I INTRODUCTION
The lymphatic vascular system (LVS) is one of the components of the lymphatic system, which includes organs such as lymph nodes, the spleen and the thymus, among others. In mammals, the LVS maintains the fluid balance between blood and interstitium, is integral to the immune defence, and plays an important role in the intake and distribution of long-chain fatty acids and fat-soluble vitamins. Besides these major functions, it fulfils several other tasks (Oliver et al., 2020; Wilting & Becker, 2022).
The LVS of different animal classes features unique adaptations resulting from their separate evolutionary trajectories (Hedrick et al., 2013). These include, for instance, the cutaneous lymph sacs and lymph hearts in amphibia (Hedrick et al., 2014). Fish display a far greater morphological heterogeneity than all other vertebrates combined (Gans & Bell, 2001). This is unsurprising, as fish are not an animal class but rather an informal grouping, comprising jawless fish, cartilaginous fish, ray-finned fish and non-tetrapod lobe-finned fish, and many of these are more closely related to tetrapods than they are to other fish groups. The placement of extant fish clades within the vertebrate phylogenetic tree is shown in Figure 3, and the term “fish” will be used to indicate this paraphyletic assemblage. Due to their morphological diversity, different descriptions of the piscine LVS as described in the past have been similarly varied, ranging from claims of complete absence (Vogel & Claviez, 1981) to implicitly assumed gross equivalence to the mammalian one (Otto Frederic Kampmeier, 1969).
The study of lymphatic vessels is more challenging than that of other vascular beds. The historical technical challenges to visualise lymphatic vessels not only led to a reduced presence in scientific research compared to the cardiovascular system, but also to several genuine scientific controversies stretching over more than a century. One of the early controversies concerned the embryonic origin of lymphatic vessels in mammals (the centrifugal versus centripetal or Sabin versus Kampmeier controversy) (Sabin, 1902; Otto Frederic Kampmeier, 1969). While the dual embryonic origin of the lymphatic vascular system is now understood (Van Der Jagd, 1932; Wilting et al., 2000; Stanczuk et al., 2015; Martinez-Corral et al., 2015; Klotz et al., 2015), reviewed by (Mattonet & Jeltsch, 2015; Ulvmar & Mäkinen, 2016), the debate is still ongoing over the relationship between the secondary vascular system (SVS, a subcompartment of the cardiovascular system exclusive to fishes) and the LVS (Vogel, 2010). Combining older observations (Jourdain, 1880; Mayer, 1919; Burne, 1929) with more recent studies (Yaniv et al., 2006; Küchler et al., 2006; Jensen et al., 2009; Rummer et al., 2014; Das et al., 2022), we aim to provide a view of the status quo and propose a co-existence and partial overlap of lymphatic vessels and SVS, which can explain why previous research results have appeared contradictory.
II THE SECONDARY VASCULAR SYSTEM OF FISH
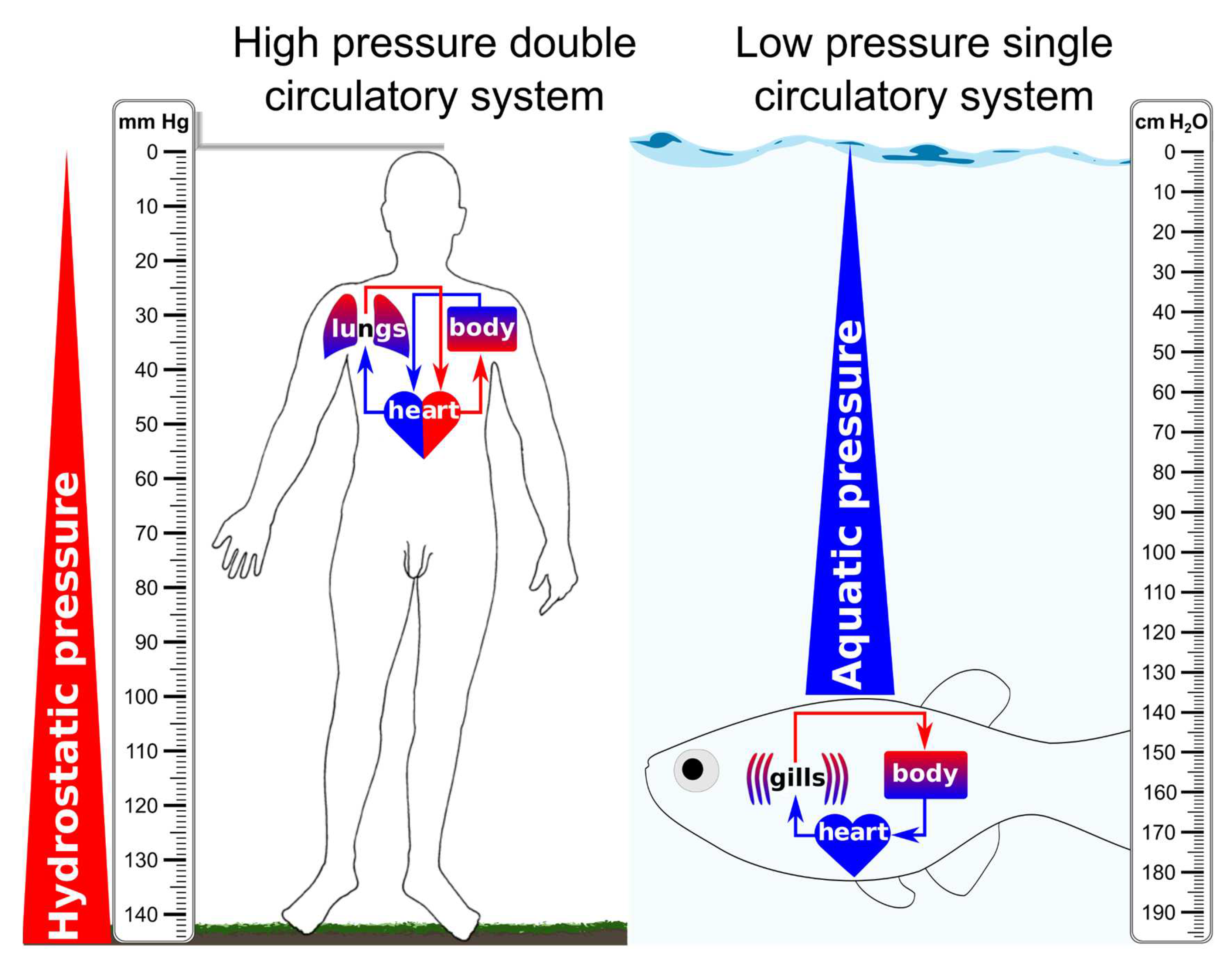
The major differences in the setup of the cardiovascular system between fishes and mammals are well described in comparative animal physiology textbooks. However, the differences do not stop at the general layout and the forces that these systems are exposed to (see
Figure 1). Many fishes feature a specialised subcompartment of the cardiovascular system that is absent in mammals: the secondary vascular system (SVS). While the larger vessels of this secondary system run in parallel with the primary circulation, the capillary networks from the primary and secondary systems usually serve different body parts, with the secondary circulation serving primarily the external body surfaces and the fins. The general setup of the SVS is described in contemporary textbooks of fish physiology (Steffensen & Lomholt, 1992; Olson & Farell, 2011; Eliason & Stecyk, 2021). The secondary vessels do not directly receive blood from the heart but via specialised connections from the primary larger arteries (
Figure 2A) (Vogel, 1981; Steffensen & Lomholt, 1992). These tortuous connections are called inter-arterial anastomoses (IAAs) in the framework of the SVS, or alternatively arterial-lymphatic conduits (ALCs). The IAAs/ALCs enable the regulated entry of red blood cells (RBCs) to the SVS (Jensen
et al., 2009; Rummer
et al., 2014). The abilities and the methods to cope with hypoxia vary between species, but the assumption that the regulated RBC entry to the SVS is related to oxygen supply and demand is a reasonable first approximation since oxygen is arguably the single most defining factor in fish evolution (Holeton, 1980). However, this hypothesis may appear counterproductive given some physiological responses, such as increasing the perfusion of superficial vascular networks with RBCs when the water becomes hypoxic (Jensen
et al., 2009). The interaction between hypoxia, exercise, and RBC perfusion of the SVS is addressed in detail in Chapter IX.
Secondary vascular networks were described in the skin, fins, gills, peritoneal lining, and oral mucosa of ray-finned fish (Actinopterygii) and some non-actinopterygian species. The SVS is by no means a small subcompartment of the cardiovascular system. Although there seems to be large inter-species heterogeneity, it can contain in some species a larger blood volume than the primary vascular system (Steffensen & Lomholt, 1992). Many scientists have noticed the morphological and functional similarities between the SVS capillaries and the mammalian lymphatic capillaries (Burne, 1929; Vogel & Claviez, 1981; Lahnsteiner, Lametschwandtner & Patzner, 1989; Steffensen & Lomholt, 1992; Olson, 1996; Lomholt & Franko-Dossar, 1998; Hedrick et al., 2013; Pavlov et al., 2017). Both are thin-walled, have overlapping junctions and a slow flow. Unsurprisingly, the notion that the SVS might be an evolutionary predecessor of the mammalian lymphatic vasculature has been put forward several times (Vogel & Claviez, 1981; Olson, 1996; Jeltsch, 2002). However, the exact nature of the relationship between the SVS and the lymphatic vasculature remains to be explained.
III THE LYMPHATIC VASCULAR SYSTEM OF FISH
The lymphatic vascular network is one of the components of the lymphatic system, together with organs such as lymph nodes, the thymus, and other lymphoid organs. These vessels are involved in a number of biological functions, including immune cell trafficking, dietary lipids absorption and regulation of fluid homeostasis.
The first descriptions of lymphatic vasculature in ray-finned fishes appear quite early (Hewson, 1769; Jourdain, 1880; Hoyer & Michalski, 1920). However, it was only with the emergence of zebrafish as the model organism of choice in vascular developmental biology (reviewed in (Lieschke & Currie, 2007; Gore et al., 2012)) - due to its embryonic transparency and ease of transgenesis - that fish lymphatics started being the subject of in-depth scientific investigation.
In 2006, Yaniv et al. (Yaniv et al., 2006) and Küchler et al. (Küchler et al., 2006) described the development of lymphatic vascular network in zebrafish embryos using time-lapse imaging of transgenic zebrafish, molecular markers, and morpholino inhibition experiments of the lymphatic-specific VEGFC/VEGFR3 signalling pathway. Since then, many follow-up publications have contributed to our understanding of the lymphatic development in zebrafish (Hogan et al., 2009a; Bussmann et al., 2010; Le Guen et al., 2014; Nicenboim et al., 2015; Koltowska et al., 2015; Jung et al., 2017; Vogrin et al., 2019; Peng et al., 2022; Hußmann et al., 2023; Grimm et al., 2023).
The lymphatic vasculature of zebrafish shares many developmental, anatomical, cellular, and molecular features with the LVS of mice or humans. Examples of the mammalian lymphatics’ close relationship to the ray-finned fishes’ lymphatics are found, e.g., in valve formation (Shin et al., 2019), growth factor activation (Hogan et al., 2009a), organotypic anatomy (Castranova et al., 2021) and development (Harrison et al., 2019). Similar to mammals (Mattonet & Jeltsch, 2015), different lymphatic beds emerge from distinct developmental origins (Eng et al., 2019). However, there are also surprising differences, e.g. the existence of perivascular cell population within the leptomeningeal layer of the zebrafish brain referred to in the literature as Fluorescent Granular Perithelial cells (FGPs) or mural LECs (muLECs) or brain LECs (BLECs). These are single endothelial cells molecularly resembling lymphatics, which arise from choroid plexus, while in mammals, the functionally corresponding cells are not considered to be of lymphatic nature (Galanternik et al., 2017; van Lessen et al., 2017; Bower et al., 2017a; Castranova et al., 2021; Huisman et al., 2022; Karam et al., 2022; Siret et al., 2022).
The intestinal lymphatics, necessary for effective lipid transport in mammals, have been reported in teleosts. Coffindaffer-Wilson (Coffindaffer-Wilson, Craig & Hove, 2011) documented the presence of a supraintestinal lymphatic vessel formed from adjacent LEC clusters in embryonic zebrafish. Later, Okuda expanded the observation by identifying lyve1b-positive supraintestinal lymphatic vessels and an extensive lymphatic network localised over the entire intestine (Okuda et al., 2012). To describe the morphological and functional characteristics of intestinal lymphatics in adult fish, Sheridan isolated and determined chylomicron particles in rainbow trout serum based on electrophoretic mobility (Sheridan, Friedlander & Allen, 1985; Sheridan, 1988). In mammals, dietary lipids are packed into chylomicron particles and delivered by intestinal lymphatics because they are too large to be taken up by adjacent blood vessels (Bernier-Latmani & Petrova, 2017). Recent studies (Ho et al., 2004; Stoletov et al., 2009; Otis et al., 2015) have extensively described lipid transport and metabolism in adult zebrafish but have not investigated the role of lymphatics in this process. Therefore, our understanding of the fundamental pathways between intestinal lymphatics and lipid transport remains incomplete. Already in 1933, Glaser regrets that the data are insufficient for conclusions, but observes that they cast doubt on the existence of fat-absorbing lacteals in cartilaginous fish, while making them a possibility in bony fish (Glaser, 1933). However, he also discusses Vialleton’s interpretation, that nutritional fat uptake by fish lymphatics never happens directly from the intestinal epithelium (Vialleton, 1902). In this scenario, the blood capillaries of the intestinal villi would perform the actual uptake but hand some of their cargo to non-villi intestinal lymphatics. Almost 100 years later, the presence and function of lacteals in fish await a thorough investigation.
While there are some striking differences in the overall lymphatic system of zebrafish and mammals, e.g. the lack of bone marrow hematopoiesis or clearly delineated lymph nodes (Menke et al., 2011), the undeniable similarities in the development of the lymphatic vasculature have made zebrafish a useful model for development, drug discovery, genetics, and associated-diseases in humans (Li et al., 2019).
IV THE LYMPHATICS/SVS CONTROVERSY
Most of the very early descriptions left no doubt about ray-finned fish having an LVS very similar to the mammalian one (Hewson, 1769; Jourdain, 1880; Hoyer & Michalski, 1920) (
Figure 2B). However, additional observations soon started casting doubts about the nature of some of the observed vessels. In 1880, Sylvian Jourdain described in young, largely still unpigmented flatfish (European plaice,
Pleuronectes platessa) the existence of both “white” and “red” blood vessels in the fins, with the white blood vessels being prominent throughout the fin, while the red blood vessels were limited to the base of the fin (Jourdain, 1880). Paul Mayer confirmed Jourdain’s findings in 1919, adding observations about the paradox proximal-to-distal direction of lymph flow (contrary to the expected distal-to-proximal flow of mammalian lymphatics) and the fin vessels' intermittent and changing RBC content. Mayer regarded his observations as incompatible with a classic lymphatic vascular system as described in mammals, and he is probably the first to propose the concept of a secondary circulation, relabelling the superficial lymphatics in ray-finned fish as “white blood vessels” due to the arterial origin of their content (Mayer, 1919). Burne also explicitly distinguished two different types of ray-finned fish lymphatic vessels that serve different body parts (Burne, 1926).
Definitive proof of the SVS in teleost fish started accumulating only in the 1980s, starting with Vogel and Claviez. They were the first to identify the inter-arterial anastomoses in vascular casts as the origin of the SVS perfusion and to show that the fluid in the vessels previously regarded as lymphatics originates from the arteries (Vogel & Claviez, 1981). They also explained the episodic absence of RBCs in these vessels by identifying specialised structures responsible for regulating RBC entry into this compartment. The debate about the nature of the described lymphatics vessels in fish seemed to be settled, and in the following years, prior reports of the piscine LVS were reinterpreted as descriptions of the SVS (Vogel & Claviez, 1981; Steffensen, Lomholt & Vogel, 1986; Lahnsteiner et al., 1989; Steffensen & Lomholt, 1992). The new paradigm also rationalised many puzzling observations, notably those by Mayer (Mayer, 1919) and Burne (Burne, 1926). Since the early 1980s, it has been implicitly acknowledged among fish physiologists that the piscine LVS is identical to the SVS. Since lymphatics were defined as vessels whose fluid originates from the interstitium, these vessels were named “secondary blood vessels”, and fish were declared devoid of a “true” lymphatic system.
However, this assumption came under scrutiny with the “rediscovery” of ray-finned fish lymphatics in the early 2000s. It must be noted that the SVS concept was established when molecular biology was still in its infancy, and lymphatic-specific immunohistochemical identification, tissue-specific reporter lines and visualisation techniques were not yet available. The seminal studies on zebrafish lymphatics (Yaniv et al., 2006; Küchler et al., 2006), and those which followed, demonstrated the presence of an embryonic lymphatic vasculature sharing developmental origin and molecular signatures with mammalian lymphatic vessels, re-opening the question of the existence of “true” lymphatics in fish.
Regrettably, the discovery of the lymphatic vasculature in zebrafish was not followed by a renewed interest in the nature of the relation between the SVS and the lymphatic vasculature. Ambiguities regarding the nature of the fish LVS remained and some scientists assumed the equivalence of the rediscovered fish LVS and the SVS implicitly. Consequently, several publications use the term lymphatics for what resembles more the vascular structures of the SVS (Jensen et al., 2009; Pavlov et al., 2017), adding to the current confusion about the relationship between these two vascular systems.
V DEFINING THE LYMPHATICS: MOLECULAR MARKERS
To start addressing the relationship between the SVS and lymphatic vessels, we must first define what makes a vessel lymphatic. There are many different ways to define lymphatics. Often, an amalgamation of a) molecular identity and b) structure/function is used:
a) Despite some heterogeneity within the lymphatic vascular network, lymphatic endothelial cells are characterised by the expression of molecular markers such as PROX1, VEGFR3, LYVE1, and Podoplanin, the pan-endothelial marker CD31 (Wilting et al., 2002) and the absence of blood vascular markers such as CD34 (Breiteneder-Geleff et al., 1999).
b) Lymphatics are functionally and anatomically defined as endothelial-cell-lined, valve-containing conduits that do not receive fluid directly from the cardiovascular system but instead by uptake of interstitial fluid.
Molecular markers such as VEGFR3 (Kaipainen et al., 1995), Podoplanin (Breiteneder-Geleff et al., 1999), LYVE1 (Banerji et al., 1999), and PROX1 (Wigle & Oliver, 1999) are routinely used in mice to identify lymphatic vessels. As the heterogeneity of endothelial cells has become increasingly evident over the last decade (Becker et al., 2023), a combination of PROX1 with one or more other markers is usually used to discriminate between blood and lymphatic endothelial cells (Wilting et al., 2002; Petrova et al., 2008; Schroedl et al., 2014). This assessment can be complemented with functional data, such as uptake of large tracer molecules or particles from the interstitium after injection (Polomska & Proulx, 2021) or the absence of erythrocytes, to definitely assert the lymphatic nature of a vessel in humans or mice (Jha, Rauniyar & Jeltsch, 2018).
Not every mammalian lymphatic marker shows the same expression pattern in zebrafish. For example, the gene for podoplanin, a widely used lymphatic marker in mice, seems to be absent in zebrafish (Mulligan & Weinstein, 2014). However, many of the factors labelling lymphatic vessels in mammals perform a similar function in zebrafish, such as Prox1a (van Impel et al., 2014; Koltowska et al., 2015), Lyve1b (Okuda et al., 2012) and Vegfr3/Flt4 (van Impel et al., 2014). Genes such as Stabilin1/stabilin and Mrc1/mrc1a are also expressed in the lymphatic endothelium of both zebrafish and mouse (Prevo et al., 2004; Salmi et al., 2004; Taylor, Gordon & Martinez-Pomares, 2005; Hogan et al., 2009a; Jung et al., 2017), although they have been used as lymphatic markers almost exclusively in the former. Very little is known about the heterogeneity of markers in other ray-finned fish species, and studies led on alternative fish models often assume the same marker distribution as in zebrafish (Jensen et al., 2009).
An important conserved pathway in lymphatic vascular development is Vascular Endothelial Growth Factor (VEGF) signalling. Two VEGF family members are crucial for establishing lymphatic vasculature: VEGFC and VEGFD. VEGFC specifically has been shown to be essential for the developing lymphatic vasculature in all vertebrates to date, including ray-finned fish (Karkkainen et al., 2004; Yaniv et al., 2006; Küchler et al., 2006). In both mouse and zebrafish, VEGFC controls the sprouting of the lymphatic progenitors from the cardinal vein (Hogan et al., 2009a; Hagerling et al., 2013) and loss of VEGFC (Karkkainen et al., 2004; Yaniv et al., 2006; Küchler et al., 2006; Hogan et al., 2009a; Hagerling et al., 2013) or its receptor VEGFR3 (Haiko et al., 2008; Hogan et al., 2009b; Hagerling et al., 2013) causes lymphatic defects in both species. As VEGFC is a secreted protein that needs to be activated by specific proteases in the extracellular environment (Künnapuu, Bokharaie & Jeltsch, 2021), loss of this molecular machinery also results in compromised lymphatics (Hogan et al., 2009a; Bos et al., 2011; Jeltsch et al., 2014; Janssen et al., 2015). The VEGFC signalling pathway is of particular interest for human studies, as several lymphatic conditions have been connected to mutations in either VEGFC (Gordon et al., 2013), VEGFR3 (Ferrell et al., 2010) or the VEGFC processing machinery (Alders et al., 2009; Jha et al., 2017; Brouillard et al., 2017). In zebrafish, loss of Vegfc can be compensated by Vegfd in specific subsets of the lymphatic vasculature (Astin et al., 2014; Vogrin et al., 2019). For an in-depth description of the interaction of VEGFC and VEGFD with their receptors VEGFR3 and VEGFR2 and the molecular machinery responsible for the processing of VEGFC, see Chapter XIII.
The degree of conservation of the factors involved in the development and maintenance of lymphatic vessels points to the common developmental origin and shared molecular programming of the mammalian and ray-finned fish lymphatics, regardless of the possible subsequent processes of transdifferentiation.
VI DEFINING THE LYMPHATICS: INTERSTITIAL FLUID UPTAKE FUNCTION
A second approach to the identification of the lymphatic vessels is by anatomy and function. Out of the many functions of the lymphatics, only interstitial drainage lends itself easily to identification purposes. High molecular weight tracers injected into the interstitium will be collected and transported by the lymphatic vessels, allowing their visualisation (Leu et al., 1994).
Both Yaniv et al. (2006) and Küchler et al. (2006) characterise the functionality of embryonic lymphatics. However, data about the uptake of interstitial fluid by adult zebrafish lymphatics are still limited. Technically, such studies are extremely difficult to perform at physiological tissue pressures. Injections will inevitably lead to pressure increases. However, Yaniv et al. show that caudal subcutaneous injection of 0.5% trypan blue into adult zebrafish specifically labels the thoracic duct, as does the subcutaneous injection of rhodamine-dextran into an 18-day-old zebrafish (Yaniv et al., 2006). Quantum dots (Q-dots) have alternatively been used for functional assays of zebrafish lymphatics (Jung et al., 2017). Q-dots are between 2 and 12 nm in size and are available with different coatings. Their large hydrodynamic radius does not prevent them per se from exiting blood vessels (Jiang et al., 2017), but PEG-coated Q-dots minimise the interaction with the vascular wall, and they seem to be retained well in the vasculature (Kamei et al., 2006), although exceptions seem to exist even in mammals (Radu et al., 2015).
Drainage from the interstitium into the lymphatic vessels has been documented in embryonic stages by multiple studies (Yaniv
et al., 2006, 2007; Küchler
et al., 2006). Under a strict SVS interpretation (
Figure 2B), one might discard such observation as pertaining to a transient embryonic structure which later completely transdifferentiates into the SVS. However, it has been shown that thoracic duct remains present after the emergence of an SVS in the fin (Yaniv
et al., 2006), and data by multiple laboratories argues for the persistence of lymphatic structures with drainage function in adult zebrafish (Isogai
et al., 2009; Venero Galanternik
et al., 2016; Castranova
et al., 2021; Elmagid
et al., 2022). Küchler et al. used an intramuscular injection of fluorescent dextran, showing accumulation in the presumed thoracic duct in 33% versus accumulation in the posterior cardinal vein in 31% of the injections (Küchler
et al., 2006). This relatively weak association might reflect that the injection method had been established and validated in mammals and needs to be further optimised for zebrafish. At the same time, it might reflect the generally high vascular permeability in fish and the fact that tissue drainage can also happen directly into the cardiovascular system. Re-absorption at the venous end of the capillary and venule network has been previously thought to be responsible for up to 90% of the extravasated fluid in mammals, but old and recent evidence does not support the notion of a general, large contribution for reabsorption at the venous end of the capillary network (Levick, 1991; Levick & Michel, 2010; Huxley & Scallan, 2011; Brenner, 2018). Notably, many of the early experiments about capillary filtration have been performed in amphibians whose transcapillary flux magnitude is closer to fish than to mammals (Hillman, Drewes & Hedrick, 2021). Capillary “filtrate” might, therefore, become re-absorbed in fish, or - more accurately - the concept of capillary filtration might vanish with increasing capillary permeability, and it has been proposed that this is the normal situation for fish and amphibians but exceptional in reptiles, birds and mammals (Hillman
et al., 2021). In mammals, capillary permeability and the resulting extravasation necessitate drainage. Despite several studies confirming the ability of fish lymphatics to drain interstitial fluid, we know surprisingly little about vascular permeability and capillary filtration in fish, which create surplus interstitial fluid in the first place.
VII VASCULAR PERMEABILITY AND INTERSTITIAL FLUID IN FISH
There is not much experimental data on the maintenance of tissue fluid balance in fish, but vascular permeability in ray-finned fish is generally greater than mammalian vascular permeability (Hargens, Millard & Johansen, 1974; Olson et al., 2003b; Olson & Farell, 2011). Mammalian blood vessels are usually relatively impermeable to large molecules since the oncotic pressure (i.e. the osmotic pressure generated by large plasma proteins) needs to counteract the high blood pressure and orthostatic pressure to prevent massive fluid extravasation across the vascular wall. On average, fish blood pressures are much below mammalian blood pressures, and orthostatic pressure is absent. Hence, fish do not need to maintain a high plasma protein concentration and low interstitial protein concentrations as mammals do (Rutili & Arfors, 1977; Kamel & Halperin, 2017). Because their plasma and interstitial fluid (ISF) protein concentrations are very similar, their baseline vascular permeabilities can be very high (Hargens et al., 1974; Olson et al., 2003b). It has been assumed that the amount of net capillary filtration is insignificant in fish, which might explain why some organs that require drainage in mammals might not require separate drainage systems in fish. However, there are no definitive studies, and the importance of the oncotic pressure for the fluid balance of fish capillaries remains controversial.
Given the similarities of the SVS with the lymphatic vasculature, such as low pressure and overlapping cell junctions (Olson, 1996), the SVS may contribute to ISF uptake in fish. Undeniably, lymphatic edema does exist in ray-finned fish, as demonstrated by embryonic edema caused by mutations in the VEGFC/VEGFR3 signalling pathway (Hogan
et al., 2009a, 2009b; Shin
et al., 2016) or by the endothelial-specific deletion of
gata2a (Shin
et al., 2023). But not all edema in fish is lymphedema; it can be cardiac (Huang
et al., 2013) or renal (Hentschel
et al., 2005) edema. Most edema in fish is likely unrelated to the inability to drain surplus tissue fluid. Fish also do not experience orthostatic pressure, which forces fluid out of the vessels. Depending on the water depth, fish experience the opposite pressure force of the water column above, essentially acting like a compression garment counteracting the accumulation of ISF (see
Figure 1). In freshwater fishes such as zebrafish, more likely causes of edema are problems in keeping the osmotic balance, which involves both gills and kidneys. After all, the gill circulation constantly straddles the balance between maximal oxygen uptake versus ion loss (freshwater fishes) or ion influx (saltwater fishes) (Evans, Piermarini & Choe, 2005). Therefore, caution must be exercised in considering all occurrences of edema as an indicator of disrupted lymphatic vasculature functionality.
VIII PHYLOGENETIC DISTRIBUTION OF THE SVS
In order to discuss the function and origin of the SVS, it is important to establish the distribution of this type of vasculature in the main groups of vertebrates, whose identity and relation to each other are shown in
Figure 3.
It is generally accepted that all ray-finned fish feature a more or less developed SVS (Skov & Bennett, 2003), although data is missing for the most distantly related group, the Cladistia. However, even within ray-finned fish, the SVS is characterised by a large number of species-specific differences (Skov & Bennett, 2003), likely contributing to the controversy about fish lymphatics. This great variability is at least partially the outcome of a different methodological approach: contemporary experimental research on mammalian lymphatics is done almost exclusively in mice, while fish physiologists deploy a wide variety of species, resulting in many reports about the large inter-species heterogeneity in the setup of the systemic (i.e. non-gill) vasculature of fish. Was the lymphatic vasculature carefully characterised in multiple mammalian species, it is certainly possible that a similar range of differences might be found, and indeed, studies in marsupials have found significant differences in the lymphatic vessels arrangement compared with placental mammals, such as a duplicated thoracic duct and the presence of lateral lymph trunks, common in vertebrates but absent in adult placentates (Bryant, 1977). The specialised gill vasculature is no less heterogeneous, and will be discussed separately in a later paragraph. In some species, such as the fast-swimming tuna, the SVS has even been co-opted for locomotion, providing fin stability and control by a hydraulic mechanism (Pavlov et al., 2017), while in other species, the system only appears to be rudimentary or vestigial (Lahnsteiner et al., 1989).
The presence of an SVS outside of ray-finned fish is more controversial. In lungfish, no signs of a systemic SVS have been found (Skov & Bennett, 2003), but instead, there is evidence of a mammalian-type LVS (Vogel & Mattheus, 1998). The presence of an SVS has also not been reported in any tetrapod clade. The only description of the presence of an SVS in lobe-finned fish (Sarcopterygii) is limited to the gills vasculature of coelacanth, addressed later in this review. Several older publications describe the “lymphatics” of sharks and rays (Robin, 1845; Jourdain, 1868; Hoyer, 1928a; Glaser, 1933; Otto Frederic Kampmeier, 1969). It is noteworthy that already Mayer and Vialleton classify the superficial vessels in the fish skin of sharks (Mayer, 1888) and Torpedo marmorata (Vialleton, 1902) as blood vessels, while at the same time reporting the existence of lymphatics in other organs of these animals. However, recent reports fail to identify any systemic SVS in cartilaginous fish (Chondrichthyes) (Skov & Bennett, 2003). This survey used the torturous-type IAA as a cardinal sign to detect the presence of an SVS (Skov & Bennett, 2003), so it might be that the anastomoses in cartilaginous fish are simpler and of a different type. In any case, the absence of chylous vessels and skin lymphatics in cartilaginous fish is explicitly discussed already by Weidenreich, Baum and Trautmann (Weidenreich, Baum & Trautmann, 1934). At the same time, these authors point out that the skin vessels in cartilaginous fish are the same vessels that are of lymphatic nature in bony fishes, basing their nomenclature - in the absence of any alternative - solely on the perfusion with RBCs. Mayer discusses the same nomenclature issue in his paper about the vascular system of sharks and rays and points out – similar to Burne (Burne, 1926) – that RBCs are frequently found in the so-called lymphatics (Mayer, 1888). Altogether, these data suggest that it might be premature to categorically deny the existence of an SVS in cartilaginous fish. In jawless fish, the existence of a mixed “venolymphatic” compartment akin to the SVS has been known since the early 20th century (Allen, 1913; Cole, 1926) and has been discussed frequently thereafter (Grodziński, 1932; Johansen, Fänge & Waage Johannessen, 1962; Casley-Smith & Casley-Smith, 1975), and after the emergence of the SVS concept, this compartment has been proposed to be part of the SVS (Lomholt & Franko-Dossar, 1998). Overall, the presence of a systemic SVS in cartilaginous and jawless fish is still controversial, and further research in these organisms would be needed to elucidate their lymphatic and SVS organisation.
IX POSSIBLE FUNCTIONS OF THE SVS
Several functions have been proposed for the SVS. Comparable to the human lymphatic vascular system, the SVS has been implicated to have a function in immune defence (Ishimatsu, Iwama & Heisler, 1995; Randolph et al., 2017; Johnson, 2021) and in pH- and osmoregulation (Ishimatsu et al., 1995; Olson, 1996; Machnik et al., 2009; Wiig et al., 2013). Many authors consider a role in aquatic respiration (Vogel & Claviez, 1981; Steffensen & Lomholt, 1992; Rummer et al., 2014). A remarkable functional difference between the SVS and the primary circulation is that the SVS can differentially regulate the blood flow-entry of RBCs and thus oxygen supply. However, RBC entry in the SVS is regulated in a complex manner depending on multiple factors. For example, exercise does stimulate RBC entry into the SVS in glass catfish (Rummer et al., 2014), but the oxygen content of the water and whether fish are allowed to exercise aquatic surface respiration (ASR) are crucial additional parameters. Hypoxic water reduces RBC entry to the SVS only if fish have no opportunity for ASR (Rummer et al., 2014). However, in zebrafish, hypoxia does trigger RBC entry into the SVS (Jensen et al., 2009). ASR opportunities during this experiment were not reported, although they were later shown to be significant (Mandic et al., 2022).
To understand these complex interactions, the oxygen exchanges between the external surfaces of the fish and the surrounding water must be analysed. Whether RBCs lose or take up oxygen in superficial capillary networks depends on the relative oxygen pressure difference between the superficial vessels and the surrounding water. Under low metabolic activity in normoxic water, the oxygen demand of the body surfaces and the fins are thought to be met solely by direct diffusion from the water, and the SVS contains only a few RBCs. During tissue hypoxia, oxygen-depleted RBCs might be able to O2-recharge in superficial capillary networks and thus supplement gill respiration. Therefore, during exercise, the specialised connections between the primary arteries and the SVS elongate and allow RBC entry to allow additional oxygen uptake. When the surrounding water is hypoxic, and haemoglobin becomes oxygen-depleted before the blood has completed the cardiovascular circuit, the RBCs might make a detour through the SVS connections to recharge in the body surfaces and fins. Skin respiration is especially important during development (Rombough, 2011), but in some species and under hypoxic conditions, also adult fish can satisfy up to 30% of their oxygen demand through skin respiration (Steffensen, Lomholt & Johansen, 1981; Sacca & Burggren, 1982). However, in some species, skin respiration might barely account for the skin’s own oxygen consumption (Kirsch & Nonnotte, 1977). Hibernating turtles deploy a similar rescue oxygenation mechanism (“cloacal respiration”) (Ultsch, 1989), and even mammals seem to be able to supplement lung respiration (Okabe et al., 2021).
Gill respiration is less efficient than lung respiration because the oxygen content of water is much lower than that of air. On top of this, diffusion in water is slower than in air. Due to these difficulties, fish have developed a broad spectrum of methods to supplement the main oxygen uptake by the gills. In addition to regulating the presence of RBCs in the SVS, ray-fish can swallow air or skim the oxygen-enriched surface water layer, and they deploy – in addition to the gills – other organs for oxygen uptake, such as the mouth, stomach, intestine, or swim bladder (Val, 1995; Nelson, 2014).
The difficult oxygen uptake is perhaps the primary reason why fish are cold-blooded animals. It is probably impossible to extract enough oxygen from water to maintain a body temperature significantly higher than the environment. There is likely a constant evolutionary pressure to optimise oxygen extraction from the surrounding water. Direct oxygen uptake via the skin is an additional pathway to increase oxygen supply when demand is high, and higher metabolically active species tend to feature a more extensive SVS (Skov & Bennett, 2003), which supports this interpretation. Overall, increasing the oxygen uptake in hypoxic conditions seems to be one of the key functions of the SVS, in response to the enormous adaptive pressure exercised by the scarcity of this resource in an aquatic environment.
X SOME RESPIRATORY GILL VESSELS HAVE SVS CHARACTERISTICS
Due to their complexity and many species-specific adaptations, the vascular networks of the gills have been subject to many discussions, including the question of whether gills feature SVS-like vessels. A generic setup is shown in
Figure 4, comprising solutions reported from different fish species. IAAs and a secondary circulation in gills have been described in Teleosts (Olson, 2002). They are also present in the gills of jawless fishes, a lineage that diverged early on during vertebrate evolution (Pohla, Lametschwandtner & Adam, 1977; Nakao & Uchinomiya, 1978; Lomholt & Franko-Dossar, 1998). In the gills of cartilaginous fishes, IAAs have been found as well (Donald, 1989; Metcalfe & Butler, 2009), although they do not always take the morphologically tortuous shape mostly seen in bony fishes (Olson, 1996). SVS-like gill vessels have also been reported in coelacanth (Vogel, Hughes & Mattheus, 1998). These IAA give rise to gill vessels, often referred to as “nutrient” vessels or “non-respiratory” vessels, and it has been debated whether they are part of the SVS. Many authors clearly distinguish between the SVS in the gills (“branchial” or “gill” SVS) and the SVS in the rest of the body (“systemic SVS”) (Skov & Bennett, 2003). Some authors recognise three anatomically different vascular pathways in the gill by further separating the non-respiratory vessels into the “interlamellar system” (alternatively sometimes called “central sinus” or “venolymphatic system”, a dense ladder-like system shown in green in
Figure 4) and the “nutrient system” (shown in magenta in
Figure 4), but these might be just different anatomic plumbings of the same network (Olson, 1996). Overall, the presence of an SVS-like vasculature in the gills is attested throughout the different fish groups, a distribution potentially even wider than that of the systemic SVS.
XI ONTOGENY OF THE LYMPHATICS
As several lines of investigation point to a developmental origin of the SVS from the LVS, a short summary of the ontogeny of these systems is in order. Historically, the venous tissue has been considered the origin of the lymphatic endothelial cells. However, in later years, additional cellular sources for the lymphatics have been described in mice (Stanczuk et al., 2015; Martinez-Corral et al., 2015; Klotz et al., 2015; Maruyama et al., 2019) and zebrafish (Eng et al., 2019). In zebrafish, all progenitor populations described thus far are marked by the expression of prox1a since the earlier stages (Dunworth et al., 2014, 2014; Koltowska et al., 2015; Eng et al., 2019). In the trunk, these progenitors sprout dorsally from the posterior cardinal vein, forming a transient parachordal lymphangioblast population at the horizontal myoseptum (Hogan et al., 2009a). This population then undergoes a second migration to form the main vessels of the trunk (Yaniv et al., 2006; Bussmann et al., 2010).
The embryonic development of lymphatic endothelial cells in bony fish presents a series of strong similarities. In mice, PROX1-positive cells are observed in the posterior cardinal vein (PCV) (Wigle & Oliver, 1999) prior to the sprouting that gives rise to the lymphatic sacs dorsally to the cardinal vein, from which the mature lymphatic vasculature will develop (Hagerling et al., 2013). A somewhat similar pattern has also been observed in Xenopus laevis, where Prox1 positive cells bud off the PCV to form the dorsal and ventral caudal lymphatic vessels (Ny et al., 2005). Interestingly, Hoyer (Hoyer, 1928b) reported a proliferation of the dorsal part of the PCV in early shark embryos, corresponding to the position of the described cardinal lymphatic ducts at later stages. The migration of the lymphatic progenitors also presents conserved characteristics. In addition to the conserved role of VEGF signalling described in Chapter XIII, NRP2 plays a role in the migration of lymphatic endothelial cells in mouse, zebrafish and Xenopus (Yuan et al., 2002; Xu et al., 2010; Hermans et al., 2010).
The embryonic lymphatic vasculature of teleosts also presents morphological similarities with that of mammals. Recently, a single pair of lymphatic valves, as well as lymphovenous valves at the interface between the lymphatics and the venous circulation, were described in zebrafish (Shin et al., 2019). Such lymphatic valves form under the control of the same factors regulating valvulogenesis in mammals (GATA2, NFATC1, FOXC1/2) and both in zebrafish and mouse the leaflet morphology is affected by the loss of integrin-a9 (Bazigou et al., 2009; Shin et al., 2019). Moreover, a conserved Prox1 enhancer labelling the lymphatic valve in mice also specifically marks the valves in zebrafish (Kazenwadel et al., 2023). Together, these data suggest lymphatic valves were already present in the ancestor of all bony fishes, and are therefore not an exclusive feature of mammalian lymphatics. As valves have not been described in the SVS, their existence implies that the lymphatic of ray-finned fish indeed needs to perform a similar function to that of mammals, at least in some of their parts.
In conclusion, the initial stages of the development of the lymphatic vasculature presents many similarities among bony fish, indicating the common origin of this structure regardless of having the potential to give rise to SVS in the anal fin at later larval developmental stages.
XII ONTOGENY OF THE SVS
Most of the older studies on the SVS/lymphatics have been performed during embryonic development due to the transparency of young fish (Jourdain, 1880; Mayer, 1919; Hoyer & Michalski, 1920). Likewise, the two seminal publications describing the lymphatics of zebrafish almost exclusively deal with early embryonic development. As a consequence, our knowledge of the later maturation of these vessels is still lacking. The only in-depth analysis of the lymphatic vasculature that systematically followed the developmental trajectory into adulthood concerns the anal and dorsal fin of zebrafish (Das
et al., 2022). This study showed that in these fins, an SVS develops by transdifferentiation from the pre-existing lymphatic network during the late larval and juvenile stages (
Figure 2C and
Figure 5). Following this transdifferentiation, the fins are a-lymphatic in adult fish. When the lymphatic vascularisation of the fins was blocked (as in
flt4/vegfr3 deficient zebrafish), the fins became vascularised by sprouts from nearby veins. However, this “rescue” vasculature was functionally compromised, and RBCs aggregated inside the fin vessels (Das
et al., 2022). The transdifferentiation of lymphatics into blood vessels of the SVS appears to be a genetic program independent of the perfusion with blood. Only at the very end of the transdifferentiation program do these new vascular networks connect to the primary vasculature to initiate blood flow. Moreover, the program of lymphatic vascularisation and transdifferentiation into SVS vessels recapitulates during fin regeneration, indicative of an ingrained developmental pathway. Extrapolating from the few existing data, the entire SVS is possibly a developmental derivative of the LVS.
Interestingly, a similar process, resembling the transdifferentiation of lymphatic-like vessel anlagen into blood vessels, also occurs in mammals and birds. The early embryonic vascular plexuses of murine and avian embryos express typical lymphatic factors such as VEGFR3, and deletion of the respective gene causes embryonic vascular failure and death already before lymphatics start to develop (Kaipainen et al., 1995; Wilting, Eichmann & Christ, 1997). Comparable to the developing SVS, embryonic mammalian vessels are not perfused with blood before they are connected to a functional heart. This seems to suggest that this transdifferentiation programme might be involved in more than just SVS development. Noticeably, studies on the VEGF signalling family have shown that VEGFA, the factor most associated with blood vessel development, appears evolutionarily later compared to the lymphangiogenic factor VEGFC, which is more ancestral (Holmes & Zachary, 2005; Kasap, 2005; Rauniyar et al., 2023). Further studies would be needed to elucidate if and what pathways act in both these processes.
From all reports on the SVS, it is clear that this system can be found mainly in the skin and the fins, and the vascular networks of visceral organs (such as intestine, mesentery and kidney) (Skov & Bennett, 2003) and skeletal muscle are reportedly devoid of secondary blood vessels. However, there is some evidence that not all fin or skin vasculature transdifferentiates from lymphatics. At least the proximal part of the anal fin and the skin of glass catfish appear to be served by the primary vasculature (Steffensen et al., 1986), emphasising the possibility of large interspecies differences even within the same order. Also, a recent study of the pectoral fin vasculature did not report any lymphatic contribution during development (Paulissen et al., 2022).
The study by Das et al. (2022) was a quantum leap regarding our understanding of the SVS by demonstrating that the transdifferentiated lymphatics connect to the arterial blood flow. However, the anatomical details of these connections have still to be worked out. Given that the zebrafish were able to regulate the entry of RBCs into the anal fin, it appears likely that the connections are functionally and/or anatomically similar to the IAAs, which have been described as the origin of the blood flow in the SVS, specifically for the anal fin (Steffensen et al., 1986). Larger SVS arteries often run in parallel to the primary vessels (Steffensen et al., 1986; Olson, 1996; Skov & Bennett, 2005), which is highly reminiscent of developing lymphatic vessels, which preferentially grow along the blood vessels (Oh et al., 1997; Breslin et al., 2018), requiring physical interaction (Bussmann et al., 2010), chemokine signalling (Cha et al., 2012) and/or sharing a similar preference for a specific environment. For the regenerating zebrafish brain, even the inverse has been reported: lymphatics guiding the growth of blood vessels (Chen et al., 2019). Such molecular and physical interaction between lymphatic endothelial cells and arteries, including mural cells (Peng et al., 2022), could be a first step in the development of ALCs/IAAs. Since lymphatic and blood vascular endothelial cells actively avoid connections both in vivo and in vitro (Mäkinen et al., 2001; Knezevic et al., 2017), this repulsion must be overcome to form anastomoses. Here, our knowledge about the mammalian lymphatico-venous connections (Welsh, Kahn & Sweet, 2016; Janardhan & Trivedi, 2019) is only of limited value since ALCs/IAAs involve arteries and the exit of blood plasma. In mammals, several proteins have been shown to be involved in the separation of blood and lymphatic vascular separation. Among these are the cytosolic tyrosine kinase Syk, the intracellular signalling molecule Slp76/LCP2 and the 7-transmembrane-spanning platelet-activating receptor are involved in the separation of blood and lymphatic vasculature (Abtahian et al., 2003; Hess et al., 2014). Interestingly, CLEC2, which is required for lymphatic development and maintenance (Bénézech et al., 2014), for maintaining venous vessel integrity during remodelling (Zhang et al., 2018), and for preventing RBCs from filling lymphatics throughout adult mouse life (Haining et al., 2021), does not exist in fish (Hughes et al., 2012). Blood clotting in fish is substantially different from that in terrestrial vertebrates since central molecules of the intrinsic (contact) pathway are for the most part absent (Mariz & Nery, 2020), and the fish equivalent of mammalian platelets (which are cell fragments), fish thrombocytes are complete nucleated cells (Stosik, Tokarz-Deptuła & Deptuła, 2019). Interestingly, the intrinsic pathway is also inactivated in the marine mammals of the cetacean lineage, where it is thought to reduce the risk of thrombus formation during diving (Huelsmann et al., 2019).
Two prior publications can help to shed at least some light on the nature of the ALCs/IAAs: Steffensen et al. (1986) and Jensen et al. (2009). Steffensen et al. describe the vasculature of the anal fin in the glass catfish. They identified both the origin of the blood flow (IAAs) and the outflow (secondary veins). Jensen et al. did the same for both glass catfish and zebrafish but also experimentally stimulated RBC entry into the secondary vasculature (by exposing fish to hypoxic water) and identified the lymphatic duct as the major outflow vessel of the secondary vasculature. Like Das et al. (2022), Jensen et al. did not discuss the SVS. Instead, they refer to the intermittently RBC-filled vessels as “lymphatics”, and modify
Figure 1 from Steffensen et al. (1986, equivalent to
Figure 6A), relabelling the SVS vessels as “lymphatics” and the IAAs as “arterial-lymphatic conduits” (ALCs,
Figure 6B).
The recent discovery of the transdifferentiation from LVS to SVS in the zebrafish fins offers us a unique model to start understanding the relationship between these two systems, and to identify the signalling pathways mediating this transition.
XIII VASCULAR ENDOTHELIAL GROWTH FACTOR SIGNALLING AND THE SVS
When trying to identify possible pathways involved in the LVS to SVS transdifferentiation, the VEGF family stands up as an obvious candidate, as its members are often involved in vascular development. For example, VEGFA (also referred to as VEGF) is crucial for the establishment of blood vessels (Carmeliet et al., 1996; Ferrara et al., 1996), and we already described the role of VEGFC and VEGFD in the lymphatics in Chapter V. However, while VEGFC and VEGFD are often regarded as specific for the lymphatic vasculature, they can replace VEGFA in activating VEGFR2, which is the primary mitogenic receptor of blood vascular endothelial cells (BECs) (Cao et al., 1998). In order to signal via VEGFR2, VEGFC and VEGFD need to be activated by specific proteases (Künnapuu et al., 2021). This binding makes VEGFC and VEGFD prime candidates for continuously sustaining vascular growth and survival during the lymphatic endothelial cell (LEC) to BEC transdifferentiation, since they can support VEGFR3 in LECs as well as VEGFR2 in BECs.
The VEGF family appears early in vertebrate evolution, and orthologs of VEGFA and VEGFC are found in all fish classes. VEGFD, VEGFB and PlGF can also be identified in all fishes except for jawless fishes (Rauniyar et al., 2023). While these three VEGF genes can be deleted without any obvious consequences in mice (Bellomo et al., 2000; Carmeliet et al., 2001; Aase et al., 2001; Baldwin et al., 2005), at least Vegfd and Vegfba are indispensable for zebrafish vascularization (Song et al., 2007; Jensen et al., 2015; Bower et al., 2017b), but their relevance for the SVS has not been analysed.
The interaction patterns of the lymphangiogenic VEGFD with its receptors are not conserved between species, not even between mice and humans (Baldwin et al., 2001). Two recombinant forms of mature zebrafish VEGFD did not interact with zebrafish VEGFR3/IgG fusion proteins (Vogrin et al., 2019), behaving similar to the Cathepsin D-activated form of human VEGFD (Leppänen et al., 2011). However, it is unknown what VEGFD forms are produced endogenously by zebrafish, and only one of the two recombinant forms deployed by Vogrin et al. (2019) has a corresponding human form. A comprehensive in-vitro comparison of the binding profiles of authentic zebrafish VEGFs to zebrafish VEGF receptors has not been performed, but the development of zebrafish facial and heart lymphatics needs clearly both VEGFC and VEGFD (Astin et al., 2014; Bower et al., 2017b; Vivien et al., 2019).
While many of the duplicated VEGF genes in zebrafish derive from the teleost genome duplication event (Rauniyar
et al., 2023), the VEGF receptor/Kdrl
(Kdr-like
) resulted from an earlier vertebrate lineage duplication (Bussmann
et al., 2008). Thus, four VEGF receptors are the rule among vertebrates, and eutheria (“placental mammals”), who lost Kdrl, are the exception. The name Kdrl might be misleading since phylogenetic analysis suggests that its closest relative is VEGFR1, and not VEGFR2 (Krd) (Bussmann, Bakkers & Schulte-Merker, 2007) (
Figure 7). Although less frequently used, the term VEGFR4 thus appears appropriate. With this context, the receptor binding pattern of the zebrafish VEGF family appears less surprising. Like in humans, VEGFD binds VEGFR2, and the only difference might be that zebrafish VEGFD has completely lost Flt4 (Vegfr3) binding, which is retained by one of the human isoforms. In fish, VEGFR4/Kdrl could work as the second VEGFA receptor, allowing VEGFR2 function to diverge. According to Vogrin et al. (2019), VEGFR2 has not lost its VEGFA affinity, but its additional ability to bind VEGFC and VEGFD makes it a possible drop-in replacement for VEGFR3.
However, these observations present a caveat. VEGF factors need to be processed intra- and extracellularly by a protease machinery to become active. While VEGFC is highly conserved in vertebrates, the polypeptide sequence recognised by the VEGFC-activating proteases is highly divergent between fish and other vertebrates (Jha et al., 2019; Rauniyar et al., 2023), possibly indicating differences in the activation of VEGFs. Many of the studies on VEGF binding in zebrafish either perform their characterisation of receptor specificities with human cDNAs in a human cell line (Wang et al., 2020), or the zebrafish-derived proteins are expressed and processed in human cells (Vogrin et al., 2019), and there is no data showing that fish cells process VEGF proteins in the same way mammalian cells do. Specifically, the requirement for proteolytic processing of VEGFC and VEGFD could render data obtained from non-piscine cell lines prone to artifactual activation by host cell proteases. As of today, receptor binding data for zebrafish VEGFs produced in a fish cell line does not exist, a gap that impairs our ability to fully characterise the role of VEGF signalling in the different vascular beds.
In conclusion, it seems likely that some members of the VEGF/VEGFR signalling pathways could have further specialised in mediating the LVS to SVS transdifferentiation.
XIV WHAT REMAINS FROM EMBRYONIC LYMPHATICS AFTER TRANSDIFFERENTIATION?
After the description of the differentiation of the lymphatics into SVS in the fin, the question remains of how much of the embryonic LVS remains in the adult.
While there is no lack of publications describing the lymphatic vessels or secondary blood vessels of fish intestines (Mayer, 1919; Grodziński, 1932; Glaser, 1933; Sire, Lutton & Vernier, 1981; Lomholt & Franko-Dossar, 1998; Hellberg et al., 2013; Astin et al., 2014; Okuda et al., 2015), it is unclear whether these are mammalian- or SVS-type lymphatics or still other vascular types. The studies performed in ray-finned fishes lack appropriate molecular characterisation (Mayer, 1919; Sire et al., 1981; Hellberg et al., 2013) or describe snapshots of embryonic vessels (Astin et al., 2014; Okuda et al., 2015), potentially subject to concurrent or later transdifferentiation. Moreover, transdifferentiation might not completely convert vessels from “embryonic mammalian-type lymphatics” into “adult SVS-type lymphatics”. For example, the data from Jensen et al. (2009) show that the lymphatic duct maintains its Prox1 expression in adult zebrafish while being functionally integrated into the SVS. This large lymphatic vessel appears to receive both lymphatic and SVS tributaries and might form a terminal hybrid network connecting both lymphatics and SVS to the primary vasculature. While Das et al. (2022) did not follow the venous return of the freshly transdifferentiated fin vasculature, Jensen et al. showed both in glass catfish and zebrafish filling of the thoracic duct with RBCs when the SVS became perfused (Jensen et al., 2009). This arrangement shows that the expression of lymphatic markers is not incompatible with RBC perfusion. Indeed, the concept of hybrid vessels is not new, and they have already been suggested to exist in jawless fishes, such as hagfish and lamprey (Allen, 1913; Cole, 1926; Lomholt & Franko-Dossar, 1998).
Despite these uncertainties, the functional studies performed in late larval and adult stages in zebrafish suggest that vessels performing the fluid-uptake function of lymphatics do persist past the early embryonic stages (Isogai
et al., 2009; Venero Galanternik
et al., 2016; Castranova
et al., 2021; Elmagid
et al., 2022). The viscera and the musculature are organs in which the lymphatics keep expressing the standard lymphatic markers, and transdifferentiation is therefore likely absent, partial or incomplete. E.g., a
flt4+/prox1+ lymphatic network has been shown in the adult zebrafish heart (Vivien
et al., 2019; Harrison
et al., 2019), and an
mrc1a+/lyve1+ lymphatic network in the meninges (Castranova
et al., 2021). Overall, a model postulating the coexistence of SVS and LVS in different compartments of adult ray-finned fishes (
Figure 2C), with the SVS concentrating on the body surface and gills and the LVS draining the deeper tissues in the body, is the one that best fits the currently available data.
Although the developmental derivation of the SVS from LVS is well supported, this does not necessarily imply that the lymphatic endothelium is the sole developmental origin of secondary vessels. If all secondary vasculature was derived from pre-existing lymphatics, one might expect regions devoid of lymphatic vessels, such as the brain parenchyma (Louveau et al., 2015), to be devoid of secondary blood vessels. The tailfin of adult zebrafish features intermittent RBC perfusion while maintaining Prox1 expression (Jensen et al., 2009), which is different from the anal and dorsal fin, where Prox1 expression is lost (Das et al., 2022). Vascular networks with hybrid marker expression (such as Schlemm’s canal) exist even in humans (Aspelund et al., 2014; Park et al., 2014). Jensen et al. (2009) suggest that ALCs/IAAs might also exist in mammals, but their reference to the hypothesis by Schmid-Schönbein (Schmid-Schönbein, 2003) appears unconvincing. Similarly, mouse knock-out models or tumours in which lymphatics become perfused by RBCs are not indicative of any physiological perfusion of mammalian lymphatics with RBCs (Jensen et al., 2009).
We are facing a highly complex situation, where variable subsets of lymphatics transdifferentiate during embryonic development to different degrees into blood vessels depending on the organ and species context, forming – perhaps together with vessels of different developmental origin – the SVS. Consequently, vascular identity always falls on a spectrum and a binary distinction (assumed by the definition of lymphatics in Chapter V) is insufficient to capture the variability seen in the complex biological world (Jeltsch & Alitalo, 2022). In mammals, most vessels are located close to the ends of the spectrum, but we should not assume that this has to be the case in other animal phyla. Neither should we assume that a comprehensive characterisation of vessel identity is easy since some integral elements of this characterisation, such as functional in-vivo assays of the SVS or the lymphatic vasculature, are challenging to automatise.
In conclusion, the available data seem to point toward a coexistence in adult ray-finned fishes of a superficial SVS, mainly located in the skin and fins, together with lymphatic-like vessels, hybrid vessels or both located deeper in the body and performing at least partially the functions of a “traditional” LVS (
Figure 5).
XV AN EVOLUTIONARY MODEL OF THE LYMPHATIC VASCULAR SYSTEM
To conclude this review, we would like to propose an evolutionary model explaining the appearance and relation between the SVS and the lymphatic vasculature.
In Chapter X we have described the similarities between the non-respiratory gill vasculature and the IAAs of the SVS. Because of such similarities, it seems reasonable to assume that they fulfil a similar function. Oxygen loss in hypoxic water is likely a problem not only in the fins and other body surfaces but also in the gills. Assuming that shunting pathways bypassing the respiratory vasculature exist in jawless vertebrates, the group phylogenetically most distant from tetrapods (Nakao & Uchinomiya, 1978; Lomholt & Franko-Dossar, 1998), we may hypothesise that the capability to deny RBC entry to specific organs originate at the base of vertebrates due to the need to optimise aquatic respiration. In other words, all fishes possess an alternative gill vascular system, and possibly a systemic one, due to the need to compartmentalise the cardiovascular system to avoid oxygen loss. Once the SVS had developed and improved RBC oxygenation, additional forces likely contributed to the evolution of this system.
A likely major evolutionary driver was the need for immune surveillance. Although the need for immune surveillance in its innate form predates the emergence of vascular systems and is found, e.g., already in
Hydra (Bosch, 2014), an adaptive immune system (AIS) requires a specific minimum body size, due to the need for a large antigen receptor repertoire. Thus, the significant increase in body size between extant vertebrates and their closest relatives could have created the need for both an AIS and aquatic respiration since the need to distribute oxygen via a cardiovascular system also results from an increased body volume (Burggren & Reiber, 2007). Interestingly, the BCR–TCR–MHC-based AIS present in jawed vertebrates (Gnathostomata) is thought to have an independent evolutionary origin from the jawless fish AIS, which is based on a different set of molecules (variable lymphocyte receptor, VLR) (Flajnik & Kasahara, 2010). Therefore, the jawed vertebrate AIS system is thought to have appeared after the split with jawless vertebrates, and, therefore, after the initial appearance of an SVS (
Figure 8).
The rise of an AIS would, in turn, require a mechanism that ensures that intruding antigens meet with their cognate antigen receptor-presenting cells, such as migratory immune cells or the draining of the interstitial fluid via a specialised vascular network. This “lymphatic” vasculature would have appeared either at the base of jawed vertebrates or that of bony fish, depending on the actual presence of the described deep lymphatics of cartilaginous fishes (Diamare, 1909; Hoyer, 1928b; Otto Frederic Kampmeier, 1969) (
Figure 8). Given the common developmental origin, this vasculature can be assumed to have evolved from a subcompartment of the SVS. Interestingly, there is data suggesting a specialised function of the SVS in immune defence (Rasmussen, Steffensen & Buchmann, 2013), which would support the close evolutionary relation between the SVS and immunity.
Later, in the tetrapod lineage, the blood pressure increase associated with the transition from poikilothermic to homeothermic temperature regulation likely required further adaptations, resulting in the loss of an SVS and the appearance of “true lymphatics”. In some of these scenarios, a true LVS could have also existed before the water-to-land transition and the emergence of the tetrapod lineage. This point is supported by the vascular arrangement of lungfish. These animals do not appear to have an SVS and instead present mammalian-like lymphatics (Vogel & Mattheus, 1998; Skov & Bennett, 2003). It is important to highlight here that although today, lungfish live a “semi-aquatic” lifestyle, the common ancestor they share with tetrapods is believed to have been fully aquatic (Long & Gordon, 2004; Long
et al., 2006). Save for a convergent loss of SVS structures and acquisition of a “true” lymphatic in both lineages, this suggests that the appearance of a mammalian-like lymphatic is not associated with the water-to-and transition, but precedes it (
Figure 8). Efficient lymph nodes have only been observed in aquatic birds and in mammals, and might be the manifestation of increased demands upon the immune system in these lineages (Boehm, Hess & Swann, 2012). Until recently, it was assumed that teleost fish only possessed diffuse lymphoid tissues (Bjørgen & Koppang, 2021), but recent data suggests more defined lymphoid organs might be present in this group as well (Resseguier
et al., 2023). This observation stresses the importance of the AIS across vertebrates and its close connection with the SVS/LVS evolution.
We are not the first to suggest an evolutionary origin of the LVS from the SVS (Steffensen & Lomholt, 1992; Vogel
et al., 1998). However, this model could potentially explain many of the character distributions we observe, such as the co-occurrence of an AIS and (deep) lymphatics within jawed vertebrates (
Figure 8). Due to the scarcity of data in many of the discussed phyla, our model had to be based on some assumptions, such as the presence of some form of SVS in jawless and cartilaginous fish, and we are aware further studies in this subject could overturn our conclusions. However, we believe it is essential to provide an evolutionary model for the emergence of the SVS and its relation with the LVS, which attempts to bring together and synthesise the vast amount of information that has been collected on this topic in more than a century, and against which new data can be compared, either strengthening the model or rebuking it.
XVI CONCLUSIONS
- 1)
Unanswered questions about the lymphatic vascular system in fishes go back more than 100 years. With recent data strongly supporting the presence of vessels with lymphatic characteristics in ray-finned fish, the question of the relationship between lymphatic vasculature and SVS in these animals is more important than ever. This question transcends simple naming discussions because the underlying physiology and evolutionary forces are at the heart of the controversy.
- 2)
While ray-finned fishes and coelacanths possess some form of SVS, the data concerning jawless and cartilaginous fish are less clear. Whether vascular specialisations similar to the SVS exist in the gills of the above-mentioned fishes and whether such could represent the evolutionary origin of the SVS also remains controversial. We speculate that capillary filtration and the resulting requirement for tissue drainage are insufficient to explain the evolutionary emergence of the SVS. Instead, the primary evolutionary pressure for SVS-like adaptations is likely the need to maximise oxygen extraction and retention faced with a variable environmental supply. From a molecular perspective, all fishes, including the jawless fishes, possess sufficient molecular diversity to account for a dual vascular setup. They all feature haemangiogenic as well as lymphangiogenic vascular endothelial growth factors (VEGFs) and their cognate receptors (VEGFRs).
- 3)
The mammalian-type lymphatics and the SVS are evolutionarily related. In piscine embryonic development, the SVS appears to develop from pre-existing lymphatics, but the SVS seems to be phylogenetically older. Future research needs to determine the extent of lymphatic-to-blood vessel transdifferentiation in the embryonic development of fishes and how much and which parts of the embryonic lymphatic vasculature are maintained into adulthood. Preliminary data suggest that transdifferentiation does vary, and does so even in closely related species.
- 4)
Similar to the lymphatic origin debate, also the SVS vs. lymphatics debate will likely be resolved by a hybrid model, which was proposed already more than 100 years ago by Cole and Allen. They proposed the term "venolymphatic system", although both Cole and Allen thought this mixed lymphatic/blood vascular system to be limited to hagfish and lampreys. This model will have to transcend the binary categorisation into the cardiovascular and lymphatic vasculature that we have become accustomed to from our focus on mammalian biology.
- 5)
We propose an evolutionary model in which the SVS evolves in response to the increased limitations to oxygen supply connected with the larger size of early vertebrates. Consequent recruitment of part of these vessels in the AIS functions led to the appearance of lymphatic-like vessels with draining functions. In lungfish and tetrapods, the SVS was lost leading to a mammalian-like lymphatic vascular network.
- 6)
As the reader will no doubt have noticed, the topic of the lymphatic and secondary vascular systems is no stranger to contradictory data. We hope this review will spark a renewed interest in this long-lived controversy, providing the scientific community with a comprehensive overview of the anatomical, molecular, physiological, developmental and evolutionary observations and hopefully motivating a new effort in answering the many questions that remain unaddressed.
Author Contributions
M. J. and J. W. conceived the idea and drafted the initial structure. The writing was performed by Z. V., V. P., J. W. and M. J. K. K. and V. P. were responsible for an essential and significant overhaul in both structure and content. The writing was coordinated by M. J. All co-authors read and commented on drafts and approved the final version.
Acknowledgements
We thank Ameera Elawad for the critical reading of the manuscript. The laboratory of Michael Jeltsch was supported during the work for this manuscript by the Academy of Finland grant #337120. Katarzyna Koltowska and Virginia Panara were supported by Wallenberg Academy Fellowship (2017.0144), Ragnar Söderbergs Fellowship (M13/17), Vetenskapsådet (VR-MH-2016-01437) and Beijer Foundation.
References
- Aase, K.; von Euler, G.; Li, X.; Pontén, A.; Thorén, P.; Cao, R.; Cao, Y.; Olofsson, B.; Gebre-Medhin, S.; Pekny, M.; et al. Vascular Endothelial Growth Factor-B–Deficient Mice Display an Atrial Conduction Defect. Circ. 2001, 104, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Abtahian, F.; Guerriero, A.; Sebzda, E.; Lu, M.-M.; Zhou, R.; Mocsai, A.; Myers, E.E.; Huang, B.; Jackson, D.G.; Ferrari, V.A.; et al. Regulation of Blood and Lymphatic Vascular Separation by Signaling Proteins SLP-76 and Syk. Science 2003, 299, 247–251. [Google Scholar] [CrossRef]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Mäkinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Hogan, B.M.; Gjini, E.; Salehi, F.; Al-Gazali, L.; A Hennekam, E.; E Holmberg, E.; Mannens, M.M.A.M.; Mulder, M.F.; A Offerhaus, G.J.; et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 2009, 41, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.F. (1913) Studies on the development of the venolymphatics in the tail region of Pollistotrema (Bdellostoma) stoutii. Quarterly Journal of Microscopical Science 59, 309–360.
- Aspelund, A. , Tammela, T., Antila, S., Nurmi, H., Leppänen, V.-M., Zarkada, G., Stanczuk, L., Francois, M., Mäkinen, T., Saharinen, P., Immonen, I. & Alitalo, K. (2014) The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. Journal of Clinical Investigation 124, 3975–3986.
- Astin, J.W.; Haggerty, M.J.L.; Okuda, K.S.; Le Guen, L.; Misa, J.P.; Tromp, A.; Hogan, B.M.; Crosier, K.E.; Crosier, P.S. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 2014, 141, 2680–2690. [Google Scholar] [CrossRef]
- Bahary, N.; Goishi, K.; Stuckenholz, C.; Weber, G.; LeBlanc, J.; Schafer, C.A.; Berman, S.S.; Klagsbrun, M.; Zon, L.I. Duplicate VegfA genes and orthologues of the KDR receptor tyrosine kinase family mediate vascular development in the zebrafish. Blood 2007, 110, 3627–3636. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Catimel, B.; Nice, E.C.; Roufail, S.; Hall, N.E.; Stenvers, K.L.; Karkkainen, M.J.; Alitalo, K.; Stacker, S.A.; Achen, M.G. The Specificity of Receptor Binding by Vascular Endothelial Growth Factor-D Is Different in Mouse and Man. J. Biol. Chem. 2001, 276, 19166–19171. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular Endothelial Growth Factor D Is Dispensable for Development of the Lymphatic System. Mol. Cell. Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef]
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-specific Receptor for Hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef]
- Bazigou, E.; Xie, S.; Chen, C.; Weston, A.; Miura, N.; Sorokin, L.; Adams, R.; Muro, A.F.; Sheppard, D.; Makinen, T. Integrin-α9 Is Required for Fibronectin Matrix Assembly during Lymphatic Valve Morphogenesis. Dev. Cell 2009, 17, 175–186. [Google Scholar] [CrossRef]
- Becker, L.M.; Chen, S.-H.; Rodor, J.; de Rooij, L.P.M.H.; Baker, A.H.; Carmeliet, P. Deciphering endothelial heterogeneity in health and disease at single-cell resolution: progress and perspectives. Cardiovasc. Res. 2022, 119, 6–27. [Google Scholar] [CrossRef]
- Bellomo, D. , Headrick, J.P., Silins, G.U., Paterson, C.A., Thomas, P.S., Gartside, M., Mould, A., Cahill, M.M., Tonks, I.D., Grimmond, S.M., Townson, S., Wells, C., Little, M., Cummings, M.C., Hayward, N.K., et al. (2000) Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circulation Research 86, E29-35.
- Bénézech, C.; Nayar, S.; Finney, B.A.; Withers, D.R.; Lowe, K.; Desanti, G.E.; Marriott, C.L.; Watson, S.P.; Caamaño, J.H.; Buckley, C.D.; et al. CLEC-2 is required for development and maintenance of lymph nodes. Blood 2014, 123, 3200–3207. [Google Scholar] [CrossRef]
- Bernier-Latmani, J. & Petrova, T.V. (2017) Intestinal lymphatic vasculature: structure, mechanisms and functions. Nature Reviews Gastroenterology & Hepatology.
- Betancur-R, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 1–40. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Boehm, T. , Hess, I. B. ( 2012) Evolution of lymphoid tissues. Trends in Immunology 33, 315–321.
- Bos, F.L.; Caunt, M.; Peterson-Maduro, J.; Planas-Paz, L.; Kowalski, J.; Karpanen, T.; van Impel, A.; Tong, R.; Ernst, J.A.; Korving, J.; et al. CCBE1 Is Essential for Mammalian Lymphatic Vascular Development and Enhances the Lymphangiogenic Effect of Vascular Endothelial Growth Factor-C In Vivo. Circ. Res. 2011, 109, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C. Rethinking the role of immunity: lessons from Hydra. Trends Immunol. 2014, 35, 495–502. [Google Scholar] [CrossRef] [PubMed]
- I Bower, N.; Koltowska, K.; Pichol-Thievend, C.; Virshup, I.; Paterson, S.; Lagendijk, A.K.; Wang, W.; Lindsey, B.W.; Bent, S.J.; Baek, S.; et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat. Neurosci. 2017, 20, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Bower, N.I. , Vogrin, A.J., Guen, L.L., Chen, H., Stacker, S.A., Achen, M.G. & Hogan, B.M. (2017b) Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development. Development 144, 507–518. Oxford University Press for The Company of Biologists Limited.
- Breiteneder-Geleff, S.; Soleiman, A.; Kowalski, H.; Horvat, R.; Amann, G.; Kriehuber, E.; Diem, K.; Weninger, W.; Tschachler, E.; Alitalo, K.; et al. Angiosarcomas Express Mixed Endothelial Phenotypes of Blood and Lymphatic Capillaries: Podoplanin as a Specific Marker for Lymphatic Endothelium. Am. J. Pathol. 1999, 154, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E. (2018) Das Lymphsystem und das Starlingsche Gleichgewicht. Lymphologie in Forschung und Praxis 22, 9–13.
- Breslin, J.W. , Yang, Y., Scallan, J.P., Sweat, R.S., Adderley, S.P. & Murfee, W.L. (2018) Lymphatic Vessel Network Structure and Physiology. In Comprehensive Physiology pp. 207–299. John Wiley & Sons, Ltd.
- Brouillard, P.; Dupont, L.; Helaers, R.; Coulie, R.; E Tiller, G.; Peeden, J.; Colige, A.; Vikkula, M. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia–lymphedema syndrome 3. Hum. Mol. Genet. 2017, 26, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.J. (1977) Chapter 6: The Development of the Lymphatic and Immunohematopoietic Systems. In The Biology of Marsupials (ed D.I. Hunsaker), pp. 349–385. Academic Press, New Yoork, San Francisco, London.
- Burggren, W.W. & Reiber, C.L. (2007) Evolution of Cardiovascular Systems and Their Endothelial Linings. In Endothelial Biomedicine (ed W.C. Aird), pp. 29–49, 1st edition. Cambridge University Press.
- Burne, R.H. I. A contribution to the anatomy of the ductless glands and lymphatic system of the angler fish ( Lophius piscatorius ). Philos. Trans. R. Soc. London. Ser. B, Contain. Pap. a Biol. Character 1927, 215, 1–56. [Google Scholar] [CrossRef]
- Burne, R.H. (1929) A System of ‘Fine’ Vessels Associated with the Lymphatics in the Cod (Gadus morrhua). Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 217, 335–366. The Royal Society.
- Bussmann, J. , Bakkers, J. & Schulte-Merker, S. (2007) Early Endocardial Morphogenesis Requires Scl/Tal1. PLOS Genetics 3, e140. Public Library of Science.
- Bussmann, J.; Bos, F.L.; Urasaki, A.; Kawakami, K.; Duckers, H.J.; Schulte-Merker, S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 2010, 137, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, J.; Lawson, N.; Zon, L.; Schulte-Merker, S. ; Zebrafish Nomenclature Committee Zebrafish VEGF Receptors: A Guideline to Nomenclature. PLOS Genet. 2008, 4, e1000064. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. , Linden, P., Farnebo, J., Cao, R., Eriksson, A., Kumar, V., Qi, J.-H., Claesson-Welsh, L. & Alitalo, K. (1998) Vascular endothelial growth factor C induces angiogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America 95, 14389–14394.
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Casley-Smith, J.R. & Casley-Smith, J.R. (1975) The fine structure of the blood capillaries of some endocrine glands of the hagfish, Eptatretus stouti:implications for the evolution of blood and lymph vessels. Revue Suisse de Zoologie 82, 35–40.
- Castranova, D.; Samasa, B.; Galanternik, M.V.; Jung, H.M.; Pham, V.N.; Weinstein, B.M. Live Imaging of Intracranial Lymphatics in the Zebrafish. Circ. Res. 2021, 128, 42–58. [Google Scholar] [CrossRef]
- Cha, Y.R.; Fujita, M.; Butler, M.; Isogai, S.; Kochhan, E.; Siekmann, A.F.; Weinstein, B.M. Chemokine Signaling Directs Trunk Lymphatic Network Formation along the Preexisting Blood Vasculature. Dev. Cell 2012, 22, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Ni, R.; Yang, Q.; Zhang, Y.; Luo, L. Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev. Cell 2019, 49, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Coffindaffer-Wilson, M.; Craig, M.P.; Hove, J.R. Determination of lymphatic vascular identity and developmental timecourse in zebrafish (Danio rerio). . 2011, 44, 1–12. [Google Scholar]
- Cole, F.J. (1926) IV.—A Monograph on the general Morphology of the Myxinoid Fishes, based on a study of Myxine. Part VI. The Morphology of the Vascular System. Earth and Environmental Science Transactions of The Royal Society of Edinburgh 54, 309–342. Royal Society of Edinburgh Scotland Foundation.
- Das, R.N.; Tevet, Y.; Safriel, S.; Han, Y.; Moshe, N.; Lambiase, G.; Bassi, I.; Nicenboim, J.; Brückner, M.; Hirsch, D.; et al. Generation of specialized blood vessels via lymphatic transdifferentiation. Nature 2022, 606, 570–575. [Google Scholar] [CrossRef]
- Diamare, V. (1909) Sui rapporti della vena porta e delle arterie splancniche in Scyllium catulus e Torpedo marmorata. Anatomischer Anzeiger 34, 552.
- Donald, J.A. (1989) Vascular anatomy of the gills of the stingarees Urolophus mucosus and U. paucimaculatus (Urolophidae, Elasmobranchii). Journal of Morphology 200, 37–46.
- Donald, J.A. & Ellis, A.G. (1983) Arterio-Venous anastomoses in the gills of australian short-finned eel, Anguilla australis. Journal of Morphology 178, 89–93.
- Dunworth, W.P.; Cardona-Costa, J.; Bozkulak, E.C.; Kim, J.-D.; Meadows, S.; Fischer, J.C.; Wang, Y.; Cleaver, O.; Qyang, Y.; Ober, E.A.; et al. Bone Morphogenetic Protein 2 Signaling Negatively Modulates Lymphatic Development in Vertebrate Embryos. Circ. Res. 2014, 114, 56–66. [Google Scholar] [CrossRef]
- Eliason, E.J. & Stecyk, J.A.W. (2021) The Cardiovascular System. In The Physiology of Fishes pp. 47–615. CRC Press, Hoboken.
- Elmagid, L.A.; Mittal, N.; Bakis, I.; Lien, C.-L.; Harrison, M.R. Intramyocardial Injection for the Study of Cardiac Lymphatic Function in Zebrafish. J. Vis. Exp. 2022, e64504. [Google Scholar] [CrossRef] [PubMed]
- Eng, T.C.; Chen, W.; Okuda, K.S.; Misa, J.P.; Padberg, Y.; E Crosier, K.; Crosier, P.S.; Hall, C.J.; Schulte-Merker, S.; Hogan, B.M.; et al. Zebrafish facial lymphatics develop through sequential addition of venous and non-venous progenitors. Embo Rep. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H. , Piermarini, P.M. & Choe, K.P. (2005) The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiological Reviews 85, 97–177. American Physiological Society.
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O'Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, R.E.; Baty, C.J.; Kimak, M.A.; Karlsson, J.M.; Lawrence, E.C.; Franke-Snyder, M.; Meriney, S.D.; Feingold, E.; Finegold, D.N. GJC2 Missense Mutations Cause Human Lymphedema. Am. J. Hum. Genet. 2010, 86, 943–948. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 2009, 11, 47–59. [Google Scholar] [CrossRef]
- Galanternik, M.V.; Castranova, D.; Gore, A.V.; Blewett, N.H.; Jung, H.M.; Stratman, A.N.; Kirby, M.R.; Iben, J.; Miller, M.F.; Kawakami, K.; et al. A novel perivascular cell population in the zebrafish brain. eLife 2017, 6. [Google Scholar] [CrossRef]
- Gans, C. & Bell (2001) IV. Vertebrate Classification. In Encyclopedia of biodiversity (ed S.A. Levin), p. Academic Press, San Diego.
- Glaser, G. (1933) Beiträge zur Kenntnis des Lymphgefäßsystems der Fische. Zeitschrift für Anatomie und Entwicklungsgeschichte 100, 433–511.
- Gordon, K.; Schulte, D.; Brice, G.; Simpson, M.A.; Roukens, M.G.; van Impel, A.; Connell, F.; Kalidas, K.; Jeffery, S.; Mortimer, P.S.; et al. Mutation in Vascular Endothelial Growth Factor-C, a Ligand for Vascular Endothelial Growth Factor Receptor-3, Is Associated With Autosomal Dominant Milroy-Like Primary Lymphedema. Circ. Res. 2013, 112, 956–960. [Google Scholar] [CrossRef]
- Gore, A.V. , Monzo, K. M. ( 2012) Vascular Development in the Zebrafish. Cold Spring Harbor Perspectives in Medicine 2, a006684.
- Grimm, L.; Mason, E.; Yu, H.; Dudczig, S.; Panara, V.; Chen, T.; I Bower, N.; Paterson, S.; Galeano, M.R.; Kobayashi, S.; et al. Single-cell analysis of lymphatic endothelial cell fate specification and differentiation during zebrafish development. EMBO J. 2023, 42, e112590. [Google Scholar] [CrossRef]
- Grodziński, Z. (1932) Bemerkungungen über das Lymphgefässsystem von Myxine glutinosa L. Bulletin International de l’Académie Polonaise des Sciences et des Lettres, Classe des Sciences Mathématiques et Naturelles 44, 221–236.
- Hägerling, R.; Pollmann, C.; Andreas, M.; Schmidt, C.; Nurmi, H.; Adams, R.H.; Alitalo, K.; Andresen, V.; Schulte-Merker, S.; Kiefer, F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013, 32, 629–644. [Google Scholar] [CrossRef]
- Haiko, P.; Makinen, T.; Keskitalo, S.; Taipale, J.; Karkkainen, M.J.; Baldwin, M.E.; Stacker, S.A.; Achen, M.G.; Alitalo, K. Deletion of Vascular Endothelial Growth Factor C (VEGF-C) and VEGF-D Is Not Equivalent to VEGF Receptor 3 Deletion in Mouse Embryos. Mol. Cell. Biol. 2008, 28, 4843–4850. [Google Scholar] [CrossRef]
- Haining, E.J.; Lowe, K.L.; Wichaiyo, S.; Kataru, R.P.; Nagy, Z.; Kavanagh, D.P.; Lax, S.; Di, Y.; Nieswandt, B.; Ho-Tin-Noé, B.; et al. Lymphatic blood filling in CLEC-2-deficient mouse models. Platelets 2020, 32, 352–367. [Google Scholar] [CrossRef]
- Hargens, A.R. , Millard, R.W. & Johansen, K. (1974) High capillary permeability in fishes. Comparative Biochemistry and Physiology Part A: Physiology 48, 675–680.
- Harrison, M.R. , Feng, X., Mo, G., Aguayo, A., Villafuerte, J., Yoshida, T., Pearson, C.A., Schulte-Merker, S. & Lien, C.-L. (2019) Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. eLife 8, e42762. eLife Sciences Publications, Ltd.
- Hedrick, M.S.; Hansen, K.; Wang, T.; Lauridsen, H.; Thygesen, J.; Pedersen, M. Visualising lymph movement in anuran amphibians with computed tomography. J. Exp. Biol. 2014, 217, 2990–2993. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, M.S.; Hillman, S.S.; Drewes, R.C.; Withers, P.C. Lymphatic regulation in nonmammalian vertebrates. J. Appl. Physiol. 2013, 115, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, H. , Bjerkås, I., Vågnes, Ø.B. & Noga, E.J. (2013) Mast cells in common wolffish Anarhichas lupus L.: Ontogeny, distribution and association with lymphatic vessels. Fish & Shellfish Immunology 35, 1769–1778.
- Hentschel, D.M.; Park, K.M.; Cilenti, L.; Zervos, A.S.; Drummond, I.; Bonventre, J.V. Acute renal failure in zebrafish: a novel system to study a complex disease. Am. J. Physiol. Physiol. 2005, 288, F923–F929. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.; Claes, F.; Vandevelde, W.; Zheng, W.; Geudens, I.; Orsenigo, F.; De Smet, F.; Gjini, E.; Anthonis, K.; Ren, B.; et al. Role of synectin in lymphatic development in zebrafish and frogs. Blood 2010, 116, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.R.; Rawnsley, D.R.; Jakus, Z.; Yang, Y.; Sweet, D.T.; Fu, J.; Herzog, B.; Lu, M.; Nieswandt, B.; Oliver, G.; et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Investig. 2013, 124, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Hewson, W. (1769) XXIX. An account of the lymphatic system in fish. Philosophical Transactions of the Royal Society of London 59, 204–215. Royal Society.
- Hillman, S.S.; Drewes, R.C.; Hedrick, M.S. Control of blood volume following hypovolemic challenge in vertebrates: Transcapillary versus lymphatic mechanisms. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2021, 254, 110878. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y. , Thorpe, J.L., Deng, Y., Santana, E., DeRose, R.A. & Farber, S.A. (2004) Lipid Metabolism in Zebrafish. In Methods in Cell Biology pp. 87–108. Academic Press.
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef]
- Hogan, B.M. , Herpers, R., Witte, M., Heloterä, H., Alitalo, K., Duckers, H.J. & Schulte-Merker, S. (2009b) Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136, 4001–4009.
- Holeton, G.F. (1980) Oxygen as an Environmental Factor of Fishes. In Environmental Physiology of Fishes (ed M.A. Ali), pp. 7–32. Springer US, Boston, MA.
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef]
- Hoyer, H. On the lymphatic vessels of Scyllium canicula. Anat. Rec. 1928, 40, 143–145. [Google Scholar] [CrossRef]
- Hoyer, H. & Michalski, L. (1920) Das Lymphgefäßsystem von Forellenembryonen nebst Bemerkungen über die Verteilung der Blutgefäße. Gegenbaurs Morphologisches Jahrbuch 51, 1–89.
- Hoyer, H.F. (1928b) Recherches sur les vaisseaux lymphatiques des Sélaciens. Bulletin L’Académie Polonaise des Science, Classe des Sciences Mathématiques et Naturelles, 79.
- Huang, C.-C.; Monte, A.; Cook, J.M.; Kabir, M.S.; Peterson, K.P. Zebrafish Heart Failure Models for the Evaluation of Chemical Probes and Drugs. ASSAY Drug Dev. Technol. 2013, 11, 561–572. [Google Scholar] [CrossRef]
- Huelsmann, M.; Hecker, N.; Springer, M.S.; Gatesy, J.; Sharma, V.; Hiller, M. Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations. Sci. Adv. 2019, 5, eaaw6671. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Radhakrishnan, U.P.; Lordkipanidzé, M.; Egginton, S.; Dijkstra, J.M.; Jagadeeswaran, P.; Watson, S.P. G6f-Like Is an ITAM-Containing Collagen Receptor in Thrombocytes. PLOS ONE 2012, 7, e52622. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.M.; Peyraud, C.; Peyraud-Waitzenegger, M.; Soulier, P. Physiological Evidence for the Occurrence of Pathways Shunting Blood Away from the Secondary Lamellae of Eel Gills. J. Exp. Biol. 1982, 98, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Huisman, Y.; Uphoff, K.; Berger, M.; Dobrindt, U.; Schelhaas, M.; Zobel, T.; Bussmann, J.; van Impel, A.; Schulte-Merker, S. Meningeal lymphatic endothelial cells fulfill scavenger endothelial cell function and cooperate with microglia in waste removal from the brain. Glia 2021, 70, 35–49. [Google Scholar] [CrossRef]
- Hußmann, M. , Schulte, D., Weischer, S., Carlantoni, C., Nakajima, H., Mochizuki, N., Stainier, D.Y., Zobel, T., Koch, M. & Schulte-Merker, S. (2023) Svep1 is a binding ligand of Tie1 and affects specific aspects of facial lymphatic development in a Vegfc-independent manner. eLife 12, e82969. eLife Sciences Publications, Ltd.
- Huxley, V.H.; Scallan, J. Lymphatic fluid: exchange mechanisms and regulation. J. Physiol. 2011, 589, 2935–2943. [Google Scholar] [CrossRef]
- van Impel, A.; Zhao, Z.; Hermkens, D.M.A.; Roukens, M.G.; Fischer, J.C.; Peterson-Maduro, J.; Duckers, H.; Ober, E.A.; Ingham, P.W.; Schulte-Merker, S. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 2014, 141, 1228–1238. [Google Scholar] [CrossRef]
- Ishimatsu, A. , Iwama, G.K. & Heisler, N. (1995) Physiological Roles of the Secondary Circulatory System in Fish. In Mechanisms of Systemic Regulation: Respiration and Circulation (ed N. Heisler), pp. 215–236. Springer, Berlin, Heidelberg.
- Isogai, S.; Hitomi, J.; Yaniv, K.; Weinstein, B.M. Zebrafish as a new animal model to study lymphangiogenesis. Anat. Sci. Int. 2009, 84, 102–111. [Google Scholar] [CrossRef]
- Janardhan, H.P. & Trivedi, C.M. (2019) Establishment and maintenance of blood-lymph separation. Cellular and molecular life sciences : CMLS 76, 1865–1876.
- Janssen, L.; Dupont, L.; Bekhouche, M.; Noel, A.; Leduc, C.; Voz, M.; Peers, B.; Cataldo, D.; Apte, S.S.; Dubail, J.; et al. ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis 2015, 19, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, M. (2002) VEGFR-3 Ligands and Lymphangiogenesis. Doctoral Thesis, University of Helsinki, Helsinki.
- Jeltsch, M.; Alitalo, K. Lymphatic-to-blood vessel transdifferentiation in zebrafish. Nat. Cardiovasc. Res. 2022, 1, 539–541. [Google Scholar] [CrossRef]
- Jeltsch, M.; Jha, S.K.; Tvorogov, D.; Anisimov, A.; Leppänen, V.-M.; Holopainen, T.; Kivelä, R.; Ortega, S.; Kärpanen, T.; Alitalo, K. CCBE1Enhances Lymphangiogenesis via A Disintegrin and Metalloprotease With Thrombospondin Motifs-3–Mediated Vascular Endothelial Growth Factor-C Activation. Circ. 2014, 129, 1962–1971. [Google Scholar] [CrossRef]
- Jensen, L.D.; Nakamura, M.; Bräutigam, L.; Li, X.; Liu, Y.; Samani, N.J.; Cao, Y. VEGF-B-Neuropilin-1 signaling is spatiotemporally indispensable for vascular and neuronal development in zebrafish. Proc. Natl. Acad. Sci. 2015, 112, E5944–E5953. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.D.E.; Cao, R.; Hedlund, E.-M.; Söll, I.; Lundberg, J.O.; Hauptmann, G.; Steffensen, J.F.; Cao, Y. Nitric oxide permits hypoxia-induced lymphatic perfusion by controlling arterial-lymphatic conduits in zebrafish and glass catfish. Proc. Natl. Acad. Sci. 2009, 106, 18408–18413. [Google Scholar] [CrossRef]
- Jha, S.K. , Rauniyar, K., Chronowska, E., Mattonet, K., Maina, E.W., Koistinen, H., Stenman, U.-H., Alitalo, K. & Jeltsch, M. (2019) KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D. eLife 8, e44478.
- Jha, S.K. , Rauniyar, K. & Jeltsch, M. (2018) Key molecules in lymphatic development, function, and identification. Annals of Anatomy - Anatomischer Anzeiger 219, 25–34.
- Jha, S.K.; Rauniyar, K.; Karpanen, T.; Leppänen, V.-M.; Brouillard, P.; Vikkula, M.; Alitalo, K.; Jeltsch, M. Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Sarsons, C.D.; Gomez-Garcia, M.J.; Cramb, D.T.; Rinker, K.D.; Childs, S.J. Quantum dot interactions and flow effects in angiogenic zebrafish ( Danio rerio ) vessels and human endothelial cells. Nanomedicine: Nanotechnology, Biol. Med. 2017, 13, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Fänge, R.; Johannessen, M.W. Relations between blood, sinus fluid and lymph in Myxine glutinosa L. Comp. Biochem. Physiol. 1962, 7, 23–28. [Google Scholar] [CrossRef]
- Johnson, L.A. In Sickness and in Health: The Immunological Roles of the Lymphatic System. Int. J. Mol. Sci. 2021, 22, 4458. [Google Scholar] [CrossRef]
- Joss, J.M. Lungfish evolution and development. Gen. Comp. Endocrinol. 2006, 148, 285–289. [Google Scholar] [CrossRef]
- Jourdain, S. (1868) Coup-d’oeil sur le système veineux et lymphatique de la Raie bouclée. Ann. Acad La Rochelle Sect. Sc. N. N. 8, 21–34.
- Jourdain, S. (1880) Sur l’existence d’une circulation lymphatique chez les Pleuronectes. Compt. rend. Acad. Sc. Paris 90, 1430–1432.
- Jung, H.M.; Castranova, D.; Swift, M.R.; Pham, V.N.; Galanternik, M.V.; Isogai, S.; Butler, M.G.; Mulligan, T.S.; Weinstein, B.M. Development of the larval lymphatic system in the zebrafish. Development 2017, 144, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.W.; Fang, G.H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. 1995, 92, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef]
- Kamel, K.S. & Halperin, M.L. (2017) Sodium and Water Physiology. In Fluid, electrolyte, and acid-base physiology: a problem-based approach pp. 216–254.
- Karam, M.; Janbon, H.; Malkinson, G.; Brunet, I. Heterogeneity and developmental dynamics of LYVE-1 perivascular macrophages distribution in the mouse brain. J. Cereb. Blood Flow Metab. 2022, 42, 1797–1812. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2003, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kasap, M. (2005) Phylogenetic Analysis of Vascular Endothelial Growth Factor Diversity. Turkish Journal of Biology 29, 217–227.
- Kazenwadel, J.; Venugopal, P.; Oszmiana, A.; Toubia, J.; Arriola-Martinez, L.; Panara, V.; Piltz, S.G.; Brown, C.; Ma, W.; Schreiber, A.W.; et al. A Prox1 enhancer represses haematopoiesis in the lymphatic vasculature. Nature 2023, 614, 343–348. [Google Scholar] [CrossRef] [PubMed]
- King, J. & Hossler, F. (1986) The Gill Arch of the Striped Bass, Morone Saxatilis. II. Microvasculature Studied with Vascular Corrosion Casting and Scanning Electron Microscopy. Scanning Electron Microscopy 1986.
- Kirsch, R.; Nonnotte, G. Cutaneous respiration in three freshwater teleosts. Respir. Physiol. 1977, 29, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dubé, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef]
- Knezevic, L.; Schaupper, M.; Mühleder, S.; Schimek, K.; Hasenberg, T.; Marx, U.; Priglinger, E.; Redl, H.; Holnthoner, W. Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices. Front. Bioeng. Biotechnol. 2017, 5, 25. [Google Scholar] [CrossRef]
- Koltowska, K.; Lagendijk, A.K.; Pichol-Thievend, C.; Fischer, J.C.; Francois, M.; Ober, E.A.; Yap, A.S.; Hogan, B.M. Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Rep. 2015, 13, 1828–1841. [Google Scholar] [CrossRef]
- Küchler, A.M.; Gjini, E.; Peterson-Maduro, J.; Cancilla, B.; Wolburg, H.; Schulte-Merker, S. Development of the Zebrafish Lymphatic System Requires Vegfc Signaling. Curr. Biol. 2006, 16, 1244–1248. [Google Scholar] [CrossRef]
- Künnapuu, J. , Bokharaie, H. & Jeltsch, M. (2021) Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs. Biology 10, 167.
- Lahnsteiner, F. , Lametschwandtner, A. & Patzner, R. (1989) The Secondary Blood Vessel System of Segmental Arteries and Dorsal Aorta in Blennius pavo and Zosterisessor ophiocephalus. Histology, Fine Structure and SEM of Vascular Corrosion Casts. Scanning Microscopy 4.
- Laurent, P.; Dunel, S.; Barthe, J.-C. Functional Organization of the Teleost Gill. Acta Zoöl. 1976, 57, 189–209. [Google Scholar] [CrossRef]
- Le Guen, L.; Karpanen, T.; Schulte, D.; Harris, N.C.; Koltowska, K.; Roukens, G.; Bower, N.I.; van Impel, A.; Stacker, S.A.; Achen, M.G.; et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 2014, 141, 1239–1249. [Google Scholar] [CrossRef]
- Leppänen, V.-M.; Jeltsch, M.; Anisimov, A.; Tvorogov, D.; Aho, K.; Kalkkinen, N.; Toivanen, P.; Ylä-Herttuala, S.; Ballmer-Hofer, K.; Alitalo, K. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 2011, 117, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- van Lessen, M. , Shibata-Germanos, S., van Impel, A., Hawkins, T.A., Rihel, J. & Schulte-Merker, S. (2017) Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. eLife 6, e25932. eLife Sciences Publications, Ltd.
- Leu, A.J.; Berk, D.A.; Yuan, F.; Jain, R.K.; Nelson, T.S.; Akin, R.E.; Weiler, M.J.; Kassis, T.; Kornuta, J.A.; Dixon, J.B. Flow velocity in the superficial lymphatic network of the mouse tail. Am. J. Physiol. Circ. Physiol. 1994, 267, H1507–H1513. [Google Scholar] [CrossRef]
- Levick, J.R. Capillary filtration-absorption balance reconsidered in light of dynamic extravascular factors. Exp. Physiol. 1991, 76, 825–857. [Google Scholar] [CrossRef]
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef]
- Li, D.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Seiler, C.; Pinto, E.; Matsuoka, L.S.; Battig, M.R.; Bhoj, E.J.; Wenger, T.L.; et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 2019, 25, 1116–1122. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, J.P. & Franko-Dossar, F. (1998) The Sinus System of Hagfishes — Lymphatic or Secondary Circulatory System? In The Biology of Hagfishes (eds J.M. Jørgensen, J.P. Lomholt, R.E. Weber & H. Malte), pp. 259–272. Springer Netherlands, Dordrecht.
- Long, J.A.; Gordon, M.S. The Greatest Step in Vertebrate History: A Paleobiological Review of the Fish-Tetrapod Transition. Physiol. Biochem. Zoöl. 2004, 77, 700–719. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Young, G.C.; Holland, T.; Senden, T.J.; Fitzgerald, E.M.G. An exceptional Devonian fish from Australia sheds light on tetrapod origins. Nature 2006, 444, 199–202. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Machnik, A.; Neuhofer, W.; Jantsch, J.; Dahlmann, A.; Tammela, T.; Machura, K.; Park, J.-K.; Beck, F.-X.; Müller, D.N.; Derer, W.; et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat. Med. 2009, 15, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Flear, K.; Qiu, P.; Pan, Y.K.; Perry, S.F.; Gilmour, K.M. Aquatic surface respiration improves survival during hypoxia in zebrafish (Danio rerio) lacking hypoxia-inducible factor 1-α. Proc. R. Soc. B: Biol. Sci. 2022, 289, 20211863. [Google Scholar] [CrossRef] [PubMed]
- Mariz, J.P.V.; Nery, M.F. Unraveling the Molecular Evolution of Blood Coagulation Genes in Fishes and Cetaceans. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Martinez-Corral, I.; Ulvmar, M.H.; Stanczuk, L.; Tatin, F.; Kizhatil, K.; John, S.W.; Alitalo, K.; Ortega, S.; Makinen, T. Nonvenous Origin of Dermal Lymphatic Vasculature. Circ. Res. 2015, 116, 1649–1654. [Google Scholar] [CrossRef]
- Maruyama, K.; Miyagawa-Tomita, S.; Mizukami, K.; Matsuzaki, F.; Kurihara, H. Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev. Biol. 2019, 452, 134–143. [Google Scholar] [CrossRef]
- Mattonet, K. & Jeltsch, M. (2015) Heterogeneity of the origin of the lymphatic system. [German]. Lymphologie in Forschung und Praxis 19, 84–88.
- Mayer, P. (1888) Über Eigentümlichkeiten in den Kreislaufsorganen der Selachier. Mitteilungen der Zoologischen Station Neapel 2, 307–373.
- Mayer, P. (1919) Über die Lymphgefäße der Fische und ihre mutmaßliche Rolle bei der Verdauung. Jena Z. Naturwiss. 55, 125–174.
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.M.; Woutersen, R.A. Normal Anatomy and Histology of the Adult Zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef]
- Metcalfe, J.D.; Butler, P.J. The functional anatomy of the gills of the dogfish (Scyliorhinus canicula). J. Zoöl. 1986, 208, 519–530. [Google Scholar] [CrossRef]
- Mulligan, T.S.; Weinstein, B.M. Emerging from the PAC: Studying zebrafish lymphatic development. Microvasc. Res. 2014, 96, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Uchinomiya, K. A study on the blood vascular system of the lamprey gill filament. Am. J. Anat. 1978, 151, 239–263. [Google Scholar] [CrossRef]
- Nelson, J.A. Breaking wind to survive: fishes that breathe air with their gut. J. Fish Biol. 2014, 84, 554–576. [Google Scholar] [CrossRef]
- Nicenboim, J.; Malkinson, G.; Lupo, T.; Asaf, L.; Sela, Y.; Mayseless, O.; Gibbs-Bar, L.; Senderovich, N.; Hashimshony, T.; Shin, M.; et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015, 522, 56–61. [Google Scholar] [CrossRef]
- Ny, A.; Koch, M.; Schneider, M.; Neven, E.; Tong, R.T.; Maity, S.; Fischer, C.; Plaisance, S.; Lambrechts, D.; Héligon, C.; et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat. Med. 2005, 11, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Jeltsch, M.M.; Birkenhäger, R.; McCarthy, J.E.; Weich, H.A.; Christ, B.; Alitalo, K.; Wilting, J. VEGF and VEGF-C: Specific Induction of Angiogenesis and Lymphangiogenesis in the Differentiated Avian Chorioallantoic Membrane. Dev. Biol. 1997, 188, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Okabe, R.; Chen-Yoshikawa, T.F.; Yoneyama, Y.; Yokoyama, Y.; Tanaka, S.; Yoshizawa, A.; Thompson, W.L.; Kannan, G.; Kobayashi, E.; Date, H.; et al. Mammalian enteral ventilation ameliorates respiratory failure. Med 2021, 2, 773–783. [Google Scholar] [CrossRef]
- Okuda, K.S.; Astin, J.W.; Misa, J.P.; Flores, M.V.; Crosier, K.E.; Crosier, P.S. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 2012, 139, 2381–2391. [Google Scholar] [CrossRef]
- Okuda, K.S.; Misa, J.P.; Oehlers, S.H.; Hall, C.J.; Ellett, F.; Alasmari, S.; Lieschke, G.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. A zebrafish model of inflammatory lymphangiogenesis. Biol. Open 2015, 4, 1270–1280. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Olson, K. (1996) Secondary circulation in fish: Anatomical organization and physiological significance. Journal of Experimental Zoology 275, 172–185.
- Olson, K.R. Vascular anatomy of the fish gill. J. Exp. Zoöl. 2002, 293, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. , Dewar, H., Graham, J.B. & Brill, R.W. (2003a) Vascular anatomy of the gills in a high energy demand teleost, the skipjack tuna (Katsuwonus pelamis). Journal of Experimental Zoology Part A: Comparative Experimental Biology 297A, 17–31.
- Olson, K.R. & Farell, A.P. (2011) Secondary Circulation and Lymphatic Anatomy. In Encyclopedia of fish physiology: from genome to environment (eds A.P. Farrell, E.D. Stevens, J.J. Cech & J.G. Richards), pp. 1161–1168. Academic Press, London ; Waltham, MA.
- Olson, K.R. , Kinney, D.W., Dombkowski, R.A. & Duff, D.W. (2003b) Transvascular and intravascular fluid transport in the rainbow trout:revisiting Starling’s forces, the secondary circulation and interstitial compliance. Journal of Experimental Biology 206, 457–467.
- Otis, J.P. , Zeituni, E.M., Thierer, J.H., Anderson, J.L., Brown, A.C., Boehm, E.D., Cerchione, D.M., Ceasrine, A.M., Avraham-Davidi, I., Tempelhof, H., Yaniv, K. & Farber, S.A. (2015) Zebrafish as a model for apolipoprotein biology: comprehensive expression analysis and a role for ApoA-IV in regulating food intake. Disease Models & Mechanisms 8, 295–309.
- Otto Frederic Kampmeier (1969) Evolution and Comparative Morphology of the Lymphatic System. Thomas.
- Park, D.-Y. , Lee, J., Park, I., Choi, D., Lee, S., Song, S., Hwang, Y., Hong, K.Y., Nakaoka, Y., Makinen, T., Kim, P., Alitalo, K., Hong, Y.-K. & Koh, G.Y. (2014) Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. The Journal of Clinical Investigation 124, 3960–3974.
- Paulissen, S.M.; Castranova, D.M.; Krispin, S.M.; Burns, M.C.; Menéndez, J.; Torres-Vázquez, J.; Weinstein, B.M. Anatomy and development of the pectoral fin vascular network in the zebrafish. Development 2022, 149. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Rosental, B.; Hansen, N.F.; Beers, J.M.; Parish, G.; Rowbotham, I.; Block, B.A. Hydraulic control of tuna fins: A role for the lymphatic system in vertebrate locomotion. Science 2017, 357, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Peng, D. , Ando, K., Hußmann, M., Gloger, M., Skoczylas, R., Mochizuki, N., Betsholtz, C., Fukuhara, S., Schulte-Merker, S., Lawson, N.D. & Koltowska, K. (2022) Proper migration of lymphatic endothelial cells requires survival and guidance cues from arterial mural cells. eLife 11, e74094.
- Petrova, T.V.; Bono, P.; Holnthoner, W.; Chesnes, J.; Pytowski, B.; Sihto, H.; Laakkonen, P.; Heikkilä, P.; Joensuu, H.; Alitalo, K. VEGFR-3 Expression Is Restricted to Blood and Lymphatic Vessels in Solid Tumors. Cancer Cell 2008, 13, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Pohla, H. , Lametschwandtner, A. & Adam, H. (1977) Die Vaskularisation der Kiemen von Myxine glutinosa L. (Cyclostomata) - Wiley Online Library. Zoologica Scripta 6, 331–341.
- Polomska, A.K. & Proulx, S. T. ( 2021) Imaging technology of the lymphatic system. Advanced Drug Delivery Reviews 170, 294–311.
- Prevo, R. , Banerji, S., Ni, J. & Jackson, D.G. (2004) Rapid Plasma Membrane-Endosomal Trafficking of the Lymph Node Sinus and High Endothelial Venule Scavenger Receptor/Homing Receptor Stabilin-1 (Feel-1/Clever-1)*. Journal of Biological Chemistry 279, 52580–52592.
- Radu, B.M.; Radu, M.; Tognoli, C.; Benati, D.; Merigo, F.; Assfalg, M.; Solani, E.; Stranieri, C.; Ceccon, A.; Pasini, A.M.F.; et al. Are they in or out? The elusive interaction between Qtracker®800 vascular labels and brain endothelial cells. Nanomedicine 2015, 10, 3329–3342. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef]
- Rasmussen, K.J. , Steffensen, J. ( Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases 36, 675–679.
- Rauniyar, K.; Bokharaie, H.; Jeltsch, M. Expansion and collapse of VEGF diversity in major clades of the animal kingdom. Angiogenesis 2023, 26, 437–461. [Google Scholar] [CrossRef]
- Resseguier, J. , Nguyen-Chi, M., Wohlmann, J., Rigaudeau, D., Salinas, I., Oehlers, S.H., Wiegertjes, G.F., Johansen, F.-E., Qiao, S.-W., Koppang, E.O., Verrier, B., Boudinot, P. & Griffiths, G. (2023) Identification of a pharyngeal mucosal lymphoid organ in zebrafish and other teleosts: Tonsils in fish? Science Advances 9, eadj0101. American Association for the Advancement of Science.
- Robin, Ch. (1845) Note sur un appareil particulier des vaisseaux lymphatiques chez les poissons. Revue de zoologie agricole et appliquée 8, 224.
- Rombough, P.J. (2011) Respiratory Gas Exchange During Development: Respiratory Transitions. In Encyclopedia of fish physiology: from genome to environment (eds A.P. Farrell, E.D. Stevens, J.J. Cech & J.G. Richards), pp. 838–845. Academic Press, London ; Waltham, MA.
- Rossi, A.; Gauvrit, S.; Marass, M.; Pan, L.; Moens, C.B.; Stainier, D.Y.R. Regulation of Vegf signaling by natural and synthetic ligands. Blood 2016, 128, 2359–2366. [Google Scholar] [CrossRef]
- Rummer, J.L. , Wang, S., Steffensen, J.F. & Randall, D.J. (2014) Function and control of the fish secondary vascular system, a contrast to mammalian lymphatic systems. Journal of Experimental Biology 217, 751–757. The Company of Biologists Ltd.
- Rutili, G.; Arfors, K. Protein Concentration in Interstitial and Lymphatic Fluids from the Subcutaneous Tissue. Acta Physiol. Scand. 1977, 99, 1–8. [Google Scholar] [CrossRef]
- Sabin, F.R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1902, 1, 367–389. [Google Scholar] [CrossRef]
- Sacca, R. & Burggren, W. (1982) Oxygen uptake in air and water in the air-breathing reedfish Calamoichthys calabaricus: role of skin, gills and lungs. Journal of Experimental Biology 97, 179–186.
- Salmi, M.; Koskinen, K.; Henttinen, T.; Elima, K.; Jalkanen, S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood 2004, 104, 3849–3857. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. The Second Valve System in Lymphatics. Lymphat. Res. Biol. 2003, 1, 25–31. [Google Scholar] [CrossRef]
- Schroedl, F.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; Lutty, G.A.; Maruyama, K.; Chen, L.; Lütjen-Drecoll, E.; et al. Consensus Statement on the Immunohistochemical Detection of Ocular Lymphatic Vessels. Investig. Opthalmology Vis. Sci. 2014, 55, 6440–6442. [Google Scholar] [CrossRef]
- Sheridan, M.A. (1988) Lipid dynamics in fish: aspects of absorption, transportation, deposition and mobilization. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 90, 679–690.
- Sheridan, M.A.; Friedlander, J.K.; Allen, W.V. Chylomicra in the serum of postprandial steelhead trout (Salmo gairdnerii). Comp. Biochem. Physiol. Part B: Comp. Biochem. 1985, 81, 281–284. [Google Scholar] [CrossRef]
- Shin, M.; Male, I.; Beane, T.J.; Villefranc, J.A.; Kok, F.O.; Zhu, L.J.; Lawson, N.D. Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors. Development 2017, 143, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Nozaki, T.; Idrizi, F.; Isogai, S.; Ogasawara, K.; Ishida, K.; Yuge, S.; Roscoe, B.; Wolfe, S.A.; Fukuhara, S.; et al. Valves Are a Conserved Feature of the Zebrafish Lymphatic System. Dev. Cell 2019, 51, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yin, H.-M.; Shih, Y.-H.; Nozaki, T.; Portman, D.; Toles, B.; Kolb, A.; Luk, K.; Isogai, S.; Ishida, K.; et al. Generation and application of endogenously floxed alleles for cell-specific knockout in zebrafish. Dev. Cell 2023, 58, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- Sire, M.F.; Lutton, C.; Vernier, J.M. New views on intestinal absorption of lipids in teleostean fishes: an ultrastructural and biochemical study in the rainbow trout. J. Lipid Res. 1981, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Siret, C.; van Lessen, M.; Bavais, J.; Jeong, H.W.; Samawar, S.K.R.; Kapupara, K.; Wang, S.; Simic, M.; de Fabritus, L.; Tchoghandjian, A.; et al. Deciphering the heterogeneity of the Lyve1+ perivascular macrophages in the mouse brain. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Skov, P.V.; Bennett, M.B. The secondary vascular system of Actinopterygii: interspecific variation in origins and investment. Zoomorphology 2003, 122, 181–190. [Google Scholar] [CrossRef]
- Skov, P.V.; Bennett, M.B. Branchial vascular pathways in two species of Tetraodontiformes and the concept of secondary vessels and nutrient arteries. Zoomorphology 2005, 124, 79–88. [Google Scholar] [CrossRef]
- Song, M.; Yang, H.; Yao, S.; Ma, F.; Li, Z.; Deng, Y.; Deng, H.; Zhou, Q.; Lin, S.; Wei, Y. A critical role of vascular endothelial growth factor D in zebrafish embryonic vasculogenesis and angiogenesis. Biochem. Biophys. Res. Commun. 2007, 357, 924–930. [Google Scholar] [CrossRef]
- Stanczuk, L.; Martinez-Corral, I.; Ulvmar, M.H.; Zhang, Y.; Laviña, B.; Fruttiger, M.; Adams, R.H.; Saur, D.; Betsholtz, C.; Ortega, S.; et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015, 10, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F. & Lomholt, J.P. (1992) The secondary vascular system. In The Cardiovascular System (eds W.S. Hoar, D.J. Randall & A.P. Farrell), pp. 185–217. Academic Press, San Diego.
- Steffensen, J.F. , Lomholt, J. ( exposed to graded hypoxia. Respiration Physiology 44, 269–275.
- Steffensen, J.F. , Lomholt, J. O.P. ( the Primary and Secondary Vessels in Fishes. Acta Zoologica 67, 193–200.
- Stoletov, K.; Fang, L.; Choi, S.-H.; Hartvigsen, K.; Hansen, L.F.; Hall, C.; Pattison, J.; Juliano, J.; Miller, E.R.; Almazan, F.; et al. Vascular Lipid Accumulation, Lipoprotein Oxidation, and Macrophage Lipid Uptake in Hypercholesterolemic Zebrafish. Circ. Res. 2009, 104, 952–960. [Google Scholar] [CrossRef]
- Stosik, M.; Tokarz-Deptuła, B.; Deptuła, W. Characterisation of thrombocytes in Osteichthyes. J. Veter- Res. 2019, 63, 123–131. [Google Scholar] [CrossRef]
- Taylor, P.R.; Gordon, S.; Martinezpomares, L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef]
- Ultsch, G.R. (1989) Ecology and Physiology of Hibernation and Overwintering Among Freshwater Fishes, Turtles, and Snakes. Biological Reviews 64, 435–515.
- Ulvmar, M.H.; Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 2016, 111, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Val, A.L. (1995) Oxygen transfer in fish: morphological and molecular adjustments. Brazilian Journal of Medical and Biological Research = Revista Brasileira De Pesquisas Medicas E Biologicas 28, 1119–1127.
- Van Der Jagd, E. (1932) The Origin and Development of the Anterior Lymph-Sacs in the Sea-Turtle (Thalasso- chelys caretta). Quarterly Journal of Microscopical Science.
- Galanternik, M.V.; Stratman, A.N.; Jung, H.M.; Butler, M.G.; Weinstein, B.M. Building the drains: the lymphatic vasculature in health and disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 689–710. [Google Scholar] [CrossRef] [PubMed]
- Vialleton, L. (1902) Les lymphatiques du tube digestif de la Torpille (Torpedo marmorata, Risso). Archives d’anatomie microscopique 5, 378--456.
- Vivien, C.J.; Pichol-Thievend, C.; Sim, C.B.; Smith, J.B.; Bower, N.I.; Hogan, B.M.; Hudson, J.E.; Francois, M.; Porrello, E.R. Vegfc/d-dependent regulation of the lymphatic vasculature during cardiac regeneration is influenced by injury context. npj Regen. Med. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W. [Structure and principles of organization of the vascular system in bony fishes]. . 1981, 127, 772–84. [Google Scholar]
- Vogel, W.O.P. (1985a) Systemic vascular anastomoses, primary and secondary vessels in fish, and the phylogeny of lymphatics. In Cardiovascular shunts: Phylogenetic, ontogenetic and clinical aspects p. Munksgaard, Copenhagen.
- Vogel, W.O.P. (1985b) The Caudal Heart of Fish: Not a Lymph Heart. Cells Tissues Organs 121, 41–45. Karger Publishers.
- Vogel, W.O.P. Zebrafish and lymphangiogenesis: a reply. Anat. Sci. Int. 2010, 85, 118–119. [Google Scholar] [CrossRef]
- Vogel, W.O.P. & Claviez, M. (1981) Vascular Specialization in Fish, but No Evidence for Lymphatics. Zeitschrift für Naturforschung 36, 490–492.
- Vogel, W.O.P.; Hughes, G.M.; Mattheus, U. Non-respiratory blood vessels in Latimeria gill filaments. Philos. Trans. R. Soc. B: Biol. Sci. 1998, 353, 465–475. [Google Scholar] [CrossRef]
- Vogel, W.O.P.; Mattheus, U. Lymphatic vessels in lungfishes (Dipnoi). Zoomorphology 1998, 117, 199–212. [Google Scholar] [CrossRef]
- Vogrin, A.J.; Bower, N.I.; Gunzburg, M.J.; Roufail, S.; Okuda, K.S.; Paterson, S.; Headey, S.J.; Stacker, S.A.; Hogan, B.M.; Achen, M.G. Evolutionary Differences in the Vegf/Vegfr Code Reveal Organotypic Roles for the Endothelial Cell Receptor Kdr in Developmental Lymphangiogenesis. Cell Rep. 2019, 28, 2023–2036. [Google Scholar] [CrossRef]
- Wang, G.; Muhl, L.; Padberg, Y.; Dupont, L.; Peterson-Maduro, J.; Stehling, M.; le Noble, F.; Colige, A.; Betsholtz, C.; Schulte-Merker, S.; et al. Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis. Nat. Commun. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Weidenreich, F. , Baum, H. & Trautmann, A. (1934) VII. Lymphgefäßsystem. In Handbuch der vergleichenden Anatomie der Wirbeltiere pp. 745–854. Urban & Schwarzenberg, Berlin & Vienna.
- Welsh, J.D.; Kahn, M.L.; Sweet, D.T. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood 2016, 128, 1169–1173. [Google Scholar] [CrossRef]
- Wigle, J.T.; Oliver, G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Wiig, H.; Schröder, A.; Neuhofer, W.; Jantsch, J.; Kopp, C.; Karlsen, T.V.; Boschmann, M.; Goss, J.; Bry, M.; Rakova, N.; et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J. Clin. Investig. 2013, 123, 2803–2815. [Google Scholar] [CrossRef]
- Wilting, J.; Becker, J. The lymphatic vascular system: much more than just a sewer. Cell Biosci. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Wilting, J.; Eichmann, A.; Christ, B. Expression of the avian VEGF receptor homologues Quek1 and Quek2 in blood-vascular and lymphatic endothelial and non-endothelial cells during quail embryonic development. Cell Tissue Res. 1997, 288, 207–223. [Google Scholar] [CrossRef]
- Wilting, J.; Papoutsi, M.; Christ, B.; Nicolaides, K.H.; Kaisenberg, C.S.; Borges, J.; Stark, G.B.; Alitalo, K.; Tomarev, S.I.; Niemeyer, C.; et al. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002, 16, 1271–1273. [Google Scholar] [CrossRef]
- Wilting, J. , Papoutsi, M. ( 2000) The lymphatic endothelium of the avian wing is of somitic origin. Developmental Dynamics 217, 271–278.
- Xu, Y. , Yuan, L., Mak, J., Pardanaud, L., Caunt, M., Kasman, I., Larrivée, B., Toro, R. del, Suchting, S., Medvinsky, A., Silva, J., Yang, J., Thomas, J.-L., Koch, A.W., Alitalo, K., et al. (2010) Neuropilin-2 mediates VEGF-C–induced lymphatic sprouting together with VEGFR3. The Journal of Cell Biology 188, 115–130.
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef]
- Yaniv, K. , Isogai, S., Castranova, D., Dye, L., Hitomi, J. & Weinstein, B.M. (2007) Imaging the Developing Lymphatic System Using the Zebrafish. In Vascular Development pp. 139–151. John Wiley & Sons, Ltd.
- Yuan, L.; Moyon, D.; Pardanaud, L.; Bréant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806. [Google Scholar]
- Zhang, Y.; Daubel, N.; Stritt, S.; Mäkinen, T. Transient loss of venous integrity during developmental vascular remodeling leads to red blood cell extravasation and clearance by lymphatic vessels. Development 2018, 145, dev156745. [Google Scholar] [CrossRef]
Figure 1.
The mammalian and the piscine circulatory systems differ in their layout (double versus single circuit) and experience different pressures. In mammals, the high blood pressure and the hydrostatic/orthostatic pressure both increase capillary filtration (leakage of fluid), whereas, in fish, the low blood pressure causes less capillary filtration, which is additionally counteracted by the aquatic pressure.
Figure 1.
The mammalian and the piscine circulatory systems differ in their layout (double versus single circuit) and experience different pressures. In mammals, the high blood pressure and the hydrostatic/orthostatic pressure both increase capillary filtration (leakage of fluid), whereas, in fish, the low blood pressure causes less capillary filtration, which is additionally counteracted by the aquatic pressure.
Figure 2.
The SVS and LVS in ray-finned fishes. The terminology and classification of fish vasculature have changed repeatedly during history. (A) The lymphatic interpretation considers the fish’s cardiovascular and lymphatic vascular systems to be separate networks that communicate only via a few lymphaticovenous junctions, where the lymph fluid returns to the venous system, being functionally and anatomically largely identical to the mammalian LVS. (B) The SVS interpretation redefines all lymphatic vessels as secondary blood vessels that receive their flow from inter-arterial anastomoses, which can regulate the influx of red blood cells (RBCs). (C) In the hybrid interpretation, a subset of lymphatic networks maintains their lymphatic nature in the adult fish (termed here “intransigent lymphatics”). However, Das et al. (2022) showed for the anal and dorsal fin of zebrafish that some lymphatics transdifferentiate during the juvenile stage into SVS blood vessels. Jensen et al. (2009) further demonstrated that the SVS is confluent with some parts of the lymphatic vascular system. These key insights explain many previous incomprehensible research results and demonstrate that vascular identity is more fluid than previously thought.
Figure 2.
The SVS and LVS in ray-finned fishes. The terminology and classification of fish vasculature have changed repeatedly during history. (A) The lymphatic interpretation considers the fish’s cardiovascular and lymphatic vascular systems to be separate networks that communicate only via a few lymphaticovenous junctions, where the lymph fluid returns to the venous system, being functionally and anatomically largely identical to the mammalian LVS. (B) The SVS interpretation redefines all lymphatic vessels as secondary blood vessels that receive their flow from inter-arterial anastomoses, which can regulate the influx of red blood cells (RBCs). (C) In the hybrid interpretation, a subset of lymphatic networks maintains their lymphatic nature in the adult fish (termed here “intransigent lymphatics”). However, Das et al. (2022) showed for the anal and dorsal fin of zebrafish that some lymphatics transdifferentiate during the juvenile stage into SVS blood vessels. Jensen et al. (2009) further demonstrated that the SVS is confluent with some parts of the lymphatic vascular system. These key insights explain many previous incomprehensible research results and demonstrate that vascular identity is more fluid than previously thought.
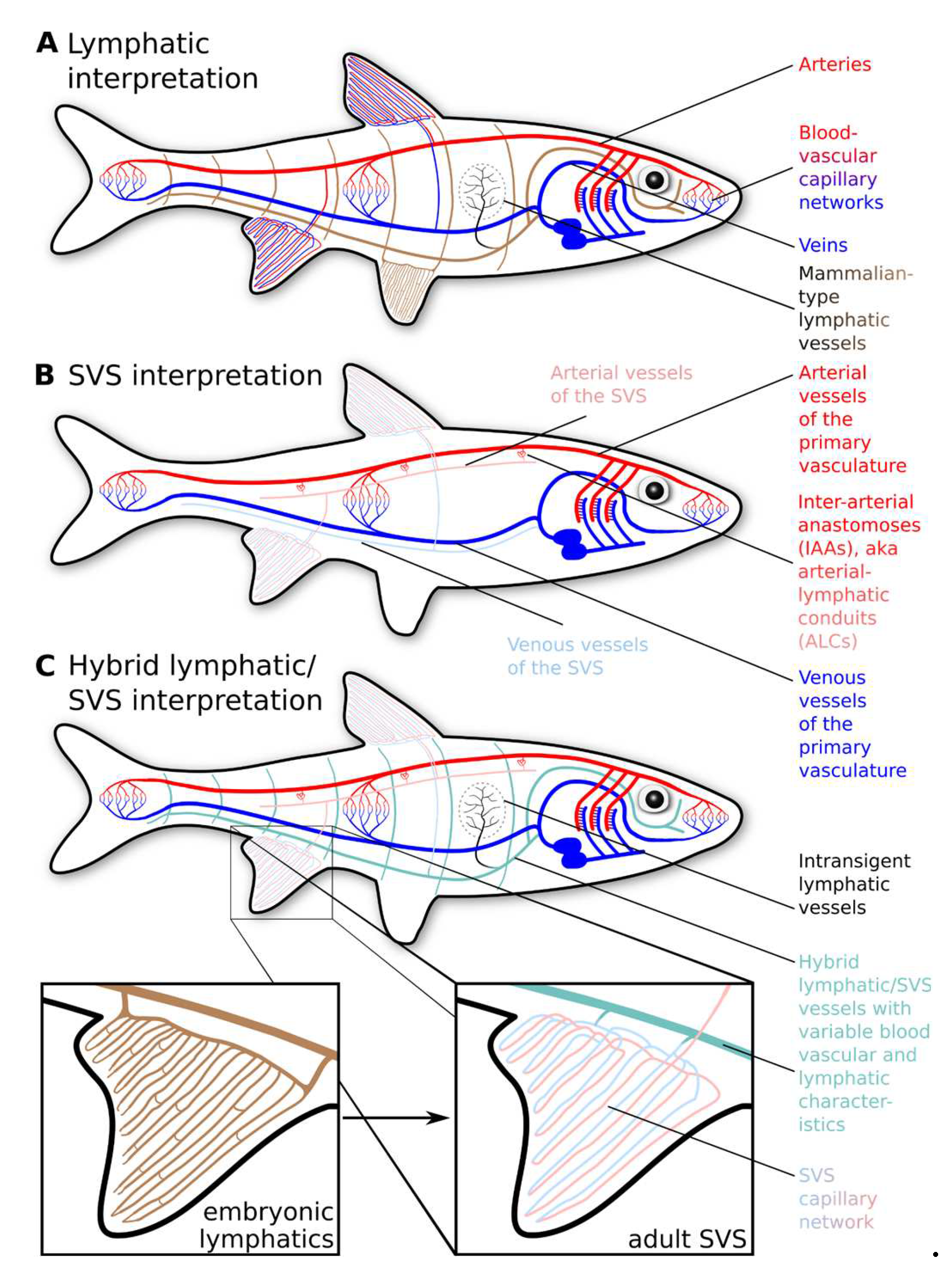
Figure 3.
Phylogenetic tree of chordates. Fish is an informal grouping of animals. From a cladistic point of view, mammals are just a tiny subclade within the larger clade of bony fishes, explaining the much larger morphological diversity among fishes compared to the rest of the vertebrates. All vertebrates feature at least two members of the VEGF family: The hemangiogenic VEGFA and the lymphangiogenic VEGFC. Thus, all vertebrates have the molecular prerequisites for some version of a lymphatic vascular system or SVS. Fish feature the same set of five VEGFs as mammals, except for jawless fishes (Cyclostomata), which lack PlGF, VEGFB, and VEGFD. The figure has been adapted from (Rauniyar, Bokharaie & Jeltsch, 2023) by adding more detailed information about the ray-finned fishes according to (Betancur-R et al., 2017). VGD1: First vertebrate whole-genome duplication. VGD2: Second vertebrate whole-genome duplication. TGD: Teleost whole-genome duplication. SaGD: Salmonid whole-genome duplication.
Figure 3.
Phylogenetic tree of chordates. Fish is an informal grouping of animals. From a cladistic point of view, mammals are just a tiny subclade within the larger clade of bony fishes, explaining the much larger morphological diversity among fishes compared to the rest of the vertebrates. All vertebrates feature at least two members of the VEGF family: The hemangiogenic VEGFA and the lymphangiogenic VEGFC. Thus, all vertebrates have the molecular prerequisites for some version of a lymphatic vascular system or SVS. Fish feature the same set of five VEGFs as mammals, except for jawless fishes (Cyclostomata), which lack PlGF, VEGFB, and VEGFD. The figure has been adapted from (Rauniyar, Bokharaie & Jeltsch, 2023) by adding more detailed information about the ray-finned fishes according to (Betancur-R et al., 2017). VGD1: First vertebrate whole-genome duplication. VGD2: Second vertebrate whole-genome duplication. TGD: Teleost whole-genome duplication. SaGD: Salmonid whole-genome duplication.
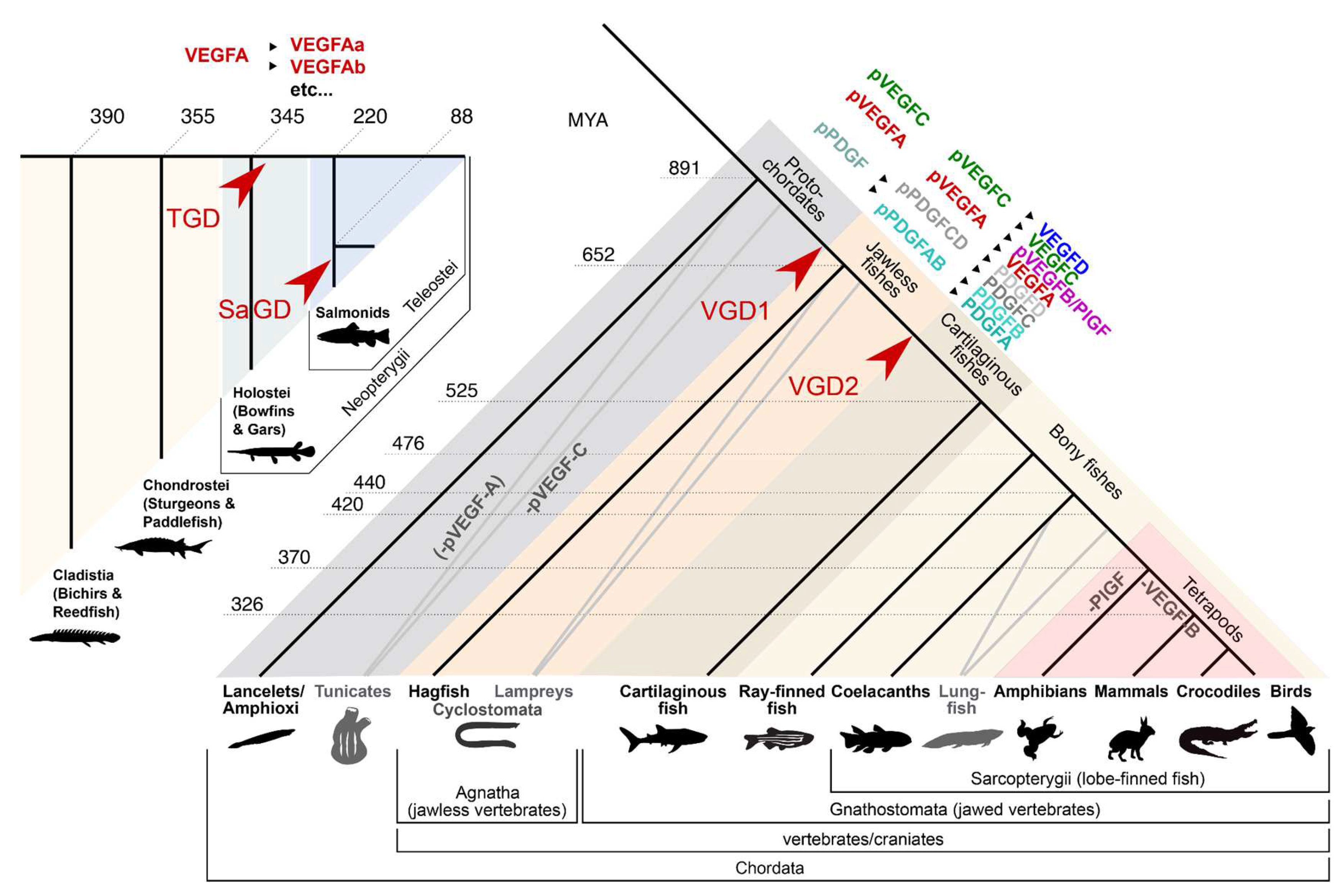
Figure 4.
Simplified schematic of the gill vascular architecture. Apart from the arterio-arterial (respiratory) vascular pathway, two morphologically distinct non-respiratory flow paths can be identified in the gills of ray-finned fishes (but perhaps in modified form also in all other fishes): the interlamellar vessels (shown in green) and the nutrient vessels (shown in magenta). Both originate from anastomoses at different places in the arterial tree. The interlamellar vessels are heterogeneous between different species but display lymphatic-like features (thin, irregular-shaped endothelium, few RBCs, and overlapping cells). While the existence of these vessels is unequivocal, the origin of their fluid content is less clear. Thus, their classification as part of the SVS has been questioned. In most fishes, at least some of the flow originates from post-lamellar anastomoses of the effort filamental artery, as shown in the figure, albeit these anastomoses are not particularly abundant (Olson, 2002). In some ray-finned fishes (Anguilla), the flow has been shown to originate also from prelamellar anastomoses, thus bypassing the lamellae and thus oxygenation (Laurent, Dunel & Barthe, 1976; Hughes et al., 1982; Donald & Ellis, 1983). In still other species, the flow originates from so-called nutrient vessels, which receive their flow in most species via torturous anastomoses from the proximal part of the efferent filament and branchial arteries (Laurent et al., 1976; King & Hossler, 1986; Olson et al., 2003a). However, not all nutrient vessel flow originates from IAAs, and some authors reserve the term “nutrient vessel” for those not derived from IAAs (Skov & Bennett, 2005). The post-lamellar anastomoses – although not tortuous – are also thought to be regulated. Outflow from the nutrient vessels (capillaries) happens, depending on the species, via a separate venous system, or they anastomose into the interlamellar vessels. Due to the large inter-species differences, this figure does not depict the gills of a specific species but is an amalgamation of observations from different species. The figure was drawn based on the description from (Olson, 2002) with the modifications mentioned in this legend. For a summary of the diversity in the anatomy of the gill vasculature, see the introduction in (Skov & Bennett, 2005).
Figure 4.
Simplified schematic of the gill vascular architecture. Apart from the arterio-arterial (respiratory) vascular pathway, two morphologically distinct non-respiratory flow paths can be identified in the gills of ray-finned fishes (but perhaps in modified form also in all other fishes): the interlamellar vessels (shown in green) and the nutrient vessels (shown in magenta). Both originate from anastomoses at different places in the arterial tree. The interlamellar vessels are heterogeneous between different species but display lymphatic-like features (thin, irregular-shaped endothelium, few RBCs, and overlapping cells). While the existence of these vessels is unequivocal, the origin of their fluid content is less clear. Thus, their classification as part of the SVS has been questioned. In most fishes, at least some of the flow originates from post-lamellar anastomoses of the effort filamental artery, as shown in the figure, albeit these anastomoses are not particularly abundant (Olson, 2002). In some ray-finned fishes (Anguilla), the flow has been shown to originate also from prelamellar anastomoses, thus bypassing the lamellae and thus oxygenation (Laurent, Dunel & Barthe, 1976; Hughes et al., 1982; Donald & Ellis, 1983). In still other species, the flow originates from so-called nutrient vessels, which receive their flow in most species via torturous anastomoses from the proximal part of the efferent filament and branchial arteries (Laurent et al., 1976; King & Hossler, 1986; Olson et al., 2003a). However, not all nutrient vessel flow originates from IAAs, and some authors reserve the term “nutrient vessel” for those not derived from IAAs (Skov & Bennett, 2005). The post-lamellar anastomoses – although not tortuous – are also thought to be regulated. Outflow from the nutrient vessels (capillaries) happens, depending on the species, via a separate venous system, or they anastomose into the interlamellar vessels. Due to the large inter-species differences, this figure does not depict the gills of a specific species but is an amalgamation of observations from different species. The figure was drawn based on the description from (Olson, 2002) with the modifications mentioned in this legend. For a summary of the diversity in the anatomy of the gill vasculature, see the introduction in (Skov & Bennett, 2005).
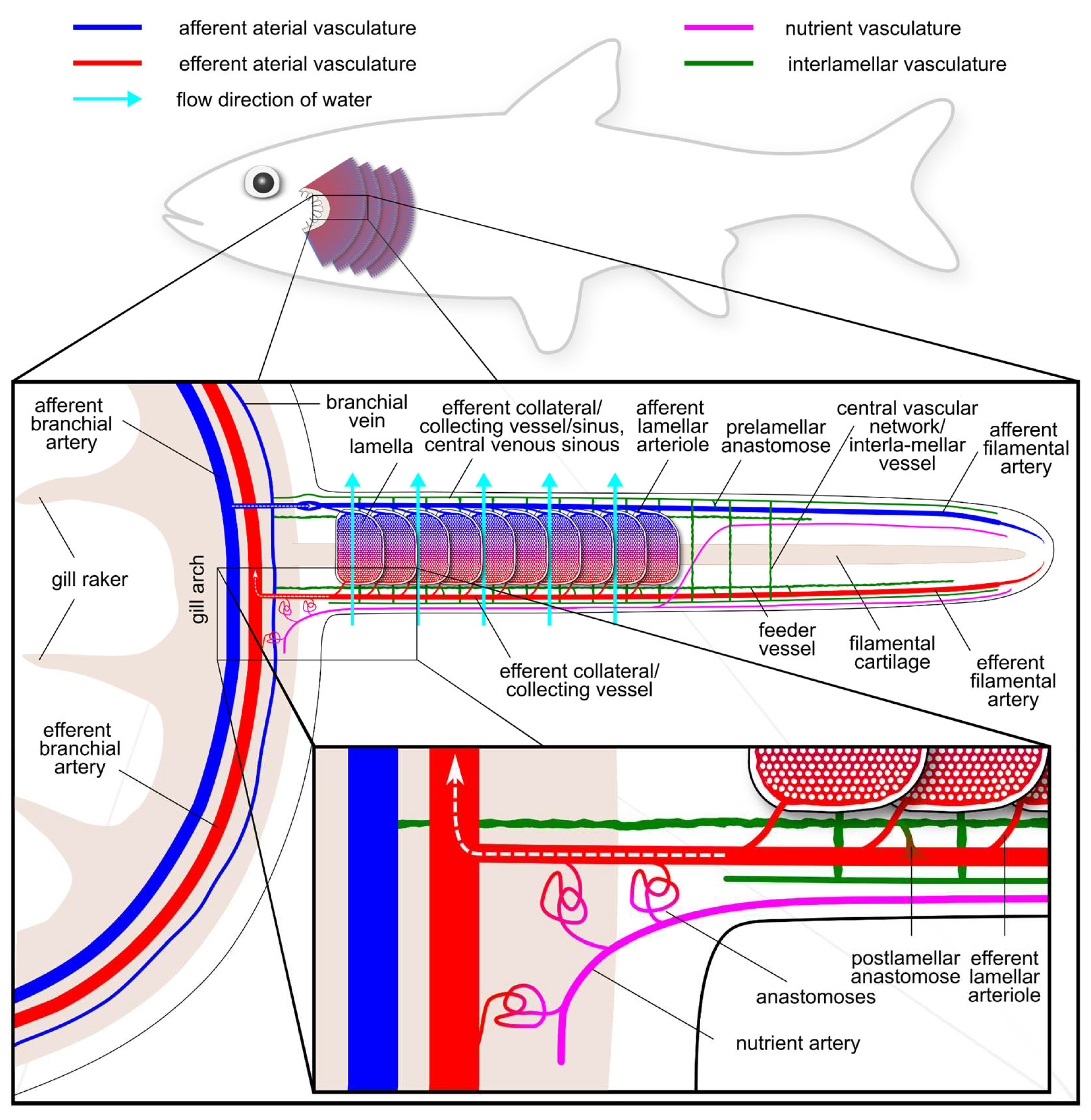
Figure 5.
The similarity between the SVS and the LVS. The SVS (A) can be topologically converted into a mammalian-type lymphatic vascular system (B) by removing the inter-arterial anastomoses. The lymphatics of the anal and dorsal fins of zebrafish transdifferentiate during embryonic development into blood vessels of the SVS, supporting the idea of a common origin. Panel (A) is simplified and adapted from (Vogel, 1985a) and thus considers the non-respiratory vasculature of the gills to be part of the SVS. For the same reason, it includes the caudal heart, which is a vascular organ in mostly larger fishes, characterised by a valved, double-chambered sinus at the base of the tail fin powered mostly by skeletal muscles. Depending on the interpretation, it has been either regarded as part of the SVS or the LVS (Vogel, 1985b). Current data suggests that both SVS and lymphatics coexist, as shown in (C), with some of the collecting vessels participating in both systems. The SVS develops in part or in its entirety by transdifferentiation from an embryonic lymphatic vasculature that appears similar to the mammalian adult LVS. Thus, the mammalian LVS can be considered an example of paedomorphism, an evolutionary phenomenon in which adults retain originally ancestral exclusive juvenile features (Joss, 2006). The vasculature of the gills is more complex and thus has been excluded from the hybrid model (C).
Figure 5.
The similarity between the SVS and the LVS. The SVS (A) can be topologically converted into a mammalian-type lymphatic vascular system (B) by removing the inter-arterial anastomoses. The lymphatics of the anal and dorsal fins of zebrafish transdifferentiate during embryonic development into blood vessels of the SVS, supporting the idea of a common origin. Panel (A) is simplified and adapted from (Vogel, 1985a) and thus considers the non-respiratory vasculature of the gills to be part of the SVS. For the same reason, it includes the caudal heart, which is a vascular organ in mostly larger fishes, characterised by a valved, double-chambered sinus at the base of the tail fin powered mostly by skeletal muscles. Depending on the interpretation, it has been either regarded as part of the SVS or the LVS (Vogel, 1985b). Current data suggests that both SVS and lymphatics coexist, as shown in (C), with some of the collecting vessels participating in both systems. The SVS develops in part or in its entirety by transdifferentiation from an embryonic lymphatic vasculature that appears similar to the mammalian adult LVS. Thus, the mammalian LVS can be considered an example of paedomorphism, an evolutionary phenomenon in which adults retain originally ancestral exclusive juvenile features (Joss, 2006). The vasculature of the gills is more complex and thus has been excluded from the hybrid model (C).

Figure 6.
The secondary vascular system/lymphatic vasculature of the anal fin in glass catfish (Kryptopterus bicirrhis). In glass catfish, the anal fin is large and extends over most of the body except for the head region. Similar to the description by Das et al., the larger (secondary) vessels run alongside the fin-supporting bones. The connection to the arterial blood flow happens at the level of the segmental artery via inter-arterial anastomoses (IAAs). However, the secondary network serves only the distal anal fin, while the primary vasculature serves the proximal anal fin. The left magnified insert (A) shows the SVS interpretation, according to Steffensen et al. (1986), while the right insert (B) shows the lymphatic interpretation, according to Jensen et al. (2009). Jensen shows that these anatomic structures are not unique to glass catfish but are also found in zebrafish. Red blood cell (RBC) entry into the anal fin vasculature is controlled via nitric oxide-mediated opening and linearisation of the IAAs/ALCs and leads to RBCs filling the collecting lymphatic vessels and the thoracic duct, which, differently from the transdifferentiating anal fin lymphatics (Das et al., 2022) maintained Prox1 expression also in the adult. The figure is based on (Steffensen et al., 1986; Jensen et al., 2009).
Figure 6.
The secondary vascular system/lymphatic vasculature of the anal fin in glass catfish (Kryptopterus bicirrhis). In glass catfish, the anal fin is large and extends over most of the body except for the head region. Similar to the description by Das et al., the larger (secondary) vessels run alongside the fin-supporting bones. The connection to the arterial blood flow happens at the level of the segmental artery via inter-arterial anastomoses (IAAs). However, the secondary network serves only the distal anal fin, while the primary vasculature serves the proximal anal fin. The left magnified insert (A) shows the SVS interpretation, according to Steffensen et al. (1986), while the right insert (B) shows the lymphatic interpretation, according to Jensen et al. (2009). Jensen shows that these anatomic structures are not unique to glass catfish but are also found in zebrafish. Red blood cell (RBC) entry into the anal fin vasculature is controlled via nitric oxide-mediated opening and linearisation of the IAAs/ALCs and leads to RBCs filling the collecting lymphatic vessels and the thoracic duct, which, differently from the transdifferentiating anal fin lymphatics (Das et al., 2022) maintained Prox1 expression also in the adult. The figure is based on (Steffensen et al., 1986; Jensen et al., 2009).
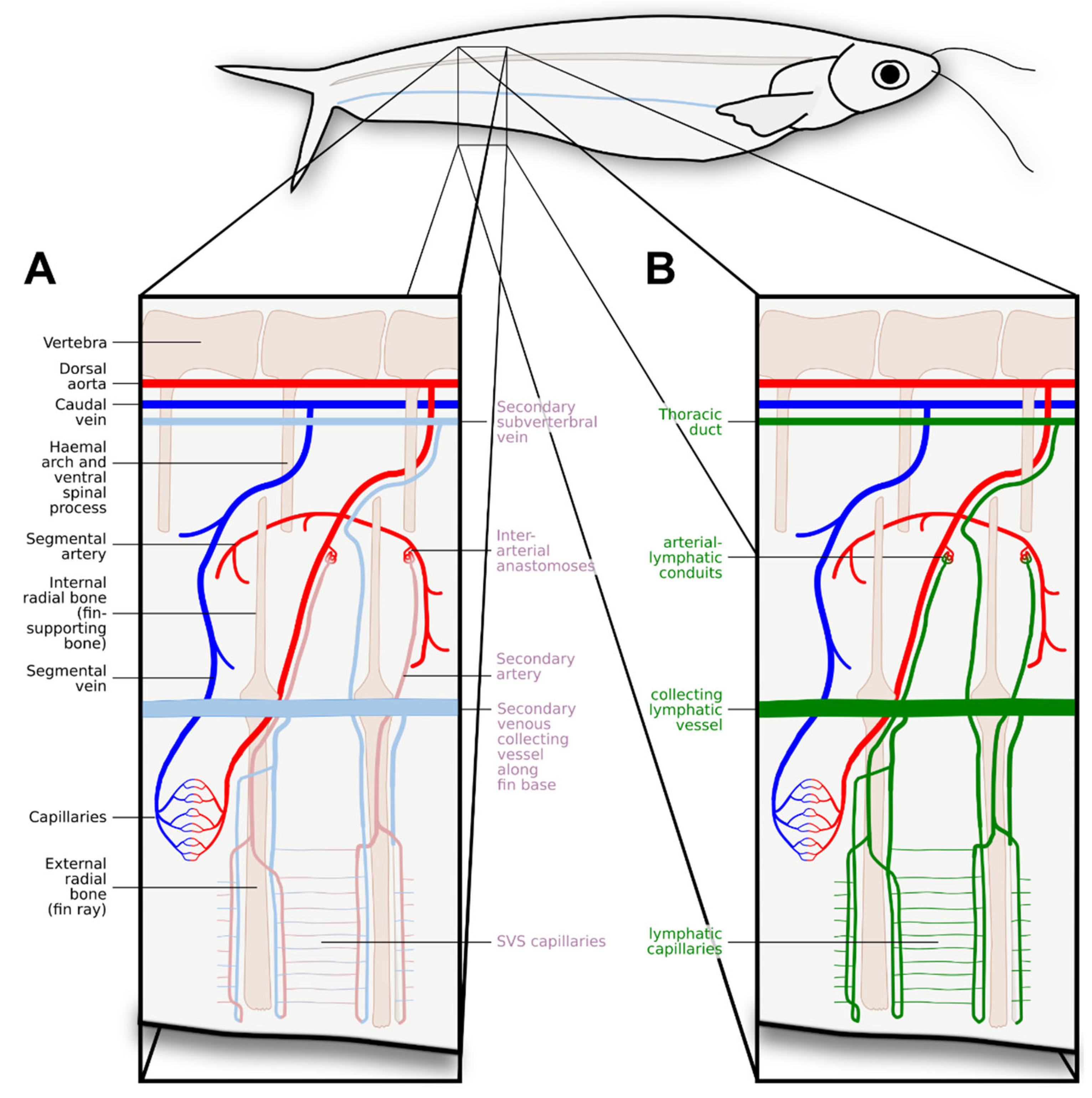
Figure 7.
(
A)
Schematic comparison of VEGFR signalling between zebrafish, mice, and humans. While in mammals, the generation of the short, soluble VEGFA isoforms is supported by differential mRNA splicing, zebrafish has undergone a duplication of its
vegfa gene and produces the short isoforms from a different gene than the long isoforms (Bahary
et al., 2007; Rossi
et al., 2016). Compared to mice, in which VEGFD exclusively interacts with VEGFR3 (Baldwin
et al., 2001), zebrafish Vegfd might exclusively interact with VEGFR2 (Vogrin
et al., 2019), while human VEGFD can interact with both receptors (Achen
et al., 1998). While there are only three VEGF receptor genes in mice and humans due to a deletion in the eutherian lineage, zebrafish feature the entire repertoire of four VEGF receptors (Bussmann
et al., 2008). (
B)
Phylogenetic tree of the four VEGF receptors with reliability bootstrap values indicated by colour. The VEGFR2/Kdr and VEGFR3/Flt4 branches share a common evolutionary history on the upper half, while the VEGFR1/Flt1 and VEGFR4/Kdrl do likewise on the lower half of the unrooted consensus tree with reliability bootstraps values of 95.9% and 97.2% for the VEGFR2/3 and VEGFR1/VEGFR4 branching, respectively. This strongly suggests that the closest relative of VEGFR2 is VEGFR3 and not VEGFR4/Kdrl. Note that eutherian mammals are entirely absent from the VEGFR4/Kdrl branch. This tree is based on the analysis available from
https://github.com/mjeltsch/vegfr and is compatible with Bussmann
et al. (2008).
Figure 7.
(
A)
Schematic comparison of VEGFR signalling between zebrafish, mice, and humans. While in mammals, the generation of the short, soluble VEGFA isoforms is supported by differential mRNA splicing, zebrafish has undergone a duplication of its
vegfa gene and produces the short isoforms from a different gene than the long isoforms (Bahary
et al., 2007; Rossi
et al., 2016). Compared to mice, in which VEGFD exclusively interacts with VEGFR3 (Baldwin
et al., 2001), zebrafish Vegfd might exclusively interact with VEGFR2 (Vogrin
et al., 2019), while human VEGFD can interact with both receptors (Achen
et al., 1998). While there are only three VEGF receptor genes in mice and humans due to a deletion in the eutherian lineage, zebrafish feature the entire repertoire of four VEGF receptors (Bussmann
et al., 2008). (
B)
Phylogenetic tree of the four VEGF receptors with reliability bootstrap values indicated by colour. The VEGFR2/Kdr and VEGFR3/Flt4 branches share a common evolutionary history on the upper half, while the VEGFR1/Flt1 and VEGFR4/Kdrl do likewise on the lower half of the unrooted consensus tree with reliability bootstraps values of 95.9% and 97.2% for the VEGFR2/3 and VEGFR1/VEGFR4 branching, respectively. This strongly suggests that the closest relative of VEGFR2 is VEGFR3 and not VEGFR4/Kdrl. Note that eutherian mammals are entirely absent from the VEGFR4/Kdrl branch. This tree is based on the analysis available from
https://github.com/mjeltsch/vegfr and is compatible with Bussmann
et al. (2008).
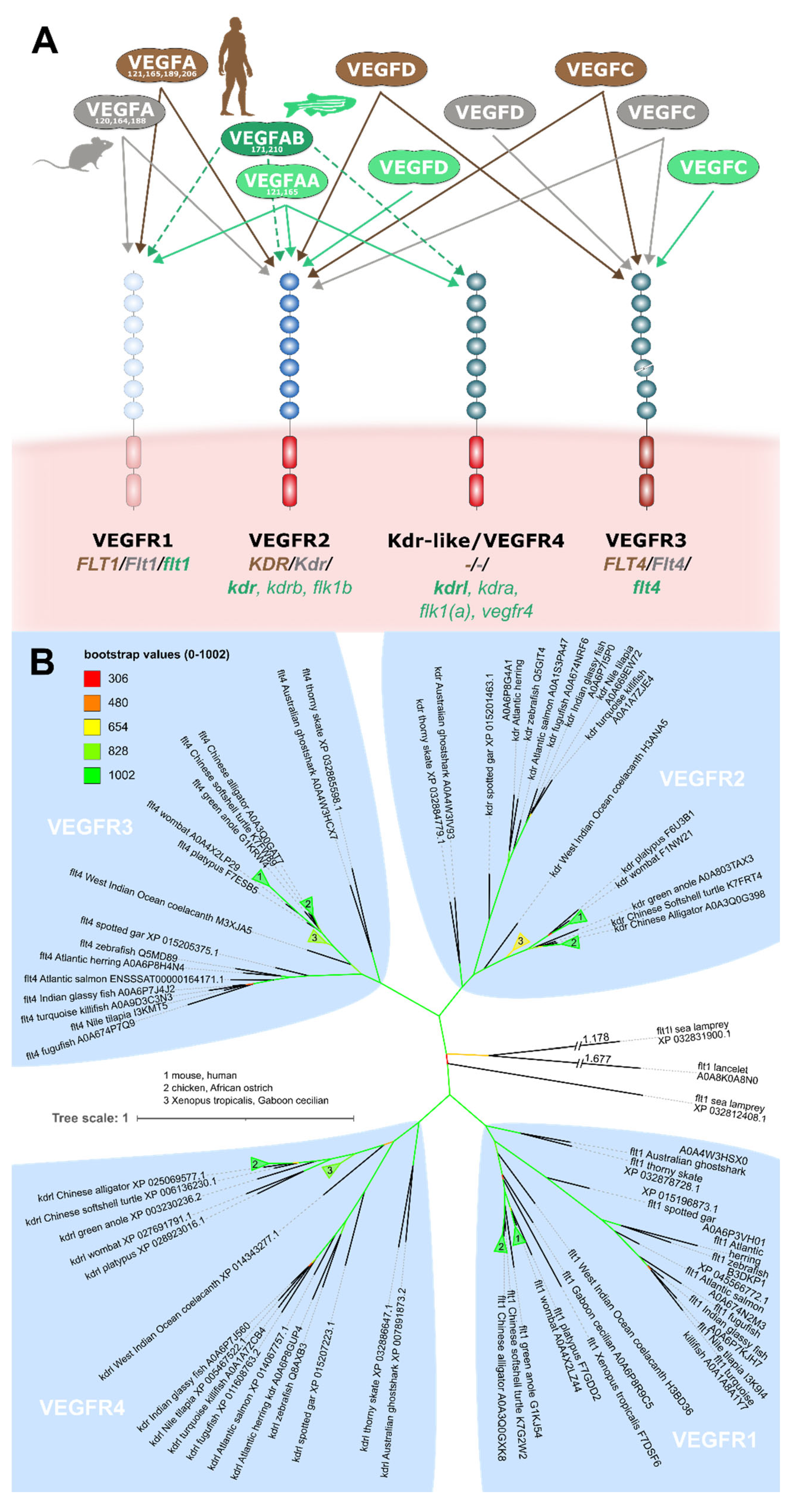
Figure 8.
The proposed model of SVS/lymphatic evolution. A phylogenetic arrangement in which lung-fish are the sister group to tetrapods and jawless fishes are a monophyletic group is assumed. Gill SVS are postulated to appear in stem vertebrates, in association with the increase in body size, followed by a systemic SVS appearing either before or after the split between jawed and jawless vertebrates, depending on the presence of a systemic SVS in the latter. The emergence of an AIS in the lineage leading to jawed vertebrates created the need for a lymphatic-like vasculature, which emerges either before or after the split between bony and cartilaginous fishes, depending on the accuracy of the reports of deep lymphatics in the latter. The AIS of jawless fishes is not homologous with the AIS of jawed fish, but is the result of convergent evolution. In the lineage leading to lung-fishes and tetrapods, the SVS was lost, and the lymphatic vasculature took on a defined “mammalian-like” organisation. As represented in this figure, missing and contradictory data on the SVS and lymphatic organisation can be found in several vertebrate groups, and further research will be needed to confirm or rebuke the proposed model. AIS: adaptive immune system. LVS: lymphatic vascular system. SVS: secondary vascular system.
Figure 8.
The proposed model of SVS/lymphatic evolution. A phylogenetic arrangement in which lung-fish are the sister group to tetrapods and jawless fishes are a monophyletic group is assumed. Gill SVS are postulated to appear in stem vertebrates, in association with the increase in body size, followed by a systemic SVS appearing either before or after the split between jawed and jawless vertebrates, depending on the presence of a systemic SVS in the latter. The emergence of an AIS in the lineage leading to jawed vertebrates created the need for a lymphatic-like vasculature, which emerges either before or after the split between bony and cartilaginous fishes, depending on the accuracy of the reports of deep lymphatics in the latter. The AIS of jawless fishes is not homologous with the AIS of jawed fish, but is the result of convergent evolution. In the lineage leading to lung-fishes and tetrapods, the SVS was lost, and the lymphatic vasculature took on a defined “mammalian-like” organisation. As represented in this figure, missing and contradictory data on the SVS and lymphatic organisation can be found in several vertebrate groups, and further research will be needed to confirm or rebuke the proposed model. AIS: adaptive immune system. LVS: lymphatic vascular system. SVS: secondary vascular system.
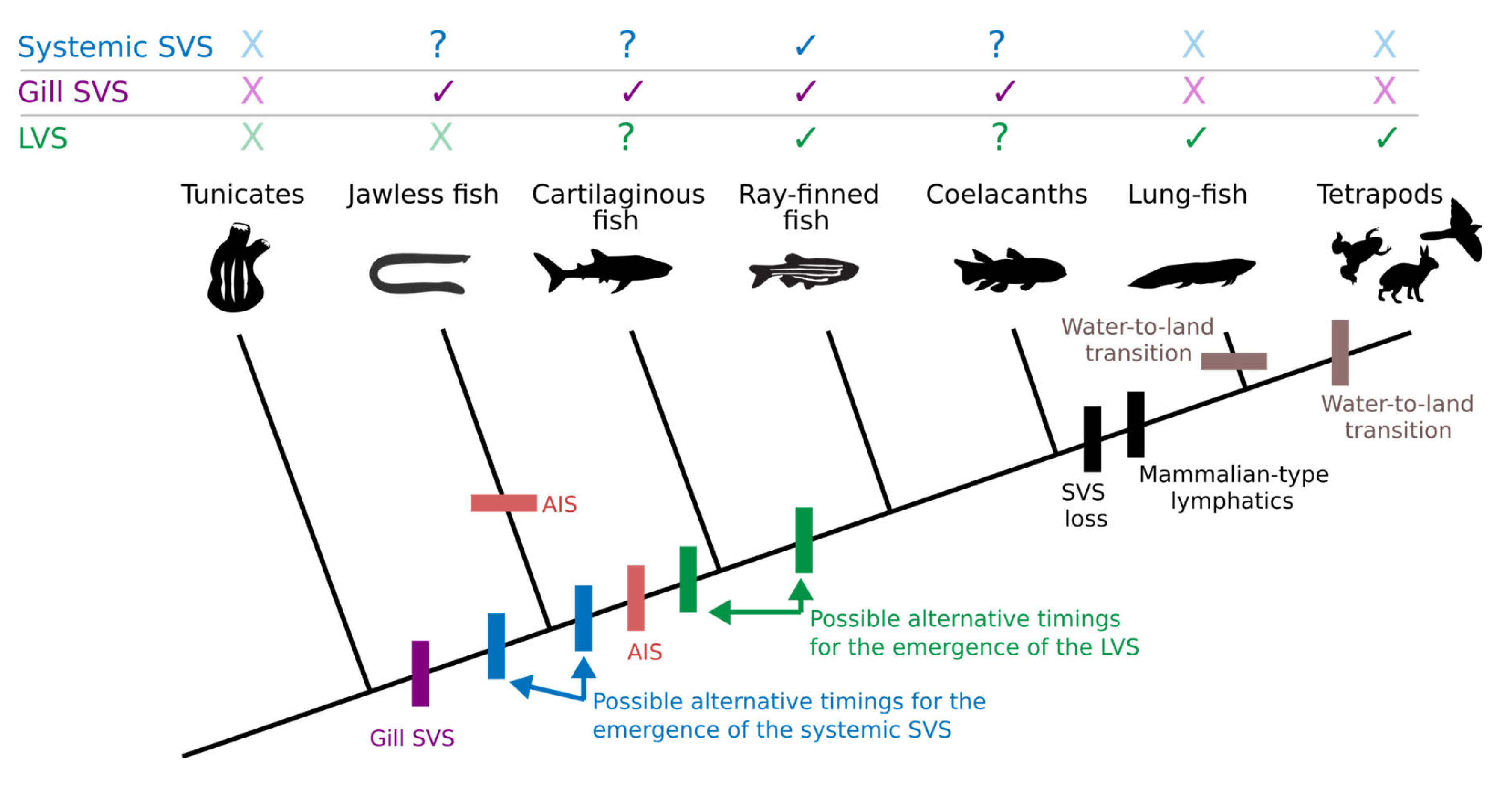
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).