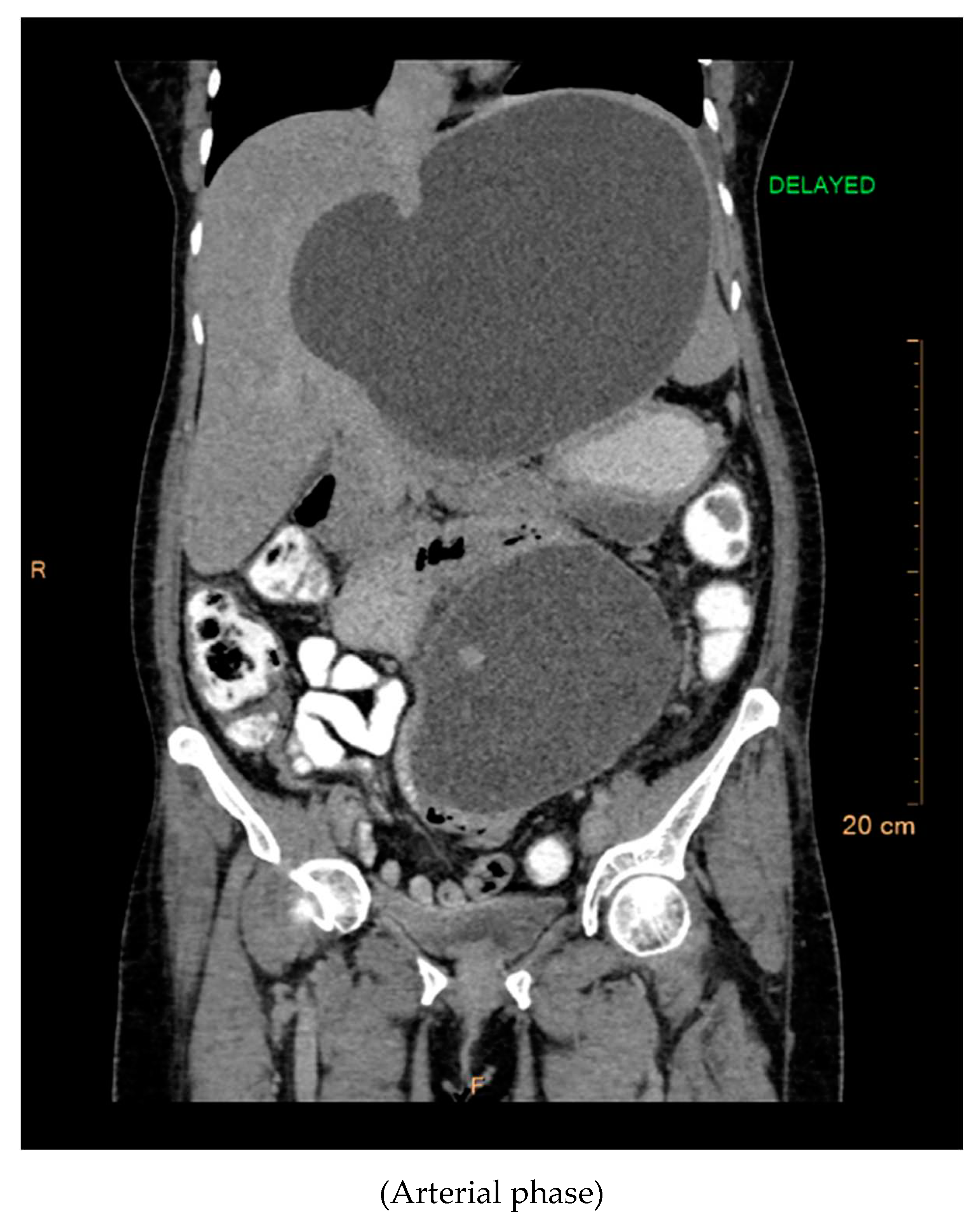
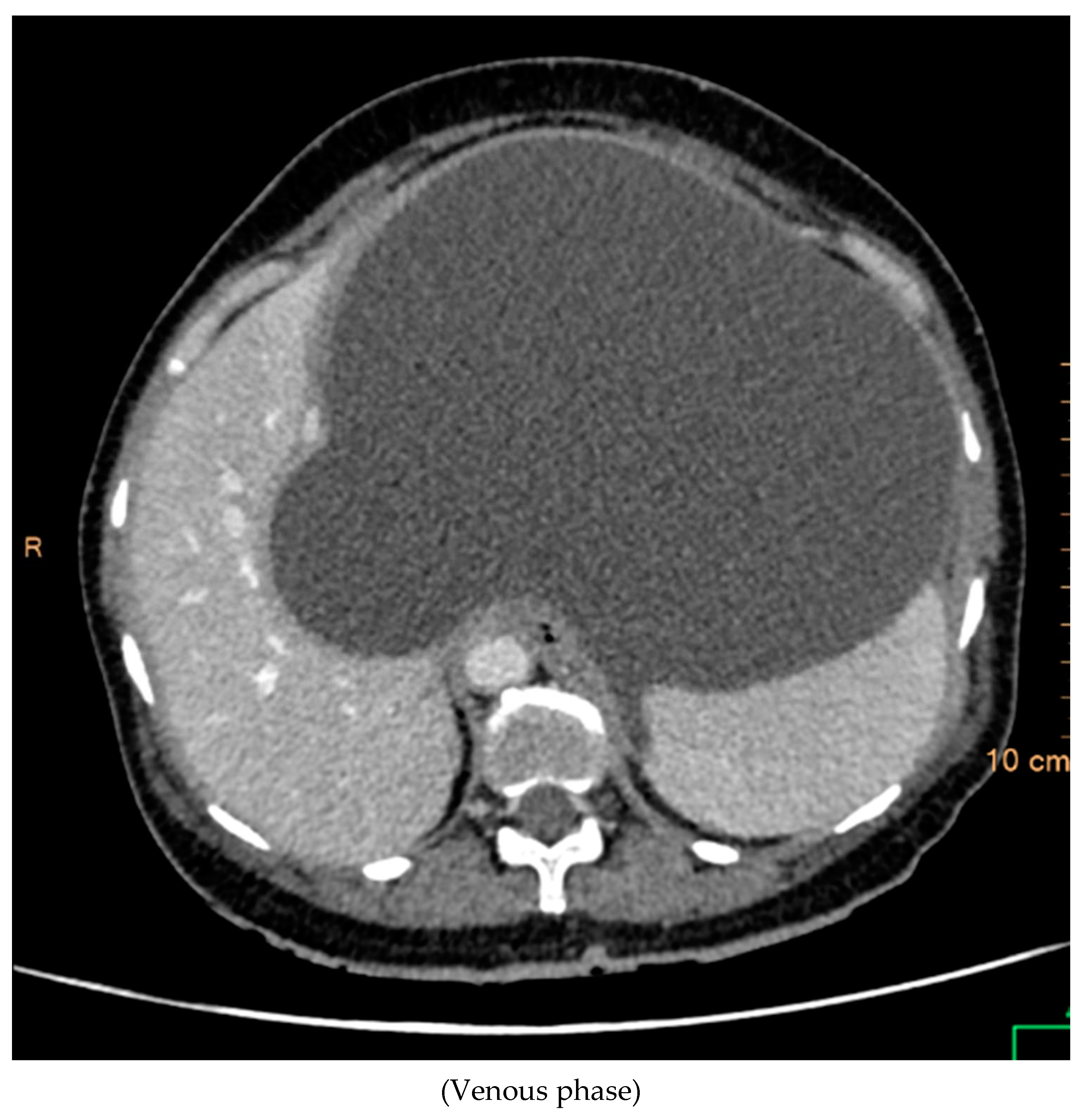
The contrast-enhanced computed tomography (CT) findings reveal a series of notable features within the abdominal region. Multiple well-defined peripherally enhancing cystic lesions, each exhibiting internal septations, are observed in distinct locations, including the epigastric, gastrohepatic, left lumbar, and greater omentum regions along the greater curvature of the stomach. These cystic formations present as significant entities in the imaging, demonstrating a complex architecture.
A large, well-defined peripherally enhancing cystic lesion with several internal enhancing septations is identified in the epigastric and gastrohepatic region. There is a possibility that this lesion originates from the left lobe of the liver. Notably, the superior extension of this lesion reaches up to the left hypochondriac and subdiaphragmatic regions. Laterally, it extends towards the left lateral abdominal wall, exerting pressure and indentation on the right lobe of the liver.
Inferiorly, the large cystic lesion exerts compression on the body of the stomach, causing displacement. Posteriorly, the lesion notably compresses the body of the pancreas. Further examination reveals compression on the common hepatic artery, portal vein, and splenic vein at the splenoportal junction, although these vessels remain patent. Anteriorly, the lesion extends up to the anterior abdominal wall, showcasing its expansive nature.
The posterior and left lateral aspects of the lesion result in the displacement and compression of the mid and upper pole of the spleen. Additionally, collateral vessels are observed in the perisplenic and left hypochondriac regions, indicating the potential impact of this cystic lesion on vascular dynamics. The comprehensive CT contrast findings provide valuable insights into the intricate anatomical alterations and potential clinical implications associated with these cystic lesions within the abdominal cavity.
Histopathology
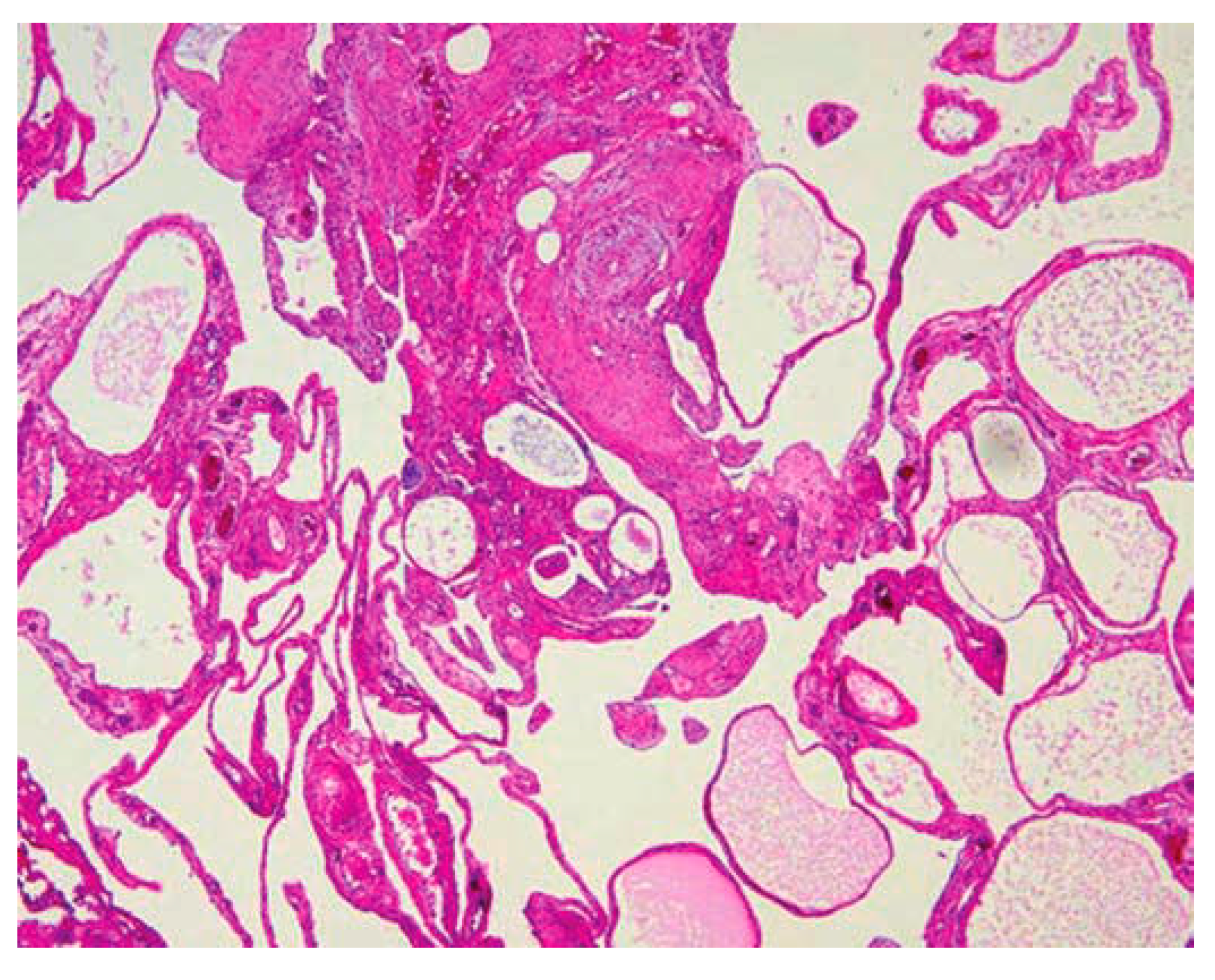
The specimen submitted for histopathological examination yielded crucial insights into the nature of the observed abnormalities. Upon careful analysis, the examination revealed the presence of neoplastic acini and micropapillae within the serosa and muscularis propria. This indicates a significant involvement of these tissues by abnormal cellular growth, suggesting a complex pathology within the affected area.
Furthermore, the examination identified lymph vascular invasion, indicating the infiltration of neoplastic cells into the lymphatic and vascular systems. This observation underscores the aggressive nature of the pathological process, with potential implications for disease progression and spread to other regions of the body.
Upon closer inspection of sections obtained from a specific nodule, additional noteworthy findings emerged. A cyst was identified, its lining composed of neoplastic cells organized into micropapillae. This distinctive architectural pattern within the cyst suggests a characteristic manifestation of the underlying pathology. Moreover, the presence of necrosis in the center of the cyst adds another layer of complexity, indicating areas of tissue death and potential implications for the overall prognosis.
In light of these comprehensive findings, a conclusive diagnosis of peritoneal mesothelioma was established. This diagnosis encapsulates the nature of the observed abnormalities and provides a critical framework for understanding the disease process. The detailed histopathological examination not only aids in confirming the presence of peritoneal mesothelioma but also provides valuable information for guiding subsequent treatment decisions and prognostic considerations.
Treatment
The patient was diagnosed with peritoneal mesothelioma and as treatment underwent an exploratory laparotomy, followed by the resection of cysts, ileoileal anastomosis, and the creation of a Paul Mikulicz ileostomy.
Discussion
A malignancy of the peritoneum, the lining that lines the abdomen, is known as peritoneal mesothelioma. Nausea, weight loss, stomach pain, and edema are typical symptoms. It is the second most common type of mesothelioma case. Over 80% of instances of mesothelioma are pleural, making it the most prevalent type. [
6].
Research has linked peritoneal mesothelioma to asbestos exposure[
7]. A person may breathe in or consume asbestos fibers as a result of exposure. It is possible for these fibers to enter the abdomen and get stuck in the peritoneum. Some also believe that the fibers may reach the abdomen via blood vessels. Over time, asbestos fibers may lead to mesothelioma tumors developing in the peritoneum. There's a possibility that the cancer could spread to other abdominal organs[
8].
Men have a slightly higher risk of developing peritoneal mesothelioma than women [
9] This may be because of the types of employment that men and women historically had. Jobs like construction and vehicle repair, where there was a greater risk of asbestos exposure, were more likely to be occupied by men. These jobs frequently exposed workers to high amounts of hazardous asbestos contact. Excessive levels of sustained exposure to tiny fibers are the most harmful kind of asbestos exposure.
SYMPTOMS -
Peritoneal mesothelioma symptoms can include:
Abdominal pain: Persistent or localized pain in the abdomen.
Abdominal swelling: Swelling or fluid buildup in the abdomen.
Inflammatory lesions: Abnormal tissue growth or lesions in the peritoneum.
Intestinal obstruction: Blockage of the intestines, leading to digestive issues.
Nausea and Vomiting
Night sweats: Excessive sweating during the night, unrelated to environmental factors.
Peritoneal fluid buildup: Accumulation of fluid in the peritoneal cavity, causing swelling.
Fever: Increased temperature as a sign of infection most probably of the peritoneum
Weight loss: Unintentional and significant loss of body weight.
It's important to note that these symptoms can be non-specific and may resemble those of other abdominal conditions[
10]. If someone experiences persistent or worsening symptoms, especially if they have a history of asbestos exposure, it's crucial to seek medical attention for a proper diagnosis and timely treatment.
The initial diagnostic process for mesothelioma typically involves a comprehensive approach[
11], encompassing various examinations and assessments. Basic imaging tests, such as X-rays and CT scans, are commonly employed to visualize potential abnormalities in the affected areas. Blood tests may also be conducted to detect specific biomarkers associated with mesothelioma. A thorough review of the patient's medical history is essential, focusing on potential asbestos exposure and other relevant factors. Additionally, a physical examination by a healthcare professional is conducted to identify any noticeable symptoms or signs of the disease. Together, these diagnostic methods contribute to a holistic evaluation, aiding in the accurate identification and assessment of mesothelioma.
Treatment Options
In order to treat peritoneal mesothelioma, doctors often combine various therapies. Multimodal therapy may be the most effective way of improving mesothelioma survival [
12]. Immunotherapy, surgery, and chemotherapy are possible components of this multimodal strategy. While determining a course of treatment, doctors frequently take various aspects into account like as the type of tumor cell type, disease stage, and health of the patient. Less aggressive therapies like peritonectomy may be beneficial for patients who possess specific characteristics or are immunocompromised to tolerate chemotherapy.
Chemotherapy may be a part of a patient's cancer treatment plan for peritoneal mesothelioma [
13]. Chemotherapy can be systemic, which means that it travels through the bloodstream of the entire body. It could also be regional, which would limit its application to the abdomen. Systemic chemotherapy may be used for patients whose peritoneal mesothelioma is inoperable. Cisplatin and pemetrexed together are the most often used chemotherapeutic medicines for peritoneal mesothelioma. Patients who got pemetrexed with or without cisplatin have reported survival times ranging from 12 to 23 months.
Also, eligible peritoneal mesothelioma cases may receive cytoreductive surgery (CRS) and Hyperthermic intraperitoneal chemotherapy (HIPEC)[
14]. CRS and HIPEC are associated with long-term peritoneal mesothelioma survival.
Conclusion
Radiological findings are of paramount importance in cases of peritoneal malignancy - mesothelioma. which enables accurate diagnosis in appropriate settings. Early diagnosis and timely operative intervention must occur to provide the best outcome for the patient.
Ethical Statement
Being a case report study, there were no ethical issues and the IRB was notified about the topic and the case. However, as this was a record-based case report, formal approval was not needed. Permission from the patient for the article has been acquired and ensured that their information or identity is not disclosed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- J. Kim, S. Bhagwandin, and D. M. Labow, “Malignant peritoneal mesothelioma: a review,” Ann Transl Med, vol. 5, no. 11, pp. 236–236, Jun. 2017. [CrossRef]
- P. Mirarabshahii, K. Pillai, T. C. Chua, M. H. Pourgholami, and D. L. Morris, “Diffuse malignant peritoneal mesothelioma – An update on treatment,” Cancer Treat Rev, vol. 38, no. 6, pp. 605–612, Oct. 2012. [CrossRef]
- K. Washimi et al., “Well-differentiated papillary mesothelioma, possibly giving rise to diffuse malignant mesothelioma: A case report,” Pathol Int, vol. 63, no. 4, pp. 220–225, Apr. 2013. [CrossRef]
- H. D. Shin and S. B. Kim, “Benign Cystic Mesothelioma Misdiagnosed as Peritoneal Carcinomatosis,” Case Rep Gastroenterol, vol. 10, no. 1, pp. 120–125, Apr. 2016. [CrossRef]
- A. Greenbaum and H. R. Alexander, “Peritoneal mesothelioma,” Transl Lung Cancer Res, vol. 9, no. S1, pp. S120–S132, Feb. 2020. [CrossRef]
- J. Tedesco, M. Jaradeh, and W. T. Vigneswaran, “Malignant Pleural Mesothelioma: Current Understanding of the Immune Microenvironment and Treatments of a Rare Disease,” Cancers (Basel), vol. 14, no. 18, p. 4415, Sep. 2022. [CrossRef]
- A. Burdorf, B. Jarvholm, and S. Siesling, “Asbestos exposure and differences in occurrence of peritoneal mesothelioma between men and women across countries,” Occup Environ Med, vol. 64, no. 12, pp. 839–842, Dec. 2007. [CrossRef]
- C. I. Wade and M. J. Streitz, Anatomy, Abdomen and Pelvis: Abdomen. 2023.
- A. Thomas, Y. Chen, T. Yu, A. Gill, and V. Prasad, “Distinctive clinical characteristics of malignant mesothelioma in young patients,” Oncotarget, vol. 6, no. 18, pp. 16766–16773, Jun. 2015. [CrossRef]
- P. H. Sugarbaker et al., “Diagnosis and treatment of peritoneal mesothelioma: The Washington Cancer Institute experience,” Semin Oncol, vol. 29, no. 1, pp. 51–61, Feb. 2002. [CrossRef]
- S. V. Jain and J. M. Wallen, Malignant Mesothelioma. 2023.
- L. Berzenji and P. Van Schil, “Multimodality treatment of malignant pleural mesothelioma,” F1000Res, vol. 7, p. 1681, Oct. 2018. [CrossRef]
- P. Mirarabshahii, K. Pillai, T. C. Chua, M. H. Pourgholami, and D. L. Morris, “Diffuse malignant peritoneal mesothelioma – An update on treatment,” Cancer Treat Rev, vol. 38, no. 6, pp. 605–612, Oct. 2012. [CrossRef]
- G. H. C. Tan, M. Cheung, J. Chanyaputhipong, K. C. Soo, and M. C. C. Teo, “Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma.,” Ann Acad Med Singap, vol. 42, no. 6, pp. 291–6, Jun. 2013.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).