Submitted:
27 December 2023
Posted:
29 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
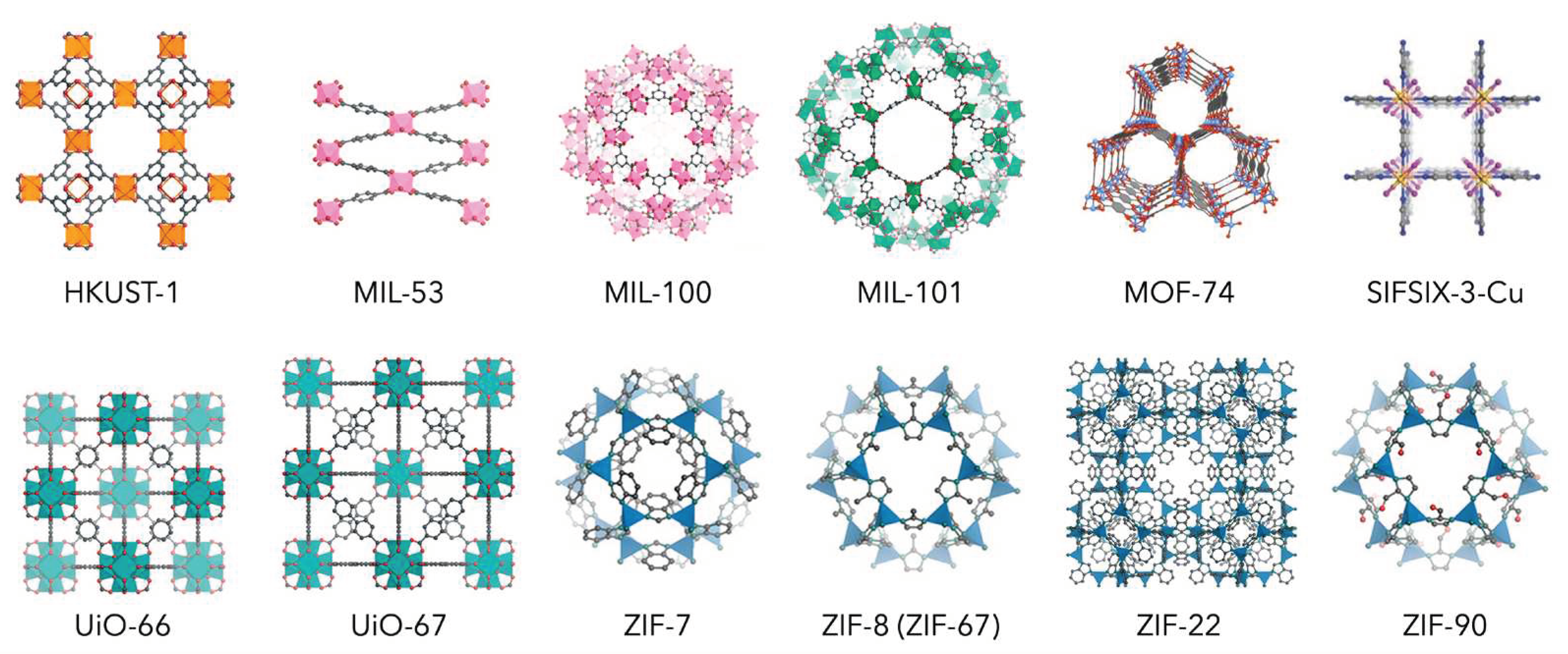
2. Characteristics of MOFs
2.1. Structural Flexibility
2.2. Structural Stability
3. Hydrocarbon Adsorption on MOFs
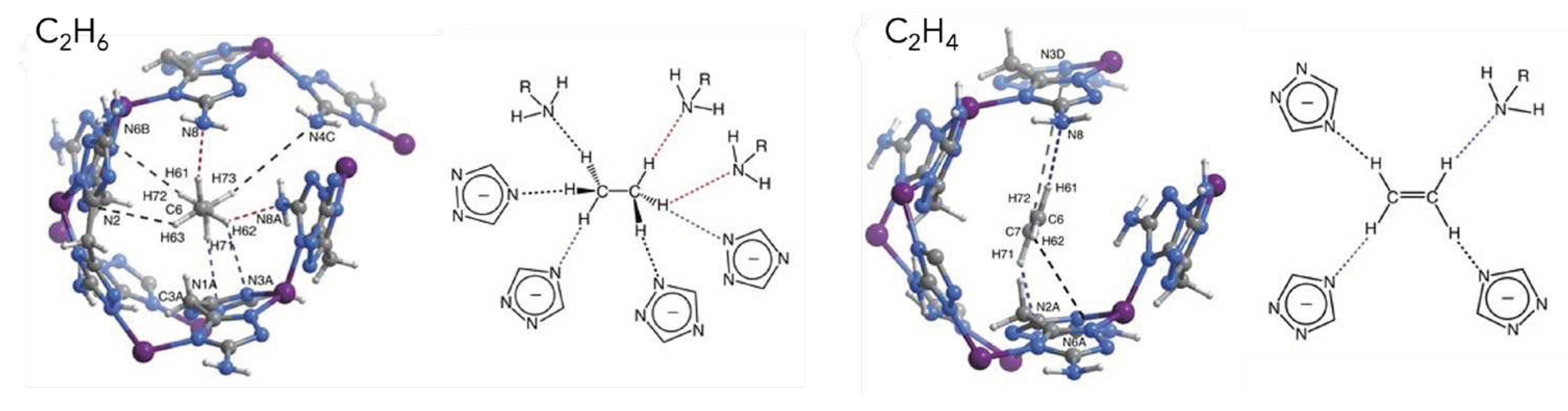
3.1. Olefins and Paraffins
3.2. Other Hydrocarbons
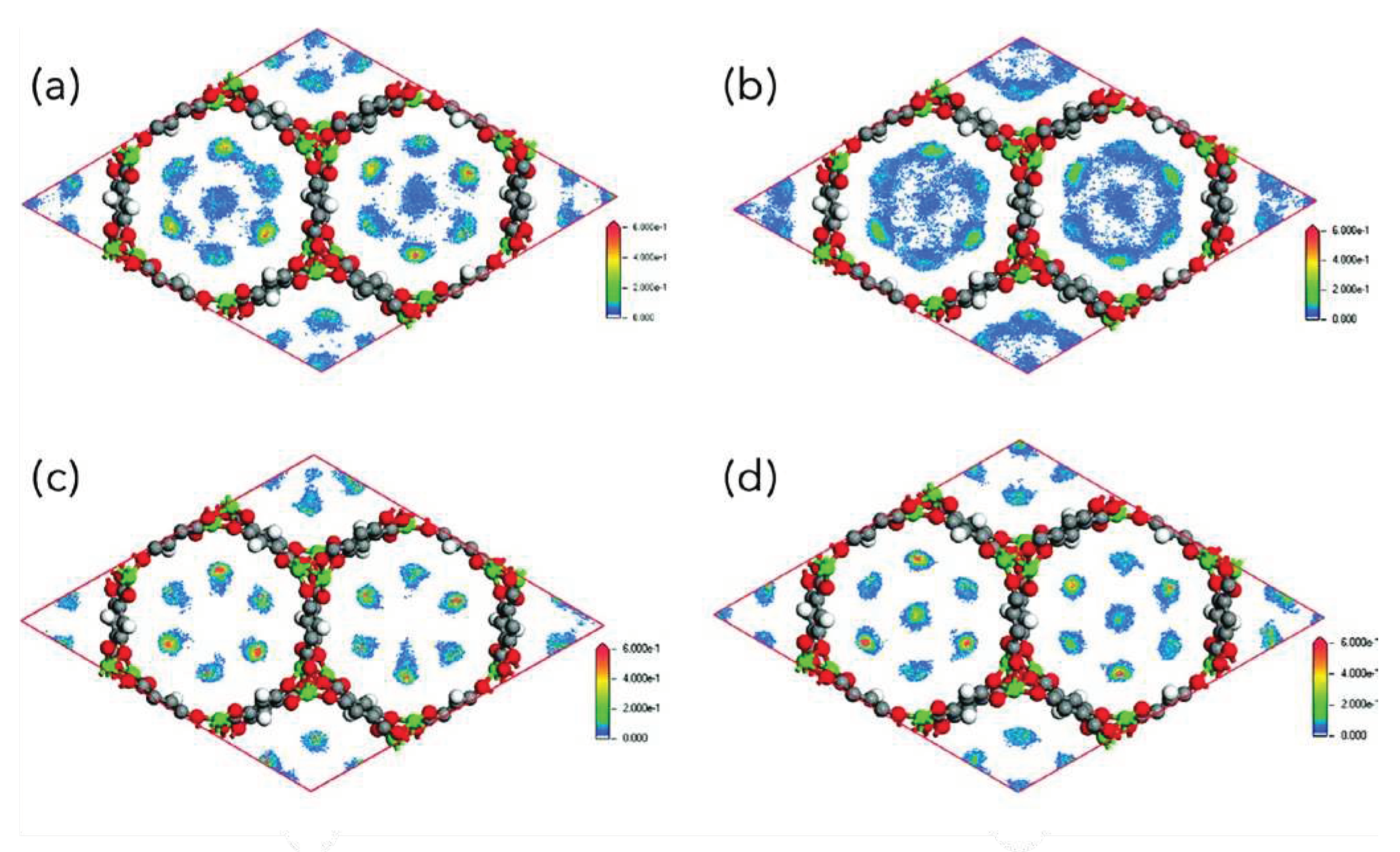
4. CO2 Separation and Capture
5. MOF-Based Membranes
5.1. Types of Membranes
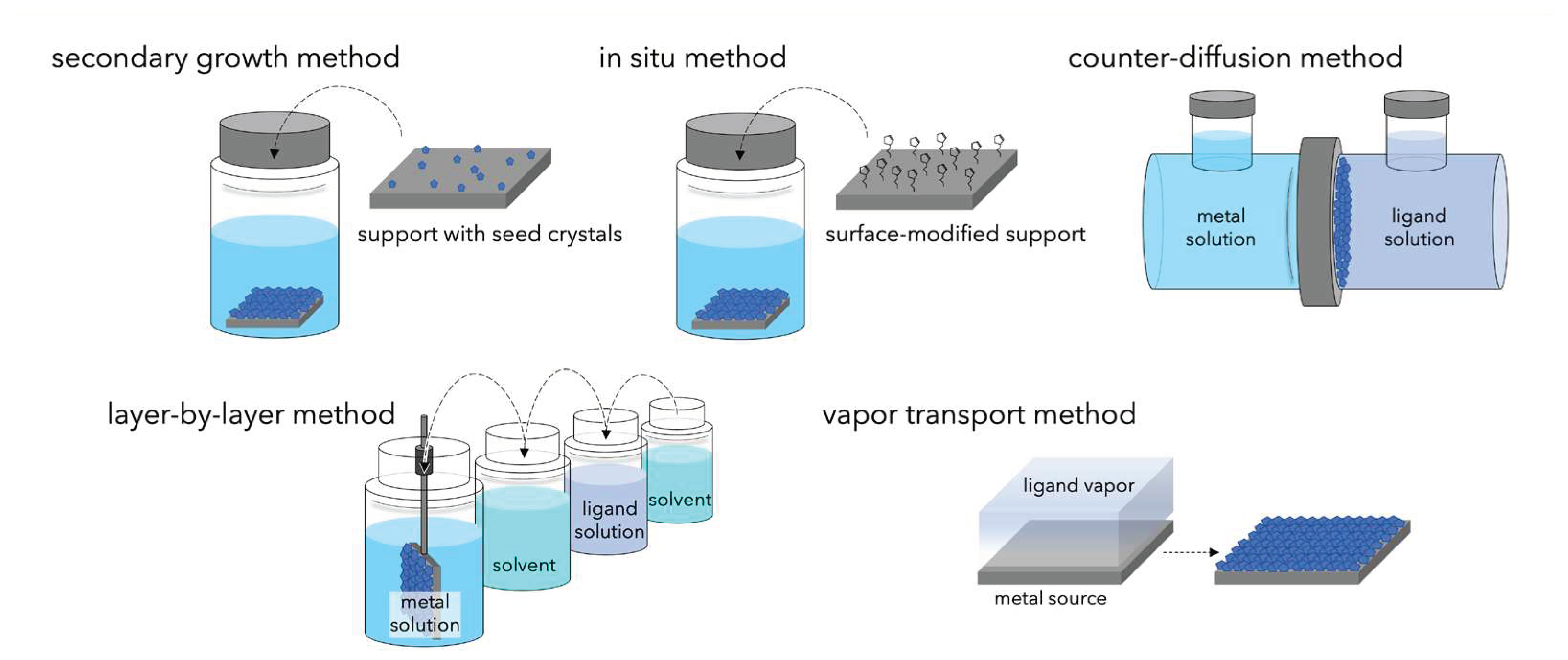
5.2. MOF Membrane Preparation Method and Points to Consider
5.3. Olefin/Paraffin Separation
5.4. Other Hydrocarbons Separation
5.5. CO2 Separation
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Tissot, A.; Serre, C. Metal-organic frameworks: From ambient green synthesis to applications. Bull. Chem. Soc. Jpn. 2021, 94, 2623–2636. [Google Scholar] [CrossRef]
- Zulkifli, Z.I.; Lim, K.L.; Teh, L.P. Metal-organic frameworks (MOFs) and their applications in CO2 adsorption and conversion. ChemistrySelect 2022, 7, e202200572. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Frameworks for commercial success. Nat. Chem. 2016, 8, 987. [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef]
- Sakata, Y.; Furukawa, S.; Kondo, M.; Hirai, K.; Horike, N.; Takashima, Y.; Uehara, H.; Louvain, N.; Meilikhov, M.; Tsuruoka, T.; Isoda, S.; Kosaka, W.; Sakata, O.; Kitagawa, S. Shape-memory nanopores induced in coordination frameworks by crystal downsizing. Science 2013, 339, 193–196. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; Van der Perre, S.; Remi, J.C.S.; Van Assche, T.; Baron, G.V.; Denayer, J.F.M. Adsorption and diffusion phenomena in crystal size engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Shen, D.M.; Bulow, M.; Lau, M.L.; Deng, S.G.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Lin, K.S.; Adhikari, A.K.; Ku, C.N.; Chiang, C.L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Lamia, N.; Jorge, M.; Granato, M.A.; Almeida, P.F.A.; Chevreau, H.; Rodrigues, A.E. Adsorption of propane, propylene and isobutane on a metal–organic framework: Molecular simulation and experiment. Chem. Eng. Sci. 2009, 64, 3246–3259. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Gohari, D.P.; Depauw, H.; Coudert, F.X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Jiang, D.; Keenan, L.L.; Burrows, A.D.; Edler, K.J. Synthesis and post-synthetic modification of MIL-101(Cr)-NH2 via a tandem diazotisation process. Chem. Commun. 2012, 48, 12053–12055. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Segakweng, T.; North, B.C.; Mathe, M.; Kang, X. Modulated synthesis of chromium-based metal-organic framework (MIL-101) with enhanced hydrogen uptake. Int. J. Hydrogen Energy 2014, 39, 12018–12023. [Google Scholar]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Latroche, M.; Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Llewellyn, P.L.; Lee, J.H.; Chang, J.S.; Jhung, S.H.; Férey, G. Hydrogen storage in the giant-pore metal–organic frameworks MIL-100 and MIL-101. Angew. Chem., Int. Ed. 2006, 45, 8227–8231. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Grenèche, J.M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 2820–2822. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K. High uptakes of CO2 and CH4 in mesoporous metal—Organic frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E. Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem., Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoon, J.W.; Seo, Y.K.; Kim, M.B.; Lee, S.K.; Lee, U.H.; Hwang, Y.K.; Bae, Y.S.; Chang, J.S. Effect of purification conditions on gas storage and separations in a chromium-based metal-organic framework MIL-101. Microporous Mesoporous Mater. 2014, 193, 160–165. [Google Scholar] [CrossRef]
- Chang, G.; Bao, Z.; Ren, Q.; Deng, S.; Zhang, Z.; Su, B.; Xing, H.; Yang, Y. Fabrication of cuprous nanoparticles in MIL-101: An efficient adsorbent for the separation of olefin–paraffin mixtures. RSC Adv. 2014, 4, 20230–20233. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Krishna, R.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Forrest, K.; Space, B.; Ma, S. Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 2015, 51, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Yoon, T.U.; Kim, E.J.; Yoon, J.W.; Kim, S.Y.; Yoon, J.W.; Hwang, Y.K.; Chang, J.S.; Bae, Y.S. Facile loading of Cu (I) in MIL-100 (Fe) through redox-active Fe (II) sites and remarkable propylene/propane separation performance. Chem. Eng. J. 2018, 331, 777–784. [Google Scholar] [CrossRef]
- Bao, Z.; Alnemrat, S.; Yu, L.; Vasiliev, I.; Ren, Q.; Lu, X.; Deng, S. Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework. Langmuir 2011, 27, 13554–13562. [Google Scholar] [CrossRef]
- Bae, Y.S.; Lee, C.Y.; Kim, K.C.; Farha, O.K.; Nickias, P.; Hupp, J.T.; Nguyen, S.T.; Snurr, R.Q. High propene/propane selectivity in isostructural metal–organic frameworks with high densities of open metal sites. Angew. Chem., Int. Ed. 2012, 51, 1857–1860. [Google Scholar] [CrossRef]
- Bachman, J.E.; Kapelewski, M.T.; Reed, D.A.; Gonzalez, M.I.; Long, J.R. M2(m-dobdc) (M = Mn, Fe, Co, Ni) metal–organic frameworks as highly selective, high-capacity adsorbents for olefin/paraffin separations. J. Am. Chem. Soc. 2017, 139, 15363–15370. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ramirez-Cuesta, A.J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S.K.; Campbell, S.I.; Tang, C.C.; Schroder, M. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 2014, 7, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/ethene separation turned on its head: Selective ethane adsorption on the metal−organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef]
- van den Bergh, J.; Gucuyener, C.; Pidko, E.A.; Hensen, E.J.M.; Gascon, J.; Kapteijn, F. Chem.-Eur. J. 2011, 17, 8832–8840. [CrossRef] [PubMed]
- Liao, P.Q.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, 8697. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Okumura, Y.; Watanabe, Y.; Mukoyoshi, M.; Bracco, S.; Comotti, A.; Sozzani, P.; Horike, S.; Kitagawa, S. Recognition of 1,3-butadiene by a porous coordination polymer. Angew. Chem., Int. Ed. 2016, 55, 13784–13788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Kapteijn, F.; Moulijn, J.A.; Jansen, J.C. Selective adsorption of unsaturated linear C4 molecules on the all-silica DD3R. Phys. Chem. Chem. Phys. 2000, 2, 1773–1779. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef]
- Lange, M.; Kobalz, M.; Bergmann, J.; Lässig, D.; Lincke, J.; Möllmer, J.; Möller, A.; Hofmann, J.; Krautscheid, H.; Staudt, R.; Gläser, R. Structural flexibility of a copper-based metal–organic framework: Sorption of C4-hydrocarbons and in situ XRD. J. Mater. Chem. A 2014, 2, 8075–8085. [Google Scholar] [CrossRef]
- Ye, Z.M.; He, C.T.; Xu, Y.T.; Krishna, R.; Xie, Y.; Zhou, D.D.; Zhou, H.L.; Zhang, J.P.; Chen, X.M. A new isomeric porous coordination framework showing single-crystal to single-crystal structural transformation and preferential adsorption of 1,3-butadiene from C4 hydrocarbons. Cryst. Growth Des. 2017, 17, 2166–2171. [Google Scholar] [CrossRef]
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of hydrocarbons with a microporous metal–organic framework. Angew. Chem., Int. Ed. 2006, 45, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Separation of C6 paraffins using zeolitic imidazolate frameworks: Comparison with zeolite 5A. Ind. Eng. Chem. Res. 2012, 51, 4692–4702. [Google Scholar] [CrossRef]
- Alaerts, L.; Kirschhock, C.E.; Maes, M.; van der Veen, M.A.; Finsy, V.; Depla, A.; Martens, J.A.; Baron, G.V.; Jacobs, P.A.; Denayer, J.F.M.; De Vos, D.E. Selective Adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem., Int. Ed. 2007, 46, 4293–4297. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Maes, M.; Jacobs, P.A.; Denayer, J.F.M.; De Vos, D.E. Activation of the metal-organic framework MIL-47 for selective adsorption of xylenes and other difunctionalized aromatics. Phys. Chem. Chem. Phys. 2008, 10, 2979–2985. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P.A.; Martens, J.A.; Denayer, J.F.M.; Kirschhock, C.E.A.; De Vos, D.E. Selective adsorption and separation of ortho-substituted alkylaromatics with the microporous aluminum terephthalate MIL-53. J. Am. Chem. Soc. 2008, 130, 14170–14178. [Google Scholar] [CrossRef]
- Finsy, V.; Verelst, H.; Alaerts, L.; De Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Pore-filling-dependent selectivity effects in the vapor-phase separation of xylene isomers on the metal−organic framework MIL-47. J. Am. Chem. Soc. 2008, 130, 7110–7118. [Google Scholar] [CrossRef] [PubMed]
- Finsy, V.; Christine, E.A.; Kirschhock, C.E.; Vedts, G.; Maes, M.; Alaerts, L.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Framework breathing in the vapour-phase adsorption and separation of xylene isomers with the metal–organic framework MIL-53. Chem. Eur. J. 2009, 15, 7724–7731. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Schlissel, D.W.D.; Wamsted, D. Holy Grail of Carbon Capture Continues to Elude Coal Industry. Institute for Energy Economics and Financial Analysis 2018. [Google Scholar]
- Refinitiv carbon market survey 2021. Available online: https://www.refinitiv.com/ (accessed on 24 June 2021).
- Kamio, E.; Yoshioka, T. Membrane separation technology for CO2 separation and recovery in Japan. Membrane 2017, 42, 2–10. [Google Scholar] [CrossRef]
- Hermes, S.; Schroder, F.; Chelmowski, R.; Wöll, C.; Fischer, R.A. Selective nucleation and growth of metal−organic open framework thin films on patterned COOH/CF3-terminated self-assembled monolayers on Au(111). J. Am. Chem. Soc. 2005, 127, 13744–13745. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Kortunov, P.; Jones, D.J.; Nedellec, Y.; Kärger, J.; Caro, J. Oriented crystallisation on supports and anisotropic mass transport of the metal-organic framework manganese formate. Eur. J. Inorg. Chem. 2007, 60–64. [Google Scholar] [CrossRef]
- Scherb, C.; Schodel, A.; Bein, T. Directing the structure of metal–organic frameworks by oriented surface growth on an organic monolayer. Angew. Chem. Int. Ed. 2008, 47, 5777–5779. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on α-alumina. Micropor. Mesopor. Mater. 2008, 113, 132–138. [Google Scholar] [CrossRef]
- Lin, R.J.; Hernandez, B.V.; Ge, L.; Zhu, Z.H. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Pan, Y.; Lai, Z. Synthesis of highly c-oriented ZIF-69 membranes by secondary growth and their gas permeation properties. J. Membr. Sci. 2011, 379, 46–51. [Google Scholar] [CrossRef]
- Zheng, B.; Pan, Y.; Lai, Z.; Huang, K.W. Molecular dynamics simulations on gate opening in ZIF-8: Identification of factors for ethane and propane separation. Langmuir 2013, 29, 8865–8872. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Carreon, M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Shin, H.; Yoo, S.J.; Kwon, H.T.; Kim, J. Zeolitic imidazolate framework ZIF-8 films by ZnO to ZIF-8 conversion and their usage as seed layers for propylene-selective ZIF-8 membranes. J. Ind. Eng. Chem. 2019, 72, 374–379. [Google Scholar] [CrossRef]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-aminopropyltriethoxysilane as covalent linker. Angew. Chem., Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Guerrero, V.V.; Barnett, G.V.; Jeong, H.K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shimada, T.; Fujita, K.; Miyake, Y.; Kida, K.; Yogo, K.; Denayer, J.F.M.; Sugita, M.; Takewaki, T. Seeding-free aqueous synthesis of zeolitic imidazolate framework-8 membranes: How to trigger preferential heterogeneous nucleation and membrane growth in aqueous rapid reaction solution. J. Membr. Sci. 2014, 472, 29–38. [Google Scholar] [CrossRef]
- Tanaka, S.; Okubo, K.; Kida, K.; Sugita, M.; Takewaki, T. Grain size control of ZIF-8 membranes by seeding-free aqueous synthesis and their performances in propylene/propane separation. J. Membr. Sci. 2017, 544, 306–311. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Zhang, G.; Fan, Z.; Meng, Q.; Shen, C.; Gao, C. Stiff metal–organic framework–polyacrylonitrile hollow fiber composite membranes with high gas permeability. J. Mater. Chem. A 2014, 2, 2110–2118. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Zhang, C.; Zhang, G. Metal–organic framework/PVDF composite membranes with high H2 permselectivity synthesized by ammoniation. Chem. Eur. J. 2015, 21, 7224–7230. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dong, D.; Li, D.; He, L.; Xu, G.; Wang, H. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Know, H.T.; Jeong, H.K. In situ synthesis of thin zeolitic–imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Guerrero, V.V.; Yoo, Y.; McCarthy, M.C.; Jeong, H.K. HKUST-1membranes on porous supports using secondary growth. J. Mater. Chem. 2010, 20, 3938–3943. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Chance, R.R.; Koros, W.J.; Sholl, D.S.; Nair, S. Exploring the framework hydrophobicity and flexibility of ZIF-8: From biofuel recovery to hydrocarbon separations. J. Phys. Chem. Lett. 2013, 4, 3618–3622. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; van Baten, J.M.; Krishna, R.; Caro, J. Novel MOF-membrane for molecular sieving predicted by IR-diffusion studies and molecular modeling. Adv. Mater. 2010, 22, 4741–4743. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lai, Z. Sharp separation of C2/C3 hydrocarbon mixtures by zeolitic imidazolate framework-8 (ZIF-8) membranes synthesized in aqueous solutions. Chem. Commun. 2011, 47, 10275–10277. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected molecular sieving properties of zeolitic imidazolate framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Brunelli, N.A.; Eum, K.; Rashidi, F.; Johnson, J.R.; Koros, W.J.; Jones, C.W.; Nair, S. Interfacial microfluidic processing of metal-organic framework hollow fiber membranes. Science 2014, 345, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Ma, C.; Rownaghi, A.; Jones, C.W.; Nair, S. ZIF-8 membranes via interfacial microfluidic processing in polymeric hollow fibers: Efficient propylene separation at elevated pressures. ACS Appl. Mater. Interfaces 2016, 8, 25337–25342. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, L.; Duan, Y.; Hou, Q.; Zhang, L.; Ding, L.X.; Xue, J.; Wang, H.; Caro, J. Paralyzed membrane: Current-driven synthesis of a metal-organic framework with sharpened propene/propane separation. Science Adv. 2018, 4, eaau1393. [Google Scholar] [CrossRef]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin metal–organic framework membrane production by gel–vapour deposition. Nat. Commun. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Mkhoyan, K.A.; Tsapatsis, M. Zeolitic imidazolate framework membranes made by ligand-induced permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [PubMed]
- Eum, K.; Ma, C.; Koh, D.Y.; Rashidi, F.; Li, Z.; Jones, C.W.; Lively, R.P.; Nair, S. Zeolitic imidazolate framework membranes supported on macroporous carbon hollow fibers by fluidic processing techniques. Adv. Mater. Interfaces 2017, 4, 1700080. [Google Scholar] [CrossRef]
- Wu, X.; Wei, W.; Jiang, J.; Caro, J.; Huang, A. High-flux high-selectivity metal–organic framework MIL-160 membrane for xylene isomer separation by pervaporation. Angew. Chem., Int. Ed. 2018, 57, 15354–15358. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Dunne, J.; Myers, A.L. Adsorption of gas mixtures in micropores: Effect of difference in size of adsorbate molecules. Chem. Eng. Sci. 1994, 49, 2941–2951. [Google Scholar] [CrossRef]
- Pakseresht, S.; Kazemeini, M.; Akbarnejad, M.M. Equilibrium isotherms for CO, CO2, CH4 and C2H4 on the 5A molecular sieve by a simple volumetric apparatus. Sep. Purif. Technol. 2002, 28, 53–60. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M.; Bozorgzadeh, H.; Mohamadalizadeh, A. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: Comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 2013, 19, 1045–1053. [Google Scholar] [CrossRef]
- Calleja, G.; Pau, J.; Calles, J.A. Pure and multicomponent adsorption equilibrium of carbon dioxide, ethylene, and propane on ZSM-5 zeolites with different Si/Al ratios. J. Chem. Eng. Data 1998, 43, 994–1003. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Synthesis of SAPO-34 crystals in the presence of crystal growth inhibitors. J. Phys. Chem. B 2008, 112, 16261–16265. [Google Scholar] [CrossRef]
- Lin, J.B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; Fylstra, N.; Iremonger, S.S.; Dawson, K.W.; Sarkar, P.; Hovington, P.; Rajendran, A.; Woo, T.K.; Shimizu, G.K.H. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O adsorption equilibrium and rates on metal−organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228. [Google Scholar] [CrossRef]
- Venna, S.R.; Zhu, M.Q.; Li, S.G.; Carreon, M.A. Knudsen diffusion through ZIF-8 membranes synthesized by secondary seeded growth. J. Porous Mater. 2014, 21, 235–240. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, X.; Sun, F.; Ren, H.; Liu, J.; Zhang, F.; Zhao, N.; Zhu, G. Development of hydrogen-selective CAU-1 MOF membranes for hydrogen purification by ‘dual-metal-source’ approach. Int. J. Hydrogen Energy 2013, 38, 5338–5347. [Google Scholar] [CrossRef]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up fabrication of two-dimensional Co-based zeolitic imidazolate framework tubular membranes consisting of nanosheets by vapor phase transformation of Co-based gel for H2/CO2 separation. J. Membr. Sci. 2019, 573, 200–209. [Google Scholar] [CrossRef]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-supported and free-standing metal–organic framework membrane. Chem. Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef]
- Kang, Z.; Xue, M.; Fan, L.; Huang, L.; Guo, L.; Wei, G.; Chen, B.; Qiu, S. Highly selective sieving of small gas molecules by using an ultra-microporous metal–organic framework membrane. Energy Environ. Sci. 2014, 7, 4053–4060. [Google Scholar] [CrossRef]
- Wang, X.; Chi, C.; Zhang, K.; Qian, Y.; Gupta, K.M.; Kang, Z.; Jiang, J.; Zhao, D. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 2017, 8, 14460. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, P.; Zhang, G.; Shen, C.; Meng, Q. Preparation of continuous NH2–MIL-53 membrane on ammoniated polyvinylidene fluoride hollow fiber for efficient H2 purification. J. Membr. Sci. 2015, 495, 384–391. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, X.; Gao, X.; Fan, S.; Sun, F.; Ren, H.; Zhu, G. Hydrogen selective NH2-MIL-53(Al) MOF membranes with high permeability. Adv. Funct. Mater. 2012, 22, 3583–3590. [Google Scholar] [CrossRef]
- Wang, N.; Mundstock, A.; Liu, Y.; Huang, A.; Caro, J. Amine-modified Mg-MOF-74/CPO-27-Mg membrane with enhanced H2/CO2 separation. Chem. Eng. Sci. 2015, 124, 27–36. [Google Scholar] [CrossRef]
- Fan, S.; Sun, F.; Xie, J.; Guo, J.; Zhang, L.; Wang, C.; Pan, Q.; Zhu, G. Facile synthesis of a continuous thin Cu(bipy)2(SiF6) membrane with selectivity towards hydrogen. J. Mater. Chem. A 2013, 1, 11438–11442. [Google Scholar] [CrossRef]
- Knebel, A.; Sundermann, L.; Mohmeyer, A.; Strauß, I.; Friebe, S.; Behrens, P.; Caro, J. Azobenzene guest molecules as light-switchable CO2 valves in an ultrathin UiO-67 membrane. Chem. Mater. 2017, 29, 3111–3117. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Li, X.; Zhang, C.; Fan, Z.; Zhang, G. Non-activation ZnO array as a buffering layer to fabricate strongly adhesive metal–organic framework/PVDF hollow fiber membranes. Chem. Commun. 2014, 50, 9711–9713. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Wang, J.; Bai, J.; Yin, H.; Yuan, B.; Lu, J.; Zhang, Y.; Zhou, L.; Duan, C. Deposition of chemically modified α-Al2O3 particles for high performance ZIF-8 membrane on a macroporous tube. Chem. Commun. 2012, 48, 5977–5979. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Caro, J. Highly hydrogen permselective ZIF-8 membranes supported on polydopamine functionalized macroporous stainless-steel-nets. J. Mater. Chem. A 2014, 2, 8246–8251. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, D.; Liu, Z.; Zhong, C. Synthesis of zeolitic imidazolate framework membrane using temperature-switching synthesis strategy for gas separation. Ind. Eng. Chem. Res. 2016, 55, 7164–7170. [Google Scholar] [CrossRef]
- Huang, A.; Wang, N.; Kong, C.; Caro, J. Organosilica-functionalized zeolitic imidazolate framework ZIF-90 membrane with high gas-separation performance. Angew. Chem., Int. Ed. 2012, 51, 10551–10555. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Chen, Y.; Wang, N.; Hu, Z.; Jiang, J.; Caro, J. A highly permeable and selective zeolitic imidazolate framework ZIF-95 membrane for H2/CO2 separation. Chem. Commun. 2012, 48, 10981–10983. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Yang, W. Two-dimensional metal–organic framework nanosheets for membrane-based gas separation. Angew. Chem., Int. Ed. 2017, 56, 9757–9761. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, J.; Xie, Z.; Yang, J.; Bai, J.; Lu, J.; Zhang, Y.; Yin, D.; Lin, J.Y.S. A highly permeable and selective amino-functionalized MOF CAU-1 membrane for CO2–N2 separation. Chem. Commun. 2014, 50, 3699–3701. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Metal–organic framework membranes with layered structure prepared within the porous support. RSC Adv. 2013, 3, 14233–14236. [Google Scholar] [CrossRef]
- Rui, Z.; James, J.B.; Kasik, A.; Lin, Y.S. Metal-organic framework membrane process for high purity CO2 production. AIChE J. 2016, 62, 3836–3841. [Google Scholar] [CrossRef]
- Dou, Z.; Cai, J.; Cui, Y.; Yu, J.; Xia, T.; Yang, Y.; Qian, G. Preparation and gas separation properties of metal-organic framework membranes. Z. Anorg. Allg. Chem. 2015, 641, 792–796. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Chen, Y.; Li, W. Polydopamine-modified metal–organic framework membrane with enhanced selectivity for carbon capture. Environ. Sci. Technol. 2019, 53, 3764–3772. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-embedded metal–organic framework membranes on polymeric substrates for efficient CO2 capture. J. Mater. Chem. A 2017, 5, 19954–19962. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Innovative layer by layer and continuous growth methods for synthesis of ZIF-8 membrane on porous polymeric support using poly(ether-block-amide) as structure directing agent for gas separation. Microporous Mesoporous Mater. 2016, 234, 43–54. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin Metal–Organic Framework Nanosheets as a Gutter Layer for Flexible Composite Gas Separation Membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Du, H.; Chen, L.; Chen, B. Nanoscale MOF/organosilica membranes on tubular ceramic substrates for highly selective gas separation. Energy Environ. Sci. 2017, 10, 1812–1819. [Google Scholar] [CrossRef]
- Nan, J.; Dong, X.; Wang, W.; Jin, W. Formation mechanism of metal–organic framework membranes derived from reactive seeding approach. Microporous Mesoporous Mater. 2012, 155, 90–98. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chai, S.P.; Zhu, P.W.; Mohamed, A.R. Development of a hybrid membrane through coupling of high selectivity zeolite T on ZIF-8 intermediate layer and its performance in carbon dioxide and methane gas separation. Microporous Mesoporous Mater. 2014, 196, 79–88. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Mao, Y.; Ying, Y.; Sun, L.; Peng, X. Zinc hydroxide nanostrands: Unique precursors for synthesis of ZIF-8 thin membranes exhibiting high size-sieving ability for gas separation. CrystEngComm 2014, 16, 9788–9791. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Wang, N.; Li, Y.; Pan, J.H.; Yang, W.; Caro, J. Significantly enhanced separation using ZIF-8 membranes by partial conversion of calcined layered double hydroxide precursors. ChemSusChem 2015, 8, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.; Ma, Q.; Mo, K.; Mao, H.; Feldhoff, A.; Cao, X.; Li, Y.; Pan, F.; Jiang, Z. A MOF glass membrane for gas separation. Angew. Chem. 2020, 132, 4395–4399. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Etxeberría-Benavides, M.; Karvan, O.; Téllez, C.; Coronas, J. Sequential amine functionalization inducing structural transition in an aldehyde-containing zeolitic imidazolate framework: Application to gas separation membranes. CrystEngComm 2017, 19, 1545–1554. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.L.; Orsi, A.F.; Lozinska, M.M.; Johnson, T.; Jansen, K.M.B.; Wright, P.A.; Carta, M.; McKeown, N.B.; Kapteijn, F.; Gascon, J. Towards high performance metal–organic framework–microporous polymer mixed matrix membranes: Addressing compatibility and limiting aging by polymer doping. Chem. Eur. J. 2018, 24, 12796–12800. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.F.; Yu, H.Y.; Xu, S.; Shen, Q.C.; Ye, H.M.; Li, N.W. Zn(II)-modified imidazole containing polyimide/ZIF-8 mixed matrix membranes for gas separations. J. Membr. Sci. 2020, 597, 117775. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 235, 116150. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial design of mixed matrix membranes for improved gas separation performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Nafisi, V.; Hagg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Sorribas, S.; Román-Marín, J.M.; Zornoza, B.; Téllez, C.; Coronas, J. Increased selectivity in CO2/CH4 separation with mixed-matrix membranes of polysulfone and mixed-MOFs MIL-101(Cr) and ZIF-8. Eur. J. Inorg. Chem. 2016, 4363–4367. [Google Scholar] [CrossRef]
- Xin, Q.P.; Ouyang, J.Y.; Liu, T.Y.; Li, Z.; Li, Z.; Liu, Y.C.; Wang, S.F.; Wu, H.; Jiang, Z.Y.; Gao, X.Z. Enhanced interfacial interaction and CO2 separation performance of mixed matrix membrane by incorporating polyethylenimine-decorated metal–organic frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef]
- Japip, S.; Xiao, Y.; Chung, T.S. Particle-size effects on gas transport properties of 6FDA-Durene/ZIF-71 mixed matrix membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Hao, L.; Liao, K.S.; Chung, T.S. Photo-oxidative PIM-1 based mixed matrix membranes with superior gas separation performance. J. Mater. Chem. A 2015, 3, 17273–17281. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; Kitagawa, S.; Sivaniah, E. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy. 2017, 2, 17086. [Google Scholar] [CrossRef]
- Yu, G.L.; Zou, X.Q.; Sun, L.; Liu, B.S.; Wang, Z.Y.; Zhang, P.P.; Zhu, G.S. Constructing connected paths between UiO-66 and PIM-1 to improve membrane CO2 separation with crystal-like gas selectivity. Adv. Mater. 2019, 31, 1806853. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; He, S.; Bai, Y.; Shao, L. Interface manipulation of CO2–philic composite membranes containing designed UiO-66 derivatives towards highly efficient CO2 capture. J. Mater. Chem. A 2018, 6, 15064–15073. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Crosslinked MOF-polymer to enhance gas separation of mixed matrix membranes. J. Membr. Sci. 2016, 520, 941–950. [Google Scholar] [CrossRef]
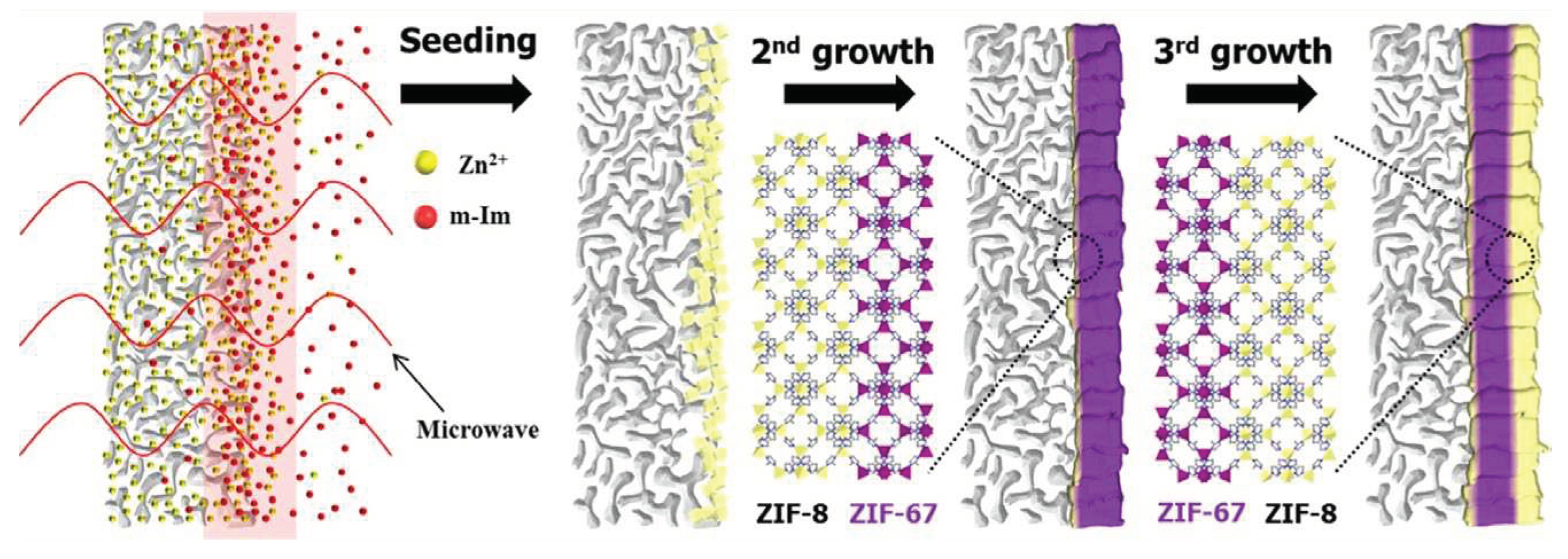
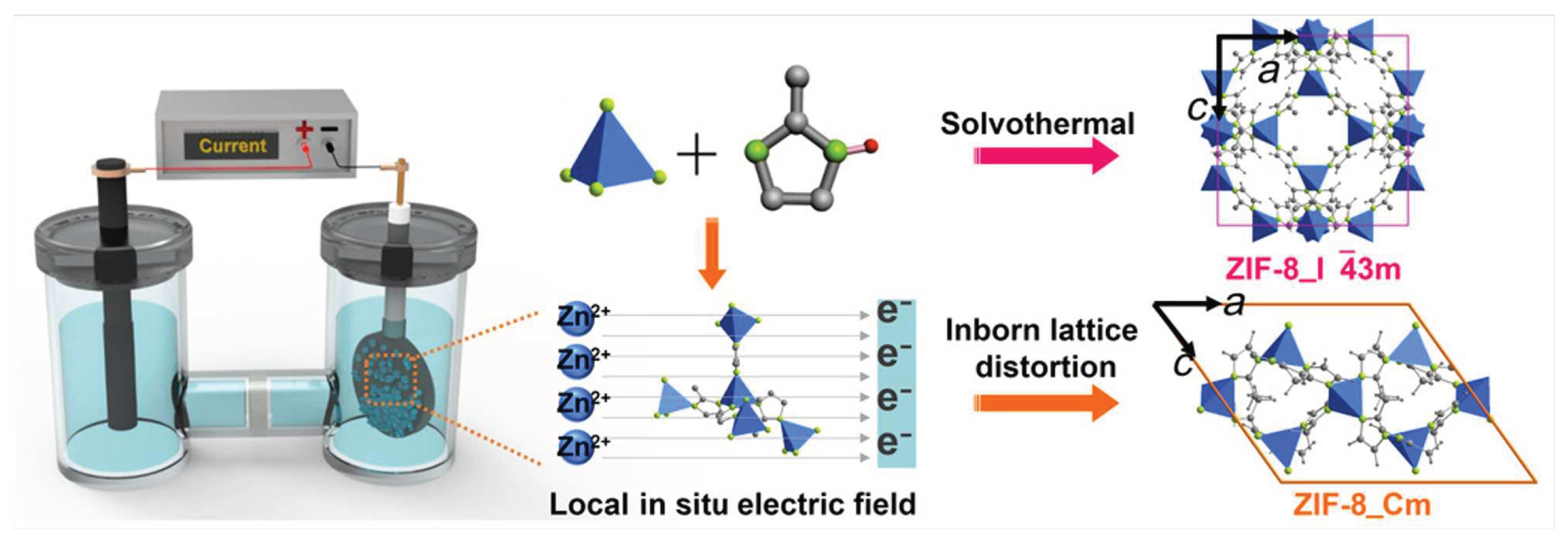
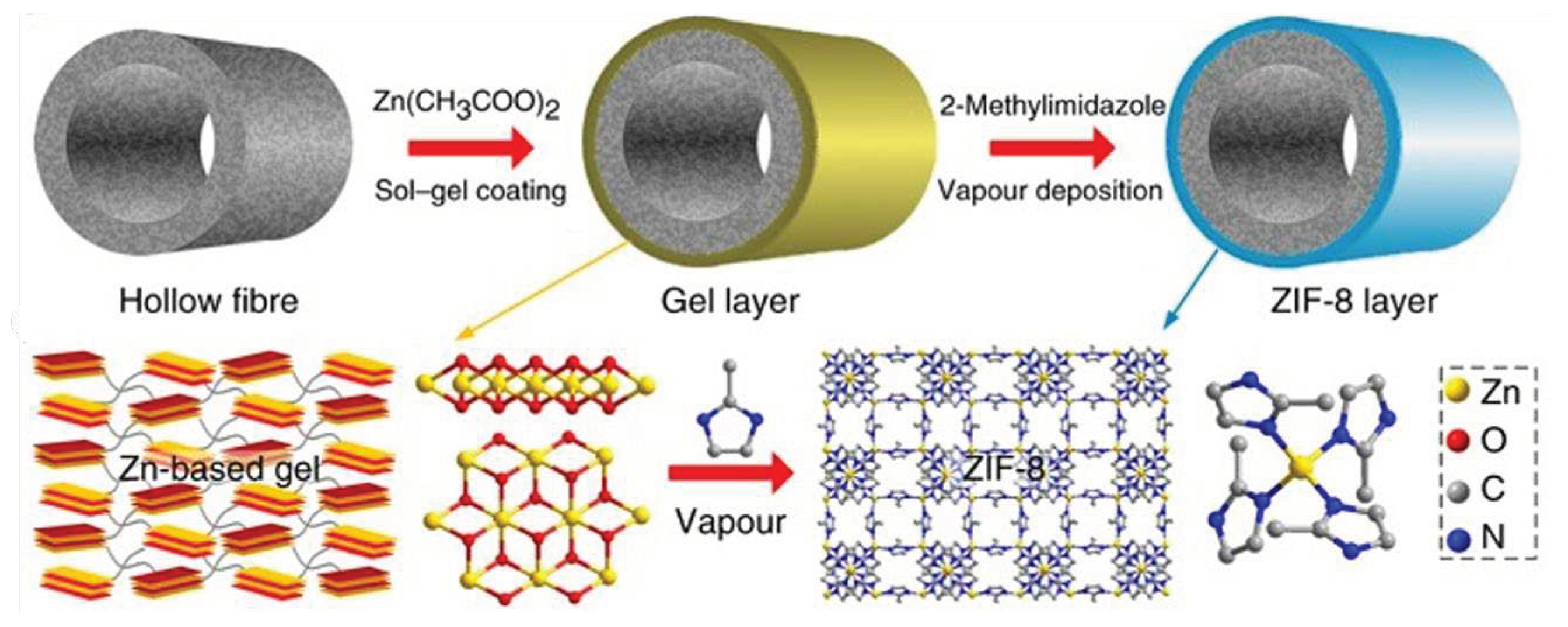
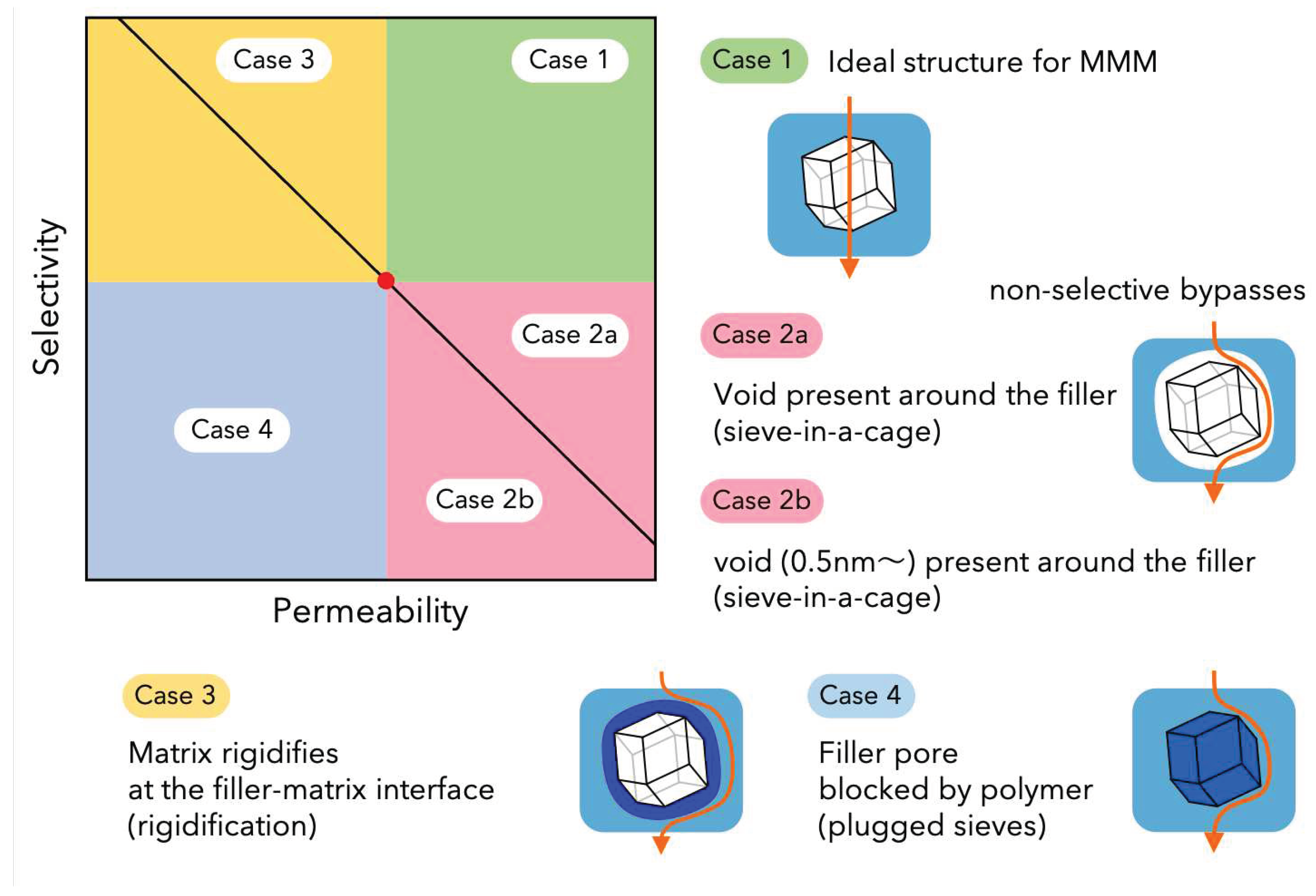
| Method | Support | Membrane Thickness |
QC3H6 (mol m−2 s−1 Pa−1) |
αC3H6/C3H8 | Ref. |
|---|---|---|---|---|---|
| secondary growth | α-Al2O3 | ~1 μm | 8.1×10−9 | 90.2 | [60] |
| in situ | α-Al2O3 | 1 μm | 8.5×10−9 | 36 | [64] |
| counter-diffusion | α-Al2O3 | ~1.5 μm | 2.1×10−8 | 50 | [68] |
| counter-diffusion | α-Al2O3 | ~80 μm | 2.3×10−8 | 57 | [69] |
| IMMP | Torlon® | 8.8 μm | 1.3×10−8 | 12 | [78] |
| IMMP | Torlon® | 8.1 μm | 1.5×10−8 | 180 | [79] |
| heteroepitaxial | α-Al2O3 | 1.0 μm | 3.7×10−8 | 209.1 | [80] |
| FCDS | Pt coated AAO | ~200 nm | 1.7×10−8 | 304.8 | [81] |
| GVD | PVDF | 114 nm | 2.1×10−7 | 67.8 | [82] |
| ALD | γ-Al2O3 | ~500 nm | 8.8×10−8 | 71 | [83] |
| Material | Conditions | CO2 Adsorption (mmol/g) | Ref. | |
|---|---|---|---|---|
| zeolite | 13X | 298 K, 21 bar | 5.2 | [87] |
| 5A | 303 K, 10 bar | 3.55 | [88] | |
| DDR | 198 K, 1.85 bar | 2.8 | [89] | |
| H-ZSM-5 | 281 K, 0.81 bar | 2.15 | [90] | |
| SAPO-34 | 293 K, 1 bar | 3 | [91] | |
| MOF | CALF-20 | 293 K, 1.2 bar | 4.07 | [92] |
| HKUST-1 | 298 K, 35 bar | 10.7 | [93] | |
| MIL-53 | 304 K, 25 bar | 10 | [94] | |
| MIL-100 | 304 K, 50 bar | 18 | [23] | |
| MIL-101 | 304 K, 50 bar | 40 | [23] | |
| MOF-5 | 298 K, 35 bar | 21.7 | [93] | |
| Ni-MOF-74 | 298 K, 1 bar | 6.68 | [95] | |
| Mg-MOF-74 | 303 K, 1 bar | 8.04 | [96] | |
| SIFSIX-3-Cu | 298 K, 0.15 bar | 2.46 | [97] | |
| ZIF-8 | 293 K, 1 bar | 2.6 | [98] | |
| MOF | Remark | Pore Size (Å) | Method | Support | QH2 (GPU) | αH2/CO2 | Ref. |
|---|---|---|---|---|---|---|---|
| CAU-1 | Al4(OH)2(OCH3)4(NH2-bdc)3 | 3.0~4.0 | secondary growth | Al2O3 | 322 | 12.34 | [99] |
| Co2(bim)4 | nanosheet | 3.4 | vapor phase | GO on Al2O3 | 564 | 42.7 | [100] |
| HKUST-1 | Cu3(btc)2 (Cu-BTC) | 9.0 | in situ | PAN | 210447 | 7.14 | [65] |
| HKUST-1 | in situ | PMMA | 3373 | 9.24 | [101] | ||
| JUC-150 | Ni2(L-asp)2(pz) | 3.8×4.7, 2.5×4.5 | secondary growth | Ni mesh | 546 | 38.7 | [102] |
| MAMS-1 | Ni8(5-bbdc)6(μ-OH)4, nanosheet | — | drop cast | AAO | 553 | 235 | [103] |
| NH2-MIL-53 | ammoniated support | 8.0 | in situ | PVDF | 12576 | 32.35 | [104] |
| NH2-MIL-53 | Al(OH)(NH2-bdc) | 8.0 | secondary growth | glass flit | 5925 | 30.9 | [105] |
| Mg-MOF-74 | amine-modified | 11 | in situ | MgO on Al2O3 | 227 | 28 | [106] |
| SIXSIX-3-Cu | Cu(bipy)2(SiF6) | 3.54 | in situ | glass flit | 806 | 8.0 | [107] |
| UiO-67 | azobenzene-loaded, light-responsive | 10 | in situ | Al2O3 | 1316 | 14.7 | [108] |
| ZIF-7 | Zn(bim)2 | 3.0 | in situ | ZnO on PVDF | 7027* | 18.43* | [109] |
| ZIF-7 | ammoniated support | in situ | Al2O3 | 3051 | 15.52 | [66] | |
| ZIF-8 | APTES-modified Al2O3 | 3.4 | in situ | Al2O3 | 171044* | 17.0* | [110] |
| ZIF-8 | PDA-modified support | in situ | Al2O3 | 71044 | 8.1 | [111] | |
| ZIF-9 | Co(bim)2 | 4.3 | in situ | Al2O3 | 22179 | 14.74* | [112] |
| ZIF-90 | APTES-modified support, post synthetic modification | 3.5 | in situ | Al2O3 | 884 | 21.6 | [113] |
| ZIF-95 | Zn(cbim)2 | 3.7 | in situ | Al2O3 | 5820 | 25.7 | [114] |
| Zn2(bim)3 | nanosheet | 2.9 | drop cast | Al2O3 | 1943 | 128.4 | [115] |
| MOF | Remark | Pore Size (Å) | Method | Support | QCO2 (GPU) | ACO2/N2 | Ref. |
|---|---|---|---|---|---|---|---|
| CAU-1 | Al4(OH)2(OCH3)4(NH2-bdc)3 | 3.0~4.0 | secondary growth | alumina | 3880 | 22.82 | [116] |
| HKUST-1 | Cu3(btc)2 (Cu-BTC) | 9.0 | counter-diffusion | alumina | 7.3* | 33.3* | [117] |
| IRMOF-1 | isoreticular MOF-1 (MOF-5) | 11.2 | secondary growth | Al2O3 | 615 | 410 | [118] |
| MIL-100(In) | In3O(H2O)2OH(btc)2 | 4.6, 8.2 | in situ | alumina | 5283 | 3.61* | [119] |
| SIFSIX-3-Cu | Cu(bipy)2(SiF6) | 3.54 | in situ | glass flit | 115 | 0.88 | [107] |
| UiO-66 | PDA-modification | 6.0 | secondary growth | AAO | 1116 | 51.6 | [120] |
| ZIF-8 | enzyme-embedded | 3.4 | in situ | PAN | 24.16* | 165.5* | [121] |
| ZIF-8 | PPSU = polyphenylsulfone, PDMS coating | LBL | PPSU | 925.4* | 15.8* | [122] | |
| ZnTCPP | nanosheet | — | filtration, spincoat | PAN | 2070* | 33* | [123] |
| MOF | Remark | Pore Size (Å) | Method | Support | QCO2 (GPU) | αCO2/CH4 | Ref. |
|---|---|---|---|---|---|---|---|
| CAU-1 | Al4(OH)2(OCH3)4(NH2-bdc)3 | 3.0~4.0 | secondary growth | alumina | 3940* | 14.8* | [116] |
| HKUST-1 | Cu3(btc)2 (Cu-BTC) | 9.0 | counter-diffusion | alumina | 7.3* | 41.5* | [117] |
| IRMOF-1 | isoreticular MOF-1 (MOF-5) | 11.2 | secondary growth | Al2O3 | 761 | 328 | [118] |
| NH2-MIL-53 | MOF/organosilica composite | 8.0 | hot-dipcoat | ceramic fiber | 430 | 18.2 | [124] |
| MIL-96 | reactive seeding | 3.6×4.5 | in situ | Al2O3 | 630* | 0.6* | [125] |
| UiO-66 | PDA-modification | 6.0 | secondary growth | AAO | 1179 | 28.9 | [120] |
| ZIF-8 | zeolite/ZIF-8 hybrid | 3.4 | secondary growth | alumina | 163 | 182 | [126] |
| ZIF-8 | PPSU = polyphenylsulfone, PDMS coating | LBL | PPSU | 925.4* | 17.3* | [122] | |
| ZIF-8 | Zn(OH)2 nanostrand precursor | crystal conversion | AAO | 3931 | 2.7 | [127] | |
| ZIF-8 | ZnAl-NO3 LDH precursor | crystal conversion | alumina | 5.7 | 16.7 | [128] | |
| ZIF-62 | Zn(Im)1.75(Bim)0.25, MOF glass membrane |
1.4 | melt-quenching | alumina | 36 | 36.6 | [129] |
| ZIF-94 | SIM-1, carboxaldehyde group |
2.6 | microfluidic | P84® | 3.5 | 37.7 | [130] |
| Polymer | MOF Filler | Loading | Pressure, Temp. | Permeability (Barrer) |
αCO2/N2 | αCO2/CH4 | ref. |
|---|---|---|---|---|---|---|---|
| CA | NH2-MIL-53(Al) | 15 wt% | 3 bar, 298 K | — | 12 | 16 | [131] |
| Pebax-1657 | NH2-MIL-53(Al) | 10 wt% | 10 bar, 308 K | 149 | 55.5 | 20.5 | [132] |
| PIM-1/Matrimid | NH2-MIL-53(Al) | 25 wt% | 2 bar, 298 K | 4390 | 25.0 | [133] | |
| 6FDA-BI | ZIF-8 | 20 wt% | 4 bar, 298 K | 20.3 | 25.9 | 57.9 | [134] |
| Pebax-1657 | ZIF-8 | 2 wt% | 11 bar, 308 K | 118 | 59 | 21.4 | [135] |
| PI | ZIF-8 | 30 wt% | 308 K | 1437 | 12 | 16 | [136] |
| Pebax-2533 | ZIF-8 | 35 wt% | 2 bar, RT | 1287 | 32.3 | 9 | [137] |
| Pebax-2533 | ZIF-8 + GO | 6 wt% | 1 bar, 298 K | 220 | 41 | — | [138] |
| Pebax-1657 | ZIF-8 + IL | 15 wt% | 1 bar, 298 K | 104.9 | 83.9 | 34.8 | [139] |
| PSF | ZIF-8 + MIL-101(Cr) | 16 wt% | 2 bar, 308 K | 14 | 40 | — | [140] |
| SPEEK | PEI + MIL-101(Cr) | 40 wt% | 1 bar, 298 K | 2490 | 80 | 71.8 | [141] |
| Pebax-1657 | ZIF-67 | 4 wt% | 11 bar, 308 K | 16 | 72.7 | 27.6 | [135] |
| 6FDA-Durene | ZIF-71 | 20 wt% | 3.5 bar, 308 K | 2560 | 13.8 | 14.2 | [142] |
| PIM-1 | ZIF-71 | 30 wt% | 3.5 bar, 308 K | 8377.1 | 18.3 | 11.2 | [143] |
| PIM-1/Matrimid | ZIF-94 | 25 wt% | 2 bar, 298 K | 3730 | 27.1 | — | [133] |
| PIM-1 | UiO-66 | 5 wt% | 4 bar, 298 K | 2952 | 26.9 | 27.3 | [144] |
| PIM-1 | UiO-66-CN | 20 wt% | 1 bar, 298 K | 12063.3 | 53.5 | — | [145] |
| Matrimid® | UiO-66-NH2 | 23 wt% | 1.4 bar, RT | 23.5 | 36.5 | — | [146] |
| PEO | UiO-66-MA | 2 wt% | 3.5 bar, 308 K | 1450 | 45.8 | — | [147] |
| PIM-1 | MOF-74 | 20 wt% | 2 bar, 298 K | 21269 | 28.7 | 19.1 | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





