Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Xenotransplantation Models in HCT Research
3. Mechanism of HSC Homing: from Tail Vein to Bone Marrow
4. Role of Bone Marrow Niche in HSC Homing
5. Measurements of Engraftment in Mice Models
6. Clonal Hematopoiesis
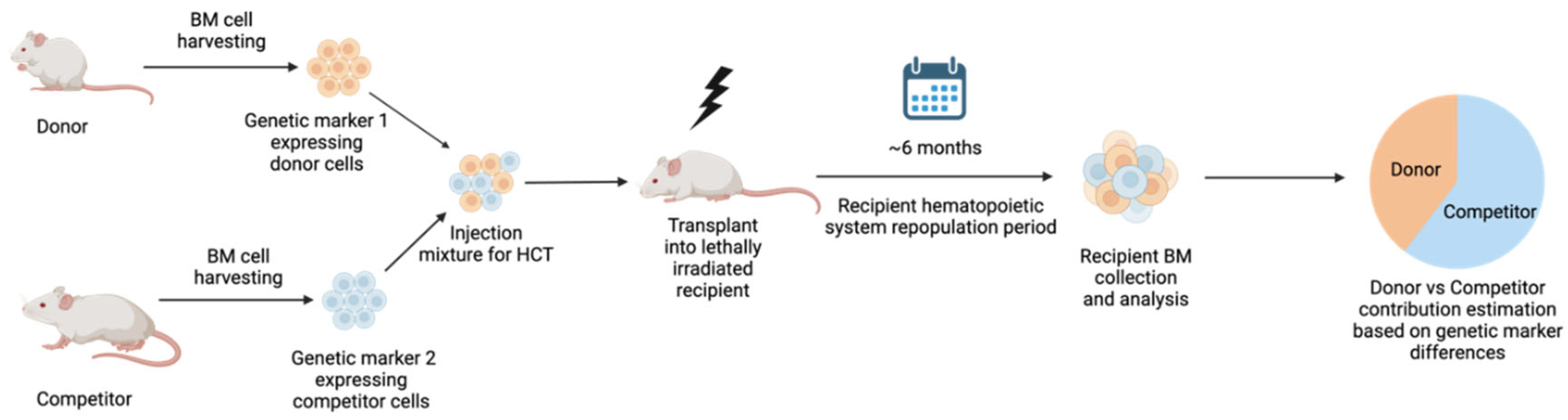
7. Competitive Repopulation
8. Long-Term Engraftment and its Influence on Leukemia Relapse
9. Strategies to Improve Engraftment
10. Conclusions
References
- Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174, 6477–6489 (2005). [CrossRef]
- Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106, 1565–1573 (2005). [CrossRef]
- Pearson, T. et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol 154, 270–284 (2008). [CrossRef]
- Hamilton, N., Sabroe, I. & Renshaw, S. A. A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models. F1000Res 7, 594 (2018). [CrossRef]
- Konantz, M., Müller, J. S. & Lengerke, C. Zebrafish Xenografts for the In Vivo Analysis of Healthy and Malignant Human Hematopoietic Cells. Methods Mol Biol 2017, 205–217 (2019). [CrossRef]
- Hopper, S. E. et al. Comparative Study of Human and Murine Aortic Biomechanics and Hemodynamics in Vascular Aging. Front Physiol 12, 746796 (2021). [CrossRef]
- Bjornson-Hooper, Z. B. et al. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates. Front Immunol 13, 867015 (2022). [CrossRef]
- Haas, S., Trumpp, A. & Milsom, M. D. Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell Stem Cell 22, 627–638 (2018). [CrossRef]
- Life expectancy at birth (years). https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years).
- Calvi, L. M. & Link, D. C. The hematopoietic stem cell niche in homeostasis and disease. Blood 126, 2443–2451 (2015). [CrossRef]
- Ratajczak, M. Z. & Suszynska, M. Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells. Stem Cell Rev Rep 12, 121–128 (2016). [CrossRef]
- Zhao, M. & Li, L. Dissecting the bone marrow HSC niches. Cell Research 2016 26:9 26, 975–976 (2016). [CrossRef]
- Bigas, A. & Espinosa, L. Hematopoietic stem cells: to be or Notch to be. Blood 119, 3226–3235 (2012). [CrossRef]
- Varnum-Finney, B. et al. Notch2 governs the rate of generation of mouse long-and short-term repopulating stem cells. J Clin Invest 121, (2011). [CrossRef]
- Maillard, I. et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2, 356–366 (2008). [CrossRef]
- Xiao, Y. et al. Current insights into the bone marrow niche: From biology in vivo to bioengineering ex vivo. Biomaterials 286, 121568 (2022). [CrossRef]
- Boulais, P. E. & Frenette, P. S. Making sense of hematopoietic stem cell niches. Blood 125, 2621–2629 (2015). [CrossRef]
- Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003). [CrossRef]
- Hosokawa, K. et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6, 194–198 (2010). [CrossRef]
- Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003 425:6960 425, 836–841 (2003). [CrossRef]
- Frenette, P. S., Subbarao, S., Mazo, I. B., Von Andrian, U. H. & Wagner, D. D. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A 95, 14423–14428 (1998). [CrossRef]
- Mazo, I. B. et al. Hematopoietic Progenitor Cell Rolling in Bone Marrow Microvessels: Parallel Contributions by Endothelial Selectins and Vascular Cell Adhesion Molecule 1. Journal of Experimental Medicine 188, 465–474 (1998). [CrossRef]
- Ibbotson, G. C. et al. Functional α4-integrin: A newly identified pathway of neutrophil recruitment in critically ill septic patients. Nature Medicine 2001 7:4 7, 465–470 (2001). [CrossRef]
- Peled, A. et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95, 3289–3296 (2000).
- Lapidot, T., Dar, A. & Kollet, O. How do stem cells find their way home? Blood 106, 1901–1910 (2005). [CrossRef]
- Plett, P., Frankovitz, S. M., Wolber, F. M., Abonour, R. & Orschell-Traycoff, C. M. Treatment of circulating CD34+ cells with SDF-1α or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential. Exp Hematol 30, 1061–1069 (2002). [CrossRef]
- Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity 25, 977–988 (2006). [CrossRef]
- Marchese, A. et al. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell 5, 709–722 (2003). [CrossRef]
- Rossi, L. et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 109, 533–542 (2007). [CrossRef]
- Adamiak, M. et al. Evidence for the involvement of sphingosine-1-phosphate in the homing and engraftment of hematopoietic stem cells to bone marrow. Oncotarget 6, 18819–18828 (2015). [CrossRef]
- Di Virgilio, F. et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97, 587–600 (2001). [CrossRef]
- Liles, W. C. et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 102, 2728–2730 (2003). [CrossRef]
- Onai, N. et al. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow–hematopoietic progenitor cells expressing SDF-1–intrakine. Blood 96, 2074–2080 (2000).
- NOTTA, F. et al. Isolation of Single Human Hematopoietic Stem Cells Capable of Long-Term Multilineage Engraftment. Science (American Association for the Advancement of Science) 333, 218–221 (2011). [CrossRef]
- Al-Amoodi, A. S. et al. Refining the migration and engraftment of short-term and long-term HSCs by enhancing homing-specific adhesion mechanisms. Blood Adv 6, 4373–4391 (2022). [CrossRef]
- Mathe et al 1965.
- Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013 502:7473 502, 637–643 (2013). [CrossRef]
- Chen, J., Hendriks, M., Chatzis, A., Ramasamy, S. K. & Kusumbe, A. P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. Journal of Bone and Mineral Research 35, 2103–2120 (2020). [CrossRef]
- Zhao, M. & Li, L. Dissecting the bone marrow HSC niches. Cell Research 2016 26:9 26, 975–976 (2016). [CrossRef]
- D, B., DJ, R. & IL, W. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169, 439–443 (2006). [CrossRef]
- Mazurier, F., Doedens, M., Gan, O. I. & Dick, J. E. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med 9, 959–963 (2003). [CrossRef]
- Al-Amoodi, A. S. et al. Refining the migration and engraftment of short-term and long-term HSCs by enhancing homing-specific adhesion mechanisms. Blood Adv 6, 4373–4391 (2022). [CrossRef]
- Uchida, N., Dykstra, B., Lyons, K. J., Leung, F. Y. K. & Eaves, C. J. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol 31, 1338–1347 (2003). [CrossRef]
- Dykstra, B. et al. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc Natl Acad Sci U S A 103, 8185 (2006). [CrossRef]
- Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, (2019). [CrossRef]
- Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371, 2477–2487 (2014). [CrossRef]
- Skead, K. et al. Interacting evolutionary pressures drive mutation dynamics and health outcomes in aging blood. Nat Commun 12, (2021). [CrossRef]
- Silver, A. J. & Jaiswal, S. Clonal hematopoiesis: Pre-cancer PLUS. Adv Cancer Res 141, 85–128 (2019). [CrossRef]
- Busque, L. et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet 44, 1179–1181 (2012). [CrossRef]
- Steensma, D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015). [CrossRef]
- Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20, 1472–1478 (2014). [CrossRef]
- Cho, R. H., Sieburg, H. B. & Muller-Sieburg, C. E. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561 (2008). [CrossRef]
- Dykstra, B. et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218–229 (2007). [CrossRef]
- Biasco, L. et al. In Vivo Tracking of Human Hematopoiesis Reveals Patterns of Clonal Dynamics during Early and Steady-State Reconstitution Phases. (2016). [CrossRef]
- Tothova, Z. et al. Multiplex CRISPR/Cas9-Based Genome Editing in Human Hematopoietic Stem Cells Models Clonal Hematopoiesis and Myeloid Neoplasia. Cell Stem Cell 21, 547-555.e8 (2017). [CrossRef]
- Jaiswal, S. et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 377, 111–121 (2017). [CrossRef]
- Yuan, N. et al. Young donor hematopoietic stem cells revitalize aged or damaged bone marrow niche by transdifferentiating into functional niche cells. Aging Cell 22, (2023). [CrossRef]
- Stewart, F., Crittenden, R., Lowry, P. & Pearson-White, S. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. (1993).
- Brecher, G., Ansell, J. D., Micklem, H. S., Tjio, J. H. & Cronkite, E. P. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc Natl Acad Sci U S A 79, 5085–5087 (1982). [CrossRef]
- Saxe, D., Boggs, S., hematology, D. B.-E. & 1984, undefined. Transplantation of chromosomally marked syngeneic marrow cells into mice not subjected to hematopoietic stem cell depletion. europepmc.orgDF Saxe, SS Boggs, DR BoggsExperimental hematology, 1984•europepmc.org.
- Shimoto, M., Sugiyama, T. & Nagasawa, T. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood 129, 2124–2131 (2017). [CrossRef]
- Sugiyama, T., Kohara, H., Noda, M., Immunity, T. N.- & 2006, undefined. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. cell.comT Sugiyama, H Kohara, M Noda, T NagasawaImmunity, 2006•cell.com. [CrossRef]
- Ding, L., Saunders, T., Enikolopov, G., Nature, S. M.- & 2012, undefined. Endothelial and perivascular cells maintain haematopoietic stem cells. nature.comL Ding, TL Saunders, G Enikolopov, SJ MorrisonNature, 2012•nature.com. [CrossRef]
- Zhang, B. et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 21, 577–592 (2012). [CrossRef]
- Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285–299 (2013). [CrossRef]
- Li, T. et al. Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Stem Cell Res Ther 12, (2021). [CrossRef]
- Arora, M. et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biology of Blood and Marrow Transplantation 10, 395–404 (2004). [CrossRef]
- Hu, L. et al. Antioxidant N-acetyl-L-cysteine increases engraftment of human hematopoietic stem cells in immune-deficient mice. Blood 124, e45–e48 (2014). [CrossRef]
- Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–1517 (2014). [CrossRef]
- Hu, Y. et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res 27, 2764–2772 (2021). [CrossRef]
- Ravandi, F. et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). 10.1200/JCO.2020.38.15_suppl.7508 38, 7508-7508 (2020). [CrossRef]
- Sheng, Y. et al. FOXM1 regulates leukemia stem cell quiescence and survival in MLL-rearranged AML. Nat Commun 11, (2020). [CrossRef]
- Zhao, C. et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779 (2009). [CrossRef]
- Agarwal, P. et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell 24, 769 (2019). [CrossRef]
- Borthakur, G. et al. Phase 1 study of combinatorial sorafenib, G-CSF, and plerixafor treatment in relapsed/refractory, FLT3-ITD-mutated acute myelogenous leukemia patients. Am J Hematol 95, 1296–1303 (2020). [CrossRef]
- Mori, T. et al. Phase 1 study of plerixafor in combination with total body irradiation-based myeloablative conditioning for allogeneic hematopoietic stem cell transplantation. Int J Hematol 113, 877–883 (2021). [CrossRef]
- Du, J., Yu, D., Han, X., Zhu, L. & Huang, Z. Comparison of Allogeneic Stem Cell Transplant and Autologous Stem Cell Transplant in Refractory or Relapsed Peripheral T-Cell Lymphoma: A Systematic Review and Meta-analysis. JAMA Netw Open 4, (2021). [CrossRef]
- Pidala, J. et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplant 48, 346–350 (2013). [CrossRef]
- Alotaibi, H. et al. Upfront Alternative Donor Transplant versus Immunosuppressive Therapy in Patients with Severe Aplastic Anemia Who Lack a Fully HLA-Matched Related Donor: Systematic Review and Meta-Analysis of Retrospective Studies, on Behalf of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Transplant Cell Ther 28, 105.e1-105.e7 (2022). [CrossRef]
- Cashen, A. et al. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant 14, 1253–1261 (2008). [CrossRef]
- Li, C. et al. Single-dose MGTA-145/plerixafor leads to efficient mobilization and in vivo transduction of HSCs with thalassemia correction in mice. Blood Adv 5, 1239 (2021). [CrossRef]
- Manesia, J. K. et al. AA2P-mediated DNA demethylation synergizes with stem cell agonists to promote expansion of hematopoietic stem cells. Cell reports methods 3, 100663 (2023). [CrossRef]
- Gao, J. et al. Enhanced in vivo motility of human umbilical cord blood hematopoietic stem/progenitor cells introduced via intra-bone marrow injection into xenotransplanted NOD/SCID mouse. Exp Hematol 37, 990–997 (2009). [CrossRef]
- Maganti, H. B. et al. Persistence of CRISPR/Cas9 gene edited hematopoietic stem cells following transplantation: A systematic review and meta-analysis of preclinical studies. Stem Cells Transl Med 10, 996–1007 (2021). [CrossRef]
- Ratajczak, M. Z. & Suszynska, M. Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells. Stem Cell Rev Rep 12, 121–128 (2016). [CrossRef]
- Janzen, V. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–426 (2006). [CrossRef]
- Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev 18, 306–319 (2004). [CrossRef]
- Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002). [CrossRef]
- Mantel, C. R. et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell 161, 1553–1565 (2015). [CrossRef]
- Capitano, M. L., Hangoc, G., Cooper, S. & Broxmeyer, H. E. Mild Heat Treatment Primes Human CD34(+) Cord Blood Cells for Migration Toward SDF-1α and Enhances Engraftment in an NSG Mouse Model. Stem Cells 33, 1975–1984 (2015). [CrossRef]
| Engraftment Factors | Function | Study model | Influence on engraftment | References |
| Stromal-cell-derived factor-1 (SDF-1) also known as CXCL12 | Chemokine isolated from stromal fibroblasts and abundantly expressed in BM. | NOD/LtSz-scid/scid (NOD/SCID) mice and MxCre-CXCR4f/null mice and C57BL/6 |
Actuate and promotes HSC maintenance and Improves engraftment | Lapidot, T. 2005 Plett, P. et al. 2002 Onai, N. et al. 2000 |
| Notch ligands | Signal through Jagged-1 generates short-term progenitor cells and long-term HSCs post-myeloablation, hindering myeloid differentiation | Transgenic Mice: Mx-Cre+ × ROSADNMAML/+ mice and C57BL/6 (B6, CD45.2+) and (B6-SJL, CD45.1+) |
Support HSC self-renewal and improves engraftment |
Varnum-Finney, B. et al. 2011 Maillard, I. et al. 2008 |
| Lepr and nestin+ reticular cells | Associated with the regulation of HSC quiescence and proliferation | Transgenic Mice: Tie2-cre and leptin receptor (LepR)-cre mice and Col1-caPPR mice |
Improves HSC frequency in the bone marrow | Xiao, Y. et al. 2022 Boulais, P. E. et al. 2015 |
| N-cadherin | Osteoblast direct interactions via N-cadherin-mediated adhesion support HSC function | Transgenic mice: Scl-tTA::TRE-BCR/ABL (BA) double-transgenic mouse - CML | Positively Regulates HSCs in BM niche | Hosokawa, K et al. 2010 Schepers, K et al 2013 |
| Osteopontin, angiopoietin-1, and thrombopoietin | Activated osteoblasts can produce osteopontin, angiopoietin-1, and thrombopoietin, which limit HSC expansion and contribute to HSC quiescence | Transgenic mice: Mx1-Cre+Bmpr1afx mutant mice |
Shown to positively impact HSC regulation | Hosokawa, K. et al. 2010 |
| Intercellular adhesion molecule-1 (ICAM-1) | Play a role in homing through mediating cellular adhesion interaction | Transgenic mice: C57BL/6 and 129S strains P/E-/- (C57/Bl6J×129S) Mice lacking the two selectins (P and E-) |
Positively Regulates HSCs in BM niche |
Frenette, P. S. et al. 1998 |
| Vascular cell adhesion molecule-1 (VCAM-1) | Play a role in homing through mediating rolling and firm adhesion of HPC in BM | Transgenic mice: C57/Bl6J×129S P/E-/- |
Positively Regulates HSCs in BM niche |
Mazo, I. B. et al. 1998 |
| α4β1/VLA-4 integrin and lectins | Primary roles in HSC attachment to marrow stromal cells | NOD/SCID | HSC homing by enabling attachment to the vascular endothelium | Peled et al 2000 |
| Adenosine triphosphate (ATP) and uridine triphosphate (UTP) | Extracellular nucleotide (eNTPs). act as potent chemotactic factors in modulating HSC migration in the presence of SDF-1 | NOD/SCID | UTP and ATP (to a lesser extent) modulate HSC motility and homing to BM niche | Rossi, L. et al. 2007 |
| Sphingosine-1-phosphate (S1P) | Extracellular nucleotide (eNTPs). act as potent chemotactic factors in modulating HSC migration in the presence of SDF-1 | Transgenic: B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J | Homing of HSPC | Adamiak, M. et al. 2015 |
| N-acetyl-L-cysteine (NAC) | Shown to restore the health of BM microenvironment | NOD/SCID and NSG mice | Increase in human HSC engraftment and multilineage hematopoietic differentiation | Hu, L. et al 2014 |
| TGF-B1, TGF-B2, and SLIT2 | TGF-B2 promote myeloid differentiation and TGF-B1/SLIT2 are HSC retention factors, all Support HSC function | BCR/ABL (BA) mice | Regulate quiescence and self-renewal of HSCs | Schepers, K et al 2013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




