Submitted:
27 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
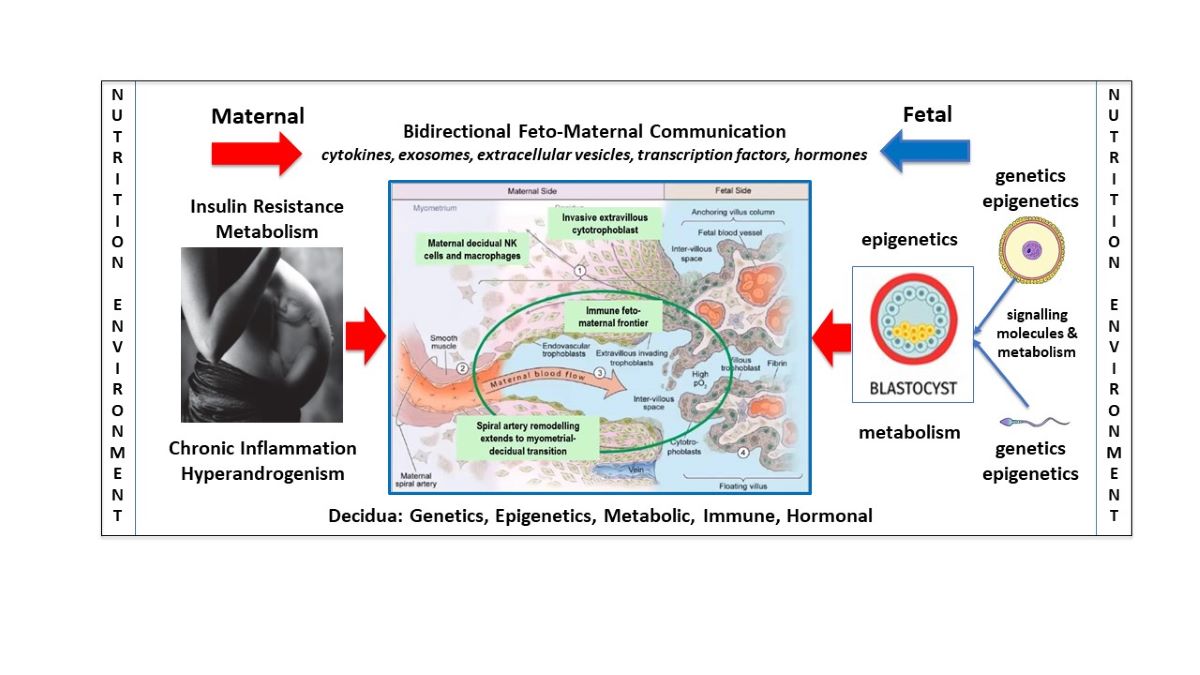
Keywords:
1. Introduction
2. Evidence for Increased Risk of PE in Women with PCOS
3. Evidence for the Role of Nutritional Factors in the Pathophysiology of PE
4. Mechanisms of Action of Nutritional Factors in the Pathophysiology of PE
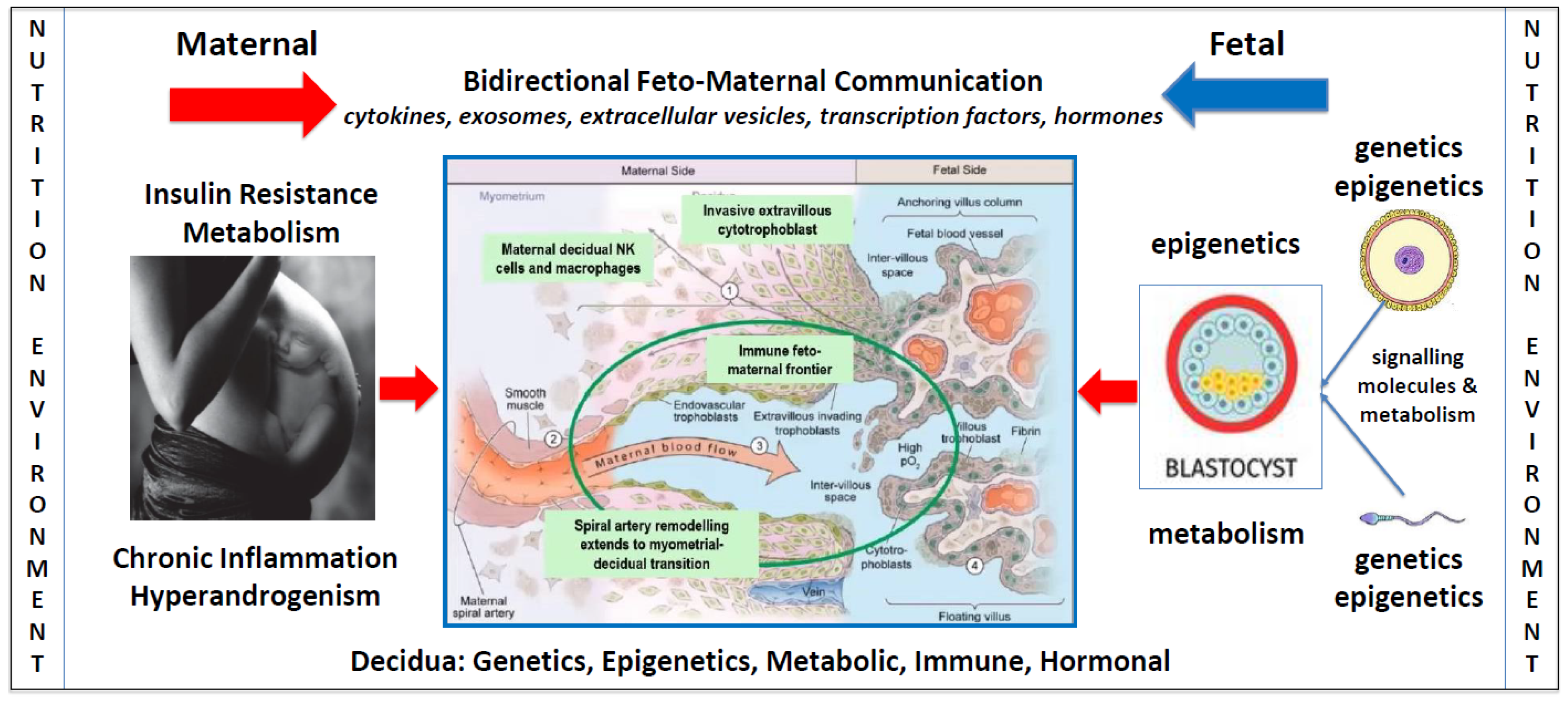
4.1. Insulin Resistance
4.2. Chronic Systemic Inflammation
4.3. Hyperandrogenism
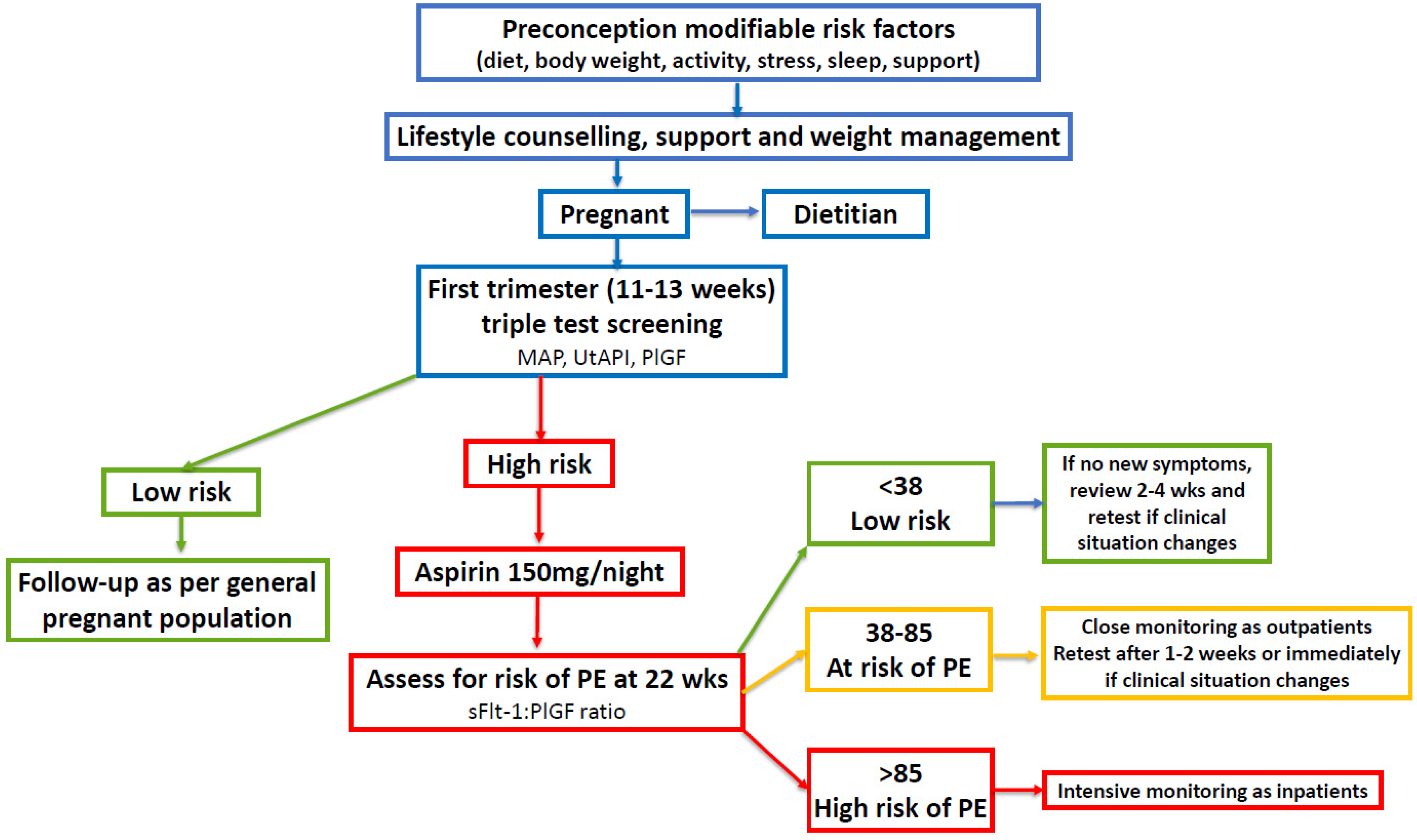
5. Identification, Assessment, and Management of Women with PCOS in Pregnancy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Parker, J.; O’brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int J Environ Res Public Health. 2022, 19, 1336. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Chazenbalk, G.D.; Scholar, G. An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome. J Clin Med. 2023, 12, 1–16. [Google Scholar]
- Parker, J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life. 2023, 13, 1056. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Padmanabhan, V.; Chazenbalk, G.D.; Abbott, D.H. Polycystic ovary syndrome as a plausible evolutionary outcome of metabolic adaptation. Reprod Biol Endocrinol [Internet]. 2022, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H.; Sanchita, S.; Chazenbalk, G.D. Endocrine–metabolic dysfunction in polycystic ovary syndrome: an evolutionary perspective. Curr Opin Endocr Metab Res [Internet]. 2020, 12, 41–48. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C. Evolutionary and genetic antecedents to the pathogenesis of polycystic ovary syndrome (PCOS). J ACNEM. 2021, 40, 12–20. [Google Scholar]
- Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013, 28, 777–784. [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.D.; Amer, S. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int J Mol Sci. 2021, 22, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int J Mol Sci. 2022, 23. [Google Scholar] [CrossRef]
- Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—A systematic review, meta-analysis, and meta-regression. Obes Rev. 2019, 20, 659–674. [CrossRef]
- Zahid S, Khan MZ, Gowda S, Faza NN, Honigberg MC, Vaught A, et al. Trends, Predictors, and Outcomes of Cardiovascular Complications Associated With Polycystic Ovary Syndrome During Delivery Hospitalizations: A National Inpatient Sample Analysis (2002–2019). J Am Heart Assoc. 2022, 11, 1–12.
- Elawad T, Scott G, Bone JN, Elwell H, Lopez CE, Filippi V, et al. Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG An Int J Obstet Gynaecol. 2022;(September):1–17.
- Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, et al. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019, 104, 2171–2183.
- Brennan, K.M.; Kroener, L.L.; Chazenbalk, G.D.; Dumesic, D.A. Polycystic Ovary Syndrome: Impact of Lipotoxicity on Metabolic and Reproductive Health. Obstet Gynecol Surv. 2019, 74, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; Dumesic, D.A.; Franks, S. Developmental origin of polycystic ovary syndrome - A hypothesis. J Endocrinol. 2002, 174, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Hoyos, L.R.; Chazenbalk, G.D.; Naik, R.; Padmanabhan, V.; Abbott, D.H. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction. 2019, 159, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; Dumesic, D.A.; Abbott, D.H. Fetal androgen excess provides a developmental origin for polycystic ovary syndrome. Expert Rev Obs Gynecol. 2009, 4, 1–7. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Gersh, F.L. Developmental origins and transgenerational inheritance of polycystic ovary syndrome. Aust New Zeal J Obstet Gynaecol. 2021, 61, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.L. An unwelcome inheritance: childhood obesity after diabetes in pregnancy. Diabetologia [Internet]. 2023, 66, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Zore, T.; Joshi, N.V.; Lizneva, D.; Azziz, R. Polycystic Ovarian Syndrome: Long-Term Health Consequences. Vol. 35, Seminars in Reproductive Medicine. 2017. p. 271–81.
- Reyes-Muñoz E, Castellanos-Barroso G, Ramírez-Eugenio BY, Ortega-González C, Parra A, Castillo-Mora A, et al. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil Steril. 2012, 97, 1467–1471. [CrossRef]
- Rodgers RJ, Avery JC, Moore VM, Davies MJ, Azziz R, Stener-Victorin E, et al. Complex diseases and co-morbidities: Polycystic ovary syndrome and type 2 diabetes mellitus. Endocr Connect. 2019, 8, R71–5. [CrossRef]
- Wu, J.; Yao, X.Y.; Shi, R.X.; Liu, S.F.; Wang, X.Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: An update meta-analysis. Reprod Health. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; De Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015, 21, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Helena Teede, Chau Thien Tay JL, Anuja Dokras, Lisa Moran TP, Michael Costello JB, Leanne Redman JB, Robert Norman, Aya Mousa AJ. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023. Natl Heal Med Res Counc. 2023;1–258.
- Du, Y.; Li, F.; Li, S.; Ding, L.; Liu, M. Causal relationship between polycystic ovary syndrome and chronic kidney disease : A Mendelian randomization study. Front Endocrinol (Lausanne). 2023, 14, 1120119. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, C.M.; Eijkemans, M.J.C.; Hughes, E.G.; Visser, G.H.A.; Fauser, B.C.J.M.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006, 12, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Kjerulff, L.E.; Sanchez-Ramos, L.; Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am J Obstet Gynecol [Internet]. 2011, 204, 558–e1. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Pang, L.H.; Li, M.J.; Fan, X.J.; Huang, R.D.; Chen, H.Y. Obstetric complications in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod Biol Endocrinol. 2013, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.F.; Chen, H.S.; Rao, D.P.; Gong, J. Association between polycystic ovary syndrome and the risk of pregnancy complications A PRISMA-compliant systematic review and meta-analysis. Med (United States). 2016, 95, e4863. [Google Scholar]
- Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens [Internet]. 2022;27(October 2021):148–69. Available from: https://doi.org/10.1016/j.preghy.2021.09.008. [CrossRef]
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018, 72, 24–43. [CrossRef] [PubMed]
- Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet. 2019;145(S1):1–33.
- Verlohren, S.; Dröge, L.A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am J Obstet Gynecol [Internet]. 2022, 226, S1048–58. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med. 2019, 8, 1–22. [Google Scholar] [CrossRef]
- Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics. 2012, 129, e1552–e1561. [CrossRef]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: A review. J Am Coll Cardiol [Internet]. 2014, 63, 1815–1822. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. The human placenta: new perspectives on its formation and function during early pregnancy. Proc R Soc B Biol Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Prim. 2023, 9, 1–22.
- Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021, 398, 341–354.
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes—a molecular link for altered immunoregulation and inflammation mediated vascular dysfunction in preeclampsia. Int J Mol Sci. 2020, 21, 1406. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Mauro, M.; Williams, Z. Direct toxicity of insulin on the human placenta and protection by metformin. Fertil Steril [Internet]. 2019, 111, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sun M, Sun B, Qiao S, Feng X, Li Y, Zhang S, et al. Elevated maternal androgen is associated with dysfunctional placenta and lipid disorder in newborns of mothers with polycystic ovary syndrome. Fertil Steril [Internet]. 2020, 113, 1275–1285. [CrossRef] [PubMed]
- Myers, J.E. What are the metabolic precursors which increase the risk of pre-eclampsia and how could these be investigated further. Placenta [Internet]. 2017, 60, 110–114. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Baker, P.N.; Tong, C. Revisiting preeclampsia: a metabolic disorder of the placenta. FEBS J. 2022, 289, 336–354. [Google Scholar] [CrossRef]
- Parker, J.; Hawrelak, J.; Gersh, F.L. Nutritional role of polyphenols as a component of a wholefood diet in the management of polycystic ovary syndrome. J ACNEM. 2021, 40, 6–12. [Google Scholar]
- Kinshella MLW, Pickerill K, Bone JN, Prasad S, Campbell O, Vidler M, et al. An evidence review and nutritional conceptual framework for pre-eclampsia prevention. Br J Nutr. 2023, 130, 1065–1076. [CrossRef] [PubMed]
- Bahri Khomami M, Moran LJ, Kenny L, Grieger JA, Myers J, Poston L, et al. Lifestyle and pregnancy complications in polycystic ovary syndrome: The SCOPE cohort study. Clin Endocrinol (Oxf). 2019, 90, 814–821. [CrossRef] [PubMed]
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017, 377, 613–622. [CrossRef] [PubMed]
- Pan H, Xian P, Yang D, Zhang C, Tang H, He X, et al. Polycystic ovary syndrome is an independent risk factor for hypertensive disorders of pregnancy: A systematic review, meta-analysis, and meta-regression. Endocrine. 2021, 74, 518–529. [CrossRef] [PubMed]
- Riestenberg, C.; Jagasia, A.; Markovic, D.; Buyalos, R.P.; Azziz, R. Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences. J Clin Endocrinol Metab. 2022, 107, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A; Tay, CT; Teede H. Technical Report for the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Monash University; 2023.
- Verlohren S, Brennecke SP, Galindo A, Karumanchi SA, Mirkovic LB, Schlembach D, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens [Internet]. 2022;27(August 2021):42–50. Available from: https://doi.org/10.1016/j.preghy.2021.12.003. [CrossRef]
- Wright, D.; Wright, A.; Nicolaides, K.H. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol [Internet]. 2020, 223, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Serrano B, Bonacina E, Rodo C, Garcia-Manau P, Sanchez-Duran MÁ, Pancorbo M, et al. First-trimester screening for pre-eclampsia and small for gestational age: A comparison of the gaussian and Fetal Medicine Foundation algorithms. Int J Gynecol Obstet. 2023, 160, 150–160. [CrossRef] [PubMed]
- ACOG Low-Dose Aspirin Use during Pregnancy. Am Coll Obstet Gynecol. 2018, 132, E44–52. [CrossRef]
- Kane, S.C.; Da Silva Costa, F. Risk factors for pre-eclampsia: Received wisdom versus reality. BJOG An Int J Obstet Gynaecol. 1732. [Google Scholar]
- Bahri Khomami, M.; Teede, H.J.; Joham, A.E.; Moran, L.J.; Piltonen, T.T.; Boyle, J.A. Clinical management of pregnancy in women with polycystic ovary syndrome: An expert opinion. Clin Endocrinol (Oxf). 2022, 97, 227–236. [Google Scholar] [CrossRef]
- Howeler, J.F. Hewson A. Dietary Fibre and Toxaemia of Pregnancy. Med J Aust. 1957;761–3.
- Hipsley, E. Dietary Fibre and Pregnancy Toxaemia. Br Med J. 1953;22 August:420–4222.
- Harding, V.J.; Van Wyck, H.B. Diet in the Treatment of Pre-Eclampsia. J Obstet Gynaecol. 1926, 33, 17–32. [Google Scholar] [CrossRef]
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [CrossRef] [PubMed]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol [Internet]. 2023, 189, G43–64. [CrossRef]
- Traore SS, Bo Y, Amoah AN, Khatun P, Kou G, Hu Y, et al. A meta-analysis of maternal dietary patterns and preeclampsia. Clin Nutr Open Sci [Internet]. 2021, 40, 15–29. [CrossRef]
- Perry, A.; Stephanou, A.; Rayman, M.P. Dietary factors that affect the risk of pre-eclampsia. BMJ Nutr Prev Heal. 2022, 5, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2019, 22, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.O.; Patriota, E.S.O.; Gonçalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2022, 14, 3242. [Google Scholar] [CrossRef] [PubMed]
- APant SGribbin PMachado AHodge LMoran SMarschner, S.Z. Association of ultra-processed foods with cardiovascular disease and hypertension in australian women. In: European Heart Journal [Internet]. Available from. [CrossRef]
- Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health. Circ Cardiovasc Qual Outcomes. 2017, 10, 1–9.
- Tuncalp Ö, Rogers LM, Lawrie TA, Barreix M, Peña-Rosas JP, Bucagu M, et al. WHO recommendations on antenatal nutrition: an update on multiple micronutrient supplements. BMJ Glob Heal. 2022, 5, 3–6.
- Marshall NE, Abrams B, Barbour LA, Catalano P, Christian P, Friedman JE, et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am J Obstet Gynecol [Internet]. 2022, 226, 607–632. [CrossRef]
- Grieger JA, Grzeskowiak LE, Bianco-Miotto T, Jankovic-Karasoulos T, Moran LJ, Wilson RL, et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum Reprod. 2018, 33, 1063–1070. [CrossRef] [PubMed]
- Sampathkumar, S.; Parkhi, D.; Ghebremichael-Weldeselassie, Y.; Sukumar, N.; Saravanan, P. Effectiveness of pre-pregnancy lifestyle in preventing gestational diabetes mellitus—a systematic review and meta-analysis of 257,876 pregnancies. Nutr Diabetes. 2023, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Erickson ML, Mey JT, Axelrod CL, Paul D, Gordesky L, Russell K, et al. Rationale and study design for lifestyle intervention in preparation for pregnancy (LIPP): A randomized controlled trial. Contemp Clin Trials [Internet]. 2020;94(May):106024. Available from: https://doi.org/10.1016/j.cct.2020.106024. [CrossRef]
- Gustaw Eriksson, Congru Li, Sanjiv Risal, Han-Pin Pui, Sara Torstensson, Angelica Linden Hirschberg, Sophie Petropoulos, Qiaolin Deng ES-V. Mapping Endometrial Cell-type-specific Disease Signatures A nd Endometrial Organoids In Polycystic Ovary Syndrome. J Endocr Soc. 2023;Oct 5;7(Suppl 1):bvad114.1587.
- Gustaw Eriksson, Congru Li, Sanjiv Risal, Han-Pin Pui ST, Angelica Linden Hirschberg, Sophie Petropoulos, Qiaolin Deng ES-V. Mapping Endometrial Cell-type-specific Disease Signatures And Endometrial Organoids In Polycystic Ovary Syndrome. J Endocr Soc. 2023;7(Supplement):A848.
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol [Internet]. 2022, 226, S907–27. [Google Scholar] [CrossRef] [PubMed]
- Schjenken JE, Sharkey DJ, Green ES, Chan HY, Matias RA, Moldenhauer LM, et al. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun Biol. 2021, 4, 1–14.
- Robertson, S.A.; Prins, J.R.; Sharkey, D.J.; Moldenhauer, L.M. Seminal Fluid and the Generation of Regulatory T Cells for Embryo Implantation. Am J Reprod Immunol. 2013, 69, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Taylor, R.N. Genetic factors in the etiology of preeclampsia/eclampsia [Internet]. Fourth Edi. Chesley’s Hypertensive Disorders in Pregnancy, Fourth Edition. Elsevier Inc.; p. Available from. [CrossRef]
- Christians, J.K.; Leavey, K.; Cox, B.J. Associations between imprinted gene expression in the placenta, human fetal growth and preeclampsia. Biol Lett. 2017, 13, 20170643. [Google Scholar] [CrossRef]
- Ashraf, U.M.; Hall, D.L.; Rawls, A.Z.; Alexander, B.T. Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin Sci. 2021, 135, 2307–2327. [Google Scholar] [CrossRef] [PubMed]
- Neubrand L, Pothmann H, Besenfelder U, Havlicek V, Gabler C, Dolezal M, et al. In vivo dynamics of pro - inflammatory factors , mucins , and polymorph nuclear neutrophils in the bovine oviduct during the follicular and luteal phase. Sci Rep [Internet]. 2023;1–14. Available from: https://doi.org/10.1038/s41598-023-49151-9. [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Turco, M.Y. Review: Histotrophic nutrition and the placental-endometrial dialogue during human early pregnancy. Placenta [Internet]. 2020;102(February):21–6. Available from: https://doi.org/10.1016/j.placenta.2020.02.008. [CrossRef]
- O’Brien, K.; Wang, Y. The Placenta: A Maternofetal Interface. Annu Rev Nutr. 2023, 43, 301–325. [Google Scholar] [CrossRef]
- Conrad, K.P.; Rabaglino, M.B.; Post Uiterweer, E.D. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta [Internet]. 2017, 60, 119–129. [Google Scholar] [CrossRef]
- Rabaglino, M.B.; Conrad, K.P. Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration. FASEB J. 2019, 33, 11682–11695. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal hyperlipidemia and the risk of preeclampsia: A meta-analysis. Am J Epidemiol. 2014, 180, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Tippetts, T.S.; Sieber, M.H.; Solmonson, A. Beyond energy and growth: the role of metabolism in developmental signaling, cell behavior and diapause. Development. 2023, 150, dev201610. [Google Scholar] [CrossRef] [PubMed]
- Kamrani A, Alipourfard I, Ahmadi-Khiavi H, Yousefi M, Rostamzadeh D, Izadi M, et al. The role of epigenetic changes in preeclampsia. BioFactors. 2019, 45, 712–724.
- Apicella, C.; Ruano, C.S.M.; Méhats, C.; Miralles, F.; Vaiman, D. The role of epigenetics in placental development and the etiology of preeclampsia. Int J Mol Sci. 2019, 20, 2837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The impact of hypoxia in early pregnancy on placental cells. Int J Mol Sci.
- Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, et al. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim Biophys Acta - Mol Basis Dis [Internet]. 2020, 1866, 165354. [CrossRef]
- Gómez de Cedrón, M.; Moreno Palomares, R.; Ramírez de Molina, A. Metabolo-epigenetic interplay provides targeted nutritional interventions in chronic diseases and ageing. Front Oncol. 2023;13(June):1–20.
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet [Internet]. 2020, 21, 782. [Google Scholar] [CrossRef] [PubMed]
- Doshani, A.; Konje, J.C. Placental dysfunction in obese women and antenatal surveillance. Best Pract Res Clin Obstet Gynaecol [Internet]. 2023, 91, 102407. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, et al. Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review. Lifestyle Genomics. 2019;12(1–6):25–44.
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin Epigenetics. 2012, 4, 1–7. [Google Scholar] [CrossRef]
- Abraham E, Rousseaux S, Agier L, Giorgis-Allemand L, Tost J, Galineau J, et al. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ Int. 2018;118(May):334–47.
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007-2013. Environ Health Perspect. 2014, 122, 775–786. [CrossRef]
- Ye, Y.; Tang, Y.; Xiong, Y.; Feng, L.; Li, X. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J. 2019, 33, 2732–2742. [Google Scholar] [CrossRef]
- Tait, S.; Tassinari, R.; Maranghi, F.; Mantovani, A. Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J Appl Toxicol. 2015, 35, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J Matern Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, et al. Epigenetic matters: The link between early nutrition, microbiome, and long-term health development. Front Pediatr. 2017;5(August):1–14.
- Kinshella MLW, Omar S, Scherbinsky K, Vidler M, Magee LA, von Dadelszen P, et al. Maternal nutritional risk factors for pre-eclampsia incidence: findings from a narrative scoping review. Reprod Health. 2022, 19, 1–13.
- Trigg NA, Skerrett-Byrne DA, Xavier MJ, Zhou W, Anderson AL, Stanger SJ, et al. Acrylamide modulates the mouse epididymal proteome to drive alterations in the sperm small non-coding RNA profile and dysregulate embryo development. Cell Rep [Internet]. 2021, 37, 109787. [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol [Internet]. 2011, 31, 363–373. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol.
- Foidart, J.M.; Hustin, J.; Dubois, M.; Schaaps, J.P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int J Dev Biol. 1992, 36, 451–453. [Google Scholar]
- Kingdom JCP, Drewlo S. Is heparin a placental anticoagulant in high-risk pregnancies? Blood [Internet]. 2011, 118, 4780–4788. [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat Rev Mol Cell Biol [Internet]. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Muniyappa, R. Iantorno, M. Quon M. An Integrated View of Insulin Resistance and Endothelial Dysfunction. Endocrinol Metab Clin North Am. 2008, 37, 685. [CrossRef] [PubMed]
- Mandal, A.K.; Leask, M.P.; Estiverne, C.; Choi, H.K.; Merriman, T.R.; Mount, D.B. Genetic and Physiological Effects of Insulin on Human Urate Homeostasis. Front Physiol.
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. Diabetologia. 1981, 21, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef] [PubMed]
- Ma Q, Fan J, Wang J, Yang S, Cong Q, Wang R, et al. High levels of chorionic gonadotrophin attenuate insulin sensitivity and promote inflammation in adipocytes. J Mol Endocrinol. 2015, 54, 161–170. [CrossRef] [PubMed]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(SUPPL. 2).
- Sonagra, A.D. Normal Pregnancy- A State of Insulin Resistance. J Clin Diagnostic Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Zuo, Y.; Gao, J.; Liu, Y.; Zheng, W. The risk factors of gestational diabetes mellitus in patients with polycystic ovary syndrome: What should we care. Med (United States). 2021, 100, E26521. [Google Scholar] [CrossRef]
- Yan, Q.; Qiu, D.; Liu, X.; Xing, Q.; Liu, R.; Hu, Y. The incidence of gestational diabetes mellitus among women with polycystic ovary syndrome: a meta-analysis of longitudinal studies. BMC Pregnancy Childbirth [Internet]. 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat Rev Endocrinol [Internet]. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.M.B.; Tsai, J.H.; Moley, K.H. Obesity and PCOS: The effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci. 2015, 22, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Scalisi, N.M.; Milluzzo, A.; Ettore, G.; Vigneri, R.; Sciacca, L. Maternal Diabetes Impairs Insulin and IGF-1 Receptor Expression and Signaling in Human Placenta. Front Endocrinol (Lausanne). 2021;12(March):1–10.
- Abhari, F.R.; Ghanbari Andarieh, M.; Farokhfar, A.; Ahmady, S. Estimating rate of insulin resistance in patients with preeclampsia using Homa-IR index and comparison with nonpreeclampsia pregnant women. Biomed Res Int. 2014, 2014, 140851. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E. Regulation of the aromatase activity of human placental cytotrophoblasts by insulin, insulin-like growth factor-I, and -II. J Steroid Biochem Mol Biol. 1993;44(4–6):449–57.
- Lassance L, Haghiac M, Leahy P, Basu S, Minium J, Zhou J, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am J Obstet Gynecol [Internet]. 2015, 212, 647–e1. [CrossRef]
- Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, et al. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr [Internet]. 2016, 103, 1064–1072. [CrossRef]
- Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017, 158, 2543–2555.
- O’Tierney-Ginn, P.; Presley, L.; Myers, S.; Catalano, P. Placental growth response to maternal insulin in early pregnancy. J Clin Endocrinol Metab. 2015, 100, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Leoni, M.; Padilla, N.; Fabbri, A.; Della-Morte, D.; Ricordi, C.I.M. Mechanisms of Insulin Resistance during Pregnancy [Internet]. Evolving Concepts in Insulin Resistance. In: Marco Infante. University of Rome. Italy, editor. Evolving Concepts in Insulin Resistance [Internet]. Intech Open; 2022. p. Downloaded 29 November 2023. Available from: https://www.intechopen.com/chapters/83989.
- Gou, R.; Zhang, X. Glycolysis: A fork in the path of normal and pathological pregnancy. FASEB J. 2023, 37, 1–20. [Google Scholar] [CrossRef]
- Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001, 103, 1410–1415. [CrossRef]
- Lambert EA, Teede H, Sari CI, Jona E, Shorakae S, Woodington K, et al. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clin Endocrinol (Oxf). 2015, 83, 812–819. [CrossRef]
- Oncul M, Albayrak M, Sozer V, Karakus B, Gelisgen R, Karatas S, et al. Polycystic ovary syndrome and endothelial dysfunction: A potential role for soluble lectin-like oxidized low density lipoprotein receptor-1. Reprod Biol. 2020, 20, 396–401. [CrossRef] [PubMed]
- Chen, L.H.; Lin, C.P.; Wu, H.M.; Chu, P.H. Endothelial dysfunction in subfertile women with polycystic ovary syndrome. Reprod Biomed Online [Internet]. 2023, 46, 391–398. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G.; Kandarakis, S.A.; Chrousos, G.P. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007, 18, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Avagliano L, Bulfamante G Pietro, Morabito A, Marconi AM. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J Clin Pathol. 2011, 64, 1064–1068. [CrossRef] [PubMed]
- Hauguel-de Mouzon, S.; Guerre-Millo, M. The Placenta Cytokine Network and Inflammatory Signals. Placenta. 2006, 27, 794–798. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, F.; Liu, F.; Zhao, J.; Huang, X. Metaflammation in glucolipid metabolic disorders : Pathogenesis and treatment. Biomed Pharmacother [Internet]. 2023;161(March):114545. Available from: https://doi.org/10.1016/j.biopha.2023.114545. [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA) - A novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses [Internet]. 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Wolf, M.; Sandler, L.; Hsu, K.; Vossen-Smirnakis, K.; Ecker, J.L.; Thadhani, R. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003, 26, 819–824. [Google Scholar] [CrossRef]
- Du M, Basu A, Fu D, Wu M, Centola M, Jenkins AJ, et al. Serum inflammatory markers and preeclampsia in type 1 diabetes: A prospective study. Diabetes Care. 2013, 36, 2054–2061. [CrossRef]
- Parchim NF, Wang W, Iriyama T, Ashimi OA, Siddiqui AH, Blackwell S, et al. Neurokinin 3 receptor and phosphocholine transferase: Missing factors for pathogenesis of C-reactive protein in preeclampsia. Hypertension. 2015, 65, 430–439. [CrossRef]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Liu S, Hong L, Mo M, Xiao S, Chen C, Li Y, et al. Evaluation of endometrial immune status of polycystic ovary syndrome. J Reprod Immunol [Internet]. 2021;144(February):103282. Available from: https://doi.org/10.1016/j.jri.2021.103282. [CrossRef]
- Matteo M, Serviddio G, Massenzio F, Scillitani G, Castellana L, Picca G, et al. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil Steril [Internet]. 2010, 94, 2222-2227.e3. Available from: http://dx.doi.org/10.1016/j.fertnstert.2010.01.049. [CrossRef]
- Redman, C.W.G.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 4: I).
- Wilson, R.C.; Lo, J.O.; Romero Jimenez, G.; Lindner, J.R.; Slayden, O.D.; Roberts, V.H.J. Utilizing Contrast-Enhanced Ultrasonography with Phosphatidylserine Microbubbles to Detect Placental Inflammation in Rhesus Macaques. Molecules. 2023, 28, 2894. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Michael Nelson, D.; Desai, M.; Ross, M.G. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Francis, E.C.; Dabelea, D.; Boyle, K.E.; Jansson, T.; Perng, W. Maternal Diet Quality Is Associated with Placental Proteins in the Placental Insulin/Growth Factor, Environmental Stress, Inflammation, and MTOR Signaling Pathways: The Healthy Start ECHO Cohort. J Nutr. 2021, 152, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am J Physiol - Regul Integr Comp Physiol. 2016, 310, R87–93. [Google Scholar] [CrossRef] [PubMed]
- Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. The healthy eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J Nutr [Internet]. 2014, 144, 399–407. [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Herrock, O.; Deer, E.; LaMarca, B. Setting a stage: Inflammation during preeclampsia and postpartum. Front Physiol. 2023;14(February):1–12.
- Daan NMP, Louwers Y V., Koster MPH, Eijkemans MJC, De Rijke YB, Lentjes EWG, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: Who is really at risk? Fertil Steril [Internet] 2014, 102, 1444–1451. [CrossRef]
- Jeanes, Y.M.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr Res Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Shroff, R.; Syrop, C.H.; Davis, W.; Van Voorhis, B.J.; Dokras, A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007, 88, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Elenis, E.; Turkmen, S.; Kramer, M.S.; Yong, E.L.; Poromaa, I.S. Higher risk of type 2 diabetes in women with hyperandrogenic polycystic ovary syndrome. Fertil Steril. 2021, 116, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Mumm H, Jensen DM, Sørensen JA, Andersen LLT, Ravn P, Andersen M, et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. 2015, 94, 204–211. [CrossRef] [PubMed]
- de Wilde MA, Lamain-de Ruiter M, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, et al. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril [Internet]. 2017, 108, 333–340. [CrossRef] [PubMed]
- Naver K V., Grinsted J, Larsen SO, Hedley PL, Jørgensen FS, Christiansen M, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG An Int J Obstet Gynaecol. 2014, 121, 575–581. [CrossRef]
- Jiang R, Yao Y, Wang T, Li B, Jiang P, Wang F, et al. Preeclampsia in pregnant women with polycystic ovary syndrome: risk factor analysis based on a retrospective cohort study. Ginekol Pol. 2023;Oct 20:Epub ahead of print. PMID: 37861221.
- Palomba S, Russo T, Falbo A, Di Cello A, Amendola G, Mazza R, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: An experimental case-control study. J Clin Endocrinol Metab. 2012, 97, 2441–2449. [CrossRef] [PubMed]
- Palomba S, Russo T, Falbo A, Di Cello A, Tolino A, Tucci L, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013, 28, 2838–2847. [CrossRef]
- Palomba S, Falbo A, Chiossi G, Tolino A, Tucci L, La Sala GB, et al. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod Biomed Online [Internet]. 2014, 29, 370–381. [CrossRef] [PubMed]
- Sathish Kumar, Geoffrey H Gordon, David H Abbott, Mishra J. Androgens in maternal vascular and placental function: Implications for Preeclampsia Pathogenesis. Reproduction. 2018, 156, R155–67.
- Sathishkumar, K.; Balakrishnan, M.; Chinnathambi, V.; Chauhan, M.; Hankins, G.D.V.; Yallampalli, C. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. J Perinatol [Internet]. 2012, 32, 328–335. [Google Scholar] [CrossRef]
- Chinnathambi, V.; Balakrishnan, M.; Ramadoss, J.; Yallampalli, C.; Sathishkumar, K. Testosterone alters maternal vascular adaptations: Role of the endothelial no system. Hypertension. 2013, 61, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, et al. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014, 64, 405–414. [CrossRef] [PubMed]
- Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M, et al. Elevated Testosterone Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Rats. Hypertension. 2016, 67, 630–639. [CrossRef] [PubMed]
- Diamond, M.P.; Grainger, D.; Diamond, M.C.; Sherwin, R.S.; Defronzo, R.A. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998, 83, 4420–4425. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Gilling-Smith, C.; Watson, H.; Willis, D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999, 28, 361–378. [Google Scholar] [CrossRef] [PubMed]
- González, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012, 77, 300–305. [Google Scholar] [CrossRef]
- Kelley, A.S.; Smith, Y.R.; Padmanabhan, V. A Narrative Review of Placental Contribution to Adverse Pregnancy Outcomes in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019, 104, 5299–5315. [Google Scholar] [CrossRef]
- Kanbour, S.A.; Dobs, A.S. Hyperandrogenism in Women with Polycystic Ovarian Syndrome: Pathophysiology and Controversies. Androgens. 2022, 3, 22–30. [Google Scholar] [CrossRef]
- Piltonen TT, Ruokojärvi M, Karro H, Kujanpää L, Morin-Papunen L, Tapanainen JS, et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS One. 2019, 14, 1–12.
- Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018, 52, 186–195. [CrossRef]
- O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016, 214, 103–e1.
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016, 374, 13–22. [CrossRef] [PubMed]
- Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC, Sennström M, et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol. 2019, 53, 367–375. [CrossRef] [PubMed]
- Bian X, Biswas A, Huang X, Lee KJ, Li TKT, Masuyama H, et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women with Suspected Preeclampsia. Hypertension. 2019, 74, 164–172. [CrossRef] [PubMed]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol [Internet]. 2022, 226, S1108–19. [Google Scholar] [CrossRef] [PubMed]
- Panagodage S, Yong HEJ, Da Silva Costa F, Borg AJ, Kalionis B, Brennecke SP, et al. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am J Pathol [Internet]. 2016, 186, 3217–3224. [CrossRef] [PubMed]
- Li, C.; Raikwar, N.S.; Santillan, M.K.; Santillan, D.A.; Thomas, C.P. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta. 2015, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Khanabdali R, Shakouri-Motlagh A, Wilkinson S, Murthi P, Georgiou HM, Brennecke SP, et al. Low-dose aspirin treatment enhances the adhesion of preeclamptic decidual mesenchymal stem/stromal cells and reduces their production of pro-inflammatory cytokines. J Mol Med. 2018, 96, 1215–1225. [CrossRef]
- Tawfeek, M.; Hassan, M.; Ahmed, M.; Mohamed, N.; Ahmed, N. Low dose Aspirin with clomid in pco. Minia J Med Res. 2021, 32, 100–106. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Su, F.; Wang, M. Effectiveness and safety of aspirin combined with letrozole in the treatment of polycystic ovary syndrome: a systematic review and meta-analysis. Ann Palliat Med. 2021, 10, 4632–4641. [Google Scholar] [CrossRef]
- Aref, N.K.; Ahmed, W.A.S.; Ahmed, M.R.; Sedik, W.F. A new look at low-dose aspirin: Co-administration with tamoxifen in ovulation induction in anovulatory PCOS women. J Gynecol Obstet Hum Reprod [Internet]. 2019, 48, 673–675. [Google Scholar] [CrossRef]
- Zhao Y, Du B, Jiang X, Ma M, Shi L, Zhang Q, et al. Effects of combining lowdose aspirin with a Chinese patent medicine on follicular blood flow and pregnancy outcome. Mol Med Rep. 2014, 10, 2372–2376. [CrossRef] [PubMed]
- Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: Design and baseline characteristics. Paediatr Perinat Epidemiol. 2013, 27, 598–609. [CrossRef]
- Naimi AI, Perkins NJ, Sjaarda LA, Mumford SL, Platt RW, Silver RM, et al. The effect of preconception-initiated low-dose aspirin on human chorionic gonadotropin-detected pregnancy, pregnancy loss, and live birth: Per protocol analysis of a randomized trial. Ann Intern Med. 2021, 174, 595–601. [CrossRef] [PubMed]
- Mendoza M, Bonacina E, Garcia-Manau P, López M, Caamiña S, Vives À, et al. Aspirin Discontinuation at 24 to 28 Weeks’ Gestation in Pregnancies at High Risk of Preterm Preeclampsia: A Randomized Clinical Trial. Jama. 2023, 329, 542–550. [CrossRef] [PubMed]
- Bonacina E, Garcia-Manau P, López M, Caamiña S, Vives À, Lopez-Quesada E, et al. Mid-trimester uterine artery Doppler for aspirin discontinuation in pregnancies at high risk for preterm pre-eclampsia: Post-hoc analysis of StopPRE trial. BJOG An Int J Obstet Gynaecol. 2023, 00, 1–9.
- Hastie, R.; Tong, S.; Wikström, A.K.; Sandström, A.; Hesselman, S.; Bergman, L. Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population-based cohort study. Am J Obstet Gynecol [Internet]. 2021, 224, 95–e1. [Google Scholar] [CrossRef]
- Bedrick, B.S.; Eskew, A.M.; Chavarro, J.E.; Jungheim, E.S. Self-Administered Questionnaire to Screen for Polycystic Ovarian Syndrome. Women’s Heal Reports. 2020, 1, 566–573. [Google Scholar] [CrossRef]
- Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015, 6, 1–7.
- Lowe, S.A. Bowyer L, McMahon, LP. Morton, LP. North, RA. Paech, MJ. Said J. Guideline for the Management of Hypertensive Disorders of Pregnancy, 2014. Society of Obstetric Medicine of Australia and New Zealand. 2014.
- Ayala, D.E.; Ucieda, R.; Hermida, R.C. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260–79.
| Author (Year) | Odds Ratio (95% CI) 1 | Studies | Reference |
|---|---|---|---|
| Boomsma et al (2006) | 3.47 (1.95-6.17) | 8 | [27] |
| Kjerulff et al (2011) | 4.23 (2.77-6.46) | 12 | [28] |
| Qin et al (2013) | 3.28 (2.06-5.22) | 15 | [29] |
| Yu et al (2016) | 2.79 (2.29-3.38) | 25 | [30] |
| Khomami (2019) | 1.87 (1.55-2.25) | 26 | [10] |
| Pan (2021) Riestenberg (2022) Mousa (2023) |
2.07 (1.91-2.24) 2.03 (1.43-2.87) 2.28 (1.88-2.77) |
20 15 36 |
[50] [51] [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





